CHAPTER 39 Osteoporosis
INTRODUCTION
Osteoporosis is a systemic skeletal disease characterized by compromised bone strength that predisposes the skeleton to fracture. The strength of bone is related to the mass of the bone, the distribution of the mass, the microarchitectural alignment, the age of the bone, and the quality of the bone. The connectivity and microarchitectural alignment of the bone matrix play a critical role in providing strength to the bone. Niebur and colleagues have clearly demonstrated that loss of connectivity or the presence of sites of limited dimension increase the likelihood of local trabecular fracture.1 At the onset of bone formation, the initial bone that is deposited is undermineralized with respect to the final bone product and over time there is increased mineralization and saturation with crystal. The strength of bone increases with aging as a result of this maturation process. High-turnover bone states are associated with increased risk of fracture. Biochemically, bone is a composite material consisting of collagen and mineral. Collagen provides tensile strength, while mineral provides compressive strength. Collagen is biased in its orientation and bone is stronger along the primary axis of the collagen fibers and weaker in the transverse axis. In disease states of altered bone mineral content, such as osteomalacia, rickets, and osteogenesis imperfecta, the strength of bone can be seriously compromised. Consequently, both the quantity and quality of bone profoundly affect the overall strength of bone. Altered performance in either of these two areas can lead to an increased risk of low-energy fracture.
FRACTURE RISK
In the year 2002 an estimated 30 million women aged 50 or older had osteoporosis or were at risk of developing this condition (National Osteoporosis Foundation data). Fifty-five percent of people aged 55 and older have either osteoporosis or low bone mass. With the aging of the population this trend will increase. The lifetime risk of fracture of the hip, wrist, and spine approximates 40%.2–4
BONE BIOLOGY
All individuals increase their bone mass after birth with a peak gain in bone mass occurring at the onset of rapid growth and during adolescence. Final peak bone mass is achieved between the ages of 25 and 30.4 Men can expect to achieve a higher peak bone mass than women. Certain ethnic groups such as African-Americans have greater bone mass than do Caucasian Americans. Once peak bone mass is achieved in women, there is a plateauing of bone mass followed by a very gradual decline in bone mass of approximately 0.3% per year until menopause. At menopause, the rate of bone loss will accelerate and women will lose 2% per year for a short period of time and then resume the gradual decline thereafter. After achieving peak bone mass, men also lose bone gradually. During the period of greatest bone loss, trabecular bone is resorbed at least eight times greater than cortical bone due to the increased surface area of trabecular bone. Fortunately, in an effort to compensate for this loss of bone, the endosteal bone is preferentially lost, leaving the bulk of the bone at the greatest distance from the axial center which takes advantage of the moments of inertia to partially compensate for loss of bone. There is also a gradual increase in the dimensions of the cortical side of bone to partially compensate for this diminished mass. These findings are most evident in the diaphysis of long bones, and are less visible in the metaphyseal regions and in bones with a high proportion of trabecular bone. As bone is resorbed, the individual trabeculae begin to narrow and sites of prominent osteoclastic resorption begin to develop. These areas eventually lead to disconnectivity of the trabeculae. Since these areas of bone loss are not recognized as fractures, there is no effort on the part of the body to heal these trabeculae. As a result, the interconnectivity of the trabeculae declines in the trabecular bone, particularly during times of rapid resorption. The collagen in bone is continuously being turned over through osteoclastic bone resorption. This process is driven by the metabolic state or the presence of a site of imperfection. Osteoclasts work to carve out an area of Howship’s lacunae along the trabeculae or penetrating into the cortical bone. Following osteoclastic resorption, osteoblasts will begin to reestablish the skeleton. After the age of 40, this metabolic bone disease unit is incompletely reconstituted, leading to a net loss of bone through every cycle.
BONE TURNOVER
Calcium and vitamin D metabolism are intimately related and are both a part of the bone turnover cycle. Vitamin D is produced in the skin from 1 hour of sunlight or can be obtained by dietary supplementation. Vitamin D itself is made in the skin and is then transported to the liver where it is converted to 25-hydroxy vitamin D. This form of vitamin D has a half-life of 3 days. Alternatively, the liver can degrade vitamin D by activating the P-450 hydrolase system. Certain drugs that activate the P-450 system can lead to enhanced vitamin D catabolism and in some cases vitamin D deficiency. In particular, this has been well documented with the barbiturates and the seizure medications.5 In the face of low serum calcium levels, the human body activates parathyroid hormone, which travels to the kidney where it stimulates the conversion of 25-hydoxy vitamin D into the active vitamin D metabolite, 1,25 dihydroxy vitamin D. Activated vitamin D will then stimulate the kidney to retain calcium and excrete phosphate. The 1,25 dihydroxy vitamin D also travels to the intestine where it activates a cascade that ultimately leads to the increased absorption of calcium from the diet. Parathyroid hormone also travels to the bone where it activates bone resorption. Osteoblasts have receptors for parathyroid hormone and initiate the Rank-L system that ultimately leads to the development of new osteoclasts via activation of preosteoclasts. Thus, calcium can be elevated by retaining calcium from the kidney, increasing calcium absorption from the gut, and releasing calcium by catabolizing the skeleton. Vitamin D has several other functions, most notably the physiological differentiation of osteoclasts. Vitamin D is also critical in calcium membrane transport in skeletal muscle. Disorders of the kidney such as renal osteodystrophy, which leads to destruction of the kidney, will lower the production of 1,25 dihydroxy vitamin D. Intestinal absorption is compromised in malabsorption syndromes, most notably celiac sprue, Crohn’s disease, and steroid overdose.6 In fact, serological evidence of celiac sprue is found in approximately 9% of osteoporotic patients.7
