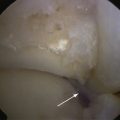
CHAPTER 11 Osteochondral Lesions of the Talar Dome
Anatomy, Etiology, and Evaluation
TERMINOLOGY
Pathologic lesions involving the talar dome have been widely reported in the orthopedic literature, along with lesions of the cartilage and subchondral bone. Osteochondritis dissecans was historically described in 1887 as an inflammatory process of the bone cartilage interface that resulted in the loss of structural integrity.1 This process has also been called osteochondrosis dissecans, and the pathogenesis was thought to be related to repeated trauma or spontaneous focal avascular osteonecrosis, resulting in the loss of structural support for the overlying articular cartilage.2 Berndt and Harty3 described the occurrence of talar articular injury in 1959 and attributed the primary cause of the traumatic lesions to these osteochondral fractures. The term osteochondral defect has been loosely used in the literature to refer to defects of the talus with traumatic or atraumatic causes. To encompass all previous terminology, these lesions are referred to as osteochondral lesions in this chapter.
NORMAL AND PATHOLOGIC ANATOMY
Ankle Anatomy and Cartilage Biology
The ankle represents a ginglymus joint, with the saddle-shaped trochlea articulating with the distal tibia. A congruent mortise is formed with the distal tibia and its malleolus and with the lateral malleolus of the fibula. The dome of the talus is trapezoidal, and on average the anterior aspect is 2.5 mm wider than the posterior aspect. Approximately 60% of the talus is covered with articular cartilage, and the thickness varies with location. The thickest area is the medial corner, and the thinnest area is in the lateral gutter.4
Topographic studies have shown significant differences in the mechanical properties of human ankle cartilage. Tibial cartilage is significantly stiffer than the corresponding talar articulating cartilage, and the softest tissue is found in the posteromedial and posterolateral talus.5 Mean cartilage thickness is inversely related to the mean compressive modulus, with thicker cartilage found medially and thinner cartilage found laterally. These properties may explain the clinically observed findings of osteochondral lesions with repetitive overuse syndromes in medial lesions and acute traumatic events resulting in lateral lesions.
Pathology
Early reports on osteochondral lesions suggested a vascular insult as the primary cause. However, the current consensus supports a traumatic event or repeated insults as the inciting cause. In most cases, a history of trauma can be elicited from the patient. In their original work, Berndt and Harty3 elicited a history of trauma in 90% of their patients studied. This finding has been reproduced by subsequent investigators.
There appears to be a discrepancy between lateral and medial lesions. Canale and Belding6 retrospectively reviewed 31 osteochondral lesions and found that all of the lateral lesions were related to trauma, whereas only 64% of the medial lesions were related to a significant traumatic event. In a comprehensive review of the early literature, including more than 500 reported cases of osteochondral lesions, Flick and Gould7 found that 98% of lateral lesions and only 70% of medial lesions were associated with a traumatic event. These findings support the theory that almost all lateral lesions occur as a result of trauma, whereas most medial lesions are traumatic in origin. The findings also suggest that there is an atraumatic cause for certain patients. In a series of 50 patients, Bauer and Ochsner8 reported no history of trauma in 33 patients with deep medial lesions. They also demonstrated deep osteolysis close to the margin of the lesion, suggesting an atraumatic cause.8 Reports of medial lesions occurring in identical twins and siblings with no history of trauma and the occurrence of bilateral talar dome lesions in up to 10% of cases further suggest an atraumatic cause in some patients.9,10
INCIDENCE AND CLASSIFICATION
Epidemiology
The incidence of osteochondral lesions depends on the associated cause. Although early studies reported an incidence of osteochondral lesions of 6.5% with ankle sprains, improved imaging techniques and the use of arthroscopy have suggested that the incidence in cases of acute ankle injury is about 70%.11–14 The prevalence is increased with associated trauma. Hinterman and colleagues14 showed that 69.4% of 288 ankle fractures requiring open reduction and fixation had associated talar articular cartilage injury. Leontaritis and coworkers13 reported a similar arthroscopic finding; the incidence of chondral injury was 74% in a series of 84 acute ankle fracture patients. The average age of occurrence is usually between 20 and 30 years, with a slight preponderance in men.15
Frequency of Lesion Location
The biomechanical forces responsible for producing medial or lateral osteochondral lesions were investigated by Berndt and Harty3 on a small number of cadavers. They were able to replicate these lesions in the laboratory using various combinations of ankle dorsiflexion and plantar flexion combined with inversion of the ankle joint. Lateral osteochondral lesions are caused by inversion with ankle dorsiflexion and subsequent internal rotation of the tibia on the talus, resulting in impaction with the face of the fibula. These lesions are usually shallow and wafer shaped, and they are thought to be caused by a tangential force vector that results in shearing forces.16 Medial osteochondral lesions occur with inversion and plantar flexion of the foot. These lesions are deeper and cup shaped, factors that are related to impaction of the posteromedial talar dome on the tibial articular surface with a more perpendicular force vector. They tend to be more stable and have less of a tendency to displace compared with the shallower lateral osteochondral lesions.
