CHAPTER 13 Osteochondral Lesions of the Talar Dome
Cartilage Replacement Using Osteochondral Autogenous Transplantation and Mosaicplasty
TERMINOLOGY
The term osteochondritis dissecans was first used in 1887, when Konig1 described an inflammatory lesion of cartilage and underlying bone in the knee joint that resulted in loss of structural integrity, causing pain and dysfunction. Since then, it has been recognized that lesions of the bone and cartilage may appear spontaneously or may be caused by a traumatic event to the joint surface.2 The general term osteochondral lesion (OCL) has gained more widespread use than osteochondritis dissecans.
ETIOLOGY, EPIDEMIOLOGY, AND CLASSIFICATION
In the ankle joint, an OCL can occur spontaneously or as the result of trauma, and there appears to be a relationship between the location of the lesion and the cause.3,4 In a comprehensive review of the early literature that included more than 500 cases of OCL, Flick and Gould5 found that 98% of lateral lesions and only 70% of medial lesions were associated with a traumatic event. This and other reports2,6 led to the associations of traumatic OCLs with lateral lesions and spontaneous OCLs with medial lesions.
The incidence of OCL depends on the etiologic factors involved. In an early study, Bosien and colleagues7 reported the incidence of OCL to be 6.5% with ankle sprains, and Hinterman and coworkers8 and Leontaritis and associates9 reported the incidence of OCL as 69% and 74%, respectively.
The most recognized classification system is that of Berndt and Harty,2 which is useful for research purposes and preoperative planning. It is based on radiographic imaging or intraoperative findings. Stage I is a small, subchondral compression; stage II is partial fragment detachment; stage III is complete fragment detachment with no displacement; and stage IV is complete fragment detachment with displacement.
PATIENT EVALUATION
History and Physical Examination
Physical examination should begin with assessment of the standing alignment to identify an associated coronal plane deformity. Inspection of the ankle with an acute injury usually reveals swelling and ecchymosis, but the ankle sometimes appears completely normal, as is often the case with delayed presentations. An attempt to elicit tenderness with palpation should be made by focusing on the common OCL sites. Posteromedial lesions may be tender in response to palpation when the ankle is dorsiflexed and the region posterior to the medial malleolus is palpated. Anterolateral lesions may be tender when the joint is palpated laterally with the ankle in plantar flexion. These findings are often nonspecific because the tenderness is likely related to joint synovitis rather than the OCL itself. The joint should be taken through a range of motion to assess stiffness and to feel for crepitus and mechanical signs of clicking or locking. Ligamentous stability should be assessed to ascertain evidence of associated laxity, particularly of the lateral ligament complex. The examination is completed with a thorough neurovascular assessment and examination of the contralateral ankle for comparison.
Diagnostic Imaging
MRI affords an accurate assessment of the ankle’s soft tissue structures, including the articular cartilage and supporting ligaments. Multiplanar evaluation is possible with no radiation exposure to the patient. Sequences can identify the size and location, and they provide information about the stability of the lesion. DeSmet and colleagues10 prospectively showed an excellent correlation between staging with MRI and arthroscopic results, correctly predicting stable and unstable lesions in 92% of cases. In some circumstances, MRI may overestimate the size of the OCL due to associated edema, and CT may be better suited for defining the dimensions of the defect.
TREATMENT
The use of non–weight-bearing articular surfaces as a donor site for osteochondral autogenous transplantation (OAT) grafts for the treatment of OCL began in the 1980s. Yamashita and coworkers11 published a case report on two patients who had an OAT graft harvested from the non–weight-bearing portion of the medial femoral condyle and transplanted to an OCL of the same knee.
In 1993, Matsusue and associates12 reported their 3-year follow-up of cases treated with a new technique that involved transplantation of multiple osteochondral plugs harvested from the same knee. They used the press-fit method of cylindrical osteochondral plugs described by Garrett13 for allografts. They harvested two osteochondral autogenous graft (OAG) plugs from the lateral femoral patellar groove and one from the anterolateral side of the intercondylar notch.
