Chapter 30 Osteoporosis
Introduction
Osteoporosis is a common skeletal condition that results in significant morbidity and mortality for men and women, with ever increasing health care costs.1 Osteoporosis afflicts 75 million people in the US, Europe and Japan and results in more than 1.3 million fractures annually in the US alone.1
In Australia, 1 in 2 women and 1 in 3 men over the age of 60 develop this disease. It is estimated that approximately 2 million Australians have osteoporosis, and this figure is expected to rise to 3 million by 2021.1 It is predicted that by 2021, 1 in 3 hospital beds in Australia will be occupied by a woman with an osteoporotic fracture. Hip fracture is a serious consequence of osteoporosis.
Risk factors
There are numerous risk factors for osteoporosis, but only those related to lifestyle behavioural factors will be discussed.2 The most important risk factors are:
Genetics
Although genes play a part in determining the risk of osteoporosis, the genetic contribution is not well understood. Two recent studies have reported gene variants associated with osteoporosis.3,4 It is estimated that genes account for 25–45% of variation in a 5-year change in bone mineral density (BMD) in women and men.5
However, prospective 25-year follow up of a nationwide cohort of elderly Finnish twins has concluded that susceptibility to osteoporotic fractures in elderly Finns is not strongly influenced by genetic factors, especially in elderly women.6
Gender/hormones/ageing
It is estimated that 30% of 50-year-old women already have osteoporosis.7 At age 65 years, 50% of women and 20% of men already have osteoporosis, and by age 75 years, 70% of men and women will have osteoporosis.7 Women are reported to usually lose approximately 1–2% of cortical bone per year after the age of 40–50 years. Moreover, 3–7 times more bone is lost in the first 7–10 years after becoming menopausal.8
In men, osteoporosis begins 5–10 years later than in women.9 Approximately 25% of men older than age 60 years will have an osteoporotic hip fracture. Mortality from fracture is higher in men.9
Using fracture as the benchmark for the risk of osteoporosis, risk for men in their lifetime is 13–25%, as compared to 50% in women.10 Bone loss is increased by oestrogen or testosterone deficiency from any cause at any time.11, 12
Moreover, a recent literature review shows that the most successful and essential strategy for improving BMD in women with functional hypothalamic amenorrhea is to increase caloric intake such that body mass is increased and there is a resumption of menses.13 However, the study also pointed out that further long-term studies to determine the persistence of this effect and to determine the size of this effect and other strategies on fracture risk are indeed warranted.
Lifestyle
Lifestyle risk factors for osteoporosis can impact growing bones in-utero resulting from maternal lifestyle and in early childhood. Epidemiological studies suggest there is a relationship of osteoporosis with birth weight, weight in infancy, hereditary, gender, diet, physical activity, sun exposure, endocrine status and smoking. Infants born to mothers who are vitamin D deficient are at risk of abnormal bone formation.14
The Mediterranean Osteoporosis Study has concluded that in both men and women lifestyle is important for bone health, and that there are a number of identifiable factors that can reverse the risk for osteoporosis.15,16
In men, the potentially reversible risk factors included low Body Mass Index (BMI), reduced physical activity, low exposure to sunlight, and low consumption of tea and alcohol and increased use of tobacco remained independent risk factors for 54% of hip fractures.15
In women, significant risk factors identified included low BMI, short fertile period, low physical activity, lack of sunlight exposure, low milk consumption, no consumption of tea, and a poor mental score.14 No significant adverse effects for coffee or smoking were reported. Moderate consumption of spirits was a protective factor in young adulthood, with no other risk effects observed for overall alcohol consumption. A low BMI and milk consumption were significant risks only in the lowest 50% and 10% of the population, respectively. A late menarche, poor mental score, low BMI and physical activity, low exposure to sunlight, and a low consumption of calcium and tea remained independent risk factors, accounting for 70% of hip fractures.14 Hence, approximately 50% of the hip fractures could be explained on the basis of potentially reversible risk factors. However, it should be noted that the use of risk factors to predict the occurrence of hip fractures had only a moderate sensitivity and specificity.
Recently, a study reported the 5-year risk of fracture among post-menopausal women of various ethnic backgrounds. The algorithm of 11 clinical factors included age, health status, weight, height, race/ethnicity, self-reported physical activity, history of fracture after age 54 years, parental hip fracture, current smoking, current corticosteroid use, and treated diabetes.17
Mind–body medicine
Stress and/or depression
There have been numerous reports that have investigated the association between depression and BMD. An early study has reported that women with depression have much lower BMD, and higher cortisol levels than controls.18 A recent review has concluded, however, that while most studies support the data that depression is associated with an increased risk for both low BMD and fractures, variations in study design, sample compositions, and exposure measurements have made the causative role played by depression in osteoporosis difficult to conclude.19
Sunlight
Sunshine is the main source of vitamin D produced by the body in response to direct skin exposure to UVB. This means that no or minimal exposure to sun can contribute to vitamin D deficiency, with risk factors including: dress codes (e.g. wearing veils, migrants, infants of migrant families); dark skin colour; living in geographically prone areas, especially over winter (southern or northern latitude); institutionalisation; elderly; being bed-bound; intellectual disability; prolonged and exclusive breastfeeding; restricted sun exposure, and certain medical conditions.20, 21 A study of 250 institutionalised patients following stroke demonstrated that regular sun exposure over 1 year increased vitamin D levels fourfold accompanied with a 3% increase in BMD, compared with the control group involving standard hospital care.22
Other than osteoporosis, osteomalacia and rickets, lack of sun exposure and vitamin D deficiency have been linked to many serious chronic diseases, including autoimmune diseases, infectious diseases, cardiovascular disease, hypertension, multiple sclerosis, depression, risk of infections, skin diseases, numerous cancers and diabetes.23–47
A recent review has proposed that sensible sun exposure can raise blood levels of 25(OH)D >30 ng/ml.48 Vitamin D deficiency is the most significant contributor to osteoporosis, fractures and falls and is a major public health issue in some parts of the world. Supplementation of Vitamin D in the form of cholecalciferol is necessary to establish normal blood levels.49
Environment
Smoking
Smoking is a risk factor for osteoporosis. A meta-analysis of the literature demonstrates an association of BMD and increased lifetime risk of vertebral fractures by 13% in women and by 32% in men, and hip fracture 31% in women and 40% in men.50 The effects were greatest in men and in the elderly, and were dose-dependent. BMD continued to significantly reduce over the time of continued smoking. Moreover, in an antioxidant vitamin supplement study it was reported that smoking had adverse effects on the skeleton.51
Physical activity/exercise
A Cochrane review has found that exercise is beneficial in the treatment of osteoporosis.51 This benefit is demonstrated in all age groups, including post-menopausal women.52, 53, 54 BMD can be improved by walking, weight-bearing and resistance exercises.17, 55 It has also been reported that resistance and weight-bearing exercises can also improve muscle strength which can help reduce falls and osteoporotic fractures.56, 57
High intensity training
A recent review has reported that several studies with post-menopausal women demonstrate modest increases in bone mineral toward the normal that are observed in a healthy population in response to high-intensity training.58 Physical activity continues to stimulate bone diameter increases throughout the lifespan. Therefore, these exercise-stimulated increases in bone diameter significantly decrease the risk of fractures by mechanically counteracting the thinning of bones and the increasing bone porosity and fragility.17
Vigorous walking
A recent study that investigated walking intensity in post-menopausal women demonstrated that exercise intensities about 115% of ventilator threshold or 74% of VO2 max, or at walking speeds greater than 6.14 km/hr mechanical loading of 1.22 times their body weight was sufficient for increases in leg muscle mass and preservation of BMD in these post-menopausal women.59 The study demonstrates vigorous speedy walking, not gentle amble walking, as helping to maintain BMD.