Calcium plays a critical role in bone homeostasis. As an intracellular signaler, calcium is tightly regulated by the body’s homeostatic mechanisms, and the body will maintain calcium levels at the expense of other tissues. The requirements of calcium vary with age and activity. Simple dairy products such as an 8-ounce glass of milk or a container of yogurt contain 280 mg of calcium. Certain foods rich in calcium, such as spinach, are unavailable for absorption. This results from the high oxalate content in spinach which binds the calcium and prevents its absorption. Calcium requirements for a growing child are 715 mg per day. From the ages of 10 to 25, 1300 mg of calcium are required per day. A healthy adult needs 800 mg per day. A pregnant woman requires 1500 mg of calcium per day, and this requirement increases to 2000 mg per day in lactating women. A postmenopausal woman needs 1500 mg per day, and any individual experiencing negative nitrogen balance or recovering from a major fracture requires 1500 mg per day.8,9
There are a number of drugs that interfere with calcium retention, including cortical steroids, heparin, isoniazid, tetracycline, furosemide, and very large doses of caffeine. Vitamin D nutrition remains critical and studies have demonstrated the efficacy of calcium in the prevention of fractures. Calcium is typically supplemented in conjunction with Vitamin D. A recent review by Dawson-Hughes has indicated that the RDA of 400 units of vitamin D may in fact be insufficient.10 Eight hundred units, particularly in the older patient, may be more reasonable and appears to be the cutoff point at which improved muscle function and a subsequent decrease in falls and fractures were identified. Vitamin D in doses of up to 2000 units per day appear to be safe except in individuals with noted history of multiple kidney stones. Vitamin D can be given either as vitamin D2 or D3 or as final pathway products (1,25 dihydroxy vitamin D). The former will last for 2 months while the latter has a 4-hour half-life. Drugs with hydroxyl groups are degraded in the liver and often activate the P-450 hydrolase system leading to premature degradation of vitamin D. Most notable in this group are the antiepileptic seizure medications. In patients taking antiepileptics or any other drug that is degraded in the liver, vitamin D metabolism may be disrupted and higher dosages of vitamin D may be warranted.11
Peak bone mass is very closely associated with menstrual status. Women who have normal menstrual cycles of 10 episodes per year of approximately 4 days per episode are able to maintain their skeletal integrity and will lose bone at a maximal rate of 0.3% per year.12 This loss can be partially compensated with better nutrition and supplemental calcium. Women who are amenorrheic or who have irregular menstrual cycles may lose bone at a rate of up to 2% per year. In the young amenorrheic individual who has yet to achieve peak bone mass, there is a diminished ability to gain bone at precisely the time when one should be increasing her peak bone mass. Primary amenorrhea is defined as the absence of menses before the age of 16; however, women who have irregular or no periods after the age of 14 are susceptible to compromises of skeletal integrity. Common disorders that affect the menstrual cycle are low body weight, eating disorders, and excessive exercise. Young athletes who lack a normal menstrual cycle have an increased rate of stress fracture compared to women who have a normal menstrual cycle.13 Birth control pills, while reestablishing cycles, may not be totally successful in protecting the skeleton.14 Low body mass is frequently the common factor in most of these disorders and good nutrition is critical, particularly during skeleton growth and development.
Mechanical factors affect bone mass. Bone responds to mechanical load by increasing bone mass and remodeling along lines of increased stress. Bone that lacks stress suffers a decline in bone mass. Normal load bearing will maintain the skeleton. Elevated loading will lead to changes in the skeleton as well. This is found primarily in bones of younger individuals with thick periosteum and decreases with aging and in those bones with limited periosteum. Very high loads overcome the ability of the body to adjust and can lead to stress fractures. In growing individuals up to the age of 30, exercise can lead to enhanced bone mass. High-impact programs have led to almost an 8% increase in bone mass compared to peers without similar programs.15 Once individuals reach a postmenopausal state, exercise rarely changes bone density. However, detailed studies have suggested that bone turnover is in fact decreased in such individuals. They have a lower rate of falling and, when corrected for the number of falls, the fracture rate is decreased. N-telopeptide, a measure of bone turnover, is similarly decreased in those individuals who exercise.16
BONE MASS
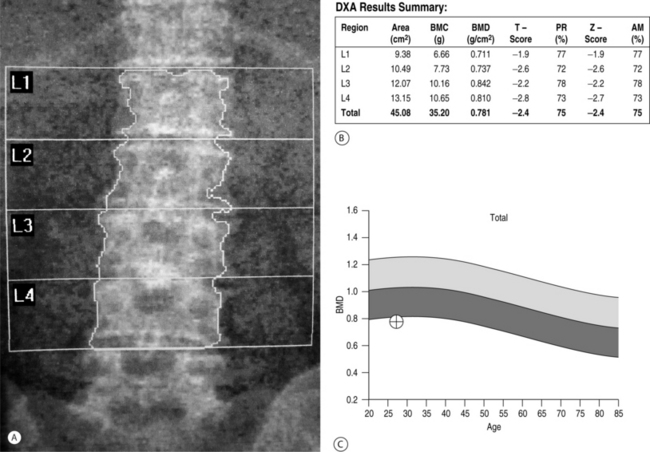