In 1996, Bobic14 was the first to publish a case series (12 patients) describing multiple OAT grafts for the treatment of knee OCLs associated with anterior cruciate ligament reconstruction. In 1997, Hangody and colleagues15 published their first report of their prospective case series. They described 44 patients with 1 to 5 years of follow-up after multiple OAG plugs were used to treat OCLs of the knee. Most patients had good or excellent outcomes. They were the first to call the procedure mosaicplasty. We now commonly use this term for the technique of using multiple OAT graft plugs to treat an OCL. Some surgeons make distinction between OAT and mosaicplasty, using the former to describe the use of only one graft plug and the latter to describe the use of multiple graft plugs. These terms can be used interchangeably, but for clarification, we use the term mosaicplasty osteochondral autogenous transplantation (MOAT) when referring to transplantation with multiple graft plugs and OAT when referring to transplantation of a single graft plug.
Evidence-Based Studies of Osteochondral Autogenous Transplantation and Mosaicplasty Osteochondral Autogenous Transplantation
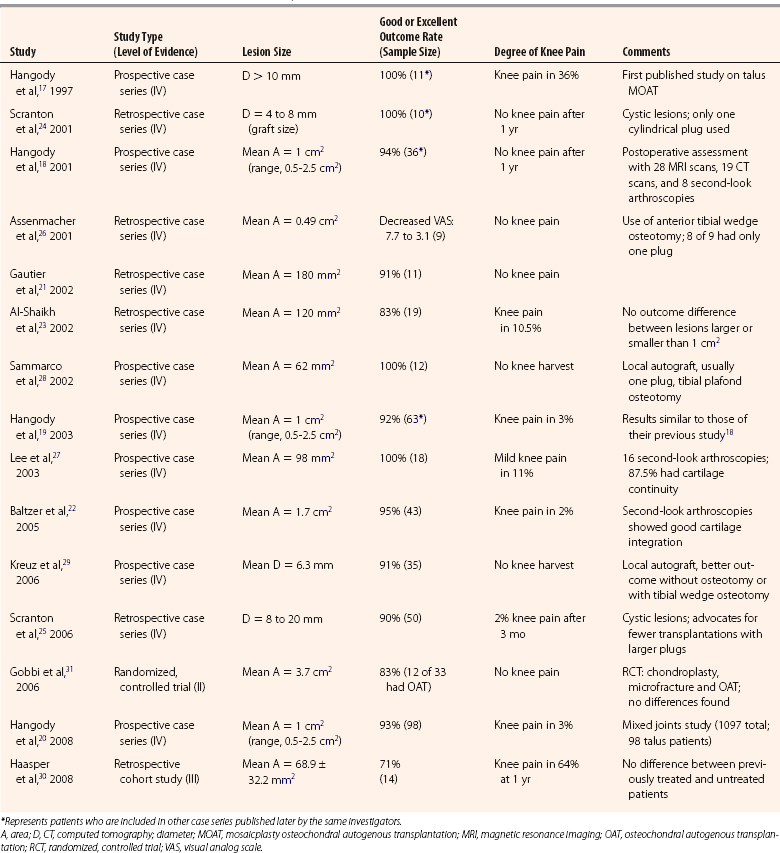
Given the success of OAT and mosaicplasty in the knee, there was a logical extension of this technique to the ankle. In reviewing the literature on OAT and MOAT in the ankle before February 2009, 15 studies were identified. All articles were reviewed and assigned a classification (i.e., I through IV) using the Journal of Bone and Joint Surgery levels of evidence for primary research questions.16 The 15 studies included 1 level II study, 1 level III study, and 13 level IV studies (Table 13-1).
