CHAPTER 12 Osteochondral Lesions of the Talar Dome
Débridement, Abrasion, Drilling, and Microfracture
Since the introduction of arthroscopy, the most common operative treatments rendered for OLT have been a continuation of what had been performed previously through standard incisions, such as excision of a loose fragment, débridement of the lesion, drilling, or abrasion and curettage. With the addition of the microfracture technique, as originally described for the knee by Steadman1 and later modified to the ankle, these forms of treatment for OLTs are collectively known as conventional surgical treatment.
INDICATIONS AND CONTRAINDICATIONS
Several factors must be taken into consideration to select the most appropriate surgical procedure for a symptomatic OLT, including the location, size or depth, and grade or stage of the lesion. Patient-specific characteristics, such as age, body mass index (BMI), degree of ankle instability, ankle alignment, duration of symptoms, and previous surgery, also influence outcomes.2–6
The lesion’s location does not technically limit the ability to perform the conventional surgical treatments discussed in this chapter as it does other restorative procedures, such as osteochondral autograft or allograft transplantation (i.e., osteoarticular transfer system [OATS]) or autologous chondrocyte implantation (ACI), which require greater access to the talar dome.7 Schimmer and colleagues8 found a trend toward a better prognosis for medial lesions, and Choi and associates9 found that anterior lesions had better results than posterior lesions, although this was disputed in a large case series that showed no correlation between outcome and lesion location.2,10 The location of the osteochondral lesion should not preclude treatment or greatly affect the outcome of lesions treated with conventional surgical treatment. It does however, have an impact on the use of specific surgical techniques (discussed later).
In contrast to the location of the lesion, size has proved to be an important prognostic indicator of the success of conventional surgical treatment. Good or excellent outcomes have been demonstrated in several studies in which the OLT was intraoperatively measured at less than 1.5 to 2.0 cm2, with later studies advocating a cut-off size of 1.5 cm2.2,6,11,12 For lesions larger than 1.5 cm2, OATS or ACI have been recommended by several surgeons.13–15 These transplantation techniques have yielded good or excellent results.16–18 Size may also be a determinant for internal fixation of acute, displaced osteochondral fragments in young patients. DeLee19 advocated internal fixation for a fragment that is larger than one third of the size of the dome. Alternatively, Stone and coworkers20 recommended that the lesion be at least 7.5 mm in diameter to warrant internal fixation.
Various grading and staging systems exist for the classification of OLTs, and each is driven by its own descriptors. Unfortunately, this has made the interpretation of results difficult for correlation of outcomes with stage or grade.12 It is widely accepted that a higher stage has a worse prognosis, as seen in a case series that established a relationship between the arthroscopic stage of Ferkel and clinical outcome.10
The literature on this topic reflects the importance of separating lesions into two distinct categories: those that have a cystic component, reflecting an underlying bony defect, and those that do not.3,4,6,21 Cystic lesions should then be further subdivided into two groups based on the presence or absence of an intact dome of overlying articular cartilage. Cystic lesions usually have poorer outcomes than noncystic lesions when treated with excision and curettage or with excision and drilling.3,4,6 However, successful treatment outcomes for cystic lesions with an intact cartilage cap have been demonstrated with the technique of retrograde drilling from the lateral talar process or sinus tarsi and bone grafting.21,22 This technique avoids violation of the intact talar dome cartilage while stimulating restoration of the underlying void. If a defect in the overlying cartilage is associated with a cystic lesion, it should be débrided and drilled or microfractured, and bone should be grafted to provide structural support for the replacement layer of fibrocartilage and any existing stable cartilage flap.
Accumulated experience in treating OLTs has taught us that factors associated with the individual patient can have a tremendous impact on the result of conventional surgical treatment.2–6 Several case series with relatively long-term follow-up have suggested that acute lesions have significantly better clinical outcome scores than chronic lesions.2–6 The threshold offered in most studies that delineates the two categories is a 12-month duration of symptoms. More controversy surrounds the influence of patient age on the outcome of OLT treatment. Three separate case series with a total of 386 patients revealed no significant correlation between patient age and outcome.3,6,9 In contrast, two case series with a total of 122 patients revealed a significant association between patient age and outcome, with success more often seen in patients younger than 30 to 35 years and failure seen in patients 45 to 50 years old.2,4
With regard to patient weight (i.e., BMI), two small case series addressing OLTs failed to establish any relationship with outcomes.3,23 The opposite viewpoint is supported by a large case series evaluating OLTs2 and another large, prospective cohort study analyzing osteochondral lesions of the knee,24 which showed a BMI grater than 28 to 30 to be a significant variable in predicting successful conventional surgical treatment.
