Chapter 5 Osteochondral Mosaicplasty
Surgical Principles and Objective
Indications
Contraindications
Patient Information
Preoperative Workup
Surgical Technique)
The following explanations relate to the figures identified.
Step 1
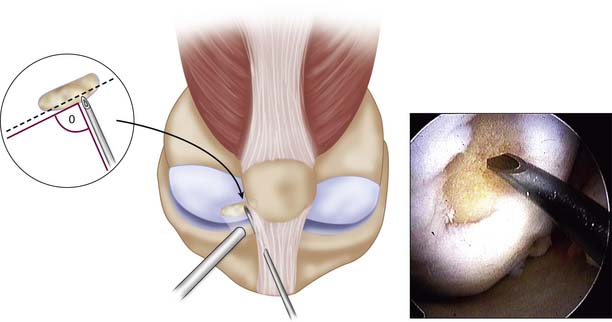
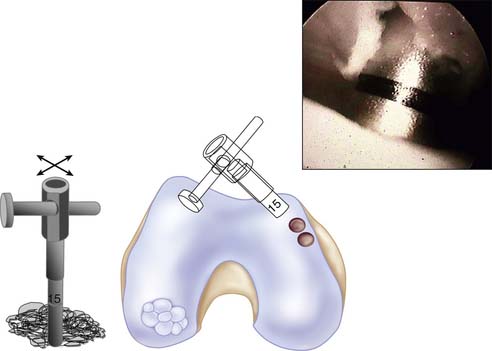
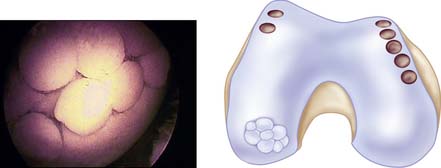
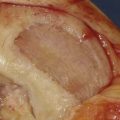
An arthroscopy is performed to examine the intraarticular conditions. The type, site, and size of the defect is determined, and the quality of other chondral surfaces as well as donor site availability are checked (Fig. 5-1).
Patellotrochlear and tibial lesions always require an open procedure.
Use a 1.2-mm K-wire or 18-gauge spinal needle initially to locate the portal sites.
Step 2
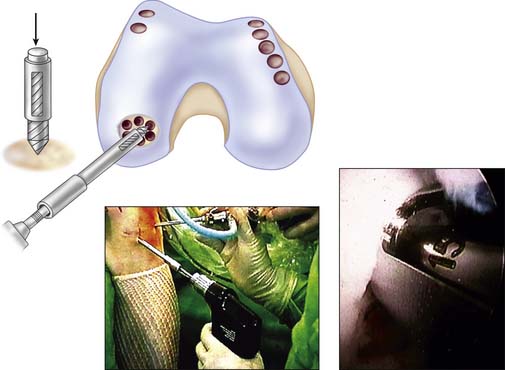
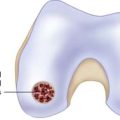
Use a full-radius resector or curette and a knife blade to bring the edges of the defect back to good hyaline cartilage at a right angle (Fig. 5-2).
Clean the base of the lesion with an arthroscopic burr or half-round rasp to viable subchondral bone. Abrasion arthroplasty of the defect site promotes fibrocartilage grouting from the bony base.
Step 3
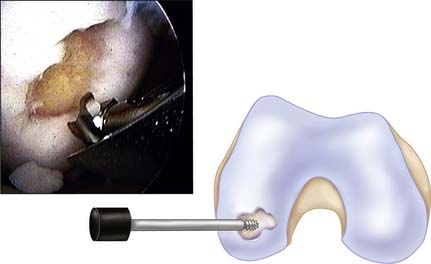
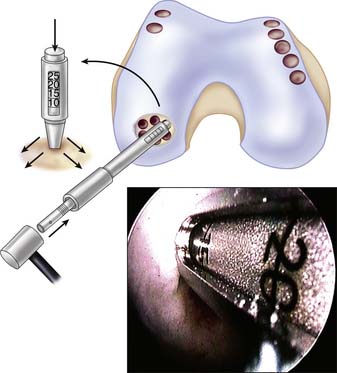
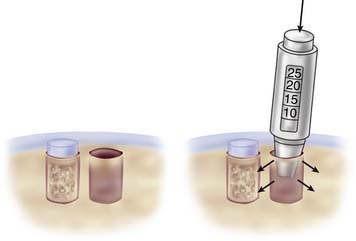
Because tapping the cutting edge of the guide into the bony base and removing it can mark the defect site, use the drill guide to determine the number and size (2.7, 3.5, 4.5, 6.5, and 8.5 mm in diameter) of grafts needed (Fig. 5-3).
