Oxygen Transport
The Basis of Cardiovascular and Pulmonary Physical Therapy
Oxygen transport is essential to life, activity, and participation in life consistent with the International Classification of Functioning, Disability and Health (ICF).1 Maximizing the efficiency of the oxygen transport pathway promotes optimal mobility and independence, the cornerstones of quality of life and well-being. Attention to oxygen transport—its deficits and threats to it—is the concern of physical therapists, irrespective of the primary clinical area of their practices. This is particularly true given the trend toward physical therapists’ direct access to patients and the prevalence of lifestyle-related conditions, all of which affect oxygen transport either directly or indirectly.
Oxygen Transport
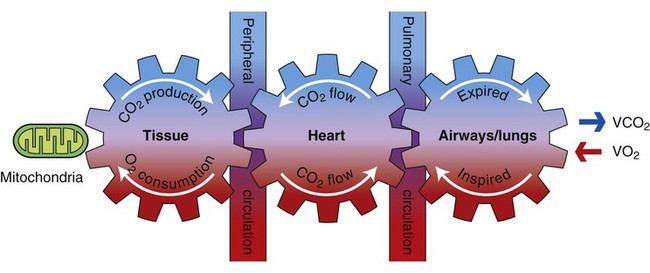
Oxygen transport refers to the delivery of fully oxygenated blood to peripheral tissues, cellular uptake of oxygen, use of oxygen within the tissue, and the return of partially desaturated blood to the lungs. The oxygen transport pathway consists of multiple steps ranging from the ambient air to the perfusion of peripheral tissues with oxygenated arterial blood (Figure 2-1). Oxygen transport has become the basis for conceptualizing cardiovascular and pulmonary function and diagnosing and managing cardiovascular and pulmonary dysfunction.2–8
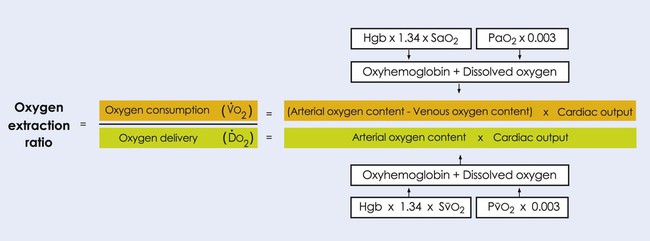
Oxygen transport variables include oxygen delivery (DO2), oxygen consumption (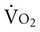 ), and the oxygen extraction ratio (OER), the utilization coefficient. Oxygen demand is the amount of oxygen required by the cells for aerobic metabolism. Oxygen demand is usually reflected by
), and the oxygen extraction ratio (OER), the utilization coefficient. Oxygen demand is the amount of oxygen required by the cells for aerobic metabolism. Oxygen demand is usually reflected by 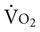 ; however, in cases of severe cardiovascular and pulmonary dysfunction and compromise to oxygen transport,
; however, in cases of severe cardiovascular and pulmonary dysfunction and compromise to oxygen transport, 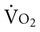 can fall short of the demand for oxygen. Oxygen transport variables, including the components of DO2,
can fall short of the demand for oxygen. Oxygen transport variables, including the components of DO2, 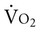 , and OER, are shown in Figure 2-2. DO2 is determined by arterial oxygen content and cardiac output,
, and OER, are shown in Figure 2-2. DO2 is determined by arterial oxygen content and cardiac output, 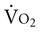 by the arteriovenous oxygen content difference and cardiac output, and oxygen extraction by the ratio of DO2 to
by the arteriovenous oxygen content difference and cardiac output, and oxygen extraction by the ratio of DO2 to 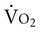 .
.
