Overview and General Considerations
This chapter provides an overview of the methods used to identify anaerobic microorganisms. The detailed technical procedures discussed should be used in conjunction with specifics provided in Chapter 42 to develop a clear understanding of the full process, from specimen collection to identification. However, readers should consider the following general objectives for the information and methods provided.
1. State the specific diagnostic purpose for the test methodology.
2. Briefly describe the test principle associated with the test methodology.
3. Outline limitations and describe a process for trouble-shooting or reporting results if a test result is equivocal or indistinguishable.
4. State the appropriate quality control organisms and results used with each testing procedure.
5. Define and differentiate obligate (strict), moderate, facultative, and aerotolerant anaerobes.
6. List suitable specimens for isolation of anaerobic bacteria and characteristics of these specimens that might suggest the presence of an anaerobic infection.
7. Explain the proper techniques for collecting, transporting, and processing clinical specimens for anaerobic bacteriology.
8. Explain the use of antigen detection methodologies in the diagnosis of anaerobic infections.
9. List the media used for cultivation of anaerobic bacteria.
10. Describe the appropriate incubation conditions for cultivation of anaerobic bacteria.
11. Describe the procedures for the identification of and antibiotic susceptibility testing for anaerobic bacteria.
General Characteristics
The organisms described in this chapter and in Chapter 42 usually do not grow in the presence of oxygen (O2); they are obligate, or strict, anaerobes (0% O2). Obligate anaerobes are killed upon brief exposure (less than a few minutes) to atmospheric oxygen. Obligate anaerobes include Prevotella sp., Fusobacterium sp., and Bacteroides spp. These chapters also include some aerotolerant organisms (5% O2), such as Actinomyces spp., Bifidobacterium spp., and Clostridium spp., which are capable of growth in the presence of either reduced or atmospheric oxygen but grow best under anaerobic conditions. Finally, facultative anaerobes do not require atmospheric oxygen but are capable of growth in oxygen and anaerobic environments.
Specimen Collection and Transport
The importance of proper collection and transport of specimens for anaerobic culture cannot be overemphasized. Because indigenous anaerobes are often present in large numbers as normal flora on mucosal surfaces, even minimal contamination of a specimen can produce misleading results. Box 41-1 shows the specimens acceptable for anaerobic culture; Box 41-2 presents specimens that are likely to be contaminated and therefore are unacceptable for anaerobic culture. In general, material for anaerobic culture is best obtained by tissue biopsy or by aspiration using a needle and syringe. Use of swabs is a poor alternative because of excessive exposure of the specimen to the deleterious effects of drying, the possibility of contamination during collection, and the easy retention of microorganisms in the fibers of the swab. If a swab must be used, it should be from an oxygen-free transport system.
Three kinds of anaerobic transport systems are shown in Figures 41-1 to 41-3. Figure 41-1 shows is a rubber-stoppered collection vial containing an agar indicator system. The vial is gassed out with oxygen-free carbon dioxide (CO2) or nitrogen. The specimen (pus, body fluid, or other liquid material) is injected through the rubber stopper after all air has been expelled from the syringe and needle. If only a swab specimen can be obtained, a special collection device with an oxygen-free atmosphere is required (see Figure 41-2). When the swab is reinserted, care must be taken not to tip the container, which would cause the oxygen-free CO2 or nitrogen to spill out and be displaced by ambient air. A tissue specimen can be immersed in a small amount of liquid to prevent it from drying and then placed in an anaerobic pouch (see Figure 41-3). All specimens should be held at room temperature pending processing in the laboratory, because refrigeration can oxygenate the specimen.
Direct Detection Methods
Antigen Detection
Gram Staining
Table 41-1 presents the cellular morphology seen with Gram staining of common anaerobes.
TABLE 41-1
| Organism | Gram Stain* | Media | Appearance |
| Actinomyces spp. | Gram-positive, branching, beaded or banded, thin, filamentous rods | Ana BAP | Colonies of most species are small, smooth, flat, convex, gray-white, translucent, with entire margins; colonies of A. israelii and A. gerencseriae are white, opaque, and may resemble a “molar tooth”; A. odontolyticus turns red after several days in ambient air and may be beta-hemolytic |
| Anaerococcus spp. | Gram-positive cocci arranged in short chains or tetrads | Ana BAP | Small, white, translucent, smooth |
| Atopobium spp. | Elongated gram-positive cocci; occur singly, in pairs, or in short chains | Ana BAP | Resemble lactobacilli |
| Bacteroides distasonis | Gram-negative, straight rods with rounded ends; occur singly or in pairs | Ana BAP | Gray-white, circular, entire, convex, smooth, translucent to opaque; nonhemolytic |
| BBE | At 48 hr, colonies are >1 mm, circular, entire, raised, and either (1) low convex, dark gray, friable, and surrounded by a dark gray zone (esculin hydrolysis) and sometimes a precipitate (bile) or (2) glistening, convex, light to dark gray, and surrounded by a gray zone | ||
| B. fragilis | Gram-negative, pale-staining, pleomorphic rods with rounded ends; occur singly or in pairs; cells often described as resembling a safety pin (see Figure 42-4) | Ana BAP | White to gray, circular, entire, convex, translucent to semiopaque; nonhemolytic (see Figure 42-5) |
