CHAPTER 88 Osteoporosis
Surgical Strategies
Osteoporosis is a systemic disease characterized by decreased bone mass and microarchitectural deterioration. The resulting decrease in bone mechanical strength typically manifests as fragility fractures, with about one half of osteoporotic fractures occurring in the spine. According to the National Osteoporosis Foundation, low bone mass currently affects more than 44 million Americans and frank osteoporosis affects 15% of postmenopausal white women and 35% of women older than 65 years of age. In addition, 50% of white women will sustain an osteoporotic fracture at some time during their lifetime. Men have a lower but still significant osteoporotic fracture risk, which peaks 10 years later than the risk in women.1
Osteoporotic Vertebral Compression Fractures
Osteoporotic vertebral compression fractures are a leading cause of disability and morbidity in the elderly.2–4 The consequences of these fractures include pain and, in many cases, progressive vertebral collapse with resultant spinal kyphosis. Osteoporotic vertebral compression fractures have been shown to adversely affect quality of life, physical function, mental health, and survival.5–7 These effects are related to the severity of the spinal deformity and are, in part, independent of pain.6,7
Nevertheless, the pain associated with acute vertebral compression fractures may be incapacitating. In a number of cases, the pain will subside over a period of weeks or months, although it is not uncommon for the pain to become chronic.7 Chronic pain after vertebral fracture most likely results from (1) incomplete vertebral healing with progressive bony collapse, (2) altered spine kinematics as a consequence of spinal deformity, or (3) the development of a pseudarthrosis at the involved vertebra. Chronic pain associated with vertebral compression fractures often leads to impaired quality of life and depression.6,7
Kyphotic deformity in the osteoporotic spine may also create a biomechanical environment favoring additional fractures. The kyphotic deformity anteriorly shifts the patient’s center of gravity, creating a longer moment arm acted on by the center of gravity. This results in greater flexion-bending moments around the apex of the kyphosis, which will promote further increases in kyphotic angulation and additional fractures.8,9 Clinical studies report that the risk of a new vertebral fracture in the first year after an incident vertebral compression fracture rises 5 to 25 times above baseline,10–12 with the vertebra adjacent to the previously fractured level at particular risk.13–14 Prevention of progressive kyphotic deformity or correction of existing deformity may therefore be important both in reducing the adjacent level fracture risk and in preventing the consequences of spinal kyphosis such as impaired pulmonary function.
Nonsurgical Treatment
Traditionally, acute osteoporotic vertebral compression fractures have been treated nonsurgically except in rare cases of fractures associated with neurologic compromise or advanced spinal instability. Spinal surgery in the osteoporotic patient is fraught with complications related to the patient’s advanced age and frequent comorbidities and due to the difficulties in securing fixation in osteoporotic bone. Thus the treatment of most patients with painful vertebral compression fractures traditionally includes bed rest, analgesic medications, bracing, antiosteoporotic drugs, or some combination thereof.15–18 Although these treatments appear to be reasonable, anti-inflammatory and narcotic medications are often poorly tolerated by the elderly and may predispose to confusion, increased risk for falling, and gastrointestinal side effects. Bed rest can lead to an overall physiologic deconditioning and acceleration of bone loss. In addition, bracing is typically poorly tolerated by older patients, is expensive, and may further restrict diaphragmatic excursion.
Vertebroplasty and Kyphoplasty
Orthopedic fracture care emphasizes restoring anatomy, correcting deformity, and preserving function. These goals have been largely ignored in the management of patients with osteoporotic spine fractures. Recently, minimally invasive procedures to address the pain and deformity associated with osteoporotic vertebral compression fractures have been developed. Vertebroplasty, involving the percutaneous fluoroscopically guided injection of polymethylmethacrylate (PMMA) directly into a fractured vertebral body, has been used to stabilize osteoporotic vertebral compression fractures. Substantial pain relief in a majority of patients treated with vertebroplasty has been reported.18–25 Kyphoplasty is a minimally invasive procedure that involves the percutaneous insertion of an inflatable bone tamp into a fractured vertebral body under fluoroscopic guidance. Inflation of the bone tamp will elevate the endplates, restoring the vertebral body back toward its original height while creating a cavity to be filled with bone void filler, most commonly PMMA. Results of kyphoplasty suggest significant pain relief, as well as the ability to improve height of the collapsed vertebral body and reduction of spinal kyphosis.15,25–32
When recommending vertebroplasty or kyphoplasty to treat a painful fracture, the vertebral compression fracture must be confirmed as the source of the patient’s back pain. This requires careful correlation of the patient’s history and clinical examination with radiographic documentation of an acute or nonhealed vertebral compression fracture. The physician should treat the symptomatic fracture(s) and should not indiscriminately treat multiple vertebral fractures seen on radiographic studies. Magnetic resonance imaging (MRI) is useful for detecting edema, which may indicate an acute vertebral fracture, and for helping to rule out malignancy or infection. Tumors resulting in vertebral compression fractures are usually associated with an ill-defined margin, enhancement, pedicle involvement, as well as a paravertebral soft tissue mass.33 Sagittal MRI with short tau inversion recovery (STIR) sequences highlights the marrow edema changes associated with acute vertebral compression fractures and is useful in determining the acuity of a vertebral compression fracture.
