CHAPTER 14 Osteochondral Lesions of the Talar Dome
Cartilage Replacement Using Autologous Chondrocyte Implantation and Allografts
Osteochondral lesions of the talus (OLTs) are rare, representing only about 4% of all such lesions in the body.1 This lesion also has been called osteochondritis dissecans, transchondral fracture, talar dome fracture, and osteochondral defect. OLTs consist of a focal cartilage deficit with associated reactive bone edema.
Several staging systems have evolved since Berndt and Harty proposed the first system based on radiographic imaging of the talus in 1959 (Table 14-1).2 Loomer and colleagues3 reviewed computed tomographic (CT) data of 92 patients with OLTs and found a previously unclassified lesion—the radiolucent lesion—in 77% of the patients in their series. In 1999, Hepple and coworkers,4 using magnetic resonance imaging (MRI) added another characteristic to the original classification system—stage 5, subchondral cyst formation.
TABLE 14-1 Staging System Proposed by Berndt and Harty
| Stage | Description |
|---|---|
| Stage 1 | Compression of the border of the talus |
| Stage 2 | Incomplete detachment of fragment |
| Stage 3 | Complete detachment, no displacement |
| Stage 4 | Displaced fragment or loose body |
From Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41A:988-1020.
NORMAL AND PATHOLOGIC ANATOMY
The articular cartilage of the talus is inconsistent with the posteromedial corner, having a greater depth of cartilage than the anterolateral. This manifests in geographic mechanical properties and may influence the rate and type of articular injury.5–7
PATIENT EVALUATION
History and Physical Examination
A history of trauma is documented in more than 85% of patients.8–12 In most cases, the mechanism of injury is an inversion injury to the lateral ligamentous complex. Although the cause of nontraumatic OLTs is unknown, a primary ischemic event may be responsible. Nontraumatic OLTs can also be familial, multiple lesions can occur in the same patient, and identical medial talar lesions have occurred in identical twins.13
Diagnostic Imaging
Patients with an acute ankle injury with hemarthrosis or substantial tenderness should first undergo weight-bearing plain radiography (i.e., anteroposterior, lateral, and mortise views). Radiographs in various degrees of plantar flexion and dorsiflexion may help in diagnosing posteromedial and anterolateral lesions, respectively.14 Plain radiographs of the contralateral ankle should be obtained, because there is a 10% to 25% incidence of a contralateral lesion.15
MRI can identify occult injuries of the subchondral bone and cartilage that may not be detected with routine radiographs.16,17 Classic MRI findings include areas of low signal intensity on T1-weighted images, which suggests sclerosis of the bed of the talus and indicates a chronic lesion.18,19 T2-weighted images reveal a rim that represents instability of the osteochondral fragment.18,20 After treatment, MRI should reveal a reduction or disappearance of the low signal intensity on T1-weighted images and the rim on T2-weighted images.
TREATMENT
Indications and Contraindications
Surgical repair of OLTs is contraindicated when the risks outweigh the perceived benefits. Risks include active infection in the operative area, the likelihood of patient noncompliance, and patients who are medically unstable. Relative contraindications include degenerative changes of the ankle involving more than an isolated OLT. Studies have shown that a trial of conservative therapy does not adversely affect surgery performed after conservative therapy has failed.1,21
Treatment Options
Arthroscopy of the Ankle
Anterior Ankle Arthroscopy.
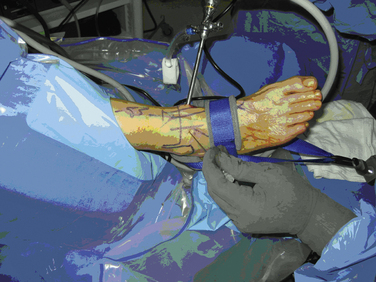
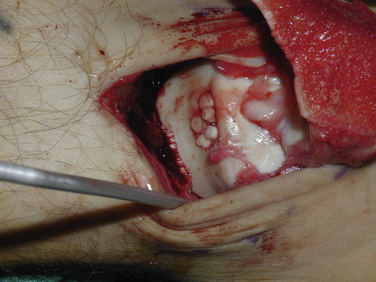
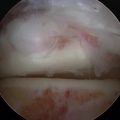
Distraction techniques facilitate the placement of the arthroscope and instruments into the tightly configured ankle joint (Fig. 14-1). Noninvasive techniques involving straps, harnesses, and outriggers are distraction methods used most commonly. These techniques minimize the risk of neurovascular injury and other complications.22
Posterior Ankle Arthroscopy.