| BBE | At 48 hr, colonies are >1 mm, circular, entire, raised, and either (1) low convex, dark gray, friable, and surrounded by a dark gray zone (esculin hydrolysis) and sometimes a precipitate (bile) or (2) glistening, convex, light to dark gray, and surrounded by a gray zone (see Figure 42-6) | ||
| B. ovatus | Gram-negative, ovoid rods with rounded ends; occur singly or in pairs | Ana BAP | Pale buff, circular, entire, convex, semiopaque; often mucoid; nonhemolytic |
| BBE | At 48 hr, colonies are >1 mm, circular, entire, raised, and either (1) low convex, dark gray, friable, and surrounded by a dark gray zone (esculin hydrolysis) and sometimes a precipitate (bile) or (2) glistening, convex, light to dark gray, and surrounded by a gray zone | ||
| B. thetaiotaomicron | Gram-negative, irregularly staining, pleomorphic rods with rounded ends; occur singly or in pairs | Ana BAP | White, circular, entire, convex, semiopaque, shiny, punctiform; nonhemolytic |
| BBE | At 48 hr, colonies are >1 mm, circular, entire, raised, and either (1) low convex, dark gray, friable, and surrounded by a dark gray zone (esculin hydrolysis) and sometimes a precipitate (bile) or (2) glistening, convex, light to dark gray, and surrounded by a gray zone | ||
| B. ureolyticus | Gram-negative, pale-staining, thin, delicate rods with rounded ends; some curved | Ana BAP | Small, translucent or transparent; may produce greening of agar on exposure to air; colonies corrode (pit) the agar (see Figure 42-9) or may be smooth and convex or spreading |
| B. vulgatus | Gram-negative, pleomorphic rods with rounded ends; occur singly, in pairs, or in short chains; swellings or vacuoles may be seen | Ana BAP | Gray, circular, entire, convex, semiopaque; nonhemolytic |
| BBE | At 48 hr, colonies are >1 mm, circular, entire, raised, glistening, convex, light to dark gray but with no gray zone (esculin not hydrolyzed) | ||
| Bifidobacterium spp. | Gram-positive diphtheroid; coccoid or thin, pointed shape; or larger, highly irregular, curved rods with branching; rods terminate in clubs or thick, bifurcated (forked) ends (“dog bones”) | Ana BAP | Small, white, convex, shiny, with irregular edge |
| Bilophila wadsworthia | Gram-negative, pale-staining, delicate rods | Ana BAP | Small, translucent |
| BBE | Grows at 3-5 days; colonies are usually gray with a black center because of production of hydrogen sulfide (H2S); black center may disappear after exposure to air | ||
| Clostridium botulinum | Gram-positive, straight rods; occur singly or in pairs; spores usually subterminal and resemble a tennis racket | Ana BAP | Gray-white; circular to irregular; usually beta-hemolytic |
| C. clostridioforme | Gram-positive rod that stains gram negative; long, thin rods; spores usually not seen; elongated football shape with cells often in pairs | Ana BAP | Small, convex, entire edge; nonhemolytic |
| C. difficile | Gram-positive straight rods; may produce chains of up to six cells aligned end to end; spores oval and subterminal | Ana BAP | Large, white, circular, matte to glossy, convex, opaque; nonhemolytic; horse stable odor; fluoresces yellow-green |
| CCFA | Yellow, ground-glass colony (see Figure 42-2) | ||
| C. perfringens | Gram-variable straight rods with blunt ends; occur singly or in pairs; spores seldom seen but if present are large and central to subterminal, oval, and swell cell; large boxcar shapes (see Figure 44-6) | Ana BAP | Gray to grayish yellow; circular, glossy, dome shaped, entire, translucent; double zone of beta-hemolysis (see Figure 42-3) |
| C. ramosum | Gram-variable straight or curved rods; spores rarely seen but are round and terminal; more slender and longer than C. perfringens | Ana BAP | Small, gray-white to colorless; circular to slightly irregular, smooth, translucent or semiopaque; nonhemolytic |
| C. septicum | Gram positive in young cultures but becomes gram negative with age; stains unevenly; straight or curved rods; occur singly or in pairs; spores subterminal, and oval and swell cells | Ana BAP | Gray; circular, glossy, translucent; markedly irregular to rhizoid margins resembling a “Medusa head”; beta-hemolytic; swarms over entire agar surface in less than 24 hr |
| C. sordellii | Gram-positive rods; subterminal spores | Ana BAP | Large colony with irregular edge |
| C. sporogenes | Gram-positive rods; subterminal spores | Ana BAP | Colonies firmly adhere to agar; may swarm over agar surface |
| C. tertium | Gram-variable rods; terminal spores | Ana BAP | Resembles Lactobacillus spp. |
| C. tetani | Gram positive, becoming gram negative after 24-hr incubation; occur singly or in pairs; spores oval and terminal or subterminal with drumstick or tennis racket appearance | Ana BAP | Gray; matte surface, irregular to rhizoid margin, translucent, flat; narrow zone of beta-hemolysis; may swarm over agar surface |
| Collinsella aerofaciens | Gram-positive chains of coccoid cells | Ana BAP | Circular, entire, white center with translucent edge |
| Eggerthella lenta | Gram-positive, small, straight rod with rounded ends | Ana BAP | Small, gray, translucent, circular, entire, convex |
| Eubacterium spp. | Gram-positive pleomorphic rods or coccobacilli; occur in pairs or short chains; E. alactolyticum has a seagull-wing shape similar to Campylobacter spp.; E. nodatum is similar to Actinomyces spp. with beading, filaments, and branching | Ana BAP | Small, gray, transparent to translucent, raised to convex; colonies of E. nodatum may resemble A. israelii |
| Finegoldia magna | Gram-positive cocci with cells > 0.6 µm in diameter; in pairs and clusters; resemble staphylococci | Ana BAP | Tiny, gray, translucent; nonhemolytic |