Low bone mass is the single most accurate predictor of increased fracture risk. Bone mineral density is used to establish a diagnosis of postmenopausal osteoporosis and can predict future fracture risk. In the National Osteoporosis Risk Assessment (NORA) study, approximately 150 000 women were evaluated for 1 year in order to define a critical role for bone density in predicting fracture risk.17 Results from this study suggested a strong continuous relationship between lower bone mineral density and higher fracture rate as expressed by the number of women who experienced fractured vertebrae. Fracture rates were highest among women with the lowest T-scores. Bone density can be determined by dual-energy X-ray absorptiometry (DEXA scan), quantitative computed tomography (q-CT), peripheral measurements, and ultrasound. Of these modalities, DEXA (Fig. 39.1) is the most accurate and is considered to be the gold standard.18 Furthermore, DEXA is the most common form of bone density utilized in centers that measure the area of bone density of the spine and several parameters within the hip, most notably, the total femur, femoral neck, and intertrochanteric area. During DEXA scanning, an intervertebral assessment of the spine (Fig. 39.2) can be performed to assess the anatomic detail of the thoracic and lumbar spine and determine if vertebral compression is present. The area of mineral content is then compared with age-corrected peers in order to obtain a Z-value and then with an ideal peak bone mass in a normal individual not corrected for age to obtain a T-value. The precision of DEXA is approximately 2% in the spine and a little over 3% in the hip. It is important to recognize that the presence of scoliosis or arthritis can artificially elevate DEXA scores in the spine. The precision of q-CT is less than that of DEXA and the radiation exposure is greater. Peripheral bone mass evaluations have only a 70% correlation with the hip and the spine. The World Health Organization has defined a normal bone density as a T-value of less than 1 standard deviation compared to normals.19 Osteopenia is defined as bone mass between 1 standard deviation and 2.4 standard deviations below normal. Osteoporosis is classified as a bone deficiency of 2.5 or more standard deviations. The risk of fracture is increased twofold in the spine and 2.5 times in the hip for each standard deviation below peak bone mass.20 In recent protocols, however, these values are used for epidemiological and demographic studies, and treatment protocols call for an earlier intervention with far less bone deficiency. The Z-value becomes critical in differentiating primary osteoporosis from secondary forms of osteoporosis. In particular, Z values of 1.5 or worse should be evaluated for secondary forms of osteoporosis. Strength of bone is also related to turnover and other aspects of bone quality. While the fracture rate is greatest in those individuals with the lowest bone mass, there are individuals with apparently relatively normal levels of bone mass who in fact have vertebral and hip fractures. Patients with hip or vertebral fractures are more likely to experience future fractures than are patients with no fractures. The risk of vertebral fracture rises rapidly with age for both men and women. In the United States women are two to three times more likely to experience a vertebral fracture than men, but when corrected for age, this disparity disappears.
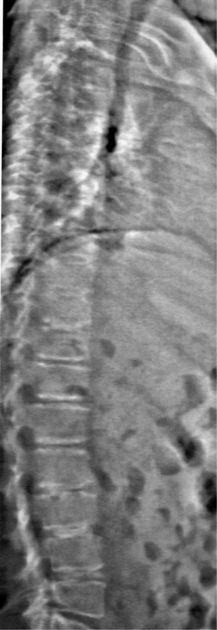
Fig. 39.2 An intervertebral assessment of the spine can be obtained during DEXA scanning to provide additional anatomic detail of the thoracic and lumbar spine and allow for the identification of vertebral compression fractures. The film is from the same patient as in Figure 39.1.
Biochemical markers have been introduced to evaluate and differentiate between high- and low-turnover osteoporosis. Bone resorption markers include collagen breakdown products – most notably, the N-telopeptides and C-telopeptides in the pyridinoline collagen cross-link products. These components are not degraded by the body and can be detected in both the serum and the urine. Bone formation markers are more popularly directed toward bone-specific alkaline phosphatase and osteocalcin. Bone resorption markers appear to have better correlation with fracture risk. The bone formation markers are disease specific and often used to test the efficacy of treatment interventions. The overall risk for a low-energy fracture is related to high-turnover states and low bone mass. Additional independent risk factors include low body weight, recent loss of body weight, personal history of fragility fracture, a first-degree relative with a fragility fracture, and smoking.21 In individuals with low body weight (persons less than 127 lb for a 5′ 5 individual), there is inadequate body fat to sustain extraovarian production of estrogen. Recent loss of body weight correlates with an increased number of bone resorption sites within the trabecular skeleton, and this weakens the trabecular microarchitecture and increases the risk of fracture. A personal history of fragility fracture or a family history might suggest genetic causes such as variations in collagen quality or quantity. Smoking is associated with inadequate nutrition and enhanced destruction of endogenous estrogen. Additional risk factors which have been identified include impaired vision, estrogen deficiency at an early age, dementia, poor health and fragility, recent falls, low calcium, lifetime history of low calcium intake, low physical activity, and alcohol intake in excess of two drinks per day. Lastly, individuals taking drugs that are known to cause osteoporosis (e.g. glucocorticoid steroids) and patients with diseases associated with osteoporosis (e.g. polio and various high bone turnover states) are also at high risk.