Occasionally, posterior lesions are not accessible from the anterior ankle. In these cases, a posterior arthroscopic approach is favored. Through the posterior scope, 54% of the talar dome surface can be visualized and treated.23
Arthroscopic Débridement with Microfracture.
Arthroscopic treatment of OLTs involves three principles: removing loose bodies, securing the OLT to the talar dome (i.e., open reduction with internal fixation), and stimulating development of fibrocartilage. Open reduction with internal fixation is reserved for large, acute OLTs. More often, the lesion is débrided to a stable articular rim, and marrow stimulation techniques are used to create the healing cartilage.
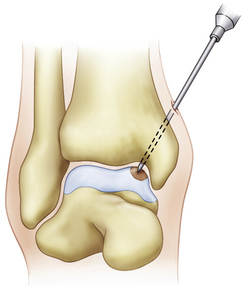
The microfracture technique for OLTs is based on the success of similar techniques in the knee (Fig. 14-2).24,25 Using awls, microfractures (i.e., perforations) are made approximately 3 to 4 mm apart in the subchondral bone while maintaining the integrity of the bone plate. The microfracture technique promotes new tissue formation by releasing substances such as mesenchymal stem cells, growth factors, and healing proteins.24 Ultimately, cartilage-like cells (i.e., fibrocartilage) form and fill the original defect.
Tol and associates26 reviewed 32 studies that reported the results of treatment for OTL. Nonoperative treatment had an average success rate of 45%, whereas the best success rate of 85% was achieved with excision, curettage, and drilling. Posteromedial lesions are difficult to access with this technique. The size of the lesion also plays a role in the success or failure of this procedure. In the meta-analysis by Tol and colleagues,26 the lesion size averaged 0.7 cm; currently, lesions larger than 1 cm are thought to have a less predictable outcome with microfracture.
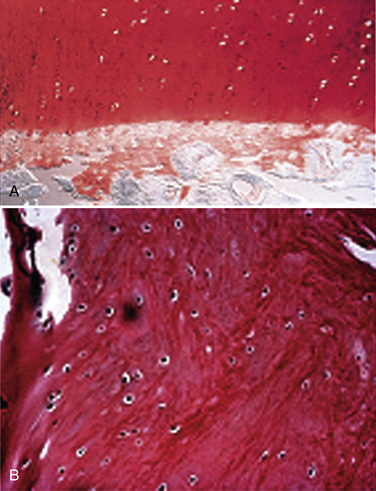
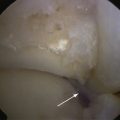
The limitation of microfracture is that fibrocartilage is created to fill the defect. This is predominantly type I cartilage, which lacks the organized structure of normal hyaline cartilage and therefore has inferior wear characteristics (Fig. 14-3).
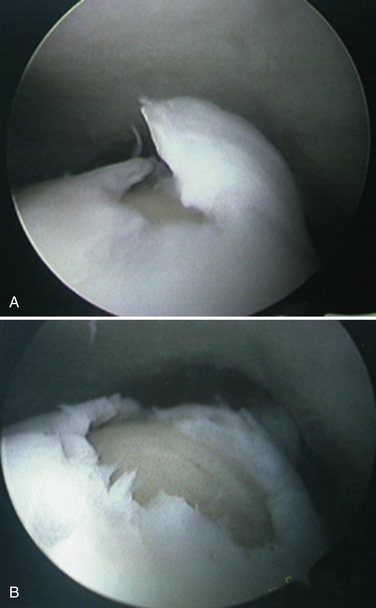
We retrospectively reviewed the results of arthroscopy and microfracture in 189 patients; MRI was used to determine the size of the lesion.27 Good results were achieved in 132 patients with an average lesion size of 0.67 cm2; 22 patients with an average lesion size of 0.76 cm2 had fair results; and 36 patients with an average lesion size of 1.09 cm2 had poor results (Fig. 14-4).