| Fusobacterium mortiferum | Gram-negative, pale-staining, irregularly stained, highly pleomorphic rods with swollen areas, filaments, and large, bizarre, round bodies | Ana BAP | Circular; entire or irregular edge, convex or slightly umbonate, smooth, translucent; nonhemolytic |
| BBE | >1 mm in diameter, flat and irregular | ||
| F. necrophorum subsp. necrophorum | Gram-negative, pleomorphic rods with round to tapered ends; may be filamentous or contain round bodies; becomes more pleomorphic with age | Ana BAP | Circular, umbonate, ridged surface, translucent to opaque; fluoresces chartreuse; greening of agar on exposure to air; some strains beta-hemolytic |
| F. nucleatum subsp. nucleatum | Gram negative; pale staining; long, slender, spindle-shaped with sharply pointed or tapered ends; occasionally cells occur in pairs end to end; resembles Capnocytophaga spp. (see Figure 42-11) | Ana BAP | Three colony types: bread crumb–like (white; see Figure 42-10), speckled, and smooth (gray to gray-white); greening of agar on exposure to air; fluoresces chartreuse; usually nonhemolytic |
| F. varium | Gram negative, unevenly staining, pleomorphic; coccoid and rod shapes; occurs singly or in pairs | Ana BAP | Gray-white center with colorless edge resembling a fried egg; circular, entire, convex, translucent; nonhemolytic |
| BBE | >1 mm in diameter, flat and irregular | ||
| Lactobacillus spp. | Gram-variable pleomorphic rods or coccobacilli; straight, uniform rods have rounded ends; short coccobacilli resemble streptococci | Ana BAP | Resemble Lactobacillus spp. colonies on aerobic blood or chocolate agar, except colonies are usually larger when incubated anaerobically |
| Leptotrichia spp. | Gram-negative, large, fusiform rods with one pointed end and one blunt end | Ana BAP | Large, raspberry-like colonies |
| Parvimonas micra | Gram-positive cocci with cells < 0.7 µm in diameter; occur in packets and short chains | Ana BAP | Tiny, white, opaque; nonhemolytic |
| Mobiluncus spp. | Gram-variable, small, thin, curved rods; the two species can be divided based on cell length | Ana BAP | Tiny colonies after 48 hr-incubation; after 3-5 days, colonies are small, low convex, and translucent |
| Peptococcus niger | Gram-positive, spherical cells; occur singly or in pairs, tetrads, and irregular masses | Ana BAP | Tiny, black, convex, shiny, smooth, circular, entire edge; becomes light gray when exposed to air |
| Peptostreptococcus anaerobius | Gram-positive, large coccobacillus; often in chains | Ana BAP | Medium, gray-white, opaque; sweet, fetid odor; colonies usually larger than most anaerobic cocci |
| Porphyromonas spp. | Gram-negative coccobacilli | Ana BAP | Dark brown to black; more mucoid than Prevotella spp.; except for P. gingivalis, fluoresces brick red |
| Prevotella disiens | Gram-negative rods; occur in pairs or short chains | Ana BAP | White, circular, entire, convex, translucent to opaque, smooth, shiny; nonhemolytic; fluoresces brick red |
| LKV | Black pigment | ||
| P. melaninogenica | Gram-negative coccobacilli | Ana BAP | Dark center with gray to light brown edges; circular, entire, convex, smooth, shiny; nonhemolytic; fluoresces brick red |
| LKV | Black pigment | ||
| Propionibacterium spp. | Gram-positive, pleomorphic, diphtheroid-like rod; club-shaped to palisade arrangements; called anaerobic diphtheroids | Ana BAP | Young colonies are small and white to gray-white and become larger and more yellowish tan with age; P. avidum is beta-hemolytic |
| Veillonella parvula | Gram-negative, tiny diplococci in clusters, pairs, and short chains; unusually large cocci, especially in clusters, suggests Megasphaera or Acidaminococcus spp. | Ana BAP | Small, almost transparent; grayish white; smooth, entire, opaque, butyrous; may show red fluorescence under UV light (360 nm) |
Ana BAP, Anaerobic blood agar plate; BBE, Bacteroides bile esculin agar; CCFA, cycloserine cefoxitin fructose agar; LKV, laked kanamycin-vancomycin blood agar; UV, ultraviolet.
*Typical Gram stain appearance is seen from broth (thioglycollate or peptone-yeast-glucose).
Specimen Processing
Anaerobe Jars or Pouches
The most frequently used system for creating an anaerobic atmosphere is the anaerobe jar. Anaerobe jars are available commercially from several companies. For example, the GasPak (Figure 41-4) is made by Becton Dickinson (Sparks, Maryland); other companies that produce these devices include EM Diagnostic Systems (Gibbstown, New Jersey) and Oxoid U.S.A. (Columbus, Maryland). All of these systems use a clear, heavy plastic jar with a lid that is clamped down to make it airtight. Anaerobic conditions can be set up by two methods. The easiest method uses a commercially available envelope containing a hydrogen and CO2 generator that is activated either by adding water (GasPak) or by the moisture on the agar plates (EM Diagnostic Systems and Oxoid USA). The production of heat within a few minutes (detected by touching the top of the jar) and subsequent development of moisture on the walls of the jar are indications that the catalyst and generator envelope are functioning properly. Reduced conditions are achieved in 1 to 2 hours, although the methylene blue or resazurin indicators take longer to decolorize. Alternatively, the “evacuation-replacement” method can be used. Air is removed from the sealed jar by drawing a vacuum of 25 inches (62.5 cm) of mercury. This process is repeated two times, with the jar being filled with an oxygen-free gas, such as nitrogen, between evacuations. The final fill of the jar is made with a gas mixture containing 80% to 90% nitrogen, 5% to 10% hydrogen, and 5% to 10% CO2. Many anaerobes require CO2 for maximal growth.