Ankle instability has been an accepted cause of OLT, and surgically correcting ankle instability in the setting of a talar OLT should correlate with outcome. This has been clearly demonstrated by two independent studies,2,8 and it is recommended that any patient with mechanical ankle instability undergo some type of anatomic ligament reconstruction at the time OLT is surgically addressed. Although it seems that the immobilization required after ligament reconstruction may impair the healing process stimulated by conventional surgical treatment and promoted by early passive motion and no weight bearing, this approach has not been confirmed.
Another patient-specific factor to take into consideration is prior conventional surgical treatment. Existing literature on this topic has exhibited inconsistent findings and led to controversy on the topic. Schuman and colleagues25 showed that revision curettage and drilling had a poorer outcome (75% good or excellent results) compared with that of primary curettage and drilling (86%), and a case series2 of 105 patients found that 17 patients who underwent repeat débridement and microfracture did not meet the criteria for a successful result, although they did improve their American Orthopaedic Foot and Ankle Society (AOFAS) scores by a mean of 17.2 points postoperatively. A different case series of 12 patients treated with repeat arthroscopic débridement and curettage reported overwhelmingly successful results.26 Based on these findings, it is reasonable to recommend repeat conventional surgical treatment to address persistently symptomatic OLTs after a primary procedure, with the exception of cystic lesions.3
After processing all of the aforementioned factors, operative intervention using conventional options can be chosen as a valid treatment for an OLT. It is therefore helpful to compartmentalize the information presented into more concrete categories: indications, contraindications, and relative contraindications (Table 12-1). Conventional surgical treatment encompasses several different surgical techniques. In the treatment of noncystic lesions, arthroscopic excision of loose fragments and débridement of the lesion are consistent approaches. Variability is introduced in the selection of drilling, abrasion and curettage, or microfracture as the additional maneuver. The medical literature shows no significant differences in outcome for these approaches. This has been demonstrated in studies directly comparing these surgical options11,27 and by numerous other studies that show successful outcomes with each individual technique.2,5,9,10,12,23 In the treatment of a lesion with a cystic component, the surgical technique directly impacts the outcome, and retrograde drilling with bone grafting should be performed when possible.21,22
| Indications | Contraindications | Relative Contraindications |
|---|---|---|
BMI, body mass index; CST, conventional surgical treatment.
TREATMENT
Conservative Management
In the evolution of treatment for osteochondral lesions, the nonoperative approach with protection from weight-bearing stresses began with the adolescent knee.28–31 This approach was extended to the ankle until the classic study by Berndt and Harty, which included a careful analysis of 24 cases of OLT and a systematic review of the literature that included 191 lesions in 183 patients.32 Their study found a 75% rate of poor results for the conservatively treated patients, and it confirmed that the results in children were no better than in adults. Over the ensuing 50 years, the nonoperative treatment of limited weight bearing and cast immobilization has continued to be the recommended treatment for Berndt and Harty stage 1 and 2 lesions, which are nondisplaced fragments that remain at least partially attached.33–35 Because there are no adverse affects on the final outcome if surgery is delayed for up to 12 months, a trial of nonoperative treatment of these lesions is warranted.35–37
Another consideration in the nonoperative management of OLT, viscosupplementation, has gained attention. Viscosupplementation has been used as an approved treatment for arthritis of the knee for some time, and although it remains controversial, several studies have shown its effectiveness for use in the treatment of ankle arthritis.38–40 This experience has been extrapolated to sporadic use for OLTs, and a prospective study has shown promising results. Mei-Dan and colleagues41 demonstrated statistically significant decreases in pain and stiffness, with increases in functional scores over a 26-week follow-up period. In a patient who is adamantly averse to surgical intervention, viscosupplementation can be introduced as a potentially viable treatment option.