Open Approaches to the Ankle: Malleolar Osteotomies
The tibiotalar articular surface can be accessed through arthrotomies and arthroscopically. For techniques in which an articular graft must be delivered to the articular defect, an open approach is often favored. Muir and colleagues28 studied nine cadavers to evaluate the accessibility of the talar dome by various approaches about the ankle. They used four arthrotomies and three osteotomies and characterized the percentage of the talar dome that could be accessed in a perpendicular fashion with respect to the articular surface. They found that without an osteotomy, up to 24% of the medial talar dome (average, 17%) and 25% of the lateral talar dome (average, 20%) could not be accessed. Osteotomies add an average of 22% exposure, although there remains a central 15% that is inaccessible in a perpendicular manner.28
Anterolateral Tibial Osteotomy for Lateral Talar Dome Access.
Unlike access to the medial talus, full access to the lateral talus requires a limited fibular osteotomy with dislocation of the ankle or an osteotomy of the tibia and the fibula. The latter provides for superior visualization of the lateral talus, and it can be incorporated to access central lesions previously believed to be inaccessible.29,30
Biologic Articular Resurfacing Options
Autologous Chondrocyte Implantation.
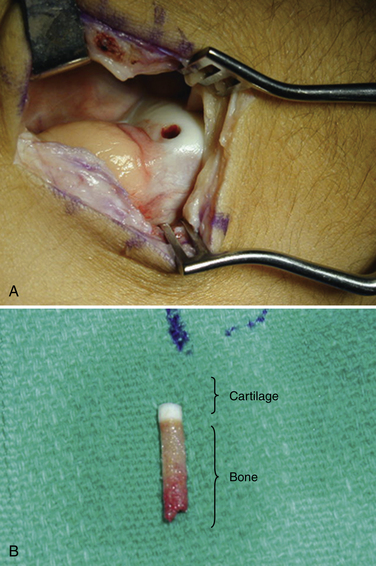
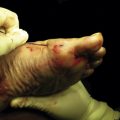
Autologous chondrocyte implantation (ACI) of the talus involves placing cultured chondrocytes underneath a periosteal patch that is placed over the OLT.31 Chondrocytes are harvested during a prior surgery from the knee or ankle (Fig. 14-5). Often, the damaged area of chondral tissue can be used for the biopsy, which obviates the need for damaging healthy tissue.32 The biopsy typically yields 2 to 3 million cells that can be stored for more than 1 year. When required, the cells are then expanded in culture for 6 to 8 weeks, resulting in at least 12 million cells available for implantation. The process of growing the cells increases the number of viable cells for implantation by at least 10-fold. The ability to culture large numbers of cells for implantation is critical for the success of the procedure.33
Whittaker and coworkers reported their results with ACI in 10 patients at 4 years’ follow-up.34 Eight of the 10 patients had failed prior arthroscopic treatment. At a mean follow-up of 24 months, 90% were “pleased” or “extremely pleased” with the surgical result. Full-thickness biopsies were obtained in five patients requiring repeat arthroscopy; two showed hyaline cartilage, and three showed fibrocartilage. The results confirmed some donor-site morbidity. The Lysholm knee score returned to normal in three patients at 1 year but remained reduced by 15% in seven patients.34
Other surgeons have found similar results.35,36 Baums and associates 37 reported the results for 12 patients treated with ACI at a mean follow-up of 63 months. There were six medial and six lateral lesions with an average size of 2.3 cm2. At 63 months’ follow-up, all patients were very satisfied or satisfied with the result. Based on the Hannover score, there were seven excellent, four good, and one satisfactory results. The preoperative American Orthopaedic Foot and Ankle Society (AOFAS) Hindfoot Score was 43.5 and the 63-month follow-up score was 88.4. MRI showed congruent graft incorporation in seven patients, and four had irregularity of the articular surface.37
Autologous Chondrocyte Implantation: Surgical Technique.
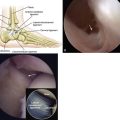
The periosteum is removed carefully using a periosteal elevator, and the cambium layer (closest to the bone) is identified because it will be placed facing the subchondral bone over the lesion (Fig. 14-6). The tibial incision is closed in a standard fashion. The periosteal patch is then sutured to the lesion using 6-0 Vicryl that has been lubricated with sterile mineral oil. Before placing the final suture at the distal portion of the lesion, the perimeter is sealed with fibrin glue, and an angiocatheter is used to place some saline in the patch to ensure that the construct is sealed.
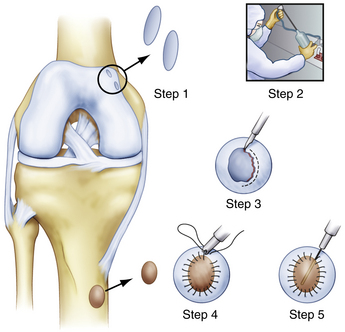
Matrix-Based Chondrocyte Implantation.
