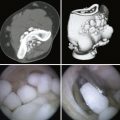
CHAPTER 6 Osteochondritis Dissecans of the Elbow
Sport-specific injuries more frequently affect young athletes with earlier and more rigorous participation in sports. The radiocapitellar compartment of the young athlete’s elbow is punished by significant stresses during repetitive throwing or during sports (e.g., gymnastics) that convert the elbow joint into a weight-bearing joint.1 Lateral compartment compression can lead to Panner’s disease (i.e., osteochondrosis) in the 6- to 10-year old patient or to various stages of capitellar osteochondritis dissecans (OCD) in the adolescent or young adult.2–5 This chapter describes Panner’s disease and OCD and outlines a treatment algorithm, including arthroscopic management using osteochondral grafting.
PANNER’S DISEASE
In 1927, Hans Jessen Panner published a description of osteochondrosis of the capitellum, likening it to Legg-Calvé-Perthes disease of the hip.6 Like other ostochondroses, it consists of non-inflammatory, disordered endochondral ossification. Its specific cause and relation to OCD remain debatable. It is known that abnormal radiocapitellar compressive forces during a period of vulnerability, typically while the physes are still open, predispose children to Panner’s disease.2,5,7 It may result from the combination of an avascular insult (likely related to the capitellum’s predominantly end-artery supply) and repetitive microtrauma.8
Epidemiology
Panner’s disease predominantly affects boys younger than age 10 years.9 Young boys may be predisposed to it for two reasons. First, compared with girls, they have a delayed appearance and maturation of their secondary growth centers. Second, boys traditionally are more prone to trauma during the more aggressive early childhood activities they select.7 This may change as more girls choose higher-risk athletic activities at younger ages. Although Panner’s disease can be confused with OCD, and the age of onset may overlap, it is distinguished by three epidemiologic characteristics. Panner’s disease does not share the strict association with repetitive throwing that OCD does, it is usually self-limited, and it resolves without any long-term sequelae.
Patient Evaluation
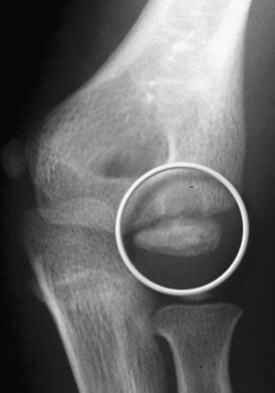
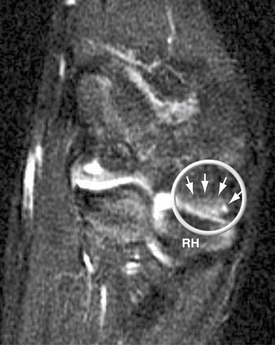
Panner’s disease initially manifests as pain and stiffness in the elbow, which is relieved by rest. On physical examination, patients have poorly localized tenderness over the lateral elbow. Radiographs initially show fissuring, lucencies, fragmentation, and irregularity of the capitellum (Fig. 6-1), particularly near or at the chondral surface. Subsequent x-ray films, taken at 3 to 5 months, demonstrate larger radiolucent areas followed by reossification of the bony epiphysis, with a corresponding resolution of symptoms. In 1 to 2 years, the epiphysis regains its contour, usually without flattening.4 As in Legg-Calvé-Perthes, radiographs often lag behind clinical symptoms. Magnetic resonance imaging (MRI) may be used effectively to document the extent of the lesion. Typically, edema is localized to the chondral surface and the bone adjacent to it, with less involvement of the deeper subchondral bone compared with the OCD (Fig. 6-2).
OSTEOCHONDRITIS DISSECANS
OCD of the capitellum is a non-inflammatory degeneration of subchondral bone occurring in the context of repetitive trauma to the lateral compartment of the elbow. Panner’s disease and OCD may represent two different stages of the same disorder,4 but they do have different characteristics: age of onset, cause, and natural history. Although Panner’s disease affects children younger than 10 years, OCD victimizes older athletes, usually between the ages of 11 and 15 years.10 Unlike Panner’s disease, OCD is thought to be directly linked to repetitive trauma. OCD is not always a self-limited disease, and if left unaddressed, it results in profound destruction of the capitellum.10
Anatomy
The elbow’s osseous anatomy and the capitellum’s idiosyncratic blood supply may predispose young athletes to OCD. The elbow is a diarthrodial joint in which the distal humerus articulates with the proximal ulna and the radial head. Its unique bony configuration allows for −15 to 0 degrees° of extension to 150 degrees of flexion. Rotation of the radial head over the stationary ulna gives an arc of almost 180 degrees of forearm rotation.11 The osseous and articular congruency of the humerus, ulna, and radial head accounts for the greater part of elbow stability, particularly at less than 20 degrees of extension or more than 120 degrees of elbow flexion.12
In young, skeletally immature athletes, the elbow possesses a greater degree of cartilaginous elasticity. Hyperextension, facilitated by this increased range of motion, can generate increased radiocapitellar compressive loads and tension of the medial capsule and ulnar collateral ligament (UCL). Overhead throwing athletes during the throwing motion and gymnasts during weight-bearing handstands in elbow hyperextension further exaggerate these stresses. Repetitive stress on this system can precipitate OCD. Medial-sided pathologies, such as medial apophysitis, UCL injury, and posteromedial impingement from the excess valgus stress, can occur concurrently.13
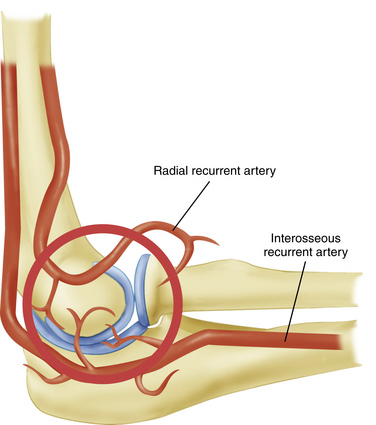
The tenuous end-artery vascular supply to the capitellum predisposes it to injury. In the young adult population, the capitellum is supplied by two end arteries coursing from posterior to anterior, which are branches of the radial recurrent and interosseous recurrent arteries (Fig. 6-3).14 As a result of the longitudinal blood supply to the capitellar epiphyseal plate and minimal collateral circulation in the area, blood flow to the capitellum may be disrupted by repetitive microtrauma resulting in an avascular state and by a single traumatic event leading to post-traumatic subchondral bone bruises.15,16
Patient Evaluation
History
OCD arises from repetitive, excessive compressive forces generated by large valgus stresses on the elbow during throwing or racket swinging or by constant axial compressive loads on the elbow, such as those endured by gymnasts.10,17,18 Specific risk factors predispose to the condition. In the case of baseball players, throwing sliders and breaking pitches, throwing more than 600 pitches per season, and increased age of the athlete increase the risk of developing OCD.18 In female gymnasts, overtraining involving excessive handstand maneuvers has been linked to OCD.19,20 There also may be a genetic predisposition to OCD.