Athletic training
Moreover, young women who exercise athletically have higher bone mass than their sedentary counterparts, and this difference may be sustained in adulthood.60 Hence, moderate physical activity during the years of peak bone acquisition may have lasting benefits for lumbar spine and proximal femoral BMD in post-menopausal women.17
Nutritional influences
Diets
The metabolism associated with bone and muscle strength, and in particular for bone formation, requires calcium and phosphorus plus vitamins D, C, B and K, as well as the minerals boron, zinc, iron, fluoride, copper, magnesium, manganese, selenium, iodine, silicon and chromium.62, 63
Any disease that causes malabsorption of any of these nutrients can impact on BMD. For instance, a study of young children with coeliac disease, demonstrated improved BMD after 4 years on a strict gluten-free diet.64
Dairy intake
Of interest, a meta-analysis of 47 studies found little relationship between dietary calcium intake in childhood and bone health.65 The review examined 37 studies that found no relationship between dairy or dietary calcium intake and measures of bone health. Only 9 studies demonstrated small positive effects on bone health from dairy foods, although 3 were confounded with milk-fortified vitamin D. These studies suggest vitamin D from safe sunshine exposure or supplementation may be the more significant source than dietary dairy intake alone. Also the study implies that once minimum calcium needs are met, extra calcium from dairy source is not required.
Caloric restriction and weight loss diets
Caloric restriction and weight loss diets may actually significantly reduce BMD according to a weight loss study of 50–60 year-old adults (BMI 23.5–29.9) which also demonstrated that the exercise group had no loss of BMD compared with control group.66
Western diets high in animal protein
An early study has reported that Western diets that consist of high levels of animal protein are acidic in character, and can lead to an acidic diet which then is believed to release alkaline salts of calcium from bone.67
A reduction in animal protein consumption has been proposed to decrease bone resorption and affect calcium balance in a favourable way. A high vegetable to animal ratio, with high dietary calcium intake, appears to protect against osteoporosis.68
Many factors are known to influence bone health. Protein can be both detrimental and beneficial to bone health and this depends on a variety of factors that include the amount of protein consumed in the diet, protein source, calcium intake, weight loss, and the acid/base balance of the overall diet.69
Alkaline diets
The notion that diets rich in vegetables, fruits and whole grains are more alkaline, and hence provide protection for bone health has not been supported by a recent randomised control trial (RCT).70 The RCT of menopausal women has demonstrated that supplementation with a potassium citrate supplement over 2 years did not reduce bone turnover or increase BMD in healthy post-menopausal women. The results suggest that alkali provision does not explain any long-term benefit of fruit and vegetable intake on bone health.
Raw food vegetarian diet
A further study with participants consuming a long-term raw food vegetarian diet has concluded that this diet was associated with low bone mass at clinically important skeletal regions and was without evidence of increased bone turnover or impaired vitamin D status.71
Mediterranean diet
In Europe, there is a noticeable difference in the severity of osteoporosis, the lowest incidence being reported in the Mediterranean area.72 The beneficial effect has been attributed mainly to a specific eating pattern. The Mediterranean diet has numerous food items that contain a complex array of naturally occurring bioactive molecules with anti-inflammatory and alkalinising properties that together may contribute to the bone-sparing effect that has been documented.70
DASH diet
The Dietary Approaches to Stop Hypertension (DASH), which is a diet rich in fruit and vegetables, has also been reported to reduce bone loss.73 Moreover, the DASH diet has been demonstrated to reduce hypertension and has subsequently been shown to reduce coronary artery disease and stroke risk.
Tea
Older women who drink tea have a reported higher BMD than those who do not drink tea.74 A Taiwanese study of 1037 people aged 30 years or more, found that those who drink green, black or oolong tea regularly over a decade have a higher BMD than those who drank tea occasionally.75 A recent epidemiological study from the Middle East reported that habitual tea drinking did not impact on bone health, rather that multi-factorial factors (high education levels, being overweight, and being treated for HT) had positive effects on BMD in this population.76
However, a study that assessed risk factors for osteoporosis in Iranian women compared with Indian women has concluded that high habitual tea consumption (4–7 cups per day) was protective in both of these populations.77
A study of impaired hip structure, assessed by dual-energy X-ray absorptiometry areal BMD, which is an independent predictor for osteoporotic hip fracture, concluded that tea drinking was associated with preservation of hip structure in elderly women.78 This study provides further evidence for the beneficial effects of daily tea consumption on the skeleton. Flavonoids in tea are thought to be responsible for their protective effects against the development of osteoporosis.79
Soft drinks
Five to 6 servings per week of soft drinks, particularly cola, are recognised as a risk factor and linked to low BMD according to a population study of over 2500 adults.80
Soy and soy isoflavones
A number of observational studies have suggested that populations with a high dietary soy intake have a lower incidence of osteoporosis and related fractures when compared to Western populations.81
Soy contains protein and also isoflavones (phytoestrogens), and has been reported to increase BMD.82 The phytoestrogens have an affinity for oestrogen receptors and can behave similar to endogenous oestrogens. A meta-analysis of randomised controlled trials has shown that soy isoflavone intake inhibits bone resorption and can stimulate bone formation in menopausal women.83 However, a recent RCT has reported that in apparently healthy early post-menopausal white European women the daily consumption of foods containing 110mg of soy isoflavone aglycone equivalents for 1 year did not prevent post-menopausal bone loss and did not affect bone turnover.84
Phytoestrogens act on both osteoblasts and osteoclasts through genomic and non-genomic pathways as evidenced through in vitro and in vivo studies.85 Given that epidemiological studies and clinical trials suggest that soy isoflavones have beneficial effects on bone mineral density, bone turnover markers, and bone mechanical strength in post-menopausal women, the conflicting results make conclusions difficult to gauge. Differences in study design, oestrogen status of the body, metabolism of isoflavones among individuals in different population groups, and other dietary factors advocate that long-term safety and efficacy of soy isoflavone supplements in the prevention of osteoporosis remains to be demonstrated.
Dietary and supplemental omega-3 fatty acids (FAs)
An imbalance of omega-3 to omega-6 FAs is associated with lower BMD at the hip in both men and women.86 Also an early study has reported that dietary omega-3 FAs can suppress production of osteoclast-activation.87
A small placebo-controlled trial using a combination of evening primrose oil and fish oil supplementation over a 3-year period increased spinal BMD.88
Reviews report that the available evidence demonstrates that increased daily intake of dietary n-3 FAs decreases the severity of autoimmune disorders, lessens the chance of developing cardiovascular disease, and protects against bone loss during post-menopause.89
A recent systematic review concludes that even though studies support the beneficial effects of n-3 FAs on bone health and osteoporosis, the dissimilar lipid metabolism in humans and animals, the various study designs, and controversies over the human clinical study outcomes make definite conclusions as to the efficacy of n-3 FAs in preventing osteoporosis difficult to interpret.90
Nutritional supplements
Vitamins
Vitamin D
Vitamin D has a pivotal role in bone metabolism, it controls intestinal calcium absorption plus its deposition into bone.91 The main source of vitamin D is sunlight exposure (see Figure 30.1).92 Ninety percent of vitamin D is produced in the skin from sunshine exposure (UVB) with only 10% from dietary sources. Dietary sources include fatty fish (e.g. mackerel), cod-liver oil, sun-exposed mushrooms and liver. The majority of women with osteoporosis have vitamin D deficiency and resulting bone loss.93 The Geelong vitamin D study on post-menopausal women found that the majority of the participants were vitamin D deficient during winter.94
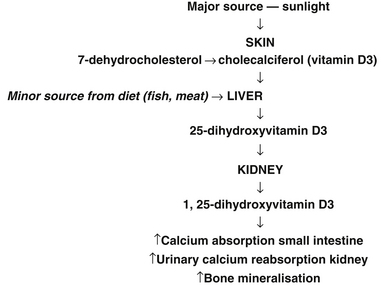
Figure 30.1 Major sources of sunlight
(Source: adapted from Nowson CA, Diamond TH, Psco JA, et al. Australian Family Physician. 2004;33(3):133–8)
Vitamin D deficiency is likely to be the commonest nutritional deficiency in Australia and many other countries, and may well be the most important one.95–100 As mentioned previously, risk factors for vitamin D deficiency include dark skin colour, dress codes (e.g. wearing veils, migrants, infants of migrant families), living in geographically prone areas especially over winter (southern or northern latitude), institutionalisation, being bed-bound, intellectual disability, elderly, prolonged and exclusive breastfeeding, restricted sun exposure, and certain medical conditions. Vitamin D deficiency has re-emerged as a major public health issue affecting at-risk groups such as the elderly, but also infants and young children potentially causing hypocalcemia, seizures, rickets, limb pain, tooth loss and poor dentition and risk of fracture.101–107 Breastfed infants of mothers who were vitamin D deficient during pregnancy were at high risk of vitamin D deficiency.108
Vitamin D has a multiplicity of roles involving virtually every body system.