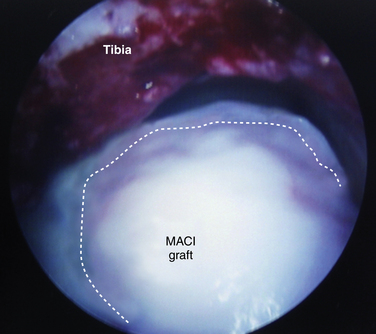
Matrix-based chondrocyte implantation (MACI) is similar to ACI; however, the chondrocytes are not placed under a periosteal patch but are embedded in a type I/III bilayer collagen membrane (Fig. 14-7). As with ACI, the cells are first harvested from the ankle or knee and then cultured to produce 15 to 20 million cells. Similar to ACI, the MACI technique allows the surgeon to increase by at least 10-fold the cells available for implantation into the defect. A second-stage operation is used to implant the membrane over the talar defect.38,39
FIGURE 14-7 In matrix-based chondrocyte implantation, a collagen or hyaluronan membrane is seeded with chondrocytes.
MACI is technically easier than ACI, and no tibial or malleolar osteotomy is required. Two operations are required, and the cells can be stored for more than 1 year after initial harvest. Cherubino and colleagues40 reported on 13 patients with short-term follow-up (i.e., 6 to 12 months) for knee osteochondral defects. No complications were reported, and repeat MRIs at 6 months demonstrated the presence of hyaline-like cartilage at the site of implantation.
Ronga and colleagues41 described six patients who were treated with the MACI technique for chondral defects in the ankle. The follow-up averaged 33.8 months, and the average age of the patients was 28.6 years. All defects were medial except for one “kissing” lesion. At the 2-year follow-up assessment, five ankles had improved AOFAS scores. All ankles had a second-look arthroscopy, and the five with improved scores had hyaline-like cartilage that was stable to probing. The ankle that did not improve had no detectable cartilage.41
Talus Matrix-Based Chondrocyte Implantation: Surgical Technique.
The size and location of the chondral defect are assessed by ankle arthroscopy, and talar articular cartilage is harvested. The harvested chondrocytes are placed in a nutrient medium tube and sent to a cell laboratory (Genzyme Biosurgery, Boston, MA) along with 100 mL of autologous blood divided among 10 tubes. The cells are enzymatically separated from the matrix and cultured for 6 to 8 weeks to produce 15 to 20 million dedifferentiated cells. These cells are embedded in a type I/III collagen membrane bilayer and returned to the surgeon for implantation.33
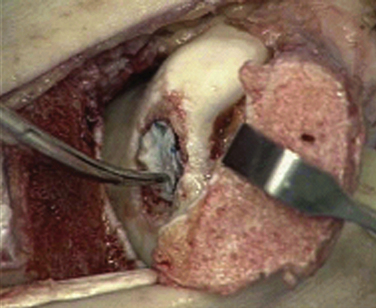
The implantation procedure is performed in a tourniquet-controlled, bloodless field. The joint is exposed with a small anterolateral or anteromedial incision, and the use of a malleolar osteotomy usually is not required. The use of a limited plafondplasty, as described by Assenmacher and associates,42 can improve access. The defect is prepared to stable margins using a curette, and a template of the lesion is prepared. The graft is cut from the MACI membrane and placed into the defect on top of a layer of fibrin tissue sealant (Fig. 14-8). The graft stability is tested with range of motion of the ankle joint.
Autologous Osteochondral Grafting: Osteochondral Autograft Transplantation and Mosaicplasty.