Unfortunately, the correlation between the radiographic grade and the actual condition of the articular cartilage is not very accurate, making it difficult to recommend treatment guidelines based solely on radiographic or CT images.42 Magnetic resonance imaging (MRI) has become the standard for evaluation of the articular cartilage in osteochondral lesions, and findings correlate well with arthroscopic findings.43 MRI studies should be obtained for all symptomatic lesions requiring treatment. The articular cartilage can then be analyzed to determine whether it remains contiguous at some point, allowing creation of a flap, or there is complete disruption of continuity, signaling an unstable pattern and warranting surgical intervention.
Arthroscopic Technique
After the OLT is evaluated and proper access is obtained, the specific treatment is based on the type of lesion. At this stage, it is essential to have the necessary instrumentation and any special equipment potentially required to address unexpected situations (Box 12-1). This is best confirmed in the preoperative setting to ensure an unhindered operation.
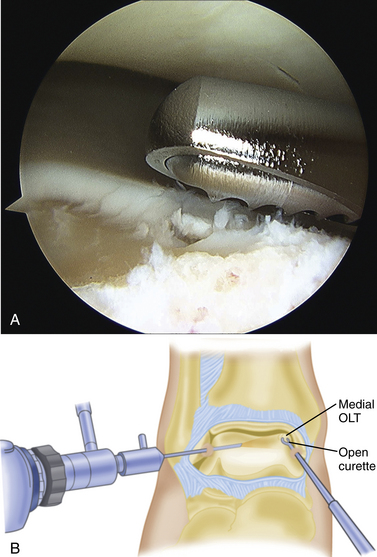
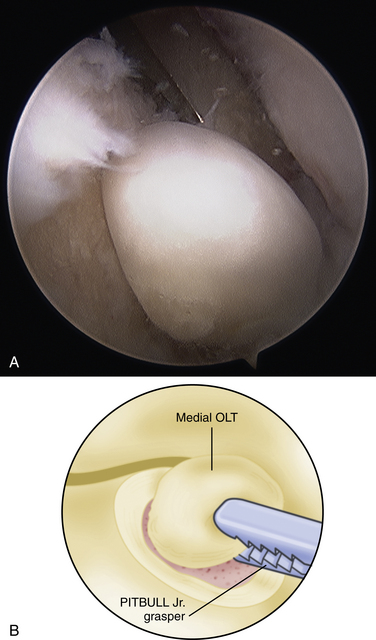
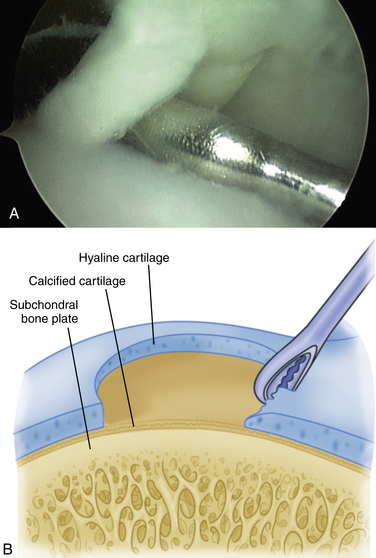
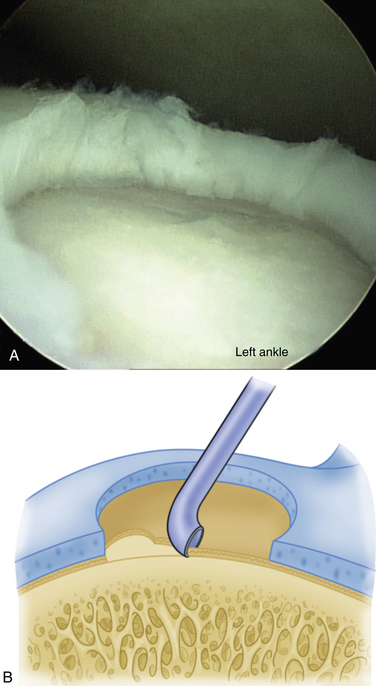
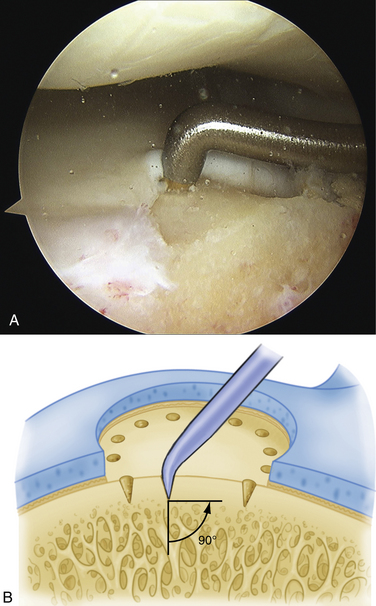
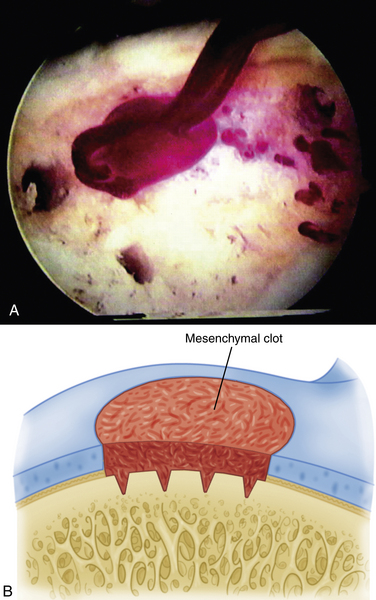
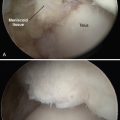
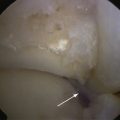
