CHAPTER 15 Osteochondral Lesions of the Talar Dome
New Horizons in Cartilage Replacement
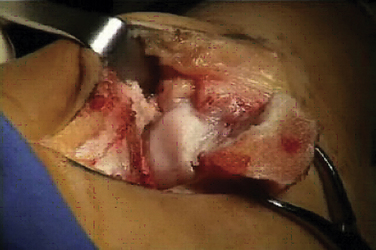
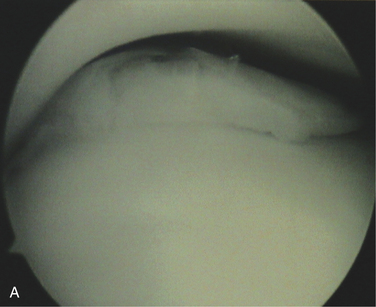
Osteochondral lesions of the talar dome larger than 2.5 to 3.0 cm2 pose a special problem in the young and middle-aged population (Figs. 15-1 to 15-3). These lesions affect the articular cartilage of the talar dome and the underlying subchondral bone.1 If encountered acutely or if a large fragment exists, some of these lesions can be stabilized and internally fixed with metallic or bioabsorbable implants. Success rates of 78% (range, 40% to 100%) have been reported with reduction and fixation of the osteochondral fragments.2 Most of the acute lesions suitable for internal fixation are laterally located and are usually anterior on the talar dome, making them relatively easy to access using a small, anterolateral arthrotomy incision or arthroscopic techniques.
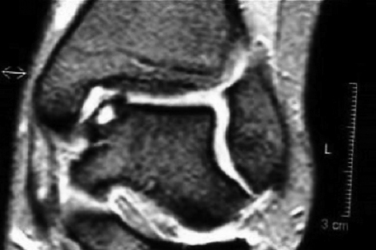
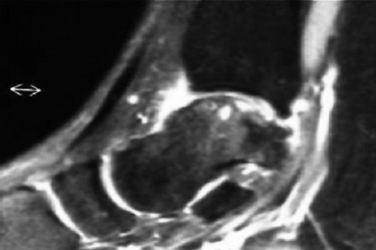
FIGURE 15-2 Anteroposterior magnetic resonance imaging shows an osteochondral lesion of the medial talus.
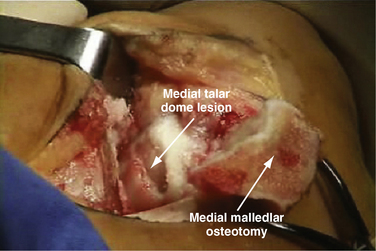
Unfortunately, patients with these lesions represent a small proportion of those who present for treatment of symptomatic osteochondral lesions of the talar dome. Most have chronic, medial lesions, which tend to occur more posteriorly on the talar dome. They are more difficult to access and usually require a medial malleolar osteotomy for open exposure. The posteromedial lesions more commonly manifest as chronic lesions that have greater depth than their lateral counterparts and that demonstrate degenerated articular cartilage with necrotic supporting subchondral bone.
COLLAGEN-COVERED AUTOLOGOUS CHONDROCYTE IMPLANTATION
In a prospective study presented at the ICRS meeting in 2004, Steinwachs17 described 163 patients treated for chondral defects in the knee with ACI using a periosteal flap or the Chondro-Gide membrane instead of the periosteal patch. At approximately 3 years of follow-up, 78% of patients in the periosteal group reported good or excellent results, and 88% of patients in the Chondro-Gide group reported good or excellent results. Statistical significance was not discussed by the investigator. There were no cases of membrane hypertrophy.17
MATRIX/MEMBRANE-INDUCED AUTOLOGOUS CHONDROCYTE IMPLANTATION
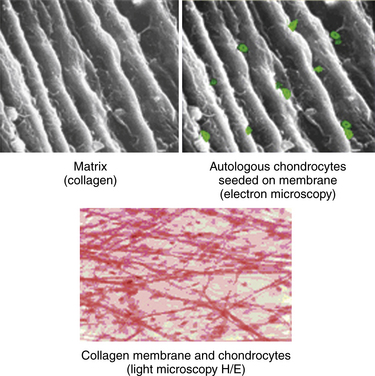
Matrix/membrane-induced autologous chondrocyte implantation (MACI) is a second-generation chondrocyte implantation process. MACI is a new biotechnology in which cultured autologous chondrocytes are impregnated onto a highly purified, porcine collagen I/III membrane (Verigen AG, Genzyme Corp., Cambridge, MA) (Fig. 15-4). The MACI implant can be fixed to the chondral defect by fibrin glue (with little or no suture necessary), suture, or bioabsorbable pins or tacks. The procedure can be performed arthroscopically or by mini- arthrotomy. No periosteal graft is needed.