WORK-UP OF A PATIENT WITH OSTEOPOROSIS
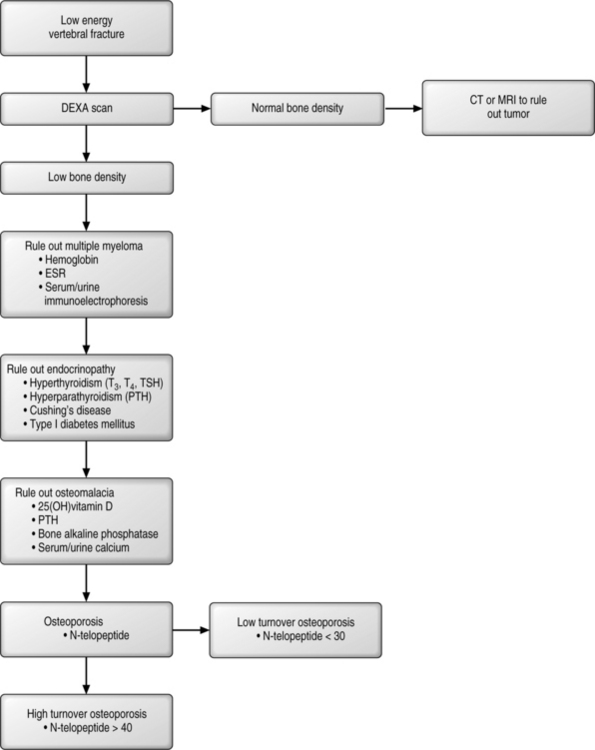
A patient who presents with a fracture should be queried as to whether it was a high-energy or low-energy event (Fig. 39.3). All low-energy fractures should be considered pathologic, stemming either from a local disease or a deranged metabolic state. If the patient does not have evidence of decreased bone density then a careful examination of the fracture should include special imaging studies (CT scan or MRI) to rule out the possibility of a primary or metastatic tumor. To begin the work-up, a complete blood count with differential, a sedimentation rate, and immunoelectrophoresis of both urine and blood should be obtained as these studies can help to rule out marrow disorders. Bone marrow disorders can account for up to 1–2% of pathologic fractures. Multiple myeloma currently affects 400 000 patients in the United States, and this number dwarfs the prevalence of osteogenic sarcomas (600 people per year). Multiple myeloma can be diagnosed by serum blood studies in 80% of patients, while the remaining 20% will have mild abnormalities on serum analysis followed by confirmatory testing of urine. Anemia and an increased sedimentation rate demands a work-up for multiple myeloma.
Among the endocrinopathies, the common forms aside from early menopause include hypoparathyroidism, hyperparathyroidism, type 1 diabetes, and Cushing’s disease. Primary hyperparathyroidism occurs in 1 in 200 women and is diagnosed with elevated calcium.22 Hyperthyroidism is often iatrogenic, typically arising in individuals who are being treated for low thyroid function who take excessive doses of thyroid replacement hormone, which shuts off TSH and increases bone turnover. High turnover is associated with loss of bone in each cycle. Type 2 diabetes is associated with high insulin, while Type 1 diabetes is associated with low insulin. Insulin is a growth factor necessary for bone health; therefore, type 1 diabetes, with low insulin and a loss of calcium in the urine, is frequently associated with osteoporosis. Excessive steroids used in the treatment of an array of diseases can lead to iatrogenic osteoporosis. Corticosteroids lead to decreased absorption of calcium across the gut, increased urinary calcium loss, decreased osteoblastic bone formation, and increased bone resorption and secondary hyperparathyroidism. Avenues of treatment include increased therapeutic doses of vitamin D, calcium-retaining diuretics, and agents that decrease bone resorption such as bisphosphonates (see below). There may be a possible role for therapeutic PTH in this disorder but this claim is premature at this period in time. Work-up for this group of patients should include a T-3, T-4, TSH-Irma, Intact-PTH, fasting glucose levels, and an investigation of present or past steroid use.
Osteomalacia, or failure of mineralization, is a common entity in the United States. Among the hip fracture patients, up to 40–50% of patients will demonstrate this disorder. Even in patients undergoing primary hip replacement without existing apparent mineral disorders, approximately 20% have evidence of osteomalacia.23 The causes of osteomalacia are nutritional, vitamin D deficiency, and disorders of intestinal absorption of vitamin D, such as celiac sprue. This latter entity occurs in 1 in 22 patients presenting with osteoporosis and at a much higher rate in those individuals with milk intolerance.24 Other diseases include renal osteodystrophy, renal tubal acidosis, and defects of vitamin D metabolism caused by drugs such as seizure medications. Laboratory tests that are diagnostic include an elevated intact PTH and a low 25-hydroxy vitamin D. An elevated bone alkaline phosphatase, decreased serum phosphate (except in renal disease), and low serum calcium also suggest abnormal vitamin D metabolism. In the setting of a fracture, alkaline phosphatase will rise and peak, usually within 5 days of the fracture. Therefore, in the setting of a new fracture, an elevated alkaline phosphatase becomes very meaningful if the evaluation is carried out 2–3 weeks after the fracture has occurred. It is important to recognize that alkaline phosphatase may be elevated simply because of the repair process itself and may not correlate with osteomalacia. Conversely, Intact-PTH and 25-hydroxy vitamin D are the markers of choice and are valid indicators of mineral metabolism. Confirmation of low vitamin D levels can be demonstrated by low urinary calcium, typically at a level of less than 100 mg per liter of urine.
Thus, the work-up for metabolic bone disease should include eliciting a history of low-energy fragility fracture. A further work-up should include a complete blood count with differential, immunoelectrophoresis, and evaluation for an underlying endocrinopathy or osteomalacia. Once this has been performed attention should then be directed to differentiating between high or low turnover since the treatment modalities are different. Bone markers, most importantly collagen breakdown products, may be of assistance in this endeavor. For example, the normal range of urinary N-telopeptide is 5–65 nm BCE/mm CRT; however, in healthy individuals the N-telopeptide is usually 20–40 nm. Values over 40 but less than 65 may indicate a high-turnover bone loss state, and values over 65 represent a pathological bone resorption state. Individuals who present with low N-telopeptide and osteoporosis suffer from a low-turnover state with the primary defect being inadequate bone formation rather than increased bone resorption.
BIOMECHANICS AND FRACTURES
Fractures are often related to injuries. Greenspan and colleagues demonstrated that the odds ratio is increased with falls to the side by 5.7 in the ambulatory patients,25 and by 2.7 for every standard deviation below peak bone mass. In a nursing home setting, however, a fall to the side is associated with a 21-fold increase risk of fracture.26 Aharonoff et al., in a series of hip fractures, found that 75% fell while standing, 3.5% fell down stairs, 7% fell out of a bed or a chair, 3% were involved in a car or a bike accident, 2.5% in assault, and 9% from other issues.27 Most falls occurred at home in the afternoon but there was no evidence of a seasonal variation. Myers and Wilson reviewed falls and injuries to the spine.28 They found that patients over the age of 60 with symptomatic vertebral fractures could recount a clear fall in 50% of cases and could report a controlled activity, such as reaching, bending or lifting, in 20% of cases. Only 30% could not identify an initiating event.