Improved Bone Mineral Density (BMD)
A large RCT of elderly people over a 5-year period demonstrated vitamin D combined with calcium supplementation has long-term benefit on BMD compared with calcium alone or control group, particularly in subjects with sub-optimal vitamin D levels.109 Moreover, it is well documented that throughout the lifecycle the skeleton requires optimum development and maintenance of its integrity to prevent fracture. The data is promising for the use of combined calcium with vitamin D and the use of vitamin K.110
Reduction of fractures
A meta-analysis of randomised controlled studies found that vitamin D supplementation prevented hip and non-vertebral fractures in the elderly.111 Of interest, in a study of osteoporotic women over 65 years of age with a history of previous hip fractures, calcium and vitamin D supplementation, up to 1 year, increased BMD, corrected secondary hyperparathyroidism, increased urinary excretion but did not reduce the risk of further fractures.112
Muscle strength and prevention of falls
Vitamin D3 supplementation can improve muscular strength and balance, which can help prevent falls and consequently fractures.113,114 An Australian study that assessed the role of vitamin D on muscle strength in patients with previous fractures reported that muscle strength was most strongly associated with serum 25 hydroxy-vitamin D levels of >50 nmol/L.115 The study concluded that there was a significant association between serum 25 hydroxy-vitamin D levels and left leg muscle strength. This association may also have benefits for BMD.
Reduction of pain
In a recent UK study that investigated the use of vitamin D and chronic widespread pain in a white middle-aged British population, it was reported that the current vitamin D status of the study population was associated with chronic widespread pain in women but not in men.116 Follow-up studies were cited as needed to evaluate whether higher vitamin D intake could abrogate chronic widespread pain.
Traditionally, vitamin D3 is preferred over vitamin D2 which has a much shorter half-life than D3, and is also about one-third as potent. However, more recent research suggests that vitamin D2 is equally effective to vitamin D3 according to a double-blind randomised study of 68 healthy adults.117 The subjects received either vitamin D2 (1000IU), vitamin D3 (1000IU), a combination of D2 plus D3 (500IU) or placebo over an 11-week period, in winter, which demonstrated mean blood levels significantly increased from baseline by 9ng/ml without any differences between groups and with no change in placebo. These findings suggest vitamin D2 and D3 supplements are equally effective in raising serum levels. Similarly a 6-week study of young children with hypovitaminosis D demonstrated equivalent outcomes in improved serum 25(OH)D3 concentration levels from supplementation with elemental calcium (50mg/kg/day) combined with either vitamin D2 (2000IU) daily, vitamin D2 (50 000IU) weekly, or vitamin D3 (2000IU) daily.118
Researchers found that a single high dose of vitamin D3 (100,000IU) every 3 months can also achieve normal serum levels in vitamin D deficient aged care residents without risks of side-effects.119
However, a recent study in JAMA shows a yearly oral dose of 500 000IU of cholecalciferol (alone) in elderly increased the risk of falls and fractures.120
If it is possible to increase sunlight exposure, then the dosage of oral vitamin D can be reduced. It is better to have the serum 25-hydroxyvitamin D in the upper half of the usual range because of the vital importance of this nutrient.
Vitamin K
Vitamin K is essential for the activation of osteocalcin which is synthesised by osteoblasts, and it is important in reducing fracture risks and maintaining bone mineral density. Patients with osteopenia and fracture have significantly decreased levels of vitamin K. This fat-soluble vitamin comes in 2 forms, vitamin K1 and K2. Vitamin K1 is found in green leafy vegetables, whereas vitamin K2 is found in meat and fermented products such as natto (fermented soybeans) and cheese.122, 123
In a systematic and meta-analysis of RCTs investigating the efficacy of vitamin K in the prevention of fractures it was suggested that supplementation with vitamin K phytonadione (vitamin K1) and menaquinone-4 (vitamin K2) reduced bone loss and fracture incidence (up to 80% reduction in hip fractures).124 In the case of menaquinone-4, there was a strong effect on incident fractures among Japanese patients.
A prospective randomised clinical study in osteoporotic patients using vitamin K2 found that it could maintain lumbar bone mineral density.125 When given together with vitamin D3 it has an additive effect in reducing post-menopausal bone loss.126 Another study over a 2-year period also demonstrated a synergistic effect of vitamin D3, calcium with vitamin K1 on BMD in 60-year-old healthy non-osteoporotic women.127 Moreover, it has been recently proposed that there is an important role for vitamin K2 when used in combination with bisphosphonates or raloxifene in the prevention of fractures in post-menopausal women with osteoporosis with vitamin K deficiency.128
A recent RCT that employed a daily dose of 5mg of vitamin K1 supplementation for 2 to 4 years did not protect against age-related decline in BMD.129 However, it was also documented that it may protect against fractures and cancers in post-menopausal women with osteopenia.
Vitamin C
A prospective study in a group of smokers with insufficient intake of vitamins C and E increased the risk of hip fracture, whereas a more adequate intake was protective.51
A recent study with high dose vitamin C has reported that it was associated with a better BMD and a lower 4-year bone loss in elderly men.130 The total amount of vitamin C ingested (dietary + supplemental) was approximately 223mg/day.
Suggested dosages: 500–1000mcg calcium ascorbate, twice daily.
Folate and B group vitamins
Elevated homocysteine levels have been linked to osteoporosis.131,132 Folic acid, vitamin B6 and B12 can reduce homocysteine levels, and hence may have a role to play in maintaining bone health.133,134 A Japanese study of 628 stroke patients (>65 years of age) with high baseline levels of homocysteine and lower levels of serum vitamin B12 compared with a healthy reference range were supplemented with folate (5mg/day) plus mecobalamin (vitamin B12 1500mg/day) or placebo.135 After 2 years, the placebo group had significantly more hip fractures than the vitamin treatment group, BMD had not changed in both groups but, as expected, was significantly lower in the hemiplegic side for both groups. More studies are required.
Vitamin A
According to the Rancho Bernardo Study, Vitamin A above the recommended daily intake (2000–2800IU/day) is a significant risk factor for reducing bone mass in the elderly, who are at greater risk of vitamin A toxicity.136 The BMD further reduced as the intake of vitamin A increased beyond this level. The authors recommended avoiding vitamin A above recommended daily intake levels in the elderly.
Minerals
Calcium
Calcium is required to support bone growth, bone healing and maintain bone strength.137 It is controversial whether calcium supplementation can prevent or treat osteoporosis. It has been reported that in populations with extremely low calcium intake correlated with extremely low rates of bone fracture, and that others with high rates of calcium intake through the consumption of milk and milk products, had a higher rate of bone fracture.65,138
In post-menopausal women, neither milk nor a high calcium diet appears to reduce the risk of osteoporotic fracture.51 Calcium supplementation does not prevent loss of bone matrix-calcium loss, nor vertebral or non-vertebral fractures.139, 140
A recent Cochrane review has documented that calcium supplements alone have a small positive effect on bone density.141 The data show a trend toward a reduction in vertebral fractures, but it is unclear if calcium supplements reduce the incidence of non-vertebral fractures. In an RCT study with men, calcium at a dose of 1200mg/day was effective on BMD in a manner comparable with those found in post-menopausal women but at a dose of 600mg/day it was ineffective for treating BMD.142 The study concluded that calcium supplements were of benefit on BMD in men. A further randomised control study of 930 elderly men and women over 80 years of age for a 4-year period demonstrated less fractures in those taking 1200mg of elemental calcium compared with the placebo group.143 The benefit dissipated when calcium supplementation stopped. A similar study also demonstrated significantly reduced risk for all-site limb fractures for elderly taking calcium supplementation over a 5-year period compared with placebo.144
However, there is also a requisite to assess the incidence of cardiovascular events with calcium supplementation so that the balance of risk and benefit can be clearly ascertained. Cardiovascular risk with calcium supplements have been recently addressed in a study with post-menopausal women.145 This study reported that calcium supplementation in healthy post-menopausal women was associated with increased trends in cardiovascular event rates. As previously discussed, this potentially detrimental effect should be balanced against the likely benefits of calcium on bone particularly in the elderly who are at higher risk of cardiovascular disease.
