15 Pacing in Neurally Mediated Syncope Syndromes
Syncope is the transient loss of consciousness with subsequent complete resolution and without focal neurologic deficits, resulting from cerebral hypoperfusion, and not requiring specific resuscitative measures. The neurally mediated syncope syndromes are a collection of clinical disorders of heart rate and blood pressure regulation, caused by autonomic reflexes.1,2 These often include bradycardia, which has led to attempts to use cardiac pacing as a therapy for carotid sinus syncope and vasovagal syncope, the most common of the neurally mediated syncopes.
There are now several expert consensus conferences and position papers on these syndromes.3,4 This chapter reviews recent progress in determining the usefulness of pacemakers in the neurally mediated syncope syndromes.*
 Carotid Sinus Syncope
Carotid Sinus Syncope
Clinical Perspective
Carotid sinus syncope (CSS) is a syndrome of syncope associated with a consistent clinical history, carotid sinus hypersensitivity, and the absence of other potential causes of syncope. Historical features that suggest the diagnosis are syncope or presyncope occurring with carotid sinus stimulation that reproduces clinical symptoms, or fortuitous Holter monitoring or other documentation of asystole during syncope following maneuvers that could presumably stimulate the carotid sinus.5–9 The incidence of carotid sinus syncope is low, about 35 per 1 million population per year.10 CSS occurs in older patients, mainly in men. It tends to occur abruptly, with minimal prodrome, and only half of patients may recognize a precipitating event. These events typically include wearing tight collars, shaving, head turning (as in looking to the back in a car), coughing, heavy lifting, and looking up.
Symptoms of CSS range from mild presyncope to profound loss of consciousness, occasionally with significant injuries. Some patients may not recall losing consciousness, instead presenting with unexplained falls. In Great Britain, fits, faints, and falls are often investigated in an integrated setting with a comprehensive clinical pathway. Elderly patients with unexplained falls may have positive carotid sinus massage (CSM) responses, suggesting that carotid sinus syncope is responsible for many unexplained or recurrent falls.11,12 However, physiologic carotid sinus hypersensitivity is much more common than carotid sinus syncope, and care should be taken in the interpretation of these results.
Natural History
Little is known about the natural history of CSS. Even though it may have a substantial effect on quality of life, CSS has not been shown to affect mortality significantly, and patients who receive therapy do not appear to have worse prognoses than the general population. Even in the absence of pacing, only 25% of patients may have a syncope recurrence.13,14
Rationale for Pacing
Table 15-1 lists recommendations and other considerations in pacing for CSS.
TABLE 15-1 Summary of Pacing for Carotid Sinus Syncope (CSS)
| Factor | Description |
|---|---|
| Goal | Prevent reflex bradycardia and compensate for reflex hypotension |
| Prevent syncope | |
| Level of evidence for success | Observational studies and open-label, randomized controlled trials (RCTs) |
| No double-blind studies | |
| Consensus recommendations | Class I: Recurrent syncope, with syncope induced by carotid sinus massage |
| Class IIa: Recurrent syncope, with profound bradycardia induced by carotid sinus massage | |
| Patient selection | Syncope and positive carotid sinus massage |
| Programming considerations | Atrioventricular sequential pacing |
Physiology
The carotid sinus reflex is an integral component of the homeostatic mechanisms of blood pressure regulation.15 Increases in intrasinus pressure stimulate mechanoreceptors, which participate in an afferent arc terminating in the brainstem. The efferent arc travels to peripheral end organs through vagal efferents, which augment cardiac vagal input and slow heart rate, and through the spinal cord to inhibit peripheral sympathetic activity in skeletal vasculature, resulting in peripheral vasodilatation. This reflex maintains blood pressure within a narrow range.
An abnormal carotid sinus reflex can cause exaggerated responses of heart rate and blood pressure. Some evidence suggests that the major defect in carotid sinus hypersensitivity does not reside in the carotid sinus or in its neural efferents,16,17 or in the brainstem. Rather, the neuromuscular structures surrounding the carotid sinus may be involved in CSS. Blanc et al.18 found similar results in 30 patients without known carotid sinus hypersensitivity or syncope. Abnormal sternocleidomastoid electromyograms were associated with abnormal responses to CSM. Because the denervated sternocleidomastoid muscle cannot provide or contribute information to the central nervous system baroreflex centers, any output from the carotid sinus is inappropriately interpreted as increased blood pressure.
Other Therapies
When CSS is the likely cause of syncopal episodes, the initial treatment recommendation should be simple elimination of any recognized maneuvers that may precipitate an event. Discontinuation of wearing tight collars and ties and shaving more carefully may help. Hypovolemia should be corrected. The addition of high salt intake, volume expanders such as fludrocortisone acetate (Florinef),19 or oral vasopressors such as midodrine (ProAmatine) may be helpful, but these interventions are frequently limited in older patients by comorbidities (e.g., hypertension, heart failure).
In the pre–pacemaker era, recalcitrant cases of carotid sinus syncope were treated with carotid sinus denervation by surgical technique.20 Surgical sinus denervation currently is reserved for cases secondary to head or neck tumors or lymphadenopathy, or it is performed in conjunction with carotid endarterectomy or in patients with severe refractory carotid sinus syncope of the purely vasodepressor type.
Evidence of Clinical Benefit
Observational Studies
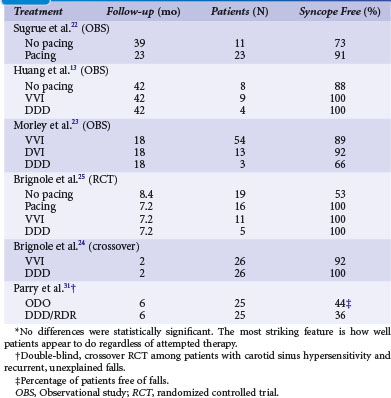
Table 15-2 summarizes studies of pacing for CSS.13,21–25 Earlier studies tended to be retrospective reports of pacing practices for CSS and therefore were inherently biased toward patients with a clear diagnosis of CSS who would truly benefit from pacing.
Randomized Trials
More recently, prospective, randomized trials have examined outcomes on the basis of presence of pacing and mode. A prospective, randomized Italian trial reaffirmed the important role of permanent pacing; 60 patients with CSS were randomized to pacing (32) or no pacing (28) therapy.14 During a follow-up of about 3 years, syncope recurred in 16 patients of the no-pacing group (51%) and in three (9%) of the pacing group (P = .002). This finding somewhat confirms the usefulness of pacing for the prevention of carotid sinus syncope, although potent placebo effects cannot be ruled out (see later).
Falls
Pacing of patients with CSS has been associated with a reduction in falls.26 Furthermore, among patients with unexplained and recurrent falls, carotid sinus hypersensitivity may be an important risk factor.27,28 In 2001, Kenny et al.29 reported the open-label SAFE PACE trial, designed to determine whether cardiac pacing reduces falls in older patients with unexplained falls and a cardioinhibitory response to CSM. The 187 patients were randomized to receive a dual-chamber pacemaker, with rate-drop responsiveness, or no intervention. Patients who received a pacemaker had a highly significant 58% reduction in falls and a 40% reduction in syncope. Although these results suggest that many unexplained falls in the elderly are caused by carotid sinus syncope, and that these can be prevented with pacing, one must remember that this was an open-label trial.
In 2010, Ryan et al.30 reported the SAFEPACE-2 study, in which 141 older patients with unexplained falls and cardioinhibitory carotid sinus hypersensitivity were randomized to receive a rate-drop responsive dual-chamber pacemaker or an implantable loop recorder (ILR). Although the relative risk (RR) of reporting a fall after implantation of a device decreased (0.23; 95% confidence interval [CI] = 0.15 to 0.37), there were no significant differences in falls reported between the paced group (67%) and the ILR group (53%) (difference RR = 1.25; 95% CI = 0.93 to 1.67). These results are at odds with the findings in SAFE PACE; possibly because of differences in patient population (patients were older and frailer). Furthermore, due to recruitment difficulties, SAFEPACE-2 may have been underpowered; according to sample size calculations, 226 patients were needed to detect a 20% difference with 80% power.
Only one double-blind, placebo-controlled crossover trial has reported pacing in patients with recurrent, unexplained falls and carotid sinus hypersensitivity.31 All study participants received a dual-chamber pacemaker with rate-drop response programmer and were randomized to DDD/RDR mode (on) versus ODO mode (off); after 6 months, patients switched to the opposite mode. Only 25 of the 34 recruited patients completed the study. The total number of falls decreased after pacemaker implantation, but risk of falling with the pacemaker on versus off was not statistically significant (RR = 0.82; 95% CI = 0.62 to 1.10). Although this suggests a placebo effect, similar to that seen in pacing of vasovagal syncope patients, the results must be interpreted with caution; the study was underpowered because of an unexpectedly high attrition rate of 26%.
Patient Selection
Careful patient selection may help provide effective and efficient therapy for CSS. Permanent pacemaker therapy is indicated for patients with recurrent, frequent, or severe CSS, particularly for predominant cardioinhibitory syncope.32,33 Predictors of success with permanent pacing include multiple episodes before implantation; episodes that occur while upright or sitting; and episodes preceded by a recognized stimulus.34 Syncope recurrence after implantation of a permanent pacemaker may be caused by a prominent vasodepressor component.