Indications and Contraindications
Vertebroplasty is designed primarily to relieve pain, and the procedure may be considered when a painful osteoporotic vertebral fracture does not respond to a reasonable period of conservative care. In addition to providing pain relief, kyphoplasty is designed to reduce the fractured vertebra so that intervening before fracture healing offers the best chance of achieving optimal fracture reduction.34 If significant kyphosis is already present at the time of presentation of an acute vertebral compression fracture, we consider kyphoplasty to improve sagittal alignment. In contrast, for patients with acute vertebral compression fractures and relatively minor degrees of vertebral collapse, an initial trial of nonsurgical care may be considered, during which serial radiographs are obtained. If the patient’s pain is incapacitating or does not respond to a period of nonsurgical care, kyphoplasty or vertebroplasty may be recommended. If progressive collapse of the vertebral body is observed during the trial of nonsurgical care, kyphoplasty is recommended. In a recent randomized controlled trial comparing kyphoplasty to nonoperative treatment, a significantly more rapid improvement in quality of life, function, mobility, and pain was observed in the kyphoplasty group.35
Techniques and Results
Vertebroplasty
Vertebroplasty may be performed in a radiology suite or operating room and is typically performed with the use of local anesthesia. The patient is positioned prone with the spine extended by chest and pelvic bolsters. Typically, an 11- to 13-gauge needle is advanced toward the center of the vertebral body using a transpedicular or extrapedicular approach and fluoroscopic guidance. If necessary, biopsy needles can be used to obtain samples before cement injection.36,37 PMMA, the bone cement most commonly used, is mixed with barium for fluoroscopic opacification. Whereas some physicians treat patients with intravenous antibiotics, some physicians add antibiotics to the cement mixture itself, especially when operating on immunocompromised patients.25,32,36,38 When the mixture attains the consistency of toothpaste, the cement is transferred to syringes or specially designed cement injection tools. Between 2 and 10 mL of cement is injected into the vertebral body under live, multidirectional fluoroscopy. Cement injection is stopped if extravertebral extravasation is detected. Ideally, the vertebral body is completely filled with cement, but pain relief has been reported when the anterior two thirds of the vertebra contains cement.32 The patient is not moved from the prone position until the cement has cured. Most patients rest supine under observation for at least 4 hours before discharge.
The mechanism of pain relief after vertebroplasty is not clear. One possible explanation is a mechanical immobilization of the fracture and support to the cortex by the cement.39 Another theory suggests that the heat produced during PMMA polymerization causes deafferentation of the fractured vertebra.
Research on the outcome for vertebroplasty has suggested that most patients experience partial or complete pain relief within 72 hours of the procedure.18–25,36,40,41 Overall, 60% to 100% of patients noted decreased pain after vertebroplasty with pain reduction maintained for months up to 10 years.21,24 In addition to decreased pain, improved functional levels and reduced analgesic medication requirements have been reported.24,36,42–45 Published studies have noted a low complication rate for vertebroplasty, with most complications resulting from extravertebral cement leakage causing spinal cord injury, nerve root compression, or pulmonary embolism.18,19,21–25,36,40,41
The limitations of the vertebroplasty technique relate to the inability of the procedure to correct spinal deformity and the risk of extravertebral cement extravasation during injection. In certain instances, some degree of postural fracture reduction is achieved with vertebroplasty.46–48 During vertebroplasty, the high-pressure injection of low viscosity cement directly into cancellous bone makes it difficult to control cement flow in the vertebral body. This creates an unpredictable risk of cement extravasation outside the vertebral body.32 In fact, extravertebral cement extravasation rates of up to 65% have been reported for vertebroplasty.40 Although a low risk of clinically relevant complications results from cement extravasation, cases of fatal pulmonary embolism and paralysis have been described.49–52
Kyphoplasty
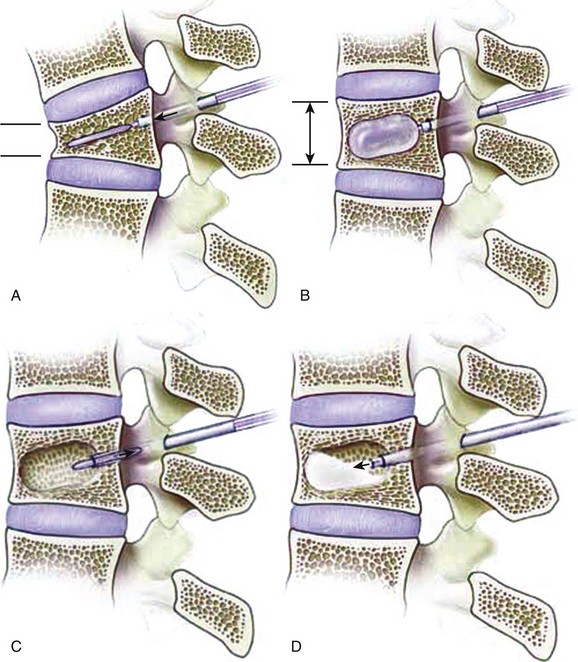
Kyphoplasty involves the percutaneous placement and expansion of an inflatable bone tamp in a fractured vertebral body. The tamp elevates the depressed vertebral body endplate(s), thereby restoring vertebral body height, and also creates a cavity within the vertebral body that is then filled with bone void filler (Fig. 88–1).15,25–32 The patient is positioned prone on a spinal frame. Fluoroscopy is used throughout the procedure, and we have found simultaneous, biplanar fluoroscopy to be advantageous. After needle positioning into the fractured vertebral body through a transpedicular or extrapedicular approach, a series of tools creates a working channel into the vertebral body. Once inserted through the cannula into the vertebral body, the balloon tamp is expanded using visual (fluoroscopy), volume, and pressure (digital manometer) controls. The inflation of the tamp continues until one of the following endpoints is reached: (1) fracture reduction is achieved, (2) the maximal balloon pressure or volume is reached, or (3) cortical wall contact occurs. The balloons are then deflated and removed. Thick cement can then be fed through the cannula under low pressure to fill the void created by the balloon tamp. The cement volume should approximate the volume of the intravertebral cavity. The patient is not moved from the prone position until the leftover cement has solidified.
