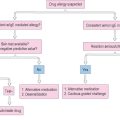
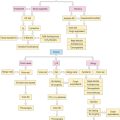
Chapter 7 Otitis Media: Background and Science
Introduction to the Problem
Otitis media (OM) is a common clinical problem that affects millions of people around the world. OM represents a spectrum of disease processes that include acute otitis media (AOM), recurrent acute otitis media (RAOM), otitis media with effusion (OME), and chronic otitis media with effusion. In the early portions of the 20th century, persistence of otitis media was termed secretory otitis media.1,2 Now considered to be equivalent to otitis media with effusion (middle ear effusion without signs of acute ear infection), this secretory otitis was noted to be resistant to typical therapeutic interventions while many of the patients were noted to have a history of atopic disease.1–6 Since that time numerous studies have attempted to establish a causal relationship between atopy and OME. Many of the early studies lacked proper controls and were fraught with misclassification bias due to poorly defined disease criteria. Over the last 10–15 years, no study has yet to definitively identify allergy as an etiologic agent in the pathogenesis of OME.6–8 However, numerous studies continue to demonstrate an association between atopy and OME.9–22 Whether research identifies absolute or implicit causality between allergy and OME, it is important that the clinician recognizes the potential role of atopy in the development of and persistence of OME to adequately approach therapeutic options and/or interventions for their patients.
Over the last decade, a change in the approach to the allergic patient has developed, in which an intimate relationship is thought to exist between the upper and lower airways in allergic disease.9,23,24 The concept of an “united airway” in the disease process holds that the middle ear, Eustachian tube, and the nasopharynx are thought to participate in the allergic Th2 immune-mediated hypersensitivity response in a similar manner as the remainder of the upper and lower airway.8,23,24 Therefore, allergic inflammation is not confined to a specific site or target organ but is considered to range throughout the common airway.8,9,23,24 This chapter reviews the epidemiology, anatomy, pathophysiology, and diagnosis of suspected allergic middle ear disease, as well as provides an evidence-based, united airway approach for the management of the patient with otitis.
Epidemiology
Otitis media (OM) and otitis media with effusion (OME) represent significant health problems with greater than 2.2 million episodes diagnosed per year in the USA.5,7 This translates into one of the most frequent health-related problems for which children receive medical care in the USA.5,7 OME is most prevalent in early childhood where screening surveys have reported point prevalence of middle-ear effusions (MEE) from infancy to 5 years of age to range between 15% and 40% with the peak incidence during the winter months.7,10,25–27 During the first year of life about half of all children will have one episode of OME, more than 60% by age two, and nearly 90% (80% of ears) will have at least one episode of OME at some time prior to school age.7,28,29
Most episodes of OME resolve within 3 months. However, about a third (30–40%) of children have recurrent OME with approximately 5–10% of episodes lasting a year or longer.3,5,7,25,28,29 The sequelae of repeated episodes or persistence of OME in children can be significant and may produce impairments in receptive language skills, balance, coordination, maintaining attention, and school readiness.7,12,30,31 The health-care-related expenditures associated with OME have been estimated to be anywhere between US$3 and 5 billion dollars annually.5,32,33 However, this may drastically underestimate the indirect impact on society. The burden of disease may also affect caregivers of those with OME. Quality of life surveys have found that more than 50% of the caregivers displayed physical problems: poor sleep, poor eating, or irritability due to recurrent AOM and chronic OME in children.34,35
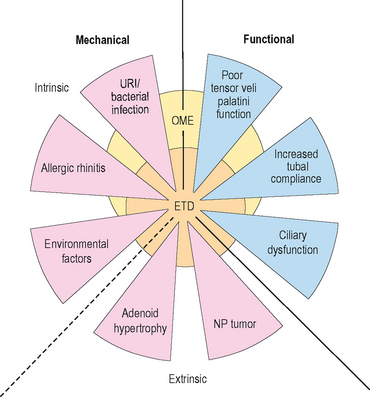
It is generally accepted that the development of OME is multifactioral. Eustachian tube dysfunction (ETD), bacterial and/or viral infection of the middle ear, and inflammation within the nose resulting from allergic rhinitis or upper respiratory infection are some of the contributors that play an important, interrelated role in the development of OME (Figure 7.1).5,6,9 Although no casual evidence directly links allergy as an etiologic agent in OME, population studies have shown a distinct relationship between allergy and OME.16,17 In Sweden, atopic infants were five times more likely to develop OME than nonatopics.16 In Japan, more than 87% of those with OME were found to have allergic symptoms and/or be atopic.17 Jero et al noted that those with allergy were 4.4 times less likely to clear acute episodes of OM.36 Other studies report that as many as 50% of all patients with otitis media have allergic rhinitis and 21% of patients with nasal allergy have otitis media.7,14 Nguyen et al showed that the allergic inflammation seen in atopics with OME occurred both in the middle ear and the nasopharynx.9
Numerous risk factors for the development of OM have identified. Risk factors for otitis media (acute or chronic) include: recurrent upper respiratory infection, allergic rhinitis, eustachian tube dysfunction, exposure to cigarette smoke, bottle-feeding/short duration of breast feeding, male gender, immunologic deficiency, ciliary dysfunction, cleft palate disease, first degree relative with allergy, attendance to day care, and seasonal changes.14,15,30,37
In adults, the exact prevalence or incidence of OME is not known. However, studies have noted the link between poorly controlled allergic rhinitis and the development of OME.17,38,39 Allergic rhinitis is a very common disease process that is thought to affect up to a cumulative frequency of 42% of the US population by age 40 and a point prevalence of 40 million.38,39 Mion et al recently confirmed a community-based study by Tomonga where about 50% of patients with chronic OME presented with allergic nasal disease.17,37
Relevant Anatomy and Physiology
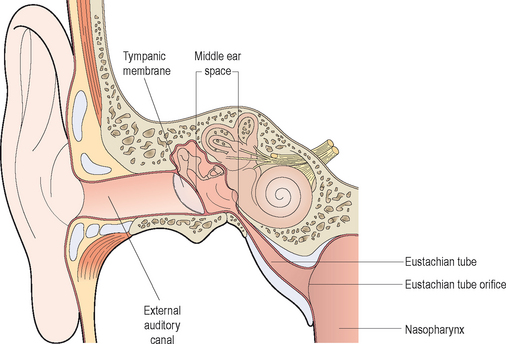
