Chapter 41 Osteoporosis
Clinicians can add quality to the years of life of patients with osteoporosis through the use of an interdisciplinary approach. This condition is the most prevalent metabolic bone disease in the United States and is a major public health problem. The direct and indirect cost of osteoporosis in the United States alone is estimated to be more than $14 billion annually.53 Much of this expense relates to hip fractures. In 15% to 20% of hip fracture cases, the outcome is fatal.57,62
The World Health Organization has defined osteoporosis as bone mineral density (BMD) of 2.5 standard deviations below the peak mean bone mass of young healthy adults.107 The T score shows the amount of one’s bone density compared with a young adult (at the age of 35) of the same gender with peak bone mass. The Z score is calculated in the same way, but the comparison is made with someone of the same age, sex, race, height, and weight. The Z score is adjusted for an individual’s age, and the T score is not. For example, a 75-year-old woman with a Z score of −1.0 is one standard deviation below the BMD of an average 75-year-old woman, but her T score may be −3.0 because she is three standard deviations below the BMD of an average 35-year-old woman. Normal BMD is a T score −1 or greater; osteopenia, a T score between −1 and −2.5; osteoporosis, a T score −2.5 or less; and severe osteoporosis, a T score −2.5 or less with fracture. In the asymptomatic stage, osteoporosis is characterized simply by decreased bone mass without fracture. Osteoporosis becomes clinically problematic only when the bone fractures.
Bone Function and Structure
Bone serves as a mechanical support for musculoskeletal structures, as protection for vital organs, and as a metabolic source of ions, especially calcium and phosphate. Despite its appearance, bone is an active tissue. To maintain its biomechanical competence, bone tissue undergoes continuous change and renewal so that older bone tissue is replaced by newly formed bone tissue. Approximately 20% of bone tissue is replaced annually by this cyclic process. There are two types of bone cells: osteoclasts, which resorb the calcified matrix, and osteoblasts, which synthesize new bone matrix.65
Bone Remodeling
The number of active remodeling units in trabecular bone is about 3 times greater than in cortical bone. The physical endurance of any bone is affected by the percentage of cortical bone involved in its structure. Trabecular bone is more active metabolically than cortical bone because of the considerable surface exposure areas. Consequently, more bone loss occurs at the trabecular areas when resorption is greater than formation. The vertebrae consist of 50% trabecular bone and 50% cortical bone, whereas the femoral neck consists of 30% trabecular bone and 70% cortical bone. When bone turnover increases, bone loss and osteoporosis occur in the vertebrae before they occur in the femoral neck.
Pathogenesis
Trabecular (or cancellous) bone represents about 20% of skeletal bone mass and makes up 80% of the turnover media. The cortex makes up only 20% of the turnover media and is made of compact bone. It represents 80% of skeletal bone mass. In both cortical and trabecular bone, bone remodeling is initiated with the activation of osteoclasts. The resulting resorption sites are then refilled by osteoblastic activities, a process called bone formation. If the amount of bone resorbed equals the amount formed, the bone loss is zero. The remodeling process does not result in zero balance after age 30 to 35 years, however, and after this age the normal process of remodeling results in bone loss.63
Certain conditions, such as hyperparathyroidism or thyrotoxicosis, can increase the rate of bone remodeling. These conditions increase the rate of bone loss, which results in high-turnover osteoporosis. The secondary causes of osteoporosis are associated with an increased rate of activation of the remodeling cycle. Although factors such as calcium intake, smoking, alcohol consumption, physical exercise, and menopause are important factors in determining BMD, genetic factors are the major determinant and contribute to 80% of the variance in peak BMD.16 Fracture incidence related to osteoporosis is lower in men than in women because the diameter of vertebral bodies and long bones is greater in men at maturity and bone loss is less (about half that of women) throughout life.67
Classification of Osteoporosis
Osteoporosis can be primary or secondary to other disorders that result in bone loss. The most common causes of osteoporosis are listed in Box 41-1. The most common type of osteoporosis is either postmenopausal or age related.56
BOX 41-1 Common Causes of Osteoporosis
Modified from Sinaki M: Metabolic bone disease. In Sinaki M, editor: Basic clinical rehabilitation medicine, ed 2, St Louis, 1993, Mosby. Used with permission of Mayo Foundation for Medical Education and Research.
Acquired (Primary and Secondary)
Generalized
Primary osteoporosis is the rare disorder of idiopathic juvenile osteoporosis. This type of osteoporosis typically occurs before puberty (between ages 8 and 14 years), and patients present with osteoporosis that is progressive over 2 to 4 years in association with multiple axial or axioappendicular fractures. Remission usually occurs by the end of the 2- to 4-year course.36 In this type of osteoporosis, the process of bone formation is normal but osteoclastic activity increases, resulting in increased bone resorption. This type of osteoporosis is most evident in the thoracic and lumbar spine and needs to be distinguished from juvenile epiphysitis or Scheuermann disease. It is usually self-limiting, but the radiographic appearance might not return to normal. The laboratory values are typically normal, and the diagnosis is made by exclusion.
Hormones and Physiology of Bone
The rate of bone remodeling can be increased by parathyroid hormone (PTH), thyroxine, growth hormone, and vitamin D (1,25-dihydroxyvitamin D3 [1,25(OH)2D3]). It can be decreased by calcitonin, estrogen, and glucocorticoids.46
Role of Sex Steroids
The main endocrine function that occurs at menopause is loss of secretion of estrogen and progesterone from the ovaries.34,46,67 The premenopausal ovary produces primarily estradiol. Progesterone secretion, which occurs cyclically after ovulation in the premenopausal stage, also decreases to very low levels in the postmenopausal stage. These changes in circulating sex steroids are gradual in a woman’s sexual reproductive life. The premenopausal ovary also produces androgens, especially testosterone. The circulating testosterone levels decrease after menopause. The major source of estrogen in postmenopausal women is conversion from dihydroepiandrostenedione. The latter is then converted into androstenedione, which changes into estrone in fat cells. Estrone is the major source of estrogen in postmenopausal women.
Men do not have the equivalent of menopause, but in some elderly men bone mass decreases along with a decline in gonadal function. The testosterone level in men decreases with age as a result of a decreased number of Leydig cells in the testes. Male hypogonadism is typically associated with bone loss.41
Other Factors Affecting Bone Mass
Several other factors can contribute to the reduction of sex-related steroid levels. In hyperprolactinemia, which is due to a prolactin-secreting pituitary tumor, failure of the gonadal axis results in a substantial loss of bone. Amenorrheic athletes who exercise excessively, such as high-mileage runners or ballet dancers who have lower-than-normal body weight, have lower circulating estradiol, progesterone, and prolactin levels. Their amenorrhea is associated with hypothalamic hypogonadism, which leads to excessive bone loss. This bone loss can be mostly reversed when training distances are decreased.14,44 With weight gain and improvement in nutrition, these young women can facilitate resumption of menses and reversal of bone loss.7,21,106 Reduction of sex steroid concentrations is not the only cause of bone loss. Other factors such as race, genetics, nutrition, physical exercise, and lifestyle can also contribute to the rate of bone loss after an ovariectomy or natural menopause.72 It is well known that bone must be physically stressed to be maintained. A considerable body of data shows that the rate of change in strain also influences bone growth and remodeling.52
Effect of Aging on Bone Mass
It appears that special forms of vitamin K therapy in elderly persons can be associated with a reduction in the rate of bone resorption, demonstrated by decreased excretion of urinary hydroxyproline. Further studies are needed in this area. Studies have shown that calcium absorption is less efficient in elderly people.32 Bone loss also has been related to deficiencies in trace metal elements, such as copper, zinc, and magnesium, but this issue is not fully resolved.
Plasma calcitonin levels are higher in men than in women. Calcitonin levels do not change with age. Studies have shown that estrogens stimulate calcitonin secretion.99,100 Thyroid hormone levels typically show no change or are slightly decreased with age. The PTH level increases with age, perhaps because of mild hypocalcemia and decreased 1,25(OH)2D3 concentration. This reduction in the active form of vitamin D can be due to decreased consumption of dietary vitamin D, decreased exposure to sunlight, decreased skin capacity for vitamin D conversion, reduced intestinal absorption, and reduced 1-α-hydroxylase activity.
Several studies have shown that the level of physical activity decreases with aging.73 This is important because physical strain and mechanical load also positively affect bone mass.23 Exercise is known to stimulate the release of growth hormone or other trophic factors that can stimulate osteoblastic activity.20 Optimal nutrition and physical activity are necessary to achieve the genetic potential for bone mass. The peak bone mass attained by young adulthood is a major determinant of bone mass in later life. Female gymnasts, both children and college-aged athletes, reportedly have higher BMD than swimmers.6,19 Nutrition also can affect both bone matrix formation and bone mineralization. In general, in estrogen-deficient women, calcium intake of 1500 mg/day and 800 international units/day of vitamin D are recommended.