Step 4
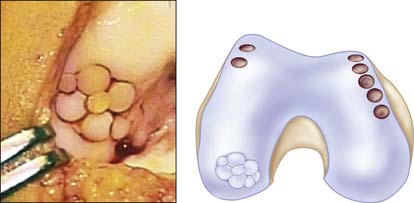
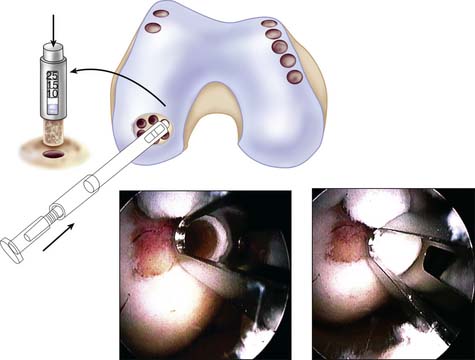
Filling of the defect by same-sized contacting rings allows a filling rate of about 70% to 80%, but use of additional sizes to cover the dead spaces and cutting the grafts into each other can improve the coverage up to 90% to 100% (Fig. 5-4).
Step 5
The medial femoral condyle periphery of the patellofemoral joint above the line of the notch is the most preferred harvest site (Fig. 5-5).
In case of arthroscopic mosaicplasty, the medial patellofemoral periphery has easier access than the lateral one as fluid distension can promote lateral positioning of the patella and may provide easier perpendicular positioning for the harvesting chisel.
Step 6
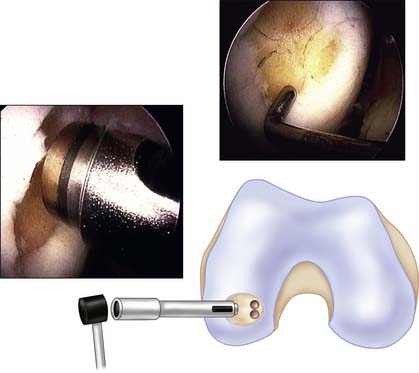
Flex the knee again and establish good distension. Reintroduce the drill guide using the dilator as an obturator. Place these tools perpendicularly to the defected surface (Fig. 5-6).
Step 7
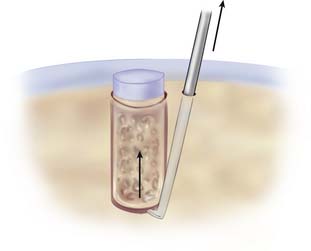
Insert the conical-shaped dilator into the drill guide again. Tap it to the desired depth, depending on the actual features of the recipient bone. Stiff bone needs more dilation than normal or soft bone. Hold the drill guide firmly, and remove the dilator from the hole (Fig. 5-7).
Step 8
Adjust the delivery tamp by turning the handle to initially allow the graft to sit slightly higher than the depth of the defect. This will minimize the likelihood of overpenetrating the graft (Fig. 5-8).
Stop the inflow to eliminate the graft being forced out of the tube by fluid flow.
Insert the graft deeper by turning the delivery tamp handle counterclockwise.
The graft should be matched to the original articular surface.
Step 9
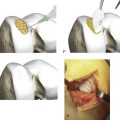
Such step-by-step graft implantation has several advantages. Dilation of the actual recipient hole allows an easy graft insertion (low insertion force on the hyaline cap), but dilation of the next hole affects surrounding bone to the previously implanted grafts, which can result in a safe press fit fixation (Fig. 5-9).
