CHAPTER 60 OTHER SECONDARY HEADACHE DISORDERS
Although most patients who present to a physician with the chief complaint of headache have primary headache disorders, the clinician must always consider the possibility of a secondary headache. Thus, when evaluating a headache patient, the clinician must search for features that may serve as evidence for an underlying disorder. Such evidence may be found during the patient’s interview and physical examination. Although a list of these “red flags” is potentially exhaustive, common worrisome features include new-onset persistent or progressive headache, change in the characteristics of prior headaches, progressive nature, older age of the patient, worsening or precipitation by changes in posture or by the Valsalva maneuver, associated systemic or neurological symptoms, sudden and severe onset, and a history of trauma (Table 60-1). Abnormalities found on examination, including alterations in blood pressure, fever, neurological deficits, papilledema, and meningismus, may also raise this suspicion.
HEADACHE SECONDARY TO CEREBROVASCULAR DISORDERS
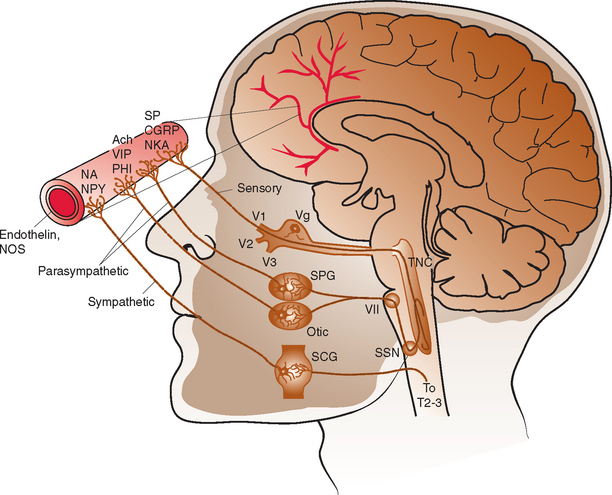
The intracranial vasculature is innervated by the sympathetic, parasympathetic, and sensory nervous systems (Fig. 60-1). The sensory system is the major conduit by which head pain is perceived after vascular stimulation. The majority of sensory nerves that innervate the anterior portion of the intracranial circulation terminate in the trigeminal nucleus caudalis, projecting via the first division of the trigeminal nerve, whereas those that innervate the posterior circulation terminate in the superior cervical ganglia and dorsal vagal ganglia.1–3 The density of sensory afferent vessels is greater in the posterior than anterior circulation, and thus stimulation of the posterior circulation is more likely to result in head pain.4–6
Direct stimulation of the intracranial vasculature and sinuses has been shown to cause activation of the trigeminal system. Stimulation of the superior sagittal sinus results in increased activity in the trigeminal nucleus caudalis, upper cervical dorsal horn, and thalamus.7 In addition, activation of orofacial excitatory receptive fields, mostly within the distribution of the first division of the trigeminal nerve, results in activation of thalamic neurons.8 The same thalamic neurons are also activated by stimulation of the superior sagittal sinus and/or the middle meningeal artery. Such evidence, in conjunction with a large body of additional data, characterizes the convergence and overlap within the trigeminovascular system. This continuity explains how nociceptive input at any part of the trigeminovascular system, including the intracranial vasculature, may result in pain in the head, face, and neck.
Ischemic Stroke
Approximately 25% of patients with stroke develop associated headaches, at least one half of which occur before the onset of neurological deficits.9 However, such headaches commonly go unrecognized, being overshadowed by more worrisome focal neurological symptoms. Headaches associated with ischemic stroke can be quite varying in their characteristics. Patients who have a primary headache disorder may develop a headache that closely resembles their usual headache. In those without such a history, headaches are most often pressing or throbbing in quality. The location of the headache may correlate with the site of the stroke. When headaches are unilateral, they are generally ipsilateral to the side of the stroke.4 Frontal pain is more common with anterior circulation strokes, and occipital pain is more common with posterior circulation strokes.
Headaches associated with stroke occur more frequently in young women and in persons with a history of migraine.10 Headaches are more common with large ischemic strokes and with strokes located in the territory of the posterior circulation, particularly those in the cerebellum.5,10 Headaches are much less common with subcortical infarctions, lacunar infarctions, and transient ischemic attacks.5,9
Cervical Artery Dissection
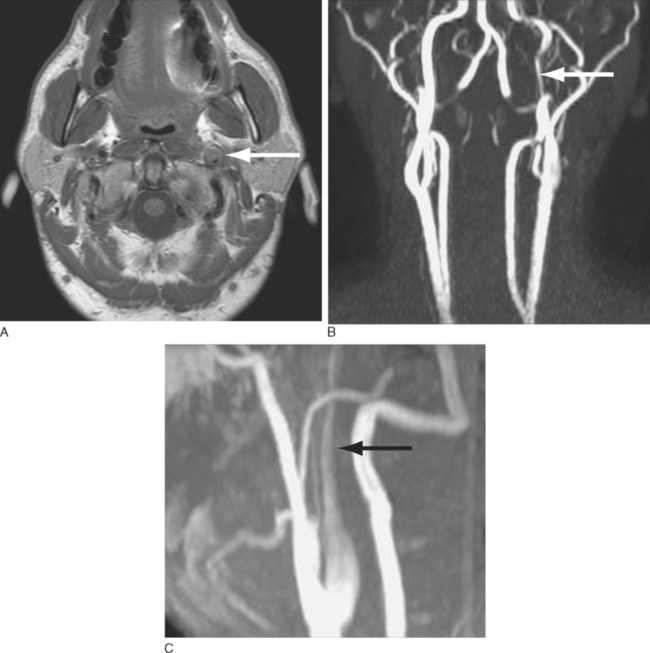
Headache is the most frequent presenting symptom of cervical artery dissection. Headache occurs with 60% to 95% of carotid artery dissections and with 70% of vertebral artery dissections.11 These headaches usually have a slow and gradual onset. However, about 20% of patients have a sudden and severe onset of pain consistent with that of a thunderclap headache.12 Headaches are typically located ipsilateral to the dissected artery. International Headache Society (IHS) diagnostic criteria stipulate that headaches considered secondary to cervical artery dissection must be ipsilateral to the dissection.13 Headaches associated with carotid dissection tend to be located in the ipsilateral frontotemporal region, lower face, jaw, or ear, whereas those of vertebral dissection are more often located in the parieto-occipital region. Patients with cervical artery dissection may also have neck pain. Neck pain occurs in 50% of patients with vertebral artery dissections and in 25% of patients with carotid artery dissections. Headaches associated with cervical artery dissections tend to be short-lived, with a median duration of 3 days. When longer lasting, headaches associated with carotid dissection usually resolve within 1 week, and those associated with vertebral dissection, by 5 weeks. Only an occasional patient develops persistent headaches after arterial dissection.