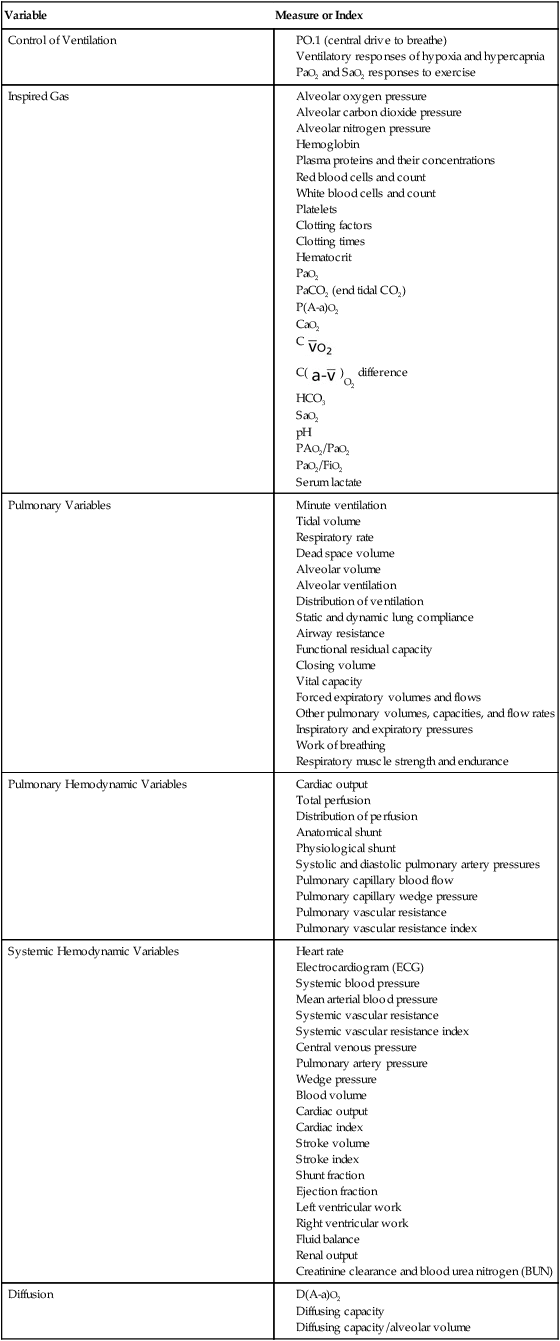
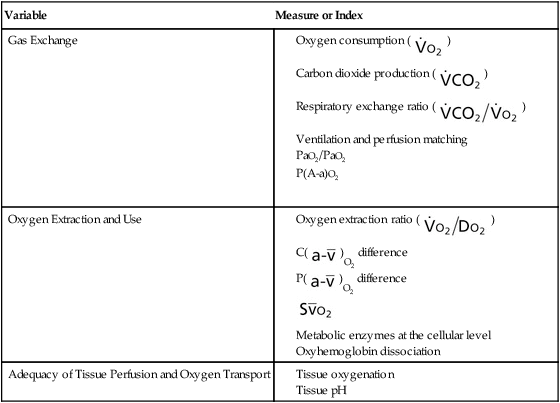
Measures and indexes of oxygen transport that reflect the function of the component steps of the oxygen transport pathway are shown in Table 2-1.
Table 2-1
Measures and Indexes of the Function of the Steps in the Oxygen Transport Pathway
| Variable | Measure or Index |
| Control of Ventilation |
Energy Transfer and Cellular Oxidation
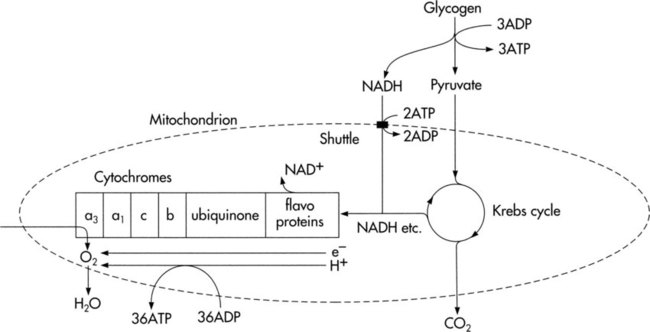
Cellular metabolism and survival depend on the continuous synthesis and degradation of adenosine triphosphate (ATP), the major source of energy for biological work. Work is performed in biological systems for contraction of skeletal, cardiac, and smooth muscle (e.g., exercise, digestion, glandular secretion, and thermoregulation) and for nerve impulse transmission (Box 2-1). These processes require a continuous supply of ATP, which is made available primarily by aerobic (oxygen-requiring) processes. In the event that oxygen delivery is inadequate, nonaerobic (anaerobic, or non–oxygen-requiring) energy-transferring processes can also supply ATP. Supplying energy anaerobically, however, is more costly metabolically; that is, it is not efficient, is limited, and cannot be sustained because of the disruptive effects of lactate (a cellular byproduct of anaerobic metabolism) on physiological processes. Metabolic acidosis is a consequence of lactate accumulation. In patients who are critically ill, the presence of metabolic acidosis secondary to anaerobic metabolism can be life threatening. Prolonged anaerobic metabolism is lethal in two respects. First, the patient is increasingly dependent on anaerobic metabolism because of inadequate DO2 to peripheral tissues; second, acidosis interferes with normal cellular processes and homeostasis, which require an optimal pH of 7.40.
