17 Overview of Solid Organ Transplantation
History
History of Solid Organ Transplantation
In the early 1950s steroids were used to suppress rejection, and this resulted in some successful kidney transplants. In 1954, Dr Joseph Murray21 performed the first truly successful kidney transplant between identical twins. Although human liver transplantation was attempted by Starzl in 1963,32 it was not successful until 1967. Also in 1967, the first successful human heart transplant was performed by Christiaan Barnard on a 55-year-old man who survived 18 days.
Evolution of Immunosuppression
In 1994, the next advance in transplantation came with the introduction of mycophenolate mofetil (MMF) and tacrolimus (another calcineurin inhibitor). Tacrolimus has gradually supplanted cyclosporine in many posttransplant protocols, because tacrolimus has been associated with lower rates of steroid-resistant acute rejection than cyclosporin.20 In contrast, MMF quickly and almost universally replaced azathioprine in posttransplant protocols.20,22
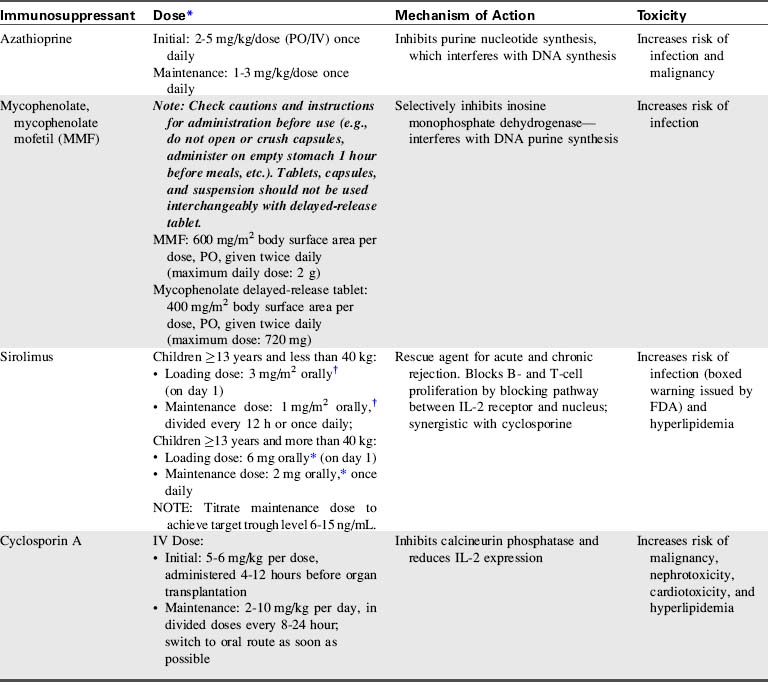
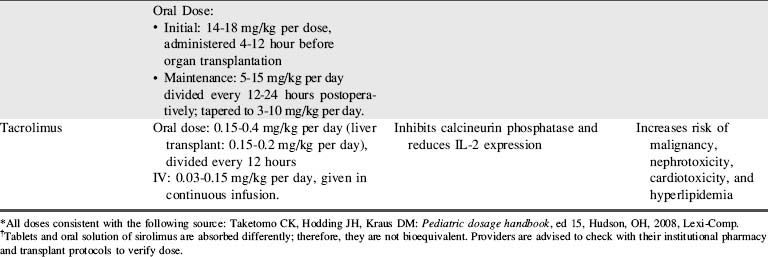
A combination of biologic agents (Table 17-1) and immunosuppressants (Table 17-2) are used to prevent and treat rejection. Because immunosuppressive regiments vary from institution to institution, providers should consult their institutional protocols and the institutional pharmacy to verify drugs and dosing regimens used.
| Biological Agent | Mechanism of Action | Toxicity |
| Antithymocyte globulin | Depletes lymphocytes | Increases risk of infection |
| Corticosteroid | Suppresses eicosanoid production: Increases TGF expression | Increases risk of infection and malignancy |
| IL-2 antibody | Selectively blocks IL-2 receptors on T helper cells | Increases risk of malignancy |
| Muromonab CD3 (OKT3) | Targets CD3 receptor complex on T cells Depletes T lymphocytes |
Increases risk of infection |
| Alemtuzumab | Targets CD52 on T cells, B cells, and NK cells causing depletion | Increases risk of infection |
| Rituximab | Binds CD20 and B cells | Increases risk of infection |
| Belatacept | Binds to T cells and prevents their activation, preventing CD28 signaling |
CD: Cluster of differentiation or cluster of designation (numerical nomenclature that identifies white blood cell surface molecules that affect cell signaling and other functions).
