14 Pacing for Atrioventricular Conduction System Disease
 Anatomy
Anatomy
The sinus (or sinoatrial, SA) node lies near the junction of the superior vena cava and the right atrium. The sinus node is supplied by the sinus nodal artery, which originates from the proximal few centimeters of the right coronary artery (RCA) in about 55% of human subjects and from the proximal few centimeters of the left circumflex (LCx) artery in the remainder1–3 (Fig. 14-1).
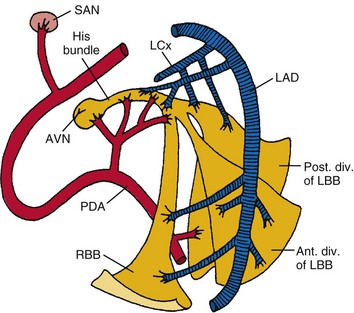
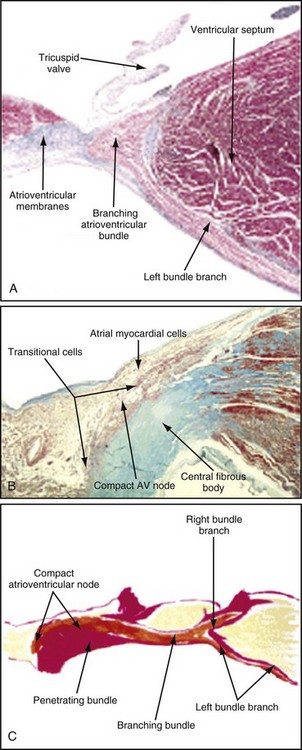
Figure 14-1 Diagrammatic representation of the conduction system and its blood supply.
(From de Guzman M, Rahimtoola SH: What is the role of pacemakers in patients with coronary artery disease and conduction abnormalities? In Rahimtoola SH, editor: Controversies in coronary artery disease. Philadelphia, 1983, Davis.)
The atrioventricular (AV) node lies directly above the insertion of the septal leaflet of the tricuspid valve and just beneath the right atrial (RA) endocardium.3 The atrioventricular junction is a structure encompassing the AV node with its posterior, septal, and left atrial (LA) approaches, as well as the His bundle and its bifurcation. The AV node is a small, subendocardial structure located within the interatrial septum, at the distal convergence of the preferential internodal conduction pathways that course through the atria from the sinus node.1 As with the SA node, the AV node has extensive autonomic innervation and an abundant blood supply. The AV node consists of three regions—the transitional cell zone, compact node, and penetrating bundle—distinguished by functional and histologic differences (Fig. 14-2). The transitional cell zone, which consists of cells constituting the atrial approaches to the compact AV node, has the highest rate of spontaneous diastolic depolarization. The compact node is composed of groups of cells that have extensions into the central fibrous body and the anulus of the mitral and tricuspid valves. These cells appear to be the site of most of the conduction delay through the AV node.4 The penetrating bundle consists of cells that lead directly into the His bundle and its branching portion.
Only the proximal two thirds of the AV node are supplied by the AV nodal artery;3 the distal segment of the AV node has a dual blood supply in 80% of human hearts from the same AV nodal artery and the left anterior descending (LAD) artery. In 90% of patients, the AV nodal artery originates from the RCA. During acute myocardial infarction (AMI), conduction disturbances in the AV node are usually the consequence of an occlusion proximal to the origin of the AV nodal artery. Therefore, the conduction abnormalities are usually associated with inferior AMI. The AV nodal tissue merges with the His bundle, which runs through the inferior portion of the membranous interventricular septum and then, in most cases, continues along the left side of the crest of the muscular interventricular septum. The His bundle usually receives a dual blood supply from both the AV nodal artery and branches of the LAD artery.5 Unlike the SA and AV nodes, the bundle of His and Purkinje system have relatively little autonomic innervation.
The right bundle branch (RBB) originates from the His bundle. It is a narrow structure that crosses to the right side of the interventricular septum and extends along the right ventricular (RV) endocardial surface to the region of the anterolateral papillary muscle of the right ventricle, where it divides to supply the papillary muscle, the parietal RV surface, and the lower part of the RV surface.5 The proximal portion of the RBB is supplied by branches from the AV nodal artery or the LAD artery, whereas the more distal portion is supplied mainly by branches of the LAD artery.
The left bundle branch (LBB) is anatomically much less discrete than the RBB. The LBB may divide immediately as it originates from the bundle of His or may continue for 1 to 2 cm as a broad band before dividing.3,5 The LBB fibers spread out over the left ventricle in a fanlike manner, a “cascading waterfall,” with many subendocardial interconnections that resemble a syncytium rather than two, anatomically discrete, distinct branches or fascicles.5
As originally proposed by Rosenbaum,6 however, it is clinically useful to consider the LBB as dividing into an anterior branch or fascicle and a larger and broader posterior branch or fascicle, both of which radiate toward the anterior and posterior papillary muscles of the left ventricle, respectively. The LBB and its anterior fascicle have a blood supply similar to that of the proximal portion of the RBB; the left posterior fascicle is supplied by branches of the AV nodal artery, the posterior descending artery, and the circumflex coronary artery.
 Diagnosis of Atrioventricular Conduction Disturbances
Diagnosis of Atrioventricular Conduction Disturbances
Electrocardiography
First-degree atrioventricular (AV) block usually is caused by conduction delay within the AV node. Much less frequently, first-degree AV block is caused by intra-atrial or infra-Hisian conduction delay.7 Localization of the site of second-degree AV block to the AV node or His-Purkinje system can be obtained by His bundle recording during invasive electrophysiologic testing, but in most cases, careful analysis of the electrocardiogram (ECG) and the effect of various pharmacologic agents on the block will suffice.8 Adherence to precise definitions of second-degree AV block, particularly in cases of 2 : 1 AV block and suspected type II AV block, is critical to avoiding diagnostic errors that could result in potentially unnecessary permanent pacemaker implantation.9 Furthermore, type I and type II designations refer only to ECG patterns and not to the anatomic site of block.
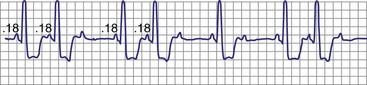
Classic type I (Wenckebach) second-degree AV block has three characteristics: (1) progressive P-R interval prolongation before the nonconducted beat, (2) progressive decrease in the increment of P-R interval prolongation, and (3) progressive decrease in the R-R interval, parallel to the progressive decrease in the increment of change in the P-R interval.10 All patterns of type I second-degree AV block not having this pattern are called “atypical” patterns, although in actuality, they may occur more often than the classic variety11 (Fig. 14-3).
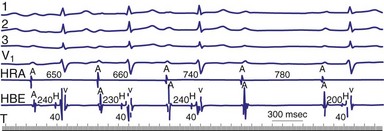
Figure 14-3 Atypical or uncommon type I second-degree atrioventricular (AV) block in the AV node.
(From Josephson ME: Clinical cardiac electrophysiology: techniques and interpretations, ed 3, Philadelphia, 2002, Lippincott–Williams & Wilkins, pp 92-109.)
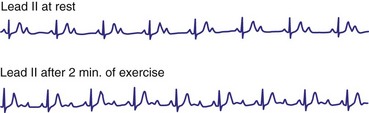
As a general rule, type I second-degree AV block with a narrow QRS complex is almost always caused by delay within the AV node. Delay within the His bundle (intra-Hisian block), however, is a rare cause of type I second-degree AV block with a narrow QRS and requires an electrophysiologic study for definitive diagnosis. Intra-Hisian block should be suspected in a patient with a narrow QRS if type I second-degree AV block is provoked by exercise. In contrast, type I AV nodal block at rest improves with exercise. When type I second-degree AV block is associated with concomitant bundle branch block (BBB), the site of delay or block is in the His-Purkinje system (infranodal) in up to 60% to 70% of cases.10
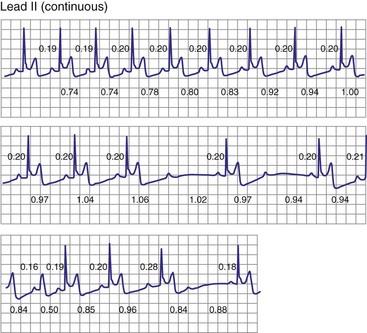
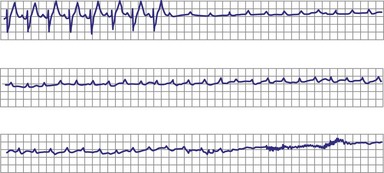
Type II second-degree AV block is defined as a single nonconducted sinus P wave associated with fixed P-R intervals before and after the blocked beat (Fig. 14-4). The sinus rate must be stable (i.e., constant P-P intervals), and there must be at least two consecutive conducted P waves to determine the P-R interval, which can be either normal or prolonged. Type II second-degree AV block should not be diagnosed in the case of a nonconducted atrial premature beat or if there is simultaneous sinus slowing and AV nodal block (e.g., hypervagotonia). Type I block with relatively long Wenckebach sequences and small increases in AV nodal conduction time may be confused with type II AV block but should not be classified as such (even though the site of block may be either AV nodal or infranodal in such cases). Type II second-degree AV block is most often encountered when the QRS complex is prolonged (in about 70% of cases) and is localized within the His-Purkinje system; in contrast, type II AV block with a narrow QRS is within the His bundle (i.e., intra-Hisian). Sudden AV block of more than one impulse with stable sinus rhythm and 1 : 1 AV conduction with constant P-R and P-P intervals before and after the block has been labeled “advanced AV block,” “paroxysmal AV block,” and “type II AV block.” Although this rhythm does not conform to the strict definition of type II block (i.e., a single block beat), it does suggest that the anatomic site of AV block is infranodal, and that permanent pacing is indicated. On the other hand, Wenckebach periodicity before the development of high-grade AV block suggests an AV nodal site.
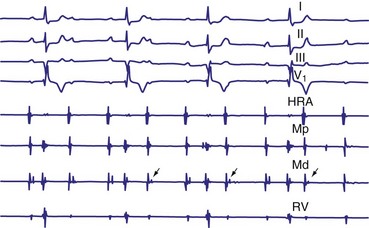
Lange et al.12 reported on their experience with a large number of patients with transient second-degree AV block and narrow QRS complexes detected on ambulatory Holter monitoring. The researchers emphasized the many ways in which second-degree AV block can be manifested. Classic, type I AV block with progressive PR prolongation to more than 40 msec during at least three beats before the blocked P waves was seen in only 50% of patients. Another pattern observed was a subtler Wenckebach periodicity with minor PR prolongation of 20 to 40 msec before a blocked P wave in 29% of patients. A third pattern, seen in 8% of patients and termed pseudo–Mobitz type II AV block, demonstrated nearly constant P-R intervals before the blocked P wave, followed by PR shortening on the subsequent conducted beat (see Fig. 14-3). Classic Mobitz type II second-degree AV block, with constant P-R intervals for at least three beats before the blocked P wave, followed by the same P-R interval after the blocked P wave, was seen in 4% of patients (see Fig. 14-4). A mixed, type I Wenckebach and pseudo–Mobitz type II AV block was seen in 6% of all patients. Of patients showing periods of pseudo–Mobitz type II block, 44% also demonstrated classic Wenckebach conduction patterns at some time.12 Slowing of the sinus cycle length often preceded the blocked P wave in both classic and pseudo–Mobitz type II AV blocks (Fig. 14-5).
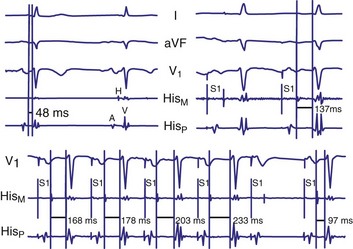
Diagnosis of the site of AV block may be problematic with 2 : 1 atrioventricular block, which should not be classified as type I or type II block. The anatomic site in 2 : 1 AV block can be in the AV node or His-Purkinje system (Fig. 14-6). The most likely site of AV block can often be determined by noting the company that the 2 : 1 AV block keeps. When 2 : 1 AV block is associated with a wide QRS complex, the block is in the His-Purkinje system in 80% and the AV node in 20% of cases (Fig. 14-7). A long P-R interval (>0.30 second) on conducted beats during 2 : 1 AV block with a narrow QRS complex suggests an AV nodal site, whereas a normal P-R interval favors intra-Hisian block.
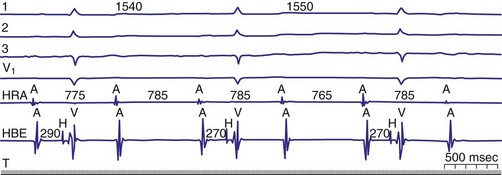
Figure 14-6 Spontaneous 2 : 1 (high-grade) atrioventricular (AV) block localized to the AV node.
(From Josephson ME: Clinical cardiac electrophysiology: techniques and interpretations, ed 3. Philadelphia, 2002, Lippincott–Williams & Wilkins, pp 92-109.)
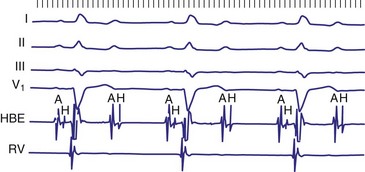
Figure 14-7 Intracardiac tracing of 2 : 1 second-degree atrioventricular (AV) block located in the His-Purkinje system.
(From Josephson ME: Clinical cardiac electrophysiology: techniques and interpretations, ed 3, Philadelphia, 2002, Lippincott–Williams & Wilkins, pp 92-109.)
In general, the response of the block, particularly 2 : 1 AV block, to pharmacologic agents may help to determine the site. Atropine generally improves AV conduction in patients with AV nodal block; however, atropine is expected to worsen conduction in patients with block localized to the His-Purkinje system, because of its effect on increasing sinus rates without improving His-Purkinje conduction (Fig. 14-8). Carotid sinus stimulation is expected to worsen block localized to the AV node, whereas it either has no effect or improves conduction in patients with His-Purkinje system disease by causing sinus node slowing. The effect of any given drug, however, may be difficult to predict because its effect on the sinus node may be greater than its effect on the AV node. For example, atropine may improve AV node conduction, but if atropine causes excessive SA node acceleration, AV conduction may improve marginally or not at all. The response to infusion of isoproterenol is less clear. Isoproterenol may improve conduction disorders localized in the AV node as well as occasionally in the His-Purkinje system.
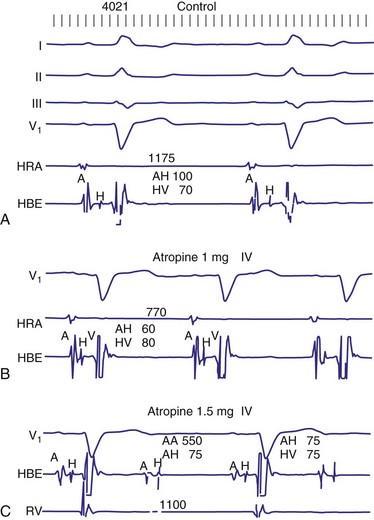
Figure 14-8 His-Purkinje atrioventricular block after atropine-induced increase in sinus rate.
(From Miles WM, Klein LS: Sinus nodal dysfunction and atrioventricular conduction disturbances. In Naccarelli GV: Cardiac arrhythmias: a practical approach. Mt Kisco, NY, 1991, Futura, pp 243-282.)