Open Technique
Chondrocytes are harvested arthroscopically from a non–weight-bearing area of the ipsilateral knee (200 to 300 mg of healthy cartilage). The chondrocytes are then cultured and expanded in vitro (3 to 5 weeks) and then impregnated on an absorbable, three-dimensional, bilayered, purified, porcine collagen I/III membrane. The bilayer structure has a smooth side that acts as a natural barrier and faces the joint. Chondrocytes are seeded on the porous side of the matrix. The membrane is tear resistant and can be templated and cut to shape. The membrane is nonantigenic (i.e., telopeptides are split during the manufacturing process), and it is bioabsorbable. The bioabsorbable membrane can be fixed to the ankle cartilage defect with fibrin glue, pins, or suture.
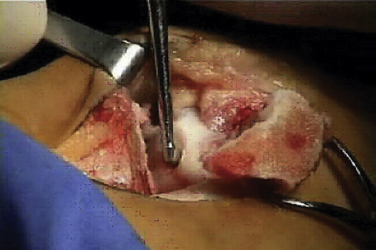
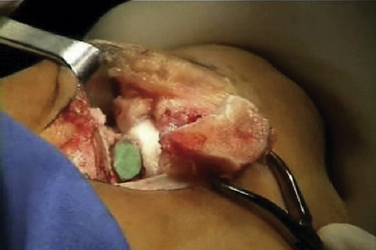
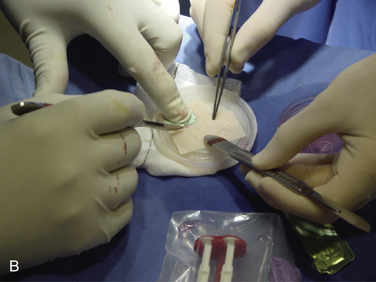
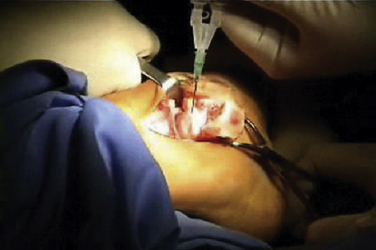
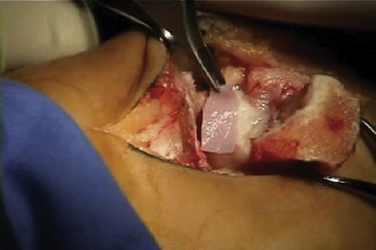
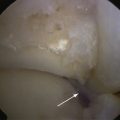
The talar dome lesion may be approached using a simple arthrotomy for an anterior lesion, or a malleolar osteotomy may be required to expose a middle to posterior talar lesion. The osteochondral defect is débrided, and the base is curetted to remove the calcified cartilage layer (Fig. 15-5). A stable cartilage rim with sharp vertical walls of healthy cartilage is created, and the chondral defect is templated for size and shape (Fig. 15-6). The MACI membrane is cut to the proper shape with a scalpel or scissors (Fig. 15-7). The membrane is then fixed with fibrin glue (Tisucol, Baxter, Spain) (Fig. 15-8). Suture or bioabsorbable pins, or both, may be used, but fibrin glue by itself is usually all that is needed (Figs. 15-9 and 15-10).18,19
Postoperatively, the patient is placed into a soft dressing, and continuous passive motion is initiated for 8 weeks, during which time the patient does not bear weight on the extremity. Patients with larger and more central lesions are not allowed to bear weight for 12 weeks.
Arthroscopic Technique
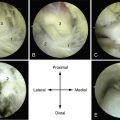
Placement of the graft is performed using a dry scope technique, which is done without fluid in the joint. Two small anchors with 5-0 absorbable sutures are then placed at opposite sides of the periphery of the cartilage lesion (i.e., 3 and 9 o’clock or 12 and 6 o’clock). The sutures are then passed through the MACI membrane outside the joint at points corresponding to the cartilage lesion (Figs. 15-11 to 15-13).