The diagnosis of cervical artery dissection may be accomplished by ultrasonography, magnetic resonance imaging (MRI), magnetic resonance angiography, computed tomographic angiography, and/or conventional catheter angiography (Fig. 60-2). MRI with diffusion sequences allows for detection of cerebral infarction that may occur secondarily to decreased perfusion or emboli distal to the dissection site. The treatment of cervical artery dissection varies according to the clinical presentation and location of disease, but it may include clinical observation, anticoagulation, and/or surgical intervention, including the use of arterial stents. Asymptomatic cervical artery dissections generally do not necessitate any intervention, although antiplatelet therapy would be recommended by some authorities. Symptomatic dissections may be treated with antiplatelet therapy or anticoagulation to prevent thrombus formation and the potential for subsequent artery-to-artery embolism.14,15 Although some studies suggest that anticoagulation may be associated with a lower rate of recurrent transient ischemic attacks and stroke, there is no controlled evidence to support the use of any particular antiplatelet or antithrombotic treatment in these patients.16,17 Anticoagulation is associated with an increased risk of hemorrhage that in select situations, such as intradural extension of the dissection, may be a contraindication to its use. Patients with aneurysmal dilation, SAH, significant arterial stenosis, or progressive neurological sequelae despite medical management may require surgical intervention.18
Intracranial Aneurysms
Headaches are common in patients with cerebral aneurysms. Headache is the presenting symptom of an unruptured intracranial aneurysm in 20% to 33% of patients.19,20 With the exception of a sentinel headache of the thunderclap type, no specific headache characteristics facilitate discovery of an unruptured aneurysm. Headaches may be focal or diffuse, unilateral or bilateral, anterior or posterior, and acute or gradual in onset.21 Therefore, it is imperative to check for associated neurological signs, such as cranial nerve palsies, that may provide evidence for an underlying lesion.
The IHS diagnostic criteria for headaches secondary to saccular aneurysms stipulate that the headache must be acute and of new onset and must resolve within 72 hours (Table 60-2).13 It is necessary to rule out SAH and intracerebral hemorrhage as underlying etiologies. Thunderclap headaches occurring in patients with an unruptured intracranial aneurysm must be recognized as warnings of impending aneurysm rupture. This type of headache is discussed in more detail later in this chapter.
TABLE 60-2 Headache Secondary to Intracranial Aneurysm: International Headache Society Diagnostic Criteria
Subarachnoid Hemorrhage
Headache is common in patients with aneurysm rupture and occurs in association with nausea and vomiting in 75% of patients.22 SAH headaches are most often of the thunderclap type, sudden and severe at onset. Although there are numerous causes of thunderclap headaches, every patient who presents with this type of headache should be evaluated for SAH (Table 60-3, Fig. 60-3). Although there may be a focal pain distribution when the headache of SAH begins, the pain usually generalizes and becomes bilateral. Severe pain is usually short-lived, lasting 1 to 2 hours, followed by a less severe headache of longer duration. With small hemorrhages, headaches tend to resolve after 2 to 3 days, whereas those associated with larger hemorrhages last an average of 8 days. Neck stiffness and pain, elevated body temperature, and alterations of consciousness are common associated features. Additional neurological symptoms and signs include nausea and vomiting, focal motor deficits, seizures, coma, cranial nerve palsies, papilledema, ocular hemorrhages, visual field deficits, and paresthesias.
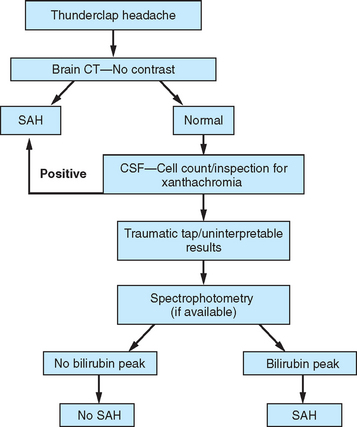
Figure 60-3 Evaluation for subarachnoid hemorrhage (SAH). CSF, cerebrospinal fluid; CT, computed tomography.
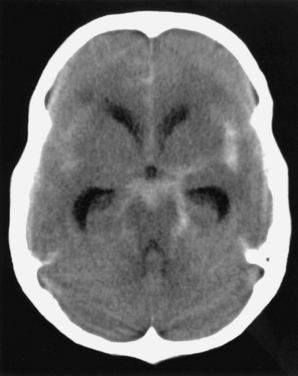
A history of a sentinel or warning headache is reported by 10% to 43% of patients with aneurysmal SAH.23 A sentinel headache is similar to the headache of SAH, but it occurs days to weeks before aneurysm rupture. Sentinel headaches generally develop over a few seconds and reach maximal intensity within minutes. Common associated features of SAH, including stiff neck, focal neurological symptoms and signs, and alterations in consciousness, are usually absent. Sentinel headaches may be caused by small aneurysmal leakages or stretching of the aneurysm wall without seepage of blood into the subarachnoid space. Identifying a sentinel headache as a warning of future SAH may allow for identification of an unruptured aneurysm and the need for surgical or endovascular intervention, thus avoiding a potentially catastrophic hemorrhage. Unfortunately, sentinel headaches are often ignored by patients and physicians, or they are misdiagnosed. According to a review by Edlow and Caplan, up to 50% of patients with SAH are initially misdiagnosed.24 Misdiagnosis occurs because the diagnostician fails to recognize the full clinical spectrum of SAH, lacks knowledge regarding the sensitivity of brain computed tomography (CT), and either fails to perform lumbar puncture or misinterprets CSF results.
CT of the brain is the first test in the evaluation of SAH (Fig. 60-4). CT is most sensitive early in the course of SAH. Its sensitivity is near 100% within the first 12 hours, but it decreases to approximately 50% by 1 week later.24–28 If CT yields negative results for SAH and does not provide an alternative diagnosis, lumbar puncture must be performed. Lumbar puncture evaluation should include measurement of opening pressure, cell count, visual inspection for xanthochromia, and spectrophotometry when available. When lumbar puncture is performed at least 12 hours after the onset of hemorrhage, the sensitivity of spectrophotometry is greater than 95%.29
Cerebral Venous Sinus Thrombosis
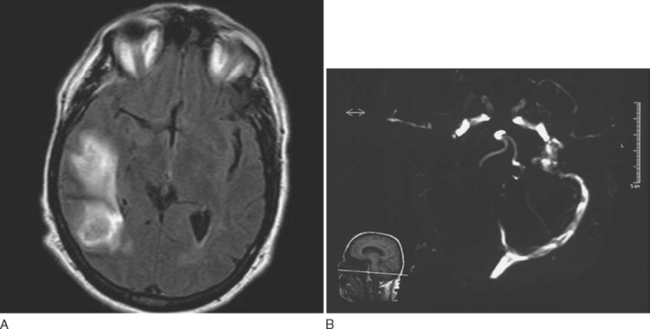
Headache is the most common presenting symptom of CVST, occurring in 75% to 95% of patients.30,31 In addition to headaches, patients with CVST usually present with papilledema, seizures, bilateral focal deficits, and/or altered level of consciousness. However, about 15% of patients present with isolated headaches.30,32 The headaches of CVST may be localized or diffuse, persistent, exacerbated by the Valsalva maneuver, and positional, with worsening on recumbency. Headaches of CVST have a gradual, subacute onset. However, approximately 10% of patients present with a headache of severe and sudden onset.33
Approximately 25% of patients with CVST who have normal neurological examination findings also have a normal CT, but CT is normal in fewer than 10% of patients who have focal neurological signs.34,35 Lumbar puncture should be performed, and opening pressure should be measured. Although 30% to 50% of patients with CVST have a combination of lymphocytic pleocytosis, elevated red blood cell count, and elevated protein levels, approximately 40% exhibit only an elevated opening pressure.36,37 MRI with venography or conventional angiography are the diagnostic modalities of choice when CVST is suspected (Fig. 60-5).