Pediatric heart transplantation
Overview
As of 2011, approximately 5437 pediatric heart transplants have been performed in the United States for recipients to the age of 17,24 and many more have been performed worldwide.7 Pediatric patients less than 17 years of age comprise approximately 10% of all heart transplant recipients.24
Heart transplantation is now an acceptable therapy for end-stage heart failure in infants, children, and adolescents (see, also, Chapter 8). One-year patient survival is 83% to 90%, with 5-year survival of 71% to 77%.24 The graft survival is somewhat lower than patient survival (82%-89% 1-year and 68%-75% 5-year graft survival), with some children requiring retransplantation.24 Unfortunately, about 20% of children and about 30% of infants less than 6 months of age who are waiting for a heart transplant die before receiving a heart.
Timing is crucial when listing a patient for a heart transplant, and the risks and benefits of transplantation must be evaluated in light of the limited donor pool. Bridge to transplant therapies such as extracorporeal membrane oxygenation (ECMO) and ventricular assist devices (VADs) are commonly needed for short- or long-term support (see Chapter 7). A recent review has demonstrated improved survival using the Berlin heart.16
Immediate Postoperative Care
If a PA catheter is not in place, evaluation of the balance between oxygen delivery and consumption is accomplished through frequent evaluation of central venous oxygen saturation (SCVO2), typically drawn from a central venous catheter placed in the superior vena cava. The difference between arterial and central venous oxygen saturation is typically 30% if cardiac output is adequate (for further information, see Chapter 6, Clinical Recognition and Management of Shock, Use of Central Venous Oxygen Saturation). If arterial oxygen content is stable, a fall in SCVO2 indicates either a fall in cardiac output or an increase in tissue oxygen consumption. If a central venous catheter is not in place, a venous sample can be drawn simultaneous with an arterial blood gas sample to assess arterial and venous oxygen saturations and evaluate the balance of oxygen delivery and consumption.
Both perioperative and immediate postoperative management focus on optimizing allograft function. Challenges to the function of a newly transplanted heart include: denervation, graft ischemia, and acclimation to the recipient’s hemodynamics.7 The newly transplanted heart has a relatively fixed stroke volume; therefore, an increased heart rate is necessary to augment cardiac output. The heart rate can be increased through administration of inotropes or with atrial pacing.
Pulmonary Hypertension and Right Ventricular Failure
Several measures can be taken to reduce pulmonary vascular resistance and decrease right ventricular afterload, including the administration of milrinone, prostaglandin, prostacyclin, nitroprusside, and inhaled nitric oxide. Milrinone is often used because it has both vasodilatory properties (that can reduce pulmonary and systemic vascular resistance) and inotropic effects.1 Alveolar hypoxia and acidosis should be avoided, because these conditions can exacerbate pulmonary artery constriction, worsening right ventricular failure (for further information, see section, Pulmonary Hypertension in Chapter 8). Some patients with significant elevation in PVR benefit from administration of neuromuscular blockers with sedation (see Chapter 5) and controlled mechanical ventilation for 24 to 48 hours posttransplant.
Treatment of right ventricular failure is largely supportive and includes judicious fluid administration to maximize right ventricular preload, and inotropic support to improve contractility. In rare instances, right heart failure may be so severe that extracorporeal membrane oxygenation (ECMO) is required to support the circulation (see Chapter 7).1 Hemodynamic monitoring and serial echocardiograms will be used to monitor right ventricular function, particularly in response to therapy and during weaning of therapy.