The technique for most osteochondral lesions (i.e., those without underlying cystic lesions) is straightforward. Any loose, unstable articular cartilage or osteochondral fragments must be débrided by shaving or curettage (Fig. 12-1) or by extracting the loose fragments from the joint with a grasper (Fig. 12-2). This leaves a defect in the articular surface with irregular margins that can be reformed into trephined edges with a small ring or regular curette (Fig. 12-3). Debris is removed from the joint with the shaver or a grasper, or both. If the basal layer of articular cartilage remains atop the defect, it is abraded or “scratched up” with a curette (Fig. 12-4). The surgeon can stop at this point or proceed with drilling or microfracture of the lesion to further expose marrow elements to create a “super clot” (Fig. 12-5).
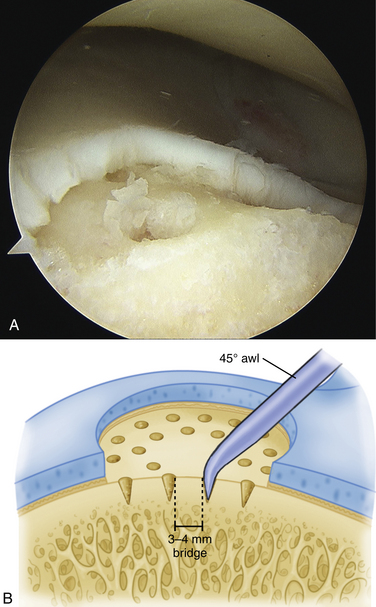
We use a 0.0625-inch Kirschner wire when drilling and standard or small microfracture awls for the microfracture technique. An assortment of angled awls is useful for penetrating the subchondral bone in a vertical orientation to the degree possible. The punctures are made to a depth of 2 mm and begin at the junction of normal cartilage with the osteochondral defect. After the perimeter of the lesion is microfractured, the pattern continues into the center of the lesion while keeping the holes 3 to 4 mm apart (Fig. 12-6). The shaver is again introduced to clean up the joint, and if used, the tourniquet is then released and the pump pressure reduced to confirm bleeding in the lesion (Fig. 12-7).