Anticoagulation is the treatment of choice for patients with CVST. Although anticoagulation carries the risk of promoting hemorrhage, it may prevent venous infarction, neurological worsening, and pulmonary embolism. Treatment with anticoagulation has been shown to be safe and to result in reduction in the risk of death or morbidity.38 If patients deteriorate despite adequate anticoagulation, thrombolysis via local infusion of thrombolytics into the occluded sinus and/or mechanical disruption of the thrombus should be considered.39
Giant Cell Arteritis
Headache is the most common manifestation. It is the presenting symptom in 48% of patients with GCA and occurs at some point during the disease course in 90%.40 The headache of GCA may be quite variable in its quality and location and may closely resemble a primary headache disorder. Associated symptoms, in descending order of frequency, are listed in Table 60-4.41,42 Ophthalmological complications include amaurosis fugax, visual loss, diplopia, ptosis, visual hallucinations, orbital bruits, and acute ocular hypotony.43 Visual loss tends to occur early in the course of the disease. If one eye is affected, the second is often affected within 2 weeks but rarely after 2 months.43
TABLE 60-4 Giant Cell Arteritis: Associated Clinical Features
Inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein level, are measured when GCA is suspected. Most patients with GCA have significant elevations in these inflammatory markers. The odds of a positive result of a temporal artery biopsy are increased 2.0 times when the ESR is 47 to 107 mm/hour, 2.7 times when the ESR is greater than 107 mm/hour, and 3.2 times when the C-reactive protein level is above 2.45 mg/dL.44 C-reactive protein level is more sensitive than ESR for the detection of GCA, and a combination of the two tests has the greatest specificity.44 Depending on the results of these laboratory tests and clinical suspicion for the disorder, temporal artery biopsy, the diagnostic “gold standard,” may be required. Although biopsy may be performed unilaterally or bilaterally, it is essential that the specimen be of adequate length in order to maximize test sensitivity. Treatment with steroids should not be delayed while biopsy is awaited. When the suspicion for GCA is high enough to plan a temporal artery biopsy, prednisone should be started immediately to decrease the risk of vascular complications, including visual loss. Furthermore, the yield of temporal artery biopsy is not significantly decreased when performed within 1 to 2 weeks of steroid initiation.45 Although temporal artery biopsy is considered the “gold standard” diagnostic test for GCA, as many as 15% of patients who meet clinical criteria for GCA have negative findings on temporal artery biopsies.46 Such patients are less likely to have jaw claudication, an abnormal temporal artery on physical examination, constitutional symptoms, and significant elevations in inflammatory markers.46,47
Primary Angiitis of the Central Nervous System and Reversible Cerebral Vasoconstriction Syndrome
PACNS is a vasculitis that is limited to the central nervous system in its distribution. Patients usually present with a headache that is subacute or slowly progressive in onset, severe, and focal or diffuse.48,49 The headache may be accompanied by nausea and vomiting. However, it is usually associated with other neurological manifestations, including hemiparesis, mental impairment, dysphasia, or seizures.50 Symptoms of PACNS may fluctuate in their severity but eventually progress over time. This often leads to delayed diagnosis; as many as 40% of cases are diagnosed after symptoms have been present for more than 3 months.50 Systemic symptoms, such as fever and weight loss, occur much less commonly than with systemic vasculitides. The diagnosis of PACNS can be made through a combination of CSF analysis, angiography, or central nervous system biopsy. CSF analysis often reveals significantly elevated protein levels and white blood cell count in patients with pathologically confirmed PACNS.51 The classic finding on cerebral angiography is a pattern of alternating areas of segmental narrowing and ectasia, producing a beaded or sausage-like appearance. Pathological specimens reveal fibrinoid necrosis and infiltration of vessel walls by lymphocytes, multinucleated giant cells, and/or histiocytes.52 PACNS tends to be an aggressive disease and is uniformly fatal without treatment. Response to cytotoxic/immunosuppressive therapy is variable; remissions are possible in some patients.
RCVS is a unifying diagnosis for a group of disorders characterized by reversible segmental cerebral vasospasm and more benign outcomes than those seen with PACNS. This includes thunderclap headache with vasospasm, benign angiopathy of the central nervous system, migrainous vasospasm or crash migraine, Call-Fleming syndrome, postpartum angiopathy, and drug-induced vasospasm.53–55 Patients with RCVS present with the acute onset of sudden and severe headache, consistent with thunderclap headache. Evaluation reveals normal or near-normal CSF findings and reversible cerebral segmental vasospasm involving arteries of the circle of Willis. The diagnostic criteria for RCVS are (1) a thunderclap headache, (2) evidence of vasospasm of one or more arteries of the circle of Willis that reverses by 12 weeks after onset, and (3) normal or near-normal CSF studies (Table 60-5). Patients may have a history of migraine, may be in the postpartum period, or have had exposure to certain drugs, including ergotamines, triptans, selective serotonin reuptake inhibitors, pseudoephedrine, cocaine, amphetamines, methylenedioxymethamphetamine (ecstasy), or bromocriptine.56–87 Patients with RCVS may differ in regard to the presence and/or severity of neurological deficits, imaging abnormalities, and circumstances at the time of symptom onset. Patients may present with thunderclap headache in isolation or in combination with changes in cognition or consciousness, motor deficits, sensory deficits, seizures, visual disturbances, ataxia, speech abnormalities, nausea, and/or vomiting.
TABLE 60-5 Reversible Cerebral Vasoconstriction Syndrome: Diagnostic Criteria
Because the angiographic appearance of segmental cerebral vasospasm in RCVS is identical to that seen in PACNS, these two entities must be differentiated, to avoid the unnecessary use of long-term immunosuppressants and cytotoxic agents in patients with RCVS. The clinical characteristic that best differentiates RCVS from PACNS is the acuity of headache onset and other clinical features. In contrast to patients with RCVS, who have a rapid onset of symptoms, patients with PACNS usually have a slowly progressive onset of disease and may accumulate new manifestations over weeks to months. Laboratory tests are also helpful in differentiating these two entities. Results of CSF analysis are markedly abnormal in approximately 80% of patients with PACNS but are generally normal in patients with RCVS.88 Cerebral imaging may appear normal in RCVS and usually appears abnormal in PACNS. In PACNS, MRI typically shows multifocal lesions secondary to ischemia or infarction distributed in the middle cerebral artery territory. Normal MRI, when diffusion and perfusion sequences are included, is uncommon in patients with symptomatic PACNS.89–92 In contrast, a greater proportion of patients with RCVS have normal brain MRI. However, abnormalities may be seen and are often consistent with posterior reversible leukoencephalopathy or watershed infarctions in the distribution of vasospastic blood vessels.93,94 RCVS cannot be differentiated from PACNS by the initial vascular imaging study, inasmuch as both show segmental vasospasm. However, even in the absence of any specific treatment, patients with RCVS have significant reversal of vasospasm within 4 weeks of symptom onset and complete normalization within several months.
Headache after Carotid Endarterectomy, Cerebral Angiography, and Coiling and Clipping of Intracranial Aneurysm
Headaches are frequently encountered during and after procedures involving the cerebral vasculature. Such headaches have been described in association with carotid endarterectomy, cerebral angiography, and coiling and clipping of aneurysms.95–97 Patients with primary headache disorders are more likely to develop headaches in association with these procedures. In some patients, stimulation of the intracranial vasculature triggers a headache that is identical to the headache of their usual primary headache disorder. In others, a new headache with different characteristics develops. Although the characteristics of postprocedure headaches differ according to the intervention, all begin in close temporal relationship to the procedure. In addition, the headache is most often located ipsilateral to the site of intervention. Although postprocedure headaches may be self-limited, others may become chronic or may be manifestations of an underlying complication. For instance, after carotid endarterectomy, a severe unilateral headache, especially if associated with seizures and contralateral focal deficits, may be the manifestation of a hyperperfusion syndrome.