Clinical Manifestations of Osteoporosis
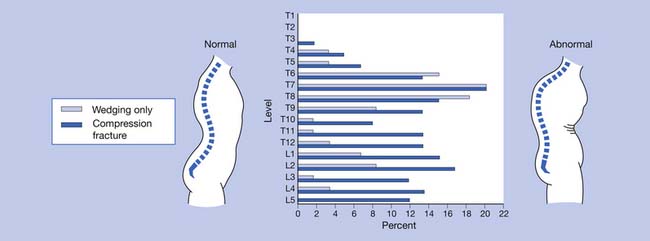
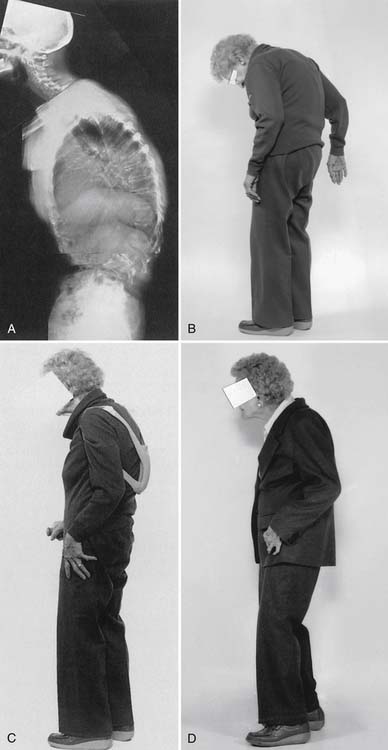
Osteoporosis is typically a “silent disease” until fractures occur. Osteoporotic vertebral fractures can go unnoticed until they are incidentally seen on a chest radiograph. Appendicular fractures, however, typically require immediate attention. The fact that a fracture resulted from osteoporosis should not affect the orthopedic method of management. The most common areas for osteoporotic fractures are the midthoracic and upper-lumbar spine (Figure 41-1),81 hip (proximal femur), and distal forearm (Colles fracture). The highest incidence of fractures is in white women. The female/male ratio is about 7:1 for vertebral fractures, 2:1 for hip fractures, and 5:1 for Colles fractures. It has been estimated that after menopause a woman’s lifetime risk of sustaining an osteoporotic fracture is 1 in 2 or 3.51
Fractures and Management
The relationship between bone mass and spinal fractures has been extensively studied, and it is known that fracture risk increases as bone mass decreases. For every standard deviation of decrease in BMD, the risk of osteoporotic fracture of the spine increases 1.5- to 2-fold, and the risk of hip fracture increases 2.6-fold.30 Another predictor of fracture risk is age itself. The risk of fracture as a result of osteoporosis doubles every 5 to 7 years.30 It is not clear whether age-related changes in bone density or bone quality are factors that increase the risk of fractures caused by falls.
Vertebral Fracture
Acute pain that occurs in the absence of a previous fracture is usually due to compression fractures of the vertebrae. Sometimes a minor fall or even an affectionate hug can cause a compression fracture. The compressed vertebrae might not be apparent on radiographs for up to 4 weeks after the injury.56 Compression fractures usually result in acute pain that later resolves (Box 41-2).71 The spinal deformity that can result from these fractures can produce chronic pain.71
BOX 41-2 Management of Acute Pain in Patients With Osteoporosis
Modified from Sinaki M: Metabolic bone disease. In Sinaki M, editor: Basic clinical rehabilitation medicine, ed 2, St Louis, 1993, Mosby. Used with permission of Mayo Foundation for Medical Education and Research.
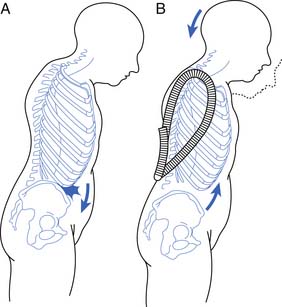
Kyphotic postural change is the most physically disfiguring and psychologically damaging effect of osteoporosis.85 The incidence of osteoporosis can be substantially decreased only by early detection and subsequent intervention in high-risk patients.
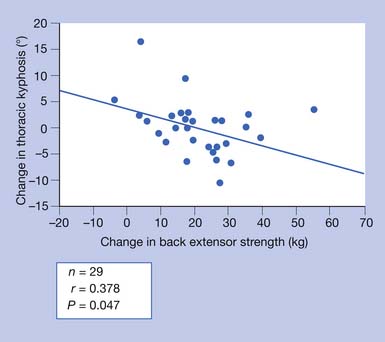
Disproportionate weakness in back extensor musculature relative to body weight or spinal flexor strength considerably increases the possibility of compressing the vertebrae in the fragile osteoporotic spine. Recognition and improvement of decreased back extensor strength can enhance the ability to maintain proper vertical alignment.69 The geriatric population has an increased risk of debilitating postural changes because of several factors, the two most apparent being a greater prevalence of osteoporosis and an involutional loss of functional muscle motor units.27,50 Development of kyphotic posture not only can predispose to postural back pain but also can increase the risk of falls.48 Several other factors also can contribute to the risk for falls (Box 41-3).
BOX 41-3 Factors Contributing to Risk for Falls
Modified from Sinaki M: Falls, fractures, and hip pads, Curr Osteoporos Rep 2:131-137, 2004, with permission.
Extrinsic
Intrinsic
Chronic spinal pain can be due to the deformity caused by vertebral wedging and compression, as well as by secondary ligamentous strain. These deformities are often difficult to distinguish from the usually associated disk deterioration. The intervertebral disks undergo the most dramatic age-related changes of all connective tissues.1 With aging, there is an increase in the number and diameter of the collagen fibrils in the disk. This change is accompanied by a progressive decrease in disk resilience. Loss of distinction between the nucleus pulposus and the annulus fibrosus eventually occurs.
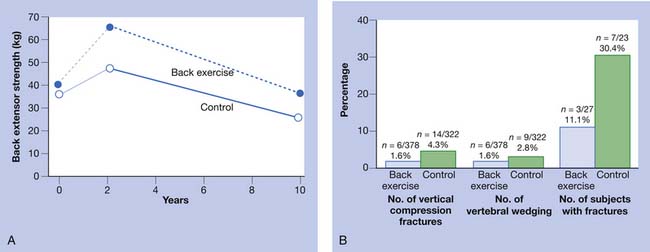
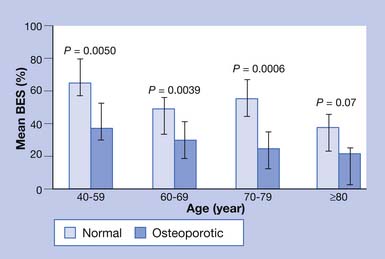
Chronic back pain secondary to osteoporosis is related to postural changes resulting from vertebral fractures.85 Strong back muscles contribute to good posture and skeletal support (Figure 41-2).33,84,89 One controlled study showed the long-term effects of back extensor resistance training 8 years after cessation of the exercise.86,91 The women in the study were not receiving hormone replacement therapy. Compared with the exercise group, the control group had a 2.7 times greater number of vertebral fractures at 10-year follow-up evaluation.91 The pain and skeletal deformity associated with osteoporosis might secondarily reduce muscle strength. The reduction in muscle strength can further exacerbate the postural abnormalities associated with this condition (Figure 41-3).
Chronic pain can also be due to microfractures that are visible only on bone scanning and which can occur continuously. Management of chronic osteoporosis-related pain is outlined in Box 41-4. Prescription of opiate analgesics, such as codeine sulfate or its derivatives, should be undertaken judiciously because their use can cause constipation.68
BOX 41-4 Management of Chronic Pain in Patients With Osteoporosis
Modified from Sinaki M: Metabolic bone disease. In Sinaki M, editor: Basic clinical rehabilitation medicine, ed 2, St Louis, 1993, Mosby. Used with permission of Mayo Foundation for Medical Education and Research.
New Hypothesis on the Most Effective Exercise to Reduce the Risk for Vertebral Fracture
After a 10-year follow-up study,91 the author developed the following hypothesis: “Back strengthening exercises performed in a prone position rather than in vertical position (nonloading) can decrease risk of vertebral fractures through improvement of horizontal trabecular connections.”79 The exercise needs to be progressive, resistive, and nonloading to avoid vertebral compression fracture.
Vertebroplasty and Kyphoplasty
Vertebroplasty and kyphoplasty procedures are used for the management of vertebral fractures. These procedures involve the injection of acrylic cement (such as polymethylmethacrylate) into a partially collapsed vertebral body. Jensen et al.35 found that 63% of osteoporotic patients who underwent vertebroplasty decreased their use of opiates and analgesics for pain control, 7% increased their use, and 30% continued on the same use. More recently, two multicenter randomized controlled trials evaluating vertebroplasty demonstrated no significant difference in pain relief when compared with a sham procedure.5,37 Vertebroplasty does not substitute for rehabilitative measures that are needed after fracture.31,59 One study showed significantly fewer vertebral refractures after vertebroplasty in patients who received instruction for back extension exercises.31 The author recommends a rehabilitation program, especially back extension exercises, for osteoporosis management.