The middle ear cleft is defined by the middle ear (ME), the Eustachian tube (ET), and the mastoid air cells. From an anatomic standpoint, the ME is limited laterally by the tympanic membrane, medially by the promontory (bone overlying the basal turn of the cochlea), and anteriorly by the carotid canal and the ET. The ME directly communicates with the mastoid air cells via the aditus ad antrum posteriorly while the floor is bound by the jugular bulb which is also covered by air cells. The ET extends from the anteroinferior portion of the ME to the nasopharynx. In adults, the nasopharyngeal orifice of the ET measures approximately 8 × 4 mm and is 15 mm below the tympanic ostium at an angle of 45 degrees from the horizontal plane.40,41 In children, the ET is positioned 10 degrees from the horizontal plane and is considerably shorter.40
Although the anatomy of the ME cleft provides a structural framework to approach ME disease, from an immunologic standpoint, it is more important to think of the physiologic components of the ME in terms of a complex interrelated system composed of the middle ear space, ET, nasopharynx (adenoids), nasal mucosa, and the mastoid air cells.42 The ET has a key role in middle ear function in that it allows for communication between the nasopharynx and middle ear. Swallowing causes the tensor veli palatini to actively open the ET and thus ventilates the middle ear with atmospheric pressure.14,40 (Approximately 1–2 μL of gas is exchanged with each swallow.) Other functions of the ET with respect to the middle ear include: providing a pathway for drainage of secretions into the nasopharynx and protection from nasopharyngeal secretions and sound pressure.18,40,43,44 Obstruction of the ET, whether functional or mechanical, alters the partial pressure of middle ear gases, resulting in negative middle ear pressure, improper ventilation, and a supportive milieu for bacteria reproduction (Figure 7.1).5,12 Children are further disadvantaged because the pediatric ET is shorter and situated in a more horizontal orientation compared to adults, which inherently impairs its protective function.40
Notably important to understanding the part allergic inflammation plays in the development and persistence of OME is the concept of gas exchange in the ME.42 Nitrogen, oxygen, and carbon dioxide are the main gases involved in exchange in the ME. Oxygen and carbon dioxide equilibrate rapidly within the ME space with each swallow. However, since the partial pressure of nitrogen is lower in the surrounding ME tissues, it slowly diffuses out of the ME space.5,40,42 The ventilation of the ME space by the ET preserves atmospheric pressure and prevents the retraction of the tympanic membrane due to the evacuation of nitrogen.42 Disruption or breakdown of these protective functions of the ET results in increased risk for the development of OME.12
Pathophysiology
To appreciate the immunologic reactions within the ME system, a brief discussion of the factors involved in these reactions is warranted. Atopic disease is characterized by allergen exposure that causes rapid degranulation of mast cells, which results in the release of histamine, cysteinyl leukotrienes (CysLTs), prostaglandins (PGD), and cytokines.45 Some of these substances lead to the infiltration of inflammatory cells such as: eosinophils, basophils, neutrophils, mast cells, and mononuclear cells.46,47 These cells subsequently stimulate further release of histamine and CysLTs, thus perpetuating the inflammatory response by signaling recruitment of more inflammatory cells, which, in turn, increase mucosal blood flow, permeability of the vasculature, as well as stimulating mucus production.45,48 Within the ME system, chronic allergic inflammation is associated with a tenfold increase in the numbers of mast cells present in mucosa, which is coupled with higher numbers of IgE receptors and surface-bound IgE.49,50 In addition, mast cells are also thought to process and present allergens to antigen-presenting cells (B lymphocytes, monocytes, and dendritic cells). Allergen presentation in this environment is a potentially important source of cytokines, including interleukin 1 (IL-1), IL-6, and tumor necrosis factor (TNF).45 Mast cells, naïve T cells, and basophils also are thought to release IL-4. At a critical threshold, IL-4 induces an isotypic switching of the activated naïve T-cells to Th2 cells. The Th2 cell then produces copious amounts of IL-4, which mediates an isotype switch of B cells to IgE production, and causes upregulation of various adhesion molecules. These Th2 lymphocytes also release IL-5, IL-9, and IL-13, which further stimulate recruitment, production, and activation of basophils, eosinophils, and mast cells.45 The imbalance in the immune response between Th1 cells and Th2 cells has been proposed as the immunologic basis for allergic rhinitis as well as asthma. Numerous clinical studies have shown Th2 dominance with excess production of both IL-4 and IL-5 in patients with allergic rhinitis and asthma.38
▪ Middle Ear Space
Over the last 20 years, many authors have attempted to isolate the ME mucosa as a target organ, a site capable of significant immunologic activity to induce OME.18–22,51,52 Hurst et al and Wright et al have demonstrated eosinophils, myeloperoxidase, eosinophil cationic protein, and mast cells to be present in ME effusions of those with chronic OME, suggesting that the ME mucosa in atopics responds differently from nonatopics.20–2251 Ebmeyer et al showed that mast cells were a major contributor to the early inflammatory response of the ME to bacteria.53 Sobol et al found T lymphocytes, eosinophils, and other Th2 cytokines in the effusions of atopic children.22 Animal models have also attempted to demonstrate the reactivity of the ME mucosa. Hardy et al, Pollock et al, and Ebert et al have demonstrated ET dysfunction via transtympanic challenge of both allergens and histamine.54–56
Despite these data there is still not definitive evidence that the ME mucosa acts as the major target organ in the genesis of OME. Because reactivity to allergens requires repeated exposure, Bernstein and others have suggested that various mechanical factors of the Eustachian tube result in low levels of antigen contact and limited exposure times.42,48,57,58 Since the ET is the primary path for antigen delivery into the ME space and opens on a limited basis, i.e. swallowing or yawning, aerosolized antigens likely have restricted access to the ME.42 In addition, allergic inflammation of the nasal mucosa likely would result in edema of the torus tubaris, further isolating and actually protecting the ME space from direct contact with inhaled antigens. However, Bernstein suggests, in a small number of patients (<10%), the ME mucosa acts as a target organ.42
▪ Eustachian Tube