HEADACHE SECONDARY TO NONVASCULAR INTRACRANIAL DISORDERS
Chiari Malformation
Chiari type I malformation consists of downward herniation, of at least 3 to 5 mm, of the cerebellar tonsils through the foramen magnum (Fig. 60-6). In studies of patients with headache, Chiari type I malformation is found in 2.7% to 5.8%.98–100 Its prevalence in patients without headache is predicted to be 2 per 1000, but this would probably be higher if modern imaging modalities were used.101 Chiari type I malformation may be associated with compression of the cervicomedullary junction, obstructive hydrocephalus, and syringomyelia.102 Patients with Chiari type I malformation can present with headache, lower cranial nerve palsies, scoliosis, sensory disturbance, or weakness.103,104
Although in most cases Chiari type I malformation is asymptomatic and diagnosed through an incidental MRI finding, headache is the most common presenting complaint.105,106 Head pain is most often described as a pressure sensation that worsens with increases in intracranial pressure and is located in the occipital or suboccipital region with radiation to the vertex, retro-orbitally, and to the neck.104,107,108 Many patients with Chiari type I malformation report headaches that occur after coughing, and more than one half of patients with cough-induced headache are found to have Chiari type I malformation.109 Headaches consistent with these characteristics, especially if associated with ocular disturbances, otoneurological dysfunction, or lower cranial nerve palsies, are suggestive of Chiari malformation. The IHS has defined diagnostic criteria for headache secondary to Chiari type I malformation (Table 60-6).13
TABLE 60-6 Chiari I Malformation Headache: International Headache Society Diagnostic Criteria
CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
Chiari malformation is diagnosed through MRI of the brain and cervical spinal cord, as indicated for evaluation for spinal cord syrinx. CSF flow studies may also be used to assess for suspected CSF obstruction at the cervicomedullary junction. The treatment of Chiari type I malformation is at times controversial, especially when patients have headache with no associated neurological features. Widely accepted indications for surgical intervention in such patients include spinal cord syrinx, cranial nerve deficits, gait instability, apnea, and torticollis.110,111 Surgical decompression may also be indicated for patients who have isolated headaches, meet criteria for Chiari headache, and are nonresponsive to conservative management. Studies have shown that most patients with headache secondary to Chiari malformation have a reduction of symptoms after surgical intervention.111–113 However, caution must be observed in interpreting these outcomes because the studies have been retrospective and involved a small number of patients.
Intracranial Neoplasm
Headache may be caused by the direct effects of an intracranial neoplasm, increased intracranial pressure secondary to such a neoplasm, carcinomatous meningitis, or secreting tumors of the hypothalamus or pituitary. Although as many as two thirds of patients with brain tumors report headaches, headache as an isolated and presenting clinical feature is uncommon, occurring in fewer than 10% of such patients.114 More often, patients present with focal neurological symptoms and signs and seizures.114 However, when headaches are associated with nausea, vomiting, or an abnormal neurological examination finding, or when there is a significant change in a patient’s prior headache pattern, the possibility of an underlying neoplasm must be investigated.115
Headaches secondary to the direct effects of an intracranial neoplasm may be progressive, localized, worse in the morning, and aggravated by increases in intracranial pressure caused by, for example, coughing and bending forward. In patients with unilateral head pain, the tumor is nearly always ipsilateral to the site of pain.115 According to IHS criteria, the headaches resolve within 7 days after surgical removal or volume reduction of the neoplasm or treatment with corticosteroids and must develop in temporal and usually spatial relation to the neoplasm.13 However, because it is often unknown when exactly a tumor first appeared, a temporal relationship may be difficult to determine in the clinical setting.
Headaches may also be secondary to increased intracranial pressure or hydrocephalus caused by a tumor. Such headaches are often accompanied by nausea and vomiting and are made worse by transient increases in intracranial pressure during coughing, sneezing, and the Valsalva maneuver. They may occur episodically in discrete and acute episodes or have a slower, progressive onset. As with other secondary headaches, there should be a temporal relationship between the onset of head pain and hydrocephalus, and the headache should resolve with reversal of the hydrocephalus.13
Carcinomatous meningitis is also commonly associated with headache. Although there are not headache features that are specific for the headache of carcinomatous meningitis, the headache develops and/or worsens with advancing disease. In addition, headache should improve with intrathecal chemotherapy.13
Hormone-secreting tumors of the hypothalamus and pituitary may also result in secondary headache. Approximately two thirds of patients with pituitary tumors report an associated headache, with the association being strongest with prolactinomas.116,117 According to IHS criteria, such headaches are most often bilateral, frontotemporal, and/or retro-orbital13; if the headache is secondary to a pituitary lesion, there is evidence of increased secretion of prolactin, growth hormone, or adrenocorticotropic hormone associated with a microadenoma; and if it is secondary to hypothalamic dysfunction, there is clinical evidence of such dysfunction, including altered temperature regulation, thirst, appetite, emotional state, or consciousness. However, a wider variation in headache features than that allowed by IHS criteria certainly exists. Patients may develop headache in association with a pituitary tumor with or without hormonal hypersecretion. Headaches most commonly mimic chronic migraine, followed by episodic migraine, primary stabbing headache, short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing, cluster headache, and hemicrania continua.118 The majority of these headaches are severe in intensity and throbbing in quality.118 Both medical treatment to counteract tumor hypersecretion and surgical resection of the tumor often lead to headache amelioration.118
HEADACHE SECONDARY TO DISORDERS OF THE NECK OR CRANIAL STRUCTURES
Cervicogenic Headache
Cervicogenic headache refers to head pain that is generated from a source in the neck. According to IHS criteria, there is clinical, laboratory, and/or imaging evidence of an abnormality within the cervical spine or soft tissues of the neck,13 and pain is attributed to the neck on the basis of either clinical signs that implicate a source in the neck or resolution of the headache after blockade of cervical structures or their nerve supply. It is important to recognize that neck pain commonly occurs in patients with headache, and thus neck pain alone does not implicate the neck as the source of the pain. Neck pain and tenderness, a history of neck trauma, unilaterality of pain, shoulder pain, and decreased range of motion in the neck are not unique features of cervicogenic headache, and thus their presence does not necessitate a diagnosis of cervicogenic headache.119 Patients with a wide range of headache disorders, including the most common headaches, such as migraine, tension-type headache, and the trigeminal autonomic cephalgias, may exhibit these symptoms. Thus, although cervicogenic headache is not a diagnosis of exclusion, it is important to evaluate patients for the presence of migrainous and autonomic features, which are less common in patients with cervicogenic headache. Although not included in the current IHS diagnostic criteria, prior proposed criteria have suggested that the head pain of cervicogenic headache is most often unilateral, fluctuating or continuous, nonthrobbing, nonlancinating, moderate to severe, and beginning in the neck with spread to other areas of the head.120,121
Although the exact pathophysiology of cervicogenic headache is unknown, it is likely that pain is generated by structures innervated by the upper three cervical nerves.122 Nociceptive stimulation of the upper cervical nerves may cause activation of the trigeminovascular pathway via convergence in the trigeminal nucleus caudalis, which extends from the brainstem to the level of C2-C3. Such a trigeminocervical interaction, with convergence of upper cervical sensory afferent vessels and trigeminal sensory afferent vessels in the brainstem, can explain the phenomenon of trigeminal nerve–distributed head pain from primary nociceptive stimulation of the upper cervical nerves.123
In order to formally diagnose cervicogenic headache, diagnostic blockades should be performed and result in resolution of head pain. These may include anesthetic blockade of the greater occipital nerve, the lesser occipital nerve, the cervical zygapophyseal joints, the cervical segmental nerves, and the intervertebral disks.124 In accordance with strict diagnostic criteria, such blocks should be placebo-controlled, to account for the high placebo response rate of interventional procedures used in the treatment of pain.
Sinus Headache
Because the sinuses and nasal mucosa are innervated by branches of the trigeminal nerve, it is easy to understand how sinus disease can result in facial and head pain.125,126 According to IHS diagnostic criteria for headache secondary to acute rhinosinusitis, headaches are frontal in location and associated with pain in the face, ears, or teeth13; there is clinical, laboratory, or radiographic evidence of acute sinusitis; and pain begins at the same time as the rhinosinusitis and resolves within 7 days of its successful treatment. Headache secondary to chronic sinusitis is not an entity recognized by the IHS.
