Chapter 2 Overview of the Microstructure of the Nervous System
The nervous system has two major divisions, the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS consists of the brain and spinal cord and contains the majority of neuronal cell bodies. The PNS includes all nervous tissue outside the CNS and is subdivided into the cranial and spinal nerves, autonomic nervous system (ANS) (including the enteric nervous system (ENS) of the gut wall) and special senses (taste, olfaction, vision, hearing and balance). It is composed mainly of the axons of sensory and motor neurones that pass between the CNS and the body. However, the ENS contains as many intrinsic neurones in its ganglia as the entire spinal cord, is not connected directly to the CNS, and may be considered separately as a third division of the nervous system.
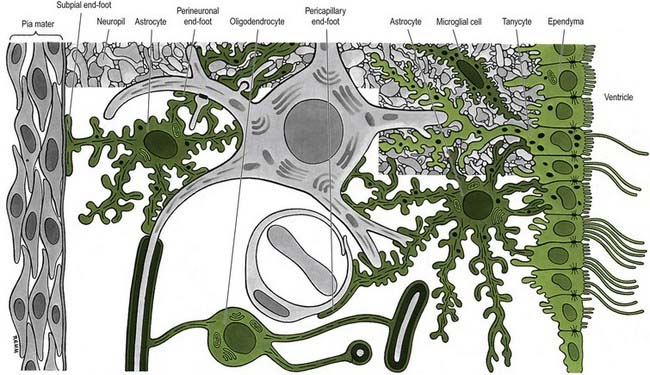
The CNS is derived from the neural tube (Ch. 3). The cell bodies of neurones are often grouped together in areas termed nuclei, or they may form more extensive layers or masses of cells collectively called grey matter. Neuronal dendrites and synaptic activity are mostly confined to areas of grey matter, and they form part of its meshwork of neuronal and glial processes that is collectively termed the neuropil (Fig. 2.1). Their axons pass into bundles of nerve fibres that tend to be grouped separately to form tracts. In the spinal cord, cerebellum, cerebral cortices and some other areas, concentrations of tracts constitute the white matter, so called because the axons are often ensheathed in myelin, which is white when fresh (see Figs. 8.16, 8.19).
The PNS is composed of the axons of motor neurones situated inside the CNS and the cell bodies of sensory neurones (grouped together as ganglia) and their processes. Sensory cells in dorsal root ganglia give off both centrally and peripherally directed processes; there are no synapses on their cell bodies. Ganglionic neurones of the ANS receive synaptic contacts from various sources. Neuronal cell bodies in peripheral ganglia are all derived embryologically from cells that migrate from the neural crest (Ch. 3).
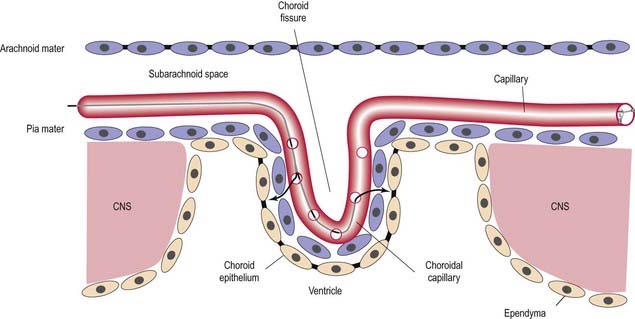
When the neural tube is formed during prenatal development, its walls thicken greatly but do not completely obliterate the cavity within. The latter remains in the spinal cord as the narrow central canal, and in the brain it becomes greatly expanded to form a series of interconnected cavities called the ventricular system. In the fore- and hindbrains, parts of the neural tube roof do not generate nerve cells but become thin, folded sheets of secretory tissue that are invaded by blood vessels and are called the choroid plexuses. The plexuses secrete cerebrospinal fluid (CSF), which fills the ventricles and subarachnoid spaces (Ch. 4). and penetrates the intercellular spaces of the brain and spinal cord to create their interstitial fluid. The CNS has a rich blood supply, which is essential to sustain its high metabolic rate. The blood–brain barrier places considerable restrictions on the substances that can diffuse from the blood stream into the nervous tissue.
Neurones
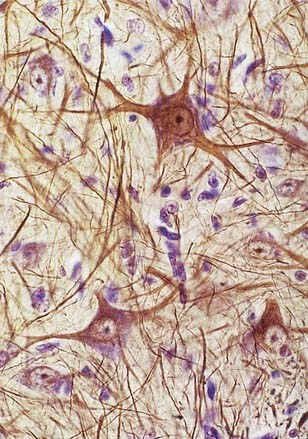
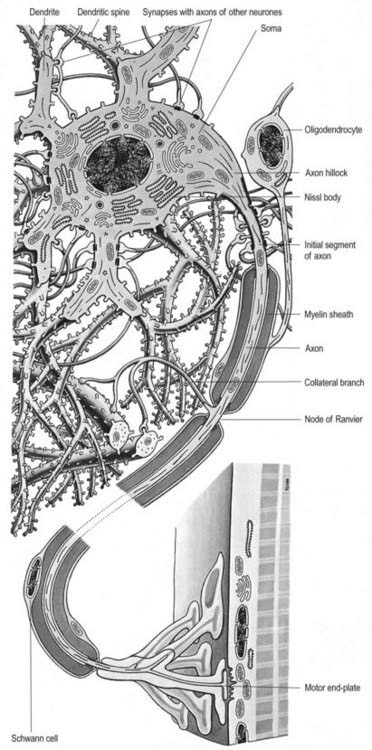
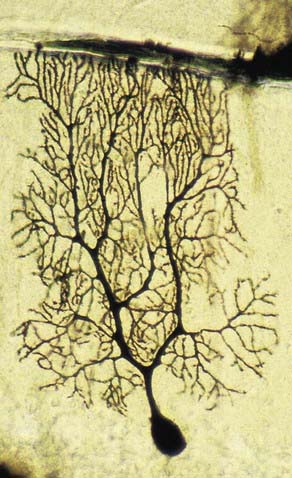
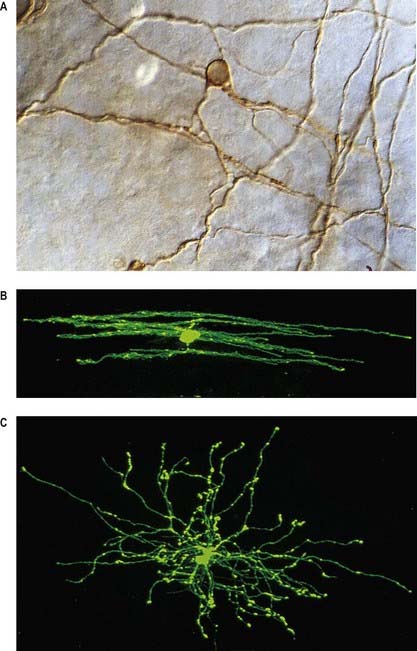
Neurones exhibit great variability in size (cell bodies range from 5 to 100 µm diameter) and shape. Their surface areas are extensive because most neurones display numerous narrow, branched cell processes. They usually have a rounded or polygonal cell body (perikaryon or soma). This is a central mass of cytoplasm that encloses a nucleus and gives off long, branched extensions, with which most intercellular contacts are made. Typically, one of these processes, the axon, is much longer than the others, the dendrites (Fig. 2.2). Dendrites conduct electrical impulses toward a soma, whereas axons conduct impulses away from it.