Allergic inflammation within or around the nasopharyngeal portion of the ET may lead to the loss of its protective functions resulting in increased risk for the development of OM and OME.12 Several mechanisms have been proposed to explain Eustachian tube obstruction due to inflammatory processes. First, poor ET function may result from a retrograde spread of edema secondary to congestion of the nasal mucosa. Second, poor mucociliary function may lead to impaired clearance of secretions and obstruction of the lumen of the ET.12,18 Third, direct allergic inflammation within the ET may cause obstruction due to intrinsic venous engorgement and hypersecretion of mucus, isolating the middle ear space, ultimately affecting gas exchange, and resulting in the diffusion of nitrogen out of the ME space and into the mucosa.48 The development of a net negative pressure in the ME develops perturbing cellular tight junctions and allows for a transudation of fluids into the middle ear space.42,48 The persistent obstruction of the Eustachian tube with mucus results in chronic ME inflammation, mucosal metaplasia, increased glandular activities of goblet cells, and an inability to clear ME fluid.42,48
▪ Nasopharynx and Nasal Mucosa
Numerous studies have supported the concept that allergic inflammation in the nose results in significant ET obstruction.17,38,42,59 In a study of 100 patients with recurrent OME, nearly one third of the cases were attributed directly to allergy. The authors concluded that the nasal allergic response in patients predisposed them to ET obstruction through the action of inflammatory mediators including cytokines released by mucosal mast cells, inflammatory cells, and epithelial cells in the nose and nasopharynx.12,42 In other studies, approximately 50% of patients with chronic OME presented with allergic rhinitis.17,37
In summary, there are four principle components of the ME system that are potential targets for allergic inflammation causing the development of OM and OME: ME space, ET, nasopharynx/adenoids, and the nasal mucosa. Of these sites, it is less understood how the ME space, with its limited physical exposure to inhaled antigens, could contribute to allergy-induced OME. By contrast, the ET, nasopharynx/adenoids, and the nasal mucosa all receive extensive airborne antigen exposure and the resulting edema, inflammation, and obstruction of the ET are all involved in the pathogenesis of OME.42
Additionally, the inflammatory response in one portion of the airway may also lead to inflammatory changes in other portions. For example, viral upper respiratory infections and/or a nasal allergic response result in a significant amount of increased nasal secretions and inflammation, which is maintained by subsequent inflammatory mediators and cytokines.14 It is thought that viral upper respiratory infections may encourage secondary bacterial infections by affecting bacterial adherence, modulating host immune and inflammatory responses throughout the airway, and possibly producing ETD, increased frequency of asthma or rhinosinusitis.14,45 In a study of atopic subjects, inhaled allergen challenge isolated to the nose produced inflammatory changes including increased adhesion molecules, increased bronchial hyperactivity, and eosinophil infiltration in both the upper and lower airways.45,60 This supports the concept that an intimate relationship exists between the upper and lower airways, whereby allergic inflammation is not isolated to a specific site or target organ but affects the common airway.8,9,14,23,24,45
Diagnosis
▪ History
OME is usually associated with a longer duration of symptoms than acute OM, including: otalgia, aural fullness, hearing loss, and popping or snapping sounds that may be aggravated by air travel.14 Although infrequent, chronic OME may also be associated with tinnitus or vertigo. Patients with allergy-induced OME may also report a history of itchy/watery or swollen eyes, prolonged and recurrent seasonal/perennial sneezing, and/or nasal congestion.14 A past medical history and family history should probe for history of allergy (food or airborne), asthma, atopic dermatitis, mucociliary dysfunction disorders (Kartagener’s and cystic fibrosis), neoplasms, and craniofacial anomalies (e.g., cleft palate, Down’s syndrome, Robin’s sequence, CHARGE association, all having a higher prevalence of chronic OME).7,14 Previous diagnoses of other inflammatory/autoimmune conditions such as Crohn’s disease, ulcerative colitis, celiac sprue, rheumatoid arthritis, etc. should be elicited. A past surgical history should solicit information regarding previous ear operations, sinus surgery, adenoidectomy, or tonsillectomy. A social history should assess for exposure to aerosolized pollutants such as smog or tobacco smoke. In children, it is critical to obtain a developmental history to help identify those at risk for speech, language, or learning problems.7
▪ Physical Examination
As with the approach to any condition, a thorough examination of the head and neck should be performed in both children and adults. First, an evaluation for any predisposing conditions such as craniofacial anomalies, palatal defects, or factors that would contribute to the development of OME should be undertaken. The rhinopharnyngeal exam should include sinonasal endoscopy (rigid or flexible) when possible to assess for septal deviation, enlarged turbinates, adenoid hypertrophy, or purulent nasal secretions. In patients with unilateral OME, the nasopharynx should be visualized to rule out the possibility of neoplasm.61 Physical features that are found in most allergic patients include clear nasal secretions as well as edematous and/or pink-bluish nasal mucosa. In children, stigmata of allergy may include facial manifestations of itching such as the allergic salute, or dorsal nasal crease, or nasal congestion including mouth breathing, “allergic shiners,” chapped lips, evidence of sleep deprivation, flattened malar eminences, “Gothic arch” appearance of the hard palate, or dental malocclusion,
Fundamental to establishing a diagnosis of any type of otitis is an appropriate physical examination to confirm the presence or absence of suspected middle ear pathology. A normal tympanic membrane (TM) should appear thin, translucent, and mobile.14 With an effusion the TM is often cloudy, has distinctly impaired mobility, and may contain a visible air–fluid level or bubbles in the middle ear (Figure 7.2).7,14 Distinct erythema of the TM is seen in about 5% of ears with OME and should be distinguished from AOM (Figure 7.3).62 A retracted TM may indicate negative pressure and atelectasis within the ME.14 Special attention should be placed on examining specifically for TM perforation, ossicular erosion, retraction pockets, or cholesteatomas.