Acute Allograft Dysfunction
Acute allograft dysfunction poses a significant clinical problem that accounts for approximately 30% of early posttransplant deaths. The causes include ischemia-reperfusion injury, problems with preservation, prolonged ischemic time, and unrecognized or underappreciated donor heart dysfunction.7 Allograft dysfunction may be detected even during the operative procedure with intraoperative transesophageal echocardiography (TEE).
A 12-lead electrocardiogram (ECG) is performed shortly after arrival from the operating room and daily after transplantation, because ECG changes may signify early rejection or graft dysfunction. Dobutamine and milrinone infusions are usually adequate to support lesser degrees of allograft dysfunction. On rare occasions, the recipient requires use of a ventricular assist device or ECMO until heart function improves (see Chapter 7).
Arrhythmias
The incidence of tachyarrhythmias after pediatric heart transplantation is about 15%, similar to that reported after adult cardiac transplantation.5 Most tachyarrhythmias resolve after a relatively brief period of medical treatment (see Arrhythmias in Chapter 8), and recurrence is uncommon.19 Although ectopic atrial tachycardia is more common in children than adults, it appears to be well tolerated in the younger age group.5 Atrial flutter tends to be associated with cardiac rejection, and atrial fibrillation is associated with a poor clinical outcome.5
Vasodilatory Hypotension
Vasodilatory hypotension can result from several factors after cardiac transplantation. Loss of vascular tone after heart transplant can be caused by the use of drugs, including angiotensin-converting-enzyme (ACE) inhibitors, amiodarone, and inodilators (e.g., milrinone). Vasodilation can also be caused by a systemic inflammatory response from inflammatory cytokine activation following cardiopulmonary bypass.7 In addition, vasodilation can result from a baroreflex-mediated depletion of arginine vasopressin (AVP). Treatment of vasodilatory hypotension requires the use of vasopressors (catecholamines).
Renal Dysfunction and Hypertension
Urine output, central venous pressure, and serum blood urea nitrogen (BUN) and creatinine are monitored closely after transplantation. Renal dysfunction can result from decreased renal perfusion associated with prolonged aortic cross-clamp time, as well as from thromboemboli or perioperative hypotension. The introduction of calcineurin inhibitors for immunosuppression (see Table 17-2) can suppress renal function further.
Hypertension is common during the early and late postoperative periods, and is often a side effect of medications. Pediatric patients typically require antihypertensive therapy after heart transplantation. Calcium channel antagonists, angiotensin-converting enzyme inhibitors, and diuretics are all acceptable first-line treatments in children with hypertension after cardiac transplantation.27 Individual patient blood pressure profiles and evaluation of drug tolerance and responsiveness are needed to determine optimal drug choice and dosing.
Posttransplant Immunosuppression
The survival of the transplanted heart relies heavily on immunosuppressive therapy. Ideally the goal of immunosuppression is to manipulate the immune response to promote tolerance to the foreign antigens, without rendering the recipient vulnerable to infection.1
Induction immunosuppression is started in the perioperative period. Many institutions begin administration of calcineurin inhibitors, such as cyclosporine or tacrolimus, before the start of the transplant surgery. High-dose corticosteroids are given intraoperatively and continued for 48 hours; they are then either discontinued or tapered to a low-dose maintenance regimen.1
Additional immunosuppressive medications may be given postoperatively in the form of polyclonal antibodies such as antithymocyte globulin (ATG). The maintenance immunosuppressive regimen uses the “triple therapy” approach: a calcineurin inhibitor (cyclosporine or tacrolimus), mycophenolate mofetil (MMF), and corticosteroids (see Tables 17-1 and 17-2).
Rejection
Most pediatric heart transplant recipients experience at least one rejection episode during their lives, with a majority of these occurring during the first 3 months after transplantation.7 Clinical evaluation of rejection is important but can be misleading, especially in the pediatric population, because the inflammatory response associated with an infection can mimic the presentation of rejection.1 Clinical signs of rejection can include vague signs such as fatigue, irritability, gastrointestinal problems (e.g., vomiting, diarrhea, or poor feeding), or the development of specific cardiovascular signs such as heart failure, low cardiac output, or arrhythmias.