Neurones can be classified according to the number and arrangement of their processes. Multipolar neurones (Fig. 2.3; see also Fig. 16.9) are common; they have an extensive dendritic tree, which arises either from a single primary dendrite or directly from the soma, and a single axon. Bipolar neurones, which typify neurones of the special sensory systems (e.g., retina), have only a single dendrite that emerges from the soma opposite the axonal pole. Unipolar neurones that transmit general sensation (e.g., dorsal root ganglion neurones) have a single short process that bifurcates into peripheral and central processes, an arrangement that arises by the fusion of the proximal axonal and dendritic processes of a bipolar neurone during development.
Neurones are postmitotic cells and, with few exceptions, are not replaced when lost.
Soma
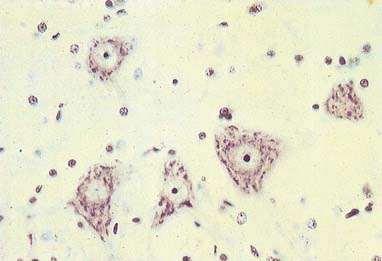
The cytoplasm of a typical soma (see Fig. 2.2) is rich in rough and smooth endoplasmic reticulum and free polyribosomes, which reveals a high level of protein synthetic activity. Free polyribosomes often congregate in large groups associated with the rough endoplasmic reticulum. These aggregates of RNA-rich structures are visible by light microscopy as basophilic Nissl (chromatin) bodies or granules (Fig. 2.4). They are more obvious in large, highly active cells such as spinal motor neurones, which contain large stacks of rough endoplasmic reticulum and polyribosome aggregates. Maintenance and turnover of cytoplasmic and membranous components are necessary in all cells; the huge total volume of cytoplasm within the soma and processes of many neurones requires a considerable commitment of protein synthetic machinery. Neurones synthesize other proteins (e.g., enzyme systems) involved in the production of neurotransmitters and in the reception and transduction of incoming stimuli. Various transmembrane channel proteins and enzymes are located at the surfaces of neurones, where they are associated with ion transport. The apparatus for protein synthesis (including RNA and ribosomes) occupies the soma and dendrites but is usually absent from axons.
The neuronal cytoskeleton is a prominent feature of its cytoplasm, and it gives shape, strength and rigidity to the dendrites and axons. Neurofilaments (the intermediate filaments of neurones) and microtubules are abundant; they occur in the soma and extend along dendrites and axons, in proportions that vary with the type of neurone and cell process. Bundles of neurofilaments constitute neurofibrils, which can be seen by light microscopy in silver stained sections. Neurofilaments are heteropolymers of proteins assembled from three polypeptide subunits: NF-L (68 kDa), NF-M (160 kDa) and NF-H (200 kDa). NF-M and NF-H have long C-terminal domains that project as side arms from the assembled neurofilament and bind to neighbouring filaments. They can be heavily phosphorylated, particularly in the highly stable neurofilaments of mature axons, and are thought to give axons their tensile strength. Some axons are almost filled by neurofilaments. Dendrites usually have more microtubules than axons.
Dendrites
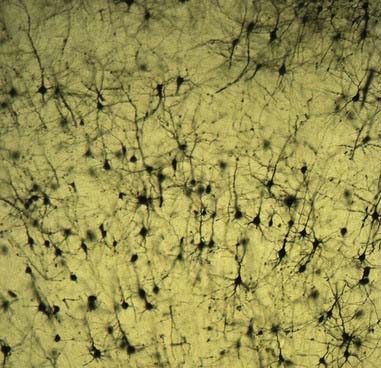
Dendrites are highly branched, usually short processes that project from the soma (see Fig. 2.2). The branching patterns of many dendritic arrays are probably established by random adhesive interactions between dendritic growth cones and afferent axons that occur during development. There is an overproduction of dendrites in early development, which is pruned in response to functional demand as the individual matures and information is processed through the dendritic tree. There is evidence that dendritic trees may be plastic structures throughout adult life, expanding and contracting as the traffic of synaptic activity varies through afferent axodendritic contacts. Groups of neurones with similar functions have a similar stereotypical tree structure (Fig. 2.5), suggesting that the branching patterns of dendrites are important determinants of the integration of afferent inputs that converge on the tree.
Dendrites differ from axons in many respects. They represent the afferent rather than the efferent system of the neurone, and they receive both excitatory and inhibitory axodendritic contacts. They may also make dendrodendritic and dendrosomatic connections (see Fig. 2.8), some of which are reciprocal. Synapses occur either on small projections called dendritic spines or on the smooth dendritic surface. Dendrites contain ribosomes, smooth endoplasmic reticulum, microtubules, neurofilaments, actin filaments and Golgi complexes. The neurofilament proteins of dendrites are poorly phosphorylated. Dendrite microtubules express the microtubule-associated protein (MAP-2) almost exclusively, in comparison with axons.
Axons
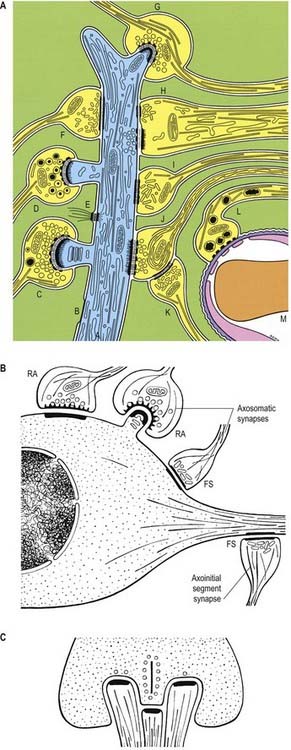
The axon originates either from the soma or from the proximal segment of a dendrite, at a specialized region called the axon hillock (see Fig. 2.2), which is free of Nissl granules. Action potentials are initiated here. The axonal plasma membrane (axolemma) is undercoated at the hillock by a concentration of cytoskeletal molecules, including spectrin and actin fibrils, which are thought to be important in anchoring numerous voltage-sensitive channels to the membrane. The axon hillock is unmyelinated and often participates in inhibitory axo-axonal synapses. This region of the axon is unique because it contains ribosomal aggregates immediately below the postsynaptic membrane.
When present, myelin begins at the distal end of the axon hillock. Myelin thickness and internodal segment lengths are positively correlated with axon diameter. In the PNS unmyelinated axons are embedded in Schwann cell cytoplasm; in the CNS they lie free in the neuropil. Nodes of Ranvier are specialized constricted regions of myelin-free axolemma where action potentials are generated and where an axon may branch. The density of sodium channels in the axolemma is highest at the nodes of Ranvier and very low along internodal membranes. In contrast, sodium channels are spread more evenly within the axolemma of unmyelinated axons. Fast potassium channels are also present in the paranodal regions of myelinated axons. Fine processes of glial cytoplasm (astrocyte in the CNS, Schwann cell in the PNS) surround the nodal axolemma. The terminals of an axon are unmyelinated. They expand into presynaptic boutons, which may form connections with axons, dendrites, neuronal somata or, in the periphery, muscle fibres, glands and lymphoid tissue. They may themselves be contacted by other axons, forming axo-axonal presynaptic inhibitory circuits. Further details of neuronal microcircuitry are given in Kandel and Schwartz (2000).