There is also evidence of an association between calcium intake and prostate cancer, a possible mechanism being the reduction of serum vitamin D by dietary calcium.146, 147
Calcium with vitamin D
Calcium appears to have a role when taken at a higher dose and in conjunction with vitamin D. A meta-analysis of 29 RCTs involving calcium and calcium plus vitamin D, supported the use of high dose calcium (1200mg or more), and vitamin D (800 IU or more), but outcomes varied depending on whether the rates of fracture or bone loss was used.148 Patients with higher compliance of treatment had better outcomes.
A Cochrane review of 45 trials of older, post-menopausal women with osteoporosis, especially frail older people confined to institutions, demonstrated fewer hip fractures if given vitamin D with calcium, but not vitamin D alone.149
Historically, a further complication in calcium and vitamin D metabolism was the reporting of calcium and vitamin D malabsorption following Roux-en-Y gastric bypass surgery.150 It was further reported that bone turnover was increased and hip bone density rapidly declined. This decline in hip BMD was strongly associated with patient weight loss itself. Adjustable gastric banding is now the most common procedure. However vigilance for nutritional deficiencies and bone loss in patients are required.
Recommended dosage is calcium citrate 800mg/day or calcium carbonate 1000mg/day.
The Position Statement released recently by the Australian and New Zealand Bone and Mineral Society, Osteoporosis Australia and the Endocrine Society of Australia is summarised in Figure 30.2.151
Sodium
Sodium causes an increase in renal calcium excretion.152 In post-menopausal women and men, a higher salt intake has been reported to lead to greater rates of bone resorption.153 In people who have a low calcium and high salt diet it is shown that they have lower BMD. If salt intake is kept below 2400mg/day, there is no negative impact on bone health. However, adequate intake of calcium or potassium can also neutralise the impact of sodium.153
Potassium
Potassium can influence calcium homeostasis especially urinary conservation and excretion of calcium. Low potassium diets increase urinary calcium losses and high potassium diets reduce it. Higher potassium intake, primarily from fruits and vegetables, has been associated with higher BMD, and less bone loss.154 There are no controlled trials that have investigated potassium in osteoporosis.
Phosphorus
Phosphorus is the second most abundant mineral in bone. The calcium/phosphorus ratio should be 2.5:1. Excess phosphorus can cause osteoporosis by decreasing calcium absorption and generating acid in the body, leading to bone loss.155 As phosphorus is common in usual diets, it is rare to have a deficiency.
Magnesium
Magnesium is the third most common mineral in bone. It can assist in calcium absorption, bone mineralisation and increases the dynamic strength of bone.156
Magnesium supplementation can increase BMD in post-menopausal women.157, 158 The recommended dosage is 400–800mg/day.
Manganese
Women with osteoporosis have been found to have decreased plasma levels of manganese, and an enhanced plasma response to an oral dose of manganese. There are no human studies that have investigated the role of manganese in the treatment of osteoporosis, but it is likely to be useful in combination with other nutrients.159 A number of animal studies demonstrate manganese together with copper can have a favourable effect on bone density.160
Copper
Copper is needed for normal bone synthesis and a 2-year controlled study found that 3mg/day of copper reduced bone loss.159–162
Fluoride
Fluoride in osteoporosis and fracture rate remains controversial with an earlier meta-analysis showing no improvement, and a more recent study showing improvement in BMD.163,164
Strontium
Strontium ranelate appears to have comparable efficacy to that of alendronate in reducing the risk of vertebral fracture in post-menopausal women with a previous fracture.165 A recent clinical trial has demonstrated that 2gm orally of strontium ranelate significantly decreased the relative risk of vertebral fractures by 45% in patients without prevalent vertebral fracture over 3 years, versus placebo. BMD was observed to linearly increase during the 3 years of treatment with strontium ranelate, in comparison with placebo. Further, strontium ranelate was well tolerated throughout the entire duration of the clinical trial.166
(Refer also to the pharmaceutical section, later this chapter.)
Other mineral micronutrients (zinc, boron, silicon, chromium, selenium)
Additional trace micronutrients play a role in bone metabolism, but it is difficult to ascertain the role of these in the treatment of osteoporosis. Boron deficiency can result in deficiency of vitamin D, magnesium and calcium deficiency and can assist in increasing BMD although there are no RCTs demonstrating benefit on bone health.167,168
Studies using a combination of zinc, manganese, magnesium, copper and calcium have shown a reduction in spinal bone loss in post-menopausal women.169–172
Boron 3mg/day may have a positive effect on bone, but there are no controlled studies.173
Herbal medicines
A study of red clover isoflavone supplementation over a 1-year period demonstrated reduced loss of BMD of the lumbar spine compared with placebo, although more studies are required to support its use.174 Isoflavones exert a mild oestrogenic effect which may explain its benefit on bone health. Further studies are warranted to replicate these findings.
The metabolism of bone occupies a complex balance between the deposition of matrix and mineralisation and resorption. It has been reported in a recent review that essential oils derived from herbs such as sage, rosemary, thyme and other herbs can inhibit osteoclast activity in vitro and in vivo and leading to an increase in BMD.175 Also, that there are various herbal medicines from the traditional herbal formulae in Chinese and Ayurvedic medicine that have demonstrated effects in pharmacological models of osteoporosis, however, clinical studies of safety and efficacy are lacking and hence warranted.
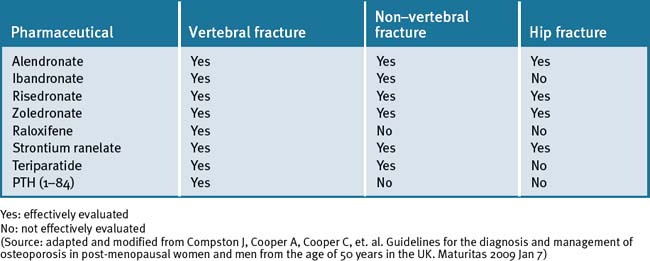
Pharmacological management of osteoporosis
The major pharmacological interventions are the bisphosphonates, strontium ranelate, raloxifene and parathyroid hormone peptides, as described in Table 30.1.179
Table 30.1 The effect of pharmacological interventions on fracture risk when co-administered with calcium and vitamin D in post-menopausal women with osteoporosis
Hormone replacement therapy
The use of combined low dose estradiol and progesterone can reduce risk of osteoporosis and rate of BMD loss.180 Percutaneous estradiol and natural progesterone were assessed in a controlled clinical trial of 57 post-menopausal women and was found to reduce bone loss.181
DHEA/testosterone hormones also play a role in the treatment of osteoporosis.