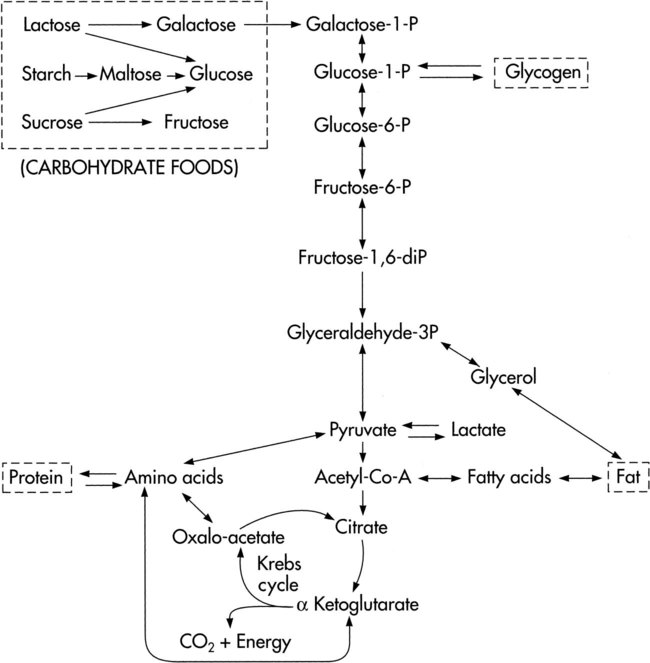
The complex, enzymatically controlled chemical reactions of metabolism are designed to form and conserve energy through the Krebs cycle and the electron transfer chain and then use this energy for biological work.9,10 Carbohydrates, fats, and proteins ingested from foodstuffs in the diet are oxidized to provide the energy for phosphorylation of adenosine diphosphate (ADP) (i.e., the formation of ATP by combining ADP with phosphate). These substances are broken down, and they access the Krebs cycle at the pyruvic acid or acetyl coenzyme A (CoA) levels (Fig. 2-3). Some amino acids can enter the Krebs cycle directly.
Cellular oxidation, or respiration, refers to the function of the electron transfer chain to release energy in small amounts and to conserve energy in the formation of high-energy bonds. It is this process that ensures a continual energy supply to meet the needs of metabolism (Fig. 2-4).
Three major systems of energy transfer exist to supply energy during all-out exercise over varying durations.10 Although these systems are discrete, they overlap. Cellular ATP and creatine phosphate (CP) are immediate energy sources for the first 10 seconds of exercise. From 30 to 60 seconds, glycolysis provides a short-term energy source. The ATP-CP system and the glycolytic system are anaerobic processes. As exercise persists for several minutes, the long-term aerobic system predominates. Thus, for sustained physical activity and exercise, energy is provided primarily by aerobic metabolism. For this process, oxygen is provided by the oxygen transport pathway. Carbon compounds that enter the body in the form of carbohydrate, fat, and protein undergo oxidative metabolism in the form of aerobic glycolysis in the mitochondria in the cytoplasm of cells.