Axoplasmic Flow
Rapid flow depends on microtubules. Vesicles with side projections line up along microtubules and are transported along them by their side arms. Two microtubule-based motor proteins with ATPase activity are involved in fast transport. Kinesin family proteins are responsible for the fast component of anterograde transport, and cytoplasmic dynein is responsible for retrograde transport. Fast anterograde transport carries vesicles, including synaptic vesicles containing neurotransmitters, from the soma to the axon terminals. Retrograde axonal transport accounts for the flow of mitochondria, endosomes and lysosomal autophagic vacuoles from the axon terminals into the soma. Retrograde transport mediates the movement of neurotrophic viruses (e.g., herpes zoster, rabies, polio) from peripheral terminals and their subsequent concentration in the neuronal soma.
Synapses
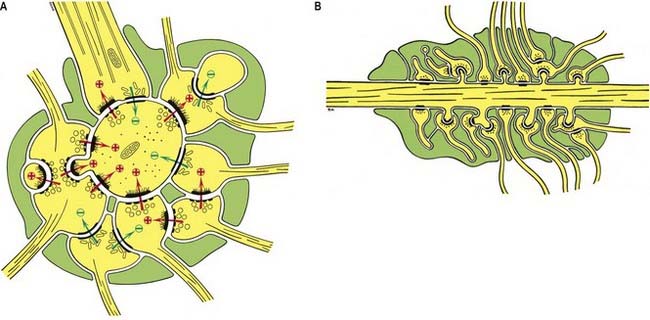
The patterns of axonal termination vary considerably. A single axon may synapse with one neurone, such as climbing fibres ending on cerebellar Purkinje neurones; more often, it synapses with many, such as cerebellar parallel fibres, which provide an extreme example of this phenomenon. In synaptic glomeruli (e.g., in the olfactory bulb) and synaptic cartridges, groups of synapses between two or more neurones form interactive units encapsulated by neuroglia (Fig. 2.6).
Classification of Chemical Synapses
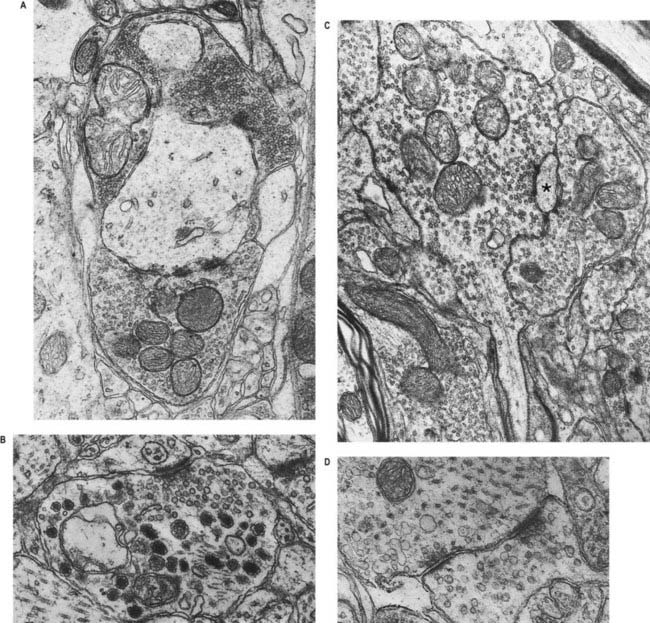
Chemical synapses have an asymmetric structural organization (Figs 2.7, 2.8), in keeping with the unidirectional nature of their transmission. Typical chemical synapses share a number of important features. They all display an area of presynaptic membrane apposed to a corresponding postsynaptic membrane; the two are separated by a narrow (20 to 30 nm) gap, the synaptic cleft. Synaptic vesicles containing neurotransmitters lie on the presynaptic side, clustered near an area of dense material on the cytoplasmic aspect of the presynaptic membrane. A corresponding region of submembrane density is present on the postsynaptic side. Together these define the active zone, the area of the synapse where neurotransmission takes place.
Chemical synapses can be classified according to a number of different parameters, including the neuronal regions forming the synapse, their ultrastructural characteristics, the chemical nature of their neurotransmitters and their effects on the electrical state of the postsynaptic neurone. The classification described here is limited to associations between neurones. Neuromuscular junctions share many (although not all) of these parameters and are often referred to as peripheral synapses. They are described separately in Chapter 22.
Synapses can occur between almost any surface regions of the participating neurones. The most common type occurs between an axon and either a dendrite or a soma, when the axon is expanded as a small bulb or bouton (see Figs. 2.7, 2.8). This may be a terminal of an axonal branch (terminal bouton) or one of a row of bead-like endings, with the axon making contact at several points and often with more than one neurone (bouton de passage). Boutons may synapse with dendrites, including dendritic spines or the flat surface of a dendritic shaft; a soma, usually on its flat surface, but occasionally on spines; the axon hillock and the terminal boutons of other axons.
Ultrastructurally, synaptic vesicles may be internally clear or dense and of different sizes (loosely categorized as small or large) and shape (round, flat or pleomorphic, i.e., irregularly shaped). The submembranous densities may be thicker on the postsynaptic than on the presynaptic side (asymmetric synapses) or equivalent in thickness (symmetric synapses). Synaptic ribbons are found at sites of neurotransmission in the retina and inner ear. They have a distinctive morphology, in that the synaptic vesicles are grouped around a ribbon- or rod-like density oriented perpendicular to the cell membrane (see Fig. 2.8).
The neurosecretory endings found in various parts of the brain and in neuroendocrine glands have many features in common with presynaptic boutons. They all contain peptides or glycoproteins within dense-core vesicles of characteristic size and appearance. These are often ellipsoidal or irregular in shape and relatively large; for example, oxytocin and vasopressin vesicles in the neurohypophysis may be up to 200 nm across.
Mechanisms of Synaptic Activity
Synaptic activation begins with the arrival of one or more action potentials at the presynaptic bouton, which causes the opening of voltage-sensitive calcium channels in the presynaptic membrane. The response time in typical fast-acting synapses is then very rapid; classic neurotransmitter (e.g., ACh) is released in less than a millisecond, which is faster than the activation time of a classic second messenger system on the presynaptic side. The influx of calcium activates Ca2+-dependent protein kinases. This uncouples synaptic vesicles from a spectrin–actin meshwork within the presynaptic ending, to which they are bound via synapsins I and II. The vesicles dock with the presynaptic membrane, through processes not yet fully understood, and their membranes fuse to open a pore through which neurotransmitter diffuses into the synaptic cleft.
Neurotransmitters
Until recently the molecules known to be involved in chemical synapses were limited to a fairly small group of classic neurotransmitters—ACh, noradrenaline, adrenaline, dopamine and histamine—all of which had well-defined rapid effects on other neurones, muscle cells or glands. However, many synaptic interactions cannot be explained on the basis of classic neurotransmitters, and it now appears that other substances, particularly some amino acids such as glutamate, glycine, aspartate, GABA and the monoamine serotonin, also function as transmitters. Substances first identified as hypophysial hormones or as part of the dispersed neuroendocrine system of the alimentary tract can be detected widely throughout the CNS and PNS, often associated with functionally integrated systems. Many of these are peptides: more than 50 (together with other candidates) function mainly as neuromodulators and influence the activities of classic transmitters.