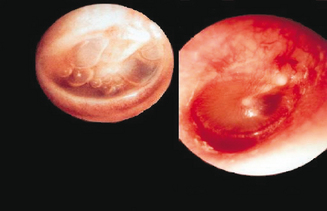
Figure 7.3 Middle ear effusions. A normal tympanic membrane (TM) should appear thin, translucent, and mobile. With an effusion the TM is often cloudy and has distinctly impaired mobility with an air–fluid level or bubble visible in the middle ear. Distinct erythema of the TM is seen in about 5% of ears with OME and should be distinguished from AOM. A retracted TM may indicate negative pressure and atelectasis within the ME.23
Pneumatic otoscopy is the recommended primary tool for diagnosis of OME, offering a superior combination of sensitivity and specificity than other diagnostic methods (a pooled sensitivity of 94% (95% CI, 91–96%) and specificity of 80% (95% CI, 75–86%) versus myringotomy as the gold standard).3,4,7,61 The main drawback with the use of pneumatic otoscopy is that the accuracy in routine clinical practice varies with clinician training and experience, as well as patient cooperation.5,7,63,64 Nevertheless, pneumatic otoscopy is available in most clinical settings, and is cost effective, and accurate in experienced hands.5,7,61
If the diagnosis of OME is unclear or the pneumatic otoscopic exam is difficult to perform, tympanometry or acoustic reflectometry could provide additional information.5,7,14,61 Tympanometry is used to measure the compliance of the TM and pressure within the ME. Studies have shown that tympanometry is a useful diagnostic tool in detection of MEE in infants and young children aged 2–24 months.61,65 Acoustic reflectometry measures the reflection of sound as a function of frequency and displays the results in terms of spectral gradients. It is reportedly less expensive than tympanometry, but it is difficult to use in those patients under age two.61 The reader should keep in mind that establishing the presence of MEE does not help identify its etiology, duration, or severity.
OME resulting in persistent MEE causes decreased mobility of the tympanic membrane and presents an impediment to sound conduction. For this reason, audiometric testing is recommended when OME persists for 3 or more months or when language delay, learning difficulties, or a significant hearing loss is suspected in a child with OME.7
Since MEE may be transient in nature, it is essential to document the side and attempt to determine the duration of effusions as well as the presence and severity of specific symptoms.7 Decisions for management typically depend on the documentation of these factors in conjunction with a detailed history and physical exam. The presence of OME does not “prove” an allergic etiology, but rather should suggest the possibility of IgE-mediated inflammation as a contributor when used in combination with a detailed, supportive history.
▪ Laboratory Tests
Since there is no ear-specific laboratory test for detecting an IgE-mediated cause of otitis, other laboratory tests may be used to confirm suspected allergies. Skin testing may be performed with several methods such as: skin prick testing (SPT), intradermal testing (IDT), and skin endpoint titration (SET). Serum tests include radioallergosorbent test (RAST or in vitro testing), eosinophil count, and total serum IgE. These and other testing methods are discussed in greater detail in Chapter 2.
▪ Food Allergy
The clinician should keep in mind the possibility of food as a source of OME. In those with allergic rhinitis (AR) or asthma, an allergy to cows’ milk may pose a significant risk factor for recurrent OME. Furthermore, a higher percentage (34%) of those with AR or asthma and cows’ milk allergy had recurrent OME compared with children with AR and no milk allergy (13%).66 These data suggest an association of recurrent OME with cows’ milk allergy in those with AR or asthma.66
Clinical suspicion of food allergy may be confirmed with a detailed clinical history and positive SPT responses to relevant food proteins combined with food challenges/elimination diets. Laboratory tests include analysis of serum for determination of food-specific IgE antibodies.67
Treatment
The ultimate goal of treatment is to alleviate symptoms of OME without causing significant adverse effects. The duration and severity of OME may dictate which of various therapeutic approaches to use, and, as patients respond to therapies differently, the selection of treatment must be individually determined. It is important to consider the possible multiple etiologic causes of OME prior to initiating therapy. For those with allergy as a contributing factor in the development of OME, there are various therapeutic options available. These approaches may be categorized as pharmacologic, nonpharmacologic, and/or complementary/alternative. Therapy is indicated if clinically significant benefits can be achieved outweighing the risks, and if it is unlikely that spontaneous resolution will occur in a timely fashion.7
▪ Pharmacologic Treatment
Multiple pharmacologic agents are available for the physician attempting to address allergic inflammation contributing to OME. Despite being theoretically efficacious, antihistamines and decongestants have not been found to be effective for OME.7 Meta-analyses have shown no benefit of antihistamine–decongestant combinations versus placebo3. However, in certain instances, treating OME with antihistamine–decongestant combinations might be beneficial. Stillwagon et al investigated the effects of anti-allergy treatment on allergen-induced ET dysfunction. In this study, adults with a history of seasonal AR to ragweed pollen received either a combination of antihistamine, and decongestants or placebo for 7 days.68 After treatment, subjects received intranasal challenge with ragweed pollen. They found that ET obstruction occurred in fewer patients receiving active treatment than those receiving placebo. They concluded that pre-exposure treatment of antihistamines in patients with AR may help decrease the risk in otherwise normal adults of developing ET dysfunction.68 As always, the use of these medications should be weighed against possible adverse effects such as insomnia, hyperactivity, blood pressure changes, and/or somnolence.
Antibiotics are thought to target the secondary bacterial infection often resulting from viral-induced inflammation causing ETD. However, the benefits conferred by antimicrobial therapy for OME only have been shown to be short-term (2–8 weeks) and diminish 2 weeks after cessation of therapy.3,7,69 The continual use of antibiotics for allergy-related OME is not inconsequential. Antibiotics may cause rashes, vomiting, diarrhea, anaphylaxis, and contribute to emerging bacterial resistance.3,7
Steroids, both oral and nasal preparations, have been investigated extensively as therapeutic options for OME. In large meta-analyses, oral steroids versus placebo did not provide long-term resolution (>2 weeks) of OME but did show short-term improvement in OME in combination with antibiotics in 33% of children treated.3,7,70–72 However, oral steroids have significant side effect profiles that include weight gain, insomnia, psychosis, adrenal suppression, immunosuppression, and avascular necrosis of the femoral head.3,7 Intranasal steroid use conveys less adverse effects but has no improved efficacy for resolving OME.71 Antileukotrienes may also address the inflammation related to nasal allergic symptoms, but have not been extensively studied for the treatment of allergy-related OME.