Physical Diagnosis with Carotid Sinus Massage
The carotid sinus is located high in the neck below the angle of the mandible. Carotid massage is contraindicated in the presence of bruits or a history of cerebrovascular disease, transient ischemic attacks (TIAs), or endarterectomy. Sequential application of CSM to the left and right carotid arteries should be performed with at least 10 to 20 seconds between applications. The duration of carotid massage should be 5 to 10 seconds, and it should be terminated with the onset of characteristic asystole or severe presyncope. In most series, the predominant responses to CSM were obtained on the right side.5 CSM should be performed while the patient is both supine and upright, either sitting or while secured safely on a tilt table. It may be difficult to document transient hypotension with standard sphygmomanometric methods, and noninvasive continuous digital plethysmography is often used.
Physiologic Responses
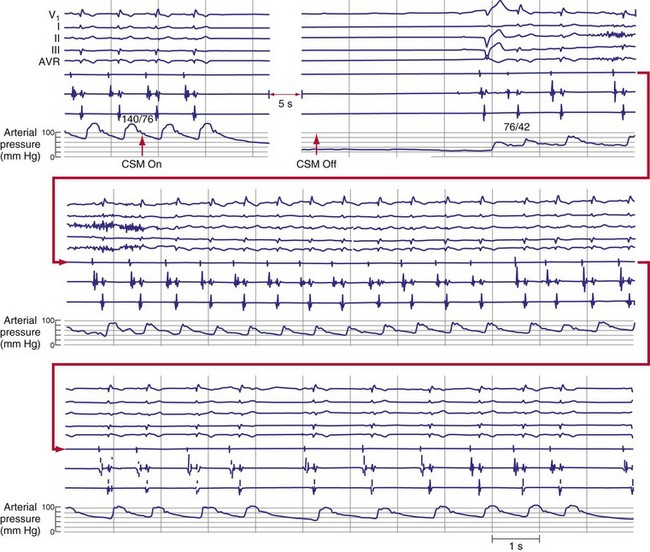
Carotid sinus massage elicits both cardioinhibitory and vasodepressor responses (Fig. 15-1). A cardioinhibitory response to CSM is defined as 3 seconds or longer of ventricular standstill or asystole. Ventricular asystole usually results from a sinus pause caused by sinus node exit block,35 but it can result from atrioventricular (AV) block as well. A vasodepressor response to CSM is defined as a drop in systolic blood pressure of 50 mm Hg or more during massage; this may be difficult to demonstrate in patients who have a significant cardioinhibitory component. In contrast to the induced cardioinhibitory component of carotid sinus hypersensitivity, the vasodepressor response may have a slower, more insidious onset and a more prolonged resolution.
Carotid Sinus Hypersensitivity and Carotid Sinus Syncope
Carotid sinus hypersensitivity denotes abnormal physiologic responses to CSM, either cardioinhibitory or vasodepressor (or both). The presence of asymptomatic carotid sinus hypersensitivity is quite common in older adults. For example, a positive cardioinhibitory response to CSM was noted in 32% of patients undergoing coronary angiography.36 CSS is the syndrome of syncope in association with carotid sinus hypersensitivity, and in the absence of other apparent causes of syncope.
Complications
Carotid sinus massage is safe if done carefully. CSM is contraindicated in patients with a history of cerebrovascular disease or carotid bruits, because it can cause cerebrovascular accident (CVA, stroke). In a review of 3100 episodes of CSM performed on 1600 patients, the seven complications (0.14%) were neurologic and transient.37 In another review of CSM on 4000 patients, complications were observed in 11 patients (0.28%);38 all were neurologic. After 1 month, nine patients had made a full recovery; neurologic symptoms persisted in two patients. Rare, arrhythmic complications include asystole and ventricular fibrillation.39
Programming
Pacing in AAI mode is contraindicated because many patients may eventually demonstrate associated reflex AV block.40 In general, patients appear to benefit most from AV sequential pacing, even when a significant component of vasodepressor CSS is present. VVI pacing should not be used in patients with intact ventriculoatrial (VA) conduction,41 because of possible pacemaker syndrome. Lack of VA conduction at a given time, however, does not ensure against its future development. Therefore, we recommend dual-chamber pacemakers for patients with CSS and normal sinus rhythm.
Few studies have examined the role of rate-responsive pacing in CSS. Patients are generally older and therefore may have bradycardic comorbidities such as sick sinus syndrome or chronotropic incompetence, either intrinsic or pharmacologic. Therefore, rate-responsive pacing might be beneficial. Similarly, few studies have prospectively examined pacing with rate-drop or hysteresis capabilities, which has the theoretical advantage of providing rapid, higher-rate AV sequential pacing to counteract the vasodepressor component during CSS attacks.42
 Vasovagal Syncope
Vasovagal Syncope
Clinical Perspective
Vasovagal syncope is the most common of the neurally mediated syncopal syndromes. Most people who faint probably do not seek medical attention for isolated events.2 Prolonged standing, sight of blood, pain, and fear are common precipitating stimuli for this, the common faint. Patients develop nausea, diaphoresis, pallor, and loss of consciousness from hypotension with or without significant bradycardia. Return to consciousness typically occurs after seconds or 1 to 2 minutes. Those with adequate warning may be able to use physical counterpressure maneuvers, or simply sit or lie down, to prevent a full faint. However, some patients have little or no prodrome, no recognized precipitating stimulus, or marked bradycardia accompanying the faint.43–47 These patients have sparked interest in permanent pacing as a therapy.
Epidemiology
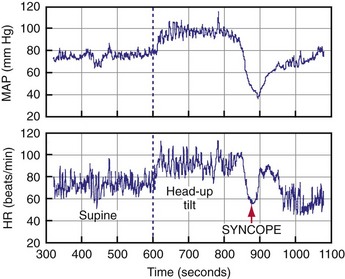
About 40% of people faint at least once in their life, and at least 20% of adults faint more than once.48,49 Fainters usually present first in their teenage years and 20s and may faint sporadically for decades. This long, usually benign, and sporadic history can make for difficult decisions about therapy. Syncope is responsible for 1% to 6% of emergency room visits and 1% to 3% of hospital admissions.50–52 Tilt tests are often used as a diagnostic tool, although they are limited by difficulties with sensitivity, specificity, and reproducibility, and with little evidence-based agreement on methodologic details and outcome criteria. Positive tilt tests are characterized by presyncope, syncope, bradycardia, and hypotension, as well as a reproduction of the patient’s perisyncopal symptoms53,54 (Fig. 15-2).
Symptom Burden and Quality of Life
The vasovagal syncope syndrome has an extremely wide range of symptoms. The symptom burden varies from a single syncopal spell in a lifetime to daily faints. Some patients have very sporadic presentations, with periods of intense symptoms interspersed with long periods of quiescence. Other patients faint at more regular intervals, although they may be months or years apart. Several observational studies and randomized clinical trials reported that patients have a median of 5 to 15 syncopal spells, with fainting episodes occurring over 2 to 60 years.55–58 Patients with recurrent syncope are impaired similar to those with severe rheumatoid arthritis or chronic low back pain and psychiatric inpatients.55 The quality of life decreases as the frequency of syncopal spells increases.56
After clinical assessment, many patients continue to do poorly. After 1, 2, and 3 years, 28%, 38%, and 49% of patients faint again, respectively.59 Interestingly, several groups reported a 90% reduction in the total number of faints in this patient population after the tilt test. The reason for this apparently great reduction in syncope frequency after assessment is unknown, but it does lead to a large number of patients who request further treatment. Therefore, when assessing syncope patients, clinicians need to be alert to the surprising impairment of quality of life that many patients endure, to provide a perspective that lasts decades, and to remember that the patient’s clinical state will probably fluctuate.
Rationale for Pacing
Table 15-3 lists recommendations and other considerations in pacing for vasovagal syncope.
| Factor | Description |
|---|---|
| Goal | Prevent reflex bradycardia and compensate for reflex hypotension |
| Prevent syncope | |
| Level of evidence for success | Limited evidence for benefit based on double-blind RCTs |
| May be a subset of patients with proved bradycardia who benefit | |
| Consensus recommendations | Class IIa: Recurrent vasovagal syncope with clinically documented bradycardia, or bradycardia induced on tilt test |
| Patient selection | Medically refractory, frequent, disabling vasovagal syncope |
| Documented pauses during syncope | |
| Tilt test results not helpful | |
| Programming considerations | Dual-chamber pacemaker |
| Benefit from specific sensor to drive rate-response or pacing algorithm (rate-drop response, ventricular impedance) not proved |
Physiology
Syncope is a transient loss of neurologic function caused by a global reduction of cerebral blood flow (CBF). Sudden cessation of CBF results in loss of consciousness within 4 to 10 seconds.60 Lesser CBF reductions may result in presyncope. Almost all vasovagal syncope occurs while the patient is in an upright position. Syncope is usually associated with heightened physiologic or psychological stress, such as prolonged orthostatic stress; arising quickly and walking; pain, fear, emotion, or seeing blood or medical procedures; and strenuous exercise.