Cellular rejection, often referred to as T-cell-mediated rejection, is most common and is characterized by an infiltration of the heart by lymphocytes. The treatment options include optimizing current immunosuppressive therapy plus the administration of high-dose corticosteroids for mild cases of rejection and antithymocyte globulin (ATG) or muromonab CD-3 (OKT3) for severe cases of rejection (see Table 17-1).
Humoral- or antibody-mediated rejection occurs when donor-specific antibodies attack the transplanted allograft. This type of rejection is B-cell mediated and is associated with a higher rate of graft loss, development of transplant vasculopathy, and decreased long-term survival.7 Treatment for humoral rejection includes the use of high-dose corticosteroids, ATG, cyclophosphamide, and plasmapheresis.
Although the most effective way to detect rejection is through an endomyocardial biopsy (see Fig. 8-72), studies are underway to develop less invasive methods for rejection surveillance. Biohumoral markers such as B-type natriuretic peptide (BNP) and vascular endothelial growth factors (VEGF) are currently being studied as markers for rejection. Elevated BNP levels at 1 year or more posttransplant are associated with worse graft survival in pediatric heart transplant patients.28
Pediatric liver transplantation
The first human liver transplant was performed by Starzl in 1963.32 Since that time, graft and recipient survival and functional outcomes have steadily improved as the result of advances in immunosuppression and improved surgical techniques. There are now approximately 100 liver transplant centers in the United States, and about 250 pediatric liver transplantations are performed each year.24 Pediatric patients comprise approximately 9% of all liver transplant recipients.24
Immediate Postoperative Care
Failure of graft functional recovery is an extremely serious complication that must be treated aggressively and immediately by infusing prostaglandin E1, adopting the necessary measures to prevent brain edema (e.g., mannitol, oxygenation and ventilation), and addressing the effects of the liver failure by infusing plasma and glucose.31 Failure of a graft in the absence of vascular compromise (primary nonfunction) requires retransplantation in almost all cases, with the outcome best in patients with the shortest duration of liver failure before retransplantation. The incidence of primary nonfunction in pediatric patients is 5% to 10%.8,31
Posttransplant Immunosuppression
Currently, most liver transplantation centers use a tacrolimus-based regimen, combined with corticosteroid therapy with or without adjunctive agents (see Tables 17-1 and 17-2).26 Cyclosporine and tacrolimus share certain acute and long-term side effects while having some that are unique to each agent. The most important side effect of these immunosuppressives is nephrotoxicity, which acutely results from vasoconstriction of the afferent renal arterioles; this nephrotoxicity is usually reversible. These drugs can also produce a more chronic nephrotoxicity marked by tubular atrophy, interstitial fibrosis, and glomerulosclerosis. The chronic nephrotoxicity is variably reversible, depending on the degree of disease. To minimize acute toxicity and to allow lower tacrolimus levels, especially in patients with pretransplant renal insufficiency, a purine antimetabolite mycophenolate mofetil (MMF) can be used as an adjunctive agent.20,22
Treatment of acute rejection uses a high-dose methylprednisolone bolus, but unresponsive cases may require use of antibody therapy (e.g., OKT-3).8 Acute rejection accounts for less than 3% of overall patient and graft loss.20 However, treatment of acute rejection is an important risk factor for the development of cytomegalovirus and Epstein-Barr virus (EBV) infections in children. The EBV, in turn, is a risk factor for the development of posttransplant lymphoproliferative disorder.13Therefore, it is necessary to achieve a balance between adequate immunosuppression to prevent acute rejection and avoiding excessive immunosuppression to reduce the risks of toxicity and other complications.
Long-term immunosuppression protocols focus on sustaining graft acceptance while limiting morbidity. Transplant centers have reported successful withdrawal of immunosuppression in patients who still achieved prolonged graft survival. Long-term clinical experience with steroid use has documented a host of adverse effects including hypertension, malignancy, and infections.31 In addition to age and growth deficit at the time of transplantation, two other major factors affecting growth after transplantation include allograft function and steroid therapy.20
Complications of Liver Transplantation
Complications of liver transplantation include the following:
These complications are addressed separately in the following.