Medial lesions occur more frequently than lateral lesions. It has been shown in cadaveric studies even without dissection of the lateral ligaments that peak pressures occur at the medial rim of the talus.17 A biomechanical topographic study has shown that the softest cartilage of the talar dome is found at the most medial and lateral edge of the talus.5 Lesions can involve the articular cartilage, underlying subchondral bone, and various depths of cancellous bone of the talus. Occasionally, a cystic component may develop beneath the articular cartilage, and this lesion may be overlooked by the inexperienced arthroscopist.
Classification
The most recognized classification system is that of Berndt and Harty.3 It is unclear from their original paper whether it was based on radiographic imaging or intra-operative findings. However, it has traditionally been adopted as a radiographic staging system (Table 11-1).
| Stage | Description |
|---|---|
| I | Small subchondral compression |
| II | Incomplete fragment avulsion |
| III | Complete fragment detachment undisplaced |
| IV | Complete fragment detachment displaced |
Adapted from Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41A:988-1020.
With the advent of more advanced imaging, various investigators have introduced other classification systems. Ferkel and Scranton18 developed a computed tomography (CT) classification system that corresponded with the original Berndt and Harty system and that included information on the amount of subchondral necrosis, cyst formation, and displacement of the fragment. A magnetic resonance imaging (MRI) classification has been introduced by Hepple and associates19. It includes information on the degree of bone and cartilage injury, amount of displacement, and subchondral cyst formation. The addition of more complicated imaging modalities has added to the description of the location of the lesion and degree of injury but has not changed operative management when the clinical course and chronicity of symptoms are included in the decision-making process. With remarkable foresight, Berndt and Harty3 referred to their classification system as arbitrary. Although it is still the most recognized classification and is useful for research purposes and preoperative planning, the staging of patients has not changed operative management based on intra-operative findings.
PATIENT EVALUATION
History
Patients with an osteochondral lesion usually present with persistent pain in the ankle, which frequently is localized to the side of the lesion. In acute cases, the pain is accompanied by swelling, ecchymosis, and decreased range of motion. In a more chronic presentation, the pain is usually a dull ache in the ankle joint with associated stiffness. Crepitation, tenderness with weight baring and range of motion, mechanical clicking, and recurrent swelling should raise the suspicion of an osteochondral lesion. Uncommonly, the ankle joint may lock because of a loose bony fragment or cartilaginous flap. A thorough history of a traumatic event or instability should be elicited, and if absent, the factors predisposing to possible vascular insufficiency should be sought, including endocrine abnormalities; vascular, hematologic, and coagulation disorders; and any relevant family history. These patients should be questioned about symptoms in the contralateral ankle.
Diagnostic Imaging
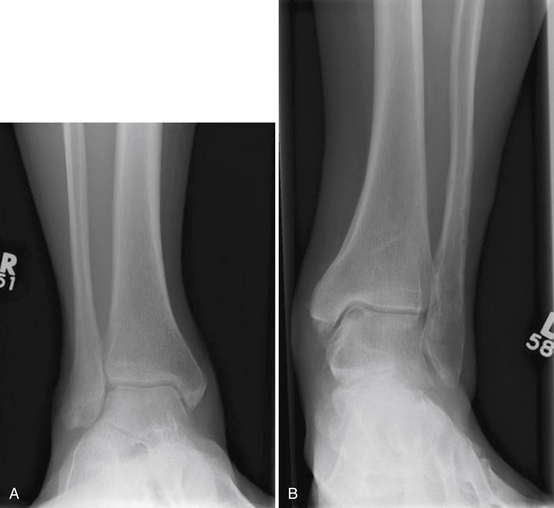
Plain radiographs are the initial investigation of choice for a suspected lesion. Anteroposterior, lateral, and mortise views should be obtained on presentation. Radiographs obtained with the ankle in various degrees of plantar flexion and dorsiflexion may help demonstrate posteromedial and anterolateral lesions, respectively (Fig. 11-1).20 Plain radiographs of the other ankle should be obtained to exclude bilateral lesions. Although useful as a primary investigation, radiographs are limited in their ability to demonstrate subtle subchondral cyst formation, and they provide no information on the status of the soft tissue.