Hip Fracture
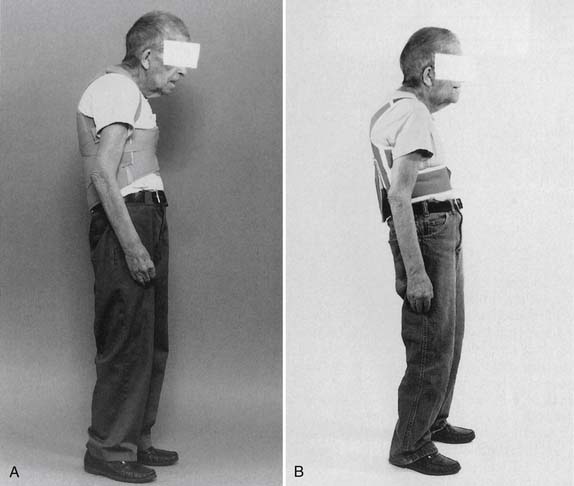
Falls and hip fractures can be life threatening.55,101 In addition to weakness of the lower limbs, one of the contributing factors to falls is disequilibrium of individuals with spinal kyphotic posture.78 The kyphotic posture places the center of gravity closer to the limit of stability.93 Measures that reduce instability, such as proper exercise program and use of a weighted kypho-orthosis (WKO), can reduce both the fear of falls and the risk of falls (Figure 41‑4).93
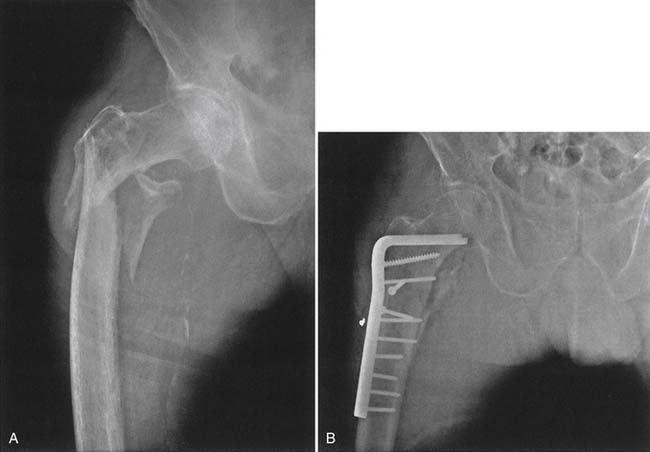
The trochanteric hip fracture creates fewer problems, despite the fact that the fracture engages more bone than the femoral neck fracture. The operative treatment of choice is internal fixation (Figure 41-5). The postoperative course for all hip fractures, regardless of whether internal fixation or joint arthroplasty is done, is less eventful if physical therapeutic measures are used postoperatively, including the use of gait aids with partial weight-bearing on the operative side. Only for patients with severely comminuted fractures or fractures in which the operative result has been unsatisfactory is the restriction of weight-bearing to no weight-bearing needed.
Hip Pads for Fracture Prophylaxis
There is conflicting evidence as to whether hip protectors can reduce the incidence of hip fractures in the elderly, high-risk population. One randomized controlled trial showed that hip protector pads can protect against hip fractures in an elderly nursing home population (average age, 81 years), but compliance with use of the hip protectors has been a concern.38 Another study showed no significant difference in the incidence of hip fractures, even in the participants who were compliant with the use of the hip protectors.103 It appears that at-risk elderly individuals, especially those with a history of falls, impaired balance, and decreased cognition, would benefit from use of hip pads, in addition to use of gait aids.77 Also, rehabilitation for patients to learn how to fall and land safely can decrease the risk of hip fracture resulting from high-impact contact during a fall. Landing on the buttocks is less traumatic to hips than landing on the greater trochanters.64
Sacral Insufficiency Fracture
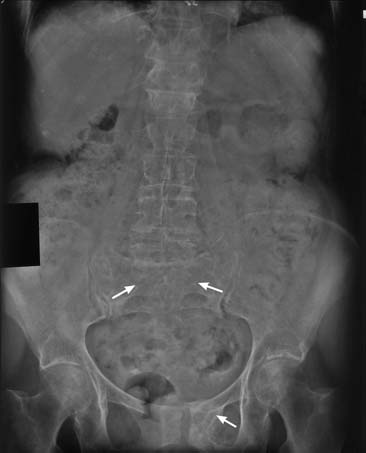
Other axial skeletal fractures, such as fractures of the sacral alae and pubic rami, can also occur (Figure 41-6). Pelvic fractures are particularly common in patients with osteoporosis. Fractures of the pubic rami can occur with minimal strain, and most patients can hardly recall having a traumatic event or an incident of severe strain. Healing typically occurs without invasive procedures. Ambulatory activities are reduced temporarily, and a wheeled walker is initially recommended to decrease pain. Later in the treatment, crutches and a cane can be used. Weight-bearing is limited, as dictated by the level of pain in the pelvic area. Fracture of the sacrum with minimal trauma can also occur, and the goal of management is to decrease weight-bearing pain with use of proper assistive devices for ambulation.71 For management of pelvic pain, physical therapeutic measures are recommended.
Diagnostic Studies in Osteoporosis
The diagnosis of osteoporosis requires a thorough history and physical examination, including family history of osteoporosis, type and location of musculoskeletal pain, general dietary calcium intake, level of physical activity, and height and weight measurements (Table 41-1).
Table 41-1 Some of the Diagnostic Evaluations for Osteoporosis
| Evaluation | Details |
|---|---|
| History and physical examination | Family history of osteoporosis, type and location of pain, general dietary calcium intake, level of physical activity, height and weight |
| Radiographs of chest and spine | To rule out lymphomas, rib fractures, compression fractures, etc. |
| Bone mineral density (spine and hip) | At menopause and every 2 years for high-risk patients and every 5 years for low-risk patients |
| Complete blood cell count | To rule out anemias associated with malignancy, etc. |
| Chemistry group (serum calcium, phosphorus, vitamin D, parathyroid hormone, bone-specific alkaline phosphatase, osteocalcin) | To assess the level of alkaline phosphatase, which may be increased in osteomalacia, Paget disease, bony metastasis and fracture, intestinal malabsorption, vitamin D deficiency, chronic liver disease, alcohol abuse, phenytoin (Dilantin) therapy, hypercalcemia of hyperparathyroidism, hypophosphatemia of hyperparathyroidism and osteomalacia, malabsorption, or malnutrition |
| Erythrocyte sedimentation rate and serum protein electrophoresis |
To determine changes indicative of multiple myeloma or other gammopathies |
| Total thyroxine | Increased total thyroxine concentration may be a cause of osteoporosis because of increased bone turnover |
| Immunoreactive parathyroid hormone | Hyperparathyroidism (accompanied by hypercalcemia) |
| 25-Hydroxyvitamin D and 1,25-dihydroxyvitamin D3 | Gastrointestinal disease, osteomalacia |
| Urinalysis and 24-hr urine | To check for proteinuria caused by nephrotic syndrome and for low pH resulting from renal tubular acidosis; a 24-hr urine test can exclude hypercalciuria (normal calcium value in men is 25-300 mg/specimen; in women, 20-275 mg/specimen62) |
| Optional: Bone scan, iliac crest biopsy | After tetracycline double-labeling for bone histomorphometry, bone marrow biopsy may be indicated to exclude multiple myeloma and metastatic malignancy |
| Biochemical markers of bone turnover (Eastell) |
Several biochemical indices also are used in the differential diagnosis of metabolic bone disease or, in some instances, for therapeutic follow-up.12 For example, biochemical markers for bone formation are calcium, phosphorus, PTH, bone-specific alkaline phosphatase, and serum osteocalcin. Resorption markers include 24-hour urinary calcium excretion (corrected by creatinine excretion), hydroxyproline, and pyridinium cross-links (in urine).24 The interpretation of these tests is unfortunately clouded in patients with osteoporosis because the intraindividual and interindividual variations are substantial for these parameters. Indices of bone turnover show seasonal and circadian variations (see Table 41-1).