Hepatic Artery and Portal Vein Thrombosis
Hepatic artery thrombosis is the most common vascular complication, with an incidence that varies from 5% to 18% depending on patient age and type of graft.8,23,31 Hepatic artery thrombosis occurring in the first week after liver transplantation is commonly associated with graft nonfunction and biliary necrosis or leak. Graft nonfunction produces acute liver failure, rising bilirubin, and mental status deterioration. Biliary leaks can develop when ischemia from the arterial thrombotic event produces disruption of the anastomosis. Duplex ultrasonography and computed tomography or conventional angiography are accepted means of diagnosis.
Thrombosis of the portal vein occurs in 2% to 4% of pediatric liver transplant procedures and is often associated with loss of the graft.23 If interventional radiology techniques are unsuccessful, prompt retransplantation is required for patient salvage.
Patients with late portal vein thrombosis usually present with recurrent variceal bleeding or ascites and can be managed medically, endoscopically, or surgically with either shunting or retransplantation. Vena caval or hepatic vein thrombosis or stenosis occurs in 3% to 6% of pediatric liver transplant patients and is usually best managed with balloon dilation in an interventional radiology unit.23
Biliary Complications
Biliary complications are the most frequent technical complication after liver transplantation. Bile leaks and anastomotic strictures account for most notable biliary problems encountered in the postoperative period. Biliary complications may result from hepatic artery thrombosis; in some series, 70% of patients with hepatic artery thrombosis have concurrent biliary complications.18 Biliary complications may also be associated with prolonged preservation time, ischemia, or surgical technique.
Infection
Infections after liver transplantation follow a rather consistent time course. In the early postoperative period, bacterial infections are most common. After approximately 2 weeks, fungal infections are the increasing concern. Beginning about 6 weeks after the transplant and for the remainder of the patient’s life, viral infections dominate the infectious disease concerns.4,25
The most frequent viral infections in liver transplant recipients are cytomegalovirus (CMV), Epstein-Barr virus (EBV), adenovirus, and respiratory syncytial virus (RSV). Epstein-Barr virus is an especially important virus to identify in this population, because increased levels of EBV in the transplant recipient can lead to posttransplant lymphoproliferative disease (PTLD).4,13,25 Lymphoproliferative disorders are summarized in a section that follows (see section, Lymphoproliferative Disorders).
A major challenge in posttransplant management is preventing viral illness secondary to immunosuppression. Antiviral prophylaxis with ganciclovir has been proved to be valuable against CMV infections. Cytomegalovirus hyperimmune globulin may also be somewhat protective against EBV. Detection is paramount in ameliorating the negative effects of EBV- and CMV-associated illness. The use of polymerase chain reaction (PCR) for this purpose has been particularly useful.25,34
Central Nervous System Toxicity
The use of tacrolimus as an immunosuppressant has been shown to induce seizures. This CNS toxicity has been attributed directly to elevated serum concentration of tacrolimus.2 As a result, when tacrolimus is initially administered, serum concentration is closely monitored.
Lymphoproliferative Disorders
An important tumor recognized to result from immunosuppression is posttransplant lymphoproliferative disease (PTLD). Posttransplant lymphoproliferative disease is most common during the first 2 years following transplantation. This disease process has been attributed to EBV secondary to drug regimens that inhibit immune surveillance.13 Epstein-Barr virus-related PTLD plays a major role in morbidity and mortality among pediatric transplant recipients.
Posttransplant lymphoproliferative disease affects 6% to 20% of posttransplant patients.8 Treatments for PTLD are largely centered on reducing the level of immunosuppression. Novel therapies involving the use of rituximab, a monoclonal antibody against CD20, may also prove beneficial.2,8,13
Psychosocial Stress
A unique set of psychosocial risk factors may be present among pediatric liver transplant recipients, leading to poor adherence to medication regimens and subsequent graft dysfunction.20 Risk factors for poor compliance include inflicted trauma (intentional injury), single-parent households, substance abuse, and the patient dropping out of school. A formal assessment of these risk factors must be performed to identify them and assess and modify their impact on patient compliance with postsurgical treatment.10,17
Pediatric kidney transplantation
History
In 1954, Dr. Joseph Murray performed a successful kidney transplant between identical twins, thus skirting the problems of immune compatibility.9 Several transplants between twins followed. However, the possibility of kidney transplantation for patients with renal failure who did not have a twin donor remained unrealized.15,21 In the early 1960s, Calne demonstrated that a derivative of 6-mercaptopurine (azathioprine) increased the success of experimental kidney transplantation in dogs.3 Human use of azathioprine followed, and long-term graft survival from nonidentical donor kidneys became a possibility.