Garfin and colleagues53 reported the initial multicenter experience with kyphoplasty to treat 2194 vertebral fractures in 1439 patients between 1998 and 2000. Ninety percent of patients reported significant pain relief within 2 weeks of the procedure.53 In this large series of kyphoplasty procedures, a 0.2% per fracture complication rate was observed. Lieberman and colleagues30 observed highly significant improvement in physical function, role physical, vitality, mental health, and social function scores of the SF-36 questionnaire after kyphoplasty. These authors reported five clinically insignificant cement leaks (8% overall).30 Phillips and colleagues34 reported on 29 patients treated with kyphoplasty. In this study, mean visual analog scale (VAS) pain scores decreased from 8.6 preoperatively to 2.6 one week postoperatively to 0.6 one year postoperatively. Cement leaks without apparent clinical consequence occurred in 6 of 61 vertebral levels with no cases of cement leakage into the spinal canal.34
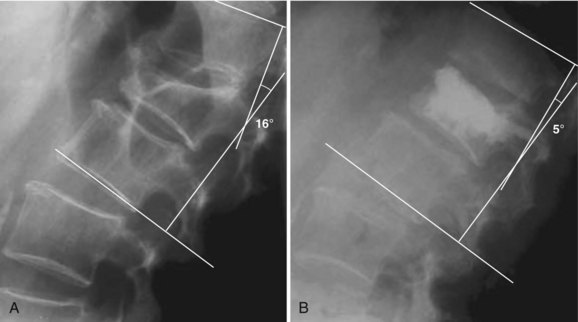
Kyphoplasty has the potential to improve spinal deformity by elevating the vertebral endplates before fixation. In an ex vivo study, Belkhoff and colleagues26 showed a 97% reversal of deformity with kyphoplasty compared with a 30% reversal with vertebroplasty. Lieberman and colleagues30 reported vertebral height restoration in 70% of 70 fractured vertebrae treated with kyphoplasty. In those patients in whom the vertebral fractures were reduced by kyphoplasty, vertebral height was increased by a mean of 46.8%. Wong and colleagues32 and Garfin and colleagues28 similarly noted increased vertebral body height after kyphoplasty. Theodorou and colleagues studied 15 patients (24 fractures) with osteoporotic vertebral compression fractures and reported a mean improvement in kyphosis of 62.4% ± 16.7%.31 Phillips and colleagues34 reported that, in their early experience with kyphoplasty, local sagittal alignment was improved by a mean of 8.8 degrees for all fractures and 14.2 degrees in those fractures that were reduced by at least 5 degrees (Fig. 88–2). Voggenreiter, in a series of 39 patients, reported a kyphosis reduction of 6.5 degrees with intraoperative prone positioning and a subsequent improvement of 3.4 degrees with inflation of the balloon tamp. However, he did note a subsequent loss of 3.1 degrees on upright standing films post operatively.47 Hulme and colleagues,54 in their systematic literature review, reported the average angular correction with kyphoplasty to be 6.6 degrees.
Three recent multistudy analyses have pooled the literature results and complications of kyphoplasty and vertebroplasty. In these analyses, clinical improvement was noted after both kyphoplasty and vertebroplasty.54,55 Kyphosis correction was noted more frequently in kyphoplasty than in vertebroplasty. Overall, complications were rare in both groups. Pooled results suggested the mortality and medical complications were comparable in both procedures.55,56 The rate of cement leakage, asymptomatic and symptomatic, was noted to be higher in vertebroplasty than in kyphoplasty in all three reviews. Lee and colleagues56 reported the symptomatic cement leak rate to be 1.48% in vertebroplasty and 0.04% in kyphoplasty (P < 0.05). These differences are likely due to subtle differences between the techniques. In vertebroplasty, the cement is usually less viscous and injected under a higher pressure and subsequently more difficult to control.
Instrumentation of the Osteoporotic Spine
Cervical Instrumentation
In the subaxial spine, anterior cervical instrumentation is frequently used to maintain alignment and improve fusion rates after anterior bone grafting. Few clinical reports specifically addressing anterior fixation in the osteoporotic spine have been published. Biomechanical studies of anterior cervical plating have shown a direct relationship between bone mineral density (BMD), screw insertion torque, and the axial force generated at the plate-screw junction.57 Because low BMD predisposed to plate pullout, Zink57 suggested that stable anterior cervical fixation with 3.5-mm screws cannot be obtained with a BMD below 150 mg/mL. The risk of anterior cervical plate failure in osteoporotic bone is reduced by increasing screw length and placing bicortical screws.57 The desire to increase stability with bicortical cervical screw fixation should be tempered by the neurologic risk associated with screw penetration of the posterior vertebral body cortex.
Anterior cervical plate-screw constructs spanning multiple levels have been reported to have a high rate of clinical failure, which usually resulted from graft-plate dislodgement.58 Biomechanical studies have confirmed that excessive screw-vertebra motion caused by fatigue at the lower end of a multiple-level corpectomy construct may explain the clinically observed failures at the caudal end of long anterior cervical plate constructs.59 This research also suggested that longer screws, larger diameter screws, and supplemental posterior fixation would potentially decrease screw-vertebra failure.59 These findings seem to be particularly relevant in osteoporotic bone where impaired bone quality would predispose to excessive screw-vertebra motion and failure. DiAngelo and colleagues60 have reported that although multiple-level cervical plating increases construct stiffness after corpectomy, the anterior plate results in the graft being excessively loaded in extension. Because these loads may exceed the adjacent endplate yield strength, pistoning and strut settling into the adjacent vertebral body may occur.60 This problem is accentuated in the osteoporotic spine, where the adjacent vertebral endplates are less able to resist graft settling. When using anterior instrumentation over multiple levels in the osteoporotic cervical spine, the surgeon should attempt to use as many points of fixation as the construct will allow. If the surgeon is concerned that anterior cervical screw purchase is compromised in osteoporotic bone, it may be advisable to proceed with segmental posterior cervical instrumentation to stabilize the anterior arthrodesis.
Dens fractures are the most common condition of the upper cervical spine requiring surgery in the elderly. This population is predisposed to dens fractures by osteoporosis of the dens coupled with the increased rigidity of the subaxial spine as a consequence of age-related degenerative spondylosis.61 Halo immobilization of these injuries has been recommended; however, this is poorly tolerated in the elderly and is associated with a high incidence of nonunion and complications.62,63 Dens fractures in this age group are frequently treated surgically using anterior or posterior approaches. For example, acceptable clinical results have been reported for the widely used technique of posterior C1-2 fusion using either wiring or screw fixation.64,65 Andersson and colleagues66 reported an unacceptable rate of anterior screw fixation failure and nonunion in eight elderly patients with dens fractures. In contrast, Berlemann and Schwarzenbach67 reported a low failure rate and successful healing of the fracture in 16 of 19 elderly patients with dens fractures treated with anterior screw fixation.