The diagnosis of complete heart block (CHB) rests on demonstration of complete dissociation between atrial and ventricular activation. Care must be taken to distinguish transient AV dissociation caused by competing atrial and junctional or ventricular rhythms with similar rates (so-called isorhythmic AV dissociation). If sufficiently long monitoring strips are available, intermittent conduction of appropriately timed atrial events is seen. Temporary atrial pacing can be performed to accelerate the atrial rate to overdrive the competing junctional or ventricular arrhythmia, demonstrating intact AV conduction. In the presence of atrial fibrillation (AF), CHB can be inferred when the ventricular rate becomes regular rather than the typical, irregular ventricular response (Fig. 14-9). Digoxin toxicity may be the cause of heart block with AF, and this and other drug toxicity should be ruled out before one assumes that structural AV conduction disease is present. In patients with chronic AF, regular R-R intervals may occasionally be caused by “concealed” sinus rhythm (i.e., no evidence of atrial activity because of very-low-amplitude P waves) and not heart block or digitalis intoxication.13
The escape rhythm in CHB may be generated by the AV junction, His bundle, bundle branches, or distal conduction system. Rarely, the underlying rhythm arises from the ventricular myocardium or, for all practical purposes, is absent. The site of AV block is important in that it determines to a great extent the rate and reliability of the underlying escape rhythm. The site of origin of the escape rhythm in cases of advanced AV block is more important than the escape rate itself.7 For example, in heart block associated with inferior AMI or congenital CHB, the escape rhythm is usually generated by the AV junction, and permanent pacing may not be required. Nevertheless, it is worth emphasizing that symptomatic AV block requires pacing regardless of the site, morphology, or rate of the escape rhythm.
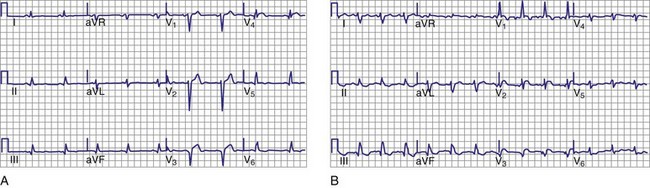
Trifascicular block is present when bifascicular block is associated with HV prolongation. However, trifascicular block also is often applied loosely to the electrocardiographic patterns of bifascicular block—right bundle branch block (RBBB) plus left anterior hemiblock, RBBB plus left posterior hemiblock, or left bundle branch block (LBBB)—plus first-degree AV block. Using “trifascicular block” to describe these AV conduction disturbances on the ECG is misleading, because the site of block in such cases may be located in the AV node or His-Purkinje system.9 The P-R interval does not identify patients who have prolonged H-V intervals in such cases. Up to 50% of patients with bifascicular block and prolonged P-R intervals have prolongation of the A-H interval (i.e., AV nodal conduction time).14 Trifascicular block should be used only to refer to alternating RBBB and LBBB, RBBB with a prolonged H-V interval (regardless of the presence or absence of left anterior or posterior fascicular block), and LBBB with a prolonged H-V interval. In addition, the term can be used in a patient with second- or third-degree AV block in the His-Purkinje system with (a) permanent block in all three fascicles, (b) permanent block in two fascicles with intermittent conduction in the third, (c) permanent block in one fascicle with intermittent block in the other two fascicles, or (d) intermittent block in all three fascicles. Thus, according to its strict definition, when interpreting an ECG in the absence of a His bundle recording, trifascicular block should be applied only to the patterns of alternating RBBB and LBBB or RBBB with intermittent left anterior and posterior hemiblocks. These situations are class I indications for permanent pacing, even in asymptomatic individuals.
Electrophysiologic Study
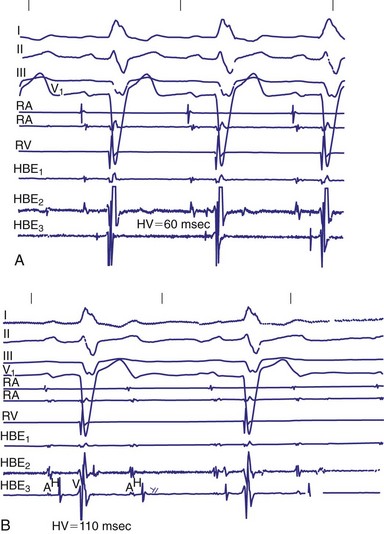
Invasive electrophysiologic study (EPS; e.g., His bundle recording) is a useful means of evaluating AV conduction in patients who have symptoms and in whom the need for permanent pacing is not obvious (Fig. 14-10). Electrophysiologic studies (strictly for evaluation of the conduction system and site of block) are not required in patients with symptomatic high-grade or complete AV block recorded on surface ECGs, ambulatory Holter monitoring, or transtelephonic recordings. The need for permanent pacing has already been established in these patients (class I indication). However, EPS may be indicated in patients with high-grade AV block if another arrhythmia is suspected or is a likely cause of symptoms. For example, even if high-grade AV block is documented on spontaneous recordings, ventricular tachycardia may still be the cause of syncope in patients with extensive AMI. In patients with alternating BBB, EPS almost invariably demonstrates a high degree of His-Purkinje system disease. These patients typically have very long H-V intervals and are at very high risk of progression to CHB in a short time. Pacing in these patients is indicated on clinical grounds, and EPS may not be necessary.7 Electrophysiologic studies are also not indicated in patients whose symptoms are shown not to be associated with a conduction abnormality or block. In addition, patients without symptoms who have intermittent AV block associated with sinus slowing, gradual PR prolongation before a nonconducted P wave, and a narrow QRS complex should not undergo EPS, given the benign prognosis of these findings.
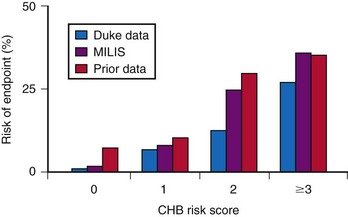
The incidence of progression of bifascicular block to CHB is variable, ranging from 2% to 6% per year. The method of patient selection affects this incidence, with patients who have asymptomatic bifascicular block progressing to CHB at a rate of 2% per year, and patients with symptoms (e.g., syncope or presyncope) progressing at a rate closer to 6% per year.15
Three large studies of patients with chronic BBB have been performed to assess the role of His bundle conduction (e.g., H-V interval) measurements in predicting progression to CHB. The measurement of the H-V interval represents the conduction time through the His bundle and bundle branches until ventricular activation begins. Because these studies included both asymptomatic and symptomatic patients, care must be taken to ensure that similar patient populations are compared for proper interpretation of these results. Dhingra et al.15 prospectively followed 517 patients with BBB and measured the time required for progression to second- and third-degree block. Only 13% of patients presented with syncope; the others had no symptoms. The cumulative 7-year incidence of progression to AV block was 10% in the group with a normal H-V interval and 20% in the group with H-V interval prolongation. The cumulative mortality rate at 7 years was 48% in patients with a normal H-V interval and 66% in patients with a prolonged H-V interval. This study emphasized that despite the high mortality associated with the presence of bifascicular block, there is only a low rate of progression to more advanced AV block.
McAnulty et al.16 studied 554 patients with “high-risk” BBB, defined as LBBB, RBBB and left-axis or right-axis deviation, RBBB with alternating left- and right-axis deviation, or alternating RBBB and LBBB. The cumulative incidence of AV block, either type II second-degree or CHB, was 4.9%, or 1% per year, in patients with a prolonged H-V interval and 1.9% in patients with a normal H-V interval (difference not significant). H-V interval prolongation did not predict a higher risk of development of CHB. After entry into the study, 8.5% of patients experienced syncope. The incidence of complete AV block was 17% in patients with syncope versus 2% in patients without a history of syncope.
Scheinman et al.17 studied 401 patients with chronic BBB for about 30 months. This study, in contrast to the Dhingra15 and McAnulty16 studies, primarily included patients with symptoms referred for EPS. About 40% of the patients in the Scheinman study had a history of syncope.17 In patients with an H-V interval of more than 70 msec, the incidence of progression to spontaneous second- or third-degree AV block was 12%. The incidence of complete AV block was 25% for those with an H-V interval of 100 msec or greater. The yearly incidence of spontaneous AV block was 3% in those with a normal H-V interval and 3.5% in those with a prolonged H-V interval.
These findings therefore suggest a relationship between a prolonged H-V interval and development of CHB during the ensuing years in patients with intraventricular conduction disturbances. It also seems that the risk varies directly with the extent of HV prolongation. Symptomatic patients with syncope or presyncope are at much higher risk than asymptomatic patients. The overall risk of CHB in an unselected, symptom-free group of patients with chronic BBB is low (<6%/yr). Current recommendations are not to perform EPS in patients with asymptomatic BBB.18
A number of studies evaluated the clinical usefulness of EPS in patients with BBB and syncope. Electrophysiologic studies were performed before the current era of ICD implantation in patients with syncope and advanced structural heart disease. In one study, 112 patients with chronic BBB and syncope or near-syncope underwent EPS.19 A normal result predicted a good long-term prognosis. About 25% of patients had significant conduction system disorder, underwent pacemaker implantation, and experienced recurrence of symptoms at a rate of only 6%. In the study reported by Morady et al.,20 28% of patients (7 of 32) had sustained, induced monomorphic ventricular tachycardia (VT), whereas about 20% had conduction disturbances at EPS. Six of the seven patients who received pacemakers had no recurrent symptoms.
Identifying Patients at Risk for AV Block
In patients with symptomatic BBB or bifascicular block, EPS with measurement of H-V intervals has been used for several decades. A “markedly prolonged” H-V interval (≥100 msec) is predictive of development of symptomatic heart block. As noted earlier, Scheinman et al.17 demonstrated that an H-V interval of 100 msec or longer identified a group of patients who had a 25% risk of development of heart block over a mean follow-up of 22 months. According to current guidelines published by the American College of Cardiology (ACC), American Heart Association (AHA) Task Force on Practice Guidelines, and North American Society for Pacing and Electrophysiology (NASPE), an H-V interval of 100 msec or longer in an asymptomatic patient documented as an incidental finding on EPS is a class IIa indication for permanent implantation of a pacemaker.21 Although the finding of a markedly prolonged H-V interval is quite specific, it is very insensitive because H-V intervals of 100 msec or longer are uncommon.
Atrial pacing to stress the His-Purkinje system may provide additional information to identify patients at risk of spontaneous AV block. Healthy subjects do not experience second- or third-degree infra-Hisian block during atrial pacing when the atrial rate is gradually increased, as would occur spontaneously. Certain pacing protocols with abrupt onset of pacing at rapid rates are more likely to induce infra-Hisian block, even in healthy subjects, but even this finding is rare at pacing rates below 150 beats per minute (bpm) in healthy individuals. Because AV nodal dysfunction is frequently seen in patients with significant His-Purkinje system disease, AV nodal block may occur at lower pacing rates than those necessary to demonstrate infra-Hisian block. This “protective” effect of AV nodal dysfunction during resting states may lead to the incorrect conclusion that significant His-Purkinje disease is not present. However, a second trial of atrial pacing after administration of atropine or isoproterenol to facilitate AV nodal conduction may demonstrate infra-Hisian block. Dhingra et al.22 reported a 50% rate of progression to type II or complete AV block in patients in whom block develops distal to the His bundle at paced rates of less than 150 bpm. In a later study, Petrac et al.23 evaluated 192 patients with chronic BBB and syncope, of whom 18 (9%) had incremental atrial pacing–induced infra-Hisian second-degree AV block at a paced rate of 150 bpm or less (mean pacing rate, 112 ±10 bpm). During a mean follow-up of 68 ±35 months, 14 of the 18 patients (78%) demonstrated spontaneous second- or third-degree AV block, confirming that this abnormal finding identifies a subgroup at a high risk for development of heart block. As with an H-V interval of 100 msec or less, however, His-Purkinje block during incremental atrial pacing at physiologic rates is uncommon in patients with BBB and syncope. According to current guidelines, if atrial pacing–induced infra-Hisian block that is “not physiologic” is demonstrated as an incidental finding on EPS, permanent pacing is recommended (class IIa indication).21
Provocative drug tests have been suggested as another means of evaluating the distal conduction system24 (Fig. 14-11). Pharmacologic stress testing may be considered in patients with BBB and syncope who have a baseline H-V interval of 70 msec or higher (but <100 msec) and no infra-Hisian block demonstrated. Data describing the experience with intravenous type Ia (procainamide, ajmaline, disopyramide) or type Ic (flecainide) antiarrhythmic drugs are limited.15,25–27 Only intravenous procainamide is available in the United States. Administration of these agents may result in a marked increase in the H-V interval (>15-20 msec), an H-V interval greater than 100 msec, or precipitation of spontaneous type II second- or third-degree AV block, all of which may indicate a higher risk for development of CHB. Intravenous disopyramide has the potential benefit of facilitating AV nodal conduction by its anticholinergic properties while accentuating underlying infra-Hisian disease through its membrane-stabilizing effects.26 Tonkin et al.27 administered procainamide at a dose of up to 10 mg/kg to 42 patients with BBB and syncope and produced intermittent second- or third-degree His-Purkinje block or H-V prolongation to more than 15 msec during sinus rhythm in 11 patients (26%).28 However, only 2 of the 11 patients (18%) with a positive result had documented high-grade AV block during 38 months of follow-up, and three of five asymptomatic control patients with BBB (60%) had a positive procainamide challenge test result. Other studies of class I antiarrhythmic drug testing to stress the His-Purkinje system likewise have limited patient numbers, lack adequate control groups or follow-up, and seem to indicate that pharmacologic testing has low predictive value. Current permanent pacing guidelines do not include a recommendation regarding the need for permanent pacing on the basis of results of pharmacologic stress testing of the His-Purkinje system.
Electrophysiologic testing has recognized limitations for identifying patients with significant AV nodal or His-Purkinje dysfunction. Although finding an abnormality on EPS may be helpful, its sensitivity is low and cannot be used alone to exclude a significant AV conduction disturbance. In a small study by Fujimura et al.,29 13 patients with documented symptomatic transient second- or third-degree AV block referred for implantation of a permanent pacemaker underwent AV conduction testing at pacemaker insertion. These tests included facilitation of AV nodal conduction with atropine and pharmacologic stress of His-Purkinje conduction with low doses of procainamide. Surprisingly, only 2 of the 13 patients showed significant abnormalities in the AV conduction system (inducible infra-Hisian block in both cases) during EPS, yielding a sensitivity of 15.4%. Two other patients had moderately prolonged H-V intervals, although much shorter than 100 msec. If these two patients are included in the diagnostic data, the sensitivity of EPS is increased to 46%, raising important questions about EPS sensitivity for identifying patients at risk for symptomatic AV block.
 Classification, Epidemiology, and Natural History of Atrioventricular Conduction Disturbances
Classification, Epidemiology, and Natural History of Atrioventricular Conduction Disturbances
First-Degree AV Block
The prognosis and natural history in patients with primary first-degree AV block and moderate PR prolongation traditionally has been thought of as being benign. Progression to CHB over time occurred in about 4% of patients in the Mymin et al.30 study, which involved only young, male U.S. Air Force pilots. Most of the patients (66%) had only mild to moderate PR prolongation, to about 0.22 to 0.23 second. In the great majority of subjects, the P-R interval remained within a narrow range, changing by less than 0.04 second. More recently, a Framingham Heart Study report challenged the long-held belief that first-degree AV block has a benign prognosis.31 In this large, community-based sample with long-term follow-up, patients with first-degree AV block (PR > 200 msec) at baseline were at substantially increased risk of future atrial fibrillation (about twofold) and pacemaker implantation (about threefold) and a moderately increased risk of all-cause mortality, compared with individuals without first-degree AV block.
Patients with “markedly prolonged” P-R interval may or may not be symptomatic at rest, but may demonstrate a pseudo–pacemaker syndrome caused by AV dyssynchrony, particularly during exertion. These events are more likely to become symptomatic during exercise, because the P-R interval may not shorten appropriately as the R-R interval decreases. Zornosa et al.32 described PR prolongation after radiofrequency ablation resulting from injury of the fast–AV nodal pathway in patients with AV nodal reentry. Symptoms caused by long P-R intervals resolved after DDD pacing was performed. Kim et al.33 also described a patient with intermittent failure of fast-pathway conduction who experienced lightheadedness, weakness, and chest fullness when the P-R interval suddenly shifted from between 160 and 180 msec to 360 msec; this patient’s condition improved after pacing.
