Chapter 14 Osteochondritis Dissecans
Background/aetiology
The most common ‘little leaguer elbow’ symptoms are on the medial side of the joint and are related to traction injury and inflammation of the medial collateral structures, especially the vulnerable medial epicondyle. Less frequently, symptoms are lateral and related to compressive overloading of the lateral bony structures, including the radial head and especially the capitellum. OCD and Panner’s disease are both seen in the capitellum and it is therefore important to define and distinguish these conditions (Table 14.1).
| Feature | Panner’s disease | Osteochondritis dissecans |
|---|---|---|
| Usual age | 10 years or less | Adolescent |
| Radiology | Capitellar sclerosis and fragmentation | Discreet capitellar change |
| Management | Usually non-operative | Non-operative and operative depending on grade |
| Prognosis | Relatively good | Variable |
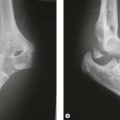
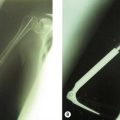
Panner’s disease is considered to be an avascular necrosis of the developing capitellar ossification centre. It is characterized initially by radiological sclerosis and then fragmentation of the capitellum (Fig. 14.1), followed by gradual restoration. It is not necessarily associated with trauma.1,2 The process is very similar to the series of changes seen in the femoral epiphysis in Perthes’ disease. Panner’s disease occurs in the relatively young (under 10 years). Management is usually non-operative and primarily involves avoidance of the causative stress together with range-of-movement therapy to maintain extension. Although it may take some time for the radiological lesion to resolve, the prognosis is relatively good. As in Perthes’ disease, the capitellar cartilaginous anlagen, devoid of its supporting bony nucleus, can deform and sometimes reossifies into a flattened oval in the sagittal plane, ultimately limiting full extension.
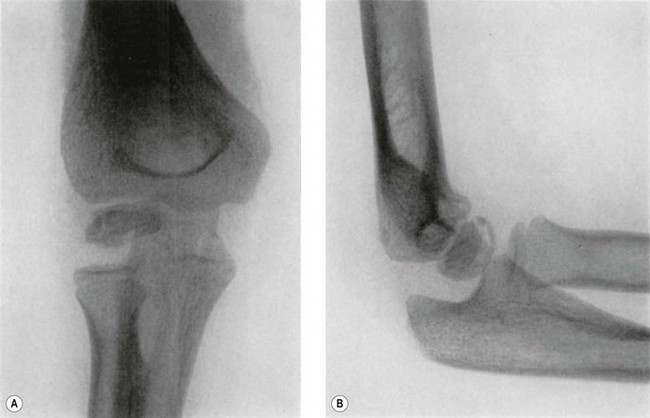
Figure 14.1 Panner’s disease. Sclerosis and fragmentation of the entire ossific nucleus of the capitellum.
From J Bone Joint Surg Am, April 1960; 42:513–516. Avascular necrosis of the capitellum humeri (Panner’s disease): a report of a case. Charles J. Heller and Leon L. Wiltse.
By contrast, OCD occurs most commonly in the adolescent age range. It is usually a more discreet condition, affecting a section of the subchondral bone and the overlying articular cartilage (Fig. 14.2). The pathology and radiology are similar to OCD seen in other joints such as the knee and ankle and there is potential for loose body formation. Management is sometimes operative and depends on the stability of the OCD lesion. Prognosis is accordingly variable and generally worse than Panner’s disease. Early recognition is important because appropriate early management can influence the prognosis.
The capitellum of the immature elbow has a tenuous vascular supply consisting of one or two posterior vessels that traverse the capitellar cartilage before entering the epiphysis. There is no metaphyseal blood supply until the physis closes at maturity.3 This tenuous circulation makes the capitellum vulnerable to avascular necrosis following lateral condyle fractures and it is postulated that it may also play a role in the genesis of Panner’s disease and OCD. Some reports suggest a genetic predisposition in some families but most evidence supports the hypothesis of repeated shear and compressive trauma, particularly in relation to OCD.