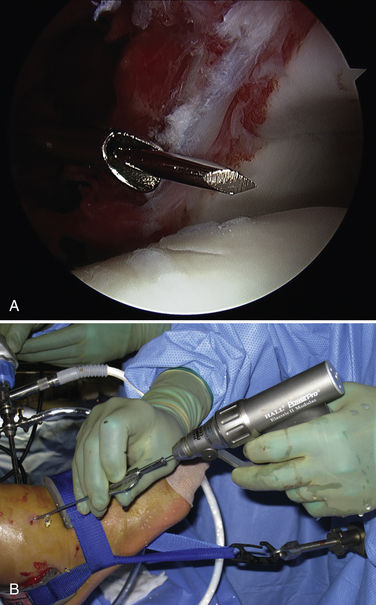
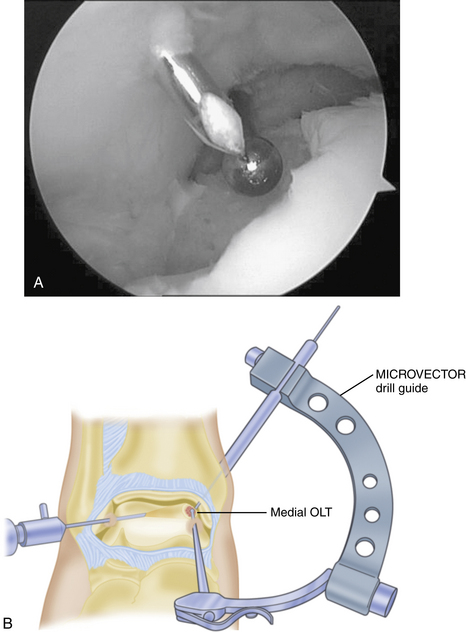
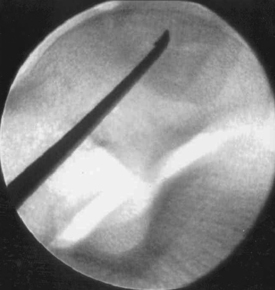
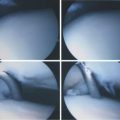
Technical aspects of antegrade drilling of OLTs depend on the location of the lesion. Anterolateral lesions are amenable to drilling through the anterolateral portal or through a separate anterior puncture in the capsule. The Kirschner wire can be introduced directly through the portal or placed through a guide, such as one of the meniscal repair cannulas used for inside-out repairs (Fig. 12-8). Plantar flexion of the ankle improves access to more posterior lesions. As in the microfracture technique described, drill holes should be placed at 3- to 4-mm intervals, beginning at the periphery of the lesion and working toward the center. Care is taken when drilling vertical walls not to perforate viable bone close to the overlying, intact subchondral plate and therefore create a stress riser that may lead to further collapse. Drilling of posteromedial lesions may be facilitated by using a commercially available drill guide with a transmalleolar approach. The aiming guide arm is introduced into the joint with the tip placed into the center of the lesion under direct arthroscopic visualization. A percutaneous incision is made over the area of the medial malleolus as indicated by the location of the external trocar, and the trocar is then advanced down to bone. A 0.062-inch Kirschner wire is drilled through the trocar in a transmalleolar fashion and visualized entering the joint (Fig. 12-9). The ankle can be dorsiflexed and plantar flexed to drill multiple holes in the lesion through the same medial malleolar drill hole.
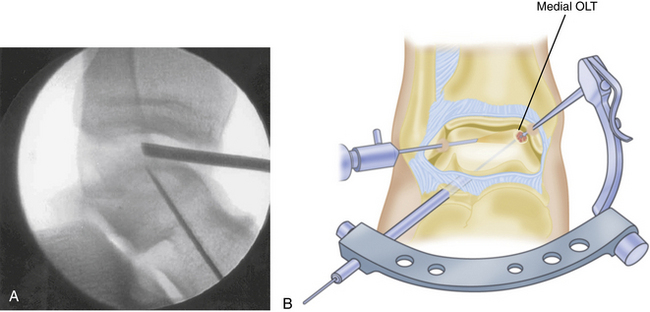
Any lesion encountered that maintains an intact cartilage roof with a cystic component and stable subchondral bone, although uncommon, is best treated with retrograde drilling and bone grafting21,22 (Fig. 12-10). This technique involves the use of a transtalar approach accessed through a more distal anterolateral incision in the area of the sinus tarsi. After the additional incision is made and blunt dissection is carried out down to the talus, the arm of a commercially available drill guide is introduced into the ankle joint through the anteromedial or posterolateral portal with the arthroscope in the anterolateral or posterolateral portal. The tip of the aiming guide is directed to rest on top of the lesion’s intact cartilage roof (Fig. 12-11). This can be assisted by the use of intraoperative C-arm fluoroscopy or, in some institutions, by computer-assisted, three-dimensional guidance systems.44 When the aiming guide is in place, a trocar is placed through the exterior arm of the drill guide into the sinus tarsi incision and down to talar bone. Using fluoroscopic guidance, a long Kirschner wire is then placed through the trocar and advanced up into the void of the cystic lesion but not through the stable subchondral bone. This position should be confirmed arthroscopically. The appropriate size of cannulated trephine drill is advanced over the Kirschner wire up to the tip, with care taken not to violate the roof. With an established tunnel leading to the lesion, the subchondral cyst can be curetted and bone grafted using autograft from the calcaneus or distal tibia (Fig. 12-12).