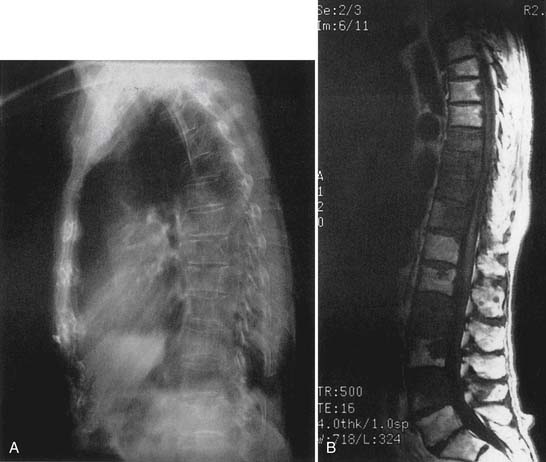
Radiographic findings of osteoporosis consist of increased lucency of the vertebral bodies with loss of horizontal trabeculae, increased prominence of the cortical end plates, vertically oriented trabeculae, reduction in cortex thickness, and anterior wedging of vertebral bodies.13,25 The degree of wedging that indicates a true fracture varies from a 15% to 25% reduction in anterior height relative to the posterior height of the same vertebra. Other morphologic changes occur, such as biconcavity of vertebral bodies and complete compression fractures (reduction in both anterior and posterior heights by at least 25% compared with adjacent normal vertebrae).25,66 Bone scan and magnetic resonance imaging can further define the cause of bone loss (Figure 41-7), if needed.
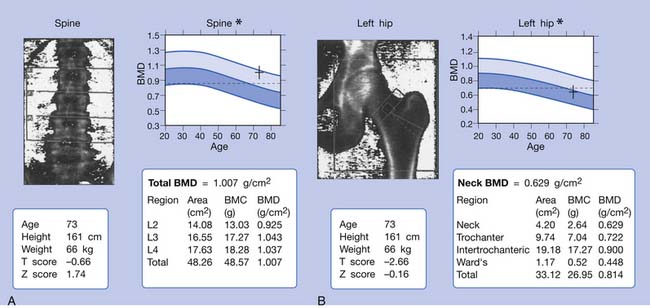
Osteoporosis is typically not visible on conventional radiographs until at least 25% to 30% of bone mineral has been lost. Consequently, evaluation of BMD through absorptiometry techniques is recommended.104 These measurements are also helpful in treatment because calculated bone loss or gain is required in therapeutic trials of agents affecting bone mass. The different methods for evaluation of bone mass have different levels of precision.105 Available methods include photon absorptiometry (single or dual), finger x-ray spectrometry, ultrasound densitometry, qualitative computed tomography, and dual-energy x-ray absorptiometry. The most commonly used technique is dual-energy x-ray absorptiometry. It has high precision and is frequently used for research and clinical evaluations to measure the BMD of the spine and hips (Figure 41-8). More commonly measured is the BMD of the femoral neck because spinal bone density can be erroneously high as a result of osteoarthritis of the spine. It also can be used to measure total-body bone mass. It is x-ray–based and has a precision of about 1%. The amount of radiation used is less than 3 mrad.104
Treatment
Osteoporosis treatment is best done with a team approach because it is a multifactorial condition.4 Endocrine consultation is needed, along with interventions from specialists in physical medicine and rehabilitation, pharmacology, psychology, and nutrition.44 The World Health Organization has defined osteoporosis as a T score of less than −2.5. Detailed definitions of normal BMD, osteopenia, and osteoporosis are provided earlier in the text of this chapter. These definitions facilitate decision making for therapeutic trials. They are also helpful for prescription of a proper exercise program.
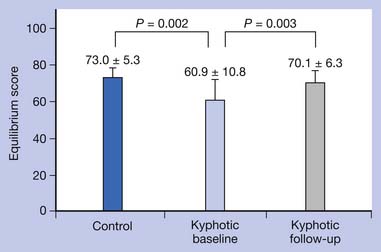
Because fractures generally occur with falls, the prevention of falls decreases the risk of fracture.66 A recent study showed that patients with osteoporosis who had kyphosis were significantly more at risk for falls than control subjects without osteoporosis-kyphosis.92 A recent controlled trial demonstrated significant improvement in risk for falls.93 The spinal proprioceptive extension exercise dynamic (SPEED) program shows decreased step width, improved steadiness of gait on gait laboratory testing, decreased risk for falls at obstacles, and increased velocity, cadence, and stride length.93 Through use of a WKO and SPEED program, back pain decreased and level of physical activity increased. The SPEED program opens a new area of investigation for reducing risk for falls in kyphotic, osteoporotic individuals with balance disorder.93
Exercise
The efficacy of exercise for improving bone mass is supported by hormonal and nutritional factors (Box 41-5). To meet the challenge of mechanical load, skeletal tissue must have enough bone mass and proper architecture to withstand the physical strain that is imposed on it. Normal musculoskeletal structure is highly adaptable and can meet the challenge of usual mechanical loads. The challenge from mechanical load and strain might not be tolerated in those with osteopenia and osteoporosis without damage to the architecture of bone. A supervised, nonstrenuous, progressive, resistive exercise program can improve bone mass in inactive individuals.88 By understanding both the benefits and the shortcomings of nutritional and exercise approaches for musculoskeletal management of osteoporosis, we can create an improved prophylactic program for patients with osteoporosis.96
BOX 41-5 Suggested Rehabilitation Guidelines Based on Bone Mineral Density T Scores∗
Reduction to −2.5 SD or More (Osteoporosis)‡
† See Figures 41-10 and 41-11 for proper exercise program and posture.
‡ Osteopenia or osteoporosis as defined by the World Health Organization.88
High rates and magnitude of bone strain are produced during high-impact sports activities, such as gymnastics, badminton, tennis, volleyball, and basketball. The high-impact bone loading results in site-specific increases in BMD. One study showed a significant difference in BMD between gymnasts and volleyball players. The lower limbs are loaded differently in these two athletic activities.19 Gymnasts had higher BMD than volleyball players, except in the pelvic bone. Swimming can improve muscle strength but not bone mass.17 According to the theory of Frost,22 a minimum threshold of mechanical loading is needed to evoke an increased level of BMD. This theory is referred to as that of the minimum effective strain stimulus. Lanyon43 suggested that the greatest osteogenic effect from mechanical loading occurs when the strain is vigorous (high strain), repeated daily, short in duration, and applied to a specific bone site. Mechanical loading, when applied properly, can stimulate osteogenic activity. Axial loading of the skeleton during lifting activities at a person’s job or in the care of children can be as osteogenic as mechanical loading exercises in a gym (Figure 41-9).94 Individuals with normal BMD can perform high-impact exercises, such as aerobics, jogging, and skiing. For persons with osteoporosis, nonstraining exercises are recommended, such as walking for 45 minutes three times a week or for 30 minutes daily. Aquatic exercises are recommended for patients who are unable to perform antigravity exercises because of pain or weakness. The nonstrenuous, low-resistance exercises can be advanced to antigravity and strengthening exercises as permitted by a patient’s musculoskeletal status.
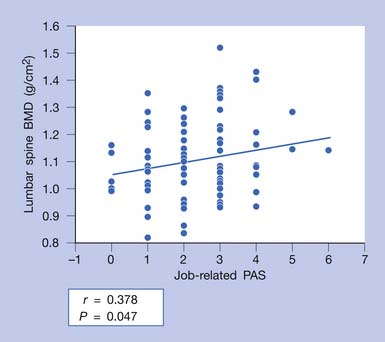
FIGURE 41-9 Job-related physical activity score (PAS) significantly correlated with spinal bone mineral density (BMD).
(Modified from Sinaki M, Fitzpatrick LA, Ritchie CK, et al: Site-specificity of bone mineral density and muscle strength in women: job-related physical activity, Am J Phys Med Rehabil 77:470-476, 1998, used with permission.)