TABLE 13-1 Clinical Outcome Studies of Mosaicplasty Osteochondral Autogenous Transplantation for Talar Osteochondral Lesions
Level IV Evidence
In 1997, Hangody and coworkers17 were the first to report on use of MOAT for treatment of OCL of the talus. In a preliminary report of a prospective case series (level IV), MOAT was done on 11 patients with OCLs larger than 10 mm diameter. The outcome results after at least 1 year of follow-up were excellent and promising. This study included second-look arthroscopies in three patients and one cartilage biopsy. Arthroscopy showed good incorporation of the grafts, with articular surface congruency and normal cartilage appearance. Biopsy showed normal articular hyaline cartilage (i.e., collagen type II) and proteoglycans and fibrocartilage bonding at the graft-recipient junction. Donor-site morbidity was a concern, because 3 of 11 patients had occasional knee pain and 2 of 11 had knee pain with activities (one with a 10-degree extension lag).
In 2001,18 2003,19 and 2008,20 Hangody and coworkers expanded their prospective case series report (level IV) after 2 to 9 years of follow-up after MOAT for OCL of the talus. The report included 98 patients with 92% to 94% good or excellent clinical results and less than 3% had knee pain after 1 year. Further assessment included 28 MRI scans, 19 CT scans, 8 second-look arthroscopies, and 4 biopsies. CT, MRI, and arthroscopy showed good incorporation of the grafts, with articular surface congruency and normal cartilage appearance. Biopsies showed normal articular cartilage collagen (type II) and proteoglycans. Three postoperative knee arthroscopies showed a donor-site defect covered by fibrocartilage congruent with the surrounding hyaline cartilage.
In smaller case series (level IV), Gauthier and colleagues21 and Baltzer and coworkers22 described 11 (retrospective) and 43 (prospective) cases, respectively, that had MOAT for OCL of the talus. More than 90% of their patients were satisfied with the results. In another retrospective case series (level IV), Al-Shaihk and associates23 performed MOAT on 19 patients with larger OCLs (mean area of 120 mm2). Eighty-three percent of patients were satisfied with the results, and two patients (10.5%) reported mild knee pain. They found no difference in outcomes between patients with lesions larger than 1 cm2 and those with lesions smaller than 1 cm.2
In 200124 and 2006,25 Scranton and colleagues confirmed earlier promising results with a retrospective case series (level IV) that evaluated 50 patients. Their technique included the transplantation of only one cylindrical plug in patients having cystic lesions that were 4 to 20 mm of diameter. The results were equally impressive, and the investigators advocated arthroscopic harvesting and the use of larger and fewer plugs to minimize peripheral graft chondrocytes death and to simplify the technique. In a retrospective case study (level IV), Assenmacher and coworkers26 reported the use of single-plug OAT in 8 of 9 patients, who had an average decrease in the visual analog scale (VAS) score of 7.7 to 3.1 after the procedure. They also described the use of an anterior tibial wedge osteotomy (i.e., plafondplasty) in five patients for lesions in the central third of the talar dome to avoid a malleolar osteotomy. Preoperative MRI showed an excellent correlation for OCL grading, and postoperative MRI showed integration of the grafts.
In a prospective case series (level IV) reported by Lee and associates,27 18 patients had 100% good or excellent outcomes. Two of the 18 patients (11%) had mild knee pain, and 14 (87.5%) of 16 second-look arthroscopies showed cartilage continuity with the native cartilage.
To minimize the risk of knee pain associated with violating an asymptomatic joint to harvest the OAG, Sammarco and colleagues28 performed a local osteochondral graft for an OCL with a mean area of 62 mm,2 and they typically used a one-plug graft. They harvested the grafts from the medial or lateral talar facet on the same side as the lesion they were treating. In the prospective case series (level IV), the investigators reported that all 12 patients were satisfied with the results and that they had no talar collapse.
In a prospective case series (level IV), Kreuz and coworkers29 reported 91% good or excellent results using local OAT grafts harvested from the medial or lateral talar facet. They subdivided their series into four subgroups: no osteotomy, medial malleolar osteotomy, tibial wedge plafond osteotomy, and posterolateral approach. They found better outcomes in the two groups not undergoing osteotomies compared with the others and found better outcomes for the tibial wedge osteotomy group compared with the medial malleolar osteotomy group.