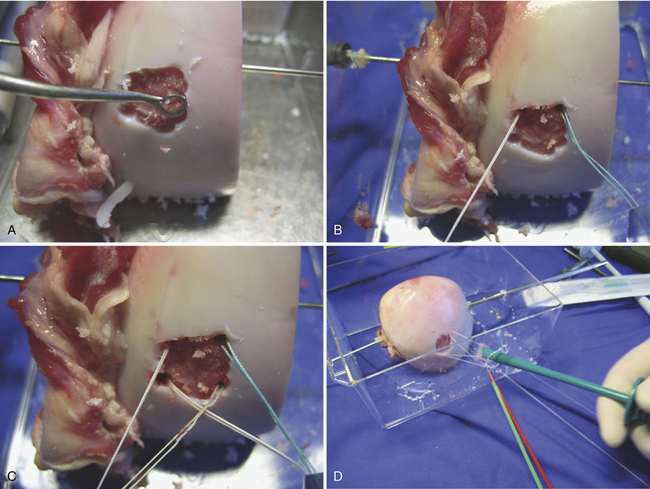
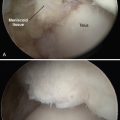
FIGURE 15-11 The lesion is curetted (A), and suture anchors are placed (B, C). The membrane is placed (D, lower right).
(From Chu C, ed. Articular cartilage surgery. Oper Tech Orthop. 2006;16:217-292.)
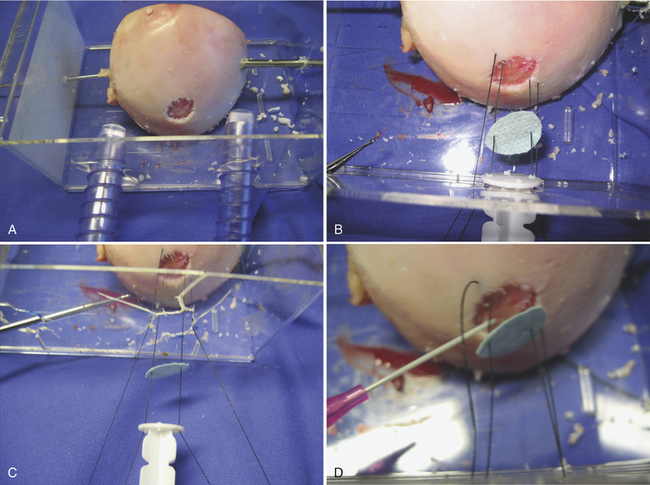
FIGURE 15-12 Placement of the membrane (A, B, C) and fibrin glue (D).
(From Chu C, ed. Articular cartilage surgery. Oper Tech Orthop. 2006;16:217-292.)
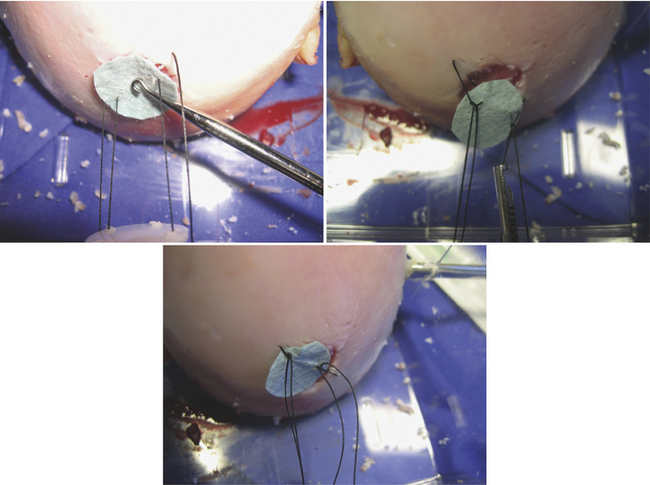
FIGURE 15-13 The membrane is secured by suture as well as fibrin glue. (From Chu C, ed. Articular cartilage surgery.
Oper Tech Orthop. 2006;16:217-292.)