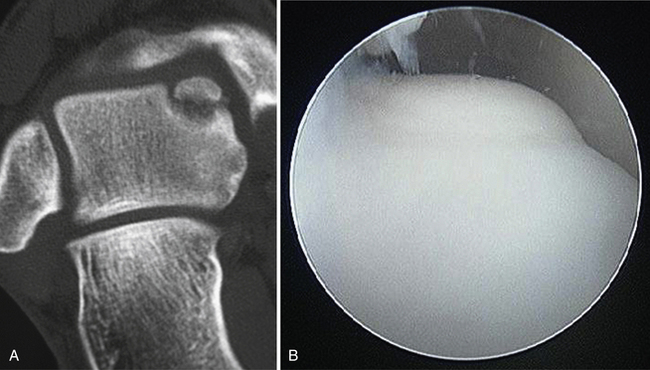
FIGURE 12-10 A, Coronal CT shows an osteochondral lesion of the medial talar dome with intact articular cartilage and an underlying cyst. B, Arthroscopic image of same osteochondral lesion shows intact articular cartilage.
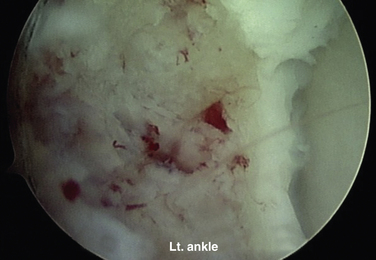
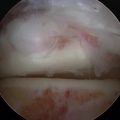
For a cystic lesion with unstable cartilage, standard or ring curettes are used to remove any loose articular cartilage flaps back to stable cartilage. The base of the lesion is inspected, and any unstable osteochondral fragment; soft, necrotic bone; or fibrous material is removed by curettage or shaving until viable bone is encountered (Fig. 12-13). The lesion must be thoroughly defined and débrided, including any portion that extends onto the vertical surfaces of the talus in the gutter.
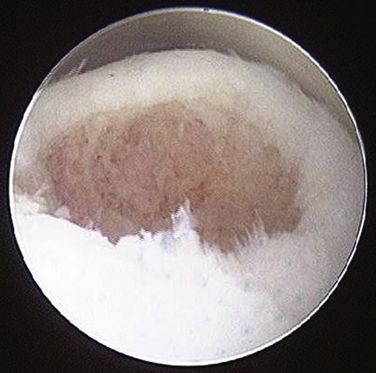
FIGURE 12-13 The arthroscopic image shows an osteochondral lesion of the talus after curettage to viable bone.
The remaining defect should be thoroughly inspected. If a hard sclerotic bony base is present that was not easily penetrated with the curettage performed, additional perforation with a microfracture awl or antegrade drilling is recommended to expose marrow elements to the defect (Fig. 12-14). Additional stimulation should be done if there is no punctate bleeding demonstrated after curettage when the inflow pressure is reduced or the tourniquet is released.
The cystic lesion may warrant open bone grafting after curettage and débridement have been done. There is no consensus regarding this issue, and no specific lesion size or depth to guide the indication of bone grafting has been established in the literature. Saxena and colleagues45 performed autogenous bone grafting by open arthrotomy on all Hepple stage 5 lesions (i.e., subchondral cyst formation seen on MRI) found in athletes with 90% good or excellent outcomes and a mean time for return to activity of 20 weeks. We prefer to obtain autogenous bone graft from the calcaneus or distal tibia by using 6- or 8-mm bone graft harvester reamers, which are commercially available. The lesion must then be approached in an open fashion by an arthrotomy or osteotomy, depending on the location of the lesion, as described in detail by Muir and associates.7 After the lesion is accessed, the bone graft should be tamped into place so that the void is tightly packed and no graft remnants are left prominent.