Physical therapies
Whole-body vibration
As physical activity plays an important role in maintaining bone health, any therapies that mechanically stimulate bone and muscle may assist people with low bone density and osteoporosis. A review of the literature found whole-body vibration may play a role for maintaining or improving bone mass, especially in individuals who have limited physical activity.182
Conclusion
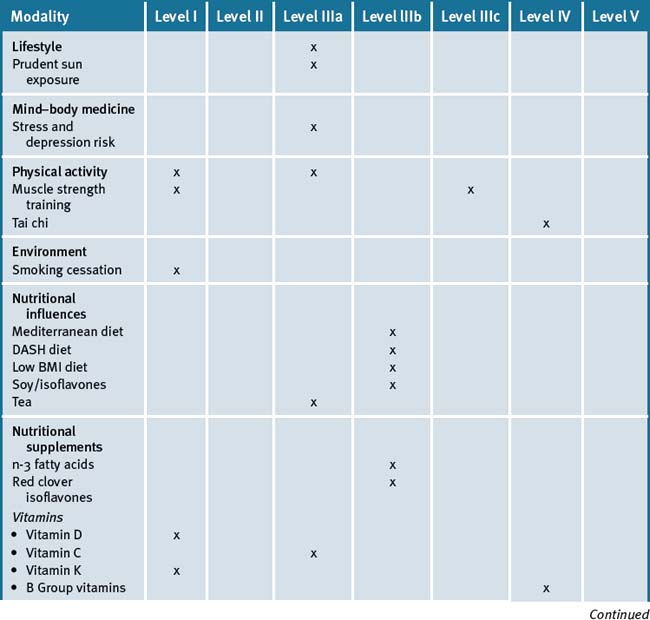
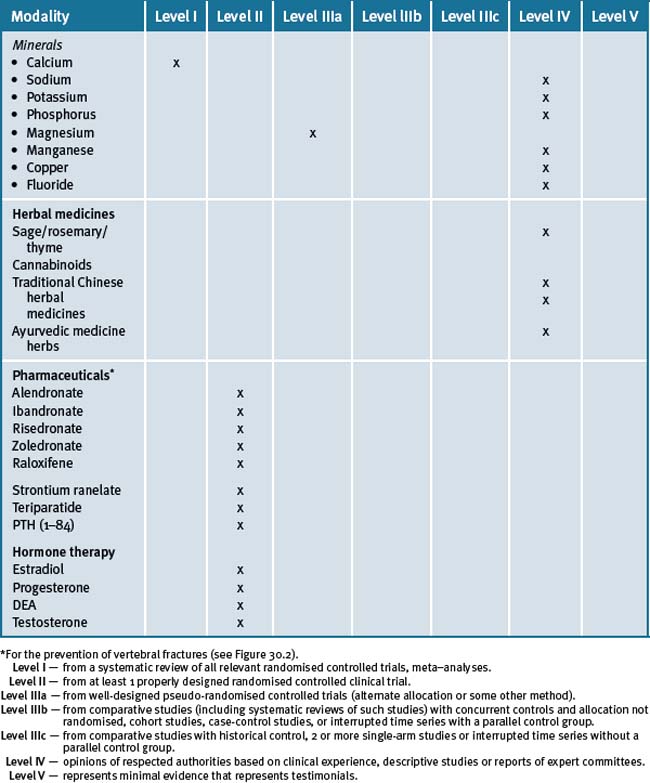
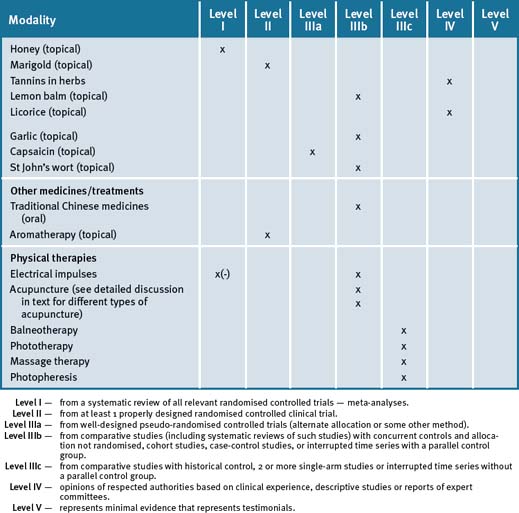
An integrative approach for the prevention and treatment of osteoporosis is centred on a lifestyle approach that further suggests that the patient maintain an adequate dietary intake of calcium, vitamin D-adequate blood levels, together with lifestyle advice such as smoking cessation, safe sun exposure and daily physical activity that particularly includes weight-bearing exercises, in conjunction with pharmacotherapy where appropriate. Table 30.2 summarises the level of evidence for some CAM therapies for the management of osteoporosis.
Clinical tips handout for patients — osteoporosis
1 Lifestyle advice
Sunshine
2 Physical activity/exercise
3 Mind–body medicine
5 Dietary changes
7 Supplements
Fish oils
Fish oils providing EPA 6.2g and DHA 3.4g/day have been used.
Evening primrose oil
Vitamins
Warning: vitamin A. Excessive intake of vitamin A above 3000IU/day contributes to bone loss.
Vitamin D3
Vitamin K
Vitamin B12
Minerals
Magnesium and calcium (best provided together)
Copper
Zinc
Other trace minerals that play a role in osteoporosis: boron, silicon, chromium, selenium, and manganese, combined with minerals discussed above.
1 South-Paul J.E. Osteoporosis: part I. Evaluation and assessment. Am Fam Physician. 2001;63(5):897-904.
2 Adapted Sali A, Vitetta L. Management of Osteoporosis and Integrative Approach. Australian Doctor article In Press Jan 2009.
3 South-Paul J.E. Osteoporosis: part II. Nonpharmacologic and pharmacologic treatment. Am Fam Physician. 2001;63(6):1121-1128.
4 Karasik D. Osteoporosis: an evolutionary perspective. Hum Genet. 2008;124(4):349-356.
5 Shaffer J.R., Kammerer C.M., Bruder J.M., et al. Genetic influences on bone loss in the San Antonio Family Osteoporosis study. Osteoporos Int. 2008 Apr 15. Epub ahead of print
6 Kannus P., Palvanen M., Kaprio J., et al. Genetic factors and osteoporotic fractures in elderly people: prospective 25 year follow up of a nationwide cohort of elderly Finnish twins. BMJ. 1999;319:1334-1337.
7 NIH Consensus Development Panel. Osteoporosis prevention, diagnosis and therapy. JAMA. 2001;285:785-795.
8 Garnero P., et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531-1538.
9 Campion J.M., Maricic M.J. Osteoporosis in men. Am Fam Physician. 2003;67(7):1521-1526.
10 Looker A.C., et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761-1768.
11 Buchanan J.R., et al. Determinants of peak trabecular bone density in women: the role of androgens, estrogens, and exercise. J Bone Miner Res. 1988;3:673-680.
12 Gasperino J. Androgenic regulation of bone mass in women. Clin Orthop. 1995;311:278-286.
13 Vescovi J.D., Jamal S.A., De Souza M.J. Strategies to reverse bone loss in women with functional hypothalamic amenorrhea: a systematic review of the literature. Osteoporos Int. 2008;19(4):465-478.
14 Thomson K., Morley R., Grover S.R., et al. Postnatal evaluation of vitamin D and bone health in women who were vitamin D-deficient in pregnancy, and in their infants. MJA. 2004;181:486-488.
15 Kanis J., Johnell O., Gullberg B., et al. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporosis Int. 1999;9(1):45-54.
16 Johnell O., Gullberg B., Kanis J.A., et al. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res. 1995;10(11):1802-1815.
17 Robbins J., Aragaki A.K., Kooperberg C., et al. Factors associated with 5-year risk of hip fracture in post-menopausal women. JAMA. 2007;298(20):2389-2398.
18 Michelson D., Stratakis C., Hill L., et al. Bone mineral density in women with depression. NEJM. 1996;335:1176-1181.
19 Mezuk B., Eaton W.W., Golden S.H. Depression and osteoporosis: epidemiology and potential mediating pathways. Osteoporos Int. 2008;19(1):1-12.
20 Brand C.A., Abi H.Y., Cough D.E., et al. Vitamin D deficiency: a study of community beliefs among dark skinned and veiled people. Intern J Rheumatic Dis. 2008;11:15-23.
21 Diamond T.H., Levy S., Smith A., et al. High bone turnover in Muslim women with vitamin D deficiency. MJA. 2002;177:139-141.
22 Sato Y., Metoki N., Iwamoto J., et al. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in stroke patients. Neurology. 2003;61:338-342.
23 Holick M.F. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008;66:S182-S194.
24 Raiten Daniel J., Frances Piccano Mary. Vitamin D and health in the 21st century: bone and beyond. Executive summary. Am J Clin Nutr. 2004;80(suppl):1673S-1677S.
25 Deeb Kristin K., Trump Donald L., Johnson Candace S. Vitamin D signaling pathways in cancer: potential for anticancer therapeutics. Nature Publishing Group. 2007;7:684-700.
26 Van Amerongen B.M., Dijkstra C.D., Lips P., et al. Multiple sclerosis and vitamin D: an update. European Journal of Clinical Nutrition. 2004;58:1095-1109.
27 Hernán Miguel A., . Olek Michael J, DO, Ascheri Alberto. Geographic variation of MS incidence in two prospective studies of US women. Neurology. 1999;53:1711.