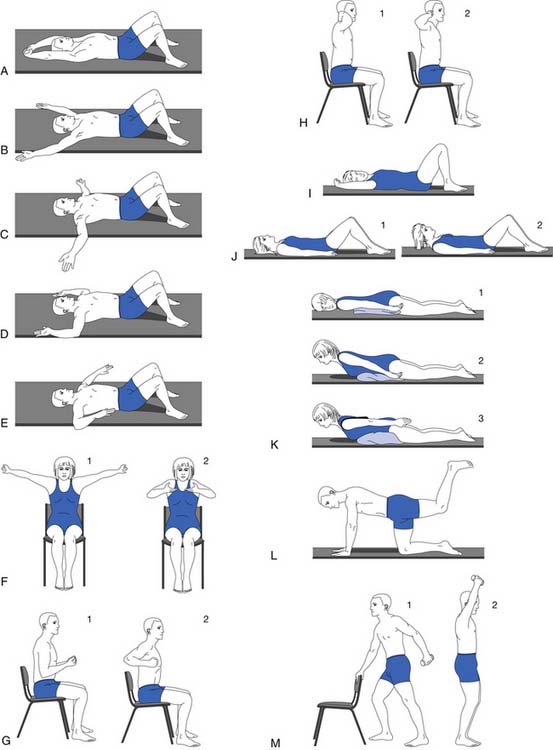
Spinal extension exercises should be used along with exercises to reduce lumbar lordosis.78,86 One study showed that progressive resistive back-strengthening exercises can improve back strength significantly.84 The most effective back-strengthening exercise continues to be progressive resistive back extension exercise.29 Weakness in abdominal muscles adds to the problems of poor posture and protruded abdomen. To complement a posture training exercise program, isometric abdominal muscle-strengthening exercises should be included (Figures 41-10 and 41-11). The author’s osteoporosis back exercise program has been studied extensively through controlled trials and has been proven to be safe and effective.29,33,81,95,91
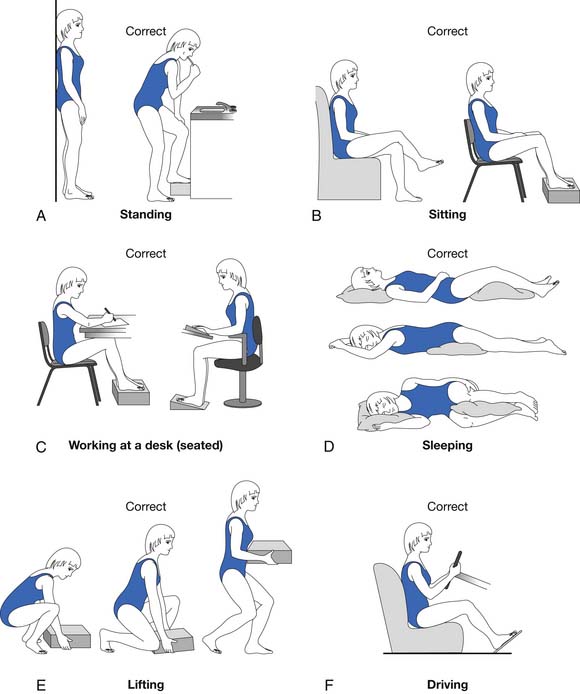
FIGURE 41-11 Static and dynamic correct postures.
(Modified from Sinaki M, Mokri B: Low back pain and disorders of the lumbar spine. In Braddom RL, editor: Physical medicine and rehabilitation, Philadelphia, 1996, WB Saunders, used with permission.)
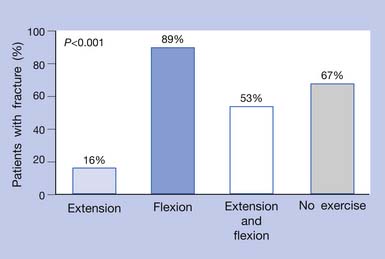
Strenuous spinal flexion and spinal flexion exercises should be avoided in patients with osteoporosis (Figure 41-12).81 In a study by Sinaki and Mikkelsen81 comparing the effect of flexion and extension exercises on the spine, it was demonstrated that, even without pharmacotherapy, patients with osteoporosis who performed back extension exercises (see Figure 41-10, K) had a significantly lower rate of fracture than those who performed spinal flexion exercises or no exercise. Osteoporotic women generally have weaker back extensors than healthy women of comparable age (Figure 41-13). The choice of physical activity is important and has to be individualized. Fitness programs, such as swimming or short periods of stationary biking, are not sufficiently osteogenic58 but can fulfill the need for cardiovascular fitness without straining the osteoporotic frame. Walking for 40 minutes at least three times a week is effective for maintaining lower-limb bone density. Knowledge of a person’s BMD is helpful before recommending a weight-training program.88
Posture Training Program and the Osteoporotic Skeletal Frame
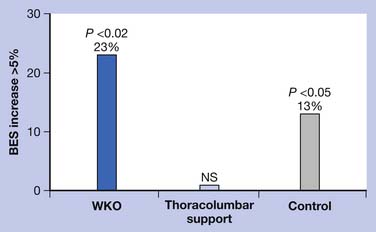
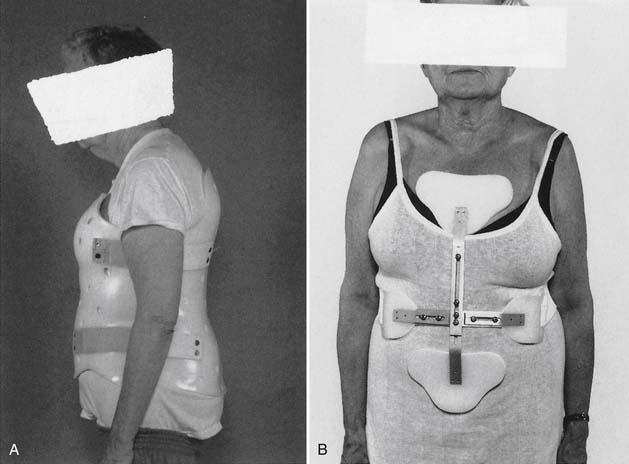
Any support that can improve posture and decrease pain-related paraspinal muscle cocontraction is desirable. The number and diameter of collagen fibrils of the intervertebral disks decrease with aging. This reduction results in loss of resiliency of the disks. In addition, reduced paraspinal muscle strength90 and forward tendency of head and trunk related to the effect of gravity can cause iliocostal friction syndrome and flank pain. This pain does not respond to the use of conventional orthoses. Indeed, orthoses such as corsets can make the pain worse through pressure over the lower rib cage (Figure 41-14). Postural training programs that are intended to decrease kyphosis can also subsequently reduce iliocostal friction syndrome.39,74 One study showed that use of a WKO and exercise increased back strength more significantly (p <0.02) than back exercise alone.39 The same study also showed that use of a thoracolumbar support interfered with improving back strength with exercise (Figure 41-15). Posture training programs, such as the application of a WKO for 20 minutes two to three times a day in cases of severe kyphosis or less frequently in milder cases while contracting the back extensors, can provide reeducation of the paraspinal muscles for improvement of kyphotic posturing and reduction of the risk of falls (Figure 41-16).83 In some severe cases, back extension is not possible without use of a WKO. The SPEED program is also based on reeducation of the back extensors and proprioceptors of the spine.93 Furthermore, proper use of WKO can decrease back and flank pavin and osteoporosis.74,76
Orthoses and the Osteoporotic Spine
Acute compression fracture usually results in severe pain and, if not managed well, can lead to prolonged immobility. The final outcome is creation of chronic pain behavior and subsequent psychologic consequences. Acute pain needs to be actively managed with proper physical measures. Sedative physical therapy, including application of initially cold and later heat, and isometric muscle contractions of the paraspinal muscles can be helpful. Rigid thoracolumbar orthoses to promote extension of the spine are helpful (Figure 41-17) (also see Chapter 16). If thoracolumbar orthoses are not tolerated because of postural changes, a thoracic WKO (Figure 41-18) or a combination of a kypho-orthosis and lower-back support (elastic abdominal support) might suffice. In some cases, long-distance ambulatory activities might require use of a cane or a wheeled walker. Temporary use of a wheelchair with a supportive back cushion is indicated in some cases. Every effort needs to be taken to prevent falls, including having the patient confined to one room or under prolonged bed rest. Immobility should be limited to avoid its resulting reactive depression. Safety during ambulation is paramount, and prevention of falls and fracture should be taught in the rehabilitative program for patients with osteoporosis. Implementation of the SPEED program can decrease posture-related back pain and increase the level of physical activity.93
Pharmacologic Interventions
Pharmacotherapy is essential for improving bone mass, but its efficacy in prevention of skeletal deformities depends on musculoskeletal rehabilitation. Over the years, through many research studies, the efficacy of measures implemented in rehabilitation of osteoporosis has been substantiated. Of course, the best osteoporosis treatment is prevention by improving back strength76,82 and peak bone mass before the age of 30 years87 and reducing bone loss thereafter. Muscle strength and exercise play significant roles in the prevention of vertebral fractures91 and falls.78 Calcium, vitamin D, and bisphosphonates are the most commonly advocated pharmacologic treatments for involutional osteoporosis. Antiresorptive agents include estrogens, androgens, calcitonin, and bisphosphonates. Osteoblast stimulator agents include fluoride and PTH. Fluoride is not approved by the U.S. Food and Drug Administration at this time for the treatment of osteoporosis because of an increased risk of appendicular fractures (Table 41-2).
Studies on the effect of PTH(1-34) (teriparatide) on fractures and BMD have been very promising. Teriparatide and PTH(1-34) decrease the risk of vertebral fractures and increase vertebral, femoral, and total body BMD.40,42 PTH is self-administered through subcutaneous injections. To maintain PTH-induced gains after an 18- to 24-month drug therapy course, antiresorptive agents need to be considered.54 There can reportedly be a 9% to 13% increase in BMD of the spine and a 65% to 69% reduction in risk of new vertebral fractures in patients receiving the treatment compared with those receiving placebo.54 PTH therapy is contraindicated in patients who have a history of cancer. Adverse effects include nausea, dizziness, leg cramps, headache, and hypercalcemia. Investigators are currently evaluating new agents that might improve bone mass. In one randomized study, receipt of a 15-minute infusion of zoledronate (5 mg) or placebo once yearly showed reduced incidence of vertebral fracture by 70% and of hip fracture by 41% in the treatment group compared with the placebo group over the 3-year trial.3 Osteonecrosis of the jaw has been reported to occur in patients taking bisphosphonates, and a warning regarding this adverse effect has recently been added to the labeling of all bisphosphonates.