The membrane is guided down the suture to the cartilage defect using the specially designed articulated passer. The membrane is then smoothed out using an articulated T smoother or tamper. Fibrin glue (Tisucol) is then placed under the membrane. The membrane over the grafted area is smoothed out to the contours of the cartilage defect. The articulated inserter is used to hold the graft in place, and the T smoother is used to remove the excess glue and ensure that the periphery of the graft is well fitted and securely glued in place. The two sutures are then tied over the graft using arthroscopic knot-tying technique. Pressure is applied for 6 or 7 minutes to allow the fibrin glue to fully set. The joint is taken through ranges of motion to ensure the graft is stable. For easily accessible lesions, the membrane can be pushed down the canula and held in place by fibrin glue and bioabsorbable pins (Figs. 15- 14 to 15-18).
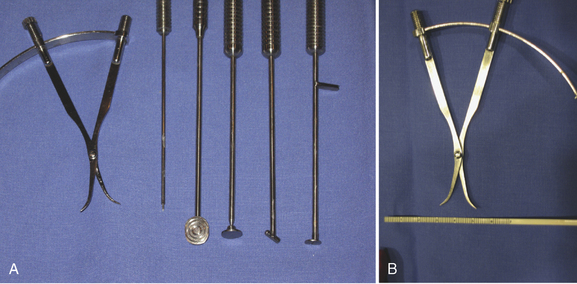
FIGURE 15-14 Arthroscopic instrumentation for matrix-based chondrocyte implantation. (A) Arthroscopic membrane inserter, articulated T smoother and articulated membrane tamp. (B) Arthroscopic caliper and flexible ruler.
(From Chu C, ed. Articular cartilage surgery. Oper Tech Orthop. 2006;16:217-292.)
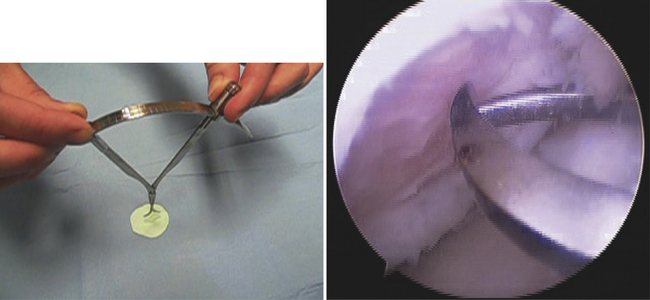
FIGURE 15-15 Arthroscopic caliper. (From Chu C, ed. Articular cartilage surgery.
Oper Tech Orthop. 2006;16:217-292.)
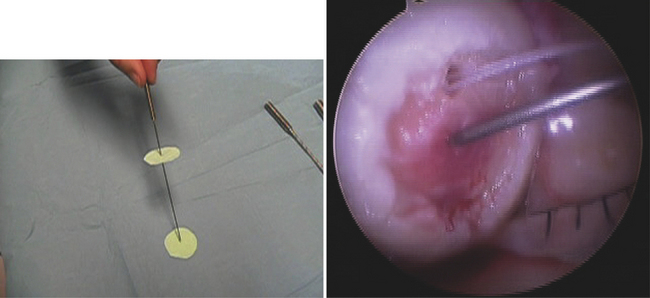
FIGURE 15-16 The graft for matrix-based chondrocyte implantation is inserted into cartilage defect.
(From Chu C, ed. Articular cartilage surgery. Oper Tech Orthop. 2006;16:217-292.)
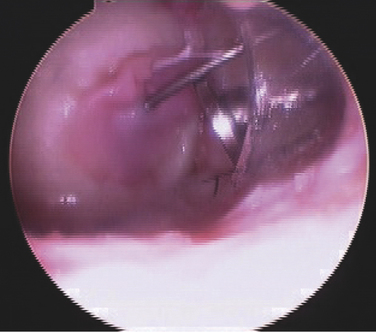
FIGURE 15-17 The graft for matrix-based chondrocyte implantation is held in place while the glue is setting.
(From Chu C, ed. Articular cartilage surgery. Oper Tech Orthop. 2006;16:217-292.)
FIGURE 15-18 Autologous chondrocyte implant for matrix-based chondrocyte implantation.
(From Chu C, ed. Articular cartilage surgery. Oper Tech Orthop. 2006;16:217-292.)
We have developed new instrumentation that allows the membrane to be pierced in its center and then placed in the center of the cartilage defect. The membrane is then pushed down the canula with a slotted articulated inserter and held in place by the arthroscopic skewer. Fibrin glue is then placed under the membrane, and the membrane is smoothed out. The excess glue is removed, and the membrane contours to the cartilage defect while the fibrin glue is setting (see Figs. 15-16 and 15-17).