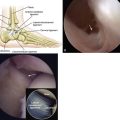
CT provides valuable information on the involved bony architecture. Scans in the coronal and axial planes should be obtained for patients with persistent pain, even when initial radiographs appeared normal (Fig. 11-2). Baseline CT scans can accurately diagnose all lesions except for early (stage I) lesions. Information can be obtained on the size and location of bone fragments, the integrity of subchondral bone, and the presence or absence of cyst formation. Intra-articular injection of contrast can add to the diagnostic accuracy of cartilage lesions and provide information on the state of attachment of the osteochondral fragment to the talus.21,22
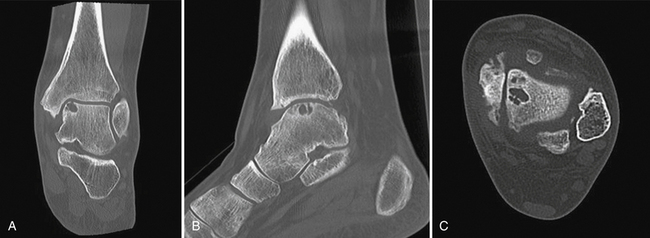
FIGURE 11-2 Coronal (A), sagittal (B), and axial (C) computed tomography views show a medial osteochondral lesion.
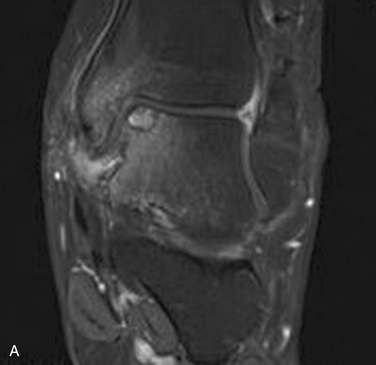
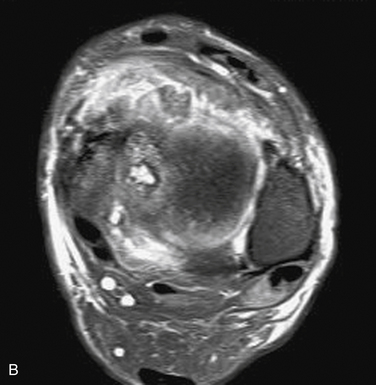
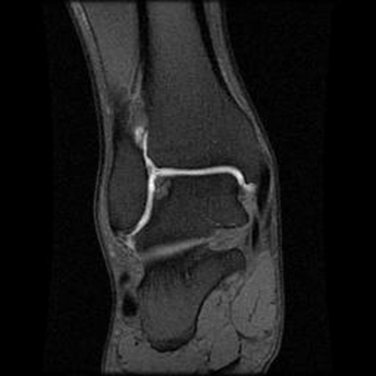
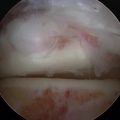
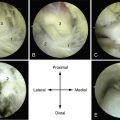
MRI affords an accurate assessment of the ankle’s soft tissue structures, including the articular cartilage and supporting ligaments. Multiplanar evaluation is possible with no radiation exposure to the patient. Sequences can identify the size and location, and they provide information on the stability of the lesion (Figs. 11-3 and 11-4). DeSmet and colleagues23 prospectively showed an excellent correlation between staging in MRI and arthroscopy and correctly predicted stable and unstable lesions in 92% of cases. MR arthrography (MRA) has become a useful adjunct to routine MRI and may provide more detail about the osteochondral lesion and afford improved preoperative planning (Fig. 11-5).