Amino acids
The best understood amino acid is GABA, which is a major inhibitory transmitter released at the terminals of local circuit neurones within the brain stem and spinal cord (e.g., recurrent inhibitory Renshaw loop; Ch. 8), cerebellum (as the main transmitter of Purkinje neurones) and elsewhere. It is stored in flattened or pleomorphic vesicles within symmetric synapses; it may be inhibitory to the postsynaptic neurone, or it may mediate either presynaptic inhibition or facilitation, depending on the synaptic arrangement.
Central Glia
Glial (neuroglial) cells vary considerably in type and number in different regions of the CNS. There are two major groups, classified according to origin. Macroglia arise within the neural plate, in parallel with neurones, and constitute the great majority of glial cells. Microglia are smaller cells, generally considered to be monocytic in origin, and are derived from haemopoietic tissue (Fig. 2.9).
Astrocytes
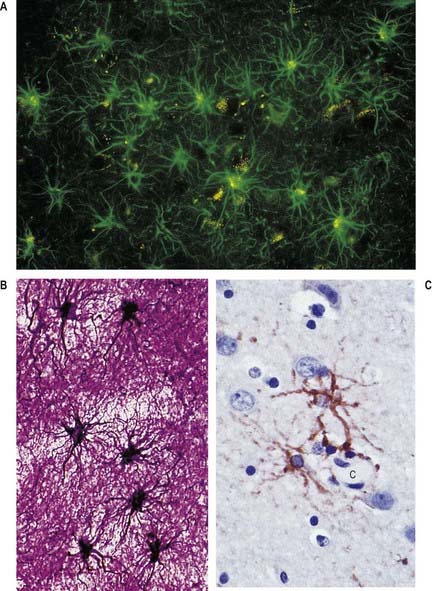
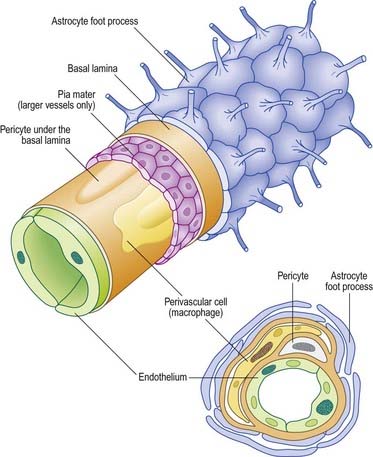
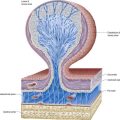
Astrocytes are star-shaped glia whose processes ramify through the entire central neuropil (see Fig. 2.9). Their processes are functionally coupled at gap junctions and form an interconnected network that ensheathes all neurones, except at synapses and along the myelinated segments of axons. Astrocyte processes terminate as end-feet at the basal lamina of blood vessels and where they form the glia limitans (glial-limiting membrane) at the pial surface (Fig. 2.10). Ultrastructurally, astrocytes typically have a pale nucleus with a narrow rim of heterochromatin, although this is variable. They have pale cytoplasm containing glycogen, lysosomes, Golgi complexes and bundles of glial intermediate filaments within their processes (the last are found particularly in fibrous astrocytes, which occur predominantly in white matter). Glial intermediate filaments are formed from glial fibrillary acidic protein (GFAP); its presence can be used clinically to identify tumour cells of glial origin. A second morphological type of astrocyte, the protoplasmic astrocyte, is found mainly in grey matter. The significance of these subtypes is unclear: there are few known functional differences between fibrous and protoplasmic astrocytes.
Blood–Brain Barrier
Proteins circulating in the blood enter most tissues of the body except those of the brain, spinal cord or peripheral nerves. This concept of a blood–brain barrier (and blood–nerve barrier) covers many substances, some of which are actively transported across the blood–brain barrier, whereas others are actively excluded. The blood–brain barrier is located at the capillary endothelium within the brain. It depends on the presence of tight junctions between endothelial cells and a relative lack of transcytotic vesicular transport. The tightness of the barrier depends on the close apposition of astrocytes to blood capillaries (Figs. 2.10C, 2.11).
Oligodendrocytes
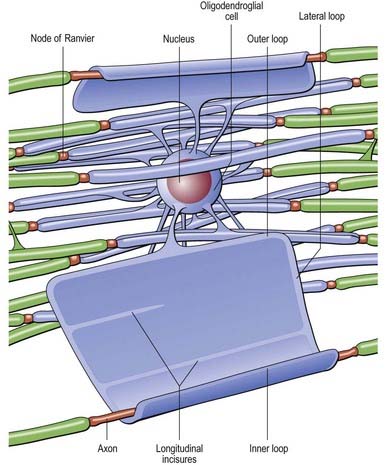
Oligodendrocytes myelinate CNS axons and are most commonly seen as intrafascicular cells in myelinated tracts (Figs 2.12, 2.13). They usually have round nuclei, and their cytoplasm contains numerous mitochondria, microtubules and glycogen. They display a spectrum of morphological variation, from large euchromatic nuclei and pale cytoplasm to heterochromatic nuclei and dense cytoplasm. Oligodendrocytes may enclose up to 50 axons in separate myelin sheaths: the largest calibre axons are usually ensheathed on a 1 : 1 basis. Some oligodendrocytes are not associated with axons and are either precursor cells or perineuronal (satellite) oligodendrocytes whose processes ramify around neuronal somata.
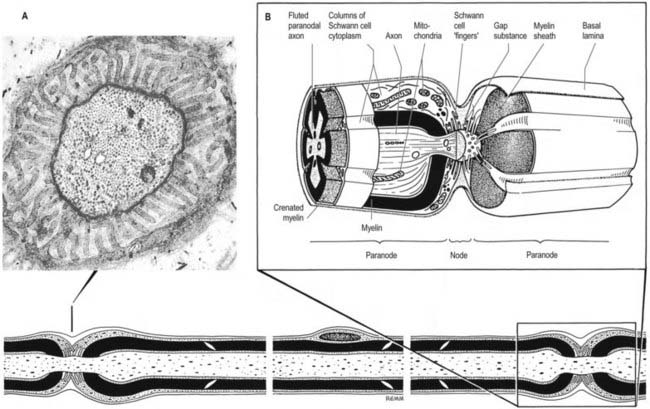
Nodes of Ranvier and Incisures of Schmidt–Lanterman
The territory ensheathed by an oligodendrocyte process defines an internode. The interval between internodes is called a node of Ranvier, and the territory immediately adjacent to the nodal gap is a paranode, where loops of oligodendrocyte cytoplasm abut the axolemma. Nodal axolemma is contacted by the end-feet of perinodal cells, which have been shown in animal studies to have a presumptive adult oligodendrocyte progenitor phenotype; their function is unknown (Butt and Berry 2000). Schmidt–Lanterman incisures are helical decompactions of internodal myelin where the major dense line of the myelin sheath splits to enclose a spiral of oligodendrocyte cytoplasm. Their function is unknown, but their structure suggests that they may play a role in the transport of molecules across the myelin sheath.