The epidemiology of vertebral fractures is continuously under evolution, often because there is a lack of universal clinical definition. There are 1.5 million low-fragility fractures in the United States each year, of which 700 000 occur in the spine, and only one-third of which are symptomatic.29 Some patients may present with either no symptoms or such trivial symptomatology that they are unaware that a fracture has occurred. Drug trials have helped to redefine vertebral fractures, which can be defined as a new fracture in which there is a 20% collapse of the vertebral body or a 40% decrease in the total area of the vertebral body. When less dramatic changes were used, the diagnostic precision decreased dramatically. The FDA has now accepted the former definition. Clinically significant fractures have a very loose description, ranging from patients with mild back pain to those whose pain requires narcotics or interventions. The majority of patients with new vertebral fractures go undiagnosed. Quite often, the recognition of the fracture occurs due to the patient’s awareness of loss of height. Height loss of up to 2 can occur naturally from narrowing of the disc space and some physiological kyphosis. Changes of more than 2 suggest the presence of osteoporosis. Other diseases include kyphosis, scoliosis, and spondylolisthesis. Vertebral fractures occur more commonly from spontaneous minor trauma such as anterior loading secondary to gravitational forces during daily activities. Myers and Wilson identified a safety factor indicating that once the load surpasses the strength of the bone, the bone is at risk.28 There is a clear correlation between decreasing bone density and the ability to handle loads anterior to the body. Risk factors associated with vertebral fractures include postmenopausal women over the age of 55, low bone mass evaluations, diagnosis of osteoporosis, prominent thoracic kyphosis, loss of 2″ of height, and glucocorticoid steroids. The most common locations for vertebral fractures are in the midthoracic range and at the thoracolumbar junction. These correspond to the most compromised regions of the spine. A history of prior vertebral fractures places a patient at risk for future vertebral fracture. Lindsay et al. showed that in those individuals who have had a fracture, a subsequent fracture within the next year occurred in 14–20%.30 Drug therapies have a profound ability to decrease the risk of vertebral fracture (see below). Fractures that occur proximal to T4 or fractures in men and women less than 50 years of age with little or no trauma should raise the clinician’s suspicion for an osteoporotic state. Similarly, fractures in a population at low risk such as men, blacks, and Latino women require special studies to rule out other etiologies, most notably tumorous conditions.
There are three forms of low-energy vertebral fractures including wedge, biconcave, and crush. The European Prospective Osteoporosis Study trial evaluated vertebral deformity and future fracture risk in men and women.31 Over 1500 men and women were evaluated in Europe. The prevalence of all deformities were 12% in females and 12% in males, and the prevalence increased with age for both sexes, while the rate of increase was larger in the female population.
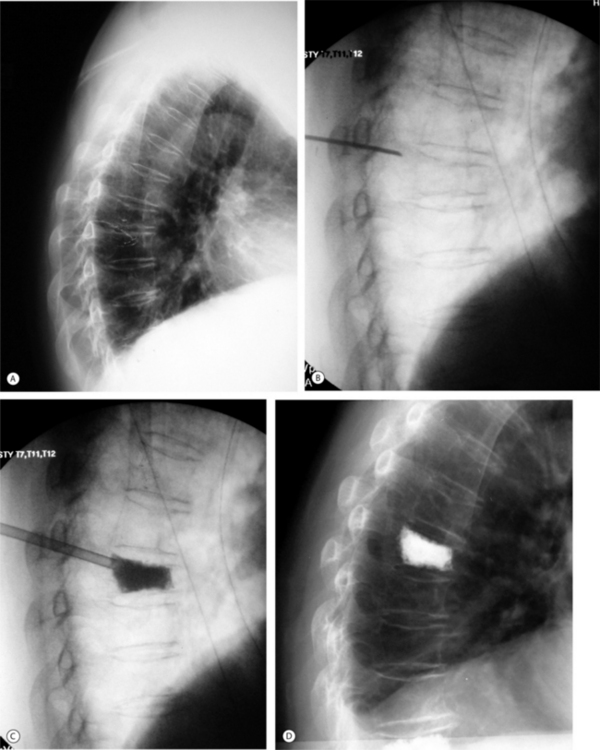
The consequences of vertebral fractures can affect the physical well-being of the patient. Vertebral fractures may cause severe pain, kyphosis deformity, loss of height, impaired physical function, and decreased lung capacity. Leidig et al. studied 63 postmenopausal women with at least one vertebral fracture and showed that pain and disability was significantly greater in women with a relatively greater height loss and a high degree of kyphosis compared to less height loss and medium kyphosis.32 With respect to pulmonary compromise, Leech et al. found that both decreased lung capacity and reduced pulmonary function are serious complications of multiple vertebral fractures and resultant kyphosis.33 Studies suggest that there may be a relationship between vertebral height loss and decreased lung function. In the review of vertebral fractures, Kado et al. showed an increased risk of death following fracture, including 11 000 excessive deaths per year for 5 years following fracture.34 Hip fracture patients have 33 000 excessive deaths in the first year, and vertebral compression fractures are associated with more deaths than hip fractures. Furthermore, the study showed that patients with a moderate fracture had a 23% increased death rate and patients with a severe fracture had a 37% increased risk of death compared to osteoporotic patients without vertebral compression fractures. The study found that the deaths were strongly associated with pulmonary disease. Other problems patients with vertebral fractures encounter include severe pain, deformity, gait alterations resulting in an increased risk of falls, social role alterations, clinical depression including discomfort, anxiety about the future, and compromise of a number of quality of life parameters. A final issue for these patients is the change in posture that accompanies vertebral wedge fractures. If the kyphosis is associated with a wedge fracture, it can be compensated by hyperlordosis. Often, these patients can function quite well in terms of gait parameters. However, if the patient has limited flexibility of the lumbar spine or preexisting spinal stenosis, the kyphosis cannot be compensated. In these instances, the head is thrust forward, the center of gravity is shifted in front of the ankle, and the patient has a decreased performance in the 6-minute walking test, the ‘get up and go’ test, and the single stance time test. In an effort to compensate for the progressive kyphosis, the patient can aggravate preexisting asymptomatic or controllable spinal stenosis due to his/her intent to acquire hyperlordosis. A relatively recent advancement in the treatment of vertebral compression fractures features minimally invasive kyphoplasty, which aims to reduce the fracture, restore vertebral height and alignment, and provide a solid foundation to maintain fracture reduction and prevent future compression fracture (Fig. 39.4).