Evidence for Bradycardia
Tilt-Table Tests
Bradycardia frequently occurs during vasovagal syncope induced by tilt-table testing.61,62 The mean heart rate during syncope induced by passive head-up tilt tests is 30 beats/min (bpm), and asystole longer than 3 seconds is often documented. However, uncertainty surrounds the relationship between the hemodynamics of tilt testing and clinical vasovagal syncope. For example, the ISSUE investigators found no relationship between the heart rate during syncope on tilt testing and during syncope in the community population. Patients with tilt test–induced bradycardia frequently do not have bradycardia during clinical syncope.63,64 Therefore, although bradycardia is the rule rather than the exception during a positive tilt test, the bradycardia evoked on a tilt test may not resemble the hemodynamics during syncope in that patient in the community.
Pacemaker Memory
Is asystole in patients in the community frequently associated with syncope? Evaluation of frequent fainters with pacemakers programmed to act as event recorders demonstrated that while transient asystole is common during documented syncope, many other asymptomatic asystolic episodes also occurred. Only 0.7% of asystolic events lasting 3 to 6 seconds and 43% of events lasting longer than 6 seconds resulted in symptoms of presyncope or syncope.65 Therefore, even asystole of several seconds’ duration does not necessarily cause syncope.
Implantable Recorders
The ILR permits prolonged electrocardiographic monitoring and is a reasonable approach to diagnosing patients with infrequent syncope. Current ILRs weigh only 17 grams and have battery life of 14 months. The ECG signal is stored in a buffer that can be frozen with a manual activator. The ILR has programmable, automatic detection parameters for high and low rates and pause. In a Canadian study of 206 patients, symptoms recurred in 69% of patients. Bradycardia was detected more frequently than tachycardia (17% vs. 6%).66,67
The European International Study on Syncope of Uncertain Aetiology (ISSUE) enrolled 111 patients with syncope and previous tilt-table testing; not all tilt tests were positive.68 Patients with positive or with negative tilt tests both had events in 34% of each group over a follow-up of 3 to 15 months. Marked sinus bradycardia (46%) or asystole (62%) were detected during syncope. Therefore, bradycardia reported during syncope varies widely; 17% to 62% of patients with vasovagal syncope had significant bradycardia during syncope in the ILR studies. The heart rate response during tilt testing did not predict spontaneous heart rate response, with more frequent asystole than expected based on tilt response. Thus, many patients with positive tilt tests may develop some degree of bradycardia at presyncope or syncope, and pacing may be a plausible treatment.
Conservative Therapy
Pacing should be tried only in patients with vasovagal syncope who have not responded to, or who are not candidates for, other treatments. Currently, most clinicians first teach patients about the causes of syncope, encourage fluid and salt intake, and coach physical counterpressure maneuvers. If this initial approach is unsuccessful, pharmacologic therapy is used (fludrocortisone, midodrine, β-blockers, SSRIs). Only after these options have been explored should permanent pacing be considered (Table 15-4).
| Area | Intervention |
|---|---|
| Diagnosis and prognosis | Confirm diagnosis with history, tilt-table tests, and loop recorder. |
| Assess likelihood of syncope recurrence (>2 spells or recent worsening). | |
| Assessment of patient needs | Insight into diagnosis |
| Cause of syncope | |
| Probability of syncope recurrences | |
| Treatment options | |
| Conservative advice | Maximizing salt and fluid intake |
| Physical counterpressure maneuvers | |
| Driving and reporting to authorities | |
| Avoidance and management of triggers | |
| Medical options | Fludrocortisone (weak evidence) |
| Midodrine (good evidence) | |
| Serotonin reuptake inhibitors (weak evidence) | |
| β-Blockers in patients age >42 (modest evidence) | |
| Permanent pacing | Weak evidence |
Salt and Fluids
Blood volume is an important factor in the pathophysiology of vasovagal syncope. Syncope almost only occurs in the upright position, and many patients faint only from orthostatic stress in situations such as attending religious services, parades, or outdoor events and taking showers. Prolonged drug-free head-up tilt provokes syncope in a large number of syncope patients; this depends on the duration and angle of head-up tilt.69 Finally, many patients report avoiding dietary salt and have low daily urinary sodium excretion.70
Physical Counterpressure Maneuvers
Physical counterpressure maneuvers may be quite helpful, although no blinded, controlled studies have been reported. Patients must have a prodrome long enough that they can react by isometrically tightening muscles using maneuvers such as squatting, leg crossing, and fist clenching. Van Dijk et al.71 reported the Physical Counterpressure Maneuvers Trial, a multicenter, randomized clinical trial to evaluate the effectiveness of physical counterpressure in preventing syncope recurrence. A total of 223 patients were randomized to receive optimal conventional therapy (lifestyle changes; e.g., avoiding triggers, increasing salt intake) with or without additional training in physical counterpressure. During the follow-up of up to 18 months, 50.9% of conventional therapy patients and 31.6% of conventional therapy with counterpressure patients experienced syncope recurrence, a relative risk reduction of 0.36 (95% CI = 0.11 to 0.53). These results provide sufficient evidence to support including the low-risk, low-cost therapy of physical counterpressure in the recommended first-line treatment of vasovagal syncope.
Medical Therapy
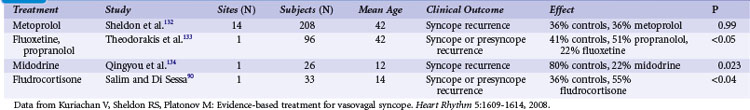
Table 15-5 summarizes major randomized clinical trials of treatment for vasovagal syncope. No therapies have proved effective in large, randomized clinical trials in preventing vasovagal syncope. Few have been subjected to rigorous clinical trials, and when interpreting open-label studies, one should remember that most patients appear to improve after assessment. There is an estimated 90% reduction in syncope in the population after tilt testing.72 The four major drug classes used are α1-adrenergic agonists, β-blockers, selective serotonin reuptake inhibitors (SSRIs), and salt-retaining mineralocorticoids.
Vasopressors (Midodrone)
Reasonably good evidence exists for the effectiveness of the α1-adrenergic agonist midodrine (ProAmatine). Midodrine, a prodrug, reduced symptoms of syncope and presyncope in three small, randomized clinical trials;73–75 overall, midodrine is probably helpful.76 The major limitations of midodrine are the need for frequent dosing and its tendency to increase supine blood pressure. The latter side effect is usually seen at higher doses (>30 mg/day). Midodrine should not be used in patients with hypertension. Side effects include piloerection and “crawling” paresthesias in the scalp.
Selective Serotonin Reuptake Inhibitors
Numerous small, open-label studies in the early 1990s reported that SSRIs prevented the induction of syncope on tilt tests and reduced symptoms in patients in the community. Paroxetine was effective in preventing syncope77 in one randomized placebo-controlled study and numerous case-report series. In contrast, Takata et al.78 reported that the same drug did not block the vasovagal reaction elicited by lower-body negative pressure. SSRIs do not appear to be used widely for the prevention of syncope, and the treatment effect is debatable.76
Beta-Adrenergic Blockers
The evidence for the effectiveness of β-blocker therapy is mixed. It has a strong physiologic rationale, and two positive and one negative open-label studies involving 42 to 153 patients.79–81 Five randomized clinical trials studied the efficacy or effectiveness of β-blockers for the prevention of syncope.82–86 Although not completely consistent, these studies indicate that metoprolol and atenolol, and possibly β-adrenergic receptor blockade in general, are ineffective in preventing vasovagal syncope in the broad patient population. A substudy showed a possible benefit in middle-aged and elderly patients.
Fludrocortisone Acetate
Fludrocortisone has mineralocorticoid activity without appreciable glucocorticoid effect at doses up to 0.2 mg, the usual clinical doses for various disorders.87 The acute actions of fludrocortisone acetate are sodium and water retention, at the expense of urinary potassium excretion. Two open-label trials examined fludrocortisone in patients with “neurocardiogenic syncope.” Both revealed clinical improvement but neither were placebo-controlled.88,89 Recently, Salim and Di Sessa90 reported a small, placebo-controlled, randomized clinical trial (RCT) of fludrocortisone (Florinef) in children with vasovagal syncope. Patients taking fludrocortisone did significantly worse (P < .04) than those taking placebo. An RCT powered to detect a 40% relative risk reduction is nearing conclusion.91 Other drugs have been studied, but not in adequately powered, placebo RCTs.
Evidence of Clinical Benefit
Four groups assessed the effect of pacing in preventing syncope induced by tilt-table testing (Table 15-6). Forty-one patients with a positive initial tilt test and a marked bradycardia underwent a second tilt test with temporary pacing at rates of 85 to 100 bpm. Taken together,61,62,65,90,92–96 the studies showed that temporary dual-chamber pacing prevented the development of syncope in 24 of the 41 patients (57%).92–94 However, almost all the conscious patients developed the vasovagal reaction and had significant presyncope. Temporary pacing may be partly effective in preventing vasovagal syncope, but it does not prevent presyncope.
