5 OSTEOGENESIS
Bone formation (osteogenesis or ossification)
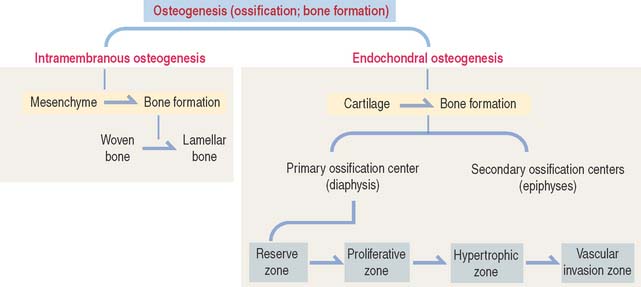
Bone develops by replacement of a preexisting connective tissue. The two processes of bone formation or osteogenesis observed in the embryo are: (1) intramembranous bone formation, in which bone tissue is laid down directly in primitive connective tissue or mesenchyme (Figures 5-1 and 5-2), and (2) endochondral bone formation, in which bone tissue replaces a preexisting hyaline cartilage, the template or anlage of the future bone (Figures 5-3 to 5-5).
Intramembranous bone formation
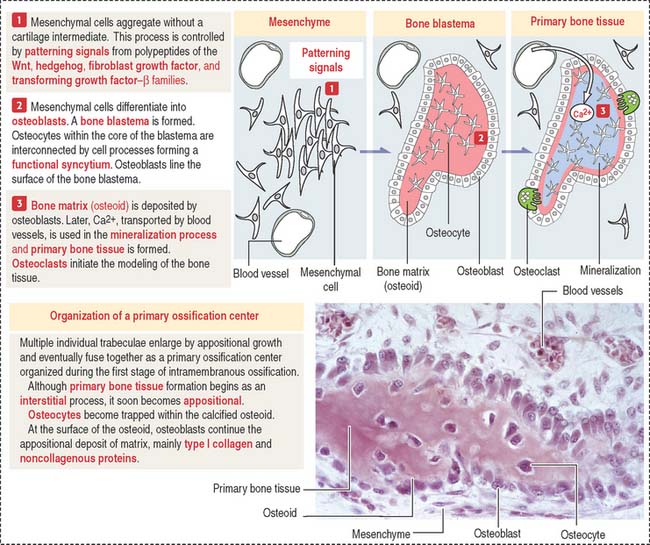
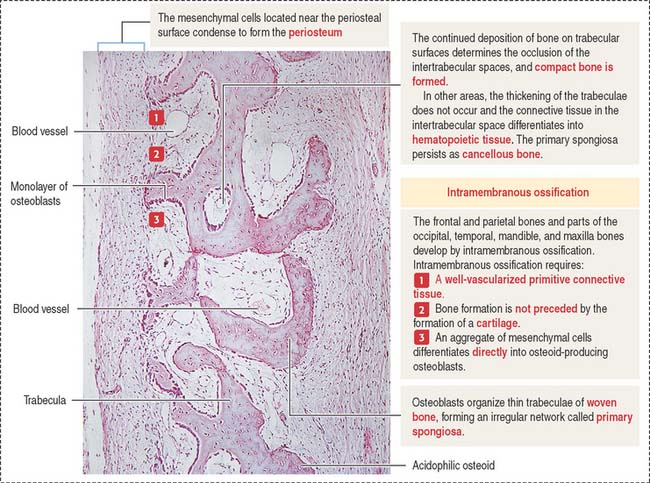
Membrane bones such as the flat bones of the skull develop by intramembranous ossification. Intramembranous ossification occurs in the following sequence (see Figure 5-1):
Box 5-A From osteoblasts to osteocytes
The final developmental events include:
Endochondral ossification
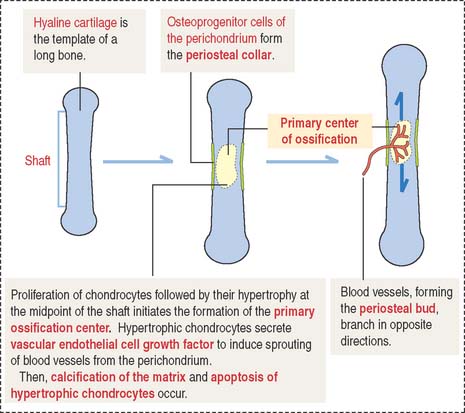
As in intramembranous ossification, a primary ossification center is formed during endochondral ossification (Figure 5-3). Unlike intramembranous ossification, this center of ossification derives from proliferated chondrocytes that have deposited an extracellular matrix containing type II collagen.