Investigation and classification
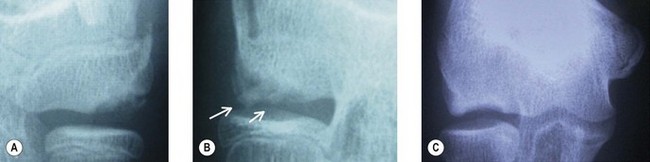
Evaluation should not overlook other potential explanations for pain. Investigation usually commences with standard anteroposterior and lateral plain radiography. The typical early appearance of OCD is anterolateral radiolucency within the capitellum, with flattening progressing to irregularity of the central or anterolateral articular surface. A separated fragment can subsequently form and either remain in its bed or become detached, forming a loose body. Radiographic findings can be classified into three grades (Fig. 14.2).4 Grade I is localized flattening or radiolucency. Grade II is an undisplaced fragment and grade III a displaced or detached fragment. Reliable grading is, however, difficult and subject to inter-observer variation.
Furthermore, plain radiography lacks sensitivity. Early grade I changes are difficult to visualize and early lesions can be easily overlooked. One study has shown that only 7 of 15 known cases of early OCD were identified with plain radiographs.5 As a result, some authors recommend supplementing the standard films with an anteroposterior view of the elbow when it is flexed to 45° to give a tangential view of the anterolateral capitellum, where the lesion is most typically located.6 Magnetic resonance imaging (MRI) is more sensitive than plain radiography and should therefore be utilized if the history and examination suggest OCD as a possible differential diagnosis.
Classification of the lesion is critical because current management guidelines are dependent on the type of lesion present and particularly on its stability. In an effort to standardize the classification of OCD lesions, the International Cartilage Repair Society (ICRS) has published a system for clinical cartilage evaluation and imaging assessment (Table 14.2).7 Prognosis deteriorates from OCD I to OCD IV. Determining the stability of the lesion is the critical issue. More stable lesions have a better prognosis and can sometimes heal completely with non-operative treatment. Unstable lesions have little or no chance of healing with non-operative management and require surgical treatment.
| International Cartilage Repair Society (ICRS) | Arthroscopic OCD lesion classification |
|---|---|
| OCD I | Lesion with a continuous but softened area covered by intact cartilage. Stable when probed |
| OCD II | Lesion with partial discontinuity of articular surface but stable when probed |
| OCD III | Complete discontinuity of articular surface but fragment not yet dislocated (‘dead in situ’) |
| OCD IV | Empty defect with loose fragment in joint or loose within bed |
| Subgroups I–IVa <10 mm in depth Subgroups I–IVb >10 mm in depth |
From Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am April 2003; 85:58–69.
By comparison with plain radiography MRI is sensitive and can accurately define the site and extent of OCD lesions. MRI assessment of stability is, however, more difficult and controversial. Some authors have suggested that unstable lesions are surrounded by a rim of high signal intensity or a fluid-filled cyst on T2-weighted images.5 Other studies have demonstrated that such high signal deep to the OCD fragment merely represents granulation tissue and the determination of instability can only be certain if a break in the articular surface is also demonstrated.8 MR arthrography, which can identify an articular surface breach more precisely, is therefore sometimes used to improve the accuracy of MR assessment.9 There is also emerging evidence that ultrasonography is a potentially useful imaging modality to evaluate OCD lesions and their stability, but interpretation requires considerable operator experience.10 Currently therefore surgical visualization and probing of the lesion, either by arthroscopy or arthrotomy, remains the gold standard for the assessment of OCD lesions.
Outcome and literature review
The long-term prognosis of advanced OCD of the capitellum is poor. Follow-up studies suggest that approximately half of all patients have long-term symptoms of pain during activities of daily living, restricted range of motion and radiological signs of degenerative change.11–13 On the other hand, there is evidence that appropriate management of early disease can prevent deterioration and result in an improved outcome.6
Stability and viability of the OCD lesion as well as the presence or absence of an open capitellar physis are key factors in predicting prognosis. In the most comprehensive review of the condition to date Takahara et al4 defined a stable lesion as one that could heal completely with non-operative elbow rest, and an unstable lesion as one that would have significantly better results if managed surgically. Observations were made regarding the determination of whether a lesion was likely to be stable or unstable (Table 14.3). Based on this study, at the time of initial presentation patients likely to have stable lesions have an open physis as well as an almost normal range of elbow motion and only early radiological flattening or radiolucency (radiological grade I). Conversely, patients likely to have an unstable lesion have a closed growth plate or a restriction of elbow motion ≥20° or radiological fragmentation (radiological grade II or III).