28 Munger K.L., Zhang S.M., O’Reilly E., et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60-65.
29 Armin Zitterman. Stefanie S Schleithoff, Reiner Koerfer. Putting cardiovascular diseases and vitamin D insufficiency into perspective. British Journal of Nutrition. 2005;94:483-492.
30 Dobnig H., Pilz S., Sharnagl H., et al. Independent association of low serum 25-hydroxyvitamin D and 1,25 dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340-1349.
31 Bischoff-Ferrari H.A., Giovannucci E., Willett W.C., et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18-28.
32 Holick M.F., Matsuoka L.Y., Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104-1105.
33 Heaney R.P. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80:1706S-1709S.
34 Nnoaham K.E., Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113-119.
35 Lui P.T., Stenger S., Li H., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773.
36 Adams J.S., Liu P.T., Chun R., et al. Vitamin D in defense of the human immune response. Ann NY Acad Sci. 2007;1117:94-105.
37 Guenther L., Van de Kerkhof P.C., Snellman E., et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol twice daily) in the treatment of psoriasis vulgaris:a randomized double-blind, vehicle-controlled clinical trial. Br J Dermatol. 2002;147:316-323.
38 Forman J.P., Giovannucci E., Holmes M.D., et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063-1069.
39 Wang T.J., Pencina M.J., Booth S.L., et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503-511.
40 Hsia J., Heiss G., Ren H., et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846-854.
41 Garland C.F., Garland F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227-231.
42 Giovannucci E., Liu Y., Rimm E.B., et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451-459.
43 Garland C.F., Gorham E.D., Mohr S.B., et al. Vitamin D and prevention of breast cancer: Pooled analysis. J Steroid Biochem Mol Biol. 2007;103:708-711.
44 Kumagai T., O’Kelly J., Said J.W., et al. Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst. 2003;95:896-905.
45 Lappe J.M., Travers-Gustafson D., Davies K.M., et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586-1591.
46 Hypponen E., Laara E., Reunanen A., et al. Intake of vitamin D and risk of type 1 diabetes: a birth cohort study. Lancet. 2001;358:1500-1503.
47 Mathieu C., Gysemans C., Giulietti A., et al. Vitamin D and diabetes. Diabetologia. 2005;48:1247-1257.
48 Holick M.F. Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol. 2008;3(5):1548-1554.
49 Victorian Government Health Information. Low vitamin D in Victoria. Key health messages for doctors, nurses and allied health. Department of Health, State Government of Victoria. Online. Available: www.health.vic.gov.au/chiefhealthofficer/publications/low_vatamin_d_med.htm (accessed 31 August 1020)
50 Ward Kenneth D., Klesges Robert C. A meta-analysis of the effects of cigarette smoking on bone mineral density Calcified Tissue International. 2001;68:259-270.
51 Melhus H., Michaëlsson K., Holmberg L., et al. Smoking, antioxidant vitamins and the risk of hip fracture. J Bone Miner Res. 1999;14:129-135.
52 Bonaiuti D., Shea B., Iovine R., et al. Exercise for preventing and treating osteoporosis in post-menopausal women. Cochrane Database Syst Rev. (3):2002. CD000333
53 Hagberg J.M., Zmuda J.M., McCole S.D., et al. Moderate physical activity is associated with higher bone mineral density in post-menopausal women. J Am Geriatr Soc. 2001;49:1411-1417.
54 Wallace B.A., Cumming R.G. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and post-menopausal women. Calcif Tissue Int. 2000;67:10-18.
55 Kelley G.A., Kelley K.S., Tran Z.V. Resistance training and bone mineral density in women: a meta-analysis of controlled trials. Am J Phys Med Rehabil. 2001;80:65-77.
56 Raisz L.G. Screening for osteoporosis. NEJM. 2005;353:164-171.
57 Sinaki M., Itoi E., Wahner H.W., et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of post-menopausal women. Bone. 2002;30:836-841.
58 Borer K.T. Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. Sports Med. 2005;35(9):779-830.
59 Borer K.T., Fogleman K., Gross M., et al. Walking intensity for post-menopausal bone mineral preservation and accrual. Bone. 2007;41(4):713-721.
60 Rideout C.A., McKay H.A., Barr S.I. Self-reported lifetime physical activity and areal bone mineral density in healthy post-menopausal women: the importance of teenage activity. Calcif Tissue Int. 2006;79(4):214-222.
61 Wolf S.L., Barnhart H.X., Kutner N.G., et al. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention Techniques. J Am Geriatr Soc. 1996;44:489-497.
62 Morgan K.T. Nutritional determinants of bone health. J Nutr Elder. 2008;27(1–2):3-27.
63 Yazdanpanah N., Zillikens M., Rivadeneira F., et al. Effect of dietary B vitamins on BMD and risk of fracture in elderly men and women: The Rotterdam Study. Bone. 2007;41:987-994.
64 Kalayci A.G., Kansu A., Girgin N., et al. Bone mineral density and importance of a gluten-free diet in patients with celiac disease in childhood. Pediatrics. 2001;108:E89.
65 Lanou A.J., Berkow S.E., Barnard N.D. Calcium, dairy products, and bone health in children and young adults: a re-evaluation of the evidence. Pediatrics. 2005;115:736-743.
66 Villareal D.T., Fontana L., Weiss E.P., et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166:2502-2510.
67 Bushinsky D.A. Net calcium efflux from a live bone during chronic metabolic, but not respiratory, acidosis. Am J Physiol. 1989;256:F836-F842.
68 Weikert C., Walter D., Hoffmann K., et al. The relation between dietary protein, calcium, and bone health in women: results from the EPIC-Potsdam cohort. Ann Nutr Metab. 2005;49:312-318.
69 Heaney R.P., Layman D.K. Amount and type of protein influences bone health. AJCN. 2008;87(5):1567S-1570S.
70 Macdonald H.M., Black A.J., Aucott L., et al. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy post-menopausal women: a randomized controlled trial. AJCN. 2008;88(2):465-474.
71 Fontana L., Shew J.L., Holloszy J.O., et al. Low bone mass in subjects on a long-term raw vegetarian diet. Arch Intern Med. 2005;165(6):684-689.
72 Puel C., Coxam V., Davicco M.J. Mediterranean diet and osteoporosis prevention. Med Sci (Paris). 2007;23:756-760.
73 Fung T., Chiuve S.E., McCullough M.L., et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713-720.
74 Hegart V., May H.M., Khaw K.T. Tea drinking and bone mineral density in older women. AJCN. 2000;71:1003-1007.
75 Wu C.H., Yang Y.C., Yao W.J., et al. Epidemiological evidence of increased bone mineral density in habitual tea drinkers. Arch Intern Med. 2002;162:1001-1006.
76 Hamdi Kara I., Aydin S., Gemalmaz A., et al. Habitual tea drinking and bone mineral density in post-menopausal Turkish women: investigation of prevalence of post-menopausal osteoporosis in Turkey (IPPOT Study). Int J Vitam Nutr Res. 2007;77(6):389-397.
77 Keramat A., Patwardhan B., Larijani B., et al. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord. 2008;9:28.
78 Devine A., Hodgson J.M., Dick I.M., et al. Tea drinking is associated with benefits on bone density in older women. AJCN. 2007;86(4):1243-1247.
79 Siddiqui I.A., Afaq F., Adhami V.M., et al. Antioxidants of the beverage tea in promotion of human health. Antioxid Redox Signal. 2004;6(3):571-582.
80 Tucker K.L., Morita K., Qiao N., et al. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study. AJCN. 2006;84:936-942.
81 Zhang Y., Chen W.F., Lai W.P., et al. Soy isoflavones and their bone protective effects. Inflammopharmacology. 2008;16(5):213-215.
82 Potter S.M., et al. Soy protein and isoflavones: their effects on blood lipids and bone density in post-menopausal women. AJCN. 1998;68(6 Suppl):1375S-1379S.
83 Ma D.F., Qin L.Q., Wang P.Y., et al. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials. Europ J Clin Nutr. 2008;62:155-161.
84 Brink E., Coxam V., Robins S., et al. PHYTOS Investigators. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early post-menopausal women: a randomized, double-blind, placebo controlled study. AJCN. 2008;87:761-770.