The atmosphere in the jars is monitored using an indicator to check anaerobiosis. Anaerobe bags or pouches are useful for laboratories processing small numbers of anaerobic specimens. A widely used anaerobic pouch, the GasPak Pouch, is shown in Figure 41-3. Besides specimen transport, the pouch also can be used to incubate one or two agar plates.
Anaerobe Chamber
Anaerobic chambers, or glove boxes, are made of molded or flexible clear plastic. The flexible clear plastic chambers are the most widely used type. Specimens and other materials are placed in the chamber through an air lock. The technologist uses gloves (Forma Scientific, Marietta, Ohio) or sleeves (Sheldon Manufacturing, Cornelius, Oregon), to form airtight seals around the arms (Figure 41-5). Media stored in the chamber are kept oxygen free, and all work on a specimen, from inoculation through workup, is performed under anaerobic conditions. A gas mixture of 5% CO2, 10% hydrogen, and 85% nitrogen, plus a palladium catalyst, maintain the anaerobic environment inside the chamber.
Anaerobic Media
Initial processing of anaerobic specimens involves inoculation of appropriate media. Table 41-2 lists commonly used anaerobic media. Primary plates should be freshly prepared or used within 2 weeks of preparation. Plates stored for longer periods accumulate peroxides and become dehydrated; this results in growth inhibition. Reduction of media in an anaerobic environment eliminates dissolved oxygen but has no effect on the peroxides. Prereduced, anaerobically sterilized (PRAS) media are produced, packaged, shipped, and stored under anaerobic conditions. They are commercially available from Anaerobe Systems (Morgan Hill, California) (Figure 41-6) and have an extended shelf life of up to 6 months.
TABLE 41-2
| Medium | Components/Comments | Primary Purpose |
| Anaerobic blood agar | May be prepared with Columbia, Schaedler, CDC, Brucella, or brain-heart infusion base supplemented with 5% sheep blood, 0.5% yeast extract, hemin, L-cystine, and vitamin K1 | Nonselective medium for isolation of anaerobes and facultative anaerobes |
| Bacteroides bile esculin agar (BBE) | Trypticase soy agar base with ferric ammonium citrate and hemin; bile salts and gentamicin act as inhibitors | Selective and differential for Bacteroides fragilis group; good for presumptive identification |
| Laked kanamycin-vancomycin (LKV) | Brucella agar base with kanamycin (75 µg/ mL), vancomycin (7.5 µg/mL), vitamin K1 (10 µg/mL), and 5% laked blood | Selective for isolation of Prevotella and Bacteroides spp. |
| Anaerobic phenylethyl alcohol agar (PEA) | Nutrient agar base, 5% blood, phenylethyl alcohol | Selective for inhibition of enteric gram-negative rods and swarming by some clostridia |
| Egg yolk agar (EYA) | Egg yolk base | Nonselective for determination of lecithinase and lipase production by clostridia and fusobacteria |
| Cycloserine cefoxitin fructose agar (CCFA) | Egg yolk base with fructose, cycloserine (500 mg/L), and cefoxitin (16 mg/L); neutral red indicator | Selective for Clostridium difficile |
| Cooked meat (also called chopped meat) broth | Solid meat particles initiate growth of bacteria; reducing substances lower oxidation-reduction potential (Eh) | Nonselective for cultivation of anaerobic organisms; with addition of glucose, can be used for gas-liquid chromatography |
| Peptone–yeast extract–glucose broth (PYG) | Peptone base, yeast extract, glucose, cysteine (reducing agent), resazurin (oxygen tension indicator), salts | Nonselective for cultivation of anaerobic bacteria for gas-liquid chromatography |
| Thioglycollate broth | Pancreatic digest of casein, soy broth, and glucose to enrich growth of most bacteria. Thioglycollate and agar reduce Eh. May be supplemented with hemin and vitamin K1 | Nonselective for cultivation of anaerobes, facultative anaerobes, and aerobes |
Approach to Identification
Subculture of Isolates
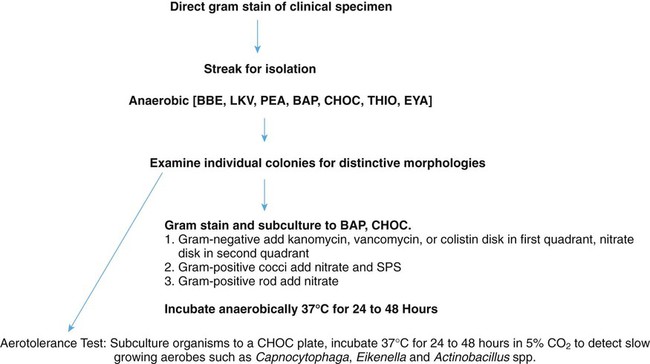
A single colony of each distinct morphotype is examined microscopically using a Gram stain and is subcultured for aerotolerance testing. Figure 41-7 presents a basic algorithm for processing isolated colonies. A sterile wooden stick or platinum loop should be used to subculture colonies to:
• A chocolate agar plate (CHOC) to be incubated in carbon dioxide (CO2) for aerotolerance
• An anaerobic blood agar plate (BAP) and a chocolate plate to be incubated anaerobically (purity plate)
The chocolate agar plate should be inoculated first, so that if only the anaerobic blood agar plate grows, there is no question of not having enough organisms to initiate growth. The following antibiotic identification disks are placed on the first quadrant of the purity plate (see Procedure 41-1 on the Evolve site):
Presumptive Identification of Isolates
Information from the primary plates in conjunction with the atmospheric requirements, Gram stain results, and colony morphology of a pure isolate provides preliminary differentiation of many anaerobic organisms. Table 41-3 summarizes the extent to which isolates can be identified using this information. Considering the specimen source and expected organisms from the site can be a useful aid in this process.