Muscle Contraction and Metabolism
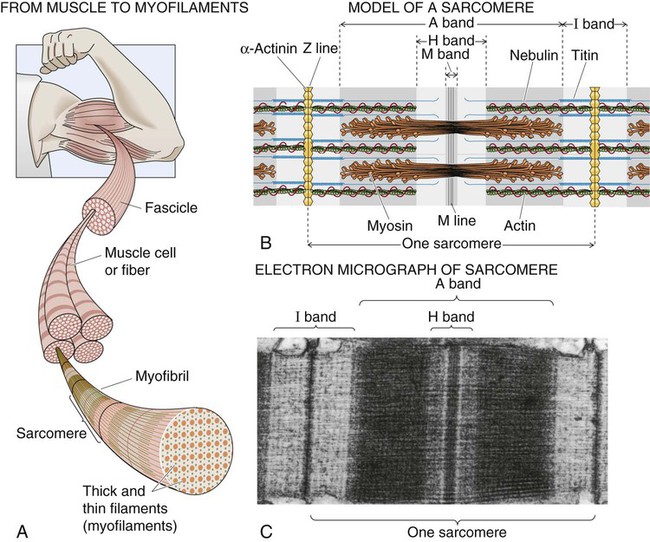
The basic mechanism for muscle contraction is excitation contraction coupling.11,12 Action potentials mediated centrally or through the spinal cord depolarize the muscle cell membrane (the sarcolemma) and stimulate the release of calcium from the lateral sacs of the sarcoplasmic reticulum. The sarcoplasmic reticulum is an extensive network of invaginations and tubular channels encasing the muscle fibers (myofibrils). The calcium floods over the myofilaments of the myofibrils within the sarcomere. Myofilaments consist of thin actin fiber and thick myosin fiber protein, which interdigitate with each other, giving the typical striated appearance to skeletal muscle (Fig. 2-5). Actin is a helical molecule with tropomyosin intertwined along its length. Tropomyosin normally inhibits the interaction of actin and myosin. Calcium causes a conformational change of the tropomyosin molecule that enables troponin, also distributed along the actin molecule, to combine with calcium. The combining of calcium and troponin triggers the interdigitation of actin and myosin (the sliding-filament theory of muscle contraction). Contraction involves myosin heads (cross bridges) attaching to and detaching from actin in a cyclical manner that causes the actin and myosin filaments to slide past each other. In this way, the muscle is shortened without shortening of the myofilaments.
The specific metabolic properties of muscle depend on the constituent muscle fiber types.13 The three primary muscle fiber types are fast-twitch fibers, slow-twitch fibers, and intermediate fibers, which have the properties of both fast-twitch and slow-twitch fibers. The fast-twitch fibers (fast glycolytic fibers) are recruited during short-term, sprint-type exercise that relies mainly on anaerobic metabolism. These fibers are well adapted for rapid, forceful contractions because they have large amounts of myosin ATPase, rapid calcium release and uptake, and a high rate of cross-bridge cycling. Slow-twitch fibers (slow oxidative fibers) are recruited during prolonged aerobic exercise. These fibers have large amounts of myoglobin, mitochondria, and oxidative enzymes. Compared with the fast-twitch fibers, slow-twitch fibers are fatigue resistant. The intermediate fibers have both anaerobic and aerobic metabolic enzymes, which makes these fibers capable of both types of muscle work. Although the characteristics of the fiber types are distinct, activity and exercise recruit both types of fibers. Depending on the particular activity or exercise, one fiber type may be preferentially recruited over the others.
Principles of Oxygen Transport
Individuals need a continuous supply of oxygen to meet moment-to-moment demands for oxygen commensurate with changing metabolic demands for energy at the cellular level as well as basal metabolic demands.14,15 Oxygen transport occurs by convection or diffusion. Convection of oxygen refers to the movement of oxygen from the alveoli to the tissue capillaries and is determined primarily by hemoglobin concentration, oxygen saturation, and cardiac output. The diffusion of oxygen refers to the movement of oxygen from the capillaries to the mitochondria and is determined by metabolic rate, vascular resistance, capillary recruitment, and tissue oxygen consumption and extraction.
At rest, regional differences in the proportion of CO normally delivered to the body organs reflect differences in organ functions and are not necessarily matched to the metabolic rate.16 For example, the distribution of CO to the kidneys is 20%; to the mesenteric, splenic, and portal tissues, 20% to 30%. Comparatively, muscle at rest receives 10% and the brain and myocardium receive less than 5% each.
Oxygen Content of the Blood
The majority of oxygen is transported in arterial blood to the tissues. Oxygen is largely combined with hemoglobin (98%) compared with a relatively minimal amount that is dissolved in the blood (2%). The oxyhemoglobin dissociation curve represents the relationship between the affinity of hemoglobin for oxygen and the arterial oxygen tension. The affinity of hemoglobin for oxygen depends on the tissues’ oxygen demand. In a healthy individual, exercising muscle increases the demand for oxygen. The heat of the working muscles and the acidic environment during exercise result in reduced oxyhemoglobin affinity and increased oxygen release. This is reflected by a shift to the right of the oxyhemoglobin dissociation curve (see Chapter 4). The affinity of hemoglobin and oxygen is increased with the cessation of exercise, which is reflected by a leftward shift of the curve.