Myelin and Myelination
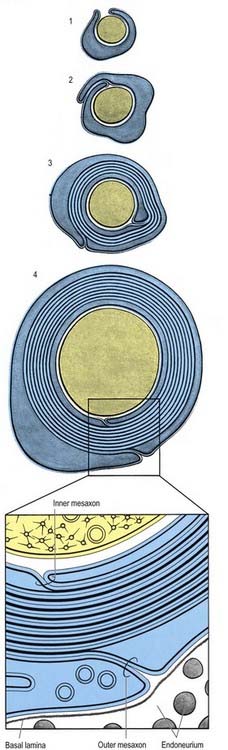
It is not known how myelin is formed in either the PNS or the CNS. The ultrastructural appearance of myelin (Fig. 2.14) is usually explained in terms of the spiral wrapping of a flat glial process around an axon and the subsequent extrusion of cytoplasm from the sheath at all points other than incisures and paranodes. In this way, it is thought that the compacted external surfaces of the plasma membrane of the ensheathing glial cell produce the minor dense lines, and the compacted inner cytoplasmic surfaces produce the major dense lines, of the mature myelin sheath (Fig. 2.15). These correspond to the intraperiod and period lines, respectively, defined in X-ray studies of myelin. The inner and outer zones of occlusion of the spiral process are continuous with the minor dense line and are called the inner and outer mesaxons.
Mutations of the major myelin structural proteins have now been recognized in a number of inherited human neurological diseases. As would be expected, these mutations produce defects in myelination and in the stability of nodal and paranodal architecture, consistent with the suggested functional roles of the relevant proteins in maintaining the integrity of the myelin sheath. The molecular organization of myelinated axons is described in Scherer and Arroyo (2002).
Ependyma
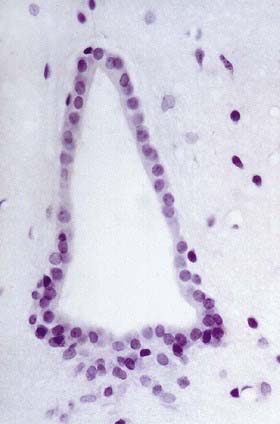
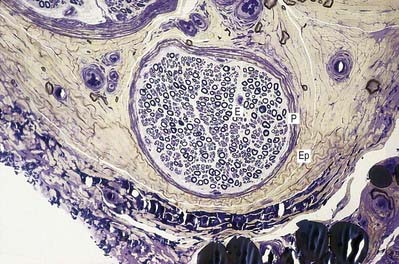
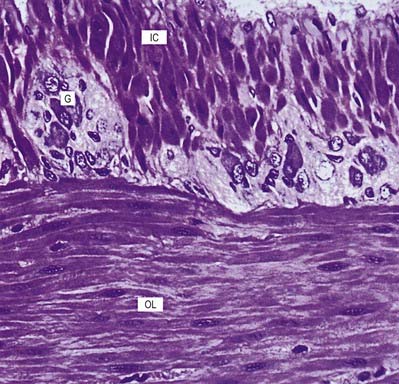
Ependymal cells line the ventricles and central canal of the spinal cord (Fig. 2.16). They form a single-layered epithelium that varies from squamous to columnar in form. At the ventricular surface, cells are joined by gap junctions and occasional desmosomes. Their apical surfaces have numerous microvilli and cilia, which contribute to the flow of CSF. There is considerable regional variation in the ependymal lining of the ventricles, but four major types have been described: the general ependyma that overlies grey matter, the general ependyma that overlies white matter, specialized areas of ependyma in the third and fourth ventricles and the choroidal epithelium.
The ependymal cells overlying areas of grey matter are cuboidal; each cell bears approximately 20 central apical cilia, surrounded by short microvilli. The cells are joined by gap junctions and desmosomes and do not have a basal lamina. Beneath them there may be a subependymal zone, two to three cells deep, consisting of cells that generally resemble ependymal cells. The capillaries beneath them have no fenestrations and few transcytotic vesicles, which is typical of the CNS. Where the ependyma overlies myelinated tracts of white matter, the cells are much flatter, and few are ciliated. There are gap junctions and desmosomes between cells, but their lateral margins interdigitate, unlike those overlying grey matter. No subependymal zone is present.
Specialized areas of ependymal cells are found in four areas around the margins of the third ventricle. These areas, called the circumventricular organs, consist of the lining of the median eminence of the hypothalamus, the subcommissural organ, the subfornical organ and the vascular organ of the lamina terminalis (Ch. 15). The area postrema, at the inferoposterior limit of the fourth ventricle, has a similar structure. In all these sites the ependymal cells are only rarely ciliated, and their ventricular surfaces bear many microvilli and apical blebs. They have numerous mitochondria, well-formed Golgi complexes and a rather flattened basal nucleus. They are joined laterally by tight junctions that form a barrier to the passage of materials across the ependyma and by desmosomes. Many of the cells are tanycytes (ependymal astrocytes) and have basal processes that project into the perivascular space surrounding the underlying capillaries. Significantly, these capillaries are fenestrated and therefore do not form a blood–brain barrier. It is believed that neuropeptides can pass from nervous tissue into the CSF by active transport through the ependymal cells in these specialized areas, giving them access to a wide population of neurones via the permeable ependymal lining of the rest of the ventricle.
The ependyma is highly modified where it lies adjacent to the vascular layer of the choroid plexus.
Choroid Plexus
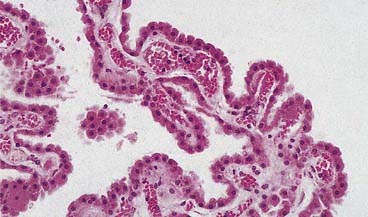
The ependymal cells in the choroid plexus resemble those of the circumventricular organs, except that they do not have basal processes; instead, they form a cuboidal epithelium that rests on a basal lamina adjacent to the enclosed fold of pia mater and its capillaries (Figs. 2.17, 2.18). Capillaries of the choroid plexus are lined by a fenestrated endothelium. Cells have numerous long microvilli, with only a few cilia interspersed between them. They also have many mitochondria, large Golgi complexes and basal nuclei, consistent with their secretory activity: they produce most components of the CSF. They are linked by tight junctions that form a transepithelial barrier (a component of the blood–CSF barrier) and by desmosomes. Their lateral margins are highly folded.
Microglia
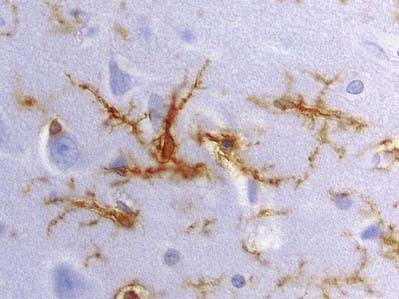
Microglia are small dendritic cells found throughout the CNS (Fig. 2.19), including the retina. Evidence largely supports the view that they are derived from fetal monocytes or their precursors, which invade the developing nervous system. An alternative hypothesis holds that microglia share a lineage with ependymal cells and are thus neural tube derivatives. According to the monocyte theory, haematogenous cells pass through the walls of neural blood vessels and invade CNS tissue prenatally as amoeboid cells. Later they lose their motility and transform into typical microglia, bearing branched processes that ramify in non-overlapping territories within the brain. All microglial domains, defined by their dendritic fields, are equivalent in size and form a regular mosaic throughout the brain. The expression of microglia-specific antigens changes with age: many are downregulated as microglia attain the mature dendritic form.