Implantation of permanent pacemakers is reasonable in patients with first-degree AV block with symptoms similar to those of pacemaker syndrome or hemodynamic compromise (class IIa indication).21 Previous versions of the ACC/AHA/NASPE guidelines required documentation of alleviation of symptoms with temporary AV pacing before permanent pacing. However, this requirement was removed in the 2002 revision of the guidelines, in part because a temporary AV pacing study may not demonstrate symptomatic improvement at rest and is often impractical to perform during exercise. Thus, it is reasonable to institute permanent pacing in symptomatic patients with extremely long P-R intervals (≥0.30 sec) that do not shorten during exercise. Permanent pacemaker implantation may also benefit patients with left ventricular (LV) systolic dysfunction, congestive heart failure, and marked first-degree AV block (>0.30 sec) in whom a shorter A-V interval results in hemodynamic improvement. An acute study in patients with first-degree AV block suggested that systolic performance measured using a Doppler echocardiography–derived aortic flow time-velocity integral improved after institution of DDD pacing at a rate of 70 bpm if the intrinsic AV conduction time (A-R interval) was longer than 0.27 second.34 The optimal AV delay in this study was 159 ± 22 msec, which is consistent with most other studies, in that the optimal AV delay is about 150 msec at rest. However, given the need for frequent ventricular pacing support to optimize the A-V interval in these patients, atrio-biventricular pacing is likely to be the optimal long-term pacing mode in these patients with LV systolic dysfunction and heart failure.
Second-Degree AV Block
Controversy surrounds the prognosis and need for permanent pacing in patients with chronic type I second-degree AV block in the presence of a narrow QRS complex. Some consider this condition benign only in young people or athletes without organic heart disease. The natural history of 56 patients with chronic type I second-degree AV block, some of whom were younger than 35 or were well-trained athletes, was described in 1981 by Strasberg et al.35 They concluded that progression to CHB is relatively uncommon in this patient population, and that this finding carries a benign prognosis in the absence of structural heart disease. In 1985, however, Shaw et al.36 suggested that patients with type I second-degree AV block have a worse prognosis than age- and gender-matched individuals, unless the patients already had permanently implanted pacemakers. The 214 patients with chronic second-degree AV block (mean age, 72) were divided into three groups—type I block (77), type II block (86), and 2 : 1 or 3 : 1 block (51)—and monitored over a 14-year period. The 3-year and 5-year survival rates were similarly poor, regardless of the type of AV block. Patients with type I block without BBB fared no better than those with type II block. Patients with type I second-degree AV block who received permanent pacemakers had survival similar to that of an age- and gender-matched control population.
In 1991 the British Pacing and Electrophysiology Group (BPEG) suggested that pacing should be considered in adults in whom type I second-degree AV block occurs during much of the day or night, regardless of the presence or absence of symptoms.37 In 2004, Shaw et al.38 reported again on the prognosis of patients with type I second-degree AV block and once again concluded that type I second-degree AV block is not a benign condition in patients 45 years or older. The majority of their patients with type I second-degree AV block who were 45 years or older progressed to higher-degree AV block, experienced symptomatic bradycardia, or died prematurely if they did not receive pacemakers. These investigators recommended pacemaker implantation in patients with type I second-degree AV block even in the absence of symptoms or structural heart disease, except in those younger than 45. According to current ACC/AHA/NASPE guidelines, however, permanent pacemaker implantation in asymptomatic type I second-degree AV block that is at the supra-His (AV node) level or is not known to be intra-Hisian or infra-Hisian is considered insupportable by current evidence (class III indication). It seems prudent, on the basis of available data, at least to monitor closely any elderly patient with asymptomatic type I AV block or 2 : 1 AV block with narrow QRS complexes, because these electrocardiographic abnormalities may be markers for progressive conduction system disease.
The natural history of asymptomatic type II second-degree AV block initially was addressed in a University of Illinois study reported in 1974 that found most patients experienced symptoms within a relatively short period.39 In 1985, Shaw et al.36 reported that 86 patients (mean age, 74) monitored between 1968 and 1982 with chronic Mobitz type II second-degree heart block had a 5-year survival rate of 61%. The 5-year survival of those who underwent permanent pacemaker implantation was significantly better than those who did not. These observations form the basis for recommendations to institute permanent pacing in all patients with type II second-degree AV block, regardless of symptoms (class IIa indication with a narrow QRS; class I recommendation with a wide QRS).21
Complete Heart Block
The natural history of spontaneously developing asymptomatic CHB in adult life predates pacemaker therapy.40–42 Currently, almost all adult patients with CHB eventually have symptoms and undergo pacemaker placement. Several studies in the 1960s emphasized the poor prognosis of patients with CHB. The 1-year survival rate of patients who experienced Stokes-Adams attacks caused by CHB and who did not receive pacing was only 50% to 75%, significantly less than that of a gender- and age-matched control population.41 The “best” prognosis was in patients with an idiopathic or unknown cause of CHB. At least 33% of deaths were related to CHB and Stokes-Adams attacks. These differences in survival persisted even after 15 years of follow-up and appear to be related to the considerably higher incidence of sudden death.42 Some debate whether the presence of syncope is associated with a worse prognosis in patients with documented CHB. The prognosis for transient CHB was poor as well, with 36% 1-year mortality reported in at least one 1970s study.41 Whether this poor prognosis applies now to patients with transient CHB who have not received pacing is unknown.
Edhag and Swahn41,42 reported in the 1960s and 1970s on the long-term prognosis of 248 patients with high-grade AV block, most of whom had complete heart block, with a mean 6.5 years of follow-up. The mean age at pacemaker implantation was 66, and the 1-year survival of patients who received pacemakers was 86%, versus 95% for an age- and gender-matched group of Swedish patients. After the first year, survival in the patients with pacemakers was similar to that in the general population. Edhag42 compared survival in different age groups, found no difference in survival between elderly patients with heart block who underwent permanent pacing and the age- and gender-matched general population. In contrast, younger patients with heart block had an increased mortality even after pacing than controls. This higher mortality likely is a reflection of the underlying structural heart disease responsible for high-grade AV block.
Complete heart block can be described as acute or chronic depending on its onset. Acute AV block associated with myocardial ischemia is rare but may occur and result in transient AV block. High-grade AV block is strictly defined as 3 : 1, 4 : 1, or higher AV ratios in which AV synchrony is intermittently present. As in complete AV block, high-grade AV block may be localized anywhere in the conduction system (Fig. 14-12). In some patients, high-grade AV block may be present at multiple levels in the conduction system. Generically, “high-grade AV block” has been used to describe any form of AV block that suggests an increased risk for CHB or symptomatic bradycardia. This typically includes type II second-degree block, 2 : 1 AV block, strictly defined high-grade AV block, and CHB. The generic use of high-grade AV block is best avoided, because the multiple forms of AV block included have variable pathogeneses and prognoses that blur the clinical usefulness of the term.
Paroxysmal AV Block
Paroxysmal AV block is defined as the sudden occurrence, during a period of 1 : 1 AV conduction, of a block of sequential atrial impulses resulting in a transient total interruption of AV conduction.43–45 It is thus the onset of a paroxysm of high-grade AV block associated with a period of ventricular asystole before conduction returns or a subsidiary pacemaker escapes. Paroxysmal AV block may occur in a variety of clinical conditions but has been described most often in association with vagal reactions, such as during vomiting, coughing, or swallowing, after urination, or with abdominal pain, carotid sinus massage, coronary angiography, or head-up tilt-table testing. Patients with neurally mediated syncopal syndromes may have transient heart block, typically with associated sinus slowing. On the other hand, paroxysmal AV block also may occur in patients with severe, distal conduction disease. This may manifest as tachycardia-dependent AV block in the His-Purkinje system (as also called phase 3 or voltage-dependent block) during or after exertion; with the abrupt onset of bradycardia or after a pause (phase 4 block); and in type II second-degree AV block. Paroxysmal AV block in these patients (not related to vagal AV block) is a marker for His-Purkinje disease, with an unpredictable escape mechanism. Permanent pacemaker implantation in these patients (with the possible exception of block mediated by acute myocardial ischemia) is almost always indicated.45
One report described the clinical experience in 20 patients (mean age, 63 + 14 years) with paroxysmal AV block seen at a single institution over a 12-year period.46 Paroxysmal AV block in these patients was related to a vagal reaction, AV-blocking drugs, or distal conduction disease. The AV block in these patients lasted from 2.2 to 36 seconds. Fifteen patients experienced syncope, and one patient had bradycardia-induced polymorphic VT that required electrical cardioversion. About one-half the patients had structural heart disease and a wide QRS duration.
Complete AV block can be classified as congenital or acquired. In patients with acquired complete AV block, the site of block is localized distal to the His bundle in about 70% to 90% of patients, to the His bundle in 15% to 20%, and within the AV node in 16% to 25%.7 In patients with congenital complete AV block, the escape rhythm is more often found in the proximal His bundle or AV node.
Bundle Branch Block
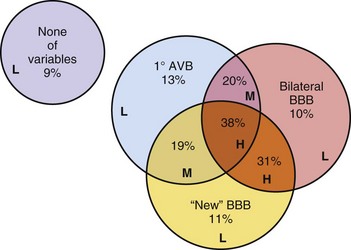
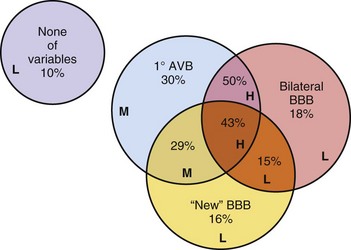
Most patients with chronic BBB or bifascicular block have underlying structural heart disease (prevalence, 50%-80%).15–1719 Historically, it was believed that progression from chronic bifascicular block to trifascicular block was common. Retrospective studies in patients with chronic bifascicular block suggested that the risk of progression to complete AV block was 5% to 10% per year. In the early 1980s, the results of several large prospective studies questioned assumptions about the incidence and clinical implications of the progression of conduction system disease in this patient population. Prospective studies of groups of symptom-free patients with bifascicular block who were found to have prolonged H-V intervals on EPS showed that such patients are at increased risk for CHB, but that the absolute risk remains very low, about 1% to 2% per year.15,17 McAnulty et al.16 found that the risk for development of CHB was 5% in 5 years. A prolonged H-V interval was associated with higher values for both total cardiovascular mortality and sudden death. Prolonged H-V interval is likely associated with more extensive structural heart disease. Furthermore, these studies demonstrated that in the absence of symptoms, routine His bundle recordings are of limited usefulness in patients with bifascicular block. Asymptomatic individuals with chronic BBB need no further evaluation than an occasional ECG.
On the other hand, patients with syncope and bifascicular block represent a different clinical problem. If a thorough clinical evaluation, including a history, physical examination, and ECG, do not uncover a cause of syncope, EPS may be useful.19,20 Linzer et al.47 found that the presence of first-degree AV block or BBB increased the odds ratio of finding abnormalities suggesting risk of bradyarrhythmia (predominantly heart block) by three- to eightfold during EPS in patients with unexplained syncope (Table 14-1). Electrophysiologic studies may uncover other causes of syncope, such as sinus node dysfunction, rapid supraventricular tachycardias, and inducible monomorphic ventricular tachycardia. In some studies, monomorphic VT was inducible in at least 30% of patients with BBB and syncope.19,20 A minority of patients are found to have a markedly prolonged H-V interval, abnormal or fragmented His bundle electrogram, or block distal to the His bundle with atrial pacing, suggesting the need for implantation of a permanent pacemaker.
TABLE 14-1 Odds Ratio for Abnormality on Electrophysiologic Testing in Patients with Syncope
| Clinical Variables | Multivariable | 95% Confidence Interval for Multivariable |
|---|---|---|
| Age | 1.01 | 0.99-1.03 |
| Duration (months) | 1.00 | 0.98-1.02 |
| Gender (male) | 1.76 | 0.79-3.93 |
| Organic heart disease | 1.53 | 0.71-3.33 |
| Sudden loss of consciousness | 1.93 | 0.89-4.16 |
| Left ventricular ejection fraction | 0.99 | 0.93-1.06 |
| Electrocardiogram | ||
| Bundle branch block | 2.97* | 1.23-7.21 |
| Sinus bradycardia | 3.47* | 1.12-10.71 |
| First-degree heart block | 7.89† | 2.12-29.31 |
| Premature ventricular contractions | 1.47 | 0.37-5.82 |
| Holter Monitoring | ||
| Sinus bradycardia | 0.68 | 0.21-2.23 |
| Sinus pause | 1.04 | 0.26-4.23 |
| Mobitz I atrioventricular block | 0.63 | 0.06-6.33 |
| Premature ventricular contractions | 0.87 | 0.35-2.13 |
Modified from Linzer M, Prystowsky EN, Divine GW, et al: Predicting the outcomes of electrophysiologic studies of patients with unexplained syncope: preliminary validation of a derived model. J Gen Intern Med 6:113, 1991.
Congenital AV Block
Congenital CHB traditionally was diagnosed in the first month after birth in a child in whom a slow heart rate was detected and certain infectious etiologies rarely seen today, such as diphtheria, rheumatic fever, and congenital syphilis, were excluded.48,49 Currently, with fetal echocardiography, many cases are diagnosed in utero and, if associated with structural heart disease, are associated with a high rate of fetal death. The incidence of congenital CHB is estimated to be 1 in 15,000 to 22,000 live births. More than one half of fetuses found to have congenital CHB have structural heart disease, including congenitally corrected transposition of the great arteries, and often have a poor prognosis in infancy. When congenital CHB is detected in utero in a child with a structurally normal heart, the condition is frequently associated with intrauterine exposure to maternal autoantibodies to Ro and La (i.e., neonatal lupus); this situation has a better prognosis than when congenital heart disease is present. The development of AV block in a child with a structurally normal heart is uncommon but should not be confused with congenital CHB. Childhood-onset heart block often is presumed to be caused by viral myocarditis. In patients with congenital CHB, the mean resting heart rate is between 40 and 60 bpm, but decreases with age.
Although controversial in the past, the indications for permanent pacemaker implantation in young patients with congenital CHB have evolved on the basis of improved understanding of the natural history of the disease. Current guidelines indicate that permanent pacemaker implantation should be performed for congenital CHB patients with a wide QRS escape, complex ventricular ectopy, ventricular dysfunction, a ventricular rate less than 55 bpm, or congenital heart disease and a ventricular rate less than 70 bpm (class I indications).21 Permanent pacemaker implantation may also be considered for patients after the first year of life with an average heart rate less than 50 bpm, abrupt pauses in heartbeat that are two or three times the basic cycle length, or associated with exercise intolerance caused by chronotropic incompetence (class IIa indications).
In asymptomatic children or adolescents with an acceptable rate, a narrow QRS complex, and normal ventricular function, a permanent pacemaker also may be considered (class IIb). Pacemaker implantation in asymptomatic patients with congenital CHB is associated with improved long-term survival and prevention of syncope. However, it must be recognized that ventricular dysfunction caused by right ventricular (RV) pacing–associated dyssynchrony may occur years or decades after pacemaker implantation. A 2004 study demonstrated that long-term transvenous RV apical pacing was associated with deleterious LV remodeling and reduced exercise capacity after 10 ±3 years of follow-up in patients with congenital CHB.50 Therefore, these patients require periodic evaluation of ventricular function after pacemaker implantation. In addition, alternative ventricular pacing sites, including RV septum and left ventricle, have been proposed in patients with congenital CHB, who may require many decades of ventricular pacing but have not been definitively defined. Ventricular dysfunction in this setting may result from myocardial autoimmune disease in association with RV pacing–induced ventricular dyssynchrony. Interestingly, a recent study suggests that the natural history of patients with isolated congenital CHB who require pacing depends on their antibody status.51 Antinuclear antibody (ANA) status was a predictor for the development of heart failure and death. Long-term RV pacing was not associated with development of congestive heart failure, deterioration in ventricular function, or reduced survival in congenital AV block patients without ANAs.