Caution must be exercised in considering the diagnosis of sinus headache. Both sinus-related symptoms and headache, which have relatively high frequencies in the general population, commonly coexist. However, their coexistence does not necessarily suggest a causative relationship. In addition, patients often inaccurately self-diagnose their head or face pain as “sinus headache.” A prospective analysis of patients with self-diagnosed “sinus headache” found that 98% of cases met IHS diagnostic criteria for migraine or migrainous headache.127 Nonetheless, patients with active signs and symptoms of sinusitis and those with headaches that are correlated temporally with sinus disease should be evaluated for headaches of sinus origin. Such an evaluation must consider clinical manifestations of sinusitis in combination with the physical examination and radiological findings. CT is the imaging modality of choice. In interpreting sinus CT findings, it is important to avoid overinterpretation of minimal abnormalities, such as mild sinus mucosal thickening. This is especially pertinent in the absence of symptoms of active sinusitis. The clinician must remember that evidence for sinus abnormalities is found in 1.3% to 13.7% of patients undergoing imaging for headache and in 27% to 42.5% of asymptomatic patients undergoing CT for other reasons.98–100,128–130 Therefore, in headache patients without a clinical history or physical examination findings suggestive of active sinus disease, there is a poor correlation between facial and/or head pain and sinus imaging findings.131,132 However, when there is a high preimaging clinical suspicion for acute sinusitis and air-fluid levels or sinus opacification on imaging, and when patients meet nonimaging criteria for active sinus disease, antibiotic treatment may be warranted.133
HEADACHE SECONDARY TO INTRACRANIAL INFECTION
Headache may occur secondary to intracranial infections, including meningitis, encephalitis, and intracranial abscess. Although headaches are quite common in patients with central nervous system infection, they are rarely the only manifestation. Associated features may include fever, focal neurological symptoms and signs, alteration in consciousness, nausea, vomiting, neck stiffness, photophobia, and back pain.134,135 However, an underlying infection must always be considered in the evaluation of a patient with headache, because of the morbidity and mortality that may be associated with a missed diagnosis. Although most patients with headaches secondary to an intracranial infection have other manifestations as well, those with indolent infections, as might be seen with fungal disease, more commonly present with isolated headaches. The diagnosis of headache secondary to an underlying intracranial infection depends on a temporal relationship between the development of the infectious process and the headache and resolution of the headache after successful treatment of the infection.13 In addition, there must be objective evidence of an underlying central nervous system infection. This is generally obtained through CSF examination, at times supplemented by neuroimaging, electroencephalography, and other laboratory tests.
HEADACHE SECONDARY TO DISORDERS OF HOMEOSTASIS
Obstructive Sleep Apnea
Headache is a common complaint of patients with obstructive sleep apnea, and “sleep apnea headache” is an IHS-defined headache type (Table 60-7). Nocturnal headaches and headaches noted on awakening are more common in patients with obstructive sleep apnea. Nondescript morning headache is reported by 36% to 58% of patients with sleep apnea, and it may be the presenting feature.136–138 More than 50% of patients with early morning headaches may be found to have identifiable sleep disorders, including obstructive sleep apnea.139,140 Headaches associated with sleep apnea tend to be mild to moderate in intensity, pressing or tightening in quality, frontal or frontotemporal in location, and unilateral or bilateral, and they last less than 2 hours.138
TABLE 60-7 Sleep Apnea Headache: International Headache Society Diagnostic Criteria
Treatment of obstructive sleep apnea headache by nocturnal oxygenation through the use of continuous positive airway pressure often eliminates these nocturnal or early morning headaches.141 Some studies but not others have identified a relationship between the apnea-hypopnea index and headache and another relationship between the nadir nocturnal oxygen concentration and headache.142 When these relationships do exist, they support the theory that physiological and cerebral hemodynamic effects of hypoxemia and/or hypercapnia associated with obstructive sleep apnea, as opposed to sleep disturbance, play a prominent role in the generation of headaches in such patients.143 However, patients with higher apnea-hypopnea indices may also have more frequent nighttime awakenings, leading to nonrestorative sleep and a form of sleep-deprivation headache.144
Hypertension
Significant controversy regarding a possible association between hypertension and headache exists in the literature. Whereas some studies have concluded that headaches are more common in hypertensive patients, others have shown no correlation, and still others have concluded that high systolic and diastolic blood pressures are associated with a reduced risk of nonmigrainous headaches.145–147 Investigators using ambulatory blood pressure monitoring have not shown a correlation between blood pressure changes and the presence or absence of headache.148 Although the exact relationship is unclear, four distinct subclassifications of headache associated with hypertension have been suggested: acute hypertensive headache, chronic hypertensive headache, headache with malignant hypertension, and headache with hypertensive encephalopathy.149
Acute hypertensive headache occurs secondary to an abrupt increase in blood pressure. Headaches occur in approximately 20% of patients with hypertensive crisis and are most often described as throbbing and nondistinct.150 On occasion, such patients present with a thunderclap headache. Other manifestations of hypertensive crisis usually coexist and include faintness, dyspnea, chest pain, psychomotor agitation, focal neurological deficits, and epistaxis.150 Chronic hypertensive headache occurs secondary to long-standing hypertension, typically manifests on awakening in the morning, is located posteriorly, and improves on arising. Headache associated with malignant hypertension occurs generally when diastolic pressures are greater than 130 mm Hg and in the presence of papilledema. Headache may be the presenting feature of hypertensive encephalopathy with reversible neurological symptoms or of posterior reversible leukoencephalopathy syndrome.93,94 These patients usually have additional symptoms and signs, including nausea, vomiting, visual changes, altered mental status, seizures, and focal neurological signs.151,152 In patients with long-standing hypertension, hypertensive encephalopathy may not develop until pressures reach 250/150 mm Hg, whereas in those without a history of hypertension, values around 160/100 mm Hg may lead to encephalopathy.153 In addition, headache may occur in conjunction with hypertension in disorders such as pheochromocytoma, preeclampsia, eclampsia, and acute pressor response to exogenous agents.
Dodick DW, Eross EJ, Parish JM. Clinical, anatomical, and physiologic relationship between sleep and headache. Headache. 2003;43:282-292.
Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29-36.
Frishberg et al Frishberg BM, Rosenberg JH, Matchar DB, et al. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. Available at: www.aan.com/professionals/practice/pdfs/g10088.pdf. (accessed March 13, 2006).
Huston KA, Hunder GG, Lie JT, et al. Temporal arteritis. A 25-year epidemiologic, clinical, and pathologic study. Ann Intern Med. 1978;88:162-167.
Lewis DW, Ashwal S, Dahl G, et al. Practice parameter: evaluation of children and adolescents with recurrent headaches. Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;59:490-498.
1 Mayberg M, Langer RS, Zervas NT, et al. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228-230.
2 Keller JT, Beduk A, Saunders MC. Origin of fibers innervating the basilar artery of the cat. Neurosci Lett. 1985;58:263-268.
3 Saito K, Moskowitz MA. Contributions from the upper cervical dorsal roots and trigeminal ganglia to the feline circle of Willis. Stroke. 1989;20:524-526.
4 Vestergaard K, Andersen G, Nielsen MI, et al. Headache in stroke. Stroke. 1993;24:1621-1624.
5 Kumral E, Bogousslavsky J, Van Melle G, et al. Headache at stroke onset: the Lausanne Stroke Registry. J Neurol Neurosurg Psychiatry. 1995;58:490-492.