Cherubino and colleagues20 reported six patients with deep chondral defects of the ankle averaging 3.4 cm2 (range, 2.5 to 4 cm2) treated using the MACI technique. At an average follow-up of 42.1 months, the American Orthopaedic Foot and Ankle Society (AOFAS) clinical-functional scores improved for five of the six patients. No complications were observed in the postoperative period. Second-look arthroscopy in the five successful ankles revealed the defect to be completely filled by hyaline-like tissue as determined by probing the surface, but biopsy of the regenerated surface was not performed. In five ankles, postoperative magnetic resonance imaging (MRI) demonstrated the presence of hyaline-like cartilage in the site of the defect according to the MRI criteria.20
MACI procedures have been used to treat 131 knees and 19 ankles at the Clinica CEMTRO in Madrid, Spain. The first 50 cases (42 knees, 8 ankles) were evaluated and reported in a level IV nonrandomized study with no control group.19 Defects were large, ICRS grade 3 (i.e., severely abnormal, 50% cartilage depth) or ICRS grade 4 (i.e., severely abnormal, extending into the subchondral bone). The size of the lesion varied from 3 to 6 cm.2 in the combined knee and ankle groups. Two years postoperatively, 88% of the patients reported no or little pain. All patients returned to their previous level of work activity. MRI showed a change in signal intensity postoperatively with a progressive decrease of subchondral edema. Biopsy of knee lesions has shown immature chondrocytes and immature cartilage. Second-look arthroscopy revealed good reparative tissue when probed (Fig. 15-19).
OTHER SCAFFOLDS AND BIOMATERIALS
Various scaffolds of a synthetic nature and hybrid materials in a variety of fibers, meshes, and gels have been applied to cartilage tissue engineering. Hyalograft C is a tissue-engineered graft consisting of autologous chondrocytes expanded in vitro and seeded onto a three-dimensional, nonwoven, hyaluronic acid scaffold, which is a benzyl ester of hyaluronic acid. The membrane is auto-adhesive and is amenable to arthroscopic implantation. This graft material has been extensively studied in the knee by Marcacci and colleagues21 at Instituti Rizzoli in Bologna, Italy, with encouraging preliminary results. A large, multicenter study in Italy of 129 patients demonstrated 91.5% improvement in their knee symptoms, functionality, and activity level. Giannini and colleagues22 reported 35 patients with osteochondral lesions of the talus treated with Hyalograft C ACI. There was no control group in the study. All patients were satisfied with their results. The AOFAS scores were improved from 50.8 ± 12 points preoperatively to 92.2 ± 9 points at 12 months’ follow-up. Three of the lesions in patients who remained symptomatic were biopsied at 1 year postoperatively. The histologic appearance was described as hyaline-like tissue with some evidence of type II collagen present.23
Chondrocelect® is a membrane-based, autologous chondrocyte implant using a set of molecular markers that predict the outcome of the in vivo cartilage-forming capacity. The chondrocytes are characterized to yield a phenotypically stable cartilage-forming cell population. By using a higher concentration of better-quality chondrocytes, a higher-quality structural repair is anticipated.24 In a multicenter, prospective, randomized study of patients with symptomatic chondral defects of the knee femoral condyle, Saris and coworkers25 compared treatment with microfracture to characterized chondrocyte implantation. They found that at short-term follow-up 1 year after treatment, characterized chondrocyte implantation resulted in regenerated tissue that was superior to that seen after microfracture in biopsy specimens. However, clinical results at 1 year of follow-up were similar for both treatments.25
Several other materials are in early phases of clinical testing. Bioseed-C® is a polyglactin/poly-p-dioxanone fleece (Biotissue Technologies, Freiberg, Germany) in which autologous chondrocytes are seeded. This can be inserted arthroscopically. Cartipatch® is a hydrogel composed of agarose and alginate for the matrix. It can be mixed with a cell suspension and can be molded. The alginate provides matrix elasticity, making it easy to handle. Hyaline cartilage with collagen II immunostaining was observed in one uncontrolled, level IV study.28 Arthromatrix® (Orthogen/Arthrex Biosystems) is an equine collagen I/III membrane seeded with chondrocytes, and Novocart 3D® is a collagen-based, biphasic carrier onto which autologous chondrocytes are seeded.30
Co.don® chondrosphere31 is a proprietary material in which chondrocytes that are processed and cultured in the presence of autologous serum form in vitro a three-dimensional chondrogenic tissue by generating their own extracellular matrix, which is similar to the matrix of hyaline cartilage. These autologous, engineered chondrospheroids serve as a basis for cartilage repair. The procedure can be performed using minimally invasive techniques. Preliminary pilot studies in humans are being conducted in Europe.