Inherited Conduction System Diseases
Inherited causes of AV conduction disturbances have been increasingly recognized and better understood over the last decade. The genetics of conduction disease represents an exciting new development in our understanding of the AV conduction system.52,53 Although at present no clear role exists for genetic testing in most forms of conduction system disease, knowledge is still limited in this area. Animal models have provided insights by elucidating molecular genetic causes of AV block. Many of the identified cases of inherited AV conduction disease previously would have been classified as idiopathic or having an unknown cause. Although inheritance makes no obvious contribution to most human conduction disease, familial clustering in cases of “idiopathic” conduction system degeneration is consistent with a hereditary basis. Some cases with familiar clustering have an autosomal dominant pattern of inheritance and associated congenital heart malformations and cardiomyopathy. However, inherited conduction system disease is not a single entity caused by a single gene mutation. Inherited conduction system disease may be associated with congenital heart diseases, neuromuscular disorders, and cardiomyopathies. Inherited conduction diseases include developmental transcription factor mutations, cardiac ion channelopathies, and mutations in genes regulating energy metabolism, gap junctions, and structural proteins.
Mutations in genes encoding cytoskeletal and nuclear membrane proteins are involved in the muscular dystrophies, including myotonic dystrophy, Emery-Dreifuss muscular dystrophy, and limb-girdle muscular dystrophy type 1B. All these neuromuscular disorders may be associated with cardiac conduction defects. Myotonic dystrophy is the most common form of muscular dystrophy. This autosomal dominant inherited disorder is caused by an expansion of cytosine-thymine-guanine repeat on chromosome 19.54 High-degree AV block and BBB are the most common conduction system defects.55 A mouse knockout model of the myotonic dystrophy protein kinase displays first-, second-, and third-degree AV block.56 The mechanism of AV nodal pathology is thought to be caused by alterations in the activation kinetics or amplitude of the ICa,L current.
Mutations in the gene encoding for the inner nuclear membrane protein lamin A/C (LMNA) cause Emery-Dreifuss muscular dystrophy with conduction system defects.57 Progressive conduction disease is present in virtually all cases and leads to the need for permanent pacing. LMNA also is implicated in a variety of other diseases including Hutchinson-Gilford syndrome, mandibuloacral dysplasia, Charcot-Marie-Tooth disease–type atypical Werner’s syndrome, and the Dunnigan type familial partial lipodystrophy, most of which also are associated with cardiac conduction system defects.53 Lamin A/C is necessary for the structural integrity of the nucleus; in the presence of LMNA mutation, myocardial cells exposed to mechanical stress undergo cell damage. LMNA mutations also are associated with dilated cardiomyopathy, with AV conduction defects referred to as “laminopathy.”58 Otomo et al.59 described a large Japanese family with 21 of 224 members affected by this genetic defect, which manifests clinically as progressive AV block, dilated cardiomyopathy, heart failure, and sudden death. EPS in affected individuals demonstrated AV nodal dysfunction (marked prolongation of A-H interval) and normal HV and QRS durations. Histologic evaluation of postmortem heart specimens from affected members showed preferential degeneration, with replacement by fibrofatty tissue of the AV nodal region. Patients may die suddenly at a young age. Of note, there may be a gender difference in the severity of the cardiac phenotypes seen in lamin A/C disease. Males often have significant cardiac disease, with moderate or severe LV dysfunction developing in the first two to three decades of life, whereas females with the same mutations are more likely to have progressive conduction disease, with less severe LV dysfunction.
Mutations in the cardiac-specific sodium channel gene (SCN5A) have been associated with progressive cardiac conduction system disease.60 Interestingly, SCN5A mutations can produce a variety of other phenotypes, including Brugada syndrome, congenital type 3 long QT syndrome (LQTS), idiopathic ventricular fibrillation, congenital sick sinus syndrome, atrial fibrillation, and dilated cardiomyopathy. An overlap syndrome involving a mutation in the cardiac sodium channel is associated with conduction system disease along with LQTS and Brugada syndrome.61,62 Homozygous mutations in the SCN5A gene can cause a highly lethal combination of LQTS and 2 : 1 AV block in infants.63 SCN5A mutations reduce cardiac sodium current, resulting in decreased action potential upstroke velocity and slowed impulse propagation, mainly in fast-conducting sodium channel–dependent conduction tissue. SCN5A mutation carriers often have long P waves and prolonged P-R and QRS intervals with normal A-H and prolonged H-V intervals, indicative of infra-Hisian delays.64 At present, at least 11 SCN5A mutations seem to be causally related to inherited cardiac conduction system disease.64–69 A novel SCN5A mutation involving a heterozygous single-nucleotide mutation resulted in an amino acid substitution (A1180V) in a three-generation Chinese family.69 The mutation causes a negative shift of voltage-dependent inactivation of the cardiac sodium channels with slower recovery, leading to a rate-dependent sodium current reduction and a moderate increase in late sodium current. The A1180V mutation was associated with familial, progressive, adult-onset AV block in the third decade of life that preceded the subsequent development of dilated cardiomyopathy. Interestingly, early signs of the sodium channel defect in unaffected carriers of the pedigree were detected in the ECG manifested by QRS widening at high heart rates and QTc prolongation at rest. This suggests a possible opportunity for early diagnosis of the mutation before development of a clinically significant conduction system defect or cardiomyopathy, even in the absence of a genetic test.
Defects in potassium channel genes may be a molecular basis for some inherited AV conduction disturbances in humans. The LQT7 syndrome, or Andersen-Tawil syndrome, is caused by mutations in the KCNJ2 encoding an inward rectifier potassium channel, Kir2.1, and is another cardiac ion channelopathy associated with conduction system disorders.70 Andersen-Tawil patients may present with conduction abnormalities, such as AV block, BBB, or intraventricular conduction delay. This syndrome is characterized by potassium-sensitive periodic paralysis, ventricular arrhythmias, and dysmorphic features. A molecular defect in KCNQ1, the pore-forming α-subunit of the IKS potassium channel, also may be a causative mutation responsible for AV conduction disease.71 An S140G mutation in KCNQ1 has been genetically linked in a Chinese family to atrial fibrillation and to a slow ventricular response in AF as a manifestation of AV conduction disease. Thirteen of 16 AF patients in the family with the KCNQ1 S140G mutation had a slow ventricular response in AF, with mean heart rate less than 60 bpm in the absence of AV nodal–blocking medications. In addition, a transgenic mouse model with myocardium-specific expression of the human KCNQ1 S140G mutation manifested frequent episodes of first-, second-, advanced-, or third-degree AV block.
Several mutations in genes encoding proteins that regulate septation of the heart have been associated with conduction system disease. In addition to conduction disease, this phenotype includes atrial or ventricular septal defects. The Nkx2.5 homeobox gene is critically involved in development of the cardiac conduction system.72–74 Loss-of-function mutations in the homeobox transcription factor Nkx2.5 cause a loss of DNA-binding activity of this gene. These mutations are associated with progressive AV node dysfunction and other diffuse conduction system abnormalities. Studies in Nkx2.5-deficient mice have shown that Nkx2.5 insufficiency perturbs the conduction system during development, resulting in hypoplasia of the AV node, His bundle, and Purkinje system. Mutations in the T-box transcription factor Tbx5, which similarly is an important early regulator of cardiac development, cause Holt-Oram syndrome.75 This syndrome is manifested by congenital heart disease (usually secundum-type atrial septal defects) associated with progressive AV block and upper-limb deformities.76 Less often, Holt-Oram patients have structurally normal hearts and clinically manifest with only AV block and subtle hand malformations. Another transcription factor associated with conduction system defects is HF-1b, an SP1-related transcription factor preferentially expressed in the cardiac conduction system and ventricular myocytes.77 Mice deficient for HF-1b are prone to sudden death and have AV block. Downregulation of the gap-junction protein connexin 40 (Cx40) has been hypothesized as a unifying genetic mechanism responsible for the AV conduction defects in Nkx2.5, Tbx5, and HF-1b transcription factor mutations.53
Mutations in the PRKAG2 gene, which encodes for a regulatory subunit of adenosine monophosphate (AMP)–activated protein kinase involved in intracellular energy metabolism, are found in association with a familial form of the Wolff-Parkinson-White (WPW) syndrome.78 However, high-grade AV block is the dominant clinical rhythm disturbance associated with this genetic defect.79 PRKAG2 gene mutations are characterized by pseudohypertrophy of the left and right ventricles caused by glycogen deposition in cardiac muscle. Ventricular preexcitation is thought to be caused by anulus fibrosis disruption and glycogen deposition, distinct from the muscular-appearing bypass tracts observed in typical WPW syndrome. Index of suspicion for PRKAG2 disease should be raised when massive LV wall thickening (>30 mm) is present in association with high-grade AV block. A mouse model carrying a mutation responsible for the human disease has been generated.80
Many of the metabolic storage disorders that have a genetic basis, including Pompe disease, Anderson-Fabry disease, and Danon disease, are associated with cardiac involvement that includes prominent AV conduction disturbances.81 These diseases may also display abnormal electrical AV connections similar to the ventricular preexcitation seen in PRKAG2 disease.
Mitochondrial disorders comprise a group of diverse genetic diseases, with cardiac conduction defects reported in 10% to 40% of the patients with these disorders.82 Kearns-Sayre syndrome (ophthalmoplegia plus), which involves large mitochondrial DNA deletions, consists of the triad of complete AV block, chronic progressive external ophthalmoplegia, and pigmentary degeneration of the retina.83 It can present with AV block and dilated cardiomyopathy, along with muscle weakness, central nervous system dysfunction, and endocrinopathies. The conduction defects typically involve the distal His bundle, bundle branches, and infranodal conduction. The accelerated and unpredictable rate of progression to complete AV block, together with an associated mortality of up to 20%, should lead to routine evaluation of patients with Kearns-Sayre syndrome for AV conduction disturbances and electrocardiographic screening of family members. Current guidelines advocate pacemaker implantation in patients with Kearns-Sayre syndrome with ECG changes indicative of conduction defects, with or without symptoms, because of the unpredictable progression of AV conduction disease in this syndrome (see Tables 14-3 and 14-4).
Acquired Causes of AV Block
Acquired AV block may be secondary to a number of causes of generalized myocardial scarring (Table 14-2). These causes include atherosclerosis, dilated cardiomyopathy, hypertension, infiltrative cardiomyopathies, inflammatory disorders, and infectious diseases. In most cases the specific etiology is clinically unknown and, with few exceptions (e.g., Lyme disease, endocarditis, sarcoidosis), is relatively unimportant from a therapeutic point of view. The most common cause of chronic acquired AV block is related to aging of the cardiac cytoskeleton. An entity known as idiopathic bilateral bundle branch fibrosis, or Lev’s disease, is characterized by slowly progressive replacement of specialized conduction tissue by fibrosis, resulting in progressive fascicular block and BBB. Lev84 proposed that damage to the proximal LBB and adjacent main bundle or main bundle alone is the result of an aging process exaggerated by hypertension and arteriosclerosis of the blood vessels supplying the conduction system. Another variant of idiopathic conduction system disorder is Lenègre’s disease, which occurs in younger patients and is characterized by loss of conduction tissue, predominantly in the peripheral parts of the bundle branch.85 As noted, some cases previously classified as “idiopathic” conduction disease, especially when there is familial clustering, may have a genetic cause for the conduction disease.
| Category | Specific Causes |
|---|---|
| Idiopathic fibrodegenerative diseases |
Patients with sick sinus syndrome are known to be at risk for concomitant symptomatic heart block.86 The block may be caused by progressive fibrodegenerative disease extending from the sinus node region to the AV conduction system. The relative frequency of this association varies between studies. Rosenqvist and Obel87 pooled the data from 28 published studies and reported a mean incidence for development of second- or third-degree AV block of 0.6% per year (range, 0-4.5%/yr) in patients in whom permanent atrial pacemakers have been implanted for symptomatic sinus node dysfunction. The total prevalence of second- or third-degree AV block was 2.1% (range, 0-11%). In a retrospective review of 1395 patients with sick sinus syndrome monitored for a mean of 34 months, Sutton and Kenny88 estimated that the annual incidence for development of conduction system diseases was 3%; such conduction diseases included significant first-degree AV block, BBB, HV prolongation, and a low Wenckebach heart rate. Thus, AV conduction system disease occurs relatively frequently in patients with sinus node dysfunction. Similarly, sinus node dysfunction, particularly chronotropic incompetence, may occur frequently in patients with acquired CHB.
Complete AV block may occur after cardiac surgical or interventional catheter-based procedures. CHB occurs more often (3%-6% incidence) after replacement of aortic, mitral, or tricuspid valves, given the proximity of their anuli to the AV junction, than after isolated coronary artery bypass graft (CABG) surgery, in which the incidence is less than 1% to 2%.89,90 CHB is often seen after surgical procedures to repair ventricular septal defects, tetralogy of Fallot, AV canal defects, or subvalvular aortic stenosis.91,92 Heart block is also a potential complication of septal myomectomy and catheter-based septal ablation to relieve LV outflow tract obstruction in hypertrophic cardiomyopathy.93,94 The incidence of heart block requiring permanent pacing after alcohol septal ablation varies from 10% to 33%.
Baerman et al.95 investigated the time course of conduction defects after bypass surgery. Surgical technique consisted of cold, hyperkalemic cardioplegia, and conduction defects resolved partially or completely in 50% of patients. Patients with conduction defects generally had longer cardiopulmonary bypass times, longer aortic cross-clamp times, and more vessels requiring bypass. In three of the four patients with CHB, the heart block eventually resolved after discharge and implantation of a permanent pacemaker. Reasons for conduction abnormalities after cardiac surgery include ischemic injury to the conduction system, direct surgical manipulation or trauma to conduction tissue, traumatic disruption of the distal conduction system, edema, dissecting hematomas, and alterations in conduction caused by cardioplegia.
Surgery for correction of valvular heart disease often leads to conduction defects. After discontinuation of cardiopulmonary bypass, a variety of cardiac rhythm disturbances may be seen, including sinus arrest, junctional rhythm, BBB, AV block, and sinus bradycardia. Many of these rhythm disturbances are transient, resolving within 5 to 7 days. Transient BBB is quite common, occurring in 4% to 35% of patients and generally resolving within 12 to 24 hours.89 In one study, newly acquired, persistent BBB developed in 15.6% of patients after aortic valve replacement and was associated with a higher adverse event rate.96 The investigators recommended that prophylactic implantation of a pacemaker be considered soon after surgery in patients who demonstrate persistent BBB. Conduction disturbances are particularly common both in patients with aortic valve disease and after aortic valve replacement, with 5% to 30% of patients experiencing some conduction abnormality after valve replacement. Most of these abnormalities are transient; however, chronic CHB may occur. Postoperative AV block that is not expected to resolve after cardiac surgery is a class I indication for permanent pacing. The incidence of conduction disorders requiring permanent pacing in patients after aortic valve replacement is 3% to 6%.97
Intraoperative heart block does not predict the need for permanent pacing.98 One study found that risk factors for irreversible AV block requiring permanent pacemaker implantation after aortic valve replacement were previous aortic regurgitation, myocardial infarction, pulmonary hypertension, and postoperative electrolyte imbalance.97 In another study, Koplan et al.99 developed and validated a preoperative risk score to predict the need for permanent pacing after cardiac valve surgery, using a large database of surgical patients at a single institution (1992–2002). Preoperative predictors of the need for permanent pacing after cardiac valve surgery were age over 70, previous valve surgery, multivalve surgery (especially tricuspid), preoperative BBB (especially RBBB), and first-degree AV block. The incidence of permanent pacemaker implantation ranged from 25% in high-risk patients to 3.6% in low-risk patients, with risk based on preoperative variables. Glikson et al.100 showed that postoperative complete AV block was the most important predictor of subsequent pacemaker dependency. They recommended earlier decisions on the timing of permanent pacemaker implantation, by the sixth postoperative day in patients with a wide QRS escape rhythm, and by the ninth day in patients with a narrow QRS rhythm. In most institutions, permanent pacing would be instituted earlier, probably by the fourth to sixth postoperative day.