Since numerous studies demonstrate a high percentage (72–100%) of those with OME to be atopic via skin testing or serum tests, immunotherapy would also theoretically be indicated in those with IgE-mediated OME.8,17,67,73,74 However, no well-controlled, prospective studies have sufficiently compared immunotherapy with observation or other management options.9 Some studies do report high rates (78–100%) of resolution of OME when allergies are treated with immunotherapy and/or dietary elimination of suspected food allergens.73–76 Subich demonstrated that patients with allergies and OME had fewer infections and episodes of otorrhea while tympanostomy tubes (TT) were in place and required 50% less TT replacement than those not treated for allergies.77 However, these studies lack sufficient control groups, and have heterogenous definitions of allergy based on SPT, medical history, nasal smears, and serum IgE.7
In patients with concurrent AR and OME, immunotherapy may be efficacious in modulating the symptoms. Immunotherapy treatment in children with AR and OME was found to increase the interval between removal and subsequent replacement of TT for recurrent OME compared with those subjects who had no treatment (11 months versus 2 months). Also, in the treated group, the time to recurrence of OME was significantly longer when compared to the untreated group.17,38
▪ Nonpharmacologic Treatment
Precipitating factors and duration of the effusion determine the likelihood of spontaneous resolution of OME. Because the majority (75–90%) of episodes of residual OME after AOM resolve and little harm is associated with observation, guidelines suggest that patients should be observed for a 3-month period prior to any other type of intervention.7 However, in atopic patients the IgE-mediated inflammatory response likely plays a critical part in the persistence and/or recurrence of OME. Doner et al found that those with allergy determined by skin testing had recurrence of OME despite drug treatment (antibiotics, systemic decongestant, and nonsteroid anti-inflammatory agents), adenoidectomy, and TT placement. They concluded a possible allergic etiology and consideration of allergen-specific therapy should be considered in those with refractory OME.72 Other factors which may influence the clinician’s decision to observe or intervene include: severity/duration of disease, parental comfort, hearing loss, reliability of follow-up, and whether or not a child is at risk for speech or language delays.7
Referral for surgical management is indicated if medical management fails to alleviate OME. Surgical placement of TT is indicated in children if OME is present for 4 or more months, if there is persistent hearing loss, speech/language delays, or evidence of middle ear pathology.7 Insertion of TT promotes drainage of persistent effusions and improves hearing.7,14 Although TT placement does not specifically address the potential cause of OME, IgE-mediated or other, it does decrease the sequelae of OME. Randomized trials have shown that TT insertion decreases the prevalence of effusion to 62% and reduces effusion presence by 128 days per child during the year following surgery.7,78 However, an estimated 20–50% of children have recurrent or persistent episodes of OME post-tube extrusion that may require additional surgery.79 If reinsertion of TT is indicated for OME, studies also support performing an adenoidectomy, as this has been shown to reduce the need for further surgical interventions. Adenoidectomy is not recommended at the time of initial surgery unless a patient has significant nasal obstruction or chronic adenoiditis. Tonsillectomy alone or myringotomy without TT placement have not been shown to be efficacious in the treatment of OME.7
Complementary/Alternative Medical Treatment
There is a growing interest in complementary and alternative medicine (CAM) by both health care providers and patients. Research in this area continues to increase in quality and frequency. Studies relevant to OME have investigated vitamin supplements, polyunsaturated fatty acids, probiotic preparations, Xylitol (gum, syrup, or lozenges), herbal remedies, yoga, relaxation therapy, chiropractic manipulations, acupuncture, and traditional Chinese medicine.7,80 However, at the time of writing, there is insufficient evidence (no randomized, controlled trials with adequate sample sizes) to support or dispute the efficacy of these interventions for the treatment of OME.7
Future Directions
Over the next several decades, the approach to the allergic patient will likely be drastically modified. The intimate relationship that exists between the upper and lower airways in allergic disease may result in therapeutic strategies that target the inflammatory processes throughout the united airway. Clinical research endeavors should focus on understanding the etiologic role allergy plays in the development of OME. It will also be essential to conduct randomized, controlled trials on the efficacy of antigen-specific therapy such as immunotherapy or dietary elimination for the treatment of OME.
From a basic science standpoint, research efforts have already begun to develop therapeutic options that utilize and modify the biomolecular factors involved in the pathogenesis of the chronic inflammatory response. Some of these approaches use molecular cloning and recombinant production of allergens focusing on genetic modification of allergens or immunomodulatory DNA sequences.81
Recent studies examining the use of immunomodulatory oligodeoxynucleotides containing synthetic immunostimulatory motifs (IMO) in reactive airway disease and allergy-induced ET inflammation have been encouraging.82–85 These oligonucleotides activate cellular signaling pathways through the Toll-like receptor 9 (TLR9), which ultimately initiates an immunomodulatory cascade resulting in the activation of B and T lymphocytes, natural killer cells, monocytes, macrophages, and dendritic cells.82,83 IMO have been shown to significantly shift the antigen-induced Th2 immune response towards a Th1-type response in antigen-sensitized and challenged mouse spleen cultures.82,83 Through enhancement of the Th1-biased immunity, IMO have demonstrated that they effectively abate both the early and late phase allergic airway responses by reducing eosinophils, IL-10, IL-4, IL-5, and IL-6 levels (in both serum and bronchioalveolar lavage specimens).82,83 In addition, Zhu et al demonstrated that IMOs suppress the production of Th2-type cytokines IL-4, IL-5, and IL-13 and also induce the production of Th1 cytokine IL-12 and IFN-γ.83 Our laboratory has recently shown that pretreatment with IMO prevent ovalbumin (OVA)-induced allergic Eustachian tube dysfunction in the rat.85 These novel methods of modifying the chronic inflammatory response have potential as treatment, and in the future may prove effective in the management of allergy-induced OME, asthma, and allergic rhinitis.