Observational Studies
Three groups reported studies of the usefulness of chronic pacing in the prevention of vasovagal syncope (see Table 15-6). Petersen et al.97 reported the first clinical study of dual-chamber pacing with rate hysteresis in 37 syncope patients. The patients had had a median of six syncopal spells, and a positive tilt test with bradycardia. Of the 37 patients, 31 received pacemakers with rate hysteresis. Over a mean follow-up of 50 months 62% of the patients remained free of syncope, and the number of syncopal spells in the total population fell from an expected number of 136 to only 11.
Benditt et al.98 reported equally encouraging results in a study of 36 patients with predominantly vasovagal syncope. The patients were very symptomatic, with a median of 10 syncopal spells over about 2 years, or about five spells annually. All patients received a pacemaker with rate-drop responsiveness. The patients were followed for a mean of 6 months. During this time, syncope recurred in only six patients, compared to expected recurrences in about 30 patients. Therefore, in this relatively short-term study, pacing may have benefited about 80% of patients.
We studied 12 extremely symptomatic patients who had had a median syncope frequency of three spells monthly.99 All had a positive tilt test and recurrent syncope while receiving medical therapy. All received a pacemaker with a rate-smoothing feature but without a high rate response. Following implantation of the pacemaker, the actuarial syncope-free survival increased 20-fold, the syncope frequency dropped by 93%, and quality of life improved highly significantly.
Open-Label Randomized Studies of Rate-Drop Responsiveness
North American Vasovagal Pacemaker Study
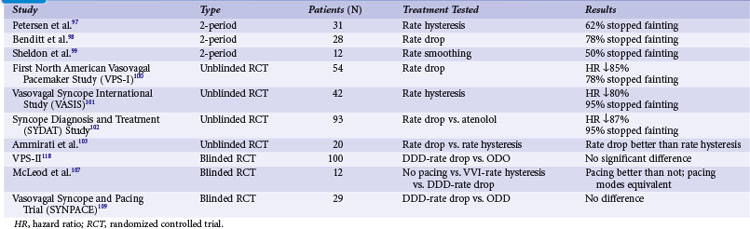
The VPS-I Study tested whether permanent pacing with rate-drop responsiveness would reduce the likelihood of syncope in patients with frequent vasovagal syncope.100 Patients were eligible if they had fainted six or more times before tilt testing, or fainted within the first year after a positive tilt test, and had a predefined degree of bradycardia. Fifty-four patients were randomized evenly to receive a pacemaker with automatic rate-drop responsiveness or the best medical therapy (no pacemaker), according to their treating physicians. Syncope recurrence was lower in the pacemaker patients (6 of 27) than in the medical patients (19 of 27). The hazard ratio for a recurrence of syncope in the paced versus medically treated patients was 0.087 (P = .000016). Figure 15-3 shows the likelihood of a first syncope recurrence among patients randomized to receive a pacemaker (or not) in VPS-I.
Ammirati et al.100 performed a small, randomized clinical trial in 20 patients with moderately frequent syncope who received a pacemaker with either rate hysteresis or rate-drop responsiveness. Three patients with rate hysteresis fainted while no patients with rate-drop responsiveness fainted (P < .05). This study suggested that rate-drop responsiveness is superior to rate hysteresis in preventing syncope, and therefore not all the pacemaker effect was caused by placebo.
Vasovagal Syncope International Study
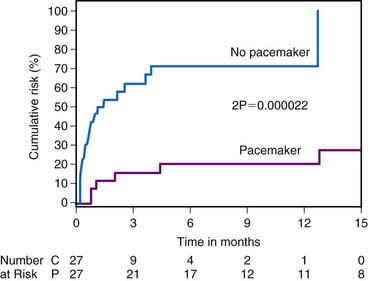
The VASIS Investigators randomly assigned 19 patients to receive a dual-chamber pacemaker with rate hysteresis and 23 patients to no pacemaker implant.101 The patients all had three or more (median six) syncopal spells over the previous 2 years and a cardioinhibitory response to tilt testing. Patients had a lower syncope burden than those of VPS-I. During a mean follow-up of 3.7 ± 2.2 years, the pacemaker group had a lower likelihood of a syncope recurrence than the no-pacemaker group (5% vs. 61%; P = .0006). Figure 15-4 shows the intent-to-treat results. Similar to VPS-I, VASIS was an open-label study that included highly select patients and could not eliminate the impact of placebo effect.
Syncope Diagnosis and Treatment Study
The SYDAT trial tested whether pacemakers or atenolol best prevented vasovagal syncope.102 Randomized to receive a DDD pacemaker with rate-drop response (46) or atenolol (47), the 93 patients were older than 35, had three or more syncopal spells in the preceding 2 years, and had a positive tilt test, with a trough heart rate of 60 bpm or less. At least one syncope recurred in 4.3% of the pacing group versus 26% in the atenolol group (odds ratio [OR] = 0.13; P = .004). This was another open-label study of pacing in vasovagal syncope using a highly select population. One confounding issue is a possible deleterious effect from the atenolol, rather than a beneficial effect from the pacemaker implantation. This seems unlikely given the overall neutral effect of β-blockers in the randomized trials previously summarized.
Summary
Observational reports42,97,99 and open-label RCTs100,101,103 strongly suggested that patients have less syncope after they receive a permanent pacemaker. The question is whether this effect is real. All these studies were unblinded to both patients and physicians. Syncope is an outcome that can be difficult to verify objectively. Also, surgical procedures can have a placebo-effect.104–106 Patients receiving a pacemaker may have benefited from the psychological effects of receiving a surgical procedure from enthusiastic health professionals. Given these uncertainties, the invasiveness and cost of pacing mandated that placebo-controlled or blinded RCTs be used to determine the true benefit of pacing.
Blinded Randomized Studies of Rate-Drop Responsiveness
McLeod Crossover Study
McLeod et al.107 reported the efficacy of rate drop–responsive dual-chamber pacing in the prevention of vasovagal syncope in 12 highly symptomatic young children who had frequent syncope associated with asystolic pauses longer than 4 seconds. In this three-way, double-blind, randomized crossover study, the pacemakers were programmed to no active pacing, ventricular pacing with rate hysteresis, or dual-chamber pacing with rate-drop responsiveness. Both pacing modes were equivalently more effective than no pacing in preventing syncope, and dual-chamber pacing was superior to ventricular pacing in preventing presyncope. This small study concluded that rate drop–responsive pacing was more efficacious than no pacing in preventing vasovagal syncope in children.
Second Vasovagal Pacemaker Study
To ascertain the therapeutic effect of permanent pacing in vasovagal syncope, we performed the Second Vasovagal Pacemaker Study (VPS-II).108 We expected that the risk of syncope in the control group would be reduced to some extent by the placebo effect of receiving a device, and we increased the study sample size accordingly. VPS-II was a multicenter, double-blind, placebo-controlled, randomized clinical trial. Patients were eligible if they had recurrent vasovagal syncope with at least six lifetime syncope spells, or at least three spells in the 2 years before enrollment, and a positive tilt-table test performed according to the protocol in each center. A requirement for a specific degree of bradycardia during tilt testing was not included because trough heart rate during tilt testing did not correlate in patients with heart rate during clinical syncope,69 and because trough heart rate during tilt testing did not appear to predict response to pacing.
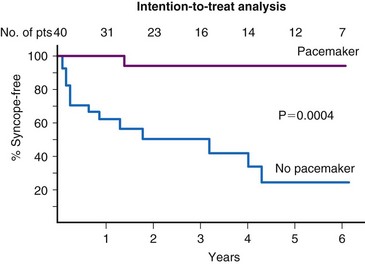
All 100 patients received a dual-chamber pacemaker and were randomized to either rate-drop responsiveness or sensing without pacing. The care providers remained blinded to treatment allocation, except for an unblinded nurse or physician who did all the programming but did not disclose any details. The study was designed to have 80% power to detect a 50% relative reduction in the risk of recurrent syncope from a rate of 60% in the control group to 30% in the treatment group. A total of 38 patients had recurrent syncope during the 6-month follow-up: 22 of 52 patients in the sensing-only group and 16 of 48 in the active pacing group. The cumulative risk of syncope at 6 months was 40% (95% Cl = 25% to 52%) for the sensing-only group and 31% (95% CI = 17% to 43%) for the rate drop–responsive group (Fig. 15-5). The relative risk reduction in time to syncope with active pacing was 30% (95% CI = −33% to 63%; P = 0.14 one-sided). A retrospective analysis found no variable that predicted benefit from pacing, other than patients who received isoproterenol during the tilt test.
Vasovagal Syncope and Pacing Trial
SYNPACE involved 29 patients who had had a median of 12 lifetime syncopal spells, a positive tilt test, and bradycardia during the syncope induced by the tilt test.109 They received a dual-chamber rate drop–responsive pacemaker and were randomized to either active pacing or no pacing. The trial was stopped early after the VPS-II results were released. Thirteen patients had at least one syncope recurrence, and there was no benefit from active pacing with rate-drop responsiveness. Although extremely underpowered, the study did not provide any support for the usefulness of pacing in preventing vasovagal syncope.
Why Were VPS-II and SYNPACE Not Positive?