Oxygen Delivery to the Tissues
The final steps in oxygen transport involve the dissociation of oxygen from hemoglobin and the diffusion of oxygen from the capillaries to the cells.17 Diffusion depends on the quantity and rate of blood flow, the difference in capillary and tissue oxygen pressures, the capillary surface area, capillary permeability, and diffusion distance.18 With increased metabolic demand by the tissues, capillary dilation and recruitment increase capillary surface area and reduce vascular resistance to flow, diffusion distance is decreased, the movement of oxygen into the cell is facilitated, and the tissue oxygen tension is increased.
Two diffusion gradients determine effective oxygen transport: one occurs between the pulmonary capillaries and the alveoli; the other occurs between the peripheral capillaries and the tissue cells. Diffusion of oxygen occurs as the blood moves from the aorta to the arterioles. The mean oxygen tension in the aorta is about 95 mm Hg and in the arterioles is 70 to 80 mm Hg. The oxygen gradient between the arterioles and the cells is the steepest. The mean oxygen pressure is less than 50 mm Hg in the capillaries. The oxygen tension in the capillaries determines the rate of diffusion to the cells. An optimal diffusion gradient maintains the oxygen tension in the cell at between 1 and 10 mm Hg; oxygen tension is less than 0.5 mm Hg in the mitochondria.16 This decremental PaO2 profile down the oxygen transport pathway, from the airways to the tissues, is termed the oxygen cascade (Figure 2-6).
Cardiac Output
In addition to arterial oxygen content, CO is a primary determinant of DO2.14 The transport of oxyhemoglobin to the tissues is dependent on convective blood flow by way of CO. CO is the volume of blood pumped from the right or left ventricle per minute. The components of CO are stroke volume (SV) and heart rate (HR) (i.e., CO = SV × HR). SV is the amount of blood ejected from the left ventricle during each ventricular systole or heartbeat and is determined by the preload, myocardial distensibility, myocardial contractility, and afterload. DO2 is optimized in patients by increasing CO through the therapeutic manipulation of preload, myocardial contractility, afterload, and heart rate.
Oxygen Debt
Tissue oxygen debt, or recovery oxygen consumption, is the difference between oxygen demand and oxygen consumption. In healthy individuals, oxygen debt can be sustained for short periods during intense exercise. Anaerobic metabolism is stimulated to produce ATP under these conditions. In patients who are critically ill, the degree of oxygen debt is correlated with survival.19
Supply-Dependent Oxygen Consumption
Normally, a decrease in DO2 does not reduce 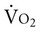 .20 With a decrement in DO2, the tissues extract a commensurate amount of oxygen from the blood. In patients who are critically ill, DO2 may be limited to the point where basic metabolic needs for oxygen (300 mL/min/M2) are not met.20–23 The critical level at which
.20 With a decrement in DO2, the tissues extract a commensurate amount of oxygen from the blood. In patients who are critically ill, DO2 may be limited to the point where basic metabolic needs for oxygen (300 mL/min/M2) are not met.20–23 The critical level at which 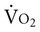 falls is associated with tissue anaerobic metabolism and the development of lactic acidosis and decreased pH (Fig. 2-7).19,24 Serum lactates correspondingly increase and provide a valid index of anaerobic metabolism in patients with multiorgan system failure. When oxygen transport data are analyzed, the effect of sedation may suggest a dependency between DO2 and
falls is associated with tissue anaerobic metabolism and the development of lactic acidosis and decreased pH (Fig. 2-7).19,24 Serum lactates correspondingly increase and provide a valid index of anaerobic metabolism in patients with multiorgan system failure. When oxygen transport data are analyzed, the effect of sedation may suggest a dependency between DO2 and 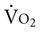 , so the impact of medication in this relationship must be considered.25
, so the impact of medication in this relationship must be considered.25
Oxygen Transport Pathway
Oxygen transport is dependent on several interconnecting steps, ranging from inhalation of oxygen-containing air through the nares to oxygen extraction at the cellular level in response to metabolic demand (see Fig. 2-1). These steps provide the mechanism for ventilatory, cardiovascular, and metabolic coupling. In addition, blood is responsible for transporting oxygen within the body; thus its constituents and consistency directly affect this process.