Posterior Spinal Instrumentation
Posterior spinal instrumentation may be achieved with plates or rods attached to the spine by means of wire, hooks, or pedicle screws. Coe and colleagues68 studied the modes of failure of these anchors in osteoporotic thoracic spines. Wire fixation failed by cutting through the bone posteriorly. Sublaminar hooks typically failed by pulling through the lamina posteriorly, although 30% of the failures occurred at the pedicle or the pedicle-body junction. Pedicle screws typically failed by screw pullout. Overall, sublaminar hooks showed superior stability compared with wire or pedicle screw constructs. These findings were supported by Butler and colleagues,69 who reported that the performance of hook fixation in the thoracic spine was not adversely affected by osteoporosis, whereas sublaminar wire cutout increased with decreasing BMD.
In current clinical practice, the large majority of posterior instrumentation spinal surgeries performed involve lumbar pedicle screw instrumentation. In the osteoporotic spine, the weak link in the instrumentation construct is the implant-bone interface. Most instrumentation failures involve screw loosening and pullout, which may lead to failure of fusion or the development of recurrent or de novo deformity. Posterior thoracolumbar instrumentation failure has been shown to correlate with BMD.68,70,71 Screw pullout and also cutout through the adjacent endplate with cyclical flexion-extension loading are directly related to BMD and may occur even at physiologic loads in the osteoporotic spine.68,70,71 In a biomechanical study, Soshi and colleagues70 concluded that pedicle screw fixation should be avoided in patients with a BMD less than 0.3 g/cm2.
At the time of pedicle screw insertion, the surgeon may recognize poor screw purchase in osteoporotic bone because of the low insertion torque required to advance the screw. Insertion torque not only correlates with BMD and screw pullout but also predicts early screw failure.72–74 If poor screw purchase is recognized intraoperatively, the surgeon should attempt to salvage the situation rather than rely on inadequate fixation to achieve the goals of instrumentation.
The surgeon may consider increasing the length or diameter of the pedicle screw in an attempt to improve the screw purchase in bone. Increasing screw length does increase screw pullout strength, although this effect may be less pronounced in osteoporotic bone.75,76 The inability to accurately gauge the anterior vertebral body cortex intraoperatively may affect the surgeon’s ability to safely place longer screws because screws extending beyond the anterior vertebral body may predispose to vascular injury. In the sacrum, optimal screw purchase is achieved by directing the screws toward the disc space anteriorly or through the sacral promontory. Increasing screw diameter will also increase pullout strength.75,77–79 Kiner and colleagues80 reported that 8-mm diameter screws demonstrated superior pullout strength than 6-mm screws with cement augmentation. However, the dimensions of the pedicle being cannulated may limit the screw diameter. In the osteoporotic spine, when the screw diameter exceeds 70% of the pedicle diameter, a high risk of pedicle fracture is created.81
In addition to appropriate screw size, optimal screw trajectory can enhance fixation in the osteoporotic spine. In the sagittal plane, screw placement so that the threads engage subchondral bone may provide superior fixation than thread engagement of cancellous bone centrally within the body. In the axial plane, medialization of the screw vector and triangulation of bilateral screws have been demonstrated to increase pullout strength.82 Bilateral triangulated pedicle screws allow the screws to, in effect, hold all of the bone between the screws rather than just the bone within the threads of the individual screws. Ruland and colleagues82 suggested that for triangulated screws to fail simultaneously, a transverse fracture through the vertebral body at the level of the tips of the pedicle screw had to occur. Triangulation of pedicle screws is limited by the bony anatomy. Lower lumbar levels generally allow for more medialization of screws, whereas upper lumbar and thoracic pedicle vectors allow for less.
Undertapping the pedicle in an osteoporotic spine can also enhance screw purchase. By tapping the pedicle with a smaller-diameter tap than the screw, cancellous bone is conserved and can be compacted around the screw threads, providing additional purchase. Although studies have shown that this technique may not be advantageous in patients with normal bone density,83 numerous authors have reported increased insertional torque and pullout resistance when undertapping by 1 mm in the osteoporotic spine.84,85
Another strategy to improve stability of the pedicle screw construct in osteoporotic bone is to distribute forces by increasing the number of fixation points to the spine by including additional levels in the construct. The advantages of this approach must be weighed against the risks and morbidity associated with the additional-level surgery, as well as the potential long-term consequences of a fusion spanning additional levels. The surgeon may also augment the pedicle screw construct with offset sublaminar hooks, which are well suited for use in the osteoporotic spine because they rely on the relatively unaffected cortical laminar bone for fixation.68,86 Biomechanical studies have supported the ability of supplemental sublaminar hooks to increase the rigidity and pullout strength of pedicle screw constructs.87,88
The bone-screw interface may also be improved by injecting PMMA bone cement into the pedicle around the pedicle screw. A twofold to threefold increase in screw pullout has been demonstrated with the use of PMMA injected into the vertebral body through a cannulated pedicle.70,76 Possible risks of this technique include cement extravasation outside the vertebra, with potential for leakage into the spinal canal or neural foramina. Other cements such as hydroxyapatite cement, calcium phosphate, and carbonated apatite have also been shown to enhance the screw-bone interface and increase pedicle screw pullout strength.79,89,90 The disadvantage of using non-PMMA cement is that it often requires 4 to 24 hours to reach maximal stiffness for screw fixation augmentation, whereas PMMA achieves immediate stiffness. In the correction of deformity with various forces applied through the screw, use of non-PMMA cement augmentation and its delayed setting may result in higher risk for immediate fixation failure. Moore and colleagues90 reported that the failure modes seen with PMMA and calcium phosphate cement differed in pullout tests. With PMMA augmentation, pedicle fracture occurred at or near the junction with the vertebral body in 80% (25 of 30) of the samples. In contrast, failure of calcium phosphate augmentation occurred at the cement-screw interface in 80% (24 of 30) of the samples. Cement augmentation of screws has been used in patients with osteoporosis and metastatic spinal tumors undergoing spinal instrumentation with acceptable clinical results and low rates of instrumentation failure.91–94
Novel variations of instrumentation have been developed to enhance pedicle screw fixation. Cannulated screws have been developed with fenestrations toward the tip of the screw. After screw placement, cement is injected into the vertebral body through the screw. Becker and colleagues95 reported that the use of cement augmented, cannulated, fenestrated pedicle screws in cadavers significantly improved pullout strength when compared with standard screw placement. However, the authors noted that cement augmentation with the fenestrated screw resulted in a more posterior final location of the cement within the vertebral body as compared with standard cement augmentation techniques. In 2 of the 10 specimens there was extravasation posteriorly. This occurrence is likely secondary to location of the perforations within the screw.