AGENTS IN THE TREATMENT OF OSTEOPOROSIS
Therapeutic agents for osteoporosis fall into two groups: those which are antiresorptive and those which stimulate bone anabolic (Table 39.1). Antiresorptive agents include the estrogens and the selective estrogen receptor modulators (SERMs), calcitonin, and the family of bisphosphonates, including alendronate and risedronate. Oral bisphosphonates include alendronate and risedronate, and intravenous agents include pamidronate and zolendronate. Bone stimulatory agents have in the past included largely the experimental fluorides and the PTH-rP analogs. The recent release by the FDA of the recombinant PTH now provides a strong anabolic agent. As might be expected, the antiresorptive agents work primarily in those patients with high resorptive rates as evidenced by increased collagen breakdown products, whereas the anabolic agents are most effective for those patients with low bone turnover states. The first line of therapy against osteoporosis remains dietary supplementation with calcium and vitamin D. These agents in combination can decrease bone resorption and can lead to a better quality of bone mineralization. Numerous studies have demonstrated that in combination these agents can decrease hip fractures on the order of 25% in nursing home populations.35 This occurs even in the situation where the bone density may not increase or could in fact decrease slightly. In the subpopulation that presents with osteomalacia, 20–40% will respond to these agents. In addition, calcium is critical for the efficacy of the antiresorptive agents, and the bisphosphonates will not work in the absence of calcium supplementation. The benefits of calcium carbonate versus calcium citrate have been discussed previously. The carbonates have the highest calcium content, but are associated with constipation and flatulence. As well, in the absence of normal gastric acidity, calcium carbonate is poorly absorbed. Consequently, patients on H2 blockers are poor candidates for calcium carbonate. In contrast, calcium citrate dissolves in all ranges of gastric acidity and clearly decreases the risk of kidney stones. Target doses of vitamin D are 800 units per day in patients older than 60 and greater than 1000 units per day in patients taking drugs that increase vitamin D degradation (e.g. antiepileptic medications).
| CALCIUM SUPPLEMENTATION | |
† Off-label use in the United States.
‡ The reduction of osteoporotic fracture risk in postmenopausal women is no longer an FDA-supported indication for estrogen replacement.
Hormone therapy
Estrogen hormone therapy has been used for over 40 years. Based on numerous studies, including the Women’s Health Initiative, there is now very clear evidence that hormone therapy with or without progesterone can decrease all fractures by approximately 35%.36 However, it carries with it some inherent problems. Specifically, it may lead to an increased risk of stroke, heart attack, phlebitis, pulmonary emboli, and a significantly increased risk of breast cancer. The breast cancer risk is increased up to 40% after 10 years of therapy and even stopping estrogen still carries the risk. Recent data would suggest that even in the absence of progestin, estrogen is deleterious, and there is some suggestion that it may increase the risk of dementia. Thus, the FDA no longer accepts estrogen in the treatment of osteoporosis, and its use should only be for those conditions that require estrogen such as postmenopausal symptomatology. From the perspective of the bone cell, SERMS, such as tamoxifen, are estrogen agonists. Seventy percent of patients on tamoxifen will experience the beneficial effects of estrogen. Tamoxifen cannot be used in the treatment of osteoporosis because it has profound postmenopausal symptomatology and has a very high rate of secondary uterine cancer. The most commonly used SERM is raloxifene. This agent has been shown to increase the bone mass in the spine and decrease the fracture rate by 40%.37 There is no risk of uterine cancer and the risk of breast cancer is decreased, but there is an increased risk of thrombophlebitis and pulmonary embolism. In addition, there is no protection against hip fracture. Therefore, this agent only works in the spine and does not appear to function in the areas of nonvertebral fracture.
Calcitonin
Calcitonin is an agent which can be delivered through a nasal administration. In a study of 100 versus 200 versus 400 international units per day, the 200-unit dose decreased spinal fractures by 33% but there was no statistical protection at the low or high dose, and at no dose was there protection against hip fractures.38 Calcitonin appears to have some role in the pain pathway and therefore is used often in those cases of painful osteoporosis but clearly its benefit is quite meager compared to the bisphosphonates and Intact-PTH. A major side effect is one of nasal irritation and occasional nosebleeds.