Patients with OCD initially complain of pain and stiffness in the elbow that is relieved by rest. The onset is usually insidious, and a history of specific trauma is often absent. If left unaddressed, the symptoms may progress to locking or catching due to intra-articular loose bodies or an inflamed plica. Throwing athletes may present with painful posterolateral clicking or catching caused by a radiocapitellar plica. These symptoms can overlap with those from the OCD lesion, and the plica itself may be responsible for chondral wear in the radiocapitellar compartment.
Physical Examination
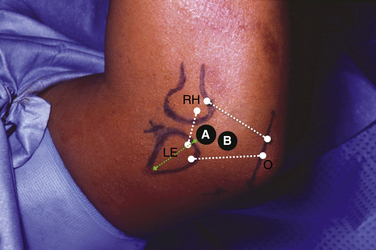
In athletes with OCD, physical examination findings tend to be remarkable for poorly localized lateral elbow tenderness over the radiocapitellar joint. Loss of range of motion with a 15- to 20-degree flexion contracture is common. Loss of extension is more common than loss of flexion. An effusion is often apparent and can be palpated by flexing the elbow and feeling the lateral portal area, triangulated by the radial head, olecranon, and lateral epicondyle. The provocative maneuver for the radiocapitellar joint we prefer is the active radiocapitellar compression test (Fig. 6-4). A positive test result elicits pain in the lateral compartment of the elbow when the patient pronates and supinates the forearm with the arm in extension. In patients with an associated symptomatic radiocapitellar plica, snapping typically occurs at greater than 90 degrees of elbow flexion with the forearm in pronation.
Diagnostic Imaging
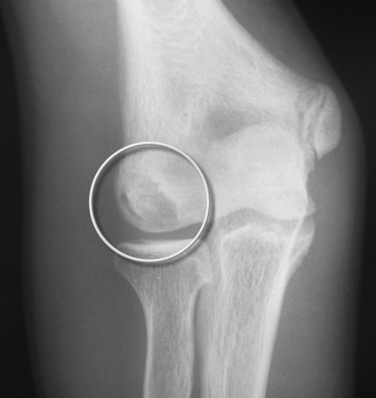
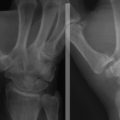
Anteroposterior radiographs in full extension, anteroposterior radiographs in 45 degrees of flexion, and lateral views of the elbow should be obtained. Radiographic findings may be negative early in the disease process. As the condition progresses, flattening and sclerosis of the capitellum, typically on its anterolateral aspect, will become apparent. Irregular areas of lucency and intra-articular loose bodies also appear. The capitellar lesions of OCD and medial-sided epicondylar fragmentation are best seen on an anteroposterior radiograph at 45 degrees of elbow flexion (Fig. 6-5).
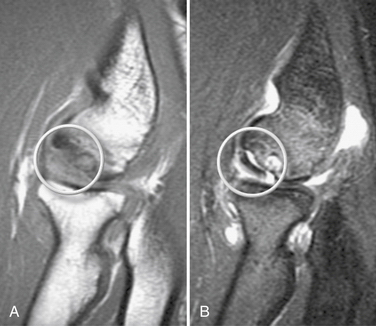
MRI should be used to assess suspected OCD. It can detect bone edema early in the disease process.21 An MR arthrogram can further delineate the extent of the injury. Contrast can show separation of a detached or partially detached piece from subchondral bone (Fig. 6-6). This is important in determining whether to proceed with operative or nonoperative management. Peiss and colleagues22 thought that fragment enhancement (Fig. 6-7B) (as opposed to the perifragment enhancement seen in Fig. 6-6) denoted viability and might be a reasonable indication for nonoperative treatment. They also suggested that enhancement of the fragment and subchondral bone interface was caused by vascular granulation tissue, indicating instability and requiring operative intervention.
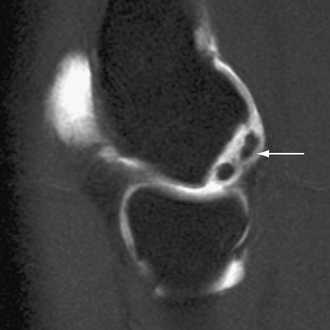
FIGURE 6-6 The MR arthrogram shows contrast surrounding an unstable osteochondritis dissecans fragment (arrow).
(Courtesy of Neal S. ElAttrache, MD, Kerlan-Jobe Orthopaedic Clinic, Los Angeles, CA.)
Pseudolesions, which appear on the posteroinferior junction of the articular and nonarticular portions of the capitellum, must be differentiated from OCD, which almost always manifests on the anterolateral aspect. The examiner also should observe whether the capitellar physis is open or closed.
Treatment
Indications, Contraindications, and Classification
Management of OCD lesions is based primarily on the status and stability of the overlying cartilage. The size and location of the lesion and the patency of the capitellar growth plate also influence decision making.23–25
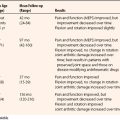
To guide treatment, detailed classification systems based on radiographic26,27 and arthroscopic23 findings have been delineated.28,29 We have simplified these algorithms into a three-stage classification that provides a template for management. Table 6-1 shows the classification.
Stage 1.