Peripheral Nerves
Widely variable numbers of peripheral nerve fibres are grouped into bundles (fasciculi). The size, number and pattern of fasciculi (Fig. 2.20) vary in different nerves and at different levels along their paths. Their number increases and their size decreases some distance proximal to a point of branching. Where nerves are subjected to pressure, such as deep to a retinaculum, fasciculi are increased in number but reduced in size, and the amount of associated connective tissue and degree of vascularity also increase. At these points, nerves may occasionally show a pink, fusiform dilatation, sometimes termed a pseudoganglion or gangliform enlargement.
Peripheral Nerve Fibres
The largest afferent axons (Aα fibres) innervate encapsulated cutaneous, joint and muscle receptors and some large alimentary enteroceptors. Aδ fibres innervate thermoreceptors and nociceptors, including those in dental pulp, skin and connective tissue. C fibres have thermoreceptive, nociceptive and interoceptive functions. The largest somatic efferent fibres (Aα) are up to 20 µm in diameter. They innervate extrafusal muscle fibres exclusively and conduct at a maximum of 120 m/s. Fibres to fast twitch muscles are larger than those to slow twitch muscles. Aβ fibres are restricted to collaterals of Aα fibres and form plaque endings on some intrafusal muscle fibres. Aγ fibres are exclusively fusimotor to plate and trail endings on intrafusal muscle fibres. C fibres are postganglionic sympathetic and parasympathetic axons. This scheme can be applied to all fibres of spinal and cranial nerves, except perhaps those of the olfactory nerve, whose fibres form a uniquely small and slow group.
Connective Tissue Sheaths
Nerve trunks, whether uni- or multifascicular, are surrounded by an epineurium. Individual fasciculi are enclosed by a multilayered perineurium, which in turn surrounds the endoneurium or intrafascicular connective tissue (see Fig. 2.20).
Schwann Cells
Unmyelinated Axons
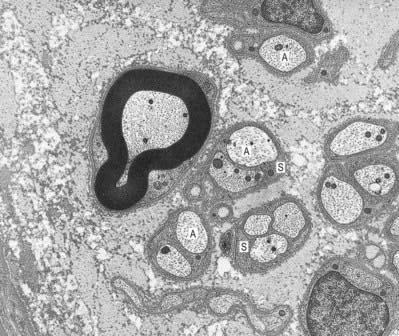
Unmyelinated axons are commonly 1 µm in diameter, although some may be 1.5 or even 2 µm in diameter. Groups of up to 10 small axons (0.15 to 2 µm in diameter) are enclosed within a chain of overlapping Schwann cells and surrounded by a basal lamina. Within each Schwann cell, individual axons are usually sequestered from their neighbours by delicate processes of cytoplasm (see Fig. 2.14). Axons move between Schwann cell chains as they pass proximodistally along a nerve fasciculus. It seems likely, on the basis of quantitative studies in subhuman primates, that axons from adjacent cord segments may share Schwann cell columns; this phenomenon may play a role in the evolution of neuropathic pain after nerve injury. In the absence of a myelin sheath and nodes of Ranvier, conduction along unmyelinated axons is not saltatory but electrotonic; the passage of impulses is therefore relatively slow (0.5 to 4 m/s).
Myelinated Axons
Myelinated axons (see Fig. 2.14) have a 1 : 1 relationship with their ensheathing Schwann cells. The territory of an individual Schwann cell defines an internode (Fig. 2.21): internodal length varies directly with the diameter of the fibre, from 150 to 1500 µm. The interval between two internodes is a node of Ranvier. In the PNS the myelin sheaths on either side of a node terminate in asymmetrically swollen paranodal bulbs. Schwann cell cytoplasm forms a continuous layer only in the perinuclear (mid-internodal) and paranodal regions. Between these sites, internodal Schwann cytoplasm forms a delicate network over the inner (abaxonal) surface of the myelin sheath. The outer (adaxonal) layer of Schwann cell cytoplasm is frequently discontinuous, and axons are surrounded by a narrow periaxonal space (15 to 20 nm), which, although nominally part of the extracellular space, is functionally isolated from it at the paranodes. For further details, see Scherer and Arroyo (2002).
Satellite Cells
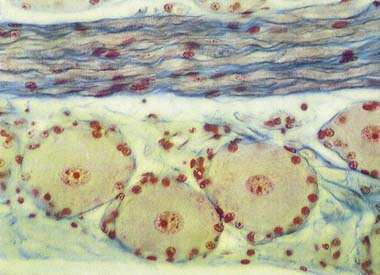
Many non-neuronal cells of the nervous system have been called satellite cells. The list includes small, round extracapsular cells in peripheral ganglia, ganglionic capsular cells and Schwann cells. The term is sometimes used to describe all non-neuronal cells, both central and peripheral, that are closely associated with neuronal somata. The name is also given to precursor cells associated with striated muscle fibres. Within the nervous system, the term is most commonly reserved for the flat, epithelioid satellite cells (ganglionic glial cells, capsular cells) that surround the neuronal somata of peripheral ganglia (Fig. 2.22). The cytoplasm of capsular cells resembles that of Schwann cells, and their deep surfaces interdigitate with reciprocal infoldings in the membranes of the enclosed neurones. The capsular layer is continuous with similar cells that enclose the initial part of the dendroaxonal process in unipolar sensory neurones of the dorsal spinal roots and, subsequently, with the Schwann cells surrounding their peripheral and central processes.
Enteric Glia
Autonomic nerves of the ENS (Ch. 21) have more in common with central tracts than with other peripheral nerves. Enteric nerves do not have the collagenous coats of other peripheral nerves, and they lack an endoneurium. The enteric ganglionic neurones are supported by glia that closely resemble astrocytes and contain more GFAP than non-myelinating Schwann cells. The enteric glia also differ from Schwann cells, in that they do not produce a surrounding basal lamina.
Ganglia
Sensory Ganglia
The sensory ganglia of dorsal spinal roots (see Fig. 2.22) and the ganglia of the trigeminal, facial, glossopharyngeal and vagal cranial nerves are enclosed in periganglionic connective tissue, which resembles the perineurium. Ganglionic neurones are unipolar. They have spherical or oval somata of varying sizes, which are aggregated in groups between fasciculi of myelinated and unmyelinated nerve fibres. For each neurone, the single axodendritic process bifurcates into central and peripheral processes; in myelinated fibres the junction occurs at a node of Ranvier. The peripheral process reaches a sensory ending, and because it conducts impulses toward the soma, strictly speaking, it functions as an elongated dendrite. However, because it has the typical structural and other functional properties of a peripheral axon, it is conventionally described as an axon.
Enteric Ganglia
The ENS is composed of ganglionic neurones and associated nerves (Fig. 2.23) serving different functions, including regulation of gut motility and mucosal transport. Extrinsic autonomic fibres supply the gut wall, and together with intrinsic enteric ganglionic neurones and the endocrine and cardiovascular systems, they integrate the activities of the digestive system as a result of either interaction with enteric neurones (e.g. via vagal fibres) or direct regulation of the local bloodflow (via postganglionic sympathetic fibres).