Autologous Matrix-Induced Chondrogenesis
Autologous matrix-induced chondrogenesis (AMIC) is a one-step procedure in which microfracture of the cartilage defect is performed (allowing bone marrow stem cells and growth factors to be released into the defect area), and the base is covered with a porcine collagen I/III matrix (Chondro-Gide matrix®) to stabilize the defect area and provide a suitable environment for the generation of new cartilage tissue. The membrane is fixed with fibrin glue and 10 mL of autologous patient serum. In a level IV, retrospective, uncontrolled case series, Behrens32 reported 25 patients with improvement of the ICRS and Cincinnati knee scores.
Platelet-Rich Plasma
Platelet-rich plasma is plasma that is rich in growth factors. These growth factors have a recognized influence on cellular activity. Autologous platelet-rich plasma can be used by itself or mixed with allograft or autograft to aid in cartilage regeneration.33
In a level IV, retrospective, uncontrolled case series, Cugat33 reported a rate of 78.2% for excellent or good results in the reduction or disappearance of pain and a rate of 77.7% for excellent or good results in improvement of function in cases of osteoarthritis. The follow-up time was short. The experience at Clinica CEMTRO in Madrid, Spain, using PRP for osteoarthritis was not encouraging.
Stem Cells
The results of ACI have shown that we are still a long way from regenerating articular cartilage. Because the chondrocyte is a rather quiescent cell, researchers are trying to develop alternative cell types, including mesenchymal stem cells (MSCs)34 to repair and regenerate tissues. An embryonic stem cell (ESC) is capable of differentiating into many tissue types, whereas differentiation of adult stem cells usually is restricted to the tissue type in which it resides. A MSC is potentially a multilineage progenitor cell that retains its capacity to divide and whose progeny can differentiate into mesodermal tissue cells such as cartilage, bone muscle, fat, tendon, and ligament.35
In principle, the goals of using stem cells are to induce and expand a group of multipotent cells down a signaled pathway into an end-stage phenotype or one that is capable of further development after implantation; to deliver the cells to the repair site using a scaffold; and to bind them to the edges of the defect.35
With current methods, autologous chondrocytes are essentially aged chondrocytes. Perhaps using stem cells may result in a higher-quality biologic and mechanical repair of cartilage tissue. MSCs have been shown to result in good repair of chondral tissue.36,37 ESCs may have offer the best repair of regenerated cartilage, but ethical and scientific issues regarding the use of ESCs in humans limits their application.
CONCLUSIONS
Full-thickness cartilage lesions pose a problem. Of the second- and third-generation autologous cultured chondrocyte technologies, none of them provides complete biomechanical or histologic restoration of native hyaline cartilage. There has not been a randomized, prospective, double-blind, statistically significant study documenting the true superiority of these tissue-engineered or bioengineered constructs in the ankle. However, promising research is occurring in one-stage MACI procedures, allogenic cartilage repair technologies, and progenitor cell and stem cell applications to cartilage regeneration.
1. Berndt AC, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41:988-1020.
2. Verhagen RA, Struijs PA, Bossmyt PM, Van Dijk CN. Systemic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8:233-243.
3. Williams SK, Amiel D, Bull S, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allograft. J Bone Joint Surg Am. 2003;85:2111-2120.
4. Ryan J, Jamali AA, Bugbee W. Fresh osteochondral all grafting. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:212-229.
5. Gross AE, Aguids Z, Hutchinson CR. Osteochondritis defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22:385-391.
6. Kim CW, Jamali A, Tontz WL, et al. Treatment of posttraumatic ankle arthrosis with bipolar tibiotalar osteochondral shell allografts. Foot Ankle Int. 2002;23:1091-1102.
7. Brittberg M, Lindahal A, Nilson A, et al. Treatment of deep cartilage defects in the knee with autologous cartilage transplantation. N Engl J Med. 1994;331:889-895.
8. Peterson L. ACI surgical techniques and results at 2-10 years. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:325-332.
9. Peterson L, Minas T, Brittberg M, et al. Two to nine year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop. 2000;374:212-234.