Table 14.3 Radiographic and clinical characteristics of stable and unstable OCD lesions
| Stable | Unstable |
|---|---|
| An open growth plate | A closed growth plate |
| Early radiological flattening or radiolucency (grade I) | Radiological fragmentation (grade II or III lesion) |
| An almost normal range of elbow motion | A restriction of elbow motion of ≥20° |
Lesions that are likely to be stable have all of the characteristics in the left-hand column at the time of initial presentation. Just one of the characteristics in the right-hand column at the time of initial presentation suggests that the lesion is likely to be unstable.
From Takahara, Nariyuki Mura, Junya Sasaki, Mikio Harada, and Toshihiko Ogino. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Masatoshi J. Bone Joint Surg Am June 2007; 89:1205–1214.
By this classification more than 90% of patients in Takahara et al’s study4 had unstable lesions. Stable lesions were relatively rare, representing less than10% of patients. Although relatively uncommon, patients with stable lesions have a good prognosis provided they follow medical advice to rest and avoid repetitive injury. Evidence suggests that failure to adhere to such recommendations leads to progression and deterioration.4,6
An open physis is an indicator of a good prognosis but does not guarantee a good outcome. Even when the physis was open, Takahara et al4 showed that two out of three radiological grade II lesions failed to heal completely. Radiological grade II lesions were therefore considered to represent partially unstable lesions, and surgical treatment, irrespective of an open physis, was recommended. Similarly, loss of range of motion equal to or greater than 20°, in spite of an open physis, was also considered to represent instability because three out of four such patients failed to respond to non-operative management and were eventually found to have unstable lesions at operation. Conversely, a closed physis is an indicator of poor prognosis. Takahara et al4 showed that in the presence of a closed physis all OCD lesions failed to heal following non-operative treatment. All lesions that were treated operatively when the physis was closed were found to be unstable at surgery and the results of surgery were significantly better than after non-operative treatment.
A practical management approach
Once a lesion is identified management is directed by the determination of stability. Takahara et al’s criteria (Table 14.3)4 can be used in combination with MRI. The determination of stability using MRI is controversial but can be enhanced with MRI arthrography. Contrast tracking into the interface between the fragment and the proximal bone suggests a break in the articular cartilage and instability.
Stable lesions
Early stable lesions are equivalent to ICRS OCD I lesions and should be managed non-operatively. This requires complete cessation of the provoking activity. Once symptoms have settled, range-of-motion and strengthening exercises can be commenced but there should be no return to the provocative sport for at least 6 months. Follow-up should continue until healing is evident using radiographs at 3- to 6-monthly intervals. It is encouraging to see radiological evidence of healing but this may take a prolonged period of time – sometimes up to 3 years or more. Management must therefore follow clinical rather than radiological progress.14 Return to pitching is not recommended until complete healing is evident but return to other positions can be considered if symptom free at 6 months. Return to other provocative activities such as gymnastics or weightlifting, where supra-physiological weight-bearing with the upper limb is unavoidable, is not recommended until complete healing. Evidence suggests that return to competitive high-level gymnastics is unlikely if lesions progress.15 Failure to respond to non-operative management with persistence of symptoms, or return of symptoms after 6 months, warrants further evaluation to reconsider the possibility of instability.
Unstable lesions
Most authors recommend fixation in situ for ICRS OCD II lesions.4,14 Various fixation methods have been described, each with its proponents, including bone peg epiphyseodesis using a dowel of bone harvested from the ulna,16 pull-out wiring17 or Herbert screw fixation.18 Fixation is also recommended for ICRS OCD III lesions with the addition of autologous bone, harvested from the ulna, being grafted to the debrided bed of the lesion before fixation.
The management of loose ICRS OCD IV lesions is the most controversial. If the lesion is less than 50% of the capitellar width then removal of the fragment and debridement and drilling of the defect give acceptable results.4 An arthroscopic approach can be used, with the advantage of being minimally invasive and allowing early mobilization and rehabilitation.19 If the OCD IV defect is greater than 50% of the capitellar width, reconstruction of the articular surface should be considered because of the very poor long-term prognosis of such lesions. Promising results are reported using autologous osteochondral mosaicplasty, harvested from the periphery of the lateral femoral condyle,20–22 or from a rib.23 As in OCD of the knee, transplantation of autologous chondrocyte cultures24 has shown some promise but this is presently best managed in specialist centres with appropriate prospective outcome assessment.