TABLE 41-3
Preliminary Grouping of Anaerobic Bacteria Based on Minimal Criteria
| Organism | Gram Stain Reaction | Cell Shape | Gram Stain Morphology | Aerotolerance | Distinguishing Characteristics |
| Bacteroides fragilis group | − | B | Can be pleomorphic with safety pin appearance | − | Grows on BBE; >1 mm in diameter; some strains hydrolyze esculin |
| Pigmented gram-negative bacilli | − | B, CB | Can be very coccoid or Haemophilus-like | − | Foul odor; black or brown pigment; some fluoresce brick red |
| Bacteroides ureolyticus | − | B | Thin; some curved | − | May pit agar or spread; transparent colony |
| Fusobacterium nucleatum | − | B | Slender cells with pointed ends | − | Foul odor; three colony types; bread crumb−like, speckled, and smooth |
| Gram-negative bacillus | − | B | − | ||
| Gram-negative coccus | − | C | Veillonella cells are tiny | − | |
| Gram-positive coccus | + | C, CB | Variable size | − | |
| Clostridium perfringens (presumptive) | + | B | Large; boxcar shape; no spores observed; may appear gram negative | − | Double-zone beta-hemolysis |
| Clostridium spp. | + | B | Spores usually observed; may appear gram negative | −* | |
| Gram-positive bacillus | + | B, CB | No spores observed; no boxcar-shaped cells | −* | |
| Actinomyces-like | + | B | Branching cells | −* | Sulfur granules on direct examination; “molar tooth” colony |
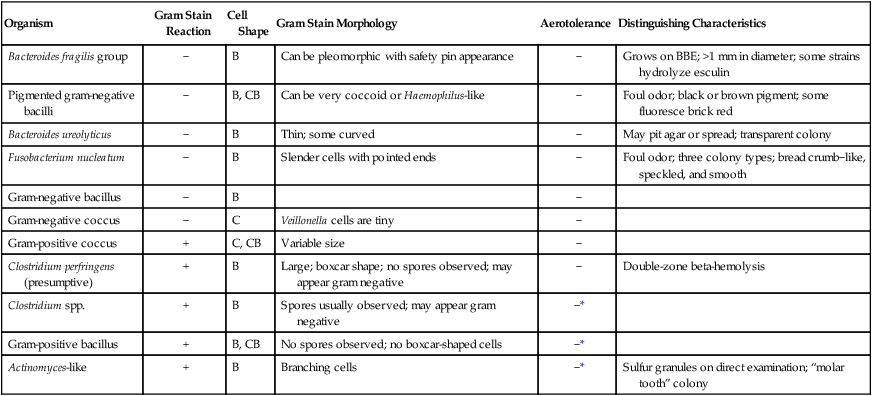
B, Bacillus; BBE, Bacteroides bile esculin agar; C, coccus; CB, coccobacillus; −, negative; + positive.
*Some strains are aerotolerant; these include Clostridium tertium, C. histolyticum, some bifidobacteria, some propionibacteria, and most Actinomyces spp.
Presumptive identification of many clinically relevant anaerobic bacteria can be accomplished using a few simple tests (Tables 41-4 and 41-5).
TABLE 41-4
Abbreviated Identification of Gram-Negative Anaerobes
| Cell Shape | Slender Cells with Pointed Ends | Kanamycin (1 mg) | Vancomycin (5 µg) | Colistin (10 mg) | Growth in Bile | Spot Indole | Catalase | Pigmented Colony | Brick Red Fluorescence | Lipase | Pits the Agar | Requires Formate/Fumarate | Nitrate Reduction | Urease | Motile | |
| Gram-Negative Rods | ||||||||||||||||
| Bacteroides fragilis group | B | − | R | R | R | + | V | V | − | − | − | − | − | − | − | − |
| Bacteroides ureolyticus | B | − | S | R | S | − | − | − | − | − | − | +− | + | + | + | − |
| Pigmented spp. | B, CB | − | R | R | V | − | V | − | +* | V | V | − | − | − | − | − |
| Prevotella intermedia | B, CB | − | R | R | S | − | + | − | + | + | + | − | − | − | − | − |
| Prevotella loescheii | B, CB | − | R | R | SRS | − | − | − | +* | + | −+ | − | − | − | −− | −− |
| Other | ||||||||||||||||
| Prevotella spp. | B, CB | − | R | R | V | − | V | − | −† | − | − | − | − | − | ||
| Porphyromona s spp. | B, CB | − | R | ‡S‡ | R | − | + | − | + | +§ | − | − | − | − | − | − |
| Bilophila sp. | B | − | S | R | S | + | − | + | − | − | − | − | − | + | +− | − |
| Fusobacterium spp. | B |