6 O’Connor TP, van der Kooy D. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J Neurosci. 1986;6:2200-2207.
7 Goadsby PJ, Zagami AS, Lambert GA. Neural processing of craniovascular pain: a synthesis of the central structures involved in migraine. Headache. 1991;31:365-371.
8 Davis KD, Dostrovsky JO. Properties of feline thalamic neurons activated by stimulation of the middle meningeal artery and sagittal sinus. Brain Res. 1988;454:89-100.
9 Ferro JM, Melo TP, Oliveira V, et al. A multivariate study of headache associated with ischemic stroke. Headache. 1995;35:315-319.
10 Tentschert S, Wimmer R, Greisenegger S, et al. Headache at stroke onset in 2196 patients with ischemic stroke or transient ischemic attack. Stroke. 2005;36:e1-e3.
11 Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45:1517-1522.
12 Mitsias P, Ramadan NM. Headache in ischemic cerebrovascular disease. Part I: clinical features. Cephalalgia. 1992;12:269-274.
13 Headache Classification Committee of the International Headache Society. The International classification of headache disorders. Cephalalgia. 2004;24(Suppl 1):1-151.
14 Thanvi B, Munshi SK, Dawson SL, et al. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81:383-388.
15 Stapf C, Elkind MSV, Mohr JP. Carotid artery dissection. Annu Rev Med. 2000;51:329-347.
16 Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Stroke. 2004;35:613-614.
17 Dziewas R, Konrad C, Drager B, et al. Cervical artery dissection—clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250:1179-1184.
18 Muller BT, Luther B, Hort W, et al. Surgical treatment of 50 carotid dissections: indications and results. J Vasc Surg. 2000;31:980-988.
19 International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
20 Solomon RA, Fink ME, Pike-Spellman J. Surgical management of unruptured intracranial aneurysms. J Neurosurg. 1994;80:440-446.
21 Raps EC, Rogers JD, Galetta SL, et al. The clinical spectrum of unruptured intracranial aneurysms. Arch Neurol. 1993;50:265-268.
22 Fontanarosa PB. Recognition of subarachnoid hemorrhage. Ann Emerg Med. 1989;18:1199-1205.
23 Polmear A. Sentinel headaches in aneurysmal subarachnoid haemorrhage: what is the true incidence? A systematic review. Cephalalgia. 2003;23:935-941.
24 Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29-36.
25 Morgenstern LB, Luna-Gonzales H, Huber JCJr, et al. Worst headache and subarachnoid hemorrhage: prospective, modern computed tomography and spinal fluid analysis. Ann Emerg Med. 1998;32:297-304.
26 van der Wee N, Rinkel GJ, Hasan D, et al. Detection of subarachnoid haemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry. 1995;58:357-359.
27 Sidman R, Connolly E, Lemke T. Subarachnoid hemorrhage diagnosis: lumbar puncture is still needed when the computed tomography scan is normal. Acad Emerg Med. 1996;3:827-831.
28 Sames TA, Storrow AB, Finkelstein JA, et al. Sensitivity of new-generation computed tomography in subarachnoid hemorrhage. Acad Emerg Med. 1996;3:16-20.
29 Vermeulen M, Hasan D, Blijenberg BG, et al. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry. 1989;52:826-828.
30 Terazzi E, Mittino D, Ruda R, et al. Cerebral venous thrombosis: a retrospective multicentre study of 48 patients. Neurol Sci. 2005;25:311-315.
31 de Bruijn SFTM, de Haan RJ, Stam J, et al. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. J Neurol Neurosurg Psychiatry. 2001;70:105-108.
32 Cumurciuc R, Crassard I, Sarov M, et al. Headache as the only neurological sign of cerebral venous thrombosis: a series of 17 cases. J Neurol Neurosurg Psychiatry. 2005;76:1084-1087.
33 de Bruijn SFTM, Stam J, Kappelle LJ. Thunderclap headache as the first symptom of cerebral venous sinus thrombosis. CVST Study Group. Lancet. 1996;348:1623-1625.
34 Rao KCVG, Knipp HC, Wagner EJ. CT findings in cerebral sinus and venous thrombosis. Radiology. 1981;140:391-398.
35 Chiras J, Bousser MG, Medler JF, et al. CT in cerebral thrombophlebitis. Neuroradiology. 1985;27:145-154.
36 Bousser MG, Chiras J, Sauron B, et al. Cerebral venous thrombosis: a review of 38 cases. Stroke. 1985;16:199-213.
37 Barinagarrementeria F, Cantu C, Arredondo H. Aseptic cerebral venous thrombosis: proposed prognostic scale. J Stroke Cerebrovasc Dis. 1992;2:34.
38 Stam J, De Bruijn SF, DeVeber G. Anticoagulation for cerebral sinus thrombosis. Cochrane Database Syst Rev. (4):2002. CD002005
39 Canhao P, Falcao F, Ferro JM. Thrombolytics for cerebral sinus thrombosis: a systematic review. Cerebrovasc Dis. 2003;15:159-166.
40 Solomon S, Cappa KG. The headache of temporal arteritis. J Am Geriatr Soc. 1987;35:163-165.
41 Huston KA, Hunder GG, Lie JT, et al. Temporal arteritis. A 25-year epidemiologic, clinical, and pathologic study. Ann Intern Med. 1978;88:162-167.
42 Hunder GG. Clinical features of GCA/PMR. Clin Exp Rheumatol. 2000;18(4, Suppl 20):S6-S8.
43 Cullen JF, Coleiro JA. Ophthalmic complications of giant cell arteritis. Surv Ophthamlol. 1976;20:247-260.
44 Hayreh SS, Podhajsky PA, Raman R, et al. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123:285-296.
45 Achkar AA, Lie JT, Hunder GG, et al. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann Intern Med. 1994;120:987-992.
46 Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, et al. Biopsynegative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum. 2001;30:249-256.
47 Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
48 Cupps TR, Moore PM, Fauci AS. Isolated angiitis of the central nervous system. Am J Med. 1983;74:97-105.
49 Calabrese LH, Mallek JA. Primary angiitis of the central nervous system. Medicine. 1987;67:20-39.
50 Calabrese LH, Furlan AJ, Gragg LA, et al. Primary angiitis of the central nervous system: diagnostic criteria and clinical approach. Cleve Clin J Med. 1992;59:293-306.
51 Calabrese LH, Gragg LA, Furlan AJ. Benign angiopathy: a distinct subset of angiographically defined primary angiitis of the central nervous system. J Rheumatol. 1993;20:2046-2050.
52 Parisi JE, Moore PM. The role of biopsy in vasculitis of the central nervous system. Neurology. 1994;14:341-348.
53 Calabrese LH, Gragg LA, Furlan AJ. Benign angiopathy: a distinct subset of angiographically defined primary angiitis of the central nervous system. J Rheumatol. 1993;20:2046-2050.
54 Hajj-Ali RA, Furlan A, Abou-Chebl A, et al. Benign angiopathy of the central nervous system: cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum. 2002;47:662-669.
55 Call GK, Fleming MC, Sealfon S, et al. Reversible cerebral segmental vasoconstriction. Stroke. 1988;19:1159-1170.
56 Walsh JP, O’Doherty DS. A possible explanation of the mechanism of ophthalmoplegic migraine. Neurology. 1960;10:1079-1084.
57 Dukes HT, Veith RG. Cerebral angiography during migraine prodrome and headache. Neurology. 1964;14:636-639.
58 Garnic JD, Schellinger D. Arterial spasm as a finding intimately associated with onset of vascular headache. Neuroradiology. 1983;24:273-276.
59 Masuzawa T, Shinoda S, Furuse M, et al. Cerebral angiographic changes on serial examination of a patient with migraine. Neuroradiology. 1983;24:277-281.