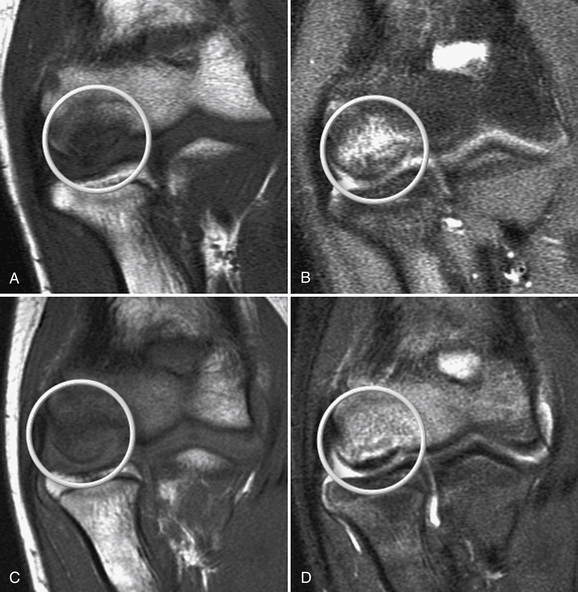
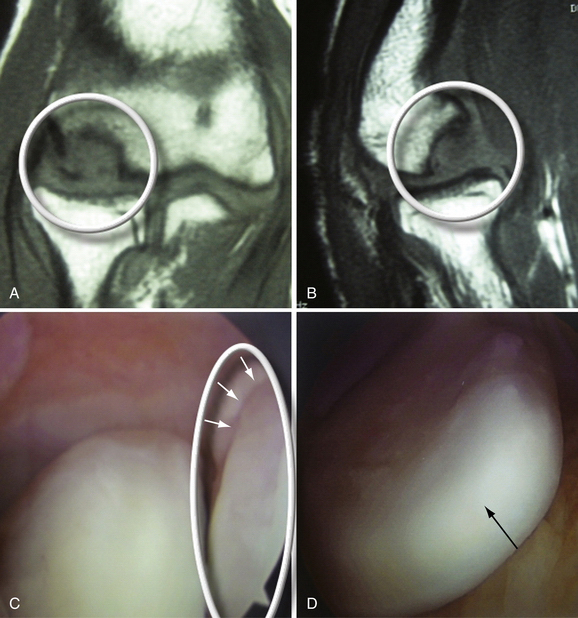
In stage 1, the osteochondral fragment is intact, stable, and not displaced. Radiographic findings are often negative. The MRI signal varies; it is typically abnormal on T1-weighted and normal on T2-weighted images, although the T2 signal may also be abnormal. Figure 6-7A and B shows a stage 1 lesion with coronal-slice signal that is abnormal on T1- and T2-weighted images. Arthroscopically, the articular cartilage is intact, with general preservation of subchondral stability.
This stage is best treated nonoperatively. All activity involving the affected arm should be stopped. The elbow should be rested in a hinged elbow brace for 3 to 6 weeks. Progressive physical therapy should ensue as symptoms abate. Return to sports usually can be expected at 3 to 6 months and should be governed by primarily by the patient’s clinical response, because radiographic changes often lag behind the clinical symptoms by several months or years. Nevertheless, follow-up radiographs and MRI scans should be obtained at 2- to 3-month intervals to track progress. Figure 6-7C and D shows the same patient as in Figure 6-7A and B at the 6-month follow-up assessment after conservative treatment, with clear reconstitution of the subchondral bone.
Stage 2.
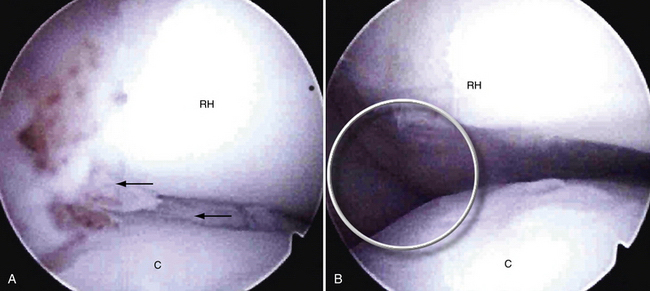
In stage 2, the osteochondral fragment is partially separated, as documented radiographically and arthroscopically. Radiographs demonstrate fissuring, lucencies, and fragmentation. On MRI, T1- and T2-weighted sequences show abnormal signal intensity and a margin around the fragment, denoting its instability (Fig. 6-8). Computed tomography (CT) may reveal the partially separated fragment. Arthroscopically, the cartilage is fractured, and the subchondral bone is unstable and partially displaced (Fig. 6-9).
When an unstable lesion is identified, conservative treatment should be bypassed. It warrants prompt surgical intervention to return the athlete to his or her sport or activities of daily living as soon as possible. In stage 2 lesions, the size and location of the lesion govern treatment. For smaller lesions, débridement is an option. Patients typically have immediate relief of symptoms, but the long-term natural history includes arthritis. Fragment fixation has been advocated by some for this stage, although questions linger concerning the long-term healing potential of fixed fragments and clinical results of the procedure.26,27,30,31 Osteochondral autografts or synthetic grafts can address large, radial head–engaging defects involving the lateral buttress of the capitellum. If the decision rests between fixation and osteochondral restoration, we prefer osteochondral or synthetic plug grafting, because it has generated more reliable results.
Size.
Takahara and coworkers32 differentiated among small (<5% of the capitellum on an anteroposterior radiograph of the elbow, <60-degree angle formed by lines drawn along the borders of the lesion on a lateral radiograph), moderate (5% to 70%), and large (>70%, >90 degrees) lesions. They concluded that large lesions should be addressed operatively. Shimada and associates33 suggested that smaller lesions (<1 cm2) could be treated with débridement, chondroplasty, and possibly microfracture or drilling as described by Bradley and Dandy.34 Larger lesions (>1 cm2) should be treated with osteochondral autografts.
Location.