Cessation of tobacco and alcohol abuse is necessary. An adequate calcium intake is required to permit normal bone development and, potentially, to decrease bone loss. Adequate calcium and vitamin D intakes appear to have only a modest effect on bone loss after menopause. Inadequate intakes of calcium and vitamin D are common, especially in elderly residents of nursing homes. One study showed the efficacy of supplementation of calcium and vitamin D for reduction of the risk of hip fracture in elderly subjects.8 Typical recommendations for women with hormone deficiency are 1500 mg of elemental calcium daily in divided doses and between 800 and 1000 international units of vitamin D daily. The recommended dose of vitamin D varies and depends on the patient’s exposure to the sun and dietary intake of vitamin D. It is necessary to determine the serum level of 1,25(OH)2D3 in some cases (normal levels are at least 30 to 35 ng/mL and preferably 25 to 75 ng/mL). These values can differ in different laboratories and locations of the United States.
Estrogen acts directly on bone cells and is an antiresorptive agent that has been shown to decrease the rate of bone loss and fractures in postmenopausal women, whether their menopause is natural or surgical. Estrogen is not as commonly used now because of the alarming results of the Women’s Health Initiative studies.2,18,26 When a patient with an intact uterus is treated with estrogen, progesterone also should be used under a proper regimen to prevent endometrial hyperplasia and possibly endometrial carcinoma. There are several regimens for estrogen and progesterone use. These agents may be used concurrently (combination pills) or cyclically. The proper regimen needs to be individualized.
Contraindications to estrogen replacement therapy include liver or gallbladder disease, recent history of thromboembolism or thrombophlebitis, and suspected breast or endometrial carcinoma. Estrogens also can have an adverse effect on existing hypertension, hyperlipidemia, migraine headaches, chronic thrombophlebitis, and endometriosis. Administration of progestins can result in uncomfortable adverse effects, such as fatigue, depression, breast tenderness, bloating, menstrual cramps, and headaches.60 Other potential adverse effects are weight gain, depression or mood change, increased serum triglyceride and glucose levels, and abnormal vaginal bleeding. The role of hormone replacement therapy for prevention and treatment of osteoporosis has been modified extensively.
Estrogen protects against both osteoporosis and cardiovascular disease. However, many postmenopausal women discontinued their use of hormone replacement therapy after the results of the Women’s Health Initiative studies became available.10,41 Of all breast cancer cases, 78% occur in women after age 50 years. There are reports that hormone replacement therapy increases the risk of breast cancer by 2.3% per year, and this risk increases to 3.5% per year after 5 years. Women who have cardiovascular disease should not use hormone replacement therapy for prevention. Estrogen therapy should also be discontinued if a woman has an acute cardiovascular event. In general, decision making for use of the therapy is better based on noncoronary benefit. The patient’s preference is important for management of menopausal symptoms. Prevention of osteoporosis is no longer considered an indication for use of estrogen therapy.
Calcitonin, an antiresorptive agent, acts directly on the osteoclasts. Calcitonin has a few disadvantages that limit its use.61 It is most effective in patients whose rate of bone turnover is high. Calcitonin is approved for treatment of established osteoporosis, but the long-term fracture-reducing efficacy of calcitonin has not been clearly demonstrated. The subcutaneous or intramuscular injection of 50 to 100 units of salmon calcitonin or 0.5 mg of human calcitonin, given every other day, is commonly used. Use of nasal calcitonin might improve a patient’s compliance; the adverse effects of parenteral use, such as flushing and nausea, and development of antibodies may limit its use. The nasal spray may cause nasal irritation, crusting, and ulcerations, which usually require discontinuation of its use.
Bisphosphonates affect trabecular bone, especially in the lumbar spine, where BMD increases of 5% to 10% occur during the first 2 years of treatment (see Table 41-2). Alendronate sodium, an aminobisphosphonate, has been shown to normalize the rate of bone turnover and increase bone mass.9 Alendronate (10 mg/day or a once-a-week dose of 70 mg) must be taken with a full glass of water on awakening. The patient should not eat or recline for 30 to 45 minutes after taking the medication because of the risk of esophageal irritation. Patient education and compliance are important for the proper use of alendronate. Risedronate is another bisphosphonate that has been used for the treatment of osteoporosis. Treatment with risedronate (5 mg/day or a once-a-week dose of 35 mg) has been shown to significantly decrease the incidence of vertebral and nonvertebral fractures in postmenopausal osteoporosis.28 Weekly doses of bisphosphonates are commonly recommended. One potential adverse effect of oral bisphosphonates is esophageal irritation, particularly in patients with reflux or other esophageal dysfunction.102 Anabolic androgenic steroids (e.g., testosterone) can increase bone and muscle mass in women with hypogonadism98 but can produce unacceptable adverse effects. Low-dose testosterone therapy has been used by some women. Anabolic steroids have an osteoblastic effect. They are used only under the most extreme circumstances, however, because they can have marked androgenic effects and induce liver function abnormalities. Thiazide diuretics inhibit urinary excretion of calcium and can retard bone loss and reduce the rate of fractures in patients with osteoporosis.
Table 41-2 FDA-Approved Medications for Use in Postmenopausal Osteoporosis
| Medication | Prevention | Treatment (Dose) |
|---|---|---|
| Bone-Antiresorptive Agents | ||
| Estrogen | ? | No |
| Alendronate (Fosamax) | Yes | 10 mg/day or 70 mg/wk orally |
| Risedronate (Actonel) | Yes | 5 mg/day or 35 mg/wk orally |
| Ibandronate sodium (Boniva) | Yes | 150 mg/mo orally or 3 mg intravenously every 3 mo |
| Zoledronic acid | No | 5 mg intravenously every 12 mo |
| Raloxifene (Evista) | Yes | 60 mg/day orally |
| Calcitonin (Miacalcin) | No | 200 international units/day intranasally |
| Bone-Forming Agent | ||
| Teriparatide (Forteo) | No | 20 mcg/day subcutaneously |
| Also Required | ||
FDA, Food and Drug Administration.
Side effects:
Estrogen: Breast cancer, endometrial cancer, etc., if used without progestin therapy.
Bisphosphonates: Esophagitis, rare occurrence of jaw osteonecrosis.
Raloxifene: Deep vein thrombosis, hot flashes, nausea, leg cramps, cerebrovascular accident.
Calcitonin: See chapter text.
∗ Includes food and supplements.
The estrogen receptor mixed agonist–antagonists tamoxifen and raloxifene protect against bone loss in ovariectomized rats. They have an antiestrogenic effect on breast tissue. These agents also are known as selective estrogen receptor modulators.11 The mechanism by which these compounds affect bone is not completely defined. Recent studies in humans have been promising,47 but the percentage increment in BMD has not been as much as that with alendronate sodium. One of the adverse effects related to tamoxifen treatment is uterine hyperplasia, but this effect is not a concern with raloxifene treatment. Raloxifene decreases serum total cholesterol and low-density lipoprotein cholesterol levels. Raloxifene (60 mg/day orally) is currently used only in the postmenopausal stage of osteoporosis. Potential adverse effects are leg cramps, hot flashes, and deep vein thrombosis.
Management of steroid-induced osteoporosis requires calcium and vitamin D supplementation, use of oral antiresorptive agents such as alendronate sodium (70 mg once a week) or risedronate (35 mg once a week), and implementation of a proper weight-bearing and weight-training exercise program. In advanced stages of bone loss and muscle weakness, when fragility occurs, use of assistive devices or a wheelchair might be necessary.68 If hyperparathyroidism or thyrotoxicosis is present, proper management should be implemented. In the case of hyperparathyroidism, surgical removal of the parathyroid adenoma is recommended.
In regard to osteoporosis, the distinct effects of nutrition, exercise, hormones, and lifestyle cannot be separated.73 A patient’s quality of life can certainly be affected by musculoskeletal challenges related to osteoporosis. Practical treatment of patients with osteoporosis requires pharmacologic interventions, physical and rehabilitative measures, and good nutrition. It also requires consideration of the psychologic consequences and reactions experienced by the patient. Public education can contribute to prevention, better understanding, and management of the consequences of osteoporosis. The National Osteoporosis Foundation and the International Osteoporosis Foundation are excellent educational sources.
1. Adams P., Eyre D.R., Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehabil. 1977;16:22-29.
2. Banks E., Canfell K. Invited commentary: Hormone therapy risks and benefits: the Women’s Health Initiative findings and the postmenopausal estrogen timing hypothesis (comment). Am J Epidemiology. 2009;170(1):24-28.