V | S | R | S | V | V | − | − | − | V | − | − | − | − | − |
| F. nucleatum subsp. nucleatum | B | + | S | R | S | − | + | − | − | − | − | − | − | − | − | − |
| F. necrophorum subsp. necrophorum | B | − | S | R | S | −+ | + | − | − | − | +− | − | − | − | − | − |
| F. mortiferum varium | B | − | S | R | S | + | V | − | − | − | − | − | − | − | − | − |
| Leptotrichia spp. | B | +¶ | S | R | S | V | − | − | − | − | − | − | − | − | − | − |
| Gram-Negative Cocci | ||||||||||||||||
| Veillonella spp. | C | − | S | R | S | − | − | V | − | −+ | − | − | − | + | − | − |
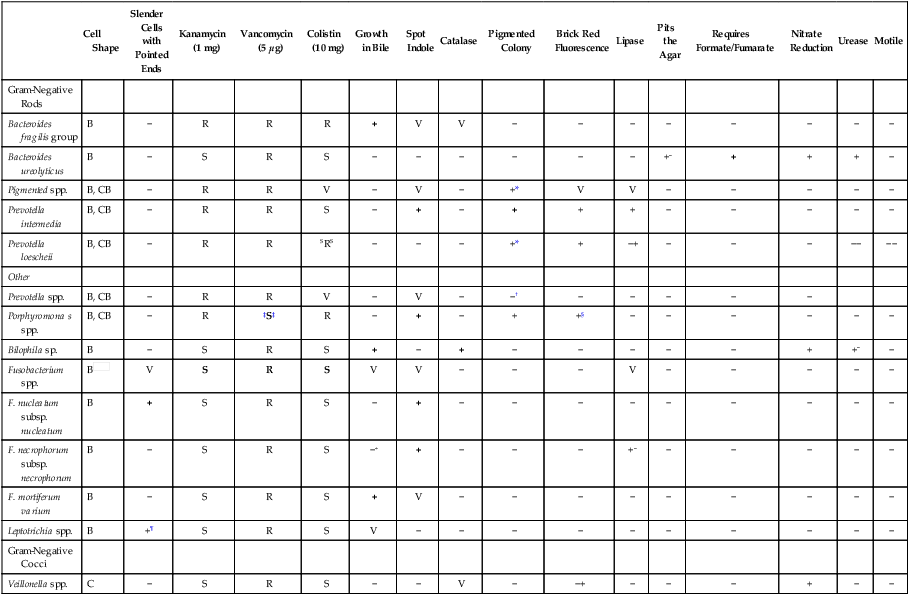
Reactions in bold type are key tests; superscripts indicate reactions of occasional strains.
B, Bacillus; C, coccus; CB, coccobacillus; R, resistant; S, sensitive; V, variable; +, positive; −, negative.
*P. melaninogenica group often requires prolonged incubation before pigment is observed.
†P. bivia produces pigment on prolonged incubation.
‡Will not grow on laked kanamycin-vancomycin (LKV) because of susceptibility to vancomycin.
TABLE 41-5
Abbreviated Identification of Gram-Positive Anaerobes
| Cell Shape | Spores Observed | Boxcar-Shaped Cells | Double-Zone Beta Hemolysis | Kanamycin (1 mg) | Vancomycin (5 mg) | Colistin (10 mg) | Spot Indole | Sodium Polyanethol Sulfonate | Catalase | Survives Ethanol Spore Test | Lecithinase | Nagler Test | Strong Reverse-Camp Test | Arginine Stimulation | Urease | Nitrate Reduction | Ground-Glass, Yellow Colonies on CCFA* Medium | Comment | |
| Gram-Positive Cocci | C, CB | − | − | − | V | S | R | V | V | V | − | − | − | − | −+ | − | |||
| Peptostreptococcus anaerobius | C, CB | − | − | − | Rs | S | R | − | S | −+ | − | − | − | − | − | − | Sweet, putrid odor; may chain | ||
| Finegoldia magna | C† | − | − | − | S | S | R | − | R | V | − | − | − | − | − | − | − | ||
| Parvimonas micros | C‡ | − | − | − | S | S | R | − | V§ | − | − | − | − | − | − | − | − | − | |
| Peptococcus niger | C | − | − | − | S | S | R | − | R | − | − | − | − | − | − | − | − | − | Black to olive green colonies |
| Gram-Positive, Spore-Forming Rods | |||||||||||||||||||
| Clostridium spp. | B | −+− | −+ | − | V | S | R | V | −+ | +− | V | − | − | V | −+ | V | |||
| NAGLER POSITIVE | |||||||||||||||||||
| C. perfringens | B | − | + | −+− | S | S | R | − | −+ | −+ | + | + | + | − | − | +− | − | ||
| C. baratii | B | + | − | − | S | S | R | − | − | + | + | +w | − | − | − | V | − | ||
| C. sordellii | B | + | − | − | S | S | R | + | −+ | + | + | +w | − | − | +− | − | − | Swarming with serpentine-edged colonies | |
| C. bifermentans | B | + | − | − | S | S | R | + | −+ | + | + | +w | − | − | − | − | − | ||
| NAGLER NEGATIVE | |||||||||||||||||||
| C. difficile | B | +− | − | − | S | S | R | − | −+ | + | − | − | − | − | − | + | Horse stable odor; fluoresces chartreuse | ||
| C. septicum | B | + | − | − | S | S | R | − | −+ | + − | − | − | − | − | − | V | − | Smoothly swarming over agar surface | |
| Non–Spore Forming | B, CB | − | − | − | V | S | R | V | V+ | − | V | − | V | V | V | − | |||