Vasodepression Cannot Be Paced
Prevention of bradycardia is the main physiologic mechanism by which a pacemaker can prevent attacks of syncope. During positive tilt tests, however, reductions in blood pressure begin earlier than the development of bradycardia.93,94 Pacing therapy might not help patients with hypotension caused by vasodepression even if bradycardia or asystole also occurs at syncope. The results of VPS-II and SYNPACE suggest that most episodes of vasovagal syncope may be associated with profound vasodepression as the cause of syncope, rather than simply bradycardia. In this light, pacing may simply be ineffective in the setting of profound vasodepression, and future progress in devices might best target implantable drug delivery systems.
Placebo Effect
The history of attempts to treat patients with implanted devices has other examples of initial promises of therapeutic success being followed by subsequent well-controlled, negative studies. For example, open-label studies suggested that dual-chamber pacing causes a marked improvement in the hemodynamics and functional status of patients with hypertrophic cardiomyopathy. Later blinded RCTs revealed evidence of a much smaller effect size. Similarly, preliminary open-label studies suggested that atrial-based pacing might prevent atrial fibrillation, but a well-controlled randomized crossover trial showed much less benefit of conventional atrial pacing in prevention of atrial fibrillation.110,111 Large open-label studies also provided strong evidence for the ability of atrial pacing to reduce stroke and death in patients with pacemakers. A large, blinded RCT showed that patients with atrial-based pacemakers versus those with single-lead ventricular pacemakers had no benefit with respect to death, stroke, quality of life, or exercise tolerance for several years after implantation.112 Therefore, care should be taken in the assessment of open-label or nonrandomized pacemaker studies. The placebo effect can be substantial.
Pacemaker therapy may be associated with an initial spurious benefit for two major reasons. First, patients receiving expensive or invasive therapy may not want to admit that such therapy may be ineffective. The placebo effect can be pronounced, particularly with surgical procedures.104–106 Second, many patients with vasovagal syncope appear to improve spontaneously after tilt testing.52,57,58 This effect may account for up to 90% of the apparent benefit. The mechanism is unknown but may involve the counseling received at the clinic visit, a regression to the mean, and the sporadic timing of vasovagal syncope. This effect is of a similar magnitude to the beneficial effect of pacing in sequential design trials. In the unblinded studies, patients who hoped to receive a pacemaker and were disappointed not to receive one may have been more prone to report syncope. Conversely, patients receiving a pacemaker may have benefited from the psychological effects of receiving a surgical procedure from enthusiastic health professionals. The double-blind trial design to a considerable extent removes this type of potential bias.
Clinical Trials of Pacing in Vasovagal Syncope: A Meta-Analysis
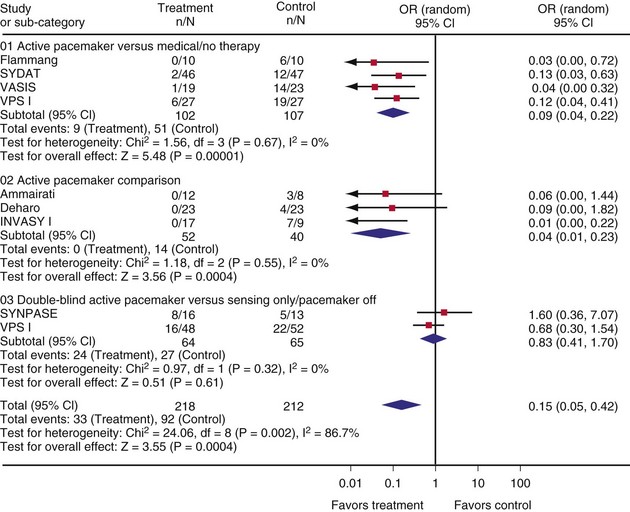
To assess the efficacy of pacing in vasovagal syndromes based on clinical trials, Sud et al.113 conducted a meta-analysis of nine randomized trials: four active pacemaker versus medical or no therapy,101,102,114,115 three active pacemaker comparisons (open label or single blind),103,116,117 and two double-blind active pacemaker versus sensing only or pacemaker off.109,118 When pooled, the nine trials showed significant heterogeneity; when based on trial methodology, however, the trials no longer showed evidence of heterogeneity. In open-label single-blind studies, risk of syncope recurrence was reduced with pacing; double-blind studies showed no effect (Figure 15-6). These results suggest that the main benefit of pacemaker insertion is an expectation effect; the expectation response appeared to explain most of the observed pacemaker response (OR = 0.16; 95% CI = 0.06 to 0.40; P = .0001).113 However, the two double-blind placebo-controlled RCTs conducted to date were small (129 total patients). A larger study is needed for definite conclusions on pacing in vasovagal syncope patients.
International Study on Syncope of Uncertain Aetiology
A multicenter double-blind RCT is in progress to study the effectiveness of pacing for the prevention of asystolic neurally mediated syncope.119 Patients 40 or older with at least three syncopal spells (suspected or confirmed as neurally mediated) over the preceding 2 years and with a severe clinical presentation requiring treatment initiation are eligible for the International Study on Syncope of Uncertain Aetiology (ISSUE-3). The exclusion criteria include carotid sinus hypersensitivity. In Phase I, participants undergo ILR until (a) first syncope with asystole of 3 seconds or longer in the loop recording; (b) asystole of 6 seconds or longer without reported syncope; or (c) 24 months of recording without (a) or (b). In Phase II, patients with (a) or (b) are randomized to receive a dual-chamber pacemaker switched on (active therapy) or off (placebo therapy). Patients, follow-up physicians, and study personnel are blinded to treatment allocation. The primary outcome is the time to first syncope recurrence in both study arms. The study will enroll up to 710 patients to randomize 60 patients to each study arm. The total study duration is estimated at 4 years.
Patient Selection
Patients should have a definite history of vasovagal syncope based on a positive tilt test, suggestive ILR findings, or scrupulous history. Given the weakness of the evidence supporting the efficacy of pacing, it is reasonable to pace only patients with documented profound bradycardia or asystole during syncope. Only patients at high risk of syncope recurrence should be paced. Any patient with at least one syncopal spell in the preceding year has almost a 50% risk of at least one syncopal spell in the next year. Otherwise, similar syncope patients with either negative or positive tilt-table tests have similar likelihood of syncope after assessment.120,121 The outcome of the tilt test (negative vs. positive) does not predict subsequent clinical outcome. Similarly, the lowest heart rate (including asystole) during tilt testing does not predict the eventual likelihood of syncope in clinical follow-up.69,97
Utility of Loop Recordings
Sud et al.122 and Brignole et al.124 assessed spontaneous bradycardia patterns from internal and external loop recordings of patients with unexplained syncope who later underwent pacemaker implantation for bradycardia. The ISSUE classification of detected rhythm from ILRs was used. Although severity of bradycardia or asystole during the loop recording did not predict response to pacemaker therapy, mechanism of bradycardia did; syncope associated with abrupt bradycardia was associated with better response to pacing therapy than syncope with gradual-onset bradycardia. These results suggest that the ISSUE classification is useful in distinguishing between bradycardia as part of vasovagal syncope and primary bradycardia, the latter of which is more responsive to pacing therapy.
Evolving Paradigms and Technology
Contractility Sensors
The search continues for alternate sensing strategies such as QT intervals, respiratory volumes or frequency, right ventricular pressure transduction (dP/dt), and indices of contractility. These are intended to sense either early hypovolemia or early rises in sympathetic activity that may precede frank syncope. Some evidence indicates that contractility can be estimated with measures of endocardial acceleration, using a microaccelerometer in the pacemaker lead to estimate right ventricular myocardial contractility,124,125 or with intracardiac impedance measurements.126,127 The theory behind contractility sensors is that vasovagal syncope might be preceded by small but significant increases in contractility caused by a sympathetic surge. These devices increase pacing rates in response to increases in apparent contractility, then slowly decrease rates after contractility subsides toward baseline. Discouragingly, Brignole et al.124 found that endocardial acceleration did not predict the occurrence of tilt table–induced syncope.
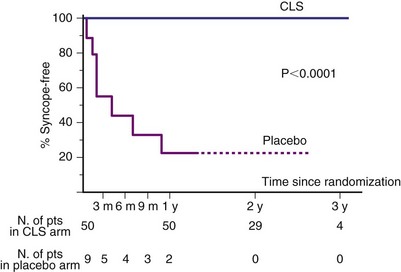
Griesbach et al.127 evaluated the use of closed-loop stimulation (CLS) in a preliminary study in 2002. CLS pacemaker technology reacts to a change in the right ventricular intracardiac impedance, felt to be a surrogate measure of contractility and therefore sympathetic tone. This study of 22 patients demonstrated that syncope sensed with this modality could be prevented on tilt testing of patients with cardioinhibitory syncope. A subsequent study used CLS prospectively in 34 patients with recurrent vasovagal syncope. Over 12 to 50 months of follow-up, 30 of 34 patients had not experienced a recurrent event.
Based on this pilot study, a larger, multicenter RCT, the Inotropy Controlled Pacing in Vasovagal Syncope (INVASY), was partly carried out in 2004.128,129 In this open-label trial, 26 patients with recurrent vasovagal syncope and a positive tilt test with induced bradycardia received a dual-chamber CLS pacemaker, then were asymmetrically randomized to simple DDI pacing (9) or dual-chamber CLS pacing (17). INVASY was stopped early after a preliminary analysis showed that, after a mean of 19 months, seven of the nine DDI patients and none of the 17 CLS patients had syncope recurrences (P < .0001) (Fig. 15-7). These positive results suggest three possible conclusions: (1) pacing can be useful in patients with vasovagal syncope; (3) CLS based on right ventricular impedance changes is effective sensing for syncope; and (3) this may be the placebo effect, again. A larger and properly blinded study is being planned.