Persistent heart block occurs infrequently (0.5%-2% of patients) after radiofrequency catheter ablation (RFA) of septal accessory pathways or the slow AV nodal pathway in patients with AV nodal reentrant tachycardia.101 Transient, intraprocedural AV block during ablation performed close to the AV septum does not necessarily indicate that permanent pacemaker implantation is required. Ablation of the fast pathway in AV nodal reentrant tachycardia in the anterior septum is associated with a higher risk of transient AV block than RFA of the slow pathway in the posterior septum. In more than 500 patients, transient second- or third-degree AV block was seen in 20% during fast-pathway ablation, in 2.3% during slow-pathway ablation, and in 42% during combined fast-and-slow pathway attempted ablation.102 Within 7 days after RFA, however, persistent AV block was seen in 3.4%, 0.2%, and 0% of patients in these groups, respectively.
Late occurrence of unexpected heart block after RFA of AV nodal reentry (using a posterior approach) or posteroseptal accessory pathways is rare (<0.5% incidence) and often resolves after a few weeks.103 In patients who experience this rare RFA complication, prolonged clinical observation and monitoring rather than immediate pacemaker implantation is a reasonable approach. The risk for development of heart block may decrease with the use of cryoablation for the treatment of AV nodal reentry and accessory pathways near the AV node. A higher incidence of heart block (1%-10% of patients) was observed when patients underwent surgical ablation for the WPW syndrome, especially when the accessory pathway was in an anteroseptal, intermediate septal, or posteroseptal location, or after surgical modification of the AV node for treatment of AV nodal reentrant tachycardia.104
Radiofrequency ablation of the AV junction should be performed only in patients with a previously implanted permanent pacemaker or at permanent pacemaker implantation (class I indication for pacing). There are no studies to assess outcome without permanent pacing after catheter RFA of the AV junction. Radiofrequency current ablation of the AV junction usually results in a junctional escape rhythm of 40 to 50 bpm. In one study, about 65% of patients had an escape rhythm with an average rate of 39 bpm, with a new RBBB in 24%, a new LBBB in 6%, and an idioventricular rhythm in 19%.105 Several studies have shown AV junction ablation with pacemaker therapy to be highly effective at controlling symptoms and to result in improved quality of life in patients with paroxysmal and chronic AF. In patients with depressed LV function, significant improvements in ventricular function are measured after ablation and pacing in 20% to 40% of patients in some series.105,106 Currently, many patients with depressed LV function who undergo AV junction ablation receive devices with biventricular pacing capabilities.
Atrioventricular nodal modification is a catheter ablation procedure designed to impair but not fully destroy AV nodal conduction in patients with rapid ventricular rates during AF.107 Radiofrequency energy is delivered in and around the slow AV nodal pathway in the posterior septum in an attempt to leave conduction intact over the fast AV nodal pathway. At present, however, AV nodal modification has fallen out of favor and is performed infrequently in patients with AF. In some patients, AV nodal modification results in a slower ventricular response during AF and possibly avoids permanent pacemaker placement, but in other patients, there is an unpredictable decrease in the ventricular rate. Patients who undergo AV nodal modification may remain symptomatic because of recurrent rapid AV conduction or the irregularity of the ventricular response during AF and may subsequently require complete AV nodal ablation. A bimodal RR histogram during AF has been suggested as indicative of dual–AV nodal physiology and may be a predictor of successful outcome after AV nodal modification.108 However, the risk of sudden death or syncope from unexpected, late CHB is a significant concern with this procedure. The rate of inadvertent AV block during the procedure may be as high as 25%.108 The incidence of long-term AV block is quite variable as well as controversial, with estimates for late development of CHB ranging from 0% to more than 20% in different series.109
In patients with blunt or penetrating chest trauma, a variety of conduction disturbances, including AV block, have been reported, but appear to be rare complications of this type of injury.110,111 The reported conduction abnormalities after chest trauma consist of BBB and varying degrees of AV block, including CHB. The most common abnormality is RBBB, followed by first-degree AV block, and the least common is CHB. Most AV conduction defects after traumatic chest injury are transient and resolve early. A few cases of persistent heart block requiring permanent pacemaker implantation have been described. Delayed development of complete AV block has been seen, occurring 15 to 30 days after injury, suggesting that patients with blunt chest trauma should be monitored for late complications. The severity of injury does not always correlate with the development of posttraumatic complications, including conduction disorders. The mechanism of conduction defects in this setting may be related to ischemia and infarction of the conduction system.
Complete heart block has been described in various infectious diseases, including bacterial, viral, fungal, protozoan, and rickettsial infections. Heart block may occur with endocarditis and may be either transient or permanent. In most infectious diseases, heart block is transient and resolves with treatment of the underlying infection. In some cases, transient heart block may recur, and permanent pacing is required. This is particularly true in patients with entities such as endocarditis, in which a valve ring abscess may erode into the conduction system, and in patients with infections such as Chagas’ disease.112–115 AV block occurs in up to 25% of patients with endocarditis complicated by perivalvular abscess, with the aortic valve involved much more than the mitral valve.
Lyme disease is the most common cause of reversible AV block in younger patients. This systemic illness, first described in 1975, was characterized later as an infection caused by a spirochete, Borrelia burgdorferi, which is transmitted to humans by a tick bite. This illness is often characterized by a rash, erythema chronicum migrans, which is followed by cardiac and neurologic abnormalities and then, in some cases, by arthritis.116 Cardiac involvement may occur in 8% to 10% of Lyme disease patients, is generally transient, and may consist of a myocarditis or a myopericarditis.117 Varying degrees of AV block are a common manifestation of carditis, occurring in about 75% of patients. More than 50% of Lyme patients with AV block have symptomatic high-grade or complete AV block that requires temporary pacing. Most often, the site of block is localized to the AV node, although occasional cases have been reported in which the site of block is intra-Hisian or infra-Hisian.118,119 Continuous cardiac monitoring is recommended in all patients with second-degree AV block and a prolonged P-R interval of more than 0.30 second, because of the risk for development of complete AV block. CHB generally resolves within 1 to 2 weeks. Recurrent AV block has not been reported. Rarely, some patients may have symptomatic AV block as the sole manifestation of Lyme disease.120 Permanent pacing is rarely required except for persistent CHB, which is uncommon. Two important axioms worth repeating are that (1) heart block associated with infectious disease usually resolves with appropriate and prompt antibiotic treatment, and (2) conduction disease is rarely the only manifesting feature of an infectious illness.
Heart block may occur rarely after radiation therapy or chemotherapy. Heart block may occur after radiation therapy if radiation is directed at the mediastinum, as for Hodgkin’s and some non-Hodgkin’s lymphomas.121 Radiation therapy may induce fibrosis of the cardiac conduction system, as well as the atrial and ventricular myocardium, and may accelerate coronary atherosclerosis. Rarely, tumors, including mesothelioma of the AV node, cardiac lymphoma, and metastatic disease to the heart from breast, lung, or skin cancer, may involve the conduction system.122 AV block has been reported as a rare complication of chemotherapeutic agents (e.g., arsenic trioxide treatment for leukemia). In general, however, it is unusual for toxicity to antineoplastic drugs, such as doxorubicin, to result in damage to the cardiac conduction system.
Certain neuromuscular diseases may give rise to progressive and insidiously developing cardiac conduction system disease. The disorders include Duchenne’s muscular dystrophy, fascioscapulohumeral muscular dystrophy, X-linked muscular dystrophy, myasthenia gravis, myotonic dystrophy, and Friedreich’s ataxia.123 Abnormalities of conduction manifest as infranodal conduction disturbances resulting in fascicular block or CHB. This has been noted particularly in Kearns-Sayre syndrome (progressive external ophthalmoplegia with pigmentary retinopathy), Guillain-Barré syndrome, myotonic muscular dystrophy, slowly progressive X-linked Becker’s muscular dystrophy, and fascioscapulohumeral muscular dystrophy. Myotonic muscular dystrophy and Kearns-Sayre syndrome are both associated with a high incidence of conduction system disease that frequently is rapidly progressive and cannot be predicted by the ECG or isolated His bundle recordings. His-Purkinje disease can culminate in fatal Stokes-Adams attacks unless anticipated by insertion of a pacemaker. In a study of 49 patients with myotonic dystrophy (46 ±9 years old) and an H-V interval of 70 msec or longer, high-grade paroxysmal AV block was recorded after pacemaker implantation in 47% of patients who had had no known bradycardia on entry into the study.124 The researchers concluded that prophylactic implantation of permanent pacing should be considered in patients with myotonic dystrophy and prolonged H-V interval (≥70 msec) even without bradycardia-related symptoms. Waiting for the development of complete AV block in patients with neuromuscular diseases may expose them to significant risk of sudden death or syncope related to AV block. Permanent pacing should be considered early in the course of neuromuscular disease and should be offered to the asymptomatic patient once any conduction abnormality is noted. A recent study found that “severe” ECG abnormalities (rhythm other than sinus, QRS >120 msec, P-R interval >240 msec, or second- or third-degree AV block) and clinical diagnosis of atrial tachyarrhythmia are independent predictors, with moderate sensitivity, of sudden death in patients with myotonic dystrophy type 1.125
Heart block may occur with amyloid and other infiltrative diseases, including hemochromatosis, porphyria, oxalosis, Refsum’s disease, carcinoid, Hand-Schüller-Christian disease, and sarcoidosis.126
Cardiac sarcoidosis should be considered in the differential diagnosis in a young patient (20-40 years old) presenting with CHB.127 Cardiac manifestations of sarcoidosis are present in at least 25% of patients with systemic sarcoidosis and can include CHB (~30%), ventricular tachyarrhythmias, intracardiac masses, ventricular aneurysms, and dilated cardiomyopathy.128–130 However, only 40% to 50% of patients with cardiac sarcoidosis at autopsy had clinical evidence of myocardial involvement during their lifetime.130 Newer modalities such as magnetic resonance imaging (MRI) may be helpful in the diagnosis of cardiac sarcoid, which can be difficult because of the lack of definitive criteria and the nonspecific clinical manifestations.131 Cardiac sarcoidosis is associated with noncaseating granulomas that tend to involve the conduction system with varying degrees of AV conduction block. The majority of patients with sarcoid heart disease have extracardiac involvement that manifests either clinically or on biopsy. Other organ systems involved in sarcoid include the lymph nodes, skin, eyes, and the nervous, musculoskeletal, renal, and endocrine systems. Isolated cardiac involvement in sarcoidosis is less common and usually precedes future systemic sarcoidosis. Although there are no large randomized trials or prospective registries of patients with cardiac sarcoidosis, the available literature indicates that cardiac sarcoidosis with heart block, ventricular arrhythmias, or LV dysfunction is associated with a poor prognosis and an increased risk of sudden death.132 Myocardial involvement in sarcoid accounts for up to 13% to 25% of deaths from sarcoidosis. In Japan, sarcoid heart disease is more common, accounting for up to 85% of mortality from sarcoidosis.127 AV block may resolve after long-term treatment with corticosteroids, alone or in combination with other immunosuppressive therapy.133 However, heart block in patients with sarcoid heart disease generally warrants a permanent pacemaker, even if AV conduction block reverses transiently, because of possible disease progression. An ICD should be considered in patients with sarcoidosis undergoing device implantation for AV block, because of the risk of ventricular tachyarrhythmias and sudden death.21 However, definitive clinical data are not available to stratify risk of sudden cardiac death among patients with cardiac sarcoidosis. Thus, decisions regarding ICD implantation must be individualized.
Connective tissue disorders giving rise to conduction system disease include periarteritis nodosa, rheumatoid arthritis, polymyositis, mixed connective tissue disorders, Reiter’s syndrome, ulcerative colitis, scleroderma, Takayasu’s arteritis, systemic lupus erythematosus, and ankylosing spondylitis.134,135 In one study of 50 consecutive patients with scleroderma, EPS demonstrated conduction abnormalities in up to 50% of patients, suggesting a much higher level of cardiac involvement in patients than readily apparent clinically. However, CHB is uncommon.136 Most patients with AV conduction disorders have other clinical manifestations of the connective tissue disease. AV block has been reported as a rare complication of antimalarial therapy used to treat systemic autoimmune diseases (e.g., chloroquine for rheumatoid arthritis or systemic lupus erythematosus).
Exercise-induced transient AV block is a relatively rare condition that is usually caused by a block in the His-Purkinje system137,138 (Fig. 14-13). The incidence of exercise-induced AV block during treadmill exercise testing is between 0.1% and 0.5%.139 Donzeau et al.140 reported 14 symptomatic patients with exercise-induced AV block, in nine of whom block was localized to an infranodal site. Other studies have confirmed that exercise-induced AV block is primarily infra-Hisian.141,142 In these studies, about 25% to 75% of patients had underlying BBB. Several reports of patients with cardiac asystole after exertion have demonstrated postexercise sinus arrest with ventricular asystole.143 Some researchers proposed that transient ischemia of the conduction system is a mechanism of exercise-induced AV block in patients with severe RCA lesions and chronic infranodal conduction disturbances.139 A case of pseudo–AV block during exercise was caused by His bundle parasystole.144 Permanent pacing is recommended in patients with exercise-induced AV block, even in the asymptomatic state, because of the high incidence of symptomatic AV block.
Drug-induced bradycardia is a common and important clinical problem.145 Many common drugs, including β-adrenergic blockers, calcium channel antagonists, digoxin, class I and III antiarrhythmic drugs, tricyclic antidepressants, phenothiazines, lithium, and donepezil (a cholinesterase inhibitor used to treat Alzheimer’s disease), can cause AV conduction disturbances. In patients presenting with drug-induced AV block, drug therapy can be stopped entirely, reduced in dosage, or continued if there is no acceptable alternative. In the last case, if drug-induced AV block results in symptomatic bradycardia, permanent pacemaker implantation is recommended (class I indication). On the other hand, if the AV block is caused by drug toxicity, is expected to resolve, and is unlikely to recur, pacemaker implantation is generally considered unnecessary, according to current guidelines. However, if AV block occurs in the setting of drug use or toxicity, and the block is expected to recur even after the drug is withdrawn, pacemaker implantation may be considered (class IIb indication).21
Supporting this guideline recommendation, Zeltser et al.146 suggest that in the majority of patients presenting with presumed drug-induced AV block, discontinuation of the offending medications does not obviate the need for pacemaker implantation. In this study, AV block persisted after drug discontinuation in most patients. Furthermore, even if AV block resolved when drugs were discontinued, patients remained at risk of recurrent AV block in the absence of offending drugs. Among a consecutive series of 169 mostly elderly patients with structural heart disease presenting with second- or third-degree AV block not related to AMI, vasovagal syncope, digitalis toxicity, or RFA, 92 patients (54%) had drug-induced AV block while receiving β-blockers and/or verapamil or diltiazem. Drug therapy was discontinued in 79 patients; in 32 (41%), AV block resolved within 48 hours. However, in 18 (56%) of the 32 patients with drug-induced AV block who experienced spontaneous resolution of AV block after drug discontinuation, AV block recurred. Ten of these patients had syncope during the subsequent 3 weeks of follow-up without drug therapy. On the basis of this experience, the researchers estimated that the medications caused only 8% of all cases of AV block and only 15% of occurrences of AV block in patients receiving medications.