The osteochondral autograft transplantation (OAT) procedure grafts a single plug from the femoral trochlea or condyle into the OLT on the talar dome (Fig. 14-9),43 whereas the mosaicplasty procedure transplants multiple plugs.44 The OAT procedure results in reduced ingrowth of the fibrocartilage, although donor-site morbidity may be greater because of harvesting a single, larger plug. The placement of multiple plugs with the mosaicplasty technique more accurately matches the contour of the talar dome and the surface area of the defect, and it enables approximately 20% to 40% of the defect to be filled with fibrocartilage (Fig. 14-10).45
Al-Shaikh and colleagues45 reported 19 patients who underwent mosaicplasty for lesions that averaged 12 × 10 mm. Plugs were harvested from the trochlear border of the ipsilateral femoral condyle. The postoperative AOFAS Ankle Score at 16 months averaged 88. Most patients had occasional mild pain but excellent function; there were no adverse effects in the knee.45
Another study reported that 94% of 36 patients undergoing mosaicplasty had good or excellent results, with follow-up ranging from 2 to 7 years.46 Prior surgical procedures had failed in 29 of the patients. Gautier and coworkers47 also reported good or excellent results for 11 patients, with an average follow-up of 24 months. Those grafts were harvested from the ipsilateral knee without adverse effects on the knee.
In another study, plugs were harvested from the ipsilateral medial or lateral articular facet of the talus in 12 patients. Significant improvement in AOFAS scores was reported (P < .0001), and no structural failures occurred in the graft or donor site.48
Synthetic Scaffolds.
The OsteoCure synthetic bone graft plugs, a poly-dl-lactic-coglycolic acid (PLGA) mixed with calcium sulfate scaffold, have been used as a synthetic osteochondral graft. This product was developed as a void filler in the donor hole when autogenous osteochondral grafts were harvested. The use of synthetic osteochondral grafts has been introduced as a means to avoid the autologous donor-site morbidity or the financial and supply issues of the allograft. Preclinical testing using a goat model showed integration of the OsteoCure into the osteochondral defect at 12 months postoperatively.49
Our experience, however, has shown various rates of resorption, with some radiographs showing radiolucent defects indicative of calcium absorption.50 There are no published reports of the use of OsteoCure, but pathologic analysis of a failed OsteoCure specimen was performed after the case was revised to an osteochondral plug. The specimen showed a central focus where the cartilage and underlying bone were disrupted and replaced by fibrous tissue containing multinucleated giant cells. There was no bony ingrowth into this focus, and there was no evident ingrowth of hyaline cartilage, although there was some fibrocartilage at the periphery of the defect, which could represent some ingrowth of chondrocytes into fibrous tissue.50
Fresh Osteochondral Allograft Transplantation
To address which OLTs are inappropriate for the use of these techniques, a modification to the classification system has been recommended to include the additional anatomic details of location and containment. Using the classification of Raikin,51 which divides the talus into nine geographic locations, an additional notation of contained or uncontained is appropriate for shoulder lesions. The most common lesion found in Raikin’s study was a midtalar medial lesion (zone 4). If this lesion extended to the shoulder with no medial wall intact, it would more appropriately be referred to as a zone 4, uncontained lesion.
For the uncontained shoulder lesions, fresh talus allografts have a role. These grafts have the potential to deliver living chondrocytes in a matrix that has little immunologic response from the host. At 21 days after harvest, the average chondrocyte viability is 50%. In the knee, osteochondral grafts can survive for 25 years, with phenotyping proving viable chondrocytes from the original donor.52
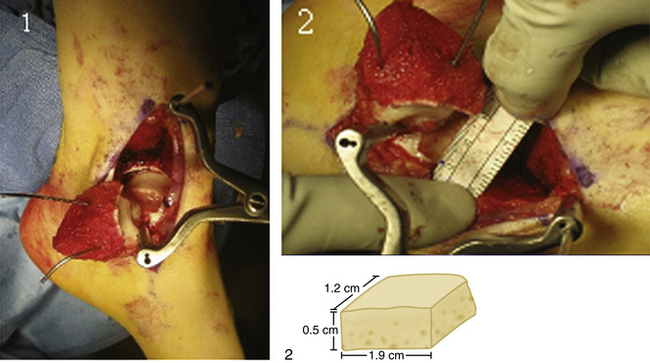
Grafts are matched for size and side, with a low tolerance for any mismatch (<2 mm). The osteochondral defect is excised with curettes and an osteotome to create a block defect. The donor graft is prepared with careful measurements from the host. The size of the graft can be large enough to include a full hemi-talus, although this is rarely necessary. The average graft size is 2 cm2. Grafts are press-fit and secured with bioabsorbable compression screws recessed below the articular surface.