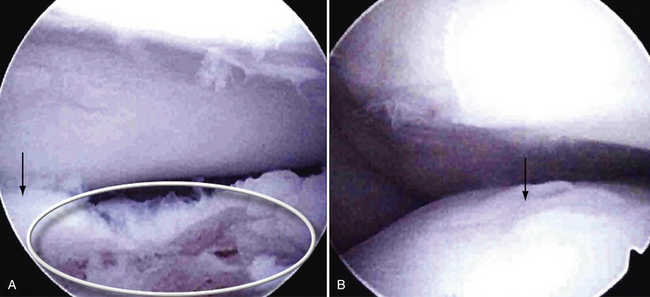
We think the location of the lesion may be more important in guiding treatment than some other factors. Extension of the lesion into the lateral margin of the capitellum, as described by Chappell and ElAttrache35 and by Ruch and colleagues,36 is associated with a potentially poorer prognosis. The lateral column of the capitellum supports large compressive forces when the elbow is stressed in valgus or with axial loading. When the lateral column is intact, a defect treated with microfracture alone is relatively protected, and fibrocartilage healing may occur. Lesions that do not involve a significant portion of the lateral buttress of the capitellum and do not engage the radial head during arthroscopic observation (i.e., pronation and supination with the elbow in extension) have been successfully treated with microfracture or retrograde or antegrade subchondral drilling. Figure 6-10 shows an OCD lesion with a predominantly intact lateral column.
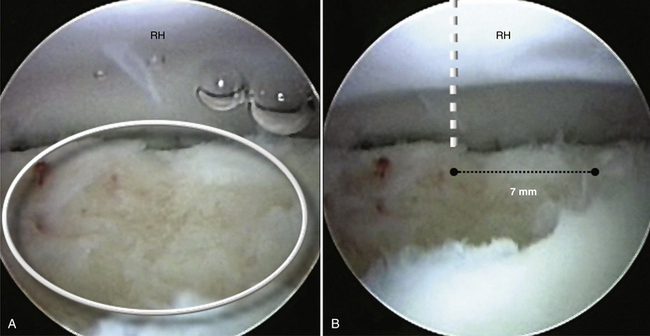
Lateral column involvement of more than about 6 to 7 mm cannot be dealt with acceptably by microfracture. The absence of a lateral buttress forestalls fibrocartilage healing by subjecting the defect to increased radiocapitellar forces. Engagement of the radial head in the defect also compromises healing and may lead to accelerated radiocapitellar arthrosis. For these larger, engaging defects or those that extend substantially into the lateral buttress (>6 to 7 mm) (Fig. 6-11), we recommend removal of the loose fragment and osteochondral restoration by means of mosaicplasty or osteochondral autograft transfer system (OATS).
For early, partially detached fragments, the detached portion (usually central) should be débrided from central to lateral aspects. After stable osteochondral borders have been obtained, the lesion is carefully evaluated arthroscopically to ascertain how much of the lateral column is involved and if the radial head engages with the defect. Chappell and ElAttrache35 reported that lesions larger than 1 cm2 (average, 1.32 cm2) and no lateral column involvement were treated successfully with microfracture, whereas those involving the lateral column did well with osteochondral grafting. Fragment fixation also is an option, but we have had superior and more consistent results with grafting.
Stage 3.
In stage 3, the fragment is fully displaced and has become or is imminently becoming a loose body. Figure 6-12 shows an example of a stage 3 lesion seen on MRI and arthroscopically. Patients may present with mechanical symptoms related to loose bodies, such as locking.
In this stage, débridement, drilling, or osteochondral replacement is indicated. If the loose osteochondral piece is acutely displaced in a patient with previously documented OCD, the surgeon can attempt to fix it to its donor site. Results of fixation are, however, inconsistent. Chronically loose bodies (documented by serial radiographs or MRI) should be removed and the donor bed débrided in preparation for one of the aforementioned treatment options, following the same algorithm. These patients often are unable to return to sports, and the long-term prognosis usually includes radiocapitellar arthrosis.
Radial Head Involvement.
Radial head involvement, in addition to capitellar pathology, indicates advanced disease and usually does not occur in athletes. If the radial lesion is less than 30% of the radial head, treatment of the capitellar OCD should proceed as delineated earlier. For radial lesions that are greater than 30% of the radial head, treatment of the capitellar lesion should be limited to débridement, drilling, and microfracture.9 Severe radiocapitellar degenerative arthritis is a relative contraindication to mosaicplasty.
Radiocapitellar Plica.
A comorbid condition that may be found in throwing athletes at the time of arthroscopy for OCD is posterolateral elbow impingement caused by a thickened radiocapitellar plica. The plica can cause chondromalacic changes on the radial head and capitellum. Symptoms may include painful clicking or catching and effusions, and they can overlap with those from the OCD lesion. Snapping often occurs at more than 90 degrees of elbow flexion with the forearm in pronation. If found during arthroscopic inspection, the plica should be resected. Kim and colleagues37 reported excellent results after plica débridement in throwing athletes and golfers.
Conservative Management
Nonoperative treatment is indicated for stage 1 OCD with a stable lesion and in patients with open capitellar growth plates. Takahara and coworkers26,27 retrospectively reviewed 106 cases of capitellar OCD with an average 7-year follow-up. They found that stable lesions that healed completely with nonoperative treatment had three common characteristics at initial presentation: an open capitellar growth plate, localized flattening or radiolucency of the subchondral bone, and good elbow motion.
Nonoperative treatment mandates complete cessation of elbow use, including activities such as throwing, gymnastics, arm wrestling, push-ups, and weightlifting. The arm may be immobilized but not for more than 3 weeks.29 Gentle range of motion should be instituted immediately after this period of immobilization if this therapeutic route is chosen. The patient is followed clinically at regular intervals (every 4 to 6 weeks) with serial radiographs. Gentle exercises are performed for the first 3 to 4 months, advancing to strengthening at 4 to 5 months. At that point, an interval throwing program can be initiated based on satisfactory clinical and radiographic findings. Return to sports should be governed by the patient’s symptoms, because radiographic changes can persist for years.38,39
Rest.
Takahara and associates32,38 observed that repetitive forces on existing OCD lesions led to an increase in lesion size. Cessation of repetitive stress on the elbow should be emphasized to the athlete’s parents, trainers, and coaches. They must be reminded that this is a potentially sport-ending injury, with degenerative arthritis as a possible outcome. The incidence of residual capitellar deformity in high-level pitchers is very low,40 and this suggests that athletes who develop a degenerative elbow from failed OCD treatment do not go on to play high-level baseball. The athletes may be able to do active controlled rest by offloading with a brace and by continuing only those activities that are pain free.