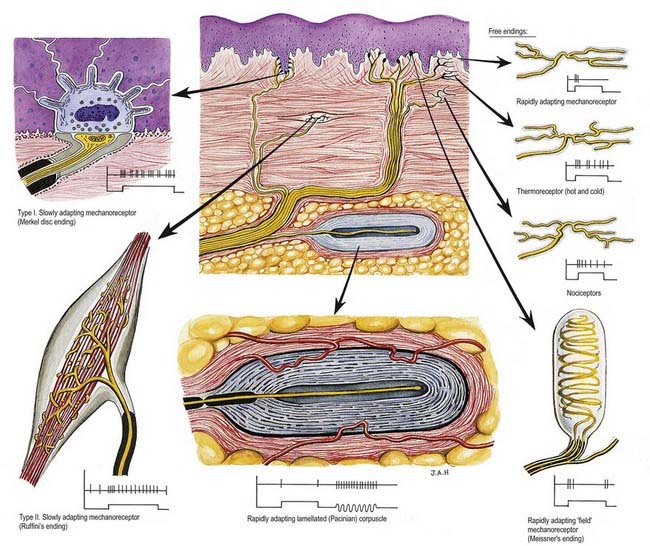
Sensory Endings (Fig. 2.24)
General Features of Sensory Receptors
There are three major forms of sensory receptor: neuroepithelial, epithelial and neuronal.
Functional Classification of Receptors
Interoceptors include vascular chemoreceptors, such as the carotid body, and baroceptors, which are concerned with the regulation of bloodflow and pressure and with the control of respiration. Irritant receptors respond polymodally to noxious chemicals or damaging mechanical stimuli and are widely distributed in the epithelia of the alimentary and respiratory tracts; they may initiate protective reflexes.
Free Nerve Endings
Sensory endings that branch to form plexuses occur in many sites (see Fig. 2.24). They occur in all connective tissues, including those of the dermis, fasciae, capsules of organs, ligaments, tendons, adventitia of blood vessels, meninges, articular capsules, periosteum, perichondrium, Haversian systems in bone, parietal peritoneum, walls of viscera and endomysium of all types of muscle. They also innervate the epithelium of the skin, corneas, buccal cavity and alimentary and respiratory tracts and their glands. Within epithelia they lack Schwann cell ensheathment and are enveloped instead by epithelial cells. Afferent fibres from free terminals may be myelinated or unmyelinated but are always of small diameter and low conduction velocity. When afferent axons are myelinated, their terminal arborizations are not. These terminals serve several sensory modalities. In the dermis, they may be responsive to moderate cold or heat (thermoreceptors); light mechanical touch (mechanoreceptors); damaging heat, cold or deformation (unimodal nociceptors) and damaging stimuli of several kinds (polymodal nociceptors). Similar fibres in deeper tissues may also signal extreme conditions, and these are experienced, as with all nociceptors, as pain. Free endings in the corneas, dentine and periosteum may be exclusively nociceptive.
Encapsulated Endings
Encapsulated endings are a major group of special endings, although they exhibit considerable variety in their size, shape and distribution. They all share a common feature, which is that the axon terminal is encapsulated by non-excitable cells. This category of ending includes lamellated corpuscles of various kinds (e.g. Meissner’s, Pacinian), Golgi tendon organs, neuromuscular spindles and Ruffini endings (see Fig. 2.24).
Meissner’s Corpuscles
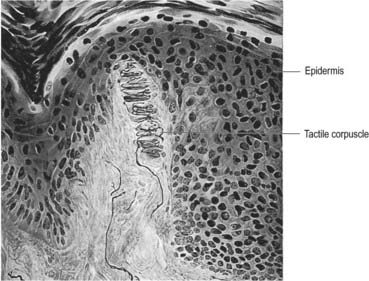
Meissner’s corpuscles are found in the dermal papillae of all parts of the hands and feet, the fronts of the forearms, lips, palpebral conjunctiva and mucous membrane of the apical part of the tongue. They are most concentrated in thick, hairless skin, especially of the finger pads, where there may be up to 24 corpuscles/cm2 in young adults. Mature corpuscles are cylindrical in shape, approximately 80 µm long and 30 µm across, with their long axes perpendicular to the skin surface. Each corpuscle has a connective tissue capsule and a central core (Fig. 2.25). Meissner’s corpuscles are rapidly adapting mechanoreceptors, sensitive to shape and textural changes in exploratory and discriminatory touch; their acute sensitivity provides the neural basis for reading Braille text.
Pacinian Corpuscles
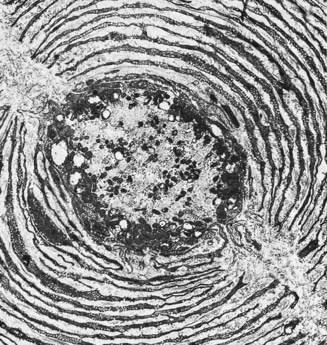
Pacinian corpuscles are situated subcutaneously in the palmar and plantar aspects of the hands and feet and their digits; in the external genitalia, arms, neck, nipple, periosteum, and interosseous membranes; near joints and in the mesentries. They are oval, spherical or irregularly coiled and are up to 2 mm long and 100 to 500 µm or more across; the larger ones are visible to the naked eye. Each corpuscle has a capsule, an intermediate growth zone and a central core that contains an axon terminal. The capsule is formed by approximately 30 concentrically arranged lamellae of flat cells approximately 0.2 µm thick (Fig. 2.26). Adjacent cells overlap, and successive lamellae are separated by an amorphous proteoglycan matrix that contains circularly oriented collagen fibres, closely applied to the surfaces of the lamellar cells. The amount of collagen increases with age. The intermediate zone is cellular, and its cells become incorporated into the capsule or core, so that it is not clearly defined in mature corpuscles. The core consists of approximately 60 bilateral, compacted lamellae that lie on both sides of a central nerve terminal.
Ruffini Endings
Ruffini endings are slowly adapting mechanoreceptors. They are found in the dermis of thin, hairy skin, where they function as dermal stretch receptors and are responsive to maintained stresses in dermal collagen. They consist of highly branched, unmyelinated endings of myelinated afferents. They ramify between bundles of collagen fibres within a spindle-shaped structure that is enclosed partly by a fibrocellular sheath derived from the perineurium of the nerve. They appear electrophysiologically similar to Golgi tendon organs, which they resemble, although they are less organized structurally. Similar structures appear in joint capsules.
Golgi Tendon Organs
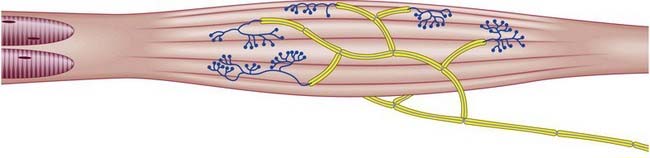
Golgi tendon organs are found mainly near musculotendinous junctions (Fig. 2.27), where more than 50 may occur at one site. Each terminal is closely related to a group of muscle fibres (up to 20) as they insert into the tendon. Golgi tendon endings are approximately 500 µm long and 100 µm in diameter and consist of small bundles of tendon fibres enclosed in a delicate capsule. The collagen bundles (intrafusal fasciculi) are less compact than elsewhere in the tendon; the collagen fibres are smaller, and the fibroblasts are larger and more numerous. One or more thickly myelinated axons enter the capsule and divide. Their branches, which may lose their Schwann cell sheaths, terminate in leaf-like enlargements containing vesicles and mitochondria, which wrap around the tendon. A basal lamina or process of Schwann cell cytoplasm separates the nerve terminals from the collagen bundles that make up the tendon. The endings are activated by passive stretch of the tendon but are much more sensitive to active contraction of the muscle. They are important in providing proprioceptive information, complementing that from neuromuscular spindles. Their responses are slowly adapting, and they signal maintained tension.