3. Black D.M., Delmas P.D., Eastell R., et al. HORIZON Pivotal Fracture Trial: once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
4. Bonner F.J.Jr., Sinaki M., Grabois M., et al. Health professional’s guide to rehabilitation of the patient with osteoporosis. Osteoporos Int. 2003;2(14 suppl):S1-S22.
5. Buchbinder R., Osborne R.H., Ebeling P.R., et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009 Aug 6;361(6):557-568.
6. Cassell C., Benedict M., Specker B. Bone mineral density in elite 7- to 9-yr-old female gymnasts and swimmers. Med Sci Sports Exerc. 1996;28:1243-1246.
7. Castelo-Branco C., Reina F., Montivero A.D., et al. Incidence of high intensity training and of dietetic and anthropometric factors on menstrual cycle disorders in ballet dancers. Gynecol Endocrinol. 2006;22(1):31-35.
8. Chapuy M.C., Arlot M.E., Duboeuf F., et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637-1642.
9. Chesnut C.H.III, McClung M.R., Ensrud K.E., et al. Alendronate treatment of the postmenopausal osteoporotic woman: effect of multiple dosages on bone mass and bone remodeling. Am J Med. 1995;99:144-152.
10. de Lignieres B. Hormone replacement therapy: clinical benefits and side-effects. Maturitas. 1996;(23 suppl):S31-S36.
11. Delmas P.D. Clinical use of selective estrogen receptor modulators. Bone. 1999;25:115-118.
12. Delmas P.D., Hardy P., Garnero P., et al. Monitoring individual response to hormone replacement therapy with bone markers. Bone. 2000;26:553-560.
13. Doyle F.H., Gutteridge D.H., Joplin G.F., et al. An assessment of radiological criteria used in the study of spinal osteoporosis. Br J Radiol. 1967;40:241-250.
14. Drinkwater B.L., Nilson K., Ott S., et al. Bone mineral density after resumption of menses in amenorrheic athletes. JAMA. 1986;256:380-382.
15. Eastell R., Hannon R.A. Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc. 2008;67(2):157-162.
16. Econs M.J., Speer M.C. Genetic studies of complex diseases: Let the reader beware. J Bone Miner Res. 1996;11:1835-1840.
17. Emslander H.C., Sinaki M., Muhs J.M., et al. Bone mass and muscle strength in female college athletes (runners and swimmers). Mayo Clin Proc. 1998;73:1151-1160.
18. Farquhar C.M., Marjoribanks J., Lethaby A., et al. Long term hormone therapy for perimenopausal and postmenopausal woman. Cochrane Database Syst Rev.. 2005 Jul 20;(3):CD004143, Review. Update in: Cochrane Database Syst Rev. 2009;(2):CD004143
19. Fehling P.C., Alekel L., Clasey J., et al. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995;17:205-210.
20. Felsing N.E., Brasel J.A., Cooper D.M. Effect of low and high intensity exercise on circulating growth hormone in men. J Clin Endocrinol Metab. 1992;75:157-162.
21. Fredericson M., Kent K. Normalization of bone density in a previously amenorrheic runner with osteoporosis. Med Sci Sports Exerc. 2005;37(9):1481-1486.
22. Frost H.M. A determinant of bone architecture: the minimum effective strain. Clin Orthop Relat Res. 1983;175:286-292.
23. Frost H.M. Why do marathon runners have less bone than weight lifters? A vital-biomechanical view and explanation. Bone. 1997;20:183-189.
24. Garnero P., Darte C., Delmas P.D. A model to monitor the efficacy of alendronate treatment in women with osteoporosis using a biochemical marker of bone turnover. Bone. 1999;24:603-609.
25. Genant H.K., Vogler J.B., Block J.E. Radiology of osteoporosis. In: Riggs B.L., Melton L.J.3rd, editors. Osteoporosis: etiology, diagnosis, and management. New York: Raven Press, 1988.
26. Grady D., Herrington D., Bittner V., et al. HERS Research Group: Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57. Erratum in: JAMA 2002 Sep 4;288(9):1064
27. Gutmann E. Age changes in the neuromuscular system and aspects of rehabilitation medicine. In: Buerger A.A., Tobis J.S., editors. Neurophysiologic aspects of rehabilitation medicine: proceedings of the International Conference on Neurophysiologic Aspects of Rehabilitation Medicine. Irvine. Springfield, IL: University of California, January 1974. Charles C Thomas, 1976
28. Harris S.T., Watts N.B., Genant H.K., et al. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group: effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344-1352.
29. Hongo M., Itoi E., Sinaki M., et al. Effects of reducing resistance, repetitions, and frequency of back-strengthening exercise in healthy young women: a pilot study. Arch Phys Med Rehabil. 2005;86:1299-1303.
30. Hui S.L., Slemenda C.W., Johnston C.C.Jr. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804-1809.
31. Huntoon E.A., Schmidt C.K., Sinaki M. Significantly fewer refractures after vertebroplasty in patients who engage in back-extensor-strengthening exercises. Mayo Clin Proc. 2008;83:54-57.
32. Ireland P., Fordtran J.S. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J Clin Invest. 1973;52:2672-2681.
33. Itoi E., Sinaki M. Effect of back-strengthening exercise on posture in healthy women 49 to 65 years of age. Mayo Clin Proc. 1994;69:1054-1059.
34. Jackson J.A., Kleerekoper M. Osteoporosis in men: diagnosis, pathophysiology, and prevention. Medicine (Baltimore). 1990;69:137-152.
35. Jensen M.E., Evans A.J., Mathis J.M., et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897-1904.
36. Jones E.T., Hensinger R.N. Spinal deformity in idiopathic juvenile osteoporosis. Spine. 1981;6:1-4.
37. Kallmes D.F., Comstock B.A., Heagerty P.J., et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569-579.
38. Kannus P., Parkkari J., Niemi S., et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506-1513.
39. Kaplan R.S., Sinaki M., Hameister M.D. Effect of back supports on back strength in patients with osteoporosis: a pilot study. Mayo Clin Proc. 1996;71:235-241.
40. Khosla S. Increasing options for the treatment of osteoporosis (editorial). N Engl J Med. 2009;361(8):818-820.
41. Khosla S. Role of hormonal changes in the pathogenesis of osteoporosis in men. Calcif Tissue Int. 2004;75:110-113.
42. Khosla S., Melton L.J. Clinical practice: Osteopenia. N Engl J Med. 2007;356(22):2293-2300.
43. Lanyon L.E. Using functional loading to influence bone mass and architecture: Objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;(18 Suppl):37S-43S.
44. Lindsay R. Estrogen deficiency. In Riggs B.L., Melton L.J.3rd, editors: Osteoporosis: etiology, diagnosis, and management, 2nd ed, Philadelphia: Lippincott-Raven Publishers, 1995.
45. Lindsay R., Cosman F., Herrington B.S., et al. Bone mass and body composition in normal women. J Bone Miner Res. 1992;7:55-63.
46. Lindsay R., Hart D.M., Sweeney A., et al. Endogenous oestrogen and bone loss following oophorectomy. Calcif Tissue Res. 1977;(22 suppl):213-216.
47. Lufkin E.G., Whitaker M.D., Nickelsen T., et al. Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res. 1998;13:1747-1754.
48. Lynn S.G., Sinaki M., Westerlind K.C. Balance characteristics of persons with osteoporosis. Arch Phys Med Rehabil. 1997;78:273-277.
49. Matkovic V., Heaney R.P. Calcium balance during human growth: evidence for threshold behavior. Am J Clin Nutr. 1992;55:992-996.
50. McComas A.J., Fawcett P.R., Campbell M.J., et al. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:121-131.
51. Melton L.J.3rd, Kan S.H., Frye M.A., et al. Epidemiology of vertebral fractures in women. Am J Epidemiol. 1989;129:1000-1011.
52. Mosley J.R., Lanyon L.E. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone. 1998;23:313-318.
53. National Osteoporosis Foundation. Capitol Hill rallies Americans to take a walk! America walks for strong women. Washington, DC: National Osteoporosis Foundation; Aug 5, 1988.
54. Neer R.M., Arnaud C.D., Zanchetta J.R., et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434-1441.
55. Nevitt M.C., Cummings S.R., Hudes E.S. Risk factors for injurious falls: A prospective study. J Gerontol. 1991;46:M164-M170.
56. Nordin B.E., Horsman A., Crilly R.G., et al. Treatment of spinal osteoporosis in postmenopausal women. Br Med J. 1980;280:451-455.
57. Peck W.A., Riggs B.L., Bell N.H., et al. Research directions in osteoporosis. Am J Med. 1988;84:275-282.
58. Petrie R.S., Sinaki M., Squires R.W., et al. Physical activity, but not aerobic capacity, correlates with back strength in healthy premenopausal women from 29 to 40 years of age. Mayo Clin Proc. 1993;68:738-742.