The success of kidney transplantation increased significantly when Goodwin and Starzl added prednisolone to azathioprine immunosuppression.33 Encouraged by this success, transplant centers began performing nonidentical living donor kidney transplantation.
Terasaki reported a marked decrease in early allograft failure from hyperacute rejection when a crossmatch between donor lymphocytes and recipient serum was performed.35 A negative crossmatch (no reaction against donor lymphocytes when incubated with recipient serum) indicated that no antibody directed against the donor’s organ was present in the recipient.
Kidney transplantation has now become the primary method of treating end-stage renal disease (ESRD) in the pediatric population (see Chapter 13). During the past decade, survival following kidney transplantation has improved in all pediatric age groups. This improvement is particularly noteworthy in children younger than 2 years of age who previously had the worst outcomes; these children now have outcomes that equal the outcomes of any age group.9,29 Recent reports demonstrate that the youngest recipients now have the longest transplant half-lives of all recipients; this is especially true if the pediatric recipient receives an adult kidney that functions immediately.12 These improvements likely reflect better donor selection, improvement in surgical techniques, better immunosuppression agents, and a better understanding of immunosuppression management in children.
Although the current short-term success of kidney transplantation in the pediatric population is encouraging, it is also important to realize that long-term graft survival in the adolescent group (ages 11-17 years) is relatively poor.12 The reasons for this significant rate of graft loss are speculative, but noncompliance likely plays a significant role.20,29 Regardless of the cause, improving the long-term outcomes in this patient population represents one of the most significant challenges in pediatric transplantation.
Immediate Postoperative Care
In patients with oliguria who appear to have adequate intravascular volume and hemodynamic stability, a dose of diuretic may be given to monitor response. It is important to administer diuretics carefully, because sudden massive urine output can cause significant intravascular volume depletion, with resulting decrease in renal perfusion. If the patient is massively volume overloaded or has significant electrolyte abnormalities, dialysis or continuous venovenous hemofiltration (CVVH) may be indicated (see Chapter 13).
Hypertension can be problematic after renal transplantation. The volume loading associated with the renal transplant procedure, as well as the use of calcineurin inhibitors for immunosuppression, can result in significant hypertension. The hypertension can be severe and require aggressive therapy to prevent seizures and other sequelae.20
Posttransplant Immunosuppression
Over the past several decades, significant advances have been made in our understanding of the immune response, and several new immunosuppressive agents have been introduced into clinical care. There are now several posttransplant immunosuppressive regimens available, but all require a balance between prevention of rejection and prevention of unwanted side effects associated with immunosuppression.12,29
Some transplant centers use a standard protocol for all renal transplant recipients, whereas other centers individualize the regimen for each patient. Immunosuppressive agents are used for induction, maintenance, and treatment of rejection episodes (see Tables 17-1 and 17-2).
Complications of Kidney Transplantation
Surgical Complications
Lymphocele
A lymphocele is an accumulation of lymphatic fluid around the kidney. Lymphoceles occur in 1% to 10% of pediatric renal transplant recipients.20 Because this complication is known to occur, some transplant surgeons prefer to place transplanted kidneys within the peritoneal cavity to allow lymph fluid to be reabsorbed by the peritoneum. Lymphoceles can produce fullness over the allograft, pain, or decreasing renal function. Large lymphoceles can compress the pelvis and ureter of the transplanted kidney and cause urinary obstruction. They can also cause venous obstruction.
Wound Infection
With the use of lower doses and more rapid tapering of steroid therapy in kidney transplantation, the risk of wound infection is decreasing. Children with augmented bladders or complete diversion of the urine (e.g., ileal conduit) are at increased risk of wound infection after transplantation.14 Signs of wound infection include swelling, erythema, or purulent drainage from the incision, usually within days of transplantation.