Stage and Management.
Because early-stage OCD responds better to nonoperative treatment than advanced stage lesions, identifying the disease promptly can have a significant impact on prognosis. Matsuura and colleagues41 found that 91% of early-stage OCD but only 53% of advanced-stage OCD improved after nonoperative treatment.
Stability.
Stability of the fragment also affects the final outcome. Mitsunaga and coworkers42 showed that less than 50% of stable fragments went on to become unstable in the long term. However, Takahara and associates26 demonstrated that fragments that did become unstable had a low rate of healing.
Patient Age and Growth Plate Status.
Age has not been correlated with the likelihood of healing.37,38 However, Mihara and colleagues25 found a significant correlation between open capitellar growth plates and healing. In their study, 94% of patients with early-stage lesions and with open growth plates healed, whereas the rate for those with closed growth plates was only 71%.
Operative Treatment
Failure of conservative treatment for early-stage, stable lesions (i.e., about 6 weeks of no improvement or 4 to 6 months of persistent symptoms) or diagnosis of an advanced-stage, unstable lesion is an indication for pursuing operative treatment. The ultimate goals of surgery are to eliminate mechanical symptoms and stimulate a healing response. Takahara and coworkers27 found that patients with unstable lesions that did well with surgery compared with elbow rest had the following common findings at presentation: a closed capitellar growth plate, radiographic fragmentation, and restriction of elbow motion of more than 20 degrees. Patients with closed capitellar physes did significantly better with surgery than with elbow rest. Larger lesions had better results with reconstruction of the articular surface than with simple fragment fixation.
Arthroscopic Positioning.
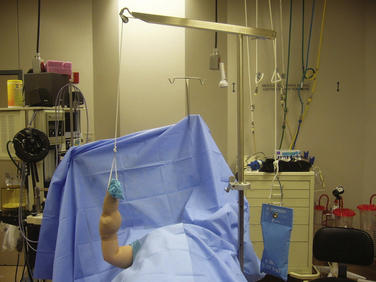
Elbow arthroscopy can proceed with the patient supine, prone, or in the lateral decubitus position. We use the supine position because it facilitates general anesthesia and enables an easy conversion to an open procedure if needed (Fig. 6-13). Structures also may lie in a more anatomic orientation in this position. In the supine position, the elbow is positioned at 90 degrees of elbow flexion and 90 degrees of shoulder abduction with the hand suspended from a pulley and using 5 pounds of traction. The lateral position gives improved posterior compartment access. The prone position also gives good posterior compartment access and does not require traction. General anesthesia provides complete muscle relaxation and obviates the need for a regional block that may prevent diagnosing a postoperative neurologic problem. A tourniquet can be used at the surgeon’s discretion.
Arthroscopic Technique.
Available instruments include 2.9- and 4.0-mm, 30-degree arthroscopes, burrs, and shavers. The elbow is distended with 30 to 50 mL of saline through the direct lateral portal. Standard arthroscopic portals are created. Diagnostic arthroscopy is performed to look for loose bodies, osteophytes, and chondral damage. The arthroscope usually is in the anteromedial portal, and working instrumentation is in the anterolateral portal. In throwers, a valgus stress test with the elbow flexed to 70 degrees can be performed during the diagnostic portion of the procedure. A 1- to 2-mm opening of the ulnohumeral joint denotes laxity of the UCL, although clinical correlation is mandatory.
After the initial arthroscopic examination is complete, a midlateral portal (i.e., lateral soft spot) is created in line with the lateral epicondylar ridge and entered with the arthroscope. The radial head, capitellum, trochlear notch, and trochlear ridge are best seen through this portal. Care should be taken to avoid the posterior antebrachial cutaneous nerve, which is at risk near this portal. A working portal is created adjacent to and slightly ulnar to the midlateral portal (Fig. 6-14). Carefully placed dual direct lateral portals do not damage lateral ligamentous structures and provide superior exposure to the capitellum.43 Patients with OCD and lateral compartment symptoms occasionally also have a thickened radiocapitellar plica (Fig. 6-15). If found, the plica should be resected.
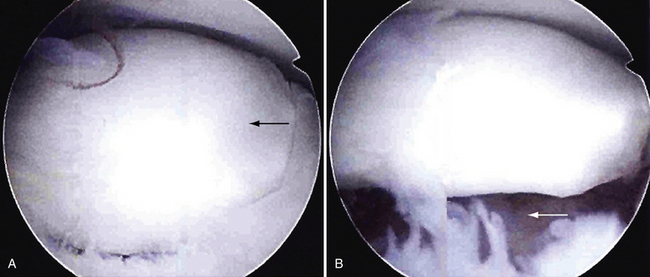
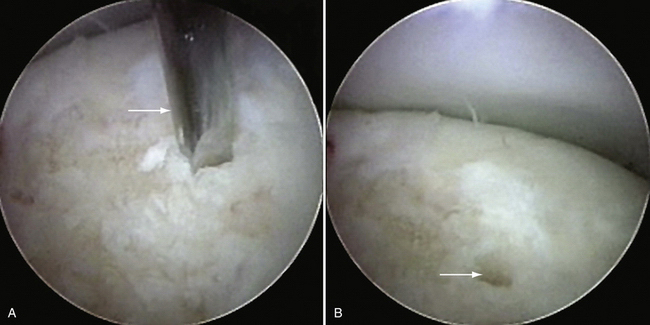
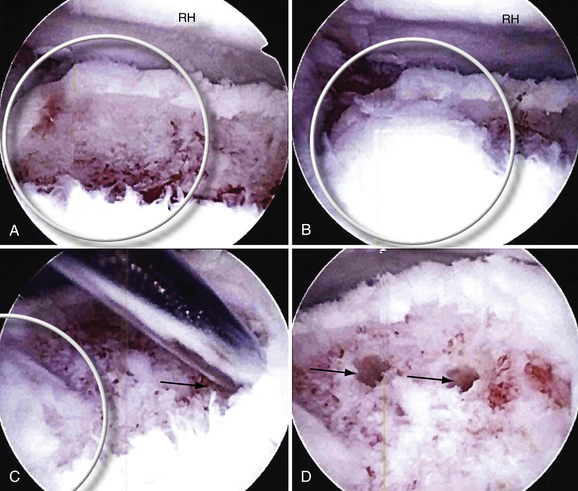
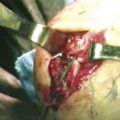
The OCD lesion is evaluated and graded. If unstable and loose, the lesion is prepared by removing any loose fragments, shaving loose fragments of cartilage down to subchondral bone and establishing healthy cartilage borders (Fig. 6-16). The size of the lesions is determined by a calibrated probe. If osteochondral grafting is planned, the portals must allow access for the required 4- to 6-mm instruments. At this point, depending on the indication, one of the following procedures can be performed: abrasion chondroplasty, drilling, microfracture, fixation of large fragments, and osteochondral autograft transfer (i.e., OATS or mosaicplasty).