Level III Evidence
In a small, retrospective cohort study (level III) of 14 patients, Haasper and coworkers30 did not find differences in outcomes between previously treated and previously untreated OCL patients receiving MOAT. The lesions were small (68.9 ± 32.2 mm2) and a surprisingly large number of patients reported knee pain at a minimum of 1-year of follow-up.
Level II Evidence
In a randomized, controlled trial (level II), Gobbi and colleagues31 compared chondroplasty, microfracture, and MOAT in 33 ankles. The American Orthopaedic Foot and Ankle Society (AOFAS) Hindfoot Score, the Ankle-Hindfoot Scale, and the Subjective Assessment Numeric Evaluation improved in all groups, and the investigators found no significant difference in outcomes among the three groups. There was an inverse correlation between size of the defect and outcome in the microfracture and OAT groups. To have comparable techniques, the central and posterior lesions that needed a malleolar osteotomy were excluded from the study.
Technique Advantages and Disadvantages
The advantages of using MOAT over other techniques to treat OCL are a one-step procedure with relatively fast recovery and hyaline cartilage covering more than 80% of the treated lesion, and consistently more than 80% to 90% good clinical results. Multiple, small autografts have the advantage of reduced complications at the donor site, better fit to the shape of the defect, and avoidance of screws or wires for fixation with the press-fit implantation technique.32
Indications and Contraindications
Débridement with subchondral bone penetration produced consistently good or excellent outcome results.31 In a prospective case series that used the microfracture technique, Chuckpaiwong and associates33 had 100% good or excellent results in patients with lesions less than 15 mm in diameter but only 3% good or excellent results in patients with lesion larger than 15 mm in diameter. The only randomized, controlled trial (level II) comparing MOAT with a form of subchondral bone penetration technique showed no difference in outcomes.31 The only level III study of MOAT showed no difference between patients with OCLs previously treated by subchondral bone penetration and untreated patients.30
Given a lack of literature to support superior outcomes for OAT and mosaicplasty for the treatment of OCL and because of the procedure’s inherently more invasive nature and the potential morbidity at the donor site, we feel that OAT and mosaicplasty should be reserved for failure of a prior attempt of arthroscopic débridement associated with any technique of subchondral bone penetration (i.e., curettage, drilling, or microfracture), at least for OCLs of the talus smaller than 15 mm in diameter. However, the literature does not provide direction about the primary treatment of OCLs larger than 15 mm in diameter. There may be a role for primary MOAT technique, especially for medial, deep, and cystic OCLs, which have worse results than lateral, more shallow, and wafer OCLs.25,34–36 Even for failed prior débridement and subchondral bone penetration, there is at least one study showing 92% good results with revision using the same procedure by the same surgeon.37
When deciding where to harvest the OAT grafts, the surgeon should consider the fact that patellofemoral peripheries allow up to 3 to 4 cm2 of graft plugs for harvest. That is sufficient for grafting most large, contained talar OCLs treated by MOAT.20 However, for smaller lesions needing only one or two plugs, some surgeons advocate local graft harvest from medial or lateral non–weight-bearing talar facets. This prevents violation of a normal knee joint and avoids chronic knee pain.
Other than obvious contraindications such as infection, tumors, rheumatoid arthritis, diabetes with neuropathy, or sympathetic dystrophy, OAT and mosaicplasty should be use with caution in patients with signs of osteoarthritis other than mild osteophytes that can be removed during the same surgical procedure. Ankle instability and alignment deformity should be addressed before or at the same time to prevent failure due to higher than normal contact stress on the transplanted grafts. Hangody and colleagues20 advise caution in treating patients older than 50 years because of their decreased repair capacity.
Surgical Technique
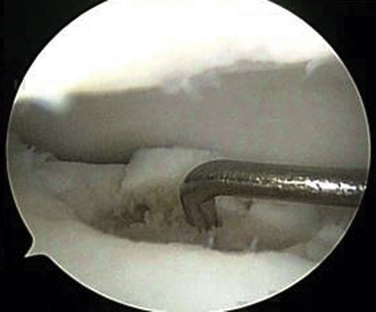
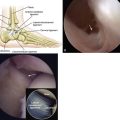
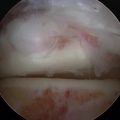
The procedure can be done under spinal or general anesthesia. The patient is positioned supine. A thigh tourniquet is used in most cases. Ankle arthroscopy may be carried out first, especially if it is a primary procedure, to confirm the location and grade of the OCL and to assess other cartilage and ankle pathology (Fig. 13-1). If the lesion is small and anterior, an all-arthroscopic technique can be used. Otherwise, an open technique is needed.