Vagally mediated AV block infrequently requires a permanent pacemaker, especially in the absence of recurrent syncope or profound asystole. AV block may occur in the setting of increased vagal tone in response to various stimuli, such as carotid sinus hypersensitivity, coughing, swallowing, and visceral distention.147–149 Because vagally mediated AV block often occurs in young, otherwise healthy patients, especially during sleep, it must be differentiated from type II second-degree AV block, because the latter patients require implantation of a permanent pacemaker.150 In most cases, vagally induced AV block occurs at the level of the AV node and is associated with a narrow QRS complex.151 As a general rule, vagally mediated AV nodal block shows obvious heart rate slowing, even if only slight, before the onset of block, because of the concomitant effect of increased vagal tone on the sinus node (Fig. 14-14). Rarely, vagal stimulation may precipitate phase IV or bradycardia-mediated block in the His-Purkinje system.43
 Indications for Permanent Pacing in Chronic Atrioventricular Block
Indications for Permanent Pacing in Chronic Atrioventricular Block
Historically, chronic or acquired AV block with syncope was the first indication for cardiac pacing. Intermittent or chronic high-grade AV block still accounts for a large but varying number of permanent pacemaker implantations, depending on the series. The proportion of pacing related to AV block without atrial fibrillation, according to world and U.S. surveys, ranged from 21% to 54% of pacemakers in 1997.152,153 Despite published guidelines, permanent pacemaker implantation continues to be underused in patients with CHB. From 1996 to 2001, there were 165,541 patients admitted to U.S. hospitals with a primary diagnosis of complete heart block. Only 74% to 83% of patients with a primary diagnosis of CHB received permanent pacemakers before hospital discharge.154 Furthermore, African-Americans and ethnic minorities were significantly less likely (68% and 60% implantation rates, respectively) than white patients (80% implantation rate) to receive pacemakers for CHB.
In 1984 a subcommittee of the ACC/AHA Task Force on Assessment of Cardiovascular Procedures formulated a set of guidelines for the indications for permanent pacing.155 These guidelines were revised in 1991, 1998, 2002, and 2008 based on major new studies that have advanced knowledge in this area, as well as new developments in the technology of devices to treat bradyarrhythmias.21,156,157 Tables 14-3 and 14-4 list permanent pacing indications for acquired AV block and chronic bifascicular and trifascicular block in the 2008 ACC/AHA/NASPE revised guidelines.21
TABLE 14-3 Indications for Permanent Pacing for Acquired Atrioventricular (AV) Block in Adults
TABLE 14-4 Indications for Permanent Pacing in Chronic Bifascicular Block
| Class I | Advanced second-degree AV block or intermittent third-degree AV block (level of evidence: B) |
| Type II second-degree AV block (level of evidence: B) | |
| Alternating bundle branch block (level of evidence: C) | |
| Class IIa | Syncope not demonstrated to be caused by AV block when other likely causes have been excluded, specifically ventricular tachycardia (level of evidence: B) |
| Incidental finding at EPS of extremely prolonged H-V interval (≥100 msec) in a symptom-free patient (level of evidence: B) | |
| Incidental finding at EPS of pacing-induced infra-Hisian block that is not physiologic (level of evidence: B) | |
| Class IIb | Neuromuscular diseases, such as myotonic muscular dystrophy, Erb’s (limb-girdle) dystrophy, and peroneal muscular atrophy with bifascicular block or any fascicular block, with or without symptoms (level of evidence: C) |
| Class III | Fascicular block without AV block or symptoms (level of evidence: B). |
| Fascicular block with first-degree AV block without symptoms (level of evidence: B) |
AV, Atrioventricular; EPS, electrophysiologic study.
Indications for pacemaker implantation are categorized into three classes as follows:
 Acute Myocardial Infarction
Acute Myocardial Infarction
Prior to the era of reperfusion therapy for AMI, AV block occurred in 12% to 25% of all patients with AMI; first-degree AV block occurred in 2% to 12%, second-degree block in 3% to 10%, and third-degree block in 3% to 7%.158–160 It is unclear whether thrombolytic therapy has altered the overall incidence of AV conduction defects in patients with AMI.161–163 In one study, use of a thrombolytic agent was associated with a higher rate of occurrence of complete AV block (odds ratio = 1.44) but a tendency toward a lower rate of occurrence of BBB (odds ratio = 0.68).162 Another study suggested that thrombolytic therapy was associated with a tendency toward a lower rate of third-degree AV block in anterior AMI but a higher rate in inferior AMI.163 Among 6657 patients admitted with AMI between 1990 and 1992 and included in the Trandolapril Cardiac Evaluation (TRACE) randomized trial in Denmark, 340 (5.1%) experienced third-degree AV block during their hospitalization.163 The incidence of third-degree AV block was higher among patients with inferior AMI (193 of 2061; 9.4%) than among those with anterior AMI (44 of 1747; 2.5%).163 Likewise, in pooled data from 75,993 patients with ST-segment elevation AMI treated with thrombolytic therapy, 5251 patients (6.9%) had second- or third-degree AV block. AV block occurred in 9.8% of those with inferior AMI and in 3.2% of those with anterior AMI.164
The onset of AV block usually occurs 2 to 3 days after the infarction but has a range of a few hours to 10 days. The mean duration is usually 2 to 3 days, and range of duration is 12 hours to 16 days. In one large study, third-degree AV block occurred within 48 hours of symptom onset in 81% of patients, with a trend toward later onset of third-degree AV block in anterior rather than inferior AMI.164
Clinicopathologic studies indicate that there is a relationship between the anatomic location of an AMI and involvement of the conduction system.165 The development of AV and intraventricular blocks during anterior AMI is related to the extent of the ischemic/infarcted area. AV block in patients with inferior AMI more often results from vagal reflexes or local metabolites occurring early within the AV node in a transient fashion. Mechanisms proposed for AV block in the presence of inferior AMI include Bezold-Jarisch reflex, reversible ischemia or injury of the conduction system, local accumulation of adenosine or its metabolites, and local AV nodal hyperkalemia. Stimulation of the Bezold-Jarisch reflex causes an abnormally increased output of vagal nerve traffic; it is initiated by ischemia of the afferent nerves in the area of the inferoposterior left ventricle. Reperfusion of the RCA with thrombolytic agents is a strong stimulus for the Bezold-Jarisch reflex.166,167 Despite this, the Second Thrombolysis in Myocardial Infarction (TIMI II) study did not show an increase in AV block in patients with inferior AMI who received thrombolytic therapy and had a patent infarct-related artery.168
In inferior or posterior AMI, obstruction of the RCA produces reversible ischemia of the AV node. In patients who experienced AV block after inferior AMI, pathologic studies demonstrated little or no necrosis, structural damage, or histologic degenerative changes in the conduction system in most cases.165,169 However, Bilbao et al.170 identified a subgroup of patients with fatal inferior or posterior AMI and AV block who had necrosis of the prenodal atrial myocardial fibers. These necrotic fibers were absent in patients without AV block. Clinically, the transient nature of the AV block supports the concept that injury to the AV node is reversible.171 The anatomic data reported by Bassan et al.158 support the concept that the blood supply of the AV node is dual. In their prospective study, 11 of 51 patients who survived an inferior AMI had some degree of transient AV block, and about 90% of the patients with AV block had simultaneous obstruction of the RCA (or left coronary artery [LCA] when it was dominant) and the proximal segment of the LAD artery. Moreover, patients with inferior AMI and LAD artery obstruction had a sixfold higher risk for development of AV block than those without LAD artery obstruction. However, the TIMI-II data do not support this finding; in study patients with inferior AMI and AV block, the incidence of disease in the LAD was low and was similar to that in patients with inferior AMI without AV block.168 The Thrombolysis and Angioplasty in Acute Myocardial Infarction (TAMI) study group also showed no increase in incidence of LAD disease in patients with inferior AMI and complete AV block.172
Local accumulation of endogenous adenosine or its metabolites also has been suggested to play a mechanistic role in AV block occurring as an early complication of inferior AMI.173 Several case reports or small series have suggested that aminophylline, a competitive adenosine antagonist, reverses atropine-resistant AV block in patients with inferior AMI.174 Current practice guidelines for management of ST-segment-elevation AMI, however, recommend against giving aminophylline to treat bradyarrhythmias because it increases myocardial oxygen demand and is arrhythmogenic.175
A higher level of potassium was found in the lymph draining from the infarcted inferior and posterior cardiac walls of dogs after experimental RCA occlusion, suggesting that local AV nodal hyperkalemia may play a role in the development of AV block in the presence of inferior AMI.176 Sugiura et al.177 found that serum potassium value was an independent predictor of the occurrence of fascicular blocks in anteroseptal AMI.
Anterior or anteroseptal AMI results from obstruction of the LAD artery. Occurrence of AV block and BBB in patients with anterior AMI is usually the result of necrosis of the septum and the conduction system below the AV node and reflects more extensive and permanent myocardial damage with severe LV dysfunction.169,178,179 However, Wilber et al.180 described two patients with anterior AMI and complete AV block in whom 1 : 1 conduction returned within minutes after late reperfusion (>40 hours) with angioplasty. Their experience suggests that reversible ischemia rather than necrosis of the conduction system occurs in some patients. Some experimental studies in dogs with anterior AMI suggest that extensive but reversible ischemia of the infranodal conduction tissue occurs, as evidenced by recovery from complete AV block. Several clinical studies also report that most patients with anterior AMI and high-grade AV block who are discharged from the hospital show late recovery of 1 : 1 AV conduction.181–185
AV Block without Bundle Branch Block
Incidence
In a series of 684 consecutive patients with AMI admitted to the Los Angeles County-University of Southern California Medical Center (LAC-USCMC) Coronary Care Unit (CCU) between 1966 and 1970, 110 had AV block (16%); 79 of 110 patients (72%) with AV block did not have BBB.186 The total percentages of patients who had first-, second-, or third-degree AV block at some time were 6%, 7%, and 4%, respectively (Table 14-5).
TABLE 14-5 Atrioventricular Block (AVB) in Acute Myocardial Infarction (AMI) without Bundle Branch Block*
| Incidence | 12% (79/684 patients) |
| First-degree AVB | 6% (44/684 patients) |
| Second-degree AVB | 7% (50/684 patients) |
| Third-degree AVB | 4% (29/684 patients) |
| Site of infarction | |
| Inferior | 79% |
| Anterior | 18% |
| Combined | 6% |
| Progression | |
| First-degree AVB to second-or third-degree AVB | 59% |
| Second-degree AVB to third-degree AVB | 36% |
| Outcome | |
| Hospital mortality | 29% |
| Return to 1 : 1 conduction in survivors | 95% |
* Data from 684 consecutive patients with AMI at Los Angeles County-University of Southern California Medical Center (LAC-USCMC), Los Angeles.
Modified from de Guzman M, Rahimtoola SH: What is the role of pacemakers in patients with coronary artery disease and conduction abnormalities? In Rahimtoola SH, editor: Controversies in coronary artery disease. Philadelphia, 1983, FA Davis, pp 191-207.
Site of Infarction
Atrioventricular block is more often associated with inferior infarction, and in those who experience second- and third-degree blocks, inferior AMI is present two to four times more frequently than anterior AMI. The site of block in inferior infarction is above the His bundle in about 90% of patients, whereas in anterior infarction, the conduction abnormality is usually localized below the His bundle in the distal conducting system.187 In the LAC-USCMC series, of the 79 patients with AV block who did not have BBB, 60 (76%) had an inferior infarction, 14 (18%) an anterior infarction, and 5 (6%) a combined infarction (Table 14-6).
TABLE 14-6 Atrioventricular Block in Anterior and Inferior-Posterior Acute Myocardial Infarction (AMI)
| Feature | Anterior AMI | Inferior-Posterior AMI |
|---|---|---|
| Pathophysiology | Extensive necrosis of septum | Reversible ischemia, injury of conduction system |
| Site of block | Infranodal | Intranodal |
| Frequency | Less frequent | Two to four times more frequent |
| Progression to complete AV block | Sudden | Gradual |
| Intraventricular conduction defect | Common | Rare |
| Escape focus | Ventricular | Junctional |
| Escape rate (per minute) | 20-40 | 40-60 |
| Prognosis | High mortality | Lower mortality |
Progression of AV Block
In patients with inferior AMI, progression of AV block typically occurs in stages, whereas in those with anterior AMI, it may occur in stages, or third-degree AV block or ventricular asystole may develop suddenly171 (Fig. 14-15).
Outcome
Atrioventricular block complicating AMI is associated with a high mortality rate (24%-48%), two to three times that for AMI without AV block (9%-16%). Even with thrombolytic therapy and primary percutaneous coronary interventions, if AV block occurs in the setting of AMI, mortality remains high, especially in anterior MI.168,172,188,189 This poor prognosis generally reflects the larger ischemic/infarcted region associated with development of AV block in the setting of AMI. Although AV block that occurs during inferior AMI predicts a higher risk of in-hospital death, it may be less predictive of long-term mortality in patients who survive to hospital discharge.168,172 Before the reperfusion era, Tans et al.160 reported that in patients with inferior AMI, those with high-grade AV block and no severe pump failure had a higher in-hospital mortality rate (17%) than those without high-grade AV block (9%).160 The major cause of death in patients who have AV block in the setting of AMI is pump failure.190 In the LAC-USCMC series, the hospital mortality rate was 29% (32 of 110 patients); 29 of the 32 deaths (91%) were caused by pump failure. Survival, therefore, is greatly influenced by the severity of the hemodynamic disturbance and is less dependent on the degree of heart block. Death is related primarily to extensive myocardial damage, but in an important minority of patients, it can be attributed to sudden ventricular asystole or severe bradycardia.
AV Block with Bundle Branch Block
Prior to the widespread use of thrombolytic therapy, BBB was present during hospitalization in 8% to 18% of patients with AMI.191–196 The presence of a persistent intraventricular conduction defect during the hospitalization increases the risk of high-grade AV block as well as other complications and is associated with poor survival in patients with AMI. In patients receiving thrombolytic therapy, the incidence of persistent intraventricular conduction defects appearing during the hospitalization is reduced to about 4% to 9%.161–163197 However, the adverse risk associated with intraventricular conduction defects (except for isolated left anterior hemiblock) has persisted even in the modern era of AMI reperfusion therapy.
In an older series of 2779 patients with AMI admitted from 1966 to 1977 to the LAC-USCMC CCU, 257 (9%) had BBB186 (Table 14-7). Of the 257 patients, 83 (32%) had LBBB, 80 (31%) had RBBB, 72 (28%) had RBBB plus left-axis deviation, 21 (9%) had RBBB plus right-axis deviation, and one had alternating BBB. The conduction abnormality was “new” in 60%; that is, the BBB developed during the infarction and was documented by serial ECGs or was present on admission and was not seen on previous ECGs or reverted to normal conduction later as documented by serial ECGs. When the site of infarction was not obscured by the BBB in the LAC-USCMC series, the block was associated with anterior AMI about three times as often as with inferior AMI.