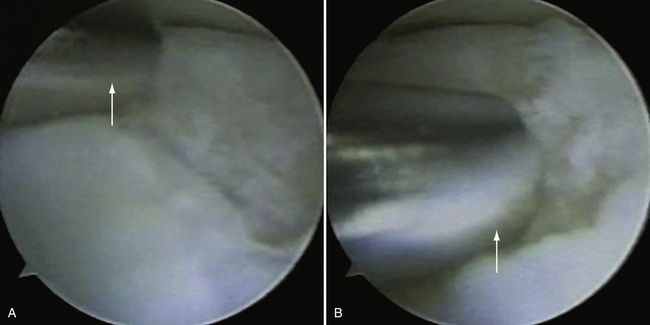
FIGURE 6-16 The osteochondritis dissecans lesion (A) is débrided with a shaver (arrow) to stable borders (B).
(Courtesy of Neal S. ElAttrache, MD, Kerlan-Jobe Orthopaedic Clinic, Los Angeles, CA.)
PEARLS& PITFALLS
PEARLS
PITFALLS
Microfracture and Subchondral Drilling.
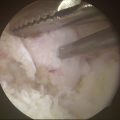
The indications for microfracture or subchondral drilling are similar: early-stage lesions with cartilage fibrillation and fissuring and small, stage 2 lesions with exposed bone that do not significantly involve the lateral column of the capitellum. The lesion bed is prepared as described earlier (see Fig. 6-16). Detached fragments or loose bodies are removed. With the arthroscope in the direct lateral portal, a 0.062-inch Kirschner wire is inserted through the accessory lateral portal and used to perforate the lesion (Fig. 6-17A). Multiple holes are made in the lesion (see Fig. 6-17B). Marrow elements released from the holes induce a fibrocartilage healing response.
If microfracture is elected, a similar approach can be employed using microfracture awls instead of pins. Bojanic and associates44 reported symptom resolution in three adolescent (13- to 15-year-old) gymnasts 5 months after arthroscopic débridement and microfracture of lesions that were about stage 2. They remained symptom free 1 year postoperatively. Using microfracture in 11 athletes with an average age of 15 years, Chappell and ElAttrache36 obtained excellent results at the 3-year follow-up, with a return to the previous level of activity by all 11. The size of the OCD lesions ranged from 7 × 6 mm to 17 × 15 mm.
Mosaicplasty.
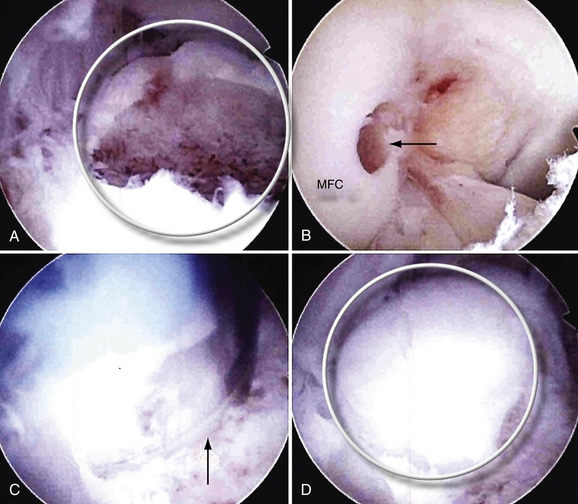
Mosaicplasty has been applied in the context of elbow OCD lesions. In this procedure, small, cylindrical osteochondral grafts are obtained from the lateral periphery or trochlear edge of the femoral condyles and transplanted to prepared osteochondral defects.45 Mosaicplasty is indicated when a large capitellar lesion engages the radial head, as observed while rotating the extended arm during arthroscopy, or when there is significant (>6 to 7 mm) lateral column involvement (Fig. 6-18A). Radial head degeneration and severe deformities of the capitellum are relative contraindications.
A midlateral working portal is used to establish healthy, stable cartilage borders. In the case of a partially detached fragment, the detached area is first evaluated. Often, the detached region is located centrally. In this situation, ElAttrache recommends débriding the partially detached portion by beginning centrally and proceeding laterally toward the lateral column. Débridement proceeds until an area of bony integrity, consisting of an osseous connection between the fragment and the subchondral bone, is encountered, if one is present. The extent of posterolateral column involvement is then determined (see Fig. 6-18A), and an arthroscopic evaluation (i.e., supination and pronation of the extended forearm) of radial head engagement in the defect is performed. If more than 6 to 7 mm of the lateral column is involved or the radial head engages in the defect, osteochondral grafting proceeds. The goal should be to restore a bony buttress to prevent radial head subluxation into the defect, not necessarily replace every millimeter of the lesion.
If osteochondral grafting is elected, the elbow is then flexed 90 to 100 degrees, and a spinal needle is introduced through the anconeus to gauge the feasibility of a perfectly perpendicular approach to the lesion (typically 3 to 4 cm distal to the midlateral portal).46 The incision is widened to provide access for a 4- to 6-mm diameter plug, so that it is in line with the perpendicular path delineated by the needle. After bluntly spreading the soft tissue to avoid neurovascular structures, the recipient site is then drilled as perpendicular to the chondral surface as possible, creating a tunnel of the necessary diameter.