Thrombosis
One of the most devastating complications of transplantation, thrombosis (either of the renal artery or the renal vein) occurs in 0.5% to 3% of kidney transplants.6 Thrombosis should be suspected in any patient who demonstrates a sudden decrease in urine production after transplantation. Thrombosis may also produce a persistently elevated serum creatinine despite an adequate urine volume.
Renal Artery Stenosis
Obstruction of arterial inflow to a transplanted kidney occurs in 1% to 23% of kidney transplants.6 Renal artery stenosis should be suspected in patients who develop hypertension that is difficult to control, and appropriate evaluation should be performed. Renal artery stenosis can result from the transplantation surgery, causing constriction at the point of anastomosis, from kinking of the renal artery, or segmental hypertrophy of the intima of the renal artery or a branch thereof.
Urologic Complications
Urologic problems are the most common surgical complications after pediatric kidney transplantation. These complications occur in approximately 7% to 13% of all kidney transplant patients.30
Obstruction
Ultrasonography is the best method of diagnosing obstruction. When the diagnosis is in question, percutaneous antegrade pyelography and a pressure-flow study (Whitaker test) is indicated.36 Treatment options include balloon dilation or open surgical revision of the ureterovesical anastomosis. When obstruction is found in the recipient of a kidney from a pediatric donor, it may be preferable to proceed with open surgical revision. Occasionally, in such cases, complete loss of the ureter may be observed. When this occurs, anastomosis of the native ureter to the transplant kidney pelvis may be possible.
Urine Leak
Use of cadaver allografts from infants is associated with an increased risk of ureteral complications (stenosis and leakage) because of the tenuous blood supply of infant ureters. Flechner et al. used a novel adaptation to the technique of en bloc infant kidney transplantation, harvesting the trigone and adjacent tissues intact and transplanting this entire segment, as a patch, onto the recipient bladder.11
1 Boucek M.M., et al. Pediatric heart transplantation. In Allen J.D., Driscoll D.J., Shaddy R.E., Feltes T.F., editors: Moss & Adam’s heart disease in infants, children, & adolescents, 7th ed, Philadelphia: Lippincott Williams & Wilkins, 2008.
2 Bucuvalas J.C., Ryckman F.C. Long-term outcome after liver transplantation in children. Pediatr Transplant. 2002;6(1):30-36. [Medline]
3 Calne R.Y., Alexandre G.P., Murray J.E. A study of the effects of drugs in prolonging survival of homologous renal transplants in dogs. Ann NY Acad Sci. 1962;99:743-761. [Medline]
4 Chang F.Y., et al. Fever in liver transplant recipients: changing spectrum of etiologic agents. Clin Infect Dis. 1998;26(1):59-65. [Medline]
5 Collins K.K., et al. Atrial tachyarrhythmias and permanent pacing after pediatric heart transplantation. J Heart Lung Transplant. 2003;22(10):1126-1133.
6 Dimitroulis D., et al. Vascular complications in renal transplantation: a single-center experience in 1367 renal transplantations and review of the literature. Transplant Proc. 2009;41(5):1609-1614.
7 Edwards N.M., Chen J.M., Mazzeo P.A. Cardiac transplantation. Totowa, NJ: Humana Press; 2004.
8 Evrard V., et al. Impact of surgical and immunological parameters in pediatric liver transplantation: a multivariate analysis in 500 consecutive recipients of primary grafts. Ann Surg. 2004;239(2):272-280. [Medline]
9 Filler G., Huang S.H. Progress in pediatric kidney transplantation. Ther Drug Monit. 2010;32(3):250-252.
10 Fine R.N., et al. Pediatric transplantation of the kidney, liver and heart: summary report. Pediatr Transplant. 2004;8(1):75-86. [Medline]
11 Flechner S.M., et al. Use of the donor bladder trigone to facilitate pediatric en bloc kidney transplantation. Pediatr Transplant. 2011;15(1):53-57. [Medline]
12 Gulati A., Sarwal M.M. Pediatric renal transplantation: an overview and update. Curr Opin Pediatr. 2010;22(2):189-196.