We conducted a prospective study of the use of fresh osteochondral allografts in 17 patients who had failed arthroscopic excision, microfracture, and débridement (Fig. 14-11).53 Preoperatively, all patients were assessed with plain radiographs, MRI, and a AOFAS Hindfoot Score. Postoperative assessment consisted of the AOFAS Hindfoot Score, radiographic assessment of osteotomy healing, graft subsidence, and radiolucency, followed by MRI assessment annually thereafter. The mean defect area was 2.0 cm2. Postoperatively, patients were allowed motion at 4 weeks and did not bear weight on the affected extremity for 6 weeks. All patients had statistically significantly higher AOFAS scores, pain subscores (P < .001), and improved function subscores. Fifteen osteotomies healed within 3 months, and the remaining two healed by 1 year.53 Radiolucencies around the transplanted graft were evaluated to reflect graft healing and the potential for later collapse. There were three cases with persistent radiolucencies at 2 years’ follow-up. Five cases had graft subsidence averaging 3.8 mm (range, 2 to 9 mm), with persistent radiolucency of the graft on the inferior surface confirmed as a substantial risk factor for later collapse. However, clinical outcomes were only slightly associated with graft subsidence. The only revision in this case series was one graft that collapsed 1 year postoperatively. The graft survival rate is 94.1% (16 patients) at the 2-year follow-up assessment.53
PEARLS& PITFALLS
POSTOPERATIVE REHABILITATION PROTOCOL
The postoperative protocol is individualized for the approach and the techniques used to re-establish the articular surface. Patients who undergo arthroscopic débridement with microfracture do not bear weight for 4 weeks. Non–weight-bearing motion is started at 2 weeks to promote molding of the joint surface and synovial lubrication. Boot walker ambulation is begun at 4 weeks, with transfer into a shoe with a protective brace occurring at 6 to 8 weeks. Physical therapy, including manual joint mobilizations, is very helpful and is begun 2 months postoperatively. An ankle effusion is an indication that the joint requires additional time and less mechanical stress, and it should be used to guide physical therapy and the patient’s return to sports or other activities. The time for return to activity after arthroscopy and microfracture was 15.1 ± 4 weeks for stage 2 to 4 osteochondral defects of the talus.54 Seijas and associates55 found that 93.5 % of soccer players had returned to full athletic participation by 3.5 years postoperatively and played at the same level they enjoyed before injury.
Osteochondral grafting (e.g., autograft, allograft, synthetic), chondrocyte transplantation (i.e., ACI or MACI ), and fresh bulk allografts all share a similar postoperative regimen. Patients are instructed not to bear weight for an average of 6 weeks to allow the osteotomy to heal. Patients without osteotomies also are instructed not to bear weight for 6 weeks. Protected ambulation in a boot walker is permitted until the patient feels comfortable, and then the patient is transferred to a shoe with an ankle brace. Physical therapy guidelines are similar to those for microfracture, with the addition of tendon mobilization around osteotomy sites. In a study of 12 patients with ACI for talar osteochondral defects, 6 patients were involved in competitive athletics, and all patients returned to full participation after treatment.37
CONCLUSIONS
Articular defects in the ankle consist primarily of lesions of the talus. The surgical treatment for talar OLT lesions has historically consisted of arthroscopy and microfracture, with anticipation of good results. However, poorer outcomes have been associated with lesions that are larger than 1 cm2. For these larger lesions and for those that have failed primary management with microfracture, there is good evidence to support the successful treatment with the evolving techniques of osteochondral grafting, chondrocyte transplantation, and bulk fresh allograft transplantation. Synthetic grafts, although attractive in theory, have not shown great promise.
The future includes continued development of scaffolds for chondrocyte transplantation, better systems for cartilage graft preparation and transplantation, and perhaps a synthetic prosthetic replacement for focal cartilage defects.
1. Alexander AH, Lichtman DM. Surgical treatment of transchondral talar-dome fractures (osteochondritis dissecans). Long-term follow-up. J Bone Joint Surg Am. 1980;62:646-652.
2. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41A:988-1020.
3. Loomer R, Fisher C, Lloyd-Smith R, et al. Osteochondral lesions of the talus. Am J Sports Med. 1993;21:13-19.
4. Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus. a revised classification, Foot Ankle Int. 201999 789-793.
5. Wan L, de Asla RJ, Rubash HE, Li G. Quantification of ankle articular cartilage topography and thickness using a high resolution stereophotography system. J Orthop Res. 2008;26:1081-1089.