TABLE 14-7 Bundle Branch Block (BBB) in Acute Myocardial Infarction (AMI)
| Incidence | 9%: (257/2779 patients)* |
| Left BBB | 32% (83/257 patients) |
| Right BBB | 31% (80/257 patients) |
| Right BBB + LAD obstruction | 28% (72/257 patients) |
| Right BBB + RAD obstruction | 9% (21/257 patients) |
| Onset of BBB | |
| New | 60% |
| Old | 40% |
| Site of Infarction | |
| Inferior | 21% |
| Anterior | 52% |
| Combined | 4% |
| Indeterminate | 18% |
| Nontransmural | 5% |
| Incidence of AVB | 29% (75/257 patients) |
| First degree | 10% (25/257 patients) |
| Second degree | 5% (13/257 patients) |
| Third degree | 14% (37/257 patients) |
| Progression of AVB | |
| First-degree AVB to second- or third-degree AVB | 32% |
| Second-degree AVB to third-degree AVB | 46% |
| Progression to high-grade AVB | 18% (46/257 patients) |
| Bilateral BBB + first-degree AVB | 50% |
| New bilateral BBB + first-degree AVB | 43% |
| First-degree AVB | 30% |
| New BBB + first-degree AVB | 29% |
| Bilateral BBB | 18% |
| New BBB | 16% |
| New bilateral BBB | 15% |
| Outcome | |
| Hospital mortality | 20% |
| Return to 1 : 1 conduction in survivors | 89% |
AVB, Atrioventricular block; LAD, left anterior descending artery; RAD, right anterior descending artery.
* Data from 2779 AMI patients seen from October 1966 to March 1977 at Los Angeles County-University of Southern California Medical Center (LAC-USCMC), Los Angeles.
Modified from de Guzman M, Rahimtoola SH: What is the role of pacemakers in patients with coronary artery disease and conduction abnormalities? In Rahimtoola SH, editor: Controversies in coronary artery disease. Philadelphia, 1983, FA Davis, pp 191-207.
Progression of AV Block
In the LAC-USCMC series conducted before the thrombolytic era, progression of AV block occurred in 75 of the 257 patients (29%) with AMI and BBB (see Table 14-7).
There were 37 patients with third-degree AV block and BBB, 13 (35%) of whom were admitted in third-degree block. Of the 24 who progressed to third-degree block, 11 (46%) had demonstrated second-degree block; 7 of the 11 had type II second-degree block. Consistent with the LAC-USCMC series, AV block occurred in other studies in about one third of patients with AMI and BBB.186,198–201
Two large studies from the 1970s and 1980s developed a data bank on patients with BBB in association with MI: a collaborative multicenter study involving five centers185 and a study conducted at LAC-USCMC.186 Both studies have limitations because (1) the data were obtained retrospectively and (2) at the time, no guidelines existed pertaining to pacemaker insertion, which was performed at the physician’s discretion in all cases. Thus, although these studies are unable to provide definitive answers about the natural history of BBB in association with AMI, they nevertheless do offer valuable clinical information.
In the multicenter study reported by Hindman et al.,185 high-grade AV block (third- or second-degree block with a type II pattern) occurred in 55 of 432 patients (22%). To determine which patients were at considerable risk for development of high-grade AV block while hospitalized with AMI, several variables were analyzed. Combinations of the three following ECG findings identified high-risk patients: (1) first-degree AV block, (2) bilateral BBB (if both bundle branches were involved [e.g., RBBB plus left- or right-axis deviation] or alternating RBBB and LBBB), and (3) “new” BBB. The absence of all variables or the presence of only one of the three defined variables was associated with a lower risk (10%-13%) for development of high-grade AV block during hospitalization. The risk was moderate for patients with first-degree AV block with either new BBB or bilateral BBB (19%-20%), and highest (31%-38%) for new bilateral BBB regardless of the P-R interval (Fig. 14-16).
In the LAC-USCMC study, high-grade AV block occurred in 46 of 257 patients (18%). The absence of all three variables (first-degree AV block, bilateral BBB, and new BBB) or the presence of either bilateral BBB or new BBB or new bilateral BBB was associated with the lowest risk (10%-18%) for development of high-grade AV block during hospitalization with AMI.186 The risk was moderate for first-degree AV block with or without new BBB (29%-30%) and highest (50%) for bilateral BBB plus first-degree AV block, regardless of whether the BBB was old or new (Fig. 14-17).
Despite some differences in the findings between the two studies, both studies found that the following subgroups of patients were at highest risk for high-grade AV block: (1) those with new bilateral BBB plus first-degree AV block (risks, 38%185 and 43%186), (2) those with bilateral BBB plus first-degree AV block (risks, 20% and 50%, respectively), and (3) new BBB plus first-degree AV block (risks, 19% and 29%, respectively). Subgroups in whom the findings of the two studies show different risks can be considered to be at moderate risk for high-grade AV block. These subgroups include patients with (1) new bilateral BBB (risks, 31% and 15%, respectively) (Fig. 14-18), (2) first-degree AV block (risks, 13% and 30%, respectively), and (3) bilateral BBB (risks, 10% and 18%, respectively). The remaining subgroups of patients with AMI and BBB can be considered to be at lowest risk (≤10%) for development of high-grade AV block.
The database assembled by the Multicenter Investigation of the Limitation of Infarct Size (MILIS) was used to develop a simplified method of predicting the occurrence of CHB. Data from 698 patients with proven MI were analyzed, and the presence or absence of ECG abnormalities of AV or intraventricular conduction was determined for each patient. Risk factors for development of CHB were as follows: first-degree AV block, Mobitz type I AV block, Mobitz type II AV block, left anterior hemiblock, left posterior hemiblock, RBBB, and LBBB. A risk score for the development of CHB was devised that consisted of the sum of each patient’s individual risk factors. Incidences of CHB of 1.2%, 7.8%, 25%, and 36% were associated with risk scores of 0, 1, 2, and 3 or more, respectively (Fig. 14-19). The risk score was subsequently tested on the published results of six studies for a combined total of 2151 patients.202 The limitations of this scoring system include the lack of differentiation between newly appearing and old BBB, a factor that has been shown to be of predictive value. It is likely that consideration of such factors would further improve the accuracy of the scoring system, but it would also add to its complexity. Another criticism of the scoring system is that it would assign a risk score of only 1 to a patient with isolated Mobitz type II AV block, a disorder usually believed to be highly predictive of progression to CHB. Isolated Mobitz type II AV block is, however, relatively rare.
Outcome
The short-term and long-term mortality and sudden death rates are higher in patients with AMI and BBB (25%-50%) than in those without BBB (15%).160,191,195,203 The one exception is the isolated finding of left anterior fascicular block in patients with AMI, which appears not to carry an unfavorable prognosis. When the infarction is extensive and produces diffuse conduction system abnormalities progressing to high-grade AV block, it is also extensive enough to damage a large amount of myocardial muscle. Therefore, affected patients often die from pump failure and from ventricular tachyarrhythmias, and the adverse prognosis is not necessarily caused by development of high-grade AV block. Nevertheless, some of these patients do not die from heart failure or ventricular arrhythmias, and in these patients, the conduction abnormality may be contributory and can be the major cause of death if prophylactic pacing is not undertaken. In some patients, sudden third-degree AV block or asystole is abrupt and fatal if untreated. It is interesting that in the LAC-USCMC study, 75% of patients with BBB and AMI had either no heart failure or, at worst, mild heart failure.186 These results as well as those of Hindman et al.185 showed that high-grade AV block influenced hospital mortality independent of pump failure.
Impact of Thrombolytic Therapy
Several prospective trials involving thrombolytic therapy of AMI provide data pertaining to the effect of such therapy on the development of high-grade (second- or third-degree) AV block and BBB.168,172
Clemmensen et al.172 examined the effect of thrombolytic therapy and adjunctive angioplasty as a treatment strategy for AMI (TAMI trial) after inferior AMI. In all patients, treatment was initiated with thrombolytic agents within 6 hours of symptom onset. There were 373 patients with an inferior AMI, of whom 50 (13%) had complete AV block; 54% of these patients had complete AV block on admission. In all but two patients, the block was manifest within 72 hours of onset of symptoms. The duration of block was less than 1 hour in 25% and less than 12 hours in 15%; the median duration of block was 2.5 hours. There was no difference in the rate of infarct vessel patency between those with and those without AV block (90% and 91%, respectively). A precipitating clinical event—vessel reperfusion, performance of percutaneous transluminal coronary angioplasty (PTCA)—or vessel reocclusion was identifiable in 38% of cases of complete AV block. At the predischarge angiogram, the vessel patency rate was 11% lower in the group with AV block than in the group without block (71% vs. 82%, respectively). Those in whom AV block developed showed a decrease in LVEF between the early postthrombolytic angiogram and the predischarge angiogram. Also, those who experienced AV block had higher in-hospital mortality, 10 of 50 (20%) versus 12 of 323 (4%; P < .001). When age, LVEF in the acute phase, number of diseased vessels, and grade of blood flow through the culprit lesion were entered into a multivariate model, the development of complete AV block still contributed significantly to the risk for in-hospital death. After a median follow-up period of 22 months, mortality rates for patients with and without AV block were equivalent (2%). These data suggest that, compared with the prethrombolytic era, use of thrombolytics and angioplasty has altered neither the incidence of complete AV block nor the associated greater ventricular dysfunction or in-hospital mortality of patients with inferior AMI.
In another study of 1786 patients with inferior AMI who received recombinant tissue-type plasminogen activator (rt-PA) within 4 hours of symptom onset, high-grade (second- or third-degree) AV block developed in 214 (12%) (TIMI-II trial).168 Of the group who had AV block, 113 (6.3% of total, or 52% of those who ever had AV block) had this finding on admission. The remaining 101 patients (5.7%) experienced heart block during the 24 hours after treatment with thrombolytics. Patients who already had high-grade AV block before receiving thrombolytic therapy tended to be older and had a higher prevalence of cardiogenic shock than those without heart block. Nevertheless, the presence of heart block did not carry a higher 21-day mortality rate independent of other variables, such as shock, and the 1-year mortality rate was similar to that in the group without heart block. Patients in this study were randomly assigned to coronary arteriography 18 to 48 hours after admission. Among those who had heart block after admission, the infarct-related artery was less frequently patent than in those without heart block (28 of 39 [72%] vs. 611 of 723 [84.5%]; P = .04]). The RCA was the infarct-related artery more often in patients who had heart block than in those who did not (36 of 69 [92.3%] vs. 542 of 723 [75.1%], respectively; P = .04). Among patients without heart block at hospital admission, death occurred within 48 hours in 4 of 9 patients (44%) with new heart block and in 8 of 68 (12%) without new heart block at 24 hours. The 21-day mortality rate was higher in the group with AV block than in the group without block (10 of 101 [9.9%] vs. 35 of 1572 [2.2%], respectively; P < .001), as was the 1-year mortality rate (15 of 101 [14.9%] vs. 65 of 1572 [4.2%], respectively; P = .001). A temporary pacemaker was inserted in about one third of patients who had heart block on admission and in almost 30% of patients who experienced heart block after institution of thrombolytic therapy, whereas only 6.5% of patients without heart block received temporary pacemakers. None of the patients who had heart block on admission or who experienced heart block later received permanent pacemakers, but four patients without heart block at 24 hours went on to receive permanent pacemakers. Heart block was not listed as a primary or contributing cause of death in any patient.
The data from these two studies of thrombolytic therapy suggest that aggressive treatment with thrombolytic agents or thrombolytic therapy plus angioplasty is not associated with a lower incidence of high-grade or complete AV block in patients with inferior AMI than was seen in the prethrombolytic era; the incidence remains about 10% to 13%, with about one half of cases appearing as new AV block during hospitalization. The infarct-related vessel is more often the RCA, and there is a lower vessel patency rate after thrombolysis among patients in whom AV block complicates inferior AMI. In-hospital and early posthospitalization mortality rates are higher in patients with block than in those without AV block, among patients treated with thrombolytics, with or without angioplasty. It has not been clear, however, that patients with acute AV block continue to be at greater risk of death over the long term if they survive the initial hospitalization. A study that pooled data from four large RCTs involving 70,000 patients with AMI treated with thrombolytic therapy evaluated the short- and long-term mortality rates associated with second- and third-degree AV blocks.164 Compared with patients with AMI and no AV block, patients with AMI and AV block were more than three times more likely to die within 30 days and 1.5 times more likely to die during 1 year of follow-up. The higher short- and long-term mortality rates were observed in the setting of inferior as well as anterior AMI (Fig. 14-20). The presumption remains, as before the era of thrombolytic therapy, that the presence or development of AV block is associated with a higher mortality because it tends to indicate the presence of more extensive infarction or injury.
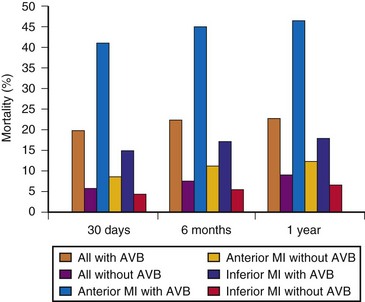
Figure 14-20 Unadjusted mortality rates in patients with and without atrioventricular block (AVB). MI, Myocardial infarction.
(From Meine TJ, Al-Khatib SM, Alexander JH, et al: Incidence, predictors, and outcomes of high-degree atrioventricular block complicating acute myocardial infarction treated with thrombolytic therapy. Am Heart J 149:670, 2005.)
In trials conducted with thrombolytic therapy in AMI, BBB is reported present on admission in up to 2% to 4% of patients.202 In the first Global Utilization of Streptokinase and Tissue-Type Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) trial, among 26,003 North American patients, 420 (1.6%) had left (n = 131) or right (n = 289) BBB on admission ECG.204 Interestingly, reversion of BBB occurred in 24% of patients during hospitalization and was associated with a 50% relative risk reduction in 30-day mortality, from 20% to 10%. Prognosis for patients who recovered normal intraventricular conduction (i.e., transient BBB) was similar to that for patients who never had BBB. Another study examined the significance of RBBB in AMI in the prethrombolytic era and compared it with the incidence and prognostic significance of new RBBB in the thrombolytic era.205 In a multicenter prospective study of 1238 patients with 1-year follow-up, a higher rate of new and transient RBBB and lower rate of bifascicular block were found in patients receiving thrombolytic therapy. The overall prognostic implications of RBBB, however, were unchanged and included a higher rate of heart failure, greater chance of needing a permanent pacemaker, and a higher 1-year mortality rate.205
Bundle Branch Block after Recovery
The subset of patients with persistent BBB and transient high-grade AV block during AMI are at increased risk of late mortality.185,206,207 It is now recognized that most of these deaths are sudden and result from ventricular tachyarrhythmias. In a previous era, however, controversy surrounded whether these patients were at high risk for sudden death from a bradyarrhythmia.183,201,208–210 Some investigators suggested that patients with persistent BBB plus transient AV block during the acute infarction had a higher risk of dying suddenly as a result of CHB. They attempted to identify the subset of patients at highest risk of late sudden death due to AV block in order to maximize the therapeutic benefit of permanently implanting a pacemaker.
The multicenter study by Hindman et al.185 supported the previous reports by Atkins206 and Ritter,207 who found that the subset of patients with chronic BBB and transient high-grade AV block during AMI were at increased risk of late sudden death. These data showed that patients who did not receive pacing had a higher incidence of sudden death or recurrent high-grade AV block during follow-up (65%) than those who were given permanent pacing (10%), suggesting that implantation of a permanent pacemaker protected against sudden death in these patients. Waugh et al.211 likewise recommended permanent pacemaker therapy to prevent syncope or sudden death in another group of high-risk patients, those with bilateral BBB plus transient high-grade AV block (type II progression).