Step 10
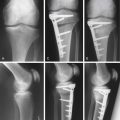
Finally, when the grafts have filled all of the holes, move the knee throughout its range of motion, provoking varus or valgus stress also, depending on the site of the resurfaced area (Fig. 5-10).
Close the portals and introduce suction drainage into the joint through a superior portal. Use an elastic bandage to fix the appropriate dressing.
Special Considerations
Mosaicplasty is often combined with concomitant procedures such as ACL reconstruction and high-tibial osteotomy. In such cases, mosaicplasty should be done first, as this procedure often requires 120-degree (or more) flexion and different stressed positions. After finishing the mosaicplasty, the fixation of the grafts is safe enough to tolerate special stresses during the concomitant procedure.
Postoperative Management
Postoperatively, the drain should be removed at 24 hours.
Postoperative thrombosis prophylaxis is recommended.
Immediate ROM exercises are encouraged, a non-weight-bearing and partial loading period of a few weeks is necessary. Usually 2 weeks non–weight bearing followed by 2 weeks partial loading (30 to 40 kg) are ordered before full weight bearing. Detailed rehabilitation recommendations are presented in Tables 5-1 and 5-2.
TABLE 5-1 Mosaicplasty Rehabilitation Protocola—General Viewpoints
| Ambulationc | |
| Two-crutch ambulation, non–weight bearing | Immediate |
| Two-crutch ambulation, partial loading (30-40 kg) | 2-4 weeks |
| Discontinue crutches, full weight bearing | 4-5 weeks |
| Functional Exercises | |
| Form walking, gait evaluation | 4-5 weeks |
| Step-up | 4-5 weeks |
| Step-down | 5-6 weeks |
| Range of Motion | |
| Early range of motion encouraged | |
| CPM in case of extended lesions 2-4 cm2 (in painless range) | Immediate (first week) |
| Full extension, flexion as tolerated | Immediate |
| Stationary bicycle | 3 weeks |
| Strength Return | |
| Quadriceps | |
| Open chain exercises, leg raises | Immediate |
| Concentric contraction to full extension | 1 week (or earlier if tolerated) |
| Concentric contraction against resistance | 2 weeks |
| Isometric exercises in different angles | Immediate |
| Eccentric exercises against resistance | 3-4 weeks |
| Hamstrings | |
| Isometric exercises in different angles | Immediate |
| Concentric and eccentric strengthening | 1-2 weeks |
| —against resistance | 3-4 weeks |
| Closed Chain Exercises d | |
| Pushing a soft rubber-ball with foot | Immediate |
| Closed chain exercises with half weight bearing | 2-3 weeks |
| —with full weight bearing | 5-6 weeks |
| Stationary bicycle with resistance | 2-4 weeks (if 90° knee flexion achieved) |
| Stairmaster | 6-8 weeks |
| Proprioception Return | |
| Balance exercises standing on both feet | 5-6 weeks |
| Standing on one foot (hard ground) | 6-8 weeks |
| Standing on one foot (trampoline or AeroStep) | 8-10 weeks |
| Return to Activity | |
| Jogging | 10 weeks |
| Straight line running | 3 months |
| Directional changes | 4-5 months |
| Shear forces | 5 months e |
| Sport specific adaptations | 5 months |
| Sport activity | 5-6 months f |
bThe main point of the rehabilitation is to ensure the early motion of treated joint to promote appropriate nutrition of transplanted cartilage. Cool therapy can be used during the first week to avoid postoperative bleeding and decrease postoperative pain. In a case of a concomitant procedure requiring external fixation of the affected joint (e.g., meniscus reinsertion), limitation of ROM for a short period by bracing can be allowed.
a Uzsoki Hospital and Sanitas Private Clinic, Budapest, Hungary.
c Extent, type (chondral or osteochondral), and location of the defect may modify weight bearing (see next page).