Bisphosphonates
Bisphosphonates are a family of drugs which are analogs of pyrophosphate. The central oxygen between the two phosphates is substituted with a carbon and then a side chain of variable length, which may or may not contain a ring structure or nitrogen group, is added. Bisphosphonates are nondegradable. They bind the surface of bone beneath osteoclasts and prevent the resorption of bone by acting as a biochemical shield. If imbibed by the osteoclasts they either interfere with the lipid membrane production or with the cell cycle leading to premature death of the osteoclast via apoptosis. Thus, they are inhibitors of osteoclasts and lead to apoptosis of the osteoclasts. The second- and third-generation bisphosphonates seem to disassociate the inhibition of resorption from the inhibition of bone formation, with resorption being more markedly affected than formation. Bisphosphonates have been shown to decrease all fractures in all bones by about 50%.39–41 They are more effective in those individuals with previous vertebral fractures but they can decrease hip fractures by 50% within 6 months of therapy. They are acidic medications and must be taken on an empty stomach, and less than 1% of the medication is absorbed. Dyspepsia initially occurred at a rate of 30%, but this side effect can be decreased dramatically by switching the medications from a daily dose to a single weekly dose in the case of risedronate and the alendronate. Bisphosphonates also have shown a benefit in the treatment of men with osteoporosis, children with osteogenesis imperfecta, osteonecrosis, prosthetic loosening, and steroid-induced osteoporosis. Alendronate has been used for the longest period of time. In a recent study, 3658 women with osteoporosis and an existing vertebral fracture or osteoporosis of the femoral neck without vertebral fracture were treated for 3–4 years with alendronate.42 The investigators found that the pooled group of patients treated with alendronate demonstrated a diminished relative risk of hip fracture (0.47 relative risk), radiographic vertebral fracture (0.52 relative risk), clinical vertebral fracture (0.55 relative risk), and all other clinical features (0.70 relative risk).
Studies in animal models have suggested treatment with high doses of bisphosphonates results in the accumulation of microdamage.43 This microdamage can be measured, with loss of toughness as the only biomechanical parameter, and has given rise to concerns about the long-term benefit of these agents. Current recommendations under consideration include a rest after 5 years of therapy once the benefit to the patient has reached a plateau, with a resumption of the medication once the bone resorption markers begin to rise again. There have been no randomized, controlled studies from which to develop clear recommendations; however, there is a clear concern by the bone biologists about long-term continued use.
A number of individuals cannot tolerate oral bisphosphonates due to poor physiology of the esophagus or clinically significant esophagitis. In those individuals, intravenous bisphosphonates, most notably pamidronate and zolendronate, appear to provide a comparable decline in resorption markers and increase in bone density to that of alendronate and risedronate. Of interest is that a 4 mg dose of the zolendronate may be given once a year leading to protection of the skeleton for at least 12 and perhaps 18 months.44 The efficacy of treatment with bisphosphonates should not be judged by increasing bone density. Studies have indicated that if one breaks down the bisphosphonate users into low-dose users who gained a low amount of bone mass and high-dose users who gained a high amount of bone, there is the same fracture potential in both groups. Therefore, only a small percentage of the protection against fractures is actually related to gains in bone density while the remainder is due to other changes in bone quality and turnover.
Parathyroid hormone
Parathyroid hormone (PTH) is the only proven anabolic agent for the treatment of osteoporosis. In continuous doses, PTH is associated with high levels of bone resorption and a gradual loss of bone. However, when given in a pulsatile fashion lasting only 15–30 minutes, it will stimulate osteoblasts and will have very little effect on the osteoclast recruitment. A shortened version of PTH that includes the N-terminal 1–34 amino acids has been on trial and is now in active use. At a dose of 20 μg per day this can increase the bone mass up to 8% in the spine and about half that level in the hip.45 Hip fracture protection occurs from 10–12 months which is slightly longer than with the bisphosphonates. PTH is a daily subcutaneous injection, and its side effects include cramps, hypertension, and headaches.
Clearly, bisphosphonates can prevent fractures and prevent disuse osteoporosis. Several studies have demonstrated that bisphosphonates do not adversely affect fracture healing in animals.46,47 However, in these studies, the data suggested that bisphosphonates have a profound effect on the fracture remodeling process. There is a delay in conversion of woven bone to lamellar bone and all markers of progression are prolonged. However, the callus is much larger, and when tested biomechanically, the fracture seems to be of equal strength to those of control subjects. These studies have been performed in animals that always heal their fractures, and there are few data in the compromised patient or in the compromised animal. PTH, on the other hand, has been shown in many studies to enhance fracture healing so that the maturation is faster than in controls, and the biomechanics of every stage of healing are superior to those in the control population.48 Therefore, PTH may augment fracture healing. Bisphosphonates, in most circumstances, probably have such a small effect on the fracture healing process that they are of little consequence, particularly in those fractures which heal in a timely manner and are located in the metaphyseal/diaphyseal area. In the compromised patient or in those patients with fractures that have a track record of poor healing, bisphosphonates may be more deleterious. An alternative would be to hold off on bisphosphonates for 3–6 weeks in those fractures that heal regularly and hold off entirely in those fractures that are compromised. There are no data regarding the effect of bisphosphonates or PTH on spine fusion. As bone healing in spine fusion is much more difficult than long bone fractures, caution should be urged in the use of bisphosphonates until further data are available, particularly in cases of long spine fusions of compromised individuals.
1 Niebur GL, Feldstein MJ, Keaveny TM. Biaxial failure behavior of bovine tibial trabecular bone. J Biomech Eng. 2002;124(6):699-705.
2 Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(Suppl 1):58-63.
3 Oden A, Dawson A, Dere W, et al. Lifetime risk of hip fractures is underestimated. Osteoporos Int. 1998;8(6):599-603.
4 Lin JT, Lane JM. Osteoporosis: a review. Clin Orthop. 2004;425:126-134.
5 Schaefer K, von Herrath D, Kraft D. Disordered calcium metabolism during anticonvulsant treatment. Ger Med. 1973;3(3–4):140-144.
6 Vestergaard P. Bone loss associated with gastrointestinal disease: prevalence and pathogenesis. Eur J Gastroenterol Hepatol. 2003;15(8):851-856.
7 Nuti R, Martini G, Valenti R, et al. Prevalence of undiagnosed coeliac syndrome in osteoporotic women. J Intern Med. 2001;250(4):361-366.