85 Atmaca A., Kleerekoper M., Bayraktar M., et al. Soy isoflavones in the management of post-menopausal osteoporosis. Menopause. 2008;15(4 Pt 1):748-757.
86 Simopoulos A.P. Human requirement for omega-3 polyunsaturated fatty acids. Poult Sci. 2000;79:961-970.
87 Endres S., Ghorbani R., Kelley V.E., et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. NEJM. 1989;320:265-271.
88 Kruger M.C., Coetzer H., de Winter R., et al. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging. 1998;10:385-394.
89 Fernandes G., Bhattacharya A., Rahman M., et al. Effects of n-3 fatty acids on autoimmunity and osteoporosis. Front Biosci. 2008;13:4015-4020.
90 Salari P., Rezaie A., Larijani B., et al. A systematic review of the impact of n-3 fatty acids in bone health and osteoporosis. Med Sci Monit. 2008;14(3):RA37-RA44.
91 Moyad M.A. Vitamin D: a rapid review. Urol Nurs. 2008;28(5):343-349.
92 Nowson C.A., Diamond T.H., Psco J.A., et al. Australian Family Physician. 2004;Vol 33(3):133-138.
93 Bischoff-Ferrari H.A., Dietrich T., Orav E.J., et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and non-active persons aged > or = 60 y. AJCN. 2004;80:752-758.
94 Nowson C.A., Margerison C. Vitamin D intake and vitamin D status of Australians. MJA. 2002;177:149-152.
95 Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
96 Van der Mei I.A., Ponsonby A.L., Engelsen O., et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007;115(8):1132-1139.
97 Guardia G., Parikh N., Eskridge T., et al. Prevalence of vitamin D depletion among subjects seeking advice on osteoporosis: a five –year cross-sectional study with public health implications. Osteoporosis Int. 2008;19(1):13-19.
98 Kull MartJr., Kallikorm Riina, Tamm Anu, et al. Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European country. BMC Public Health; 2009. 9:22doi:10.1186/1471–2458-9–22. Online. Available: www.biomedcentral.com/1471–;2458/9/22 (accessed 14 Oct 2009)
99 John Livesey, et al. Seasonal variation in vitamin D levels in the Canterbury, New Zealand population in relation to available UV radiation. The New Zealand Medical Journal. Vol. 120 No 1262:1–13.
100 Weisber P., Kelley S.S., Ruowei Li, et al. Nutritional rickets among children in the United States: review of cases reported between 1986–2003. Am J Clin Nutr. 2004;80(suppl):1697S-1705S.
101 Munns C., Zacharin M.R., et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. MJA. 2006;185:268-272.
102 Van der Meer I.M., Karamali N.S., Boeke J.P., et al. High prevalence of vitamin D deficiency in pregnant non-Western women in the Hague, Netherlands. Am J Clin Nutr. 2006;84:350-353.
103 Erbas Bircan, Ebeling Peter R., Couch Dianne, et al. Suburban clustering of vitamin D deficiency in Melbourne, Australia. Asia Pac J Clin Nutr. 2008;17(1):63-67.
104 Diamond Terrence H., Levy Sherel, Smith Angelina, et al. High bone turnover in Muslim women with vitamin D deficiency. MJA. 2002 Aug 5;177:139-141.
105 Grover S.R., Morley M. Vitamin D deficiency in veiled or dark skinned pregnant women. MJA. 2001;175:251-252.
106 Tohill Carmel, Laverty Anne. Sunshine, diet and mobility for healthy bones-an interventional study designed to implement these standards into the daily routine in an at risk population of adults with intellectual disability. Journal of Intellectual & Developmental Disability. 2001;26(3):217-231.
107 Mosekilde Leif. Vitamin D and the elderly. Clinical Endocrinology. 2005;62:265-281.
108 Thompson K., Morley R., Grover S.R., et al. Postnatal evaluation of vitamin D and bone health in women who were vitamin D-deficient in pregnancy, and in their infants. MJA. 2004;181(9):486-488.
109 Zhu K., Devine A., Dick I.M., et al. Effects of calium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a 5-year randomized controlled trial. J Clin Endocrino Metabol. 2007;93:743-749.
110 Lanham-New S.A. Importance of calcium, vitamin D and vitamin K for osteoporosis prevention and treatment. Proc Nutr Soc. 2008;67(2):163-176.
111 Bischoff-Ferrari H.A., et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257-2264.
112 Sosa M., Láinez P., Arbelo A., et al. The effect of 25-dihydroxyvitamin D on the bone mineral metabolism of elderly women with hip fracture. Rheumatology. 2000;39:1263-1268.
113 Bischoff-Ferrari H.A., Willett W.C., Wong J.B., et al. Fracture prevention with vitamin D supplementation. A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257-2264.
114 Jackson C., Gaugris S., Sen S.S., et al. The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta-analysis. Q J Med. 2007:1-8.
115 Inderjeeth C.A., Glennon D., Petta A., et al. Vitamin D and muscle strength in patients with previous fractures. NZ Med J. 2007;120(1262):U2730.
116 Atherton K., Berry D.J., Parsons T., et al. Vitamin D and chronic widespread pain in a white middle-aged British population: evidence from a cross-sectional population survey. Ann Rheum Dis. 2008 Aug 12. Epub ahead of print
117 Holick MF, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metabol. 2008;93:677-681.
118 Gordon C.M., LeBoff Williams A., Feldman H.A., et al. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrin and Metabol. 2008;93:2716-2721.
119 Wigg Alison E.R., Prest Caroline, Slobodian Peter, et al. A system for improving vitamin D nutrition in residential care. Medical Journal of Australia. 2006;185:195-198.
120 Diamond Terrence H., Ho Kenneth W., Rohl Peter G., et al. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. Medical Journal of Australia. 2005;183:10-12.
121 Sanders K.M., Stuart A.L., Williamson E.J., et al. Annual high-dose oral vitamin D and falls and fractures in older women. A randomised control trial. JAMA. 2010;3030(18):1815-1822.
122 Ikeda Y., Iki M., Morita A., et al. Intake of fermented soybeans, natto, is associated with reduced bone loss in post-menopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J Nutr. 2006 May;136(5):1323-1328.
123 Garber A.K., Binkley N.C., Krueger D.C., et al. Comparison of phylloquinone bioavailability from food sources or a supplement in human subjects. J Nutr. 1999 Jun;129(6):1201-1203.
124 Cockayne S., Adamson J., Lanham-New S., et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(12):1256-1261.
125 Shiraki M. (Vitamin K2) Nippon Rinsho. 1998;56:1525-1530.
126 Iwamoto J., Takeda T., Ichimura S. Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in post-menopausal women with osteoporosis. J Orthoped Sci. 2005:546-551.
127 Bolton-Smith C., McMurdo M.E., Paterson C.R., et al. Two year randomized controlled trial of vitamin K (1) (phylloquinone) and vitamin D3 plus plus calcium on the bone health of older women. J Bone Miner Res. 2007 Apr 22;4:509-519..
128 Iwamoto J., Takeda T., Sato Y. Role of vitamin K2 in the treatment of post-menopausal osteoporosis. Curr Drug Saf. 2006;1(1):87-97.
129 Cheung A.M., Tile L., Lee Y., et al. Vitamin K supplementation in post-menopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med. 2008;5(10):e196.
130 Sahni S., Hannan M.T., Gagnon D., et al. High vitamin C intake is associated with lower 4-year bone loss in elderly men. J Nutr. 2008;138(10):1931-1938.
131 McLean R., et al. Homocysteine is a predictive factor for hip fracture in older persons. New England Journal of Medicine. 2004;352:2042-2049.
132 McLean R.R., Jacques P.F., Selhub J., et al. Homocysteine as a predictive factor for hip fracture in older persons. NEJM. 2004;350:2042-2049.
133 Homocysteine-lowering Trialists’ Collaboration. Lowering blood homocysteine with folic acid-based supplements: meta-analysis of randomized trials. BMJ. 1998;316:894-898.
134 Gjesdal C.G., Vollset S.E., Ueland P.M., et al. Plasma total homocysteine level and bone mineral density: the Hordaland Homocysteine Study. ArchIntern Med. 2006;166:88-94.