Alternatively, expandable pedicle screws have been proposed as an alternative technique to enhancing fixation. With these screws, the distal two thirds of the screw is split lengthwise by two perpendicular slots to form four anterior fins when expanded. An expansion peg (a smaller-gauge screw) is threaded into the inner core of the pedicle screw. As the expansion peg advances into the slotted portion of the screw, it spreads and opens up the slotted tip of the screw, creating fins. Withdrawal of the expansion peg collapses the fins, allowing for removal of screw. Ngu and colleagues96 tested expandable pedicle screws and found that while expandable pedicle screws resulted in superior fixation compared with initial standard screw purchase, they were inferior to PMMA augmented pedicle screw fixation. Cook and colleagues97 reported a twofold increase in pullout strength with the expandable pedicle screw and a 250% increase with cement augmentation. In a clinical study of 145 patients who received expandable screw fixation, 21 of these patients were osteoporotic. Expandable screw breakage occurred in 3% of all patients studied, with osteoporotic patients demonstrating a higher screw breakage rate of 5% (1/21 cases, 5/97 screws). Broken expandable screws were difficult to remove. Screw breakage most frequently occurred at the level of the prongs.
Failure of posterior instrumentation in the osteoporotic spine usually occurs through loss of screw fixation by screw toggling, loosening, and eventually pulling out. This may result in a relatively large void around the loose screw that can preclude reusing the same pedicle for revision screw fixation. If revision instrumentation is required, all previously mentioned strategies for enhancing posterior fixation should be considered. In addition, strong consideration should be given to including anterior column structural support and fusion as part of the revision strategy. The anterior vertebral endplates provide a wide surface area that is advantageous for promoting fusion and also for improving the performance of structural struts.98,99 Anterior column support will also help reduce flexion-bending moments on the posteriorly placed instrumentation, thereby reducing risks of instrumentation failure.
Anterior Thoracolumbar Instrumentation
Anterior approaches to the thoracolumbar spine may be indicated in elderly patients with neurologic deficit resulting from anterior pathologic processes such as fracture or tumor, for anterior release with deformity, and to allow for shorter segment fixation. Anterior arthrodesis allows reconstruction of both the anterior and middle spinal columns, as well as placement of bone graft under compression, which provides a favorable mechanical environment for fusion.98–99
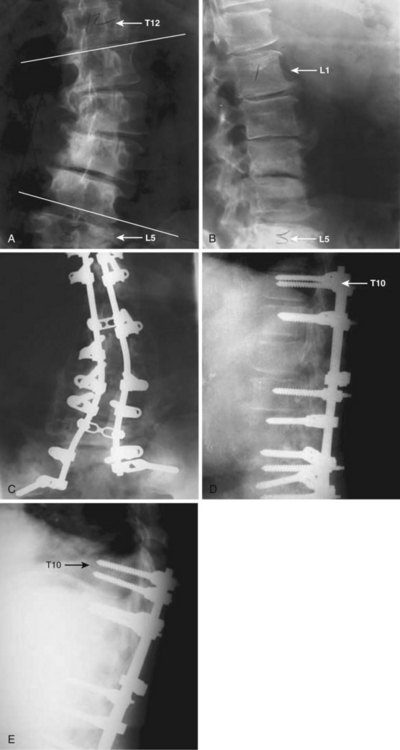
Anterior plate or rod-screw fixation may be used for anterior column reconstruction at the thoracolumbar junction and in the upper lumbar spine. These implants are customarily applied to the lateral aspect of the vertebral body and are less suited for use in the lower lumbar spine because the local vascular anatomy and the pelvis may impede the surgeon’s ability to apply instrumentation. Anterior instrumentation is designed to be load sharing and is typically used in combination with a longitudinally orientated bone graft, strut, or cage to reinforce the deficient anterior column. The use of anterior instrumentation may allow for fusion of fewer mobile segments than might be required with posterior fixation. In osteoporotic patients, achieving adequate fixation with anterior instrumentation is challenging and, in many instances in which anterior column reconstruction is required, combining an anteriorly placed strut with posterior multiple-level segmental fixation may provide a more reliable construct (Fig. 88–3).
In osteoporotic bone, anterior construct failure typically occurs with implant loosening or with subsidence of the intervertebral strut-implant into the cancellous bone of the adjacent vertebral body, which may lead to failure of fusion and recurrence of deformity. Also, intravertebral screws may loosen if fixation is poor, as is common in osteoporotic bone. Lim and colleagues100 correlated anterior screw insertion torque with BMD and correlated BMD with pullout strength of bicortical Kaneda screws placed in the vertebral body. Fixation can be improved by obtaining bicortical purchase of the vertebral body with wide-diameter screws.101 Care must be taken not to penetrate an unfused disc space with the screws. PMMA or biodegradable cement augmentation can also be used to improve screw pullout strength.102,103 The risk of construct failure resulting from the settling of the longitudinal strut or cage into the adjacent vertebral bodies may be reduced by maintaining the integrity of the vertebral endplates during their preparation.104 In addition, small-diameter struts or cages should be avoided because these tend to cut into the vertebral endplates and piston into the vertebral body. If the stability of the anterior reconstruction is questionable, the construct should be supplemented with posterior instrumentation.
