65. Osteogenesis Imperfecta
Definition
Osteogenesis imperfecta (OI), one of the most common skeletal dysplasias, is inherited via autosomal dominant mutation(s). It is produced by defective biosynthesis of type I collagen, which results in brittle, osteoporotic bones that are easily fractured. Osteogenesis imperfecta is also known as Lobstein disease, brittle-bone disease, blue sclera disease, and fragile bone disease.
Incidence
Osteogenesis imperfecta is estimated to occur in 1:20,000 to 1:30,000 births.
Etiology
Type I collagen occurs naturally as a triple helix whose constituents are two copies of the α 1-chain and one copy of the α 2-chain. The pro–α 1-chain is encoded by the COL1A gene located on chromosome17, whereas the pro–α 2-chain is encoded by the COL2A gene on chromosome 2. Quantitative defects of type I collagen are the result of mutations on the COL1A gene. Qualitative defects of type I collagen result from mutations of either the COL1A or COL2B gene, producing a mixture of both normal and abnormal collagen chains. The disease does not exhibit any predilection for a particular race, ethnic group, or gender.
Signs and Symptoms
• Barrel chest
• Blue sclera
• Constipation
• Defective dentition
• Fractures
• Growth retardation
• Hearing loss
• Joint laxity
• Kyphosis
• Limb deformities
• Macrocephaly
• Scoliosis
• Sweating
• Triangular face
• Wormian bones
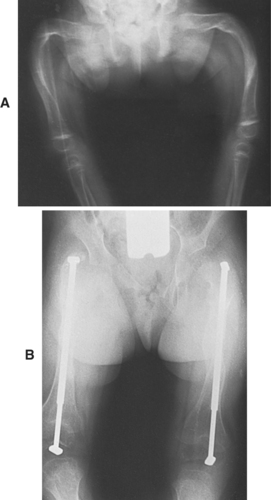 |
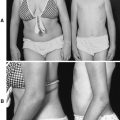
| Osteogenesis Imperfecta. A, Severe deformity of both femurs. B, Same individual after multiple osteotomies with telescoping medullary rod fixation. |
Medical Management
In the past, the primary interventions for OI consisted of deformity correction by surgical intervention, physiotherapy, orthotic support(s), and mobility-assistance devices, such as wheelchairs. As the molecular nature of this disease has become better understood, pharmacotherapy is being increasingly employed to augment bone mass and strength. Surgical intervention is kept in reserve for functional improvement.
Synthetic pyrophosphate analogues—such as bisphosphonates (particularly pamidronate)—inhibit osteoclast-mediated bone resorption. Because osteoblastic new bone formation is unopposed, cortical thickness increases, yielding stronger bone tissue. Intravenous administration of pamidronate is effective in infants, and its administration may ameliorate the natural course of the disease. In particular, pamidronate decreases the rate of fractures, increases bone mineral density, decreases bone pain, and contributes to increased height. Growth hormone may be given to act on the growth plate and stimulate osteoblast function. The patient with a quantitative collagen defect may derive somewhat greater benefit from growth hormone administration; however, the role of growth hormone has yet to be clearly delineated.
Teriparatide is a recombinant-DNA–derived form of human parathyroid hormone that has potential application in the management of OI, but that potential application has not been defined. In addition, the risk of osteosarcoma induction has thus far prevented FDA approval of administering teriparatide to children and adolescents.
Orthotics continue to occupy a limited role in the treatment of OI—as a means of stabilizing lax joints and halting progression of deformities and fractures. Walking aids, specialized wheelchairs, and home adaptation devices help the patient maintain and improve mobility. Lower limb contractures, especially when the Achilles’ tendon is involved, may require soft tissue surgical correction. Painful and recurrent bone deformities are ideally treated using intramedullary stabilization devices, such as Sheffield rods, Bailey-Dubow rods, Rush pins, or Kirschner wires. Intermedullary stabilization is superior to fixation using plates/screws or solid nails because of the high risk of new fractures above or below the device and poor fixation.