135 Sato, et al. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. JAMA. 2005;293:1082-1088.
136 Promislow J.H., Goodman-Gruen D., Slymen D.J., et al. Retinol intake and bone mineral density in the elderly: the Rancho Bernardo Study. J Bone Min Res. 2002;17:1349-1358.
137 Morgan K.T. Nutritional determinants of bone health. J Nutr Elder. 2008;27:3-27.
138 Feskanich D., Willett W.C., Stampfer M.J., et al. Milk, dietary calcium, and bone fractures in women: a 12-year prospective study. Am J Public Health. 1997;87:992-997.
139 Kreiger N., Gross A., Hunter G. Dietary factors and fracture in post-menopausal women: a case-controlled study. Int J Epidemiol. 1992;21:953-958.
140 Shea B., Wells G., Cranney A., et al. Calcium supplementation on bone loss in post-menopausal women: Cochrane Database. Syst Rev (1) CD004526. 2004.
141 Shea B., Wells G., Cranney A., et al. Osteoporosis Methodology Group; Osteoporosis Research Advisory Group. Calcium supplementation on bone loss in post-menopausal women. Cochrane Database Syst Rev. ((1):2004. CD004526. Review. Update in: Cochrane Database Syst Rev 2006;(1):CD004526
142 Reid I.R., Ames R., Mason B., et al. Randomized controlled trial of calcium supplementation in healthy, nonosteoporotic, older men. Arch Intern Med. 2008;168(20):2276-2282.
143 Bischoff-Ferrari H.A., Rees J.R., Grau M.V., et al. Effect of calcium supplementation on fracture risk: a double-blind randomized controlled trial. AJCN. 2008;87:1945-1951.
144 Prince R.A., Devine A., Dhaliwal S.S., et al. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Int Med. 2006;166:869-875.
145 Bolland M.J., Barber P.A., Doughty R.N., et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336(7638):262-266.
146 Heaney R., McCarron D.A., Dawson-Hughes B., et al. Dietary changes favourably affect bone remodeling in older adults. J Am Diet Assoc. 1999;99:1228-1233.
147 Giovannucci E., Rimm E.B., Wolk A., et al. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998;58:442-447.
148 Tang B.M., Eslick G.D., Nowson C., et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657-666.
149 Avenell A., Gillespie W.J., Gillespie L.D., et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database of Systematic Reviews. (Issue 3):2005. Art. No.: CD000227. doi: 10.1002/14651858.CD000227.pub2
150 Fleischer J., Stein E.M., Bessler M., et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735-3740.
151 Sanders Kerrie M., Nowson Caryl A., Kotowicz Mark A., et al. Position Statement. Calcium and bone health: position statement for the Australian and New Zealand Bone and Mineral Society, Osteoporosis Australia and the Endocrine Society of Australia. MJA. 2009;190(6):316-320. Online. Available: http://www.mja.com.au/public/issues/190_06_160309/san10083_fm.html (accessed 14 Oct 2009)
152 Teucher B., Fairweather-Tait S. Dietary sodium as a risk factor for osteoporosis: where is the evidence? Proc Nutr Soc. 2003;62:859-866.
153 Harrington M., Cashman K.D. High salt intake appears to increase bone resorption in post-menopausal women, but high potassium intake ameliorates this adverse effect. Nutr Rev. 2003;61:179-183.
154 Tucker K.L., Hannan M.T., Kiel D.P. The acid-base hypothesis: diet and bone in the Frammingham Osteoporosis Study. Eur J Nutr. 2001;40:231-237.
155 Jüppner H. Novel regulators of phosphate homeostasis and bone metabolism. Ther Apher Dial. 2007;11(Suppl 1):S3-S22.
156 Sojka J.E., Weaver C.M. Magnesium supplementation in osteoporosis. Nutr Rev. 1995;53:71-74.
157 Mutlu M., Argun M., Kilic E., et al. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res. 2007;35(5):692-695.
158 Stendig-Lindberg G., Tepper R., Leichter I. Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporosis. Magnes Res. 1993;6:155-163.
159 Odabasi E., Turan M., Aydin A., et al. Magnesium, zinc, copper, manganese, and selenium levels in post-menopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann Acad Med Sing. 2008;37(7):564-567.
160 Rico H. Effects on bone loss of manganese alone or with copper supplement in ovariectomized rats A morphometric and densitomeric study. Eur J Obst Gynecol Reprod Biol. 2002;90:97-101.
161 Eaton-Evans J., et al. Copper supplementation and bone mineral density in middle-aged women. Proc Nutr Soc. 1995;54:191A.
162 Palacios C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr. 2006;46(8):621-628.
163 Haguenuaer D., Welch V., Shea B., et al. Fluoride for the treatment of post-menopausal osteoporotic fractures: a meta-analysis. Osteoporosis Int. 2000;11:727-738.
164 Pak C.Y., Sakhaee K., Zerwekh J.E., et al. Treatment of post-menopausal osteoporosis with slow-release of sodium fluoride. Final report of randomized controlled trial. Ann Int Med. 1995;123:401-408.
165 Reginster J.Y., Meunier P.J., Roux C., et al. Strontium ranelate: an anti-osteoporotic treatment demonstrated vertebral and nonvertebral antifracture efficacy over 5 years in post-menopausal osteoporotic women. International Osteoporosis Foundation Osteoporosis International. 2006;17(Suppl 2):OC24.
166 Ortolani S., Vai S. Strontium ranelate: an increased bone quality leading to vertebral antifracture efficacy at all stages. Bone. 2006;38(2 Suppl. 1):19-22.
167 McCoy H., Kenney M.A., Montgomery C., et al. Relation of boron to the composition and mechanical properties of bone. Environ Health Perspect. 1994;102(7):59-63.
168 Meacham S.L., Taper L.J., Volpe S.L. Effect of boron supplementation on blood and urinary calcium, magnesium, and phosphorus, urinary boron in athletic and sedentary women. AJCN. 1995;61:341-345.
169 Gur A. The role of trace minerals in the pathogenesis of post-menopausal osteoporosis and a new effect of calcitonin. J Bone Miner Metab. 2002;20:39-53.
170 Volte S.L., et al. The relationship between boron and magnesium status and bone mineral density in the human: a review. Magnes Res. 1993;6:291-296.
171 Evans G.W., et al. Chromium picolinate decreases calcium excretion and increases dehydroepiandiosterone (DHEA) in post-menopausal women. FASEB J. 1995;9:A449.
172 Tamaki J., Iki M. Evidence-based, best-practice guidelines for primary prevention of osteoporosis and osteoporotic fractures. Clin Calcium. 2005;15(8):1312-1318.
173 Nielsen F.H. Studies on the relationship between boron and magnesium which possibly affects the formation and maintenance of bones. Mag Tr Elem. 1990;9:61-69.
174 Atkinson Charlotte, Compston Juliet E., Day Nicholas E., Dowsett Mitch, Bingham Sheila A. The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. American Journal of Clinical Nutrition. 2004;79:326-333.
175 Putnam S.E., Scutt A.M., Bicknell K., et al. Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother Res. 2007;21(2):99-112.
176 Shen L., Du J.Y., Yang J.Y. Preliminary clinical study on prevention of bone loss in post-menopausal women with kidney invigoration. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1994;14(9):515-518.
177 Ding G.Z., Zhang Z.L., Zhou Y. Clinical study on effect of bushen jiangu capsule on post-menopausal Osteoporosis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15(7):392-394.
178 Bab I., Zimmer A., Melamed E. Cannabinoids and the skeleton: from marijuana to reversal of bone loss. Ann Med. 2009;41(8):560-567.
179 Compston J., Cooper A., Cooper C., et al. Guidelines for the diagnosis and management of osteoporosis in post-menopausal women and men from the age of 50 years in the UK. Maturitas. 2009 Jan 7. Epub ahead of print
180 Prestwood K.M., Kenny A.M., Kleppinger A., et al. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-1048.
181 Riis B.J., Thomsen K., Strom V., et al. The effect of percutaneous estradiol and natural progesterone on post-menopausal bone loss. Am J Obstet Gynecol. 1987;156:61-65.
182 Totosy de Zepetnek J.O., Giangregorio L.M., Craven B.C. Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: a review. J Rehab Res Dev. 2009;46(4):529-542.