| Propionibacterium acnes | B, CB | − | − | − | S | S | R | +− | ++ | − | − | − | − | − | − | + | − | May branch; diphtheroid | |
| Eggerthella lenta | B | − | − | − | S | S | R | − | −+ | − | − | − | − | + | − | + | − | Small rod | |
| Bifidobacterium spp. | B|| | − | − | − | S | S | R | V | − | − | Some strains are aerotolerant (e.g., B. adolescentis) | ||||||||
| Eubacterium spp. | B | − | − | − | S | S | R | V | − | − |
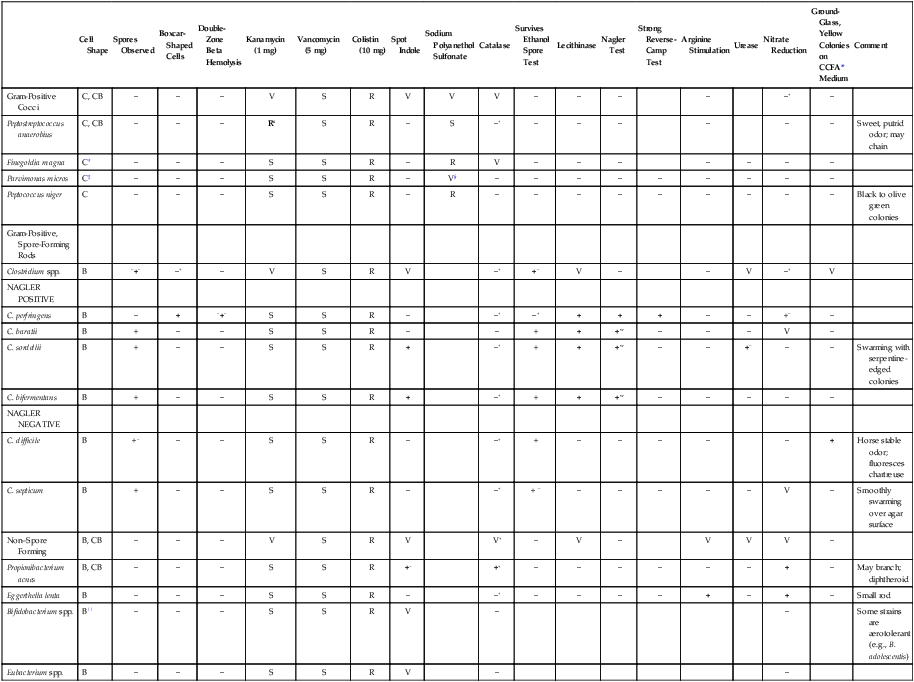
Reactions in bold type are key tests; superscripts indicate reactions of occasional strains.
B, Bacillus; C, coccus; CB, coccobacillus; R, resistant; S, sensitive; V, variable; w, weak; +, positive; −, negative.
See Procedure 41-2.
*Cycloserine cefoxitin fructose agar.
Definitive Identification
• Miniaturized biochemical systems (e.g., API 20A [bioMérieux, St. Louis, Missouri])
• Rapid, preformed enzyme detection panels (e.g., AnIdent [bioMérieux]; RapID-ANA II [Remel, Lenexa, Kansas]; BBL Brand Crystal Anaerobe ID [Becton Dickinson]; Rapid Anaerobe Identification Panel [Dade MicroScan, West Sacramento, California]; Vitek ANI card [bioMérieux]).
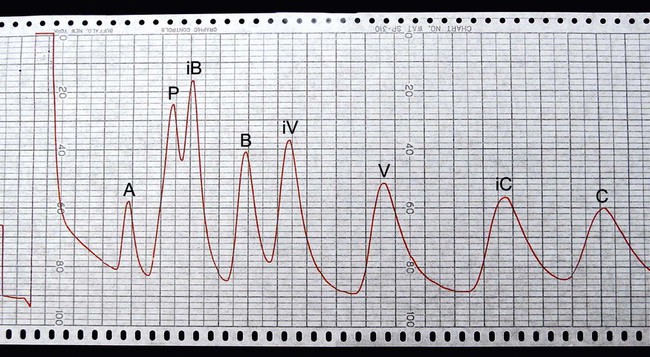
• Gas-liquid chromatography (GLC) for end products of glucose fermentation (Supelco, Bellefonte, Pennsylvania). GLC is used to separate and identify anaerobic metabolic end products (i.e., volatile fatty acids [Figure 41-9] and nonvolatile organic acids) of carbohydrate fermentation and amino acid degradation. Chromatograms produced with anaerobic bacteria can greatly facilitate identification of certain genera and species not readily identified based on other phenotypic characteristics.
• High-resolution GLC for cellular fatty acid analysis. GLC application has been expanded for the analysis of longer chain fatty acids (i.e., 9 to 20 carbons in length) to produce chromatograms for identifying organisms often without the need for other phenotypic information (e.g., Gram stain morphology). One such commercial system (MIDI Microbial Identification System, Inc.; Newark, Del.) has more than 600 bacteria in the chromatographic database. Although this approach may not be practical for identification of many commonly encountered bacterial species, it has great potential for use as a reference method for organisms that are difficult to identify by conventional methods.
Antimicrobial Susceptibility Testing and Therapy
When mixed infections are encountered, definitive information about the identification of each species present usually does not affect therapeutic management. Because most clinically relevant anaerobes are susceptible to first-line antimicrobials (Table 41-6), knowledge of their presence and Gram stain morphologies in mixed cultures is usually sufficient for guiding therapy. Therefore, definitive identification methods that follow the schemes outlined should be judiciously applied to clinical situations in which an anaerobic organism is isolated in pure culture from a normally sterile site (e.g., clostridial myonecrosis).