6. Millington SA, Grabner M, Wozelka R, et al. Osteoarthritis. Cartilage. 2007;15:205-211.
7. Sugimoto K, Takakura Y, Tohno Y, et al. Cartilage thickness of the talar dome. Arthroscopy. 2005;21:401-404.
8. Anderson IF, Crichton KJ, Grattan-Smith T. Osteochondral fractures of the dome of the talus. J Bone Joint Surg Am. 1989;71:1143-1152.
9. Baker CL, Andrews JR, Ryan JB. Arthroscopic treatment of transchondral talar dome fractures. Arthroscopy. 1986;2:82-87.
10. Parisien JS. Arthroscopic treatment of osteochondral lesions of the talus. Am J Sports Med. 1986;14:211-217.
11. Pettine KA, Morrey BF. Osteochondral fractures of the talus. A long-term follow-up. J Bone Joint Surg Br. 1987;69:89-92.
12. Van Buecken K, Barrack RL, Alexander AH. Arthroscopic treatment of transchondral talar dome fractures. Am J Sports Med. 1989;17:350-355.
13. Woods K, Harris I. Osteochondritis dissecans of the talus in identical twins. J Bone Joint Surg Br. 1995;77:331.
14. Stroud CC, Marks RM. Imaging of osteochondral lesions of the talus. Foot Ankle Clin. 2000;5:119-133.
15. Stone JW. Osteochondral lesions of the talar dome. J Am Acad Orthop Surg. 1996;4:63-73.
16. Loredo R, Sanders TG. Imaging of osteochondral injuries. Clin Sports Med. 2001;20:249-278.
17. Elias I, Jung JW, Raikin SM, et al. Osteochondral lesions of the talus. change in MRI findings over time in talar lesions without operative intervention and implications for staging systems, Foot Ankle Int. 272006 157-166.
18. Higashiyama I, Kumai T, Takakura Y. Follow-up study of MRI for osteochondral lesion of the talus. Foot Ankle Int. 2000;21:127-133.
19. Mesgarzadeh M, Sapega AA, Bonakdarpour A, et al. Osteochondritis dissecans. analysis of mechanical stability with radiography, scintigraphy, and MR imaging, Radiology. 1651987 775-780.
20. De Smet AA, Fisher DR, Burnstein MI. Value of MR imaging in staging osteochondral lesions of the talus (osteochondritis dissecans). results in 14 patients, AJR Am J Roentgenol. 1541990 555-558.
21. Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus). review of the literature and new surgical approach for medial dome lesions, Foot Ankle. 51985 165-185.
22. Frey C. Foot and ankle arthroscopy and endoscopy. In: Myerson MS, editor. Foot and Ankle Disorders. Philadelphia, PA: WB Saunders; 2000:1477-1511.
23. Sitler DF, Amendola A, Bailey CS, et al. Posterior ankle arthroscopy. an anatomic study, J Bone Joint Surg Am. 84A2002 763-769.
24. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture. surgical technique and rehabilitation to treat chondral defects, Clin Orthop. 391 Suppl. 2002 S362-S369.
25. Sledge SL. Microfracture techniques in the treatment of osteochondral injuries. Clin Sports Med. 2001;20:365-377.
26. Tol JL, Struijs PAA, Bossuyt PM, et al. Treatment strategies in osteochondral defects of the talar dome. a systematic review, Foot Ankle Int. 212000 119-126.
27. Berlet GC, Smith. Successful use of fresh-frozen osteochondral allograft for the management of osteochondral lesions of the talus. a prospective study. June 26, 2008 American Orthopaedic Foot and Ankle Society Denver, CO Paper presented at the 2008 Summer Meeting of the
28. Muir D, Saltzman CL, Tochigi Y, Amendola N. Talar dome access for osteochondral lesions. Am J Sports Med. 2006;34:1457-1463.
29. Bluman EM, Antosh IJ. Technique tip. tibiofibular osteotomy for increased access to the lateral ankle joint, Foot Ankle Int. 292008 735-738.
30. Tochigi Y, Amendola A, Muir D, Saltzman C. Surgical approach for centrolateral talar osteochondral lesions with an anterolateral osteotomy. Foot Ankle Int. 2002;23:1038-1039.
31. Peterson L, Minas T, Brittberg M, Nilsson A. Two to nine year outcome after autologous chondrocytes transplantation of the knee. Clin Orthop Relat Res. 2000;374:212-234.