Automatic Drug Delivery Systems
Current pacemaker therapies focus only on heart rate support. This might not be as useful in patients with a predominantly vasodepressor response. Giada et al.130 described an acute study assessing a novel implantable system that delivers phenylephrine when activated at the onset of syncope (prodrome with blood pressure drop). When treated with the phenylephrine, 15 of 16 patients had an immediate blood pressure rise and their tilt-induced syncope terminated, despite ongoing tilt testing. In contrast, none of the patients was able to abort the episodes when a placebo infusion was delivered. The one patient who fainted despite the phenylephrine experienced a severe cardioinhibitory response to the tilt test. This small study suggests the promise of combined approach using pacing for the cardioinhibitory component, as well as acute pharmacologic support for the vasodepressor component of syncope.
Guidelines for Pacing in Vasovagal Syncope
Pacing at least should not be used early in patients with vasovagal syncope. First, pacing should be reserved for patients with frequent, highly symptomatic vasovagal syncope whose quality of life is greatly diminished and who have not responded to lifestyle and dietary changes, education, reassurance, counterpressure maneuvers,3,131 and at least three medication attempts. Second, pacing might be more effectively used in patients with documented asystole at syncope. Third, all patients considering pacing should have frank and detailed education about the limited (at best) evidence for its efficacy.
According to the 2009 recommendations of the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology, (1) cardiac pacing should be considered in patients with frequent, recurrent reflex syncope, age over 40, and documented spontaneous cardioinhibitory response during monitoring; (2) cardiac pacing may be indicated in patients with tilt-induced cardioinhibitory response with recurrent frequent unpredictable syncope and age over 40, after alternative therapy has failed; and (3) cardiac pacing is not indicated in the absence of a documented cardioinhibitory reflex.4
1 Benditt DG, Remole S, Milstein S, et al. Causes, clinical evaluation and current therapy. Annu Rev Med. 1992;43:283.
2 Brignole M, Alboni P, Benditt DG, et al. Guidelines on mangement (diagnosis and treatment) of syncope: update 2004—exective summary. Eur Heart J. 2004;25:2054-2072.
3 Gregoratos G, Cheitlin MD, Conill A, et al. ACC/AHA Guidelines for implantation of cardiac pacemakers and antiarrhythmic devices: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Committe on Pacemaker Implantation. Circulation. 1998;97:1325-1335.
4 Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). The Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J. 2009;30:2631-2671.
5 Thomas JE. Hyperactive carotid sinus reflex and carotid sinus syncope. Mayo Clin Proc. 1969;13:2065.
6 Volkmann H, Schnerch B, Kuhnert B. Diagnosis value of carotid sinus hypersensitivity. Pacing Clin Electrophysiol. 1990;13:2065.
7 Hartzler GO, Maloney JD. Cardioinhibitory carotid sinus hypersensitivity. Arch Intern Med. 1977;137:727.
8 Weiss S, Baker JP. The carotid sinus reflex in health and disease: Its role in the causation of fainting and convulsions. Medicine. 1933;12:297.
9 Zee-Cheng CS, Gibbs HR. Pure vasodepressor carotid sinus hypersensitivity. Am J Med. 1986;81:1095.
10 Morley CA, Sutton R. Carotid sinus syncope. Int J Cardiol. 1984;6:287.
11 Kenny RA, Traynor G. Carotid sinus syndrome: clinical characteristics in elderly patients. Age Ageing. 1991;20:499.
12 Richardson DA, Bexton RS, Shaw FE, Kenny RA. Prevalence of cardioinhibitory carotid sinus hypersensitivity in patients 50 years or over presenting to the accident and emergency department with “unexplained” or “recurrent” falls. Pacing Clin Electrophysiol. 1997;15:820.
13 Huang SKS, Ezi MD, Hauser RG. Carotid sinus hypersensitivity in patients with unexplained syncope: clinical, electrophysiological, and long-term follow-up observations. Am Heart J. 1988;116:989.
14 Brignole M, Menossi C, Lolli G. Long-term outcome of paced and non-paced patients with severe carotid sinus syndrome. Am J Cardiol. 1992;69:1039.
15 Lown B, Levine SA. The carotid sinus: clinical value of its stimulation. Circulation. 1961;23:766.
16 Baig MW, Kaye GC, Perrins EJ. Can central neuropeptides be implicated in carotid sinus reflex hypersensitivity? Med Hypotheses. 1989;28:255.
17 Strasburg B, Sagie A, Erdman S. Carotid sinus hypersensitivity and carotid sinus syndrome. Prog Cardiovasc Dis. 1989;31:376.
18 Blanc JJ, L`Heveder G, Mansourati J. Assessment of a newly recognized association, carotid sinus hypersensitivity and denervation of sternocleidomastoid muscles. Circulation. 1997;95:2548.
19 DaCosta D, Mclntoch S, Kenny RA. Benefits of fludrocortisone in the treatment of symptomatic vasodepressor carotid sinus syndrome. Br Heart J. 1993;69:308.
20 Trout HH, Brown LL, Thompson JE. Carotid sinus syndrome: treatment by carotid sinus denervation. Ann Surg. 1979;189:575.
21 Katritsis D, Ward DE, Camm AJ. Can we treat carotid sinus syndrome? Pacing Clin Electrophysiol. 1991;14:1367.
22 Sugrue DD, Gersh BJ, Holmes DR. Symptomatic “isolated” carotid sinus hypersensitivity: natural history and results of treatment with anticholinergic drugs or pacemaker. J Am Coll Cardiol. 1986;158:1986.
23 Morley CA, Perrins EJ, Grant P. Carotid sinus syncope treated by pacing. Br Heart J. 1982;47:411.
24 Brignole M, Menozzi C, Lolli G. Validation of a method for choice of pacing mode in carotid sinus syndrome with or without sinus bradycardia. Pacing Clin Electrophysiol. 1991;14:196.
25 Brignole M, Menossi C, Lolli G. Natural and unnatural history of patients with severe carotid sinus hypersensitivity: a preliminary study. Pacing Clin Electrophysiol. 1988;11:1678.
26 Bexton RS, Davies A, Kenny RA. The rate-drop response in carotid sinus syndrome: the Newcastle experience. Pacing Clin Electrophysiol. 1997;20(3 Pt 2):840.
27 Davies AJ, Kenny RA. Falls presenting to the accident and emergency department: types of presentation and risk factor profile. Age Ageing. 1996;25:362-366.
28 Kenny RA, Traynor G. Carotid sinus syndrome: clinical characteristics in elderly patients. Age Ageing. 1991;20:449-454.
29 Kenny RA, Richardson DA, Steen N, et al. Carotid sinus syndrom: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol. 2001;38:1491-1496.
30 Ryan DJ, Nick S, Colette SM, Roseanne K. Carotid sinus syndrome, should we pace? A multicentre, randomised control trial (SAFEPACE-2). Heart. 2010;96:347-351.
31 Parry SW, Steen N, Bexton RS, et al. Pacing in elderly recurrent fallers with carotid sinus hypersensitivity: a randomised, double-blind, placebo controlled crossover trial. Heart. 2009;95:405-409.
32 Madigan NP, Flaker GC, Curtis JJ. Carotid sinus hypersensitivity: beneficial effects of dual-chamber pacing. Am J Cardiol. 1984;53:1034.
33 Stryjer D, Friedensohn A, Schlesinger Z. Ventricular pacing as the preferable mode for long-term pacing in patients with carotid sinus syncope of the cardioinhibitory type. Pacing Clin Electrophysiol. 1986;9:705.
34 Walter F, Crawley IS, Dorney ER. Carotid sinus hypersensitivity and syncope. Am J Cardiol. 1978;42:396.
35 Gang ES, Oseran DS, Mandel WJ. Sinus node electrogram in patients with the hypersensitivity carotid syndrome. J Am Coll Cardiol. 1985;5:1484.
36 Brown KA, Maloney JD, Smith HC. Carotid sinus reflex in patients undergoing coronary angiography: relationship of degree and location of coronary artery disease to response to carotid sinus massage. Circulation. 1980;62:697.
37 Munro NC, McLntoch S, Lawson J. Incidence of complications after carotid sinus massage in older patients with syncope. J Am Geriatr Soc. 1994;42:1248.
38 Davies AJ, Kenny RA. Frequency of neurologic complications following carotid sinus massage. Am J Cardiol. 1998;81:1256-1257.
39 Alexander S, Ding WC. Fatal ventricular fibrillation during carotid sinus stimulation. Am J Cardiol. 1966;18:289.
40 Probst P, Muhlberger V, Lederbauer M. Electrophysiologic findings in carotid sinus massage. Pacing Clin Electrophysiol. 1983;6:689.
41 Alicandri C, Fouad FM, Tarazi RC. Three cases of hypotension and syncope with ventricular pacing. Am J Cardiol. 1978;42:137.