Quality and Quantity of Blood
Blood volume is compartmentalized within the intravascular compartment such that 70% is contained within the venous compartment, 10% in the systemic arteries, 15% in the pulmonary circulation, and 5% in the capillaries.26 The large volume of blood contained within the venous circulation permits adjustments to be made as CO demand changes. The veins constrict, for example, when CO needs to be increased. When blood volume is normal and body fluids are appropriately distributed between the intravascular and extravascular compartments, fluid balance is considered normal. When they are disrupted, a fluid balance problem exists. In addition, fluid imbalance affects the concentration of electrolytes, particularly sodium, which is present in the highest concentration in the extracellular fluid. Four primary fluid problems that have implications for oxygen transport are water deficit, water excess, sodium deficit, and sodium excess (see Chapter 16). Other ions that are affected often in fluid and electrolyte imbalance deficits include potassium, chloride, calcium, and magnesium. These electrolyte disturbances also contribute to impaired oxygen transport by affecting the electrical and mechanical behavior of the heart and blood vessels, thereby also affecting CO and the distribution of oxygenated arterial blood to the periphery.
In adults, red blood cells are produced in the marrow of the membranous bones, such as the vertebrae, sternum, ribs, and pelvis. The production of red blood cells at these sites diminishes with age. Tissue oxygenation is the basic regulator of red blood cell production. Hypoxemia stimulates red blood cell production through erythropoietin production in bone.26
Hemoglobin is contained within red blood cells in a concentration of up to 34 g/dL of cells. Each gram of hemoglobin is capable of combining with 1.34 mL of oxygen (see Figure 2-2). In healthy women, 19 mL of oxygen can be carried in the blood (given that the whole blood of women contains an average of 14 g/100 mL of blood); in healthy men, the blood can carry 21 mL of oxygen (assuming the average concentration in the whole blood of men is 16 g/dL).
Oxyhemoglobin Dissociation
Demand for oxygen at the cellular level changes from moment to moment. The properties of oxyhemoglobin dissociation ensure that there is a continuous supply of oxygen at the cellular level. Oxygen combines with hemoglobin molecules in the pulmonary circulation and then is released in the tissue capillaries in response to a reduced arterial oxygen tension. The S-shaped oxyhemoglobin dissociation curve (see Chapter 4) shifts to the right in response to reduced tissue pH, increased CO2, increased temperature, and increased diphosphoglycerate (DPG), a constituent of normal blood cells.
Steps in the Oxygen Transport Pathway
Step One: Inspired Oxygen and Quality of the Ambient Air
Step Three: Lungs and Chest Wall
Air entry into the lungs depends on the integrity of the respiratory muscles, in particular the diaphragm, the lung parenchyma, and the chest wall. The contraction and descent of the diaphragm generate a negative intrapleural pressure that inflates the lungs. The distribution of ventilation is determined primarily by the negative intrapleural pressure gradient down the lungs. The negative intrapleural pressure gradient results in uneven ventilation down the lungs and in interregional differences (see Chapter 4). There are, however, other factors that contribute to uneven ventilation within regions of the lung. These intraregional differences reflect regional differences in lung compliance and airway resistance.27 In patients with partially obstructed airways, reduced lung compliance and increased airway resistance increase the time needed for alveolar filling. Gas exchange is compromised if there is inadequate time for alveolar filling or emptying (i.e., increased time constants).18 Different time constants across lung units contribute to uneven patterns of ventilation during inspiration. A lung unit with a long time constant is slow to fill and empty, and it may continue filling when surrounding units are emptying. Another factor that contributes to uneven ventilation is altered diffusion distance. In diseases in which diffusion distance is increased, ventilation among lung units is uneven.
The lungs and the parietal pleura are richly supplied with thin-walled lymphatic vessels.16 Lymphatic vessels have some smooth muscle and thus can actively contract to propel lymph fluid forward. This forward motion is augmented by valves along the lymphatic channels. The rise and fall of the pleural pressure during respiration compress lymphatic vessels with each breath, which promotes a continuous flow of lymph. During expiration and increased intrapleural pressure, fluid is forced into the lymphatic vessels. The visceral pleura continuously drains fluid from the lungs. This creates a negative pressure in the pleural space, which keeps the lungs expanded. This pressure exceeds the elastic recoil pressure of the lung parenchyma, which counters the tendency of elastic recoil to collapse the lungs.
Step Four: Diffusion
Diffusion of oxygen from the alveolar sacs to the pulmonary arterial circulation depends on four factors: the area of the alveolar capillary membrane, the diffusing capacity of the alveolar capillary membrane, the pulmonary capillary blood volume, and the ventilation and perfusion ratio.9 The transit time of blood at the alveolar capillary membrane is also an important factor that determines diffusion. The blood remains in the pulmonary capillaries for 0.75 second at rest. Within 0.25 second, one-third of that time, the blood is completely saturated. This provides a safety margin during exercise or other conditions in which CO is increased and pulmonary capillary transit time is reduced. The blood can normally be fully oxygenated even with reduced transit time.