d Partial loading promotes to transform connecting tissue (between transplanted plugs) into fibrocartilage, so these exercises are mainly important in the half-weight-bearing period. On the other hand, with some closed chain exercises (e.g., cycling), it is possible to ensure cyclic loading that makes the fluid and nutrition transport much more efficient between synovial-fluid and hyaline cartilage.
e Approximately 4 to 5 months are needed to form a composite hyaline-like surface on transplanted area, which tolerates shear forces.
f Depending on depth and extent of the defect. If strength, power, endurance, balance, and flexibility are not satisfying, sport activity is allowed only later.
TABLE 5-2 Mosaicplasty Rehabilitation Protocol—Special Viewpoints
| Weight Bearing at Different Defects of Knee | |
| Femur or tibia condyle, chondral defect, d < 15 mm | |
| Non–weight bearing | 1 week |
| Partial weight bearing | 1-3 weeks |
| Femur or tibia condyle, chondral defect, d ≥ 15 mm | |
| Non–weight bearing | 2 weeks |
| Partial weight bearing | 2-4 weeks |
| Femur or tibia condyle, osteochondral defect | |
| Non–weight bearing | 3 weeks |
| Partial weight bearing | 3-5 weeks |
| Patellar defect, d < 15 mm | |
| Partial weight bearing | 2 weeks |
| Patellar defect, d ≥ 15 mm | |
| Partial weight bearing | 3 weeks |
| Quadriceps Strengthening and Patellar Mobilization—Differences at Patellar Defects | |
| Vastus medialis strengthening | |
| Isometric exercises in extension | Immediate |
| Patellar mobilization | Immediate |
| Isometric exercises in different angles | 1 week |
| Open chain exercises | 2 weeks |
| —against resistance | 3-4 weeks |
| Eccentric exercises against resistance | 4-5 weeks |
| Closed chain exercises | 2-3 weeks |
| The treatment of underlying causes can also modify the rehabilitation program. The most frequent combinations at knee applications are the following: | |
| LCA-reconstruction combined with mosaicplasty: | |
| 2-4 weeks non–weight bearing (up to the mosaicplasty) | |
| 2 more weeks partial weight bearing | |
| 5-90° ROM for 4 weeks | |
| Mainly closed chain exercises for quadriceps strengthening | |
| Hamstring strengthening in open and closed chain | |
| Proprioceptive training | |
| Meniscus reinsertion combined with mosaicplasty: | |
| 4 weeks non–weight bearing | |
| 2 more weeks partial weight bearing | |
| 5-45° ROM for 4 weeks | |
| Retinaculum patellae reconstruction combined with mosaicplasty: | |
| 2-4 weeks non–weight bearing (up to the mosaicplasty) | |
| 2 more weeks partial weight bearing | |
| 0-45° ROM for 4 weeks | |
| High tibial osteotomy (HTO) combined with mosaicplasty: | |
| Weight bearing (for 4 weeks only with crutches and only in extension) is up to the mosaicplasty, pain, and degree of the correction of the varus (lower correction: non–weight bearing; overcorrection: early weight bearing) | |
Errors, Hazards, and Complications
Septic or thromboembolic complications may result in a negative influence on the clinical outcome. Correct aseptic conditions, one-shot antibiotics, and thrombosis prophylaxis can decrease the chance of these complications.