Other studies from the same era, however, questioned whether these patients are at high risk for sudden death from a bradyarrhythmia.183,203,208–210 For example, Nimetz et al.201 reported that late sudden death occurred in 4 of 13 (31%) survivors with BBB and second- or third-degree AV block and in 14 of 41 (34%) survivors without AV block. Ginks et al.183 found that of patients with anterior AMI complicated by CHB with return to normal sinus rhythm but with persistent BBB, 4 of 14 hospital survivors (29%) with anterior MI, persistent BBB, and transient AV block died, and 2 of 4 (50%) with permanent pacemakers died during a follow-up period averaging 49 months. In a study by Murphy et al.209 of patients surviving AMI complicated by BBB, none of the deaths resulted from heart block, even in patients with transient AV block during the AMI. Lie et al.210 reported on a group of 47 patients who had survived anterior infarction complicated by BBB and who were kept for 6 weeks in the monitoring area; 17 of the 47 patients (36%) sustained late ventricular fibrillation. Likewise, the Birmingham Trial of permanent pacing in patients with persistent intraventricular defects after AMI showed no significant difference in survival between patients with and without heart block during up to 5 years of follow-up.212 Finally, in a prospective long-term study, Talwar et al.213 monitored 18 patients with anterior AMI, intraventricular defects, and transient complete AV block for a mean of 2 years; pacemakers were permanently implanted in eight patients. There was only one death, in the unpaced group, caused by a cerebrovascular accident. Clearly, these older studies were limited by the small number of patients with AMI enrolled and monitored and may not be applicable to the current era of post-AMI management. Despite the controversy regarding whether late sudden death in patients with BBB is caused by heart block, permanent ventricular pacing is indicated for transient advanced second- or third-degree infranodal AV block and associated BBB after AMI, according to current practice guidelines (class I indication).21
Electrophysiologic Studies in AV Block and Bundle Branch Block
Electrophysiologic studies with recording of the His bundle electrogram (HBE) are not performed routinely at present for risk stratification of patients with AV block after AMI. HBE studies after AMI were used primarily in the 1970s and 1980s to identify the sites of AV conduction disturbances, which were shown to be either in the AV node (proximal block) or in the distal conduction system (distal block). The presence of distal block identifies patients at high risk for development of high-grade AV block. In individual cases in which the diagnosis is uncertain (e.g., type I second-degree AV block in patients with BBB) or infranodal or multilevel block is suspected, EPS is helpful in identifying the sites of AV block (Fig. 14-21).
In patients with inferior AMI, the site of AV block is usually proximal. Harperet al.214 showed that 30 of 32 patients (94%) with inferior AMI and third-degree AV block had AV nodal block during HBE; the remaining two patients were in normal sinus rhythm during the study and had a normal P-R interval and normal A-H and H-V intervals. Thus, in this group of patients, HBE offered no advantage over conventional ECG criteria in localizing the site of block.
In anterior AMI, the block is frequently in the distal conduction system. In the study by Harper et al.,214 50% of patients (9 of 18) with BBB and a normal P-R interval on ECG had a prolonged H-V interval.214 Of the 22 patients who experienced AV block and BBB, five had proximal block, 14 had distal block, and three had both proximal and distal blocks. Thus, in both groups of patients, HBE was the only means of localizing the block in the proximal or distal portion of the conduction system. Distal block indicates disease in either the His bundle or the remaining bundle branches; clinically, this finding is a common antecedent to sudden asystole and a poorer prognosis. Despite that a prolonged H-V interval could identify a group of patients who may be at high risk for high-grade AV block, several studies have shown that it does not help in assessing the short- or long-term prognosis of patients after AMI.214,215
Intracardiac EPS has been evaluated as a means of attempting to predict which patients with MI and BBB are most likely to die. Harper et al.208 reported on 72 patients with AMI complicated by AV block, BBB, or both who underwent His bundle recording or electrophysiologic (HBE) studies during their CCU stay. Thirty of 32 patients (94%) with AV block and narrow QRS had a proximal block. Hospital mortality was low (13%), and HBE provided no additional information to the surface ECG. Of 18 patients with BBB and a normal P-R interval, nine had distal block, but there were no hospital deaths in this group. Again, of 22 patients with BBB and AV block, five had proximal, 14 distal, and three proximal and distal blocks. Hospital mortality in these patients, who progressed to second- or third-degree AV block, was higher (9 of 12 patients, 75%) than in those who remained in first-degree AV block (2 of 10 patients, 20%). Lichstein216 and Lie184 also concluded that patients with BBB and AMI who had HBE evidence of a distal block had higher hospital mortality (73% and 81%, respectively) than those with normal H-V intervals (25% and 47%, respectively). On the other hand, Gould et al.217 found that the presence or absence of a prolonged H-V interval did not affect mortality.
Indications for Pacing
Temporary Pacing
The use of temporary transvenous pacing in the post-AMI period has diminished in recent years with the greater and more widespread reliance on transcutaneous pacing. Table 14-8 lists situations in which temporary transvenous pacing is recommended or should be considered, according to practice guidelines.175 Current practice guidelines and recommendations for temporary pacing in the setting of AMI, however, are based primarily on clinical experience rather than well-controlled clinical trials. Essentially, no trials have evaluated the risks versus the benefits of temporary pacing during the current era of AMI treatment. Furthermore, RV pacing (VVI) may have potential deleterious hemodynamic effects even when compared with spontaneous intrinsic bradycardic rhythms in the absence of BBB (e.g., sinus node dysfunction or heart block with junctional escape rhythms). In addition, there is little scientific evidence of an advantage of temporary RV pacing over intrinsic rhythm in patients with bradycardia after AMI. Thus, temporary ventricular pacing should not be used for hemodynamic support, but rather should be used primarily as “backup pacing” for prophylactic indications to prevent catastrophic bradycardia or to treat sudden CHB without an adequate ventricular escape mechanism.
TABLE 14-8 Recommended Indications for Temporary Pacing in Patients with Acute Myocardial Infarction (AMI)
| Category | Recommended Indications |
|---|---|
| Symptomatic AVB |
AVB, Atrioventricular block; BBB, bundle branch block; LAHB, left anterior hemiblock; LBBB, left bundle branch block; LPHB, left posterior hemiblock; RBBB, right bundle branch block.
After temporary pacing is instituted, the temporary pacing generator should be programmed to minimize RV pacing (i.e., prolonging the AV delay). Atrial or dual-chamber pacing (AV synchronous) leads to better cardiac output than temporary ventricular pacing.218–220 Thus, when hemodynamic support is required in patients with AMI who need temporary pacing, physiologic pacing should be considered. In general, however, because of the risks of infection, limitation in venous access, and difficulties in maintaining stability of atrial pacing during temporary pacing for prolonged periods, and unless there are contraindications, patients who need permanent pacing after AMI should undergo permanent rather than temporary pacemaker implantation as soon as feasible. The requirement for temporary pacing in AMI does not by itself mandate an indication for permanent pacing.
Administration of thrombolytic therapy is a priority in AMI patients and should not be delayed by the need to insert a temporary pacing wire. If necessary in the bradycardic patient, temporary transcutaneous pacing can be instituted while thrombolytic therapy is being given. Pharmacologic therapies (with isoproterenol, aminophylline) to treat bradycardia are not recommended in AMI because of their arrhythmogenic effects and adverse effects on myocardial oxygen demand.175 Atropine is generally well tolerated in inferior AMI, but in some cases of inferior AMI, AV block does not respond to atropine. When infranodal conduction disturbances are present, especially in anterior infarction, atropine raises the sinus rate and may worsen AV conduction and decrease the ventricular rate. When temporary pacing is required because of continued, hemodynamically significant bradycardia or profound asystole after thrombolytic therapy or in fully anticoagulated patients, the temporary pacing wire should be inserted by an experienced operator. It also is best to avoid the left subclavian approach for temporary pacemaker insertion because this is the most popular site for permanent pacemaker implantation.
In almost all cases, patients with a normal QRS complex or old or new fascicular block (left anterior fascicular block [LAFB] or left anterior hemiblock [LAHB]) and first-degree AV block or type I second-degree AV block with normal hemodynamics and patients with inferior AMI and block above the His bundle (i.e., normal QRS width) do not require insertion of a temporary pacemaker. These patients are not at risk for sudden asystole. It must be appreciated that a small number of patients with type I second-degree AV block and narrow QRS complexes have block in the His bundle and not in the AV node. Nevertheless, some clinicians believe that the relatively uncommon clinical manifestation of intra-Hisian block in inferior AMI is almost always reversible and rarely requires pacing.221
Insertion of a temporary transvenous pacemaker is recommended in patients with an anterior AMI complicated by the following: (1) type I second-degree AV block with wide QRS in which block is presumed to be below His bundle (class IIa or IIb indication, depending on whether BBB is new or old), (2) type II second-degree AV block with a normal (class IIa indication) or wide QRS (class I or IIa indication, depending on whether BBB is new or old), or (3) complete or advanced AV block (class I indication).175 These patients are at risk for sudden asystole in the setting of anterior AMI.
In the setting of a nonanterior wall AMI, temporary transvenous pacing likewise is recommended in patients with (1) type I second-degree AV block with wide QRS in whom block is presumed to be below His bundle (class IIa or IIb indication, depending on whether BBB is new or old), (2) type II second-degree AV block with a normal (class IIa indication) or wide QRS complex (class I or IIa indication, depending on whether the BBB is new or old), and (3) complete or advanced AV block (class I indication).175 As noted previously, however, in most cases of AV block complicating inferior AMI, AV block is almost always located in the AV node, is reversible, is associated with a junctional (narrow complex) escape rhythm, and does not predict the development of sudden asystole. Thus, temporary pacing can almost always be avoided in these patients.
Prophylactic temporary transvenous pacing in the early postinfarction period also is indicated for patients considered at high risk for development of sudden high-grade AV block.175 Such patients are identified by (1) new or indeterminate-age bilateral BBB (alternating LBBB and RBBB or RBBB with alternating left anterior and left posterior hemiblock) with or without first-degree AV block (class I indication), (2) new BBB with first-degree AV block (class IIa indication), or (3) indeterminate-age RBBB with fascicular and first-degree AV blocks (class IIa indication). Because of the risk of sudden asystole, these subgroups of patients probably should receive temporary pacemakers or should be considered for early implantation of permanent pacemakers. Even in these high-risk patients, however, it must be recognized that prophylactic temporary ventricular pacing has not gained widespread acceptance, because it does not improve in-hospital survival and may be associated with serious complications. Thus, the use of standby transcutaneous pacing may be a preferred alternative.
Other patient subgroups at moderate risk for development of high-grade AV block who may have to be considered for temporary prophylactic transvenous pacing are those with (1) new BBB without first-degree AV block (class IIb indication), (2) old BBB with first-degree AV block (class IIb indication), or (3) indeterminate-age RBBB with fascicular block but without first-degree AV block (class IIb indication). However, placement of external pacing pads with the ability to provide prophylactic transcutaneous pacing as standby with close telemetry monitoring may be preferred in these patient subgroups during the acute phase of MI.175 Furthermore, in the absence of higher-grade AV block, patients with persistent first-degree AV block in the presence of old or indeterminate-age BBB do not require permanent ventricular pacing after AMI.
Permanent Pacing
Table 14-9 lists the ACC/AHA/HRS current guidelines for permanent pacemaker implantation after AMI.21 All patients who have an indication for permanent pacing after AMI should also be evaluated for ICD and cardiac resynchronization therapy (CRT) indications.
TABLE 14-9 Indications for Permanent Pacing after Acute Phase of Myocardial Infarction
| Class I | Persistent second-degree AV block in the His-Purkinje system with alternating BBB or third-degree AV block within or below the His-Purkinje system after ST-segment-elevation myocardial infarction (level of evidence: B) |
| Transient advanced second- or third-degree infranodal AV block and associated BBB; if site of block is uncertain, electrophysiologic study may be necessary (level of evidence: B) | |
| Persistent and symptomatic second or third-degree AV block (level of evidence: C) | |
| Class IIa | None |
| Class IIb | Persistent second- or third-degree AV block at the AV node level, even in the absence of symptoms (level of evidence: B) |
| Class III | Transient AV block in the absence of intraventricular conduction defects (level of evidence: B) |
| Transient AV block in the presence of isolated left anterior fascicular block (level of evidence: B) | |
| New BBB or fascicular block in the absence of AV block (level of evidence: B) | |
| Persistent asymptomatic first-degree AV block in the presence of BBB or fascicular block (level of evidence: B) |
AV, Atrioventricular; BBB, bundle branch block.
Modified from Epstein AE, DiMarco JP, Ellenbogen KA, et al: ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol 51:e1-e62, 2008.
Patients with AV conduction disturbances after AMI in whom documented, symptomatic bradyarrhythmias develop and persist should receive permanent pacemakers. In patients with persistent and symptomatic second- or third-degree AV block, permanent ventricular pacing has a class I indication. Of patients who have second-or third-degree block in the hospital, regardless of infarction or block location, return to 1 : 1 conduction is seen in 95% of those without BBB and in 89% of those with BBB who survive the infarction and are discharged eventually from the hospital.186 Thus, in patients recovering from AMI who demonstrate AV block, the major determinant of the need for prophylactic permanent pacing beyond symptomatic bradycardia is the presence of intraventricular conduction defects. Patients with persistent second-degree AV block in the His-Purkinje system with bilateral BBB and those with persistent third-degree AV block located within the His-Purkinje system after AMI should receive permanent pacemakers (class I indication).
Patients with persistent second- or third-degree AV block at the AV nodal level without BBB may also be considered for permanent pacing (class IIb indication). However, permanent pacing is rarely needed in patients with an inferior wall AMI and narrow QRS. It may take up to 16 days for AV conduction to return. Some conservative clinicians recommend pacemaker implantation only when second- or third-degree AV block is present for more than 3 weeks after inferior wall MI.222 After reviewing the literature, Barold and Hayes223 asserted that patients who reportedly had “type II AV block” with a narrow QRS in the setting of inferior AMI were misclassified according to incorrect criteria for the diagnosis of type II second-degree AV block.223 Thus, transient or persistent second-degree AV block with inferior AMI is virtually always AV nodal in origin and not an indication for permanent pacing. Development of complete AV block during inferior AMI is associated with a higher in-hospital mortality, which is not altered by temporary or permanent pacing, but a favorable long-term prognosis in those who survive to hospital discharge.172
When transient advanced second- or third-degree infranodal AV block is associated with persistent BBB, permanent ventricular pacing is indicated (class I indication).21,172 EPS may be helpful if the site of block is uncertain. Development of a new intraventricular conduction delay in the setting of an anterior or inferior infarction reflects extensive myocardial damage. Patients with AMI who demonstrate BBB have an unfavorable prognosis and a higher risk of sudden death. Although these patients may be at risk for serious bradyarrhythmias in the posthospitalization period, their adverse prognosis is not necessarily related to the development of high-grade AV block. These patients are at high risk for other post-MI complications, including pump failure and ventricular tachyarrhythmias. In the past, the decision to implant a permanent pacemaker in a survivor of AMI complicated by transient complete or second-degree AV block and persistent BBB during the hospitalization was not always straightforward. In a previous edition of this textbook, it was suggested that there were at least three possible ways of managing these patients: (1) all should receive permanent pacemakers, (2) only those with documented bradyarrhythmias should receive permanent pacemakers, or (3) patients should undergo a diagnostic evaluation, including LVEF measurement, ambulatory 24-hour ECG monitoring, and possibly His bundle studies, and then the need for permanent pacemaker implantation may be considered in some of the patients.
In the current era, however, the decision to implant a permanent pacemaker to provide bradycardia support in these patients is less of an issue. Most patients with AMI with AV block and BBB have LV dysfunction and thus are eligible for implantation of an ICD for primary prevention of sudden death and/or a biventricular pacing system to provide CRT. However, the timing of ICD implantation may complicate management of such patients, because Medicare reimbursement guidelines for ICD implantation established in 2005 indicate that patients must not have had an AMI within the past 40 days.224 Furthermore, an important consideration in selection of the mode of pacing and the need for an ICD is the recognition that the extent of myocardial dysfunction in patients with ischemic heart disease may not be a permanent condition. Stunning and hibernation of the myocardium, leading to apparent dysfunction, may resolve as the ischemia resolves and after adequate time has passed for recovery.225,226