Neuromuscular Spindles
Neuromuscular spindles are essential for the control of muscle contraction. Each spindle contains a few small, specialized intrafusal muscle fibres innervated by both sensory and motor nerve fibres (Figs. 2.28, 2.29). The whole is surrounded equatorially by a fusiform spindle capsule of connective tissue, consisting of an outer perineurial-like sheath of flattened fibroblasts and collagen and an inner sheath that forms delicate tubes around individual intrafusal fibres. A gelatinous fluid rich in glycosaminoglycans fills the space between the two sheaths.
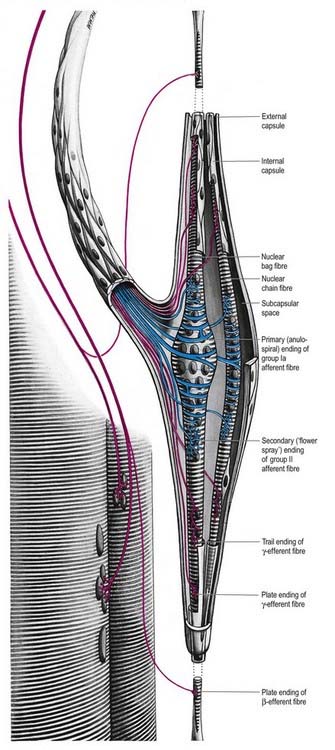
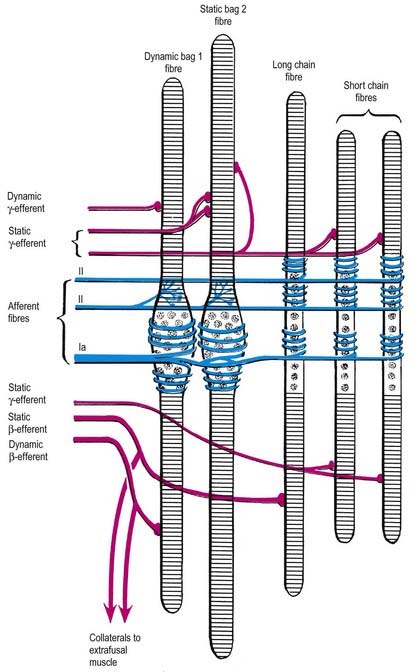
Fig. 2.28 Schematic three-dimensional representation of a neuromuscular spindle, showing nuclear bag and nuclear chain fibres; these are innervated by the sensory anulospiral and ‘flower spray’ terminals (blue) and by the γ- and β-fusimotor terminals (red). See also Fig. 2.29.
The intrafusal fibres resemble typical skeletal muscle fibres, except that the zone of myofibrils is thin around the nuclei. One subtype of nuclear bag fibre (dynamic bag 1) generally lacks M lines, possesses little sarcoplasmic reticulum and has an abundance of mitochondria and oxidative enzymes but little glycogen. A second subtype of bag fibre (static bag 2) has distinct M lines and abundant glycogen. Nuclear chain fibres have marked M lines, sarcoplasmic reticulum and T-tubules, abundant glycogen, but few mitochondria. These variations reflect, as they do in muscle generally, the contractile properties of different intrafusal fibres (Boyd 1985).
Joint Receptors
Type III endings are identical to Golgi tendon organs in structure and function; they occur in articular ligaments but not in joint capsules. They are high-threshold, slowly adapting receptors that apparently serve, at least in part, to prevent excessive stresses at joints by reflex inhibition of the adjacent muscles. They are innervated by large myelinated afferent axons.
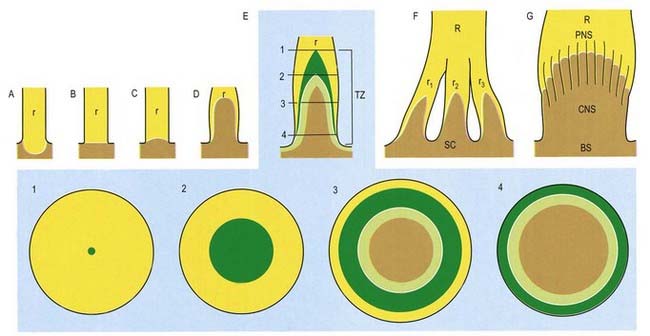
CNS–PNS Transition Zone
The transition between CNS and PNS usually occurs some distance from the point at which nerve roots emerge from the brain or the spinal cord. The segment of root that contains components of both CNS and PNS tissue is called the CNS–PNS transition zone. All axons in the PNS, other than postganglionic autonomic neurones, cross such a transition zone. Macroscopically, as a nerve root is traced toward the spinal cord or the brain, it splits into several thinner rootlets that may, in turn, subdivide into minirootlets. The transition zone is located within either a rootlet or a minirootlet (Fig. 2.30). The arrangement of roots and rootlets varies according to whether the root trunk is ventral, dorsal or cranial. Thus, in dorsal roots, the main root trunk separates into a fan of rootlets and minirootlets that enter the spinal cord in sequence along the dorsolateral sulcus. In certain cranial nerves, the minirootlets come together central to the transition zone and enter the brain as a stump of white matter.
Microscopically, the transition zone is characterized by an axial CNS compartment surrounded by a PNS compartment. The zone lies more peripherally in sensory than in motor nerves, but in both, the apex of the transition zone is described as a glial dome whose convex surface is directed distally. The centre of the dome consists of fibres with a typical CNS organization, surrounded by an outer mantle of astrocytes (corresponding to the glia limitans). From this mantle, numerous glial processes project into the endoneurial compartment of the peripheral nerve, where they interdigitate with its Schwann cells. The astrocytes form a loose reticulum through which axons pass. Peripheral myelinated axons usually cross the zone at a node of Ranvier, which is here termed a PNS–CNS compound node.
A cell type, the boundary cap cell, has recently been described in avian and mammalian species. Such cells transiently occupy the presumptive dorsal root transition zone of the embryonic spinal cord. Boundary cap cells are derived from the neural crest and are thought to prevent cell mixing at this interface and to help dorsal root ganglion afferents navigate their path to targets in the spinal cord. Further details are given in Golding and Cohen (1997).
Boyd, 1985Boyd I.A. Muscle spindles and stretch reflexes, Swash M., Kennard C. Scientific Basis of Clinical Neurology. 1985. Churchill Livingstone. Edinburgh. 74-97.
A detailed account of the functional aspects of neuromuscular spindles.
Butt, Berry, 2000Butt A.M. Berry M. Oligodendrocytes and the control of myelination in vivo: new insights from the rat anterior medullary velum. J. Neurosci. Res. 59:2000;477-488.
Describes the characteristics of a glial cell that contacts nodes of Ranvier in the CNS.
Golding, Cohen, 1997Golding J. Cohen J. Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Mol. Cell Neurosci. 9:1997;381-396.
Kandel E.R., Schwartz J.H. Principles of Neural Science, fourth ed. New York: McGraw-Hill; 2000.
Scherer, Arroyo, 2002Scherer S.S. Arroyo E.J. Recent progress on the molecular organization of myelinated axons. J. Periph. Nerv. Syst. 7:2002;1-12.
Review of the molecular architecture of myelinated peripheral axons and their myelin sheaths.