TABLE 41-6
Antimicrobial Therapy and Susceptibility Testing of Anaerobic Bacteria
| Organism Group | Therapeutic Options | Potential Resistance to Therapeutic Options | Validated Testing Methods* |
| Bacteroides fragilis group, other Bacteroides spp., Porphyromonas spp., Prevotella spp., and Fusobacterium spp. | Highly effective agents include most beta-lactam/beta-lactamase– inhibitor combinations, imipenem, metronidazole, and chloramphenicol Cefoxitin (cephalosporin) Moxifloxacin (fluoroquinolone) |
Beta-lactamase production does occur but generally does not significantly affect imipenem or beta-lactamase–inhibitor combinations. However, isolates of B. fragilis are known to produce beta-lactamases capable of hydrolyzing imipenem. Metronidazole resistance has been reported; resistance to various cephalosporins or clindamycin does occur, and susceptibility to these agents cannot be assumed. Decreasing susceptibility to ampicillin-sulbactam and amoxicillin-clavulanate has been reported. | Yes; see Table 41-7 |
| Clostridium spp. | Penicillins, with or without beta-lactamase–inhibitor combinations and imipenem; metronidazole or vancomycin for C. difficile–induced gastrointestinal disease; antimicrobial therapy is not indicated for botulism and C. perfringens food poisoning | Resistance to therapeutic options is not common, but cephalosporins and clindamycin show uncertain clinical efficacy | Yes; see Table 41-7 |
| Actinomyces spp., Propionibacterium spp., Bifidobacterium spp., Eubacterium spp. | Penicillins, with or without beta-lactamase–inhibitor combinations, imipenem, cefotaxime, and ceftizoxime | Resistance to therapeutic options not common; generally resistant to many cephalosporins and metronidazole | Yes; see Table 41-7 |
| Peptostreptococcus spp., and Peptococcus niger | Penicillins, most cephalosporins, imipenem, vancomycin, clindamycin, and chloramphenicol | Resistance to therapeutic options is not common | Yes; see Table 41-7 |
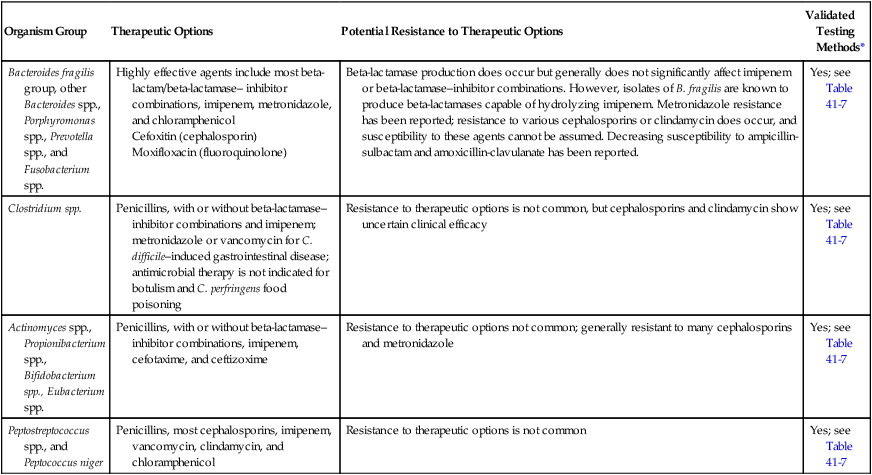
*Validated testing methods include standard methods recommended by the Clinical and Laboratory Standards Institute (CLSI) and commercial methods approved by the U.S. Food and Drug Administration (FDA).
Data from the Clinical Laboratory Science Institute (CLSI): Abbreviated identification of bacteria and yeast, Approved guideline, M35-A2, Wayne, Pa, 2002, CLSI; and Versalovic J: Manual of clinical microbiology, ed 10, Washington, DC, 2011, ASM Press.
The therapeutic options listed in Table 41-6 for each of the major groups of anaerobic bacteria are rapidly changing; therefore, therapeutic use of the antimicrobial agents listed generally requires the performance of antimicrobial susceptibility testing with anaerobic isolates. Although standard susceptibility testing methods have been established for testing anaerobic bacteria against various antimicrobial agents (Table 41-7), the fastidious nature of many species and the labor intensity involved in using these methods indicate that testing should be done only under recommended circumstances (Box 41-3).
TABLE 41-7
Summary of Antimicrobial Susceptibility Testing Methods for Anaerobic Bacteria
| Test Conditions | TEST METHODS | ||
| Agar Dilution | Broth Microdilution | Etest | |
| Medium | Brucella agar supplemented with hemin (5 µg/mL), vitamin K (1 µg/mL), and 5% (V/V) laked sheep blood | Brucella broth supplemented with hemin (5 µg/mL), vitamin K (1 µg/mL), and lysed horse blood (5%) | Brucella blood agar |
| Inoculum size | 1 × 105 CFU/spot | 1 × 106 CFU/mL | 0.1-1 McFarland standard, swab plate |
| Incubation conditions | Anaerobic, 35°-37° C | Anaerobic, 35°-37° C | Anaerobic, 35°-37° C |
| Incubation duration | 48 hr | 48 hr | 24-48 hr |
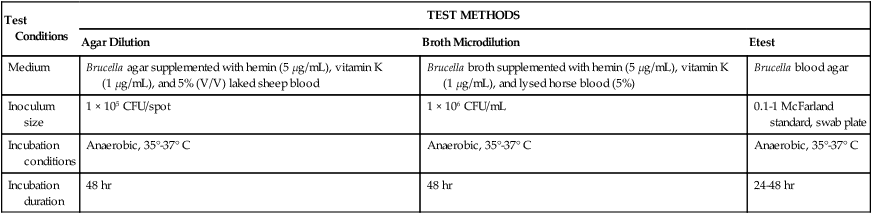
CFU, Colony forming units; V/V, volume/volume.
Data from the National Committee for Clinical Laboratory Standards (NCCLS): Abbreviated identification of bacteria and yeast, Approved guideline, M35-A2, Wayne, Pa, 2002, NCCLS; and Versalovic J: Manual of clinical microbiology, ed 10, Washington, DC, 2011, ASM Press.
Although certain commercial methods (e.g., Etest, Spiral Gradient; see Chapter 12) may facilitate anaerobic susceptibility testing in some way, the difficulty of assigning clinical significance to many anaerobic isolates and the availability of several highly effective empiric therapeutic choices significantly challenge a laboratory policy of routinely performing susceptibility testing with these organisms.