Results
Femoral, tibial, and patellar implantations were evaluated by the modified Hospital for Special Surgery (HSS), modified Cincinnati, Lysholm, and International Cartilage Repair Society (ICRS) scoring systems, whereas possible donor-site disturbances and morbidity were evaluated by the Bandi scoring system. Patients with talar lesions were subjected to Hannover ankle evaluations. Analysis of clinical scores has shown good to excellent results in 92% of patients with femoral condylar implantations, 87% of tibial resurfacings, 74% of patellar or trochlear mosaicplasties, and 93% of talar procedures. Moderate and severe donor-site disturbances were present in 3% of patients according to the Bandi score1 (evaluations were done in a 1- to 10-year interval). Postoperative complications were four deep infections and 56 painful hemarthroses. Arthroscopic or open debridement resolved all deep infections, and 12 cases of hemorrhage also required arthroscopic or open debridement. The remaining patients with hemarthroses were treated by aspiration and cryotherapy. Four patients had minor thromboembolic complications.1–8
Several independent centers have published retrospective or comparative studies about the clinical outcome of autologous osteochondral mosaicplasty technique. Marcacci et al. (2005) (in a 2-year follow-up publication), Chow et al. (2004), Gudas et al. (2005), and Solheim et al. (1999) reported the same clinical efficacy.9,10,11 Horas et al. (2003) reported outstanding clinical results of mosaicplasty in a comparative, prospective study of mosaicplasty versus autologous chondrocyte transplantation.12 Nakagawa et al. (2004) published trochlear results, and Matsusue et al. (2001) reported successful tibial outcomes.13,14 Duchow et al. (2000) and Kordás et al. (2005) discuss important details of press-fit implantation.15,16
1. Hangody L., Kish G., Kárpáti Z., Eberhart R. Osteochondral plugs – Autogenous osteochondral mosaicplasty for the treatment of focal chondral and osteochondral articular defects. Operative Techniques in Orthopaedics. 1997;7(4):312-322.
2. Hangody L., Kish G., Kárpáti Z., Szerb I., Udvarhelyi I. Arthroscopic autogeneous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5(4):262-267.
3. Hangody L., Miniaci A., Kish G. MosaicPlasty™ Osteochondral Grafting – Technique Guide. Smith and Nephew Inc. 1997.
4. Hangody L., Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(suppl 2):25-32.
5. Hangody L., Duska Z.S., Kárpáti Z. Autologous osteochondral mosaicplasty. Techniques in Knee Surgery. 2002;1(1):13-22.
6. Hangody L., Duska Z.S., Kárpáti Z. Osteochondral plug transplantation. In: Jackson D., editor. Master Techniques in Orthopaedic Surgery. Reconstructive Knee Surgery. 2nd ed. Lippincott-Williams-Wilkins; 2003:337-352.
7. Hangody L., Feczkó P., Bartha L., et al. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res. 2001;391(Suppl):328-336.
8. Hangody L., Ráthonyi G., Duska Z.S., et al. Autologous osteochondral mosaicplasty – Surgical technique. J Bone Joint Surg. 2004;86-A(suppl I):65-72.
9. Marcacci M., Kon E., Zaffagnini S., Iacono F., Neri M.P., Vascellari A., Visani A., Russo A. Multiple osteochondral arthroscopic grafting (mosaicplasty) for cartilage defects of the knee: Prospective study results at 2-year follow-up. Arthroscopy. 2005;21:462-470.
10. Chow J.C.Y., Hantes M.E., Houle J.B., Zalavras C.G. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: A 2- to 5-year follow-up study. Arthroscopy. 2004;20:681-690.
11. Gudas R., Kalesinskas R.J., Kimtys V., et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-1075.
12. Horas U., et al. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. J Bone Joint Surg.. 2003;85-A:185-192.
13. Matsusue Y., Kotake T., Nakagawa Y., et al. Arthroscopic osteochondral autograft transplantation for chondral lesion of the tibial plateau of the knee. Arthroscopy. 2001;17(6):653-659.
14. Nakagawa Y., Matsusue Y., Suzuki T., Kuroki H., Nakamura T. Osteochondral grafting for cartilage defects in the patellar grooves of bilateral knee joints. Arthroscopy. 2004;20:32-38.
15. Duchow J., Hess T., Kohn D. Primary stability of press fit-implanted osteochondral grafts: Influence of graft size, repeated insertion and harvesting technique. Am J Sports Med. 2000;28:24-27.
16. Kordás G., Szabó J.S., Hangody L. The effect of drill-hole length on the primary stability of osteochondral grafts in mosaicplasty. Orthopedics. 2005;28:401-404.