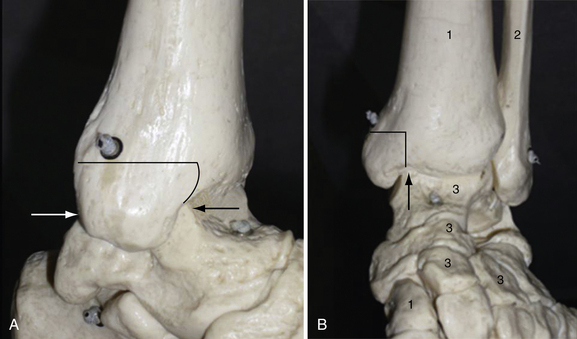
For medial OCLs, a medial approach with a medial osteotomy (Fig. 13-2) is usually needed because most of those OCLs are more posterior. The osteotomy may be a step cut or an oblique osteotomy to provide a good perpendicular view of the talar dome. To achieve this view, the talus must be rotated into valgus, and a pin can be inserted into the talar body19 or a noninvasive ankle distractor can be used.38 Some surgeons advocate an anterior tibial wedge osteotomy for smaller and middle-third lesions instead of a malleolar osteotomy,26,28,29 but we cannot confirm this approach because we have no experience with the technique.
For lateral OCLs, an anterolateral approach is usually needed. Because these OCLs often have a more anterior location, extreme plantar flexion usually can expose the lesions. If a large, posterior lesion needs more exposure, a lateral malleolar osteotomy21 (Fig. 13-3) or a release of the anterior talofibular ligament and calcaneofibular ligament by a periosteal flap at their origin on the distal fibula may be used.39 A good perpendicular view (see Fig. 13-3) of OCL of the talar surface is mandatory to allow perpendicular insertion of the graft plugs.
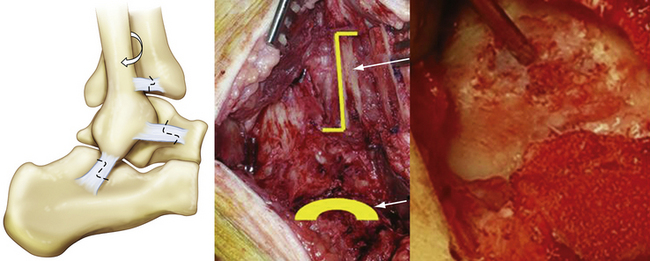
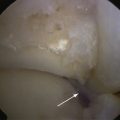
After good exposure, débridement and removal of all diseased cartilage and bone debris with a curette or knife is done until a sharply defined, viable cartilage rim and a clean bony bed of the lesion are obtained. Using MOAT-specific instruments (various companies offer complete sets of instruments), the defect is exactly sized, and the number, diameter, depth, and location of the graft plugs and recipient holes are determined (Fig. 13-4). The intended location of the planned mosaic is marked on the recipient bed with the appropriate sizer-tamp or cutting chisels.

FIGURE 13-4 An example of industry-specific instruments used for osteochondral allograft transplantation.
(Courtesy of Smith & Nephew, Andover, MA.)