10. Peterson L, Brittberg M, Lindahal A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin. 2003;8:291-303.
11. Nan EK, Ferkel RD. Autologous chondrocytes implantation of the ankle. two to five year follow up. March 13, 2004 Presented at the American Orthopaedic Society for Sports Medicine Meeting San Francisco, CA
12. Ferkel RD, Hommen JP. Arthroscopy of the foot and ankle. In: Coughlin MJ, Mann RA, Saltzman CL, editors. Surgery of the Foot and Ankle. Philadelphia, PA: Mosby; 2007:1667-1677.
13. Koulalis D, Schultz W, Heydu M. Autologous chondrocyte transplantation for osteochondritis dissecans of the talus. Clin Orthop. 2002;395:286-292.
14. Brittberg M. Talus cartilage lesions treated with autologous chondrocyte implantation. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:333-340.
15. Giannini S, Buda R, Grigolo B, Vannini F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22:513-517.
16. Whittaker JP, Smith G, Makwana N, et al. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87B:179-183.
17. Steinwachs MR. ACI and resorbable collagen membrane. Chondro-Gide surgical technique and results. Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. 2006 Timeo Editore Bologna, Italy 389-392.
18. Abelow SP, Guillen P, Ramos T. Arthroscopic techniques for matrix-induced autologous chondrocyte implantation for treatment of large chondral defects in the knee and ankle. Oper Tech Orthop. 2006;16:257-261.
19. Abelow SP, Guillen P, Fernandez T, Guillen I. Autologous chondrocyte implantation for the treatment of large chondral defects in the knee and ankle. Vancouver, BC: Presented at the Arthroscopy Association of North America Annual Meeting; 2005.
20. Cherubino P, Ronga M, Grassi FA. Clinical results with MACI. In: Zansi S, Brittberg M, Marcacci M, et al, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:565-569.
21. Marcacci M, Kon E, Zaffagnini S, Iacono F. Surgical transplantation of autologous chondrocytes. simple procedure by arthroscopic technique. Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. 2006 Timeo Editore Bologna, Italy 457-461.
22. Zansi S, Brittberg M, Marcacci M. Multicentric study and results in Italy. In: Kon E, Reggiani LM, Delcogliano M, Filardo G, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:509-514.
23. Giannini S, Buda R, Vannini F, Grigolo B. Treatment of ankle defects with Hyalograft C. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:475-480.
24. Vanlauwe J, Bijlstra A, Bellemans J, Luyten F. Chondrocelct, an improved ACI product. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:369-386.
25. Saris D, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-246.
26. Mrosek EH, Erggelet C. Autologous chondrocyte implantation with a resorbable 3D polymer matrix (Bioseed-C). In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:413-417.
27. Erggelet C, Sittinger M, Lahm A. The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects in the knee joint. Arthroscopy. 2003;19:108-110.
28. Selmi TAS. Barnouin L, Bussiere C, Neyret P. Cartipatch. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:431-438.
29. Braun K, Imhoff AB. Arthromatrix (Orthagen/Arthrex). In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:403-405.
30. Gaissmaier C, Fritz J, Schewe B, et al. Development of NOVOCART 3D, a novel system for scaffold augmented transplantation of autologous chondrocytes. In: Zansi S, Brittberg M, Marcacci M, et al, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:573-585.
31. Libera J, Luethi U, Alasevic OJ. Co.don chondrosphere (Co.don AG) autologous matrix engineered cartilage transplantation. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:591-600.
32. Behrens P, Rogan IM. AMIC, autologous matrix induced chondrogenesis. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:767-770.
33. Cugat R, Carrillo JM, Serra I, Soler C. Articular cartilage defects reconstruction by plasma rich growth factors. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:801-807.
34. Derbies A, Magalia M, Mastrogiacomo M, Cancedda R. Human mesenchymal stem/progenitor cells. isolation, characterization and chondrogenic differentiation. Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. 2006 Timeo Editore Bologna, Italy 773-780.
35. Lee EH, Hui JH. Stem cells in the treatment of partial and full thickness cartilage defects. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:791-798.
36. Wakitani S, Goto T, Young RG, et al. Mesenchymal cell-based repair of large full thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76A:579.
37. Hue JH, Lee EH. Stem cells in the treatment of partial and full thickness cartilage defects. In: Zansi S, Brittberg M, Marcacci M, editors. Basic Science, Clinical Repair, and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore; 2006:791-798.