Agrawal DK, Edwan J, Kandimalla ER, et al. Novel immunomodulatory oligonucleotides prevent development of allergic airway inflammation and airway hyperresponsiveness in asthma. Int Immunopharmacol. 2004;4:127-138.
Barenkamp SJ, Kurono Y, Ogra PL, et al. Recent advances in otitis media. 5. Microbiology and immunology. Ann Otol Rhinol Laryngol Suppl. 2005;194:60-85.
Baroody FM. Allergic rhinitis: broader disease effects and implications for management. Otolaryngol Head Neck Surg. 2003;128:616-631.
Bernstein JM. Allergic disease and the middle ear. In: Krouse JH, editor. Allergy and immunology. An otolaryngic approach. 1st edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:192-200.
Borish L. Allergic rhinitis: systemic inflammation and implications for management. J Allergy Clin Immunol. 112. 2003. 1021-1031.
Butler CC, van der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2002;4:CD001935.
Casselbrant ML. Epidemiology. In: Rosenfeld RMBC, editor. Evidence-based otitis media. 2nd edn. Hamilton, Ontario: BC Decker; 2003:147-162.
Daly KA, Rovers MM, Hoffman HJ, et al. Recent advances in otitis media. 1. Epidemiology, natural history, and risk factors. Ann Otol Rhinol Laryngol Suppl. 2005;194:8-15.
Gravel JS, Karma P, Casselbrant ML, et al. Recent advances in otitis media. 7. Diagnosis and screening. Ann Otol Rhinol Laryngol Suppl. 2005;194:104-113.
Hurst DS, Venge P. Evidence of eosinophil, neutrophil, and mast-cell mediators in the effusion of OME patietns with and without atopy. Allergy. 2000;55:435-441.
Johansson SG, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813-824.
Passalacqua G, Canonica GW. Treating the allergic patient: think globally, treat globally. Allergy. 2002;57:876-883.
Rosenfeld RM, Bluestone CD. Clinical efficacy of surgical therapy. In: Rosenfeld RM, Bluestone CD, editors. Evidence-based otitis media. Hamilton, Canada: BC Decker; 2003:227-240.
Rosenfeld RM, Culpepper L, Doyle KJ, et al. Clinical practice guideline: otitis media with effusion. Otolaryngol Head Neck Surg. 2004;130(5 suppl):S95-S118.
Shubich I. Otitis media with effusion and allergy control in children: a prospective study. In: Lim DJ, Bluestone CD, editors. Proceedings of the Sixth International Symposium on Recent Advances in Otitis Media. Hamilton, Canada: BC Decker; 1995:173-174.
1 Lewis ER. Otitis media and allergy. Ann Otol Rhinol Laryngol. 1929;38:185-188.
2 Proetz AW. Allergy in the middle and internal ear. Ann Otol Rhinol Laryngol. 1931;40:67-76.
3 Stool SE, Berman S, et al. Otitis media with effusion in young children. In Clinical practice guideline, Number 12. Rockville, MDs: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services; 1994.
4 Shekelle P, Takata G, Chan LS, et al. Diagnosis, natural history, and late effects of otitis media with effusion. Evid Rep Technol Assess (Summ). 2002;55:1-5.
5 Daly KA, Hunter LL, Giebink GS. Chronic otitis media with effusion. Pediatr Rev. 1999;20:85-93. quiz 4.
6 Doyle WJ. The link between allergic rhinitis and otitis media. Curr Opin Allergy Clin Immunol. 2002;2:21-25.
7 Rosenfeld RM, Culpepper L, Doyle KJ, et al. Clinical practice guideline: otitis media with effusion. Otolaryngol Head Neck Surg. 2004;130(5 suppl):S95-S118.
8 Barenkamp SJ, Kurono Y, Ogra PL, et al. Recent advances in otitis media. 5. Microbiology and immunology. Ann Otol Rhinol Laryngol Suppl. 2005;194:60-85.
9 Nguyen LHP, Manoukian JJ, Sobol SE, et al. Similar allergic inflammation in the middle ear and the upper airway: evidence linking otitis media with effusion to the united airways concept. J Allergy Clin Immunol. 2004;114:1110-1115.
10 Zielhuis GA, Rach GH, van den Broek P. Predisposing factors for otitis media with effusion in young children. Adv Otorhinolaryngol. 1988;40:65-69.
11 Chantzi FM, Kafetzis DA, Bairamis T, et al. IgE sensitization, respiratory allergy symptoms, and heritability independently increase the risk of otitis media with effusion. Allergy. 2006;61:332-336.
12 Bernstein JM. Role of allergy in eustachian tube blockage and otitis media with effusion: a review. Otolaryngol Head Neck Surg. 1996;114:562-568.
13 Bernstein JM, Reisman R. The role of acute hypersensitivity in secretory otitis media. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:120-127.
14 Fireman P. Otitis media and eustachian tube dysfunction: connection to allergic rhinitis. J Allergy Clin Immunol. 1997;99:787-797.
15 Alho OP, Oja H, Koivu M, Sorri M. Risk factors for chronic otitis media with effusion in infancy. Each acute otitis media episode induces a high but transient risk. Arch Otolaryngol Head Neck Surg. 1995;121:839-843.
16 Irander K, Borres MP, Bjorksten B. Middle ear diseases in relation to atopy and nasal metachromatic cells in infancy. Int J Pediatr Otorhinolaryngol. 1993;26:1-9.
17 Tomonaga K, Kurono Y, Mogi G. The role of nasal allergy in otitis media with effusion. A clinical study. Acta Otolaryngol Suppl (Stockh). suppl 458. 1988. 41-47.
18 Lazo-Saenz LG, Galvan-Aguilera AA, Martinez-Ordaz, et al. Eustachian tube dysfunction in allergic rhinitis. Otolaryngol Head Neck Surg. 2005;132:626-631.