Larger graft plugs typically are used first to fill most of the defect, and smaller grafts are used to fill the remaining dead space not covered by the primary plugs (Fig. 13-5). Analysis of a cadaveric contact model study showed that four 4-mm-diameter graft plugs better restored joint contact area and pressure to normal levels than two 6-mm-diameter graft plugs in a talar OCL made in the center of the contact zone.40 More grafts may also allow for better contouring to fit the original surface anatomy and minimize the unfilled space. However, the use of more grafts in transplantation is more technically demanding and may signify a larger area of nonviable hyaline cartilage because of the rate of possible peripheral graft chondrocytes death of up to 24%.41 This is why some arthroscopists advocate using fewer larger graft plugs.24,25
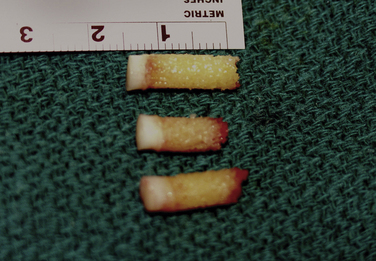
FIGURE 13-5 Intraoperative picture showing the harvest site for osteochondral allograft transplantation.
OAT graft plugs may be harvested from the ipsilateral knee (see Fig. 13-5). This can be done by arthroscopy or by miniarthrotomy, depending on the number and size of grafts needed and the ability of the surgeon. We prefer the open technique. The medial, proximal, non–weight-bearing surface of the medial femoral condylar edge is our preferred donor site. Although Marymont and coworkers42 showed with an MRI study that the best donor site for filling a medial talar lesion is the superolateral femur and although we found no literature to support our approach, we think there are more risks of patellofemoral symptoms from lateral harvesting.
For talar defects, Demirci and colleagues43 showed that the grafts should be taken from condylar edges, where the chondral thickness is decreased. Flexing the knee allows up to four graft plugs to be harvested. For more graft plugs, the lateral femoral condylar edge can be used. The graft plugs are harvested with the appropriately sized, tubular, cutting harvester chisels. It is important to be perpendicular to the articular surface in both planes when harvesting the graft. Each graft length is recorded. Expect up to 0.2 mm of normal expansion of the cartilage plugs after removing them from the harvester.19
Next, the recipient bed is cleaned again. Sequential drilling, dilation, and delivery are performed for each graft using the appropriate drill guide–delivery cylinder (see Fig. 13-4). Remaining perpendicular to the articular surface is mandatory. Drilling should be 3 to 4 mm deeper than the graft plug length.19 When delivering the grafts, care should be taken to set them as flush as possible with the surrounding articular surface of the native cartilage (Fig. 13-6). Huang and associates44 showed that small incongruities could remodel, provided they did not exceed 1 mm in either direction. Pearce and coworkers45 showed that grafts left 2 mm proud and repositioned by weight bearing caused perigraft fissuring, fibroplasia, and subchondral cavitations. They concluded these complications were caused by excessive motion between the graft and recipient site in the proud grafts. However, the graft plugs also must not be sunk too deeply. After all grafts are transplanted, the ankle is reduced and moved through its entire range of motion with slight axial compression. This ensures congruency of the MOAT.
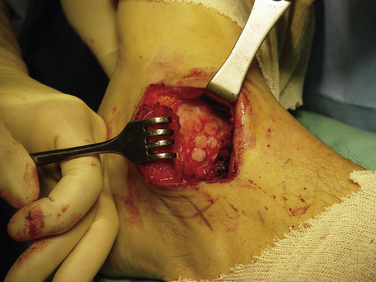
FIGURE 13-6 Intraoperative picture showing the recipient site after osteochondral allograft transplantation.
PEARLS& PITFALLS
Postoperative Management and Rehabilitation
Some surgeons advocate immediate ankle range of motion,18,19 and others advocate no motion for 3 weeks.24,25 We usually start controlled range of motion after stitches are removed at 10 to 14 days if the wound is clean. The patient is kept in a removable cast boot to prevent weight bearing for 6 weeks. We then allow progressive weight bearing over a 4-week period. Formal physiotherapy usually starts at 6 weeks. Unprotected weight bearing is allowed at 10 weeks, and athletic activities should be postponed until 6 months.