Postfusion Junction Failure
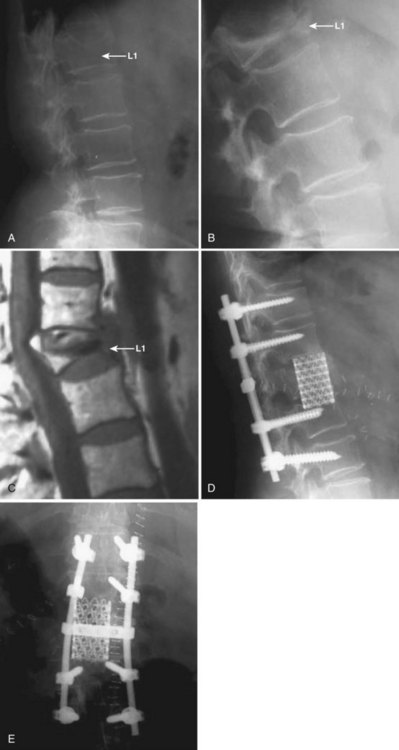
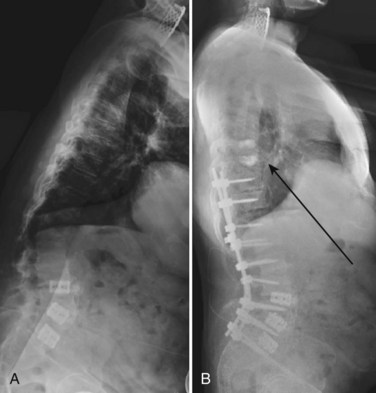
Another concern in the osteoporotic spine is the fate of the vertebra adjacent to an instrumented fusion or the terminal vertebra within a fusion construct. These vertebrae are subject to large forces and may be at increased risk for fracture. The osteoporotic sacrum or pelvis may also fracture after multiple-level, instrumented lumbosacral fusions (Fig. 88–4). A strategy for reducing the risk of adjacent level failure includes the avoidance of ending instrumentation within kyphotic segments.105 Although reports of success are anecdotal at this time, prophylactic PMMA augmentation of the vertebrae adjacent to an instrumented arthrodesis has been championed in at-risk osteoporotic spines (Fig. 88–5).
If fractures are present at vertebral levels that require decompression for spinal stenosis, cement augmentation of the fractured vertebra may prevent postoperative kyphosis and may avoid the need for a concomitant arthrodesis. Singh and colleagues106 reported on 16 patients with lumbar stenosis and osteoporotic compression fractures successfully treated with decompression and vertebral cement augmentation without fusion. Thirteen of the 16 patients reported good to excellent results, with 10 reporting excellent results.
Summary
Pearls
Pitfalls
Key Points
1 Coe JD, Warden KE, Herzig MA, et al. Influence of bone mineral density on the fixation of thoracolumbar implants: A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine. 1990;15:902-907.
2 Garfin SR, Yuan HA, Reiley MA. New technologies in spine: Kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511-1515.
3 Hu SS. Internal fixation in the osteoporotic spine. Spine. 1997;22:43S-48S.
4 Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: Technical aspects. AJNR Am J Neuroradiol. 1997;18:1897-1904.
This report includes a detailed discussion of techniques for performing vertebroplasty procedures.
5 Phillips FM, Ho E, Campbell-Hupp M, et al. Early radiographic and clinical results of balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Spine. 2003;28:2260-2265. discussion 2265-2267
6 Soshi S, Shiba R, Kondo H, et al. An experimental study on transpedicular screw fixation in relation to osteoporosis of the lumbar spine. Spine. 1991;16:1335-1341.
1 Ross PD. Osteoporosis: epidemiology and risk assessment. J Nutr Health Aging. 1998;2:178-183.
2 Iqbal MM, Sobhan T. Osteoporosis: a review. Mo Med. 2002;99:19-24.
3 Johnell O. Advances in osteoporosis: better identification of risk factors can reduce morbidity and mortality. J Intern Med. 1996;239:299-304.
4 Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. Milbank Q. 1989;67:450-484.
5 Gold D, Lyles K. Fractures: effects on quality of life. San Diego. London: Academic; 1999.
6 Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(3 Suppl):185S-189S.
7 Silverman SL. The clinical consequences of vertebral compression fracture. Bone. 1992;13(Suppl 2)):S27-S31.
8 Belmont PJJr, et al. The effects of hook pattern and kyphotic angulation on mechanical strength and apical rod strain in a long-segment posterior construct using a synthetic model. Spine. 2001;26:627-635.
9 White AA3rd, Panjabi MM, Thomas CL. The clinical biomechanics of kyphotic deformities. Clin Orthop Relat Res. 1977;128:8-17.
10 Nevitt MC, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793-800.
11 Nevitt MC, et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Fracture Intervention Trial Research Group. Arch Intern Med. 2000;160:77-85.
12 Wasnich RD. Vertebral fracture epidemiology. Bone. 1996;18(3 Suppl):179S-183S.
13 Haczynski J, Jakimiuk A. Vertebral fractures: a hidden problem of osteoporosis. Med Sci Monit. 2001;7:1108-1117.
14 Ross PD, et al. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919-923.
15 Eck JC, Hodges SD, Humphreys SC. Vertebroplasty: a new treatment strategy for osteoporotic compression fractures. Am J Orthop. 2002;31:123-127. discussion 128
16 Lukert BP. Vertebral compression fractures: how to manage pain, avoid disability. Geriatrics. 1994;49:22-26.
17 Meunier PJ, et al. Diagnosis and management of osteoporosis in postmenopausal women: clinical guidelines. International Committee for Osteoporosis Clinical Guidelines. Clin Ther. 1999;21:1025-1044.
18 Rapado A. General management of vertebral fractures. Bone. 1996;18(3 Suppl):191S-196S.
19 Chiras J, et al. [Percutaneous vertebral surgery. Technics and indications]. J Neuroradiol. 1997;24:45-59.
20 Evans AJ, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology. 2003;226:366-372.
21 Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol. 1994;15:83-86.
22 Hardouin P, et al. Should percutaneous vertebroplasty be used to treat osteoporotic fractures? An update. Joint Bone Spine. 2001;68:216-221.
23 Lapras C, et al. [Percutaneous injection of methyl-metacrylate in osteoporosis and severe vertebral osteolysis (Galibert’s technic)]. Ann Chir. 1989;43:371-376.