42 Benditt DG, Sutton R, Gammage MD, et al. Clinical experience with Thera DR rate-drop response pacing algorithm in carotid sinus syndrome and vasovagal syncope. Pacing Clin Electrophysiol. 1997;20:832-839.
43 Maloney JD, Jaeger FJ, Fouad-Tarazi FM. Malignant vasovagal syncope: prolonged asystole provoked by head-up tilt—case report and review of diagnosis, pathophysiology and therapy. Cleve Clin J Med. 1988;55:543.
44 Sutton R. Vasovagal syncope: could it be malignant? Eur Heart J. 1992;2:89.
45 Fitzpatrick AP, Ahmed R, Williams S. A randomized trial of medical therapy in “malignant vasovagal syndrome” or “neurally mediated bradycardia/hypotension syndrome”. Eur J Card Pacing Electrophysiol. 1991;2:99.
46 Fitzpatrick AP, Sutton R. Tilting toward a diagnosis in recurrent unexplained syncope. Lancet. 1989;1:658.
47 Grubb BP, Temesy-Armos P, Moore J. Head-upright tilt-table testing in the evaluation and management of the malignant vasovagal syndrome. Am J Cardiol. 1990;69:904.
48 Ganzeboom KS, Colman N, Reitsma JB, et al. Prevalence and triggers of syncope in medical students. Am J Cardiol. 2003;91:1006-1008.
49 Sheldon R, Sheldon A, Connolly SJ, et al. Investigators of the Syncope Symptom Study and the Prevention of Syncope Trial. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49.
50 Day SC, Cook EF, Funkenstein H, Goldman L. Evaluation and outcome of emergency room patients with transient loss of consciousness. Am J Med. 1982;73:15-23.
51 Savage DD, Corwin L, McGee DL, et al. Epidemiologic feature of isolated syncope: the Framingham Study. Stroke. 1985;16:626-629.
52 Dermkasian G, Lamb LE. Syncope in a population of healthy young adults. J Am Med. 1958;168:1200.
53 Benditt DG, Ferguson DW, Grubb BP, et al. Tilt-table testing for assessing syncope. J Am Coll Cardiol. 1996;28:263-275.
54 Mosqueda-Garcia R, Furlan R, Tank J, Femandez-Violante R. The elusive pathophysiology of neurally mediated syncope. Circulation. 2000;102:2898-2906.
55 Linzer M, Pontinen M, Gold DT, et al. Impairment of physical and psychosocial function in recurrent syncope. J Clin Epidemiol. 1991;44:1037-1043.
56 Rose MS, Koshman ML, Spreng S, Sheldon RS. The relationship between health-related quality of life and frequency of spells in patients with syncope. J Clin Epidemiol. 2000;53:1209-1216.
57 Lamb L, Green HC, Combs JJ, et al. Incidence of loss of consciousness in 1980 Air Force personnel. Aerospace Med. 1960;12:973-988.
58 Murdoch BD. Loss of consciousness in healthy South African men: incidence, causes and relationship to EEG abnormality. S Afr Med J. 1980;57:771-774.
59 Sheldon R, Flanagan P, Koshman ML, Killam S. Risk factors for syncope recurrence after a positive tilt-table test in patients with syncope. Circulation. 1996;93:973-981.
60 Rossen R, Kabat H, Anderson JP. Acute arrest of cerebral circulation in man. Arch Neurol Psych. 1943;50:510-528.
61 Morillo CA, Eckberg DL, Ellenbogen KA, et al. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997;96:2509-2513.
62 Mosqueda-Garcia R, Furlan R, Tank J, et al. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997;11:2736-2744.
63 Thomson HL, Atherton JJ, Khafagi FA, Frenneaux MP. Failure of reflex venoconstriction during exercise in patients with vasovagal syncope. Circulation. 1996;93:953-959.
64 Thomson HL, Wright K, Frenneaux MP. Baroreflex sensitivity in patients with vasovagal syncope. Circulation. 1997;95:395-400.
65 Menozzi C, Brignole M, Lolli G, et al. Follow-up of asystolic episodes in patients with cardioinhibitory, neurally mediated syncope and VVI pacemaker. Am J Cardiol. 1993;72:1152-1155.
66 Krahn AD, Klein GJ, Fitzpatrick A, et al. Predicting the outcome of patients with unexplained syncope undergoing prolonged monitoring. Pacing Clin Electrophysiol. 2002;25:37-41.
67 Krahn AD, Klein GJ, Yee R. Randomized Assessment of Syncope Trial: conventional diagnostic testing versus a prolonged monitoring strategy. Circulation. 2001;104:46-51.
68 Krahn AD, Klein GJ, Yee R, et al. Cost implication of testing in patients with syncope: Randomized Assessment of Syncope Trial. J Am Coll Cardiol. 2003;42:495-501.
69 Sheldon R. Tilt testing for syncope: a reappraisal. Curr Opin Cardiol. 2005;1:38-41.
70 El-Syed H, Hainsworth R. Salt supplement increases plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart. 1996;75:134-140.
71 Van Dijk N, Quartieri F, Blanc JJ, et al. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the Physical Counterpressure Manoeuvres Trial (PC-Trial). J Am Coll Cardiol. 2006;48:1652-1657.
72 Sheldon RS, Rose S, Flanagan P, et al. Risk factors for syncope recurrence after a positive tilt-table test in patients with syncope. Circulation. 1996;93:973-981.
73 Ward CR, Gray JC, Gilroy JJ, Kenny RA. Midodrine: a role in the management of neurocardiogenic syncope. Heart. 1998;79:45-49.
74 Perez-Lugones A, Schweikert R, Pavia S, et al. Usefulness of midodrine in patients with severly symptomatic neurocardiogenic syncope: a randomized controlled study. J Cardiovasc Electrophysiol. 2001;12:935-938.
75 Kaufmann H, Saadia D, Voustianiouk A. Midodrine in neurally mediated syncope: double-blind, randomized, crossover study. Ann Neurol. 2002;52:342-345.
76 Kuriachan V, Sheldon RS, Platonov M. Evidence-based treatment for vasovagal syncope. Heart Rhythm. 2008;5:1609-1614.
77 Di Girolamo E, Di Lorio C, Sabatini P, et al. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1999;33:1227-1230.
78 Takata TS, Wasmund SL. Serotonin reuptake inhibitor (Paxil) does not prevent vasovagal reaction: association with carotid sinus massage and/or lower body negative pressure in healthy volunteers. Circulation. 2002;106:1500.
79 Sheldon R, Rose S, Flanagan P, et al. Effect of beta blockers on the time to first syncope recurrence in patients after a positive isoproterenol tilt table test. Am J Cardiol. 1996;78:536-539.
80 Cox MM, Perlman BA, Mayor MR, et al. Acute and long-term beta-adrenergic blockade for patients with neurocardiogenic syncope. J Am Coll Cardiol. 1995;26:1293-1298.
81 Alegria JR, Gersh BJ, Scott CG, et al. Comparison of frequency of recurrent syncope after beta-blocker therapy versus conservative management for patients with vasovagal syncope. Am J Cardiol. 2003;92:82-84.
82 Mahanonda N, Bhuripanyo K, Kangkagate C. Randomized, double-blind, placebo-controlled trial of oral atenolol in patients with unexplained syncope and positive upright tilt table test results. Am Heart J. 1995;130:1250-1253.
83 Madrid AH, Ortega J, Rebollo JG, et al. Lack of efficacy of atenolol for the prevention of neurally mediated syncope in a highly symptomatic population: a prospective, double-blind, randomized and placebo-controlled study. J Am Coll Cardiol. 2001;37:5554-5559.
84 Flevari P, Livanis EG, Theodorakis GN, et al. Vasovagal syncope: a prospective, randomized, crossover evaluation of the effect of propranolol, nadolol and placebo on syncope recurrence and paients’ well-being. J Am Coll Cardiol. 2002;40:499-504.
85 Ventura R, Maas R, Zeidler D. A randomized and controlled pilot trial of beta-blockers for the treatment of recurrent syncope in patients with a positive or negative response to head-up tilt test. Pacing Clin Electrophysiol. 2002;25:816-821.
86 Sheldon R, Rose S, Connlly S. Prevention of Syncope Trial (POST): a randomized clinical trial of beta-blocker in the prevention of vasovagal syncope—rationale and study design. Europace. 2003;5:71-75.
87 Schimmer BP, Parker KL. Adrenocorticotropic hormone. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. New York: McGraw-Hill; 2001:1649-1677.
88 Balaji S, Oslizlok PC, Allen MC, et al. Neurocardiogenic syncope in children with a normal heart. J Am Coll Cardiol. 1994;23:779-785.
89 Scott WA, Pongiglione G, Bromberg BI, et al. Randomized comparison of atenolol and fludrocortisone acetate in the treatment of pediatric neurally mediated syncope. Am J Cardiol. 1995;76:400-402.
90 Salim MA, Di Sessa TG. Effectiveness of fludrocortisone and salt in preventing syncope recurrence in children. J Am Coll Cardiol. 2005;45:484-488.