Complications
The most common complication and the main drawback of the technique is knee pain. In a review of the literature, we found 274 patients who have had OAT grafts harvested from the knee for the treatment of talar OCLs. Eighteen of them had some form of knee symptoms, ranging from mild and occasional pain to activity-related pain with an extension lag. The overall rate of knee pain was 6.6%. In a retrospective case series that specifically addressed knee pain, Reddy and colleagues46 reported that 4 (36%) of 11 patients had poor knee outcomes according the Lysholm criteria, and they concluded that donor-site morbidity can be significant and interfere with daily living activities. We agree that chronic knee pain is a significant potential complication that should be considered by the surgeon and discussed with the patient.
Other complications may include neuroma, symptomatic hardware, wound healing problems, infection, incomplete relief of pain, chronic pain syndrome, degenerative osteoarthritis of the ankle or patellofemoral joint, and recurrence of the OCL.47
1. Barrie HJ. Osteochondritis dissecans 1887.1987. A centennial look at Konig’s memorable phrase. J Bone Joint Surg Br. 1987;69:693-695.
2. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41A:988-1020.
3. Anderson IF, Crichton KJ, Grattan-Smith T, et al. Osteochondral fractures of the dome of the talus. J Bone Joint Surg Am. 1989;71:1143-1152.
4. Bruns J, Rosenbach B, Kahrs J. Etiopathogenetic aspects of medial osteochondrosis dissecans tali. Sportverletz Sportschaden. 1992;6:43-49.
5. Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus). review of the literature and new surgical approach for medial dome lesions, Foot Ankle. 51985 165-185.
6. Canale ST, Belding RH. Osteochondral lesions of the talus. J Bone Joint Surg Am. 1980;62:97-102.
7. Bosien WR, Staples OS, Russell SW. Residual disability following acute ankle sprains. J Bone Joint Surg Am. 1955;37A:1237-1243.
8. Hintermann B, Regazzoni P, Lampert C, et al. Arthroscopic findings in acute fractures of the ankle. J Bone Joint Surg Br. 2000;82:345-351.
9. Leontaritis N, Hinojosa L, Panchbhavi VK. Arthroscopically detected intra-articular lesions associated with acute ankle fractures. J Bone Joint Surg Am. 2009;91:333-339.
10. De Smet AA, Fisher DR, Burnstein MI, et al. Value of MR imaging in staging osteochondral lesions of the talus (osteochondritis dissecans). results in 14 patients, AJR Am J Roentgenol. 1541990 555-558.
11. Yamashita F, Sakakida K, Suzu F, Takai S. The transplantation of an autogeneic osteochondral fragment for osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1985;201:43-50.
12. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9:318-321.
13. Garrett JC. Osteochondral allografts. Instr Course Lect. 1993;42:355-358.
14. Bobic V. Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction. a preliminary clinical study, Knee Surg Sports Ttraumatol Arthrosc. 31996 262-264.
15. Hangody L, Kish G, Kárpáti Z, et al. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Ttraumatol Arthrosc. 1997;5:262-267.
16. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85A:1-3.
17. Hangody L, Kish G, Kárpáti Z, et al. Treatment of osteochondritis dissecans of the talus. use of the mosaicplasty technique—a preliminary report, Foot Ankle Int. 181997 628-634.
18. Hangody L, Kish G, Modis L, et al. Mosaicplasty for the treatment of osteochondritis dissecans of the talus. two to seven year results in 36 patients, Foot Ankle Int. 222001 552-558.
19. Hangody L. The mosaicplasty technique for osteochondral lesions of the talus. Foot Ankle Clin. 2003;8:259-273.
20. Hangody L, Vásárhelyi G, Hangody LR, et al. Autologous osteochondral grafting—technique and long-term results. Injury. 2008;39(Suppl 1):S32-S39.
21. Gautier E, Kolker D, Jakob RP. Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg Br. 2002;84:237-244.
22. Baltzer AW, Arnold JP. Bone-cartilage transplantation from the ipsilateral knee for chondral lesions of the talus. Arthroscopy. 2005;21:159-166.
23. Al-Shaikh RA, Chou LB, Mann JA, et al. Autologous osteochondral grafting for talar cartilage defects. Foot Ankle Int. 2002;23:381-389.
24. Scranton PE, McDermott JE. Treatment of type V osteochondral lesions of the talus with ipsilateral knee osteochondral autografts. Foot Ankle Int. 2001;22:380-384.