PEARLS& PITFALLS
PEARLS
PITFALLS
Postoperative Rehabilitation Protocol
Postoperative rehabilitation of cystic lesions entails a slightly more conservative approach with regard to weight bearing. If bone grafting of the cystic lesion is performed, the patient is told to not bear weight for a minimum of 6 weeks. With radiographic evidence of incorporation of the bone graft into the lesion, consolidation of the bone graft donor site, and healing of any necessary osteotomies, the patient can begin progressive weight bearing in a boot over a 4-week period, followed by transition to a sneaker and brace as previously described. A patient with a cystic lesion with an intact cartilage roof that has undergone retrograde drilling is prevented from bearing weight for 2 to 4 weeks if the lesion is smaller than 1.5 cm2 and for 4 to 6 weeks if the lesion is larger than 1.5 cm2. Otherwise, early ROM with a therapy program as described is allowed, with the exception of a proportional delay in impact activities, walking, and running. As with noncystic lesions, healing can be assessed around the sixth postoperative month. However, CT is the more appropriate imaging modality to evaluate reconstitution of bony architecture.
CONCLUSIONS
OLTs continue to present a treatment challenge to the orthopedic surgeon. By following the indications from contemporary literature, an appropriate surgical treatment plan can be developed. Attention to detail in the preoperative setting allows the surgeon to strategically design the arthroscopic approach or open surgical approach, or both, that will be best suited for the lesion’s location, size, and cystic characteristics. Necessary special equipment and tools for the case should be confirmed with the operating room. The postoperative rehabilitation is tailored according to the type of procedure performed. Ultimately, treatment of OLTs with the methods outlined in this chapter should yield favorable outcomes for a high percentage of patients, lead to a better quality of life, and allow return to a desired, preinjury level of activity.
1. Steadman JR, Rodkey WG, Singleton SB, Briggs KK. Microfracture technique for full-thickness chondral defects. technique and clinical results, Oper Tech Orthop. 71997 300-304.
2. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle. outcome analysis and outcome predictors of 105 cases, Arthroscopy. 242008 106-112.
3. Robinson DE, Winson IG, Harries WJ, Kelly AJ. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br. 2003;85:989-993.
4. Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81:1229-1235.
5. Kelberine F, Frank A. Arthroscopic treatment of osteochondral lesions of the talar dome. a retrospective study of 48 cases, Arthroscopy. 151999 77-84.
6. Smith BW, Cuttica D, Hyer CF, et al. Osteochondral lesions in the talus. predictors of outcome and treatment algorithm. July, 2008 American Orthopaedic Foot and Ankle Society Denver, CO Presented at the 23rd Annual Meeting of the
7. Muir D, Saltzman CL, Tochigi Y, Amendola N. Talar dome access for osteochondral lesions. Am J Sports Med. 2006;34:1457-1463.
8. Schimmer RC, Dick W, Hintermann B. The role of ankle arthroscopy in the treatment strategies of osteochondritis dissecans lesions of the talus. Foot Ankle Intl. 2001;22:895-900.
9. Choi WJ, Lee JW, Kim BS, Han SH. Correlation of defect size and clinical outcome in osteochondral lesion of the talus. Denver, CO: American Orthopaedic Foot and Ankle Society; July 2008. Presented at the 23rd Annual Meeting of the
10. Ferkel RD, Zanotti RM, Komenda GA, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus. long-term results, Am J Sports Med. 362008 1750-1761.
11. Lahm A, Erggelet C, Steinwachs M, Reichlet A. Arthroscopic management of osteochondral lesion of the talus. results of drilling and usefulness of the magnetic resonance imaging before and after treatment, Arthroscopy. 162000 299-304.
12. Tol JL, Struijs PAA, Bossuyt PMM, et al. Treatment strategies in osteochondral defects of the talar dome. a systematic review, Foot Ankle Int. 212000 119-126.
13. Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome. current concepts review, Foot Ankle Int. 252004 168-175.
14. Baurns MH, Heidrich G, Shultz H, et al. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am. 2006;88:303-308.
15. Whittaker JP, Smith G, Makwana N, et al. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87:179-183.
16. Giannini S, Buda R, Vannini F, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus. surgical technique and results, Am J Sports Med. 362008 873-880.
17. Lee CH, Chao KH, Huang GS, Wu SS. Osteochondral autografts for osteochondritis dissecans of the talus. Foot Ankle Int. 2003;85:989-993.