At the same time, the inner perichondrial cells exhibit their osteogenic potential, and a thin periosteal collar of bone is formed around the midpoint of the shaft, the diaphysis. Consequently, the primary ossification center ends up located inside a cylinder of bone. The periosteal collar formed under the periosteum by intramembranous ossification consists of woven bone. As we will discuss later on, the periosteal collar is converted into compact bone.
The following sequence of events defines the next steps of endochondral ossification (Figure 5-4):
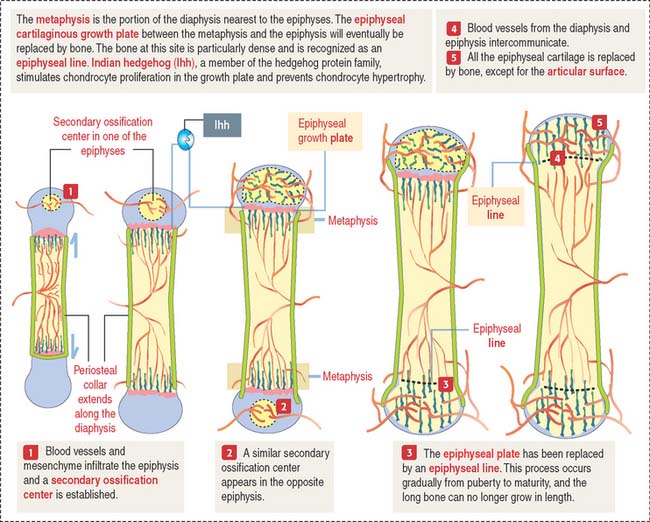
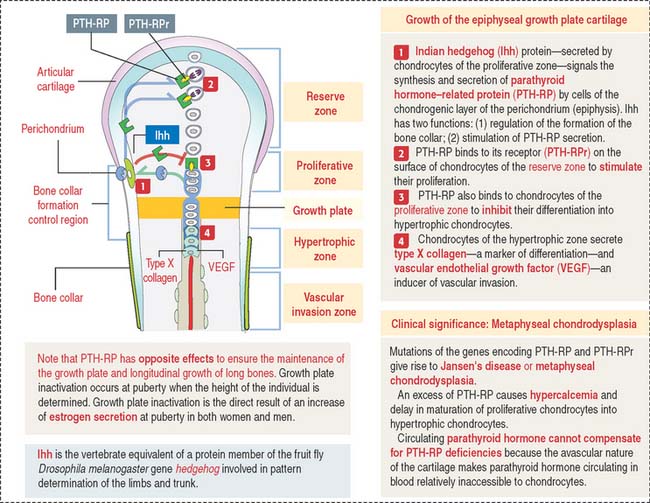
Secondary centers of ossification and the epiphyseal growth plate
After birth, secondary centers of ossification develop in the epiphyses (see Figure 5-4). As in the diaphysis, the space occupied by hypertrophic chondrocytes is invaded by blood vessels and osteoprogenitor cells from the perichondrium. Most of the hyaline cartilage of the epiphyses is replaced by the spongy bone, except for the articular cartilage and a thin disk, the epiphyseal growth plate, located between the epiphyses and the diaphysis. The epiphyseal growth plate is responsible for subsequent growth in length of the bone.
Clinical significance: The epiphyseal growth plate and dwarfism
Indian hedgehog (Ihh), a member of the hedgehog family of proteins secreted by chondrocytes, regulates chondrocyte proliferation of the growth plate in a paracrine fashion and delays chondrocyte hypertrophy (see Figure 5-9). Ihh also regulates bone formation in the perichondrial collar. A lack of expression of Ihh protein in mutant mice results in dwarfism and lack of endochondral ossification. Essentially, Ihh maintains the pool of proliferating chondrocytes in the growth plate by delaying their hypertrophy. In addition, Ihh stimulates the expression of parathyroid hormone-related peptide (PTH-RP) in perichondrial chondrocytes adjacent to the articular surface. A feedback loop between Ihh and PTH-RP regulates the balance between proliferating and hypertrophic chondrocytes.