24 Mathis JM, Petri M, Naff N. Percutaneous vertebroplasty treatment of steroid-induced osteoporotic compression fractures. Arthritis Rheum. 1998;41:171-175.
25 Watts NB, Harris ST, Genant HK. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int. 2001;12:429-437.
26 Belkoff SM, et al. An ex vivo biomechanical evaluation of an inflatable bone tamp used in the treatment of compression fracture. Spine. 2001;26:151-156.
27 Garfin S. Vertebroplasty and kyphoplasty. In: Savitz MH, Chiu JC, Yeung AT, editors. The Practice of minimally invasive spinal technique. Richmond, VA: AAMISMS Education, 2000.
28 Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511-1515.
29 Ledlie JT, Renfro M. Balloon kyphoplasty: one-year outcomes in vertebral body height restoration, chronic pain, and activity levels. J Neurosurg. 2003;98(1 Suppl):36-42.
30 Lieberman IH, et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631-1638.
31 Theodorou DJ, et al. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging. 2002;26:1-5.
32 Wong W, Reiley M, Garfin S. Vertebroplasty/kyphoplasty. Journal of Women’s Imaging. 2000;2:117-124.
33 Shih TT, Huang KM, Li YW. Solitary vertebral collapse: distinction between benign and malignant causes using MR patterns. J Magn Reson Imaging. 1999;9:635-642.
34 Phillips FM, et al. Early radiographic and clinical results of balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Spine. 2003;28:2260-2265. discussion 2265-2267
35 Wardlaw D, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373:1016-1024.
36 Jensen ME, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897-1904.
37 Minart D, et al. Percutaneous coaxial transpedicular biopsy of vertebral body lesions during vertebroplasty. Neuroradiology. 2001;43:409-412.
38 Mathis JM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol. 2001;22:373-381.
39 Belkoff SM, et al. An in vitro biomechanical evaluation of bone cements used in percutaneous vertebroplasty. Bone. 1999;25(2 Suppl):23S-26S.
40 Cortet B, et al. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol. 1999;26:2222-2228.
41 Cyteval C, et al. Acute osteoporotic vertebral collapse: open study on percutaneous injection of acrylic surgical cement in 20 patients. AJR Am J Roentgenol. 1999;173:1685-1690.
42 Amar AP, et al. Percutaneous transpedicular polymethylmethacrylate vertebroplasty for the treatment of spinal compression fractures. Neurosurgery. 2001;49:1105-1114. discussion 1114-1115
43 Kim AK, et al. Unilateral transpedicular percutaneous vertebroplasty: initial experience. Radiology. 2002;222:737-741.
44 Martin JB, et al. Vertebroplasty: clinical experience and follow-up results. Bone. 1999;25(2 Suppl):11S-15S.
45 Tsou IY, et al. Percutaneous vertebroplasty in the management of osteoporotic vertebral compression fractures: initial experience. Ann Acad Med Singapore. 2002;31:15-20.
46 Chin DK, et al. Efficacy of postural reduction in osteoporotic vertebral compression fractures followed by percutaneous vertebroplasty. Neurosurgery. 2006;58:695-700. discussion 695-700
47 Voggenreiter G. Balloon kyphoplasty is effective in deformity correction of osteoporotic vertebral compression fractures. Spine. 2005;30:2806-2812.
48 Teng MM, et al. Kyphosis correction and height restoration effects of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2003;24:1893-1900.
49 Chen HL, et al. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg. 2002;95:1060-1062. table of contents
50 Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate: a case report. Spine. 2002;27:E419-E422.
51 Moreland DB, Landi MK, Grand W. Vertebroplasty: techniques to avoid complications. Spine J. 2001;1:66-71.
52 Padovani B, et al. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 1999;20:375-377.
53 Garfin S, et al. Retrospective analysis of the outcomes of balloon kyphoplasty to treat vertebral body compression fracture (VCF) refractory to medical management. Eur Spine J. 2001;10(Suppl 1):S7.
54 Hulme PA, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31:1983-2001.
55 Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine. 2006;31:2747-2755.
56 Lee M, et al. Percutaneous Treatment of Vertebral Compression Fractures: A Meta-Analysis of Complications. Spine. 2009 May 15;34:1228-1232.
57 Zink PM. Performance of ventral spondylodesis screws in cervical vertebrae of varying bone mineral density. Spine. 1996;21:45-52.
58 Vaccaro AR, et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11:410-415.
59 Panjabi MM, Isomi T, Wang JL. Loosening at the screw-vertebra junction in multilevel anterior cervical plate constructs. Spine. 1999;24:2383-2388.
60 DiAngelo DJ, et al. Anterior cervical plating reverses load transfer through multilevel strut-grafts. Spine. 2000;25:783-795.
61 Blauth M, et al. [Spinal fractures in the elderly and their treatment]. Orthopade. 2000;29:302-317.
62 Lennarson PJ, et al. Management of type II dens fractures: a case-control study. Spine. 2000;25:1234-1237.
63 Seybold EA, Bayley JC. Functional outcome of surgically and conservatively managed dens fractures. Spine. 1998;23:1837-1845. discussion 1845-1846
64 Anderson LD, D’Alonzo RT. Fractures of the odontoid process of the axis. J Bone Joint Surg Am. 1974;56:1663-1674.
65 Jeanneret B, Magerl F. Primary posterior fusion C1/2 in odontoid fractures: indications, technique, and results of transarticular screw fixation. J Spinal Disord. 1992;5:464-475.
66 Andersson S, Rodrigues M, Olerud C. Odontoid fractures: high complication rate associated with anterior screw fixation in the elderly. Eur Spine J. 2000;9:56-59.
67 Berlemann U, Schwarzenbach O. Dens fractures in the elderly. Results of anterior screw fixation in 19 elderly patients. Acta Orthop Scand. 1997;68:319-324.
68 Coe JD, et al. Influence of bone mineral density on the fixation of thoracolumbar implants. A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine. 1990;15:902-907.