8 Heaney RP. Calcium intake and bone health throughout life. J Am Med Womens Assoc. 1990;45(3):80-86.
9 Nordin BE. Calcium and osteoporosis. Nutrition. 1997;13(7–8):664-686.
10 Dawson-Hughes B. Calcium and vitamin D nutritional needs of elderly women. J Nutr. 1996;126(4 Suppl):1165S-1167S.
11 Mikati M, Wakim RH, Fayad M. Symptomatic antiepileptic drug associated vitamin D deficiency in noninstitutionalized patients: an under-diagnosed disorder. J Med Liban. 2003;51(2):71-73.
12 Beals KA, Brey RA, Gonyou JB. Understanding the female athlete triad: eating disorders, amenorrhea, and osteoporosis. J Sch Health. 1999;69(8):337-340.
13 Sanborn CF, Horea M, Siemers BJ, et al. Disordered eating and the female athlete triad. 2000;19(2):199-213.
14 Warren MP, Stiehl AL. Exercise and female adolescents: effects on the reproductive and skeletal systems. J Am Med Womens Assoc. 1999;54(3):115-120. 138
15 MacKelvie KJ, Petit MA, Khan KM, et al. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. Bone. 2004;34(4):755-764.
16 Iwamoto J, Takeda T, Ichimura S. Relationships among physical activity, metacarpal bone mass, and bone resorption marker in 70 healthy adult males. J Orthop Sci. 2002;7(1):6-11.
17 Barrett-Connor E, Wehren LE, Siris ES, et al. Recency and duration of postmenopausal hormone therapy: effects on bone mineral density and fracture risk in the National Osteoporosis Risk Assessment (NORA) study. Menopause. 2003;10(5):412-419.
18 Slemenda CW, Hui SL, Longcope C, et al. Predictors of bone mass in perimenopausal women. A prospective study of clinical data using photon absorptiometry. Ann Intern Med. 1990;112(2):96-101.
19 World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Report of a WHO Study Group. World Health Organ Tech Repo Ser. 1994;843:1-129.
20 Lane JM, Russell L, Khan SN. Osteoporosis. Clin Orthop. 2000;372:139-150.
21 Heinemann DF. Osteoporosis: An overview of the National Osteoporosis Foundation clinical practice guide. Geriatrics. 2000;55:31-36.
22 Bilezikian JP, Silverberg SJ. Clinical practice. Asymptomatic primary hyperparathyroidism. N Engl J Med. 2004;350(17):1746-1751.
23 Arnala I, Kyrola K, Kroger H, et al. Analysis of 245 consecutive hip fracture patients with special reference to bone metabolism. Ann Chir Gynaecol. 1997;86(4):343-347.
24 Green PH, Jabri B. Coeliac disease. Lancet. 2003;362(9381):383-391.
25 Greenspan SL, Myers ER, Maitland LA, et al. Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA. 1994;271(2):128-133.
26 Greenspan SL, Myers ER, Kiel DP, et al. Fall direction, bone mineral density, and function: risk factors for hip fracture in frail nursing home elderly. Am J Med. 1998;104(6):539-545.
27 Aharonoff GB, Dennis MG, Elshinawy A, et al. Circumstances of falls causing hip fractures in the elderly, 1998. J Orthop Trauma. 2003;17(8 Suppl):S22-S26.
28 Myers ER, Wilson SE. Biomechanics of osteoporosis and vertebral fracture. Spine. 1997;22(24 Suppl):25S-31S.
29 Riggs BL, Melton LJIII. The worldwide problem of osteoporosis: Insights afforded by epidemiology. Bone. 1995;17:505S-511S.
30 Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320-323.
31 Reeve J, Lunt M, Felsenberg D, et al. Determinants of the size of incident vertebral deformities in European men and women in the sixth to ninth decades of age: the European Prospective Osteoporosis Study (EPOS). J Bone Miner Res. 2003;18(9):1664-1673.
32 Leidig G, Minne HW, Sauer P, et al. A study of complaints and their relation to vertebral destruction in patients with osteoporosis. Bone Miner. 1990;8(3):217-229.
33 Leech JA, Dulberg C, Kellie S, et al. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141(1):68-71.
34 Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern. 1999;159(11):1215-1220.
35 Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327(23):1637-1642.
36 Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;29(14):1701-1712.
37 Maricic M, Adachi JD, Sarkar S, et al. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med. 2002;162(10):1140-1143.
38 Chestnut CHIII, Silverman S, Andriano K, et al. A randomized trial of nasal spray calcitonin in postmenopausal women with established osteoporosis: The Prevent Recurrence of Osteoporotic Fractures Study. PROOF Study Group. Am J Med. 2000;109:267-276.
39 Lips P. Prevention of hip fractures: drug therapy. Bone. 1996;18(3 Suppl):159S-163S.
40 Seeman E. Osteoporosis: trials and tribulations. Am J Med. 1997;103(2A):74S-87S. discussion 87S–89S
41 Chrischilles EA, Dasbach EJ, Rubenstein LM, et al. The effect of alendronate on fracture-related healthcare utilization and costs: the fracture intervention trial. Osteoporos Int. 2001;12(8):654-660.
42 Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11):4118-4124.
43 Komatsubara S, Mori S, Mashiba T, et al. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19(6):999-1005.
44 Reid IR. Bisphosphonates: new indications and methods of administration. Curr Opin Rheumatol. 2003;15(4):458-463.
45 Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434-1441.
46 Koivukangas A, Tuukkanen J, Kippo K, et al. Long-term administration of clodronate does not prevent fracture healing in rats. Clin Orthop. 2003;408:268-278.
47 Li J, Mori S, Kaji Y, et al. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. Bone Miner Res. 1999;14(6):969-979.
48 Nakajima A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34). J Bone Miner Res. 2002;17(11):2038-2047.