59. Pfeifer M., Sinaki M., Geusens P., et al. ASBMR Working Group on Musculoskeletal Rehabilitation: Musculoskeletal rehabilitation in osteoporosis—review. J Bone Miner Res. 2004;19:1208-1214.
60. Prelevic G.M., Bartram C., Wood J., et al. Comparative effects on bone mineral density of tibolone, transdermal estrogen and oral estrogen/progestogen therapy in postmenopausal women. Gynecol Endocrinol. 1996;10:413-420.
61. Riggs B.L. Overview of osteoporosis. West J Med. 1991;154:63-77.
62. Riggs B.L., Melton L.J.3rd. Involutional osteoporosis. N Engl J Med. 1986;314:1676-1686.
63. Riggs B.L., Wahner H.W., Melton L.J.3rd, et al. Rates of bone loss in the appendicular and axial skeletons of women: evidence of substantial vertebral bone loss before menopause. J Clin Invest. 1986;77:1487-1491.
64. Robinovitch S.N., Inkster L., Maurer J., et al. Strategies for avoiding hip impact during sideways falls. J Bone Miner Res. 2003;18:1267-1273.
65. Rodan G.A., Rodan S.B. The cells of bone. In Riggs B.L., Melton L.J.3rd, editors: Osteoporosis: etiology, diagnosis, and management, ed 2, Philadelphia: Lippincott-Raven Publishers, 1995.
66. Sartoris D.J., Clopton P., Nemcek A., et al. Vertebral-body collapse in focal and diffuse disease: patterns of pathologic processes. Radiology. 1986;160:479-483.
67. Seeman E. The dilemma of osteoporosis in men. Am J Med. 1995;98:76S-88S.
68. Sinaki M. exercise and physical therapy. In: Riggs B.L., Melton L.J.3rd, editors. Osteoporosis: etiology, diagnosis, and management. New York: Raven Press, 1988.
69. Sinaki M. Beneficial musculoskeletal effects of physical activity in the older woman. Geriatr Med Today. 1989;8:53-59.
70. Sinaki M. Exercise and osteoporosis. Arch Phys Med Rehabil. 1989;70:220-229.
71. Sinaki M. Musculoskeletal rehabilitation. In Riggs B.L., Melton L.J.3rd, editors: Etiology, Diagnosis, and Management, ed 2, Philadelphia: Lippincott-Raven Publishers, 1995. Osteoporosis
72. Sinaki M. Effect of physical activity on bone mass. Curr Opin Rheumatol. 1996;8:376-383.
73. Sinaki M: Spectrum and management of musculoskeletal changes in osteoporosis. In The Official Program, IX Congresso Nazionale Societa Italiana dell’Osteoporosi e delle Malattie Metaboliche dell’Osso, Parma, Italy, October 1-4, 1997.
74. Sinaki M. Musculoskeletal challenges of osteoporosis. Aging Clin Exp Res. 1998;10:249-262.
75. Sinaki M. Metabolic bone disease. In Sinaki M., editor: Basic clinical rehabilitation medicine, 2nd ed, St. Louis: Mosby, 1993.
76. Sinaki M. Nonpharmacologic interventions: exercise, fall prevention, and role of physical medicine. Clin Geriatr Med. 2003;19:337-359.
77. Sinaki M. Falls, fractures, and hip pads. Curr Osteoporos Rep. 2004;2:131-137.
78. Sinaki M. Critical appraisal of physical rehabilitation measures after osteoporotic vertebral fracture. Osteoporos Int. 2003;14:773-779. Erratum in: Osteoporos Int. 17(11):1702, 2006
79. Sinaki M. The role of physical activity in bone health: a new hypothesis to reduce risk of vertebral fracture. Phys Med Rehabil Clin N Am. 2007;18:593-608.
80. Sinaki M: Spinal Proprioception Extension Exercise Dynamic (S.P.E.E.D.) Program for relief of back and illocosial fricion syndrome: a painful challenge of spinal osteoporosis, [abstract], ASBMR 834, 2010
81. Sinaki M., Mikkelsen B.A. Postmenopausal spinal osteoporosis: Flexion versus extension exercises. Arch Phys Med Rehabil. 1984;65:593-596.
82. Sinaki M., Offord K.P. Physical activity in postmenopausal women: Effect on back muscle strength and bone mineral density of the spine. Arch Phys Med Rehabil. 1988;69:277-280.
83. Sinaki M., Lynn S.G. Reducing the risk of falls through proprioceptive dynamic posture training in osteoporotic women with kyphotic posturing: A randomized pilot study. Am J Phys Med Rehabil. 2002;81:241-246.
84. Sinaki M., Wahner H.W., Offord K.P., et al. Efficacy of nonloading exercises in prevention of vertebral bone loss in postmenopausal women: a controlled trial. Mayo Clin Proc. 1989;64:762-769.
85. Sinaki M., Grubbs N.C. Back strengthening exercises: quantitative evaluation of their efficacy for women aged 40 to 65 years. Arch Phys Med Rehabil. 1989;70:16-20.
86. Sinaki M., Itoi E., Rogers J.W., et al. Correlation of back extensor strength with thoracic kyphosis and lumbar lordosis in estrogen-deficient women. Am J Phys Med Rehabil. 1996;75:370-374.
87. Sinaki M., Limburg P.J., Wollan P.C., et al. Correlation of trunk muscle strength with age in children 5 to 18 years old. Mayo Clin Proc. 1996;71:1047-1054.
88. Sinaki M., Wahner H.W., Bergstralh E.J., et al. Three-year controlled, randomized trial of the effect of dose-specified loading and strengthening exercises on bone mineral density of spine and femur in nonathletic, physically active women. Bone. 1996;19:233-244.
89. Sinaki M., Wollan P.C., Scott R.W., et al. Can strong back extensors prevent vertebral fractures in women with osteoporosis? Mayo Clin Proc. 1996;71:951-956.
90. Sinaki M., Nwaogwugwu N.C., Phillips B.E., et al. Effect of gender, age, and anthropometry on axial and appendicular muscle strength. Am J Phys Med Rehabil. 2001;80:330-338.
91. Sinaki M., Itoi E., Wahner H.W., et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836-841.
92. Sinaki M., Brey R.H., Hughes C.A., et al. Balance disorder and increased risk of falls in osteoporosis and kyphosis: significance of kyphotic posture and muscle strength. Osteoporos Int. 2005;16:1004-1010.
93. Sinaki M., Brey R.H., Hughes C.A., et al. Significant reduction in risk of falls and back pain in osteoporotic-kyphotic women through a Spinal Proprioceptive Extension Exercise Dynamic (SPEED) program. Mayo Clin Proc. 2005;80:849-855.
94. Sinaki M., Fitzpatrick L.A., Ritchie C.K., et al. Site-specificity of bone mineral density and muscle strength in women: job-related physical activity. Am J Phys Med Rehabil. 1998;77:470-476.
95. Sinaki M., Borgo M.J., Itoi E. An effective progressive resistive exercise program from prone position for paravertebral muscles to reduce risk of vertebral fractures [abstract]. J Bone Miner Res. (23 suppl 1):2008. s495
96. Sinaki M, Hurley D, Wermers R, et al: Vertebral compression fracures resulting from strenuous decreational exercise: when good intentons cirumble; [abstract], Proc ASBMR 834, 2010
97. Sinaki M: Pfeifer M, Preisinger E, et al: The role of exercise in the treatment of osteoporosis, Curr Osteoporos Rep 8(3):138-144, 2010
98. Snyder P.J., Peachey H., Hannoush P., et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
99. Stevenson J.C. Regulation of calcitonin and parathyroid hormone secretion by oestrogens. Maturitas. 1982;4:1-7.
100. Stevenson J.C., Abeyasekera G., Hillyard C.J., et al. Calcitonin and the calcium-regulating hormones in postmenopausal women: effect of oestrogens. Lancet. 1981;1:693-695.
101. Tinetti M.E., Doucette J., Claus E., et al. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43:1214-1221.
102. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004.
103. van Schoor N.M., Smit J.H., Twisk J.W., et al. Prevention of hip fractures by external hip protectors: a randomized controlled trial. JAMA. 2003;289:1957-1962.
104. Wahner H.O., Fogelman I. The Evaluation of osteoporosis: dual energy X-ray absorptiometry in clinical practice. London: M. Dunitz; 1994.
105. Wahner H.W., Dunn W.L., Brown M.L., et al. Comparison of dual-energy x-ray absorptiometry and dual photon absorptiometry for bone mineral measurements of the lumbar spine. Mayo Clin Proc. 1988;63:1075-1084.
106. Warner M.P., Chua A.T. Exercise-induced amenorrhea and bone health in the adolescent athlete. Ann N Y Acad Sci. 2008;135:244-252.
107. WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser. 1994;843:1-129.