19 Hurst DS, Amin K, Seveus L, Venge P. Evidence of mast cell activity in the middle ears of children with otitis media with effusion. Laryngoscope. 1999;109:471-477.
20 Hurst DS, Venge P. Levels of eosinophil cationic protein and myeloperoxidase from chronic middle ear effusion in patients with allergy and/or acute infection. Otolaryngol Head Neck Surg. 1996;114:531-544.
21 Hurst DS, Venge P. Evidence of eosinophil, neutrophil, and mast-cell mediators in the effusion of OME patients with and without atopy. Allergy. 2000;55:435-441.
22 Sobol SE, Taha R, Schloss MD, et al. T(H)2 cytokine expression in atopic children with otitis media with effusion. J Allergy Clin Immunol. 2002;110:125-130.
23 Passalacqua G, Canonica GW. Treating the allergic patient: think globally, treat globally. Allergy. 2002;57:876-883.
24 Johansson SG, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813-824.
25 Williamson IG, Dunleavey J, Bain J, Robinson D. The natural history of otitis media with effusion – a three-year study of the incidence and prevalence of abnormal tympanograms in four South West Hampshire infant and first schools. J Laryngol Otol. 1994;108:930-934.
26 Thomsen J, Tos M. Spontaneous improvement of secretory otitis. A long-term study. Acta Otolaryngol. 1981;92:493-499.
27 Zelhuis GA, Rach GH, van den Broek P. Screening for otitis media with effusion in preschool children. Lancet. 1989;1:311-314.
28 Casselbrant ML. Epidemiology. In: Rosenfeld RMBC, editor. Evidence-based otitis media. 2nd edn. Hamilton, Ontario: BC Decker; 2003:147-162.
29 Tos M. Epidemiology and natural history of secretory otitis. Am J Otol. 1984;5:459-462.
30 Daly KA, Rovers MM, Hoffman HJ, et al. Recent advances in otitis media. 1. Epidemiology, natural history, and risk factors. Ann Otol Rhinol Laryngol Suppl. 2005;194:8-15.
31 Rosenfeld RM, Goldsmith AJ, Tetlus L, et al. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg. 1997;123:1049-1054.
32 Bondy J, Berman S, Glazner J, Lezotte D. Direct expenditures related to otitis media diagnoses: extrapolations from a pediatric medicaid cohort. Pediatrics. 2000;105:E72.
33 Rovers MM, Schilder AG, Zielhuis GA, Rosenfeld RM. Otitis media. Lancet. 2004;363(9407):465-473.
34 Asmussen L, Sullivan SA, Olson LM, et al. Ear Infection Survey: a condition-specific functional outcomes measure for families of children with chronic otitis media. AHSR FHSR Annu Meet Abstr Book. 13, 1996. 14:abstract.
35 Brouwer CNM, Maille AR, Rovers MM, et al. Health-related quality of life in children with otitis media. Int J Pediatr Otorhinolaryngol. 2005;69:1031-1041.
36 Jero J, Virlainen A, Virtanen M, Eskola J, et al. Factors predicting poor outcome of acute otitis media in children. In: Lim DJ, Bluestone CD, Casselbrant M, Klein JO, Ogra PL, editors. Proceedings of the Sixth International Symposium on Recent Advances in Otitis Media. Hamilton, Canada: BC Decker; 1996:78-80.
37 Mion O, De Mello JF, Lessa MM, et al. The role of rhinitis in chronic otitis media. Otolaryngol Head Neck Surg. 2003;128:27-31.
38 Baroody FM. Allergic rhinitis: broader disease effects and implications for management. Otolaryngol Head Neck Surg. 2003;128:616-631.
39 Settipane RA. Complications of allergic rhinitis. Allergy Asthma Proc. 1999;20:209-213.
40 Bluestone CD, Doyle WJ. Anatomy and physiology of eustachian tube and middle ear related to otitis media. J Allergy Clin Immunol. 1988;81(5 Pt 2):997-1003.
41 Janfaza P, Nadol LB. Temporal bone and ear. In: Janfaza P, editor. Surgical anatomy of the head and neck. 1st edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:420-479.
42 Bernstein JM. Allergic disease and the middle ear. In: Krouse JH, et al, editors. Allergy and immunology. An otolaryngic approach. 1st edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:192-200.
43 Takahashi H, Hayashi M, Sato H, Honjo I. Primary deficits in eustachian tube function in patients with otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1989;115:581-584.
44 Miller GFJr. Eustachian tubal function in normal and diseased ears. Arch Otolaryngol. 1965;81:41-48.
45 Borish L. Allergic rhinitis: systemic inflammation and implications for management. J Allergy Clin Immunol. 2003;112:1021-1031.
46 Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147-S334.
47 Bascom R, Pipkorn U, Lichtenstein LM, et al. The influx of inflammatory cells into nasal washings during the late response to antigen challenge. Effect of systemic steroid pre-treatment. Am Rev Respir Dis. 1988;138:406-412.
48 Bernstein JM, Doyle WJ. Role of IgE-mediated hypersensitivity in otitis media with effusion: pathophysiologic considerations. Ann Otol Laryngol Suppl. 1994;16:15-19.
49 Viegas M, Gomez E, Brooks J, et al. Changes in nasal mast cell numbers in and out of the pollen season. Int Arch Allergy Appl Immunol. 1987;82:275-276.
50 Smurthwaite L, Walker SN, Wilson DR, et al. Persistent IgE synthesis in the nasal mucosa of hay fever patients. Eur J Immunol. 2001;12:3422-3431.
51 Wright ED, Hurst D, Miotto D, Giguere C, et al. Increased expression of major basic protein (MBP) and interleukin-5 (IL-5) in middle ear biopsy specimens from atopic patients with persistent otitis media with effusion. Otolaryngol Head Neck Surg. 2000;123:533-538.
52 Yin RY, Nahal A, Lee M, et al. Clincal predictors of nasal secretory cell quantities in allergy clinic patients. Ann Allergy Asthma Immunol. 1998;80:477-482.