32. Giannini S, Buda R, Grigolo B, et al. The detached osteochondral fragment as a source of cells for autologous chondrocytes implantation (ACI) in the ankle joint. Osteoarthritis Cartilage. 2005;13:601-607.
33. Williams K. Personal communication. Perth, Australia: Genzyme Corporation; 2001.
34. Whittaker JP, Smith G, Makwana N, et al. Early results of autologous chondrocytes implantation in the talus. J Bone Joint Surg Br. 2005;87:179-183.
35. Giannini S, Buda R, Grigolo B, et al. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22:513-517.
36. Koulalis D, Schultz W, Psychogios B, Papagelopoulos PJ. Articular reconstruction of osteochondral defects of the talus through autologous chondrocyte transplantation. Orthopedics. 2004;27:559-561.
37. Baums MH, Heidrich G, Schultz W, et al. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am. 2006;88:303-308.
38. Marlovits S, Striessnig G, Kutscha-Lissberg F, et al. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005;13:451-457.
39. Zheng MH, Willers C, Kirilak L, et al. Matrix-induced autologous chondrocyte implantation (MACI). biological and histological assessment, Tissue Eng. 132007 737-746.
40. Cherubino P, Grassi FA, Bulgheroni P, Ronga M. Autologous chondrocyte implantation using a bilayer collagen membrane. a preliminary report, J Orthop Surg. 112003 10-15.
41. Ronga M, Grassi FA, Montoli C, et al. Treatment of deep cartilage defects of the ankle with matrix-induced autologous chondrocyte implantation (MACI). Foot Ankle Surg. 2005;11:29-33.
42. Assenmacher JA, Kelikian AS, Gottlob C, Kodros S. Arthroscopically assisted autologous osteochondral transplantation for osteochondral lesions of the talar dome. an MRI and clinical follow-up study, Foot Ankle Int. 222001 544-551.
43. Gobbi A, Francisco RA, Lubowitz JH, et al. Osteochondral lesions of the talus. randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation, Arthroscopy. 222006 1085-1092.
44. Haasper C, Zelle BA, Knobloch K, et al. No mid-term difference in mosaicplasty in previously treated versus previously untreated patients with osteochondral lesions of the talus. Arch Orthop Trauma Surg. 2008;128:499-504.
45. Al-Shaikh RA, Chou LB, Mann JA. Autologous osteochondral grafting for talar cartilage defects. Foot Ankle Int. 2002;23:381-389.
46. Hangody L, Kish G, Modis L. Mosaicplasty for the treatment of osteochondritis dissecans of the talus. two to seven year results in 36 patients, Foot Ankle Int. 222001 552-558.
47. Gautier E, Kolker D, Jakob RP. Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg Br. 2002;84:237-244.
48. Sammarco GJ, Makwana NK. Treatment of talar osteochondral lesions using local osteochondral graft. Foot Ankle Int. 2002;23:693-698.
49. Sharma B, Fermanian S, Cascio B. Chondral lesion repair in a goat model using an integrated hydrogel scaffold and marrow stimulation. xxxx, xx: American Academy of Orthopaedic Surgeons; 2006. Poster presented at the
50. Berlet GC, Penney N, Hyer C, et al. Microscopic analysis of a failed synthetic osteochondral graft of the talar dome. a case presentation. July 2009 American Orthopaedic Foot and Ankle Society Vancouver, Canada Presented at the 2009 Summer Meeting of the
51. Elias I, Zoga AC, Morrison WB, et al. Osteochondral lesions of the talus. localization and morphologic data from 424 patients using a novel anatomical grid scheme, Foot Ankle Int. 282007 154-161.
52. Gross AE, Kim W, Las Heras F, et al. Fresh osteochondral allografts for posttraumatic knee defects. long-term followup, Clin Orthop Relat Res. 4662008 1863-1870.
53. Berlet GC, Hyer CF, Philbin TM, et al. Successful management of talar osteochondral lesions using fresh osteochondral allograft. Presented at the. Vancouver, Canada: Summer Meeting of the American Orthopaedic Foot and Ankle Society, July 2009; 2009.
54. Saxena A, Eakin C. Articular talar injuries in athletes. results of microfracture and autogenous bone graft, Am J Sports Med. 352007 1680-1687.
55. Seijas R, Alvarez P, Ares O, et al. Osteocartilaginous lesions of the talus in soccer players. Arch Orthop Trauma Surg. 2008; Dec 3. [Epub ahead of print]