18. Sammarco JG, Makwana NK. Treatment of talar osteochondral lesions using local osteochondral graft. Foot Ankle Int. 2002;22:693-698.
19. DeLee JC. Fractures and dislocations of the foot. In: Mann RA, Coughlin MJ, editors. Surgery of the Foot and Ankle. 6th ed. St. Louis, MO: Mosby; 1991:1465-1518.
20. Stone JW. Osteochondral lesions of the talar dome. J Am Acad Orthop Surg. 1996;4:63-73.
21. Taranow WS, Bisignani GA, Towers JD, et al. Retrograde drilling of osteochondral lesions of the medial talar dome. Foot Ankle Int. 1999;20:474-480.
22. Kono M, Takao M, Naito K, et al. Retrograde drilling for osteochondral lesions of the talar dome. Am J Sports Med. 2006;34:1450-1456.
23. Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26:583-589.
24. Mithoefer K, Williams RJ, Warren RF, et al. The microfracture technique for treatment of articular cartilage lesions of the knee. a prospective cohort study, J Bone Joint Surg Am. 872005 1911-1920.
25. Schuman L, Struijs PAA, Van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus. results at follow up at 2 to 11 years, J Bone Joint Surg Br. 84B2002 364-368.
26. Savva N, Jabur M, Davies M, Saxby T. Osteochondral lesions of the talus. results of repeat arthroscopic débridement, Foot Ankle Int. 282007 669-673.
27. Gobbi A, Francisco RA, Lubowitz JH, et al. Osteochondral lesions of the talus. randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation, Arthroscopy. 222006 1085-1092.
28. Green, JP. Osteochondritis dissecans of the knee. J Bone Joint Surg Br. 1966;48B:82-91.
29. Green WT, Banks HH. Osteochondritis dissecans in children. J Bone Joint Surg Am. 1953;35A:26-47, 64.
30. Van Demark RE. Osteochondritis dissecans with spontaneous healing. J Bone Joint Surg Am. 1952;34A:143-148.
31. Wiberg G. Spontaneous healing of osteochondritis dissecans in the knee joint. Acta Orthop Scand. 1943;14:270-277.
32. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41A:988-1020.
33. Canale ST, Belding RH. Osteochondral lesions of the talus. J Bone Joint Surg Am. 1980;62:97-102.
34. Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fracture of the talus). review of the literature and new surgical approach for medial dome lesions, Foot Ankle Int. 51985 165-185.
35. Pettine KA, Morrey BF. Osteochondral fractures of the talus. a long-term follow-up, J Bone Joint Surg Br. 691987 89-92.
36. Van Beucken K, Barrack RL, Alexander AH, et al. Arthroscopic treatment of transchondral talar dome fractures. Am J Sports Med. 1989;17:350-356.
37. O’Farrell TA, Costello BG. Osteochondritis dissecans of the talus. the late results of surgical treatment, J Bone Joint Surg Br. 641982 494-497.
38. Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo-controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33:951-956.
39. Salk RS, Chang TJ, D’Costa WF, et al. Sodium hyaluronate in the treatment of osteoarthritis of the ankle. a controlled, randomized, double-blind pilot study, J Bone Joint Surg Am. 882006 295-302.
40. Sun S, Chou Y, Hsu C, et al. Efficacy of intra-articular hyaluronic acid in patients with osteoarthritis of the ankle. a prospective study, Osteoarthritis Cartilage. 142006 867-874.
41. Mei-Dan O, Maoz G, Swatzon M, et al. Treatment of osteochondritis dissecans of the ankle with hyaluronic acid injections. a prospective study, Foot Ankle Int. 292008 1171-1178.
42. Pritsch M, Horoshovski H, Farine I. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1986;68A:862-865.
43. Mintz DN, Tashijian GS, Connell DA, et al. Osteochondral lesions of the talus. a new magnetic resonance grading system with arthroscopic correlation, Arthroscopy. 192003 353-359.
44. Bale RJ, Hoser C, Rosenberger R, et al. Osteochondral lesions of the talus. computer-assisted retrograde drilling—feasibility and accuracy in initial experiences, Radiology. 2182001 278-282.
45. Saxena A, Eakin C. Articular talar injuries in athletes. Am J Sports Med. 2007;35:1680-1687.