| Stability of lesion | Grade | Treatment |
|---|---|---|
| Stable | I | Non-operative with cessation of provoking activity for at least 6 months |
| Unstable | II | In situ fixation |
| Unstable | III | Fixation with bone grafting of the denuded bed of the lesion |
| Unstable | IV – less than 50% of capitellum affected | Remove fragment, debride and drill defect |
| IV – more than 50% of capitellum affected | Reconstruction of the articular surface – mosaicplasty, autologous chondrocytes |
Conclusions
Given the potential morbidity of OCD of the capitellum, awareness of the condition among medical practitioners, parents and the coaches of children and adolescents participating in provocative sporting disciplines is critical for appropriate management and prevention. In the interests of prevention of this condition, the American Academy of Orthopaedic Surgeons (AAOS)25 has offered guidance to little league baseball coaches and players. The AAOS recommends that pitchers should play no more than three to four innings each game and limit pitches to no more than 200 in each week. This includes both practice and competitive play.
1 Panner HJ. A peculiar affection of the capitellum humeri, resembling Calve–Perthes disease of the hip. Acta Radiol. 1929;10:234-242.
2 Heller CJ, Wiltse LL. Avascular necrosis of the capitellum humeri (Panner’s disease): a report of a case. J Bone Joint Surg Am. 1960;42:513-516.
3 Harraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;38(Suppl.):1-232.
4 Takahara M, Mura N, Sasak J, et al. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89:1205-1214.
5 Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271.
6 Takahara M, Shundo M, Kondo M, et al. Early detection of osteochondritis dissecans of the capitellum in young baseball players: report of three cases. J Bone Joint Surg Am. 1998;80:892-897.
7 Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85:58-69.
8 O’Connor MA, Palaniappan M, Khan N, Bruce CE. Osteochondritis dissecans of the knee in children: a comparison of MRI and arthroscopic findings. J Bone Joint Surg Br. 2002;84-B:258-262.
9 Kramer J, Stiglbauer R, Engel A, et al. MR contrast arthrography (MRA) in osteochondrosis dissecans. J Comput Assist Tomogr. 1992;16:254-260.
10 Takahara M, Ogino T, Tsuchida H, et al. Sonographic assessment of osteochondritis dissecans of the humeral capitellum. AJR. 2000;174:411-415.
11 Bauer M, Jonsson K, Josefsson PO, et al. Osteochondritis dissecans of the elbow: a long-term follow-up study. Clin Orthop Relat Res. 1992;284:156-160.
12 Takahara M, Ogino T, Fukushima S, et al. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27:728-732.
13 Takahara M, Ogino T, Sasaki I, et al. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363:108-115.
14 Bradley JP, Petrie RS. Osteochondritis dissecans of the humeral capitellum: diagnosis and treatment. Clin Sports Med. 2001;20(3):565-590.
15 Jackson DW, Silvino N, Reiman P. Osteochondritis in the female gymnast’s elbow. Arthroscopy. 1989;5(2):129-136.
16 Oka Y, Ohta K, Fukuda H. Bone-peg grafting for osteochondritis dissecans of the elbow. Int Orthop. 1999;23(1):53-57.
17 Takeda H, Watarai K, Matsushita T, et al. A surgical treatment for unstable osteochondritis dissecans lesions of the humeral capitellum in adolescent baseball players. Am J Sports Med. 2002;30:713-717.
18 Kuwahata Y, Inoue G. Osteochondritis dissecans of the elbow managed by Herbert screw fixation. Orthopedics. 1998;21(4):449-451.
19 Brownlow HC, O’Connor-Read LM, Perko M. Arthroscopic treatment of osteochondritis dissecans of the capitellum. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):198-202.
20 Iwasaki N, Kato H, Ishikawa J, et al. Autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans in teenaged patients. Am J Sports Med. 2006;34:1233-1239.
21 Shimada K, Yoshida T, Nakata K, et al. Reconstruction with an osteochondral autograft for advanced osteochondritis dissecans of the elbow. Clin Orthop Relat Res. 2005;435:140-147.
22 Ansah P, Vogt S, Ueblacker P, et al. Osteochondral transplantation to treat osteochondral lesions in the elbow J. Bone Joint Surg Am. 2007;89:2188-2194.
23 Sato YK, Nakamura T, Toyama Y, Ikegami H. Costal osteochondral grafts for osteochondritis dissecans of the capitulum humeri. Tech Hand Up Extrem Surg. 2008;12(2):85-91.
24 Yamamoto Y, Ishibashi Y, Tsuda E, et al. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34:714-720.
25 American Academy of Orthopaedic Surgeons website. Online. Available http://orthoinfo.aaos.org/topic.cfm?topic=A00328, 20 January 2011.