Spinal deformities such as scoliosis and/or kyphosis are not amenable to application of braces for OI patients. The patient’s rib cage is too fragile to withstand the requisite pressure of the “counter traction” produced by the brace. In addition, the pressure from the brace could exacerbate any chest deformity(-ies) that may be present. Surgical correction is undertaken when there is acceptably “solid” bone and more than 45 degrees of curvature in mild forms of OI or if there is 30 to 35 degrees of curvature in severe manifestations of OI.
Complications
• Adverse effects from bisphosphonate administration (acute febrile reaction, mild hypocalcemia, leukopenia, transient increased bone pain)
• Basilar invagination
• Complications of rod placement (breakage, rotational deformities, rod migration)
• Congenital heart disease
• Contractures
• Cor pulmonale
• Fractures (above or below fixation site)
• Hydrocephalus
• Hyperthermia
• Platelet dysfunction
• Respiratory depression due to brainstem compression
Anesthesia Implications
Bleeding time and coagulation parameters should be determined preoperatively. The patient with OI is prone to platelet dysfunction, and thus more susceptible to increased blood loss. It may be necessary to transfuse platelets perioperatively to lessen the degree of operative bleeding.
The patient with OI may have a relatively large head and tongue, making airway management difficult. The anesthetist must exercise great care to guard against mandibular and/or facial fractures. Neck extension during mask ventilation and direct laryngoscopy must be minimized to reduce the potential for trauma or fractures and all the concomitant untoward sequelae of cervical trauma.
This disease is characterized by “soft” or brittle bone; therefore, the patient with OI is highly prone to fractures, especially if not treated with bisphosphonates. The anesthetist should be extremely careful when using tourniquets for intravenous access, because the pressure may be sufficient to produce a fracture. In a similar fashion, the patient’s arm (humerus) should be adequately padded before application of the blood pressure cuff. The anesthetist must also take great care to ensure that bony prominences and pressure points are well padded, because of the propensity for fractures resulting from seemingly very minor trauma in a patient with OI. Transfers to and from the operating room bed and stretcher must be accomplished with equal gentleness to minimize the potential for fractures.
The patient with OI should be examined visually to assess the degree of scoliosis and/or kyphosis present. Pulmonary function testing should be obtained for a patient with significant scoliosis or kyphosis to determine the degree of pulmonary encroachment the associated anomalies may have produced.
As an associated anomaly, the patient with OI is prone to hyperthermia, and so is more susceptible to a hyperthermic episode during anesthesia. Both hyperthermia without muscle rigidity and malignant hyperthermia (MH) have been documented intraoperatively in patients with OI. Therefore, the anesthetist is cautioned to treat the patient with OI in a fashion similar to that of a patient with a documented family history of MH. Known MH triggers, such as succinylcholine or halothane, should be avoided. In preparation, the anesthetist should have MH treatment medications readily available and be prepared to mechanically cool the patient before induction of general anesthesia.
Despite the above cautionary measures, no particular anesthetic technique is endorsed or recommended more than another. Regional anesthesia is acceptable; however, the nature of this disorder may make needle placement relatively complicated. Bone puncture with a spinal or epidural needle during the insertion process may result in vertebral fractures in the perioperative period as well as the pain and potential untoward sequelae attendant to vertebral fractures. In addition, interosseous injection of local anesthetic is a possibility—one that is difficult to recognize and may contribute to local anesthetic toxicity. Total intravenous anesthesia (TIVA) is an excellent choice for the conduction of anesthesia for the patient who requires general anesthesia. The choice of the medications will depend somewhat on the overall condition of the patient combined with the nature of the proposed surgical intervention.
Finally, the anesthetist must be particularly cautious when a patient with OI must be placed in any but the supine position.The patient with OI is particularly susceptible to retinal artery occlusion, and this becomes a crucial consideration when placing the patient in the prone position. In this case, hypotension, whether deliberate or incidental, should be avoided. Perioperative hypotension in the patient with OI may result in or contribute to perioperative vision loss (POVL). The anesthetist must be particularly careful to avoid external pressure on the eye globe throughout the course of the anesthesia.