13 Guthery S.L., et al. Determination of risk factors for Epstein-Barr virus-associated posttransplant lymphoproliferative disorder in pediatric liver transplant recipients using objective case ascertainment. Transplantation. 2003;75(7):987-993. [Medline]
14 Hatch D.A., et al. Kidney transplantation in children with urinary diversion or bladder augmentation. J Urol. 2001;165(6 Pt 2):2265-2268. [Medline]
15 Hume D.M., et al. Experiences with renal homotransplantation in the human: report of nine cases. J Clin Invest. 1955;34(2):327-382. [Medline]
16 Imamura M., et al. Bridge to cardiac transplant in children: berlin heart versus extracorporeal membrane oxygenation. Ann Thorac Surg. 2009;87:1894-1901.
17 Kamath B.M., Olthoff K.M. Liver transplantation in children: update 2010. Pediatr Clin North Am. 2010;57(2):401-414.
18 Kling K., Lau H., Colombani P. Biliary complications of living related pediatric liver transplant patients. Pediatr Transplant. 2004;8(2):178-184. [Medline]
19 LaPage M.J., Rhee E.K., Canter C.E. Tachyarrhythmias after pediatric heart transplantation. J Heart Lung Transplant. 2010;29(3):273-277. Epub Sept 26, 2009
20 La Rosa C., Jorge Valuarte H., Meyers K.E.C. Outcomes in pediatric solid-organ transplantation. Pediatr Transplant. 2011;15:128-141.
21 Murray J.E., Merrill J.P., Harrison J.H. Kidney transplantation between seven pairs of identical twins. Ann Surg. 1958;148(3):343-359. [Medline]
22 Nobili V., et al. Mycophenolate mofetil in pediatric liver transplant patients with renal dysfunction: preliminary data. Pediatr Transplant. 2003;7(6):454-457. [Medline]
23 Ooi C.Y., et al. Thrombotic events after pediatric liver transplantation. Pediatr Transplant. 2010;14(4):476-482. Epub October 22, 2009
24 Organ Procurement and Transplantation Network: National Data Reports. OPTN. Available at http://optn.transplant.hrsa.gov/latestData/viewDataReports.asp Accessed 09.05.11
25 Paya C.V., et al. Risk factors for cytomegalovirus and severe bacterial infections following liver transplantation: a prospective multivariate time-dependent analysis. J Hepatol. 1993;18(2):185-195. [Medline]
26 Reding R. Tacrolimus in pediatric liver transplantation. Pediatr Transplant. 2002;6(6):447-451. [Medline]
27 Roche S.L., O’sullivan J.J., Kantor P.F. Hypertension after pediatric cardiac transplantation: detection, etiology, implications and management. Pediatric Transplantation. 2010;14(2):159-168. Epub July 16, 2009
28 Rossano J.W., et al. B-type natriuretic peptide levels late after transplant predict graft survival in pediatric heart transplant patients. J Heart Lung Transplant. 2010;29(3):385-386. Epub September 26, 2009
29 Shapiro R., Sarwal M. Pediatric kidney transplantation. Pediatr Clin North Am. 2010;57(2):393-400.
30 Shoskas D.A., et al. Urologic complications in 1,000 consecutive renal transplant recipients. J Urol. 1995;153:18-21.
31 Spada M., et al. Pediatric liver transplantation. World J Gastroenterol. 2009;15(6):648-674.
32 Starzl T.E., et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [Medline]
33 Starzl T.E., Marchioro T.L., Waddell W.R. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385-395. [Medline]
34 Takada Y., Tanaka K. Living related liver transplantation. Transplant Proc. 2004;36(2 Suppl.):271S-273S. [Medline]
35 Terasaki P.I., et al. Serotyping for homotransplantation. V. Evaluation of a matching scheme. Transplantation. 1966;4(6):688-699. [Medline]
36 Whitaker R.H. Clinical assessment of pelvic and ureteral function. Urology. 1978;12(2):146-150. [Medline]

 Be sure to check out the supplementary content available at
Be sure to check out the supplementary content available at