Step Five: Perfusion
The distribution of blood perfusing the lungs is primarily gravity dependent, so the dependent lung fields are perfused to a greater extent than are the nondependent lung fields. In upright lungs, the bases are better perfused than the apices (see Chapter 4). Ventilation and perfusion matching is optimal in the midzones of upright lungs.18 In healthy individuals, the ventilation-to-perfusion ratio is a primary determinant of arterial oxygenation. In upright lungs, this ratio is 0.8 in the midzone.
Step Six: Myocardial Function
Optimal myocardial function and CO depend on the synchronized coupling of electrical excitation of the heart and its mechanical contraction. The sinoatrial node, located in the right atrium, is the normal pacemaker for the heart and elicits the normal sinus rhythm with its multiple-component P-QRS-T configuration (see Chapter 12). This wave of electrical excitation spreads throughout the specialized neural conduction system of the atria, the interventricular septum, and the ventricles and is followed by the contraction of the atria and then of the ventricles. The contraction of the right and left ventricles ejects blood into the pulmonary and systemic circulations, respectively.
Step Seven: Peripheral Circulation
The microcirculation consists of the precapillary arterioles, capillaries, and venules. The Starling effect governs the balance of hydrostatic and oncotic pressures within the capillaries and surrounding tissues. The balance of these pressures is 0.3 mm Hg; its net effect is a small outward filtration of fluid from the microvasculature into the interstitial spaces (see Chapter 4). Any excess fluid or loss of plasma protein is drained into the surrounding lymphatic vessels, which usually have a small negative pressure, as does the interstitium. Integrity of the microcirculation is essential to regulate the diffusion of oxygen across the tissue capillary membrane and removing CO2 and waste products.
Step Eight: Tissue Extraction and Use of Oxygen
Perfusion of the tissues with oxygenated blood commensurate with metabolic demand is the principal goal of the oxygen transport system.3 Oxygen is continuously being used by all cells in the body; thus it diffuses rapidly out of the circulation and through cell membranes to meet varying metabolic needs. Diffusion occurs down a gradient from areas of high to low oxygen pressure. The distances between capillaries and cells are variable, so a significant safety factor is required to ensure adequate arterial oxygen tensions. Intracellular PO2 ranges from 5 to 60 mm Hg, with an average of 23 mm Hg.16 Given that only 3 mm Hg of oxygen pressure is needed to support metabolism, 23 mm Hg of oxygen pressure provides an adequate safety margin. These mechanisms ensure an optimal oxygen supply over a wide range of oxygen demands during healthy functioning and in the event of impaired oxygen delivery because of illness. Normally, the rate of oxygen extraction by the cells is regulated by the oxygen demand of cells (i.e., the rate at which ADP is formed from ATP) rather than by the availability of oxygen.
To detect tissue hypoxia, particularly in patients who are critically ill, regional assessments such as gastric mucosal PCO2 and pH have been proposed as being superior to global assessment of 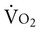 and DO2.28 Regional measures of oxygenation could help to enhance the specificity of treatment based on tissue indicators.29
and DO2.28 Regional measures of oxygenation could help to enhance the specificity of treatment based on tissue indicators.29
Factors that Normally Perturb Oxygen Transport
Several factors related to disease can significantly increase oxygen consumption and metabolic rate over and above the BMR. Such factors include fever, the disease process itself, the process of healing and recovery from injury or disease, thermoregulatory disturbance, reduced arousal, increased arousal resulting from anxiety or pain, sleep loss, medical and surgical interventions, fluid imbalance, and medications.5 These factors may contribute to a systemic increase in the BMR or may reflect local changes in tissue metabolism. Autoregulation of the regional vascular beds promotes increased regional blood flow in accordance with their local tissue metabolic demand.
Because gravitational stress and exercise stress are fundamental to normal cardiovascular and pulmonary function and oxygen transport, the effects of gravity and exercise are highlighted. These two factors augment arousal through stimulation of the reticular activating system in the brainstem and the autonomic nervous system (ANS), which, when depressed, significantly compromises oxygen transport. Emotional stress also has a marked effect on the stimulation of the ANS (the fight-or-flight response) and hence on oxygen transport. These concepts and their clinical implications are described in detail in Chapters 18 and 19.