91 Raj SR, Rose S, Ritchie D, Sheldon RS. The Second Prevention of Syncope Trial (POST II): a randomized clinical trial of fludrocortisone for the prevention of neurally mediated syncope—rationale and study design. Am Heart J. 2006;151:1186. e11-e17
92 El-Bedawi KM, Wahba MA, Hainsworth R. Cardiac pacing does not improve orthostatic tolerance in patients with vasovagal syncope. Clin Auton Res. 1994;4:233-237.
93 Fitzpatrick AP, Theodorakis G, Ahmed R, et al. Dual-chamber pacing aborts vasovagal syncope induced by head-up 60 degrees tilt. Pacing Clin Electrophysiol. 1991;14:13-19.
94 Samoil D, Grubb BP, Brewster P, et al. Comparison of single- and dual-chamber pacing techniques in prevention of upright tilt-induced vasovagal syncope. Eur J Card Pacing Electrophysiol. 1991;1:36-41.
95 Sra JS, Jazayeri MR, Avitall B. Comparison of cardiac pacing with drug therapy in the treatment of neurocardiogenic (vasovagal) syncope with bradycardia or asystole. N Engl J Med. 1993;328:1085-1109.
96 Moya A, Brignole M, Menozzi C, et al. International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Circulation. 2001;104:1261-1267.
97 Petersen ME, Chamberlain-Webber R, Fitzpatrick AP, et al. Permanent pacing for cardioinhibitory malignant vasovagal syndrome. Br Heart J. 1994;71:274-281.
98 Benditt DG, Sutton R, Gammage MD. Clinical experience with Thera DR rate drop response pacing algorithm in carotid sinus syndrome and vasovagal syncope. Pacing Clin Electrophysiol. 1997;20:832.
99 Sheldon R, Wilson W, Kiesser T, Rose S. Effect of dual-chamber pacing automatic rate-drop sensing on recurrent neurally mediated syncope. Am J Cardiol. 1998;81:158-162.
100 Connolly SJ, Sheldon RS, Roberts RS, Gent M. The North American Vasovagal Pacemaker Study. J Am Coll Cardiol. 1999;33:16-20.
101 Sutton R, Brignole M, Menozzi C, et al. Dual-chamber pacing in the treatment of neurally mediated tilt-positive cardioinhibitory syncope: pacemaker versus no therapy: a multicenter randomized study. The Vasovagal Syncope International Study (VASIS). Circulation. 2000;102:294-299.
102 Ammirati F, Colivicchi F, Santini M. Permanent cardiac pacing versus medical treatment for the prevention of recurrent vasovagal syncope: a multicenter randomized, controlled trial. Circulation. 2001;104:52-57.
103 Ammirati F, Colivicchi F, Toscano S. DDD pacing with rate drop response function versus DDI with rate hysteresis pacing for cardioinhibitory vasovagal syncope. Pacing Clin Electrophysiol. 1998;21:2178-2181.
104 Beecher HK. Surgery as placebo. JAMA. 1961;176:1102-1107.
105 Moerman DE, Jonas WB. Deconstructing the placebo effect and finding the meaning response. Ann Intern Med. 2002;136:471-476.
106 Redelmeier DA, Tu JV, Schull MJ, et al. Problems for clinical judgement. 2. Obtaining a reliable past medical history. Can Med Assoc J. 2001;164:809-813.
107 McLeod KA, Wilson N, Hewitt J. Cardiac pacing for severe childhood neurally mediated syncope with reflex anoxic seizures. Heart. 1999;82:721-725.
108 Connolly SJ, Sheldon R, Thorpe KE, et al. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope. JAMA. 2003;289:2224-2228.
109 Raviele A, Giada F, Menpzzi C, et al. A randomized, double-blind, placebo-controlled study of permanent cardiac pacing for the treatment of recurrent tilt-induced vasovagal syncope. The Vasovagal Syncope and Pacing Trial (SYNPACE). Eur Heart J. 2004;25:1741-1748.
110 Gillis AM, Connolly SJ, Lacombe P, et al. Randomized crossover comparison of DDDR versus VDD pacing after atrioventricular junction ablation for prevention of atrial fibrillation. The Atrial Pacing Peri-Ablation for Paroxysmal Atrial Fibrillation Study Investigators. Circulation. 2000;102:736-741.
111 Gillis AM, Connolly SJ, Lacombe P, et al. Atrial pacing periablation for prevention of paroxysmal atrial fibrillation. Circulation. 1999;99:2553-2558.
112 Connolly SJ, Kerr CR, Gent M, et al. Effects of physiological pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physioloic Pacing Investigators. N Engl J Med. 2000;342:1385-1391.
113 Sud S, Massel D, Klein GJ, et al. The expectation effect and cardiac pacing for refractory vasovagal syncope. Am J Med. 2007;120:54-62.
114 Flammang D, Antiel M, Church T, et al. Is a pacemaker indicated for vasovagal patients with severe cardioinhibitory reflex as identified by the ATP test? A preliminary randomized trial. Europace. 1999;1:140-145.
115 Connolly SJ, Sheldon R, Roberts RS, Gent M. The North American Vasovagal Pacemaker Study (VPS): a randomized trial of permanent cardiac pacing for the prevention of vasovagal syncope. J Am Coll Cardiol. 1999;33:16-20.
116 Deharo JC, Brunetto AB, Bellocci F, et al. DDDR pacing driven by contractility versus DDI pacing in vasovagal syncope: a multicenter, randomized study. Pacing Clin Electrophysiol. 2003;26:447-450.
117 Occhetta E, Bortnik M, Audoglio R, Vassanelli C. Closed loop stimulation in prevention of vasovagal syncope. Inotropy Controlled Pacing in Vasovagal Syncope (INVASY): a multicentre randomized, single-blind, controlled study. Europace. 2004;6:538-547.
118 Connolly SJ, Sheldon R, Thorpe KE, et al. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope: Second Vasovagal Pacemaker Study (VPS II): a randomized trial. JAMA. 2003;289:2224-2229.
119 Brignole M. International Study on Syncope of Uncertain Aetiology 3 (ISSUE 3): pacemaker therapy for patients with asystolic neurally mediated syncope—rationale and study design. Europace. 2007;9:25-30.
120 Sheldon R, Rose S, Koshman ML. Comparison of patients with syncope of unknow cause having negative or positive tilt-table tests. Am J Cardiol. 1997;80:581-585.
121 Grimm W, Degenhardt M, Hoffman J, et al. Syncope recurrence can better be predicted by history than by head-up tilt testing in untreated patients with suspected neurally mediated syncope. Pacing Clin Electrophysiol. 1997;18:1465-1469.
122 Sud S, Klein GJ, Skanes AC, et al. Implications of mechanism of bradycardia on response to pacing in patients with unexplained syncope. Europace. 2007;9:312-318.
123 Brignole M, Moya A, Menozzi C, et al. Proposed electrocardiographic classification of spontaneous syncope documented by an implantable loop recorder. Europace. 2005;7:14-18.
124 Brignole M, Menozzi C, Corbucci G. Detecting incipient vasovagal syncope: intraventricular acceleration. Pacing Clin Electrophysiol. 1997;20:801-808.
125 Osswald S, Cron T, Gradel C. Closed-loop stimulation using intracardiac impedance as a sensor principle: correlation of right ventricular dP/dtmax and intracardiac impedance during dobutamine stress test. Pacing Clin Electrophysiol. 2000;23:1502-1508.
126 Binggeli C, Duru F, Corti R. Autonomic nervous system–controlled cardiac pacing: a comparison between intracardiac impedance signal and muscle sympathetic nerve activity. Pacing Clin Electrophysiol. 2000;23:1632-1637.
127 Griesbach L Huber T, Knote B, et al. Closed-loop stimulation: therapy for malignant neurocardiogenic syncope. Prog. Biomed Res. 2002;7:242-247.
128 Occhetta EBM, Vassanelli C. The DDDR closed-loop stimulation for the prevention of vasovagal syncope: results from the INVASY Prospective Feasibility Registry. Europace. 2003;5:153-162.
129 Ochetta E, Bortnik M, Audoglio R, Vassanelli C. Closed-loop stimulation in prevention of vasovagal syncope. Inotropy Controlled Pacing in Vasovagal Syncope (INVASY): a multicentre randomized single-blind controlled study. Europace. 2004;6:538-547.
130 Giada F, Raviele A, Gasparini G. Efficacy of a patient-activated drug delivery system using phenylephrine as active drug in aborting tilt-induced syncope [abstract]. Pacing Clin Electrophysiol. 2001;24:573.
131 Brignole M, Alboni P, Benditt DG, et al. Task Force on Syncope, European Society of Cardiology. Guidelines on managemnet (diagnosis and treatment) of syncope. Eur Heart J. 2001;22:1256-1306.
132 Sheldon R, Connolly S, Rose S, et al. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation. 2006;113:1164-1170.
133 Theodorakis GN, Leftheriotis D, Livanis EG, et al. Fluoxetine vs. propranolol in the treatment of vasovagal syncope: a prospective, randomized, placebo-controlled study. Europace. 2006;8:193-198.
134 Qingyou Z, Junbao D, Chaoshu T. The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. J Pediatr. 2006;149:777-780.