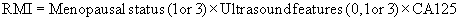Chapter 21 Ovarian neoplasms
Risk of Malignancy Index (RMI)
Screening for ovarian cancer
• Bimanual pelvic examination: low-cost, requires no special equipment but not specific or sensitive in the detection of ovarian cancer.
• Ultrasound scan: malignant features include semisolid or cystic areas, thick septa, surface papillary growths, neovascularisation, bilateral masses and/or ascites.
• Cytology: routine cervical cytological tests give abnormal results in 10%–30% of advanced ovarian cancer cases. Peritoneal cytology is not specific or sensitive, and is time-consuming and painful to perform.
• Prophylactic salpingo-oophorectomy: this should be considered in women with a strong family history of ovarian cancer or women who are BRCA carriers; 7% of women with ovarian cancer have had a previous laparotomy; 0.2% of women who have had their ovaries conserved during operations for benign gynaecological disorders develop ovarian cancer.
Tumour markers for ovarian cancer
• The four types of potential markers for ovarian cancer are oncodevelopmental markers, carcinoplacental, metabolic and tumour-specific/tumour-associated antigens. None has sufficiently high specificity for screening.
• CA125 tumour-associated antigen is a high molecular weight glycoprotein expressed in coelomic epithelium during embryogenic development; 80% of patients with ovarian carcinoma have levels >35 IU/mL. It is elevated in about 1% of healthy women and also in non-malignant conditions such as endometriosis, inflammation, benign tumours and pregnancy. CA125 levels fall with age. It is a good marker for epithelial carcinoma, except mucinous tumours, but has poor specificity (not useful for screening).
Ovarian cancer
Risk factors
Pathology of ovarian cancer
• sex cord stromal tumours: granulosa cell, Sertoli-Leydig cell (androblastoma, arrhenoblastoma), fibroma, sex cord tumours with annular tubules
Investigations for ovarian masses
FIGO staging for ovarian cancer
Staging is based on clinical examination, laparotomy, histology and cytology findings.
• stage 1: growth limited to ovaries
• stage 3: tumour involving one or both ovaries with peritoneal implants outside the pelvis and/or positive retroperitoneal or inguinal nodes; superficial liver metastasis equals stage 3; tumour is limited to the true pelvis but with histologically proven malignant extension to the small bowel or omentum
• 3A: tumour grossly limited to the true pelvis with negative nodes, but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces
Epithelial ovarian tumours
These account for 90% of ovarian cancer.
Prognostic factors for epithelial ovarian cancer
Management
Surgical treatment
Sex cord stromal tumours
Granulosa cell tumours
• Histopathology: Call-Exner bodies are granulosa cells arranged around small spaces containing nuclear fragments.
• They are mostly diagnosed at stage 1 disease, are usually a slow-growing tumour, and late recurrences are not uncommon.
Androblastoma
Germ cell tumours
• Tumour markers: alpha-fetoprotein is produced by yolk sac cells; hCG is produced by syncytiotrophoblast.
Dysgerminoma
Management
• Stage 1A: unilateral salpingo-oophorectomy, peritoneal cytology, exploration of abdomen and removal of retroperitoneal lymph nodes, biopsy of contralateral ovary if suspicious. No adjuvant chemotherapy is required.
• All other stages: conservative surgery can be offered, but will need chemotherapy. BEP (bleomycin, etoposide and cisplatin) is recommended.
Choriocarcinoma
• Choriocarcinoma in the ovary can occur as metastasis from gestational trophoblast disease in the uterus, malignant change in an ovarian ectopic pregnancy or as a primary ovarian germ cell tumour.
Embryonal carcinoma
Endodermal sinus tumour
• Presentation: acute abdomen, a large mass with haemorrhage and necrosis; 50% of patients complain of symptoms for <24 hours, and rarely symptoms are present for >2 weeks.
• Management: radical surgery produces no better results than local tumour removal. Irradiation is not helpful. Combination chemotherapy may be curative. The ideal management is conservative surgery and combination chemotherapy. Alpha-fetoprotein is the marker to gauge the woman’s response to treatment and is also used as a marker for recurrence.
Borderline (low malignant potential) tumours
Management
• Management of early-stage disease: when fertility is desired and disease is localised, conservative surgery is indicated. This includes unilateral salpingo-oophorectomy and biopsy of the contralateral ovary if suspicious and omental biopsy. In older patients, or if fertility is not required, treatment is with total abdominal hysterectomy/bilateral salpingo-oophorectomy.
• Recurrence in stage 1 disease: if managed by cystectomy alone, this is associated with a 25% recurrence rate; with oophorectomy of the affected ovary alone, a 10% recurrence rate. If total pelvic clearance, the recurrence rate is <5%.