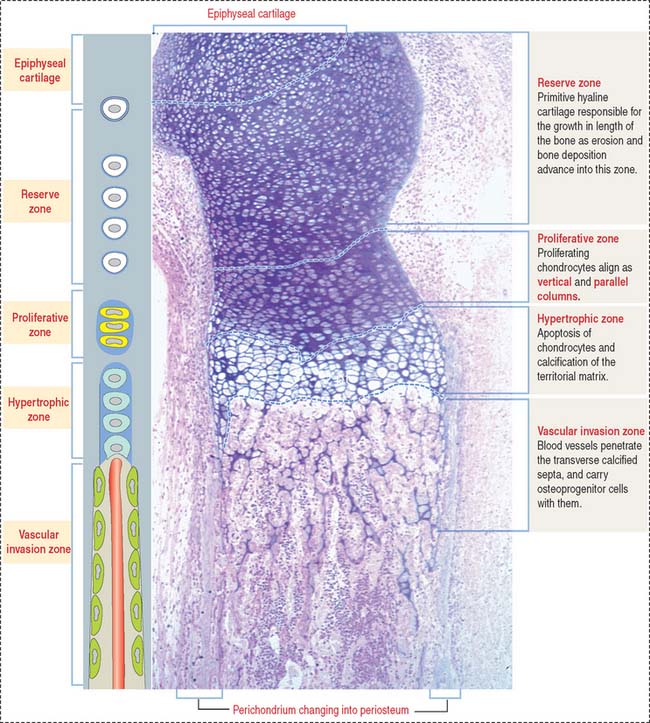
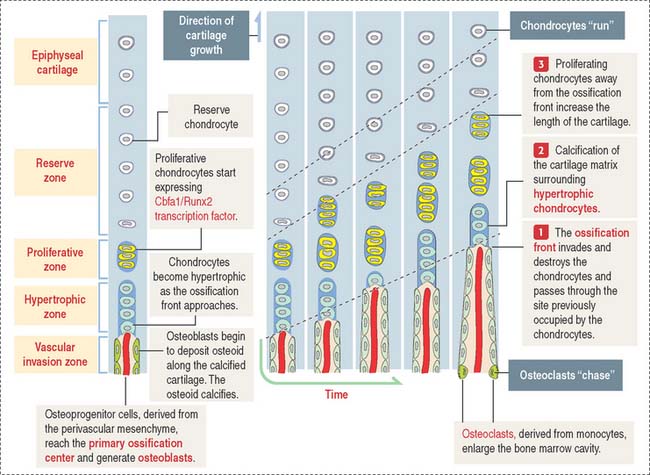
Zones of endochondral ossification
As we have seen, the deposition of bone in the center of the diaphysis is preceded by an erosion process in the hyaline cartilage template (see Figure 5-4). This center of erosion, defined as the primary ossification center, extends in both directions of the template, in parallel with the formation of a bony collar.
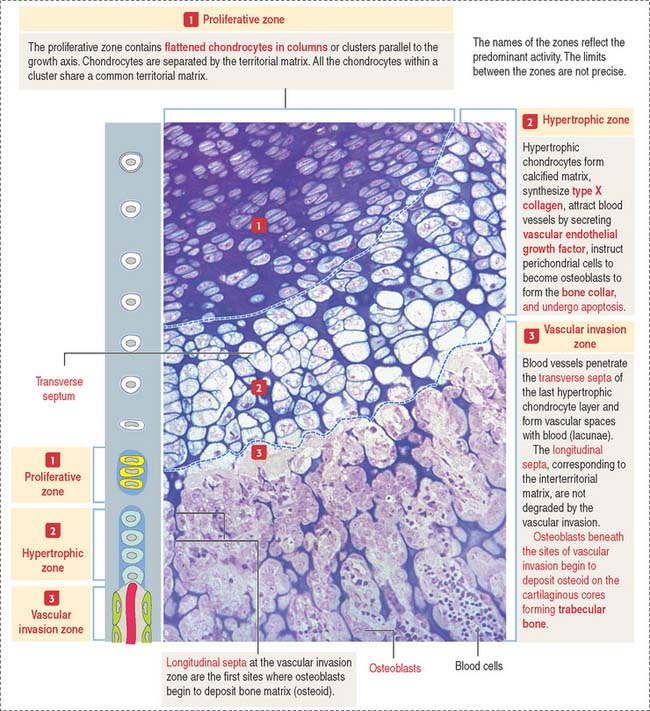
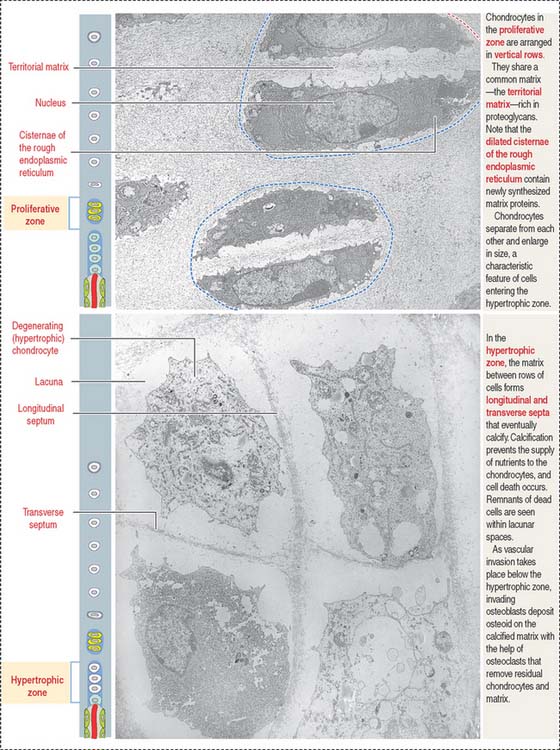
The continuing process of cartilage erosion and bone deposition can be visualized histologically (Figure 5-5). Four major zones can be distinguished, starting at the end of the cartilage and approaching the zone of erosion:
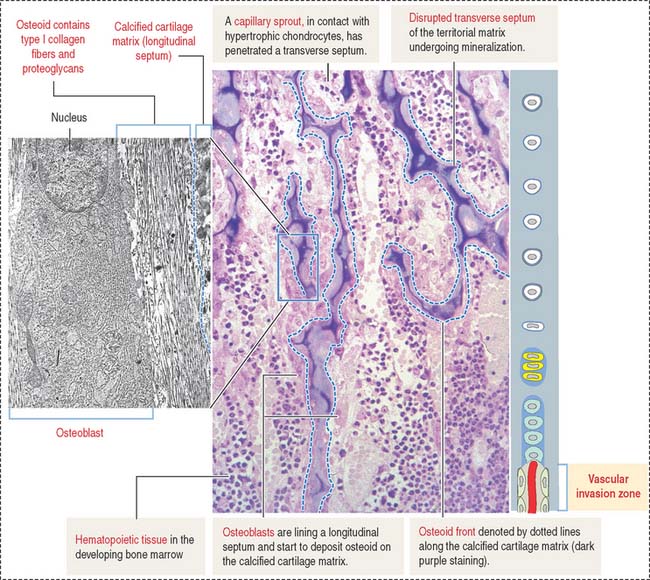
Chondrocytes in this zone are significantly enlarged (hypertrophic). As a result, the septa separating adjacent columns appear thinner due to a compression effect mediated by the hypertrophic chondrocytes. A provisional calcification begins in the longitudinal septa. The deepest layer, proximal to the vascular invasion zone, displays the blind end of capillary sprouts (Figure 5-8) derived from the developing bone marrow cavity occupied by hematopoietic cells (see Chapter 6, Blood and Hematopoiesis).
Osteoprogenitor cells give rise to osteoblasts that begin lining the surfaces of the exposed cores of calcified cartilage (stained blue—basophilic—in the light microscopy photograph in Figure 5-8) and initiate the deposition of osteoid (stained pink—acidophilic—in Figure 5-8). The osteoid contains abundant type I collagen fibers embedded in the extracellular matrix.
As the ossification process advances toward the adjacent proliferative zones (a “chase” effect), the bone marrow cavity increases in size owing to loss of cartilage and erosion of newly formed bone spicules by osteoclasts (Figure 5-10).
The periosteal collar grows in length and thickness (by appositional growth) at the midsection of the shaft and compensates for the loss of endochondral bone, while also strengthening the gradually eroding cartilage template.