60 Lieberman AN, Jonas S, Hass WK, et al. Bilateral cervical and intracerebral vasospasm causing cerebral ischemia in a migrainous patient: a case of “diplegic migraine.”. Headache. 1984;24:245-248.
61 Serdaru M, Chiras J, Cujas M, et al. Isolated benign cerebral vasculitis or migrainous vasospasm? J Neurol Neurosurg Psychiatry. 1984;47:73-76.
62 Monteiro P, Carneiro L, Lima B, et al. Migraine and cerebral infarction: three case studies. Headache. 1985;25:429-433.
63 Schon F, Harrison MJH. Can migraine cause multiple segmental cerebral artery constrictions? J Neurol Neurosurg Psychiatry. 1987;50:492-494.
64 Rothrock JF, Walicke P, Swenson MR, et al. Migrainous stroke. Arch Neurol. 1988;45:63-67.
65 Solomon S, Lipton RB, Harris PY. Arterial stenosis in migraine: spasm or arteriopathy? Headache. 1990;30:52-61.
66 Gomez CR, Gomez SM, Puricelli MS, et al. Transcranial Doppler in reversible migrainous vasospasm causing cerebellar infarction: report of a case. Angiology. 1991;42:152-156.
67 Sanin LC, Mathew NT. Severe diffuse intracranial vasospasm as a cause of extensive migrainous cerebral infarction. Cephalalgia. 1993;13:289-292.
68 Schluter A, Kissig B. MR angiography in migrainous vasospasm. Neurology. 2002;59:1772.
69 Farine D, Andreyko J, Lysikiewicz A, et al. Isolated angiitis of brain in pregnancy and puerperium. Obstet Gynecol. 1984;63:586-588.
70 Bogousslavsky J, Despland PA, Regli F, et al. Postpartum cerebral angiopathy: reversible vasoconstriction assessed by transcranial Doppler ultrasounds. Eur Neurol. 1989;29:102-105.
71 Geraghty JJ, Hoch DB, Robert ME, et al. Fatal puerperal cerebral vasospasm and stroke in a young woman. Neurology. 1991;41:1145-1147.
72 Janssens E, Hommel M, Mounier-Vehier F, et al. Postpartum cerebral angiopathy possibly due to bromocriptine therapy. Stroke. 1995;26:128-130.
73 Sugiyama Y, Muroi A, Ishikawa M, et al. A benign form of isolated angiitis of the central nervous system in puerperium: an identical disorder to postpartum cerebral angiopathy? Intern Med. 1997;36:931-934.
74 Ursell MR, Marras CL, Farb R, et al. Recurrent intracranial hemorrhage due to postpartum cerebral angiopathy: implications for management. Stroke. 1998;29:1995-1998.
75 Modi M, Modi G. Postpartum cerebral angiopathy in a patient with chronic migraine with aura. Headache. 2000;40:677-681.
76 Kyung L, Sohn YH, Kim SH, et al. Basilar artery vasospasm in postpartum cerebral angiopathy. Neurology. 2000;54:2003-2005.
77 Ihara M, Yanagihara C, Nishimura Y. Serial transcranial color-coded sonography in postpartum cerebral angiopathy. J Neuroimaging. 2000;10:230-233.
78 Kubo S, Nakata H, Tatsumi T, et al. Headache associated with postpartum cerebral angiopathy: monitoring with transcranial color-coded sonography. Headache. 2002;42:297-300.
79 Geocadin RG, Razumovsky AY, Wityk RJ, et al. Intracerebral hemorrhage and postpartum cerebral vasculopathy. J Neurol Sci. 2002;205:29-34.
80 Konstantinopoulos PA, Mousa S, Khairallah R, et al. Postpartum cerebral angiopathy: an important diagnostic consideration in the postpartum period. Am J Obstet Gynecol. 2004;191:375-377.
81 Song JK, Fisher S, Seifert TD, et al. Postpartum cerebral angiopathy: atypical features and treatment with intracranial balloon angioplasty. Neuroradiology. 2004;46:1022-1026.
82 Henry PY, Larre P, Aupy M, et al. Reversible cerebral arteriopathy associated with the administration of ergot derivatives. Cephalalgia. 1984;4:171-178.
83 Meschia JF, Malkoff MD, Biller J. Reversible segmental cerebral arterial vasospasm and cerebral infarction: possible association with excessive use of sumatriptan and Midrin. Arch Neurol. 1998;55:712-714.
84 Singhal AB, Caviness VS, Begleiter AF, et al. Cerebral vasoconstriction and stroke after use of serotonergic drugs. Neurology. 2002;58:130-133.
85 Levine SR, Washington JM, Jefferson MI, et al. “Crack” cocaine–associated stroke. Neurology. 1987;37:1849-1853.
86 Reneman L, Habraken JB, Majoie CB, et al. MDMA (“ecstasy”) and its association with cerebrovascular accidents: preliminary findings. Am J Neuroradiol. 2000;21:1001-1007.
87 Janssens E, Hommel M, Mounier-Vehier F, et al. Postpartum cerebral angiopathy possibly due to bromocriptine therapy. Stroke. 1995;26:128-130.
88 Calabrese LH, Mallek JA. Primary angiitis of the central nervous system—report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine. 1987;67:20-39.
89 Pomper MG, Miller TJ, Stone JH, et al. CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography. AJNR Am J Neuroradiol. 1999;20:75-85.
90 Harris KG, Tran DD, Sickels WJ, et al. Diagnosing intracranial vasculitis: the roles of MR and angiography. AJNR Am J Neuroradiol. 1994;15:317-330.
91 Duna GF, Calabrese LH. Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J Rheumatol. 1995;22:662-667.
92 Wasserman BA, Stone JH, Hellmann DB, et al. Reliability of normal findings on MR imaging for excluding the diagnosis of vasculitis of the central nervous system. AJR Am J Roentgenol. 2001;177:455-459.
93 Tang-Wai DF, Phan TG, Wijdicks EFM. Hypertensive encephalopathy presenting with thunderclap headache. Headache. 2001;41:198.
94 Dodick DW, Eross EJ, Drazkowski JF, et al. Thunderclap headache associated with reversible vasospasm and posterior leukoencephalopathy syndrome. Cephalalgia. 2003;23:994.
95 Tehindrazanarivelo AD, Lutz G, PetitJean C, et al. Headache following carotid endarterectomy: a prospective study. Cephalalgia. 1992;12:380-382.
96 Ramadan NM, Gilkey SJ, Mitchell M, et al. Postangiography headache. Headache. 1995;35:21-24.
97 Schwedt TJ, Samples S, Rasmussen P, et al. New headache after endovascular or microsurgical treatment of intracranial aneurysm. Neurology. 2005;64(Suppl 1):A401.
98 Lewis DW, Dorbad D. The utility of neuroimaging in the evaluation of children with migraine or chronic daily headache who have normal neurological examinations. Headache. 2000;40:629-632.
99 Bass NE, Ruggieri PM, Cohen BH, et al. Clinical usefulness of magnetic resonance imaging in pediatric headache. Ann Neurol. 1995;38:527.
100 Schwedt TJ, Guo Y, Rothner AD. “Benign” imaging abnormalities in children and adolescents with headache. Headache. 2006;46:387-398.
101 Khurana RK. Headache spectrum in Arnold-Chiari malformation. Headache. 1991;31:151-155.
102 Carmel PW. Management of the Chiari malformations in childhood. Clin Neurosurg. 1983;30:385-406.
103 Greenlee JD, Donovan KA, Hasan DM, et al. Chiari I malformation in the very young child: the spectrum of presentations and experience in 31 children under age 6 years. Pediatrics. 2002;110:1212-1219.