At this point, the knee, which has been prepared, undergoes an arthroscopic harvest of an identically sized chondral plug from the intercondylar notch. The arthroscope is placed into an anterolateral portal. Instrumentation is inserted through an anteromedial portal. Using the harvesting instrumentation, a 6-mm-diameter, 1-cm-deep plug is harvested from the trochlear edge off the medial femoral condyle (see Fig. 6-18B). Usually, one plug is sufficient because of the small size of the capitellar lesions.
The plug is introduced into the recipient site and impacted flush with the surrounding cartilage (see Fig. 6-18C and D). The goal is to reconstitute the lateral buttress (Fig. 6-19A and B) so that the radial head does not subluxate into the defect. The process of osteochondral grafting is repeated until the lateral column integrity is adequately restored. If some corners of the lesion cannot be fully replaced, they are treated with drilling or microfracture (see Fig. 6-19C and D). If autograft is unavailable, allograft or synthetic scaffolding can be used.
With this method, Iwasaki and associates45 obtained good or excellent results in 7 of 8 teenage baseball players with OCD. Yamamoto and colleagues48 found that 6 of 9 adolescents with grade 3 and 8 of 9 with grade 4 OCD returned to competitive baseball after an OATS procedure. Chappell and ElAttrache35 treated five baseball players with OCD using OATS. All five returned to competitive baseball and were still playing 5 years postoperatively. The investigators recommended the procedure, particularly when more than 6 to 7 mm of the lateral column is involved and when the radial head is seen to be engaging the lesion with a careful arthroscopic examination during supination and pronation and during flexion and extension of the forearm.
Fragment Fixation.
Fragment fixation has been performed in patients with unstable, partially open OCD lesions.30 Kuwahata and coworkers31 described using cancellous bone grafts and a Herbert screw in an open technique. At 32 months of follow-up, they reported pain-free return to sports and an improvement in range of motion of 18 degrees in all seven patients who underwent the procedure. Takahara and colleagues26,27 described using bone pegs harvested from the lateral olecranon to fix partially attached lesions. This was also done through an open approach. Newer bioabsorbable implants may allow fragment fixation to be performed routinely arthroscopically. Although these surgeons have reported encouraging results, data on fixation are still preliminary. Our recommendation remains excision and drilling or grafting for partially detached lesions.
Postoperative Rehabilitation
Return to Sport.
Return to sports has varied. Historically, gymnasts have had inferior outcomes compared with throwers, perhaps related to significantly increased axial loads borne by their elbows. Jackson and associates3 treated 10 female gymnasts with removal of loose bodies and drilling after failure of nonoperative treatment. Only one returned to sport. Although surgery for lesions refractory to conservative treatment may improve symptoms, the investigators concluded that a return to gymnastics is unlikely.
Newer technical advancements and lesion-specific management may be improving these outcomes. Bojanic and colleagues44 found that 3 of 3 female gymnasts successfully returned to their previous level after loose body removal and microfracture. Although Byrd and coworkers47 suggested that arthroscopic surgery reliably improved symptoms but returned only 4 of 10 adolescent baseball players to competitive baseball, Yamamoto and associates48 reported a return to competitive levels for 14 of 18 male baseball players with unstable fragments (in situ or displaced) after osteochondral autografting. Chappell and ElAttrache36 had 8 of 8 male baseball players return to their sport and previous level at an average of 3 years of follow-up after microfracture or osteochondral autografting.
Prognosis.
The prognosis for OCD of the capitellum is good when caught early at stage 1. Unfortunately, most cases are diagnosed at stage 2. Although surgery usually alleviates symptoms and allows a return to play, these patients have less favorable long-term outcomes. Longitudinal studies have documented that 50% of radiocapitellar OCD patients eventually develop osteoarthritis.49 Nevertheless, newer techniques, including osteochondral grafting (mosaicplasty), may mitigate the onset of long-term degenerative joint disease.
CONCLUSIONS
Young throwers and gymnasts are at risk for Panner’s disease and OCD as performance demands and expectations escalate. For Panner’s disease and early OCD, nonoperative treatment, consisting primarily of strict activity cessation, is the mainstay of management. Advanced OCD lesions require operative intervention, if feasible arthroscopically. The size and location of the lesion and its functional relationship to the radial head help guide management. Prevention, by monitoring and limiting pitch counts and excessive training and by educating athletes, parents, and coaches on early warning signs, provides the most reproducible solution for these potentially sport-ending conditions.
1. Brown R, Blazina ME, Kerlan RK, et al. Osteochondritis of the capitellum. J Sports Med. 1974;2:27-46.
2. Douglas G, Rang M. The role of trauma in the pathogenesis of the osteochondroses. Clin Orthop Relat Res. 1981;158:28-32.
3. Jackson DW, Silvino N, Reiman P. Osteochondritis in the female gymnast’s elbow. Arthroscopy. 1989;5:129-136.
4. Ruch DS, Poehling GG. Arthroscopic treatment of Panner’s disease. Clin Sports Med. 1991;10:629-636.
5. Singer KM, Roy SP. Osteochondrosis of the humeral capitellum. Am J Sports Med. 1984;12:351-360.
6. Panner H. An affection of the capitulum humeri resembling Calvé-Perthes disease of the hip. Acta Radiol. 1927;8:617-618.
7. Duthie RB, Houghton GR. Constitutional aspects of the osteochondroses. Clin Orthop Relat Res. 1981;158:19-27.
8. Yamaguchi K, Sweet FA, Bindra R, et al. The extraosseous and intraosseous arterial anatomy of the adult elbow. J Bone Joint Surg Am. 1997;79:1653-1662.
9. Kobayashi K, Burton KJ, Rodner C, et al. Lateral compression injuries in the pediatric elbow: Panner’s disease and osteochondritis dissecans of the capitellum. J Am Acad Orthop Surg. 2004;12:246-254.
10. Voloshin I, Schena A. Elbow injuries. In: Schepsis AA, Busconi BD, editors. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins, 2006.
11. Do T, Herrera-Soto J. Elbow Injuries in children. Curr Opin Pediatr. 2003;15:68-73.
12. Morrey BF, Tanaka S, An KN. Valgus stability of the elbow. A definition of primary and secondary constraints. Clin Orthop Relat Res.. 1991;265:187-195.