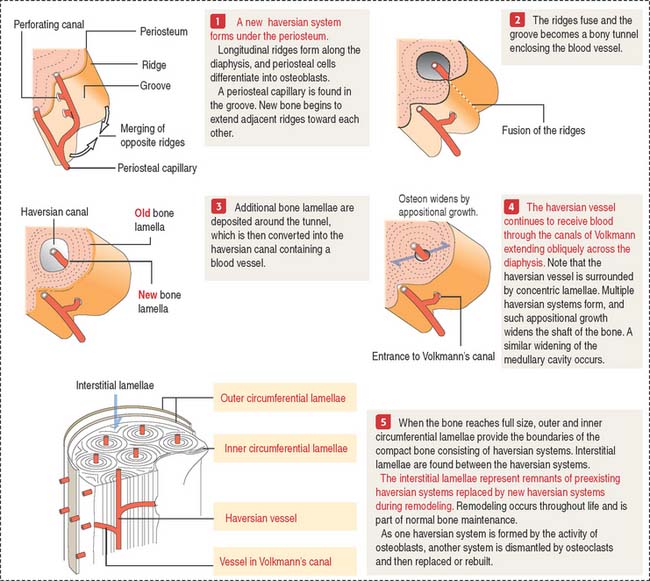
Growth in width of the diaphysis
The following sequence is observed (Figure 5-11):
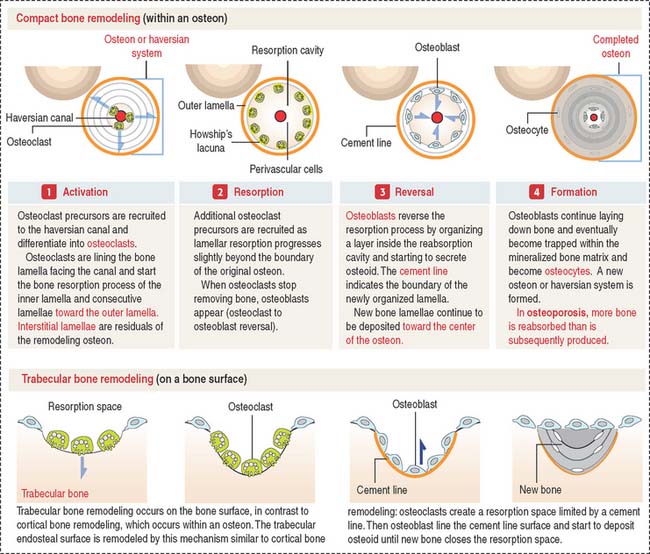
Bone remodeling
There are two forms of bone remodeling: (1) cortical bone remodeling, and (2) trabecular bone remodeling. Cortical bone remodeling is the resorption of an old haversian system followed by the organization of a new haversian system (Figure 5-12). Trabecular bone remodeling occurs on the bone surface (see Figure 5-12), in contrast to cortical bone remodeling, which occurs inside an osteon. The trabecular endosteal surface is remodeled by a mechanism similar to cortical bone remodeling.
Clinical significance: Hereditary and degenerative bone disorders
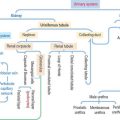
Osteopetrosis (“stonelike bone”) includes a group of hereditary diseases characterized by abnormal osteoclast function. The bone is abnormally brittle and breaks like a soft stone. The marrow canal is not developed, and most of the bone is woven because of absent remodeling.
We have already discussed a mutation in the colony-stimulating factor–1 gene whose expression is required for the formation of osteoclasts (see Bone in Chapter 4, Connective Tissue). A clinical variant of osteopetrosis, also known as marble bone disease, or Albers-Schönberg disease, is caused by a deficiency in carbonic anhydrase II, required by osteoclasts to accumulate H+ in Howship’s resorption lacunae and acidify the environment for the activation of secretory cathepsin K enzyme.