104 Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-1017.
105 Dure LS, Percy AK, Cheek WR, et al. Chiari type I malformation in children. J Pediatr. 1989;115:573-576.
106 Park JK, Gleason PL, Madsen JR, et al. Presentation and management of Chiari I malformation in children. Pediatr Neurosurg. 1997;26:190-196.
107 Kesler R, Mendizabal JE. Headache in Chiari malformation: a distinct clinical entity? J Am Osteopath Assoc. 1999;99:153-156.
108 Sansur CA, Heiss JD, DeVroom HL, et al. Pathophysiology of headache associated with cough in patients with Chiari I malformation. J Neurosurg. 2003;98:453-458.
109 Pascual J, Iglesias F, Oterino A, et al. Cough, exertional, and sexual headaches: an analysis of 72 benign and symptomatic cases. Neurology. 1996;46:1520-1524.
110 Haines SJ, Berger M. Current treatment of Chiari malformations types I and II: a survey of the Pediatric Section of the American Association of Neurological Surgeons. Neurosurgery. 1991;28:353-357.
111 Weinberg JS, Freed DL, Sadock J, et al. Headache and Chiari I malformation in the pediatric population. Pediatr Neurosurg. 1998;29:14-18.
112 Nohria V, Oakes WJ. Chiari I malformation: a review of 43 patients. Pediatr Neurosurg. 1990;16:222-227.
113 Dyste GN, Menezes AH, VanGilder JC. Symptomatic Chiari malformations. An analysis of presentation, management, and long-term outcome. J Neurosurg. 1989;71:159-168.
114 Vasquez-Barquero A, Ibanez FJ, Herrera S, et al. Isolated headache as the presenting clinical manifestation of intracranial tumors: a prospective study. Cephalalgia. 1994;14:270-271.
115 Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology. 1993;43:1678-1683.
116 Levy MJ, Jager HR, Powell M, et al. Pituitary volume and headache. Arch Neurol. 2004;61:721-725.
117 Abe T, Matsumoto K, Kuwazawa J, et al. Headache associated with pituitary adenomas. Headache. 1998;43:1678-1683.
118 Levy MJ, Matharu MS, Meeran K, et al. The clinical characteristics of headache in patients with pituitary tumours. Brain. 2005;128:1921-1930.
119 Taylor FR. Distinguishing primary headache disorders from cervicogenic headache: clinical and therapeutic implications. Headache Curr. 2005;2:37-41.
120 Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic headache: diagnostic criteria. Headache. 1990;30:725-726.
121 Antonaci F, Ghirmai S, Bono S, et al. Cervicogenic headache: evaluation of the original diagnostic criteria. Cephalalgia. 2001;21:573-583.
122 Silverman SB. Cervicogenic headache: interventional, anesthetic, and ablative treatment. Curr Pain Headache Rep. 2002;6:308-314.
123 Bartsch T, Goadsby PJ. Anatomy and physiology of pain referral patterns in primary and cervicogenic headache disorders. Headache Curr. 2005;2:42-48.
124 van Suijlekon JA, Weber WEJ, van Kleef M. Cervicogenic headache: techniques of diagnostic nerve blocks. Clin Exp Rheumatol. 2000;18(Suppl 19):S39-S44.
125 Ng YT, Butler IJ. Sphenoid sinusitis masquerading as migraine headaches in children. J Child Neurol. 2001;16:882-884.
126 Clerico DM. Sinus headaches reconsidered: referred cephalgia of rhinologic origin masquerading as refractory primary headaches. Headache. 1995;35:185-192.
127 Cady RK, Schreiber CP, Billings C. Subjects with self-described “sinus” headache meet IHS diagnostic criteria for migraine [Abstract]. Cephalalgia. 2001;21:298.
128 Maytal J, Benkowski RS, Patel M, et al. The value of brain imaging in children with headaches. Pediatrics. 1995;96:413-416.
129 Medina LS, Pinter JD, Zurakowski D, et al. Children with headache: clinical predictors of surgical space-occupying lesions and the role of neuroimaging. Radiology. 1997;202:819-824.
130 Alehan FK. Value of neuroimaging in the evaluation of neurologically normal children with recurrent headache. J Child Neurol. 2002;17:807-809.
131 Mudgil SP, Wise SW, Hopper KD, et al. Correlation between presumed sinusitis-induced pain and paranasal sinus computed tomographic findings. Ann Allergy Asthma Immunol. 2002;88:223-226.
132 Shields G, Seikaly H, Leboeuf M, et al. Correlation between facial pain or headache and computed tomography in rhinosinusitis in Canadian and U.S. subjects. Laryngoscope. 2003;113:943-945.
133 Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997;117:S1-S7.
134 Lamonte M, Silberstein SD, Marcelis JF. Headache associated with aseptic meningitis. Headache. 1995;35:520-526.
135 Scelsa SN, Lipton RB, Sander H, et al. Headache characteristics in hospitalized patients with Lyme disease. Headache. 1995;35:125-130.
136 Dexter JD. Headache as a presenting complaint of the sleep apnea syndrome [Abstract]. Headache. 1984;24:171.
137 Guilleminault C, van den Hoed J, Mitler MM. Clinical overview of the sleep apnea syndromes. In: Guilleminault C, Dement WC, editors. Sleep Apnea Syndromes. New York: Alan R. Liss; 1978:1-12.
138 Alberti A, Mazzotta G, Gallinella E, et al. Headache characteristics in obstructive sleep apnea syndrome and insomnia. Acta Neurol Scand. 2005;111:309-316.
139 Paiva T, Batista A, Martins P, et al. The relationship between headaches and sleep disturbances. Headache. 1995;35:590-596.
140 Paiva T, Farinha A, Martins A, et al. Chronic headaches and sleep disorders. Arch Intern Med. 1997;157:1701-1705.
141 Poceta JS, Dalessio DJ. Identification and treatment of sleep apnea in patients with chronic headache. Headache. 1995;35:586-589.
142 Rains J, Penzien D, Mohammed Y. Sleep and headache: morning headache associated with sleep disordered breathing [Abstract]. Cephalalgia. 2001;21:520.
143 Dodick DW, Eross EJ, Parish JM. Clinical, anatomical, and physiologic relationship between sleep and headache. Headache. 2003;43:282-292.
144 Blau JN. Sleep deprivation headache. Cephalalgia. 1990;10:157-160.
145 Fuchs FD, Gus M, Moreira LB, et al. Headache is not more frequent among patients with moderate to severe hypertension. J Hum Hypertens. 2003;17:787-790.
146 Cirillo M, Stellato D, Lombardi C, et al. Headache and cardiovascular risk factors: positive association with hypertension. Headache. 1999;39:409-416.
147 Hagen K, Stovner LJ, Vaffen L, et al. Blood pressure and risk of headache: a prospective study of 22,685 adults in Norway. J Neurol Neurosurg Psychiatry. 2002;72:463-466.
148 Kruszewski P, Bieniaszewski L, Neubauer J, et al. Headache in patients with mild to moderate hypertension is generally not associated with simultaneous blood pressure elevation. J Hypertens. 2000;18:437-444.
149 Spierings ELH. Acute and chronic hypertensive headache and hypertensive encephalopathy. Cephalalgia. 2002;22:313-316.
150 Zampaglione B, Pascale C, Marchisio M, et al. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension. 1996;27:144-147.
151 Healton E, Burst J, Feinfield D, et al. Hypertensive encephalopathy and the neurologic manifestations of malignant hypertension. Neurology. 1982;32:127-132.
152 Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
153 Cortelli P, Grimaldi D, Guaraldi P, et al. Headache and hypertension. Neurol Sci. 2004;25:S132-S134.