25. Scranton PE, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88:614-619.
26. Assenmacher JA, Kelikian AS, Gottlob C, Kodros S. Arthroscopically assisted autologous osteochondral transplantation for osteochondral lesions of the talar dome. an MRI and clinical follow-up study, Foot Ankle Int. 222001 544-551.
27. Lee CH, Chao KH, Huang GS, Wu SS. Osteochondral autografts for osteochondritis dissecans of the talus. Foot Ankle Int. 2003;241:815-822.
28. Sammarco GJ, Makwana NK. Treatment of talar osteochondral lesions using local osteochondral graft. Foot Ankle Int. 2002;23:693-698.
29. Kreuz PC, Steinwachs M, Erggelet C, et al. Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management. a prospective study with a 4-year follow-up, Am J Sports Med. 342006 55-63.
30. Haasper C, Zelle BA, Knobloch K, et al. No mid-term difference in mosaicplasty in previously treated versus previously untreated patients with osteochondral lesions of the talus. Arch Orthop Trauma Surg. 2008;128:499-504.
31. Gobbi A, Francisco RA, Lubowitz JH, et al. Osteochondral lesions of the talus. randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation, Arthroscopy. 222006 1085-1092.
32. Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome. current concepts review, Foot Ankle Int. 252004 168-175.
33. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle. outcome analysis and outcome predictors of 105 cases, Arthroscopy. 242008 106-112.
34. Robinson DE, Winson IG, Harries WJ, Kelly AJ. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br. 2003;85:989-993.
35. Kelberine F, Frank A. Arthroscopic treatment of osteochondral lesions of the talar dome. a retrospective study of 48 cases, Arthroscopy. 151999 77-84.
36. Schimmer RC, Dick W, Hintermann B. The role of ankle arthroscopy in the treatment strategies of osteochondritis dissecans lesions of the talus. Foot Ankle Int. 2001;22:895-900.
37. Savva N, Jabur M, Davies M, Saxby T. Osteochondral lesions of the talus. results of repeat arthroscopic débridement, Foot Ankle Int. 282007 669-673.
38. Dela Cruz EL, Brockbank GR. Use of the noninvasive ankle distractor with talar dome osteochondral graft transplantation. J Foot Ankle Surg. 2005;44:311-312.
39. Kish G, Modis L, Hangody L. Osteochondral mosaicplasty for the treatment of focal chondral and osteochondral lesions of the knee and talus in the athlete. Rationale, indications, techniques, and results. Clin Sports Med. 1999;18:45-66. vi
40. Choung D, Christensen JC. Mosaicplasty of the talus. a joint contact analysis in a cadaver model, J Foot Ankle Surg. 412002 65-75.
41. Huntley JS, Bush PG, McBirnie JM, et al. Chondrocyte death associated with human femoral osteochondral harvest as performed for mosaicplasty. J Bone Joint Surg Am. 2005;87:351-360.
42. Marymont JV, Shute G, Zhu H, et al. Computerized matching of autologous femoral grafts for the treatment of medial talar osteochondral defects. Foot Ankle Int. 2005;26:708-712.
43. Demirci S, Jubel A, Andermahr J, Koebke J. Chondral thickness and radii of curvature of the femoral condyles and talar trochlea. Int J Sports Med. 2008;29:327-330.
44. Huang FS, Simonian PT, Norman AG, Clark JM. Effects of small incongruities in a sheep model of osteochondral autografting. Am J Sports Med. 2004;32:1842-1848.
45. Pearce SG, Hurtig MB, Clarnette R, et al. An investigation of 2 techniques for optimizing joint surface congruency using multiple cylindrical osteochondral autografts. Arthroscopy. 2001;17:50-55.
46. Reddy S, Pedowitz DI, Parekh SG, et al. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35:80-85.
47. Nakagawa Y, Suzuki T, Matsusue Y, et al. Bony lesion recurrence after mosaicplasty for osteochondritis dissecans of the talus. Arthroscopy. 2005;21:630.