69 Butler TEJr, et al. The strength and stiffness of thoracic implant anchors in osteoporotic spines. Spine. 1994;19:1956-1962.
70 Soshi S, et al. An experimental study on transpedicular screw fixation in relation to osteoporosis of the lumbar spine. Spine. 1991;16:1335-1341.
71 Yamagata M, et al. Mechanical stability of the pedicle screw fixation systems for the lumbar spine. Spine. 1992;17(3 Suppl):S51-S54.
72 WW Lu, et al. Loosening of sacral screw fixation under in vitro fatigue loading. J Orthop Res. 2000;18:808-814.
73 Okuyama K, et al. Stability of transpedicle screwing for the osteoporotic spine. An in vitro study of the mechanical stability. Spine. 1993;18:2240-2245.
74 Zdeblick TA, et al. Anterior spinal fixators. A biomechanical in vitro study. Spine. 1993;18:513-517.
75 Polly DWJr, Orchowski JR, Ellenbogen RG. Revision pedicle screws. Bigger, longer shims—what is best? Spine. 1998;23:1374-1379.
76 Zindrick MR, et al. A biomechanical study of intrapeduncular screw fixation in the lumbosacral spine. Clin Orthop Relat Res. 1986;203:99-112.
77 Brantley AG, et al. The effects of pedicle screw fit. An in vitro study. Spine. 1994;19:1752-1758.
78 McLain RF, et al. The effect of bone quality on pedicle screw loading in axial instability. A synthetic model. Spine. 1997;22:1454-1460.
79 Yerby SA, Toh E, McLain RF. Revision of failed pedicle screws using hydroxyapatite cement. A biomechanical analysis. Spine. 1998;23:1657-1661.
80 Kiner DW, Wybo CD, Sterba W, et al. Biomechanical analysis of different techniques in revision spinal instrumentation: larger diameter screws versus cement augmentation. Spine. 2008;33:2618-2622.
81 Hirano T, et al. Fracture risk during pedicle screw insertion in osteoporotic spine. J Spinal Disord. 1998;11:493-497.
82 Ruland CM, et al. Triangulation of pedicular instrumentation. A biomechanical analysis. Spine. 1991;16(6 Suppl):S270-S276.
83 Halvorson TL, et al. Effects of bone mineral density on pedicle screw fixation. Spine. 1994;19:2415-2420.
84 Carmouche JJ, et al. Effects of pilot hole preparation technique on pedicle screw fixation in different regions of the osteoporotic thoracic and lumbar spine. J Neurosurg Spine. 2005;3:364-370.
85 Kuklo TR, Lehman RAJr. Effect of various tapping diameters on insertion of thoracic pedicle screws: a biomechanical analysis. Spine. 2003;28:2066-2071.
86 Chiba M, et al. Short-segment pedicle instrumentation. Biomechanical analysis of supplemental hook fixation. Spine. 1996;21:288-294.
87 Hasegawa K, et al. An experimental study of a combination method using a pedicle screw and laminar hook for the osteoporotic spine. Spine. 1997;22:958-962. discussion 963
88 Hilibrand AS, Moore DC, Graziano GP. The role of pediculolaminar fixation in compromised pedicle bone. Spine. 1996;21:445-451.
89 Lotz JC, et al. Carbonated apatite cement augmentation of pedicle screw fixation in the lumbar spine. Spine. 1997;22:2716-2723.
90 Moore DC, et al. Restoration of pedicle screw fixation with an in situ setting calcium phosphate cement. Spine. 1997;22:1696-1705.
91 Jang JS, Lee SH, Rhee CH. Polymethylmethacrylate-augmented screw fixation for stabilization in metastatic spinal tumors. Technical note. J Neurosurg. 2002;96(1 Suppl):131-134.
92 Noorda RJ, et al. Severe progressive osteoporotic spine deformity with cardiopulmonary impairment in a young patient. A case report. Spine. 1999;24:489-492.
93 Wuisman PI, et al. Augmentation of (pedicle) screws with calcium apatite cement in patients with severe progressive osteoporotic spinal deformities: an innovative technique. Eur Spine J. 2000;9:528-533.
94 Chang MC, Liu CL, Chen TH. Polymethylmethacrylate augmentation of pedicle screw for osteoporotic spinal surgery: a novel technique. Spine. 2008;33:E317-E324.
95 Becker SCA, Spitaler R, Kropik K, et al. Assessment of different screw augmentation techniques and screw designs in osteoporotic spines. Eur Spine J. 2008;17:1462-1469.
96 Ngu BB, Belkoff SM, Gelb DE, Ludwig SC. A biomechanical comparison of sacral pedicle screw salvage techniques. Spine. 2006;31:E166-E168.
97 Cook SD, Salkeld SL, Stanley T, et al. Biomechanical study of pedicle screw fixation in severely osteoporotic bone. Spine J. 2006;4:402-408.
98 Lund T, et al. Interbody cage stabilisation in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br. 1998;80:351-359.
99 McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81:859-880.
100 Lim TH, et al. Strength of anterior vertebral screw fixation in relationship to bone mineral density. J Spinal Disord. 1995;8:121-125.
101 Spiegel DA, et al. Anterior vertebral screw strain with and without solid interspace support. Spine. 2000;25:2755-2761.
102 Bai B, Kummer FJ, Spivak J. Augmentation of anterior vertebral body screw fixation by an injectable, biodegradable calcium phosphate bone substitute. Spine. 2001;26:2679-2683.
103 Schultheiss M, et al. Enhanced primary stability through additional cementable cannulated rescue screw for anterior thoracolumbar plate application. J Neurosurg. 2003;98(1 Suppl):50-55.
104 McBroom RJ, et al. Prediction of vertebral body compressive fracture using quantitative computed tomography. J Bone Joint Surg Am. 1985;67:1206-1214.
105 Hu SS. Internal fixation in the osteoporotic spine. Spine. 1997;22(24 Suppl):43S-48S.
106 Singh K, et al. Open vertebral cement augmentation combined with lumbar decompression for the operative management of thoracolumbar stenosis secondary to osteoporotic burst fractures. J Spinal Disord Tech. 2005;18:413-419.