Gravitational Stress
Humans are designed to function in a 1-g gravitational field. Given that 60% of the body weight is fluid contained within the intravascular and extravascular compartments and that this fluid has considerable mass, changes in body position result in significant instantaneous fluid shifts that can threaten hemodynamic stability.6,31 To maintain consciousness and normal body function during changes in body position, the heart and peripheral vasculature are designed to detect these fluid shifts and accommodate quickly to avoid deleterious functional consequences (e.g., reduced SV, CO, circulating blood volume, and cerebral perfusion). Preservation of the fluid-regulating mechanisms is essential to counter the hemodynamic effects of changing body position. This capacity is impaired with recumbency, which has long been associated with bed rest deconditioning in patients and older populations.32,33 Restoration of adaptation to gravitational stress by upright positioning of a patient is the only means by which these fluid-regulating mechanisms can be maintained and orthostatic intolerance and its short- and long-term sequelae averted.
Summary
In healthy individuals, the most significant factors that perturb oxygen transport are changes in gravitational stress secondary to changes in body position, exercise stress secondary to the increased oxygen demand of working muscles, and arousal and emotional stress. A thorough understanding of the normal effects of gravitational and exercise stress and arousal is essential to understanding deficits in oxygen transport. Numerous factors can impair and threaten oxygen transport, including underlying pathophysiology, restricted mobility, recumbency, factors related to the patient’s care, and factors related to the individual (see Chapters 2 and 17). The physical therapist needs a detailed understanding of these concepts to diagnose such threats and deficits, to understand their impact on activity and participation, and to prescribe effective treatments.

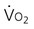 ), and oxygen extraction ratio (OER).
), and oxygen extraction ratio (OER). 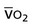
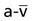 )
)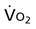 )
)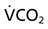 )
)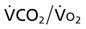 )
)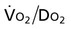 )
)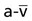 )
)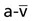 )
)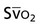






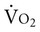 is not directly dependent on D
is not directly dependent on D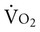 can increase 20 times. In response to increased muscle metabolism, blood flow increases to the peripheral muscles through vasodilation and capillary recruitment, thereby increasing the availability of oxygen to working tissues and its extraction from the arterial blood. As D
can increase 20 times. In response to increased muscle metabolism, blood flow increases to the peripheral muscles through vasodilation and capillary recruitment, thereby increasing the availability of oxygen to working tissues and its extraction from the arterial blood. As D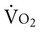 increase, venous return, stroke volume, and heart rate also increase, thereby increasing cardiac output (CO). CO can increase by more than five times during strenuous exercise. The capacity to increase CO reflects a person’s overall aerobic power.
increase, venous return, stroke volume, and heart rate also increase, thereby increasing cardiac output (CO). CO can increase by more than five times during strenuous exercise. The capacity to increase CO reflects a person’s overall aerobic power.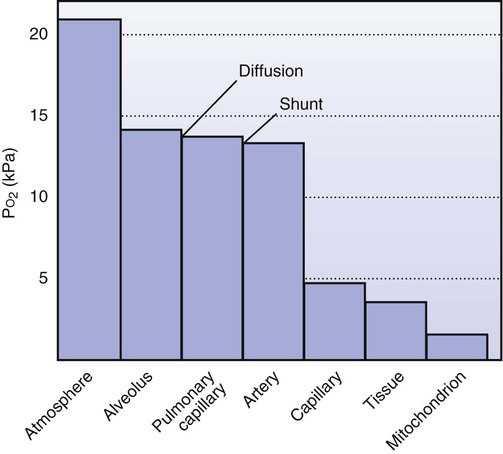
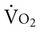 by D
by D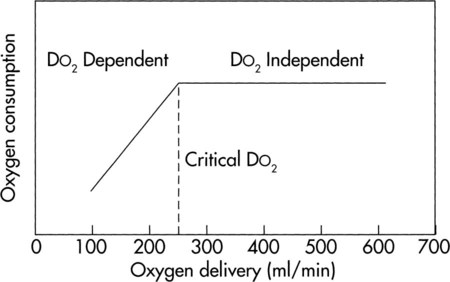
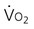 ) and oxygen delivery (D
) and oxygen delivery (D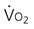 on D
on D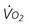 depend on D
depend on D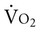 and energy expenditure from moment-to-moment and thus increase rate of metabolism.
and energy expenditure from moment-to-moment and thus increase rate of metabolism.