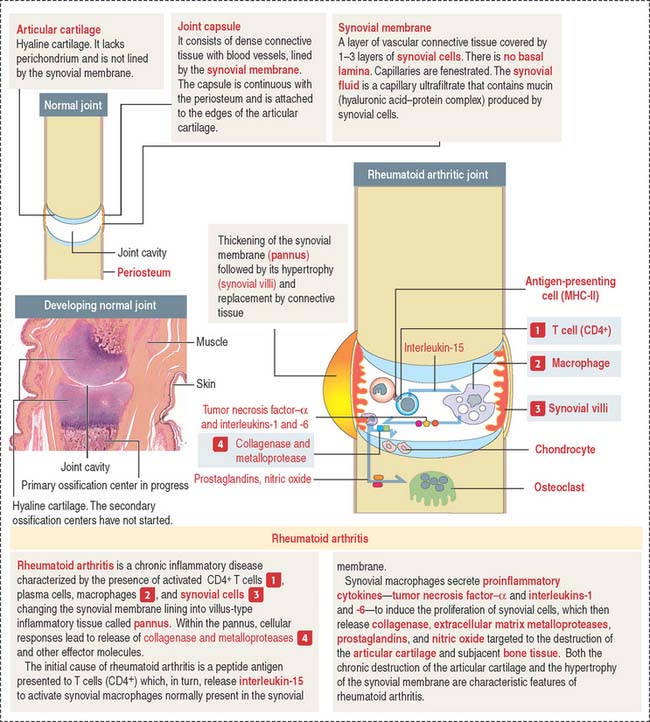
Joints
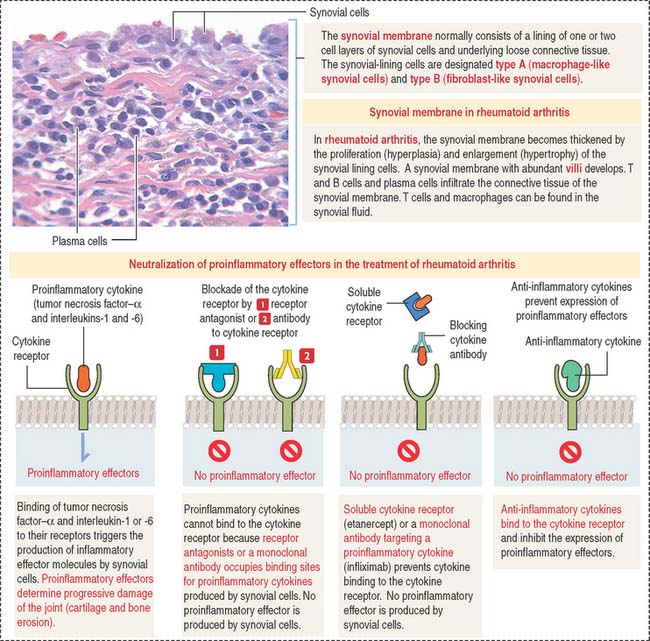
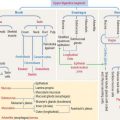
The joint capsule consists of two layers: an outer layer of dense connective tissue with blood vessels and nerves, and an inner layer, called the synovial membrane. The inner surface of the synovial membrane is covered by one to two layers of synovial cells overlying the connective tissue (Figure 5-13). There are two classes of synovial cells: (1) type A macrophage-like synovial cells, and (2) type B fibroblast-like synovial cells. There is no basal lamina separating synovial cells from the connective tissue. The connective tissue contains a rich network of fenestrated capillaries.
Clinical significance: Rheumatoid arthritis
The initial event is the activation of CD4+ T cells by an undetermined antigen. Activated CD4+ T cells stimulate the production of tumor necrosis factor–α (TNF-α), interleukin-2 (IL-2), and interleukin-6 (IL-6), and the secretion of collagenase and metalloproteinases by monocytes, macrophages, and fibroblast-like synovial cells. Activated CD4+ T cells stimulate B cells to differentiate into plasma cells to produce immunoglobulins and rheumatoid factor.
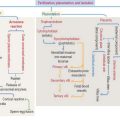
TNF-α, IL-1, and IL-6 are key cytokines in driving inflammation in rheumatoid arthritis (Figure 5-14). TNF-α and IL-1 can be detected in synovial fluid of patients with rheumatoid arthritis. TNF-α and IL-1 stimulate fibroblast-like synovial cells, osteoclasts, and chondrocytes to release cartilage and bone-destroying matrix metalloproteinases.
The neutralization of proinflammatory cytokines by soluble receptors or monoclonal antibodies is currently used in the treatment of patients with rheumatoid arthritis. Figure 5-14 provides a summary of the major therapeutic strategies for suppressing inflammation and preventing joint damage.
Osteogenesis