13. Kocher MS, Waters PM, Micheli LJ. Upper extremity injuries in the paediatric athlete. Sports Med. 2000;30:117-135.
14. Haraldsson S. On osteochondrosis deformas juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand Suppl. 1959;38:1-232.
15. Fa K, E B, U H. Are bone bruises a possible cause of osteochondritis dissecans of the capitellum? A case report and review of the literature. Arch Orthop Trauma Surg. 2005;125:545-549.
16. Yang Z, Wang Y, Gilula LA, Yamaguchi K. Microcirculation of the distal humeral epiphyseal cartilage: implications for post-traumatic growth deformities. J Hand Surg Am. 1998;23:165-172.
17. Lord J, Winell JJ. Overuse injuries in pediatric athletes. Curr Opin Pediatr. 2004;16:47-50.
18. Lyman S, Fleisig GS, Waterbor JW, et al. Longitudinal study of elbow and shoulder pain in youth baseball pitchers. Med Sci Sports Exerc. 2001;33:1803-1810.
19. Caine D, Howe W, Ross W, Bergman G. Does repetitive physical loading inhibit radial growth in female gymnasts? Clin J Sport Med. 1997;7:302-308.
20. Caine DJ, Nassar L. Gymnastics injuries. Med Sport Sci. 2005;48:18-58.
21. Griffith JF, Roebuck DJ, Cheng JC, et al. Acute elbow trauma in children: spectrum of injury revealed by MR imaging not apparent on radiographs. AJR Am J Roentgenol. 2001;176:53-60.
22. Peiss J, Adam G, Casser R, et al. Gadopentetate-dimeglumine-enhanced MR imaging of osteonecrosis and osteochondritis dissecans of the elbow: initial experience. Skeletal Radiol. 1995;24:17-20.
23. Baumgarten TE, Andrews JR, Satterwhite TE. The arthroscopic classification and treatment of osteochondritis dissecans of the capitellum. Am J Sports Med. 1998;26:520-523.
24. DiFelice GS, Meunier M, Paletta GJ. Elbow injury in the adolescent athlete. In: Altchek AJ, editor. The Athlete’s Elbow. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:231-248.
25. Mihara K, Tsutsui H, Nishinaka N, Yamaguchi K. Nonoperative treatment for osteochondritis dissecans of the capitellum. Am J Sports Med. 2009;37:298-304.
26. Takahara M, Mura N, Sasaki J, et al. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89:1205-1214.
27. Takahara M, Mura N, Sasaki J, et al. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2, pt 1):47-62.
28. Petrie R, Bradley JP. Osteochondritis dissecans of the humeral capitellum. In: De Lee J, Drez D, Miller M, editors. Orthopaedic Sports Medicine: Principles and Practice. Philadelphia, PA: WB Saunders, 2003.
29. Bradley JP, Petrie RS. Osteochondritis dissecans of the humeral capitellum. Diagnosis and treatment. Clin Sports Med. 2001;20:565-590.
30. Larsen MW, Pietrzak WS, DeLee JC. Fixation of osteochondritis dissecans lesions using poly(l-lactic acid)/poly(glycolic acid) copolymer bioabsorbable screws. Am. J Sports Med. 2005;33:68-76.
31. Kuwahata Y, Inoue G. Osteochondritis dissecans of the elbow managed by Herbert screw fixation. Orthopedics. 1998;21:449-451.
32. Takahara M, Ogino T, Fukushima S, et al. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27:728-732.
33. Shimada K, Yoshida T, Nakata K, et al. Reconstruction with an osteochondral autograft for advanced osteochondritis dissecans of the elbow. Clin Orthop Relat Res. 2005;435:140-147.
34. Bradley J, Dandy DJ. Results of drilling osteochondritis dissecans before skeletal maturity. J Bone Joint Surg Br. 1989;71:642-644.
35. Chappell JD, ElAttrache NS. Clinical outcome of arthroscopic treatment of OCD lesions of the capitellum. Orlando, FL: Presented at the meeting of the American Orthopaedic Society for Sports Medicine; 2008.
36. Ruch DS, Cory JW, Poehling GG. The arthroscopic management of osteochondritis dissecans of the adolescent elbow. Arthroscopy. 1998;14:797-803.
37. Kim DH, Gambardella RA, ElAttrache NS, et al. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34:438-444.
38. Takahara M, Ogino T, Sasaki I, et al. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363:108-115.
39. Takahara M, Shundo M, Kondo M, et al. Early detection of osteochondritis dissecans of the capitellum in young baseball players. Report of three cases. J Bone Joint Surg Am. 1998;80:892-897.
40. Mihara K. Osteoarthritic elbow caused by sports [in Japanese]. Rinsho Seikei Geka. 2000;35:1243-1249.
41. Matsuura T, Kashiwaguchi S, Iwase T, et al. Conservative treatment for osteochondrosis of the humeral capitellum. Am J Sports Med. 2008;36:868-872.
42. Mitsunaga MM, Adishian DA, Bianco AJJr. Osteochondritis dissecans of the capitellum. J Trauma. 1982;22:53-55.
43. Davis JT, Idjadi JA, Siskosky MJ, et al. Dual direct lateral portals for treatment of osteochondritis dissecans of the capitellum: an anatomic study. Arthroscopy. 2007;23:723-728.
44. Bojanic I, Ivkovic A, Boric I. Arthroscopy and microfracture technique in the treatment of osteochondritis dissecans of the humeral capitellum: report of three adolescent gymnasts. Knee Surg Sports Traumatol Arthrosc. 2006;14:491-496.
45. Iwasaki N, Kato H, Ishikawa J, et al. Autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans in teenaged patients. Am J Sports Med. 2006;34:1233-1239.
46. Ahmad CS, El Attrache NS. Treatment of capitellar osteochondritis dissecans. Tech Shoulder Elbow Surg. 2006;7:169-174.
47. Byrd JW, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30:474-478.
48. Yamamoto Y, Ishibashi Y, Tsuda E, et al. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34:714-720.
49. Bauer M, Jonsson K, Josefsson PO, Lindén B. Osteochondritis dissecans of the elbow. A long-term follow-up study. Clin Orthop Relat Res. 1992;284:156-160.