53 Ebmeyer J, Furukawa M, Pak K, et al. Role of mast cells in otitis media. J Allergy Clin Immunol. 2005;116:1129-1135.
54 Hardy SM, Heavner SB, White DR, McQueen CT, Prazma J, Pillsbury HC. Late-phase allergy and eustachian tube dysfunction. Otolaryngol Head Neck Surg. 2001;125:339-345.
55 Pollock HW, Ebert CS, Dubin MG, et al. The role of soluble interleukin-4 receptor and interleukin-5 antibody in preventing late-phase allergy-induced eustachian tube dysfunction. Otolaryngol Head Neck Surg. 2002;127:169-176.
56 Ebert CSJr, Pollock HW, Dubin MG, et al. Effect of intranasal histamine challenge on Eustachian tube function. Int J Pediatr Otorhinolaryngol. 2002;63:189-198.
57 Miglets A. The experimental production of allergic middle ear effusions. Laryngoscpoe. 1973;83:1355-1384.
58 Doyle WJ, Takahara T, Fireman P. The role of allergy in the pathogenesis of otitis media with effusion. Arch Otolaryngol. 1985;111:502-506.
59 Doyle WJ, Friedman R, Fireman P, Bluestone CD. Eustachian tube obstruction after provocative nasal antigen challenge. Arch Otolaryngol. 1984;110:508-511.
60 Braunstahl GJ, Overbeek SE, Kleinjan A, et al. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in the upper and lower airways. J Allergy Clin Immunol. 2001;107:469-476.
61 Gravel JS, Karma P, Casselbrant ML, et al. Recent advances in otitis media. 7. Diagnosis and screening. Ann Otol Rhinol Laryngol Suppl. 2005;194:104-113.
62 Karma PH, Penttila MA, Sipila MM, et al. Otoscopic diagnosis of middle ear effusion in acute and non-acute otitis media. Int J Pediatr Otorhinolaryngol. 1989;17:37-49.
63 Pichichero ME, Poole MD. Assessing diagnostic accuracy and tympanocentesis skills in the management of otitis media. Arch Pediatr Adolesc Med. 2001;155:1137-1142.
64 Steinbach WJ, Sectish TC. Pediatric resident training in the diagnosis and treatment of acute otitis media. Pediatrics. 2002;109:404-408.
65 Palmu A, Puhakka H, Rahko T, et al. Diagnostic value of tympanometry in infants in clinical practice. Int J Pediatr Otorhinolaryngol. 1999;49:207-213.
66 Juntti H, Tikkanen S, Kokkonen J, et al. Cow’s milk allergy is associated with recurrent otitis media during childhood. Acta Otolaryngol. 1999;119:867-873.
67 Nsouli TM, Nsouli SM, Linde RE, et al. Role of food allergy in serous otitis media. Ann Allergy. 1994;73:215-219.
68 Stillwagon PK, Doyle WJ, Fireman P. Effect of an antihistamine/decongestant on nasal and eustachian tube function following intranasal pollen challenge. Ann Allergy. 1987;58:442-446.
69 Williams RL, Chalmers TC, Stange KC, et al. Use of antibiotics in preventing recurrent otitis media and otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA. 1993;270:1344-1351.
70 Butler CC, van der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2002;4:CD001935.
71 Mandel EM, Casselbrant ML, Rockette HE, et al. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002;110:1071-1080.
72 Doner F, Yariktas M, Demirci M. The role of allergy in recurrent otitis media with effusion. J Invest Allergol Clin Immunol. 2004;14:154-158.
73 Hall LJ, Lukat RM. Results of allergy treatment on the Eustachian tube in chronic serous otitis media. Am J Otol. 1981;3:116-121.
74 Draper WL. Secretory otitis media in children: a study of 540 children. Laryngoscope. 1967;77:636-653.
75 Fernandez AA, McGovern JP. Secretory otitis media in allergic infants and children. South Med J. 1965;58:581-586.
76 Psifidis A, Hatzistilianou M, Samaras K, et al. Atopy and otitis media in children. In: Ruben RJ, Karma PH, editors. Proceedings of the Seventh International Congress of Pediatric Otorhinolaryngology. Amsterdam, Netherlands: Elsevier Science; 1998:205.
77 Shubich I. Otitis media with effusion and allergy control in children: a prospective study. In: Lim DJ, Bluestone CD, editors. Proceedings of the Sixth International Symposium on Recent Advances in Otitis Media. Hamilton, Canada: BC Decker; 1995:173-174.
78 Rosenfeld RM, Bluestone CD. Clinical efficacy of surgical therapy. In: Rosenfeld RM, Bluestone CD, editors. Evidence-based otitis media. Hamilton, Canada: BC Decker; 2003:227-240.
79 Mandel EM, Rockette HE, Bluestone CD, et al. Efficacy of myringotomy with and without tympanostomy tube for chronic otitis media with effusion. Pediatr Infect Dis J. 1992;11:270-277.
80 Blazek-O’Neill B. Complementary and alternative medicine in allergy, otitis media, and asthma. Curr Allergy Asthma Rep. 2005;5:313-318.
81 Ferreira F, Briza P, Infuhr D, et al. Modified recombinant allergens for safer immunotherapy. Inflamm Allergy Drug Targets. 2006;5:5-14.
82 Klinman DM. Use of CpG oligodeoxynucleotides as immunoprotective agents. Expert Opin Biol Ther. 2004;4:937-946.
83 Agrawal DK, Edwan J, Kandimalla ER, et al. Novel immunomodulatory oligonucleotides prevent development of allergic airway inflammation and airway hyperresponsiveness in asthma. Int Immunopharmacol. 2004;4:127-138.
84 Zhu FG, Kandimalla ER, Yu D, Tang JX, et al. Modulation of ovalbumin-induced Th2 responses by second-generation immunomodulatory oligonucleotides in mice. Int Immunopharmacol. 2004;4:851-862.
85 Ebert CS, Rose AS, Patel MR, et al. The role of immunomodulatory oligonucleotides in prevention of allergy-induced Eustachian tube dysfunction. Abstract. American Academy of Otolaryngic Allergy Annual Meeting. Los Angeles, CA; September, 2005.