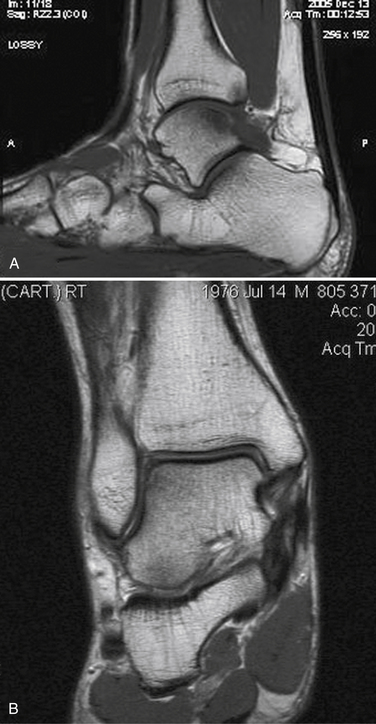
FIGURE 11-4 Coronal, T1-weighted (A) and sagittal, T2-weighted (B) MRI scans show a lateral osteochondral lesion.
Bone scintigraphic results correlate with osteochondral injury and can be used to discern which joint is causing clinical discomfort if other imaging modalities have not been helpful in locating the lesion.24 It may also have a role in localizing CT to the specific area of uptake. More advanced imaging has largely replaced scintigraphy, but it still provides a valuable tool in the workup of persistent ankle pain for the surgeon without immediate access to CT or MRI.
Verhagen and coworkers25 prospectively compared the patient’s history and physical examination, plain radiographs, CT, MRI, and arthroscopy for diagnosing an osteochondral fracture of the talus or tibial plafond in 104 painful ankles with 35 lesions. Plain radiographs had a sensitivity of 70% and a specificity of 94%. CT had a sensitivity of 81% and a specificity of 99%. MRI showed a sensitivity of 96% and a specificity of 99%, whereas arthroscopy showed a sensitivity of 100% and a specificity of 97%. There was no statistically significant difference found between CT, MRI, and arthroscopy. CT had a better positive predictive value than other modalities, and MRI had a better negative predictive value.25 Arthroscopy tended to miss the lesions on the tibial side, so the specificity of this diagnostic modality would like trend closer to 100% if only talar lesions were studied.
Stone26 recommended an imaging protocol that may prove beneficial for the general orthopedist. On initial presentation, plain radiographs are obtained, and if acute pathology is recognized, a CT scan is obtained to delineate the size, location, and degree of displacement of the osteochondral fragment. If no pathology is seen, nonoperative management for a presumed soft tissue injury is initiated. If symptoms persist despite normal radiographs, MRI is recommended because of its ability to detect bony and soft tissue lesions that may be causing symptoms. CT and MRI are equivalent sources of information in the decision-making pathway for recognized lesions, but MRI can help to diagnose osteochondral lesion and other pathology without increased radiation exposure and should be the investigation of choice after plain film radiography for evaluation of persistent foot and ankle pain.
TREATMENT
Natural History and Recommended Treatment
The natural history of osteochondral lesions remains controversial because most patients receive some form of nonoperative or operative treatment. In a small series of 10 ankles with osteochondral lesions followed for more than 15 years, 4 of 6 ankles treated nonoperatively failed to heal, but the ankles remained asymptomatic. Osteoarthritis developed in only 2 of 10 ankles and was reported as an uncommon complication.27 Further study by Bruns28 showed that development of osteoarthritis was uncommon and that it occurred only to a mild degree in adult patients with osteochondral lesions.
Most published sources recommend nonoperative treatment for Berndt and Harty stage I and II lesions. Controversy exists for stage III lesions and lesions in the skeletally immature patient. In a systematic review of 210 patients, Tol and associates29 found a success rate of 45% for nonoperative treatment of stage I and II lesions and medial stage III lesions. This nonoperative intervention included a period of immobilization and limited or no weight bearing.29 Canale and Belding6 showed that nonoperative and operative treatment of stage III lesions was equivalent, but the results of operatively treated lateral lesions were superior. This finding likely reflects the fact that medial lesions are deeper and inherently more stable than lateral lesions.
Results of operative treatment are varied and depend on the lesion’s characteristics and the intervention provided. Tol and colleagues29 found that excision, curettage, and drilling had an 85% success rate, whereas excision and curettage without drilling had a success rate of 78%, a difference that was not statistically significant. Operative intervention is recommended for Berndt and Harty stage III and IV lesions and for smaller lesions with ongoing clinical symptoms. Stage V lesions (cystic) remain controversial, with evidence for and against operative intervention. Shearer and coworkers30 showed that 71% of these lesions had good or excellent results at an average follow-up of 38 months. They suggested that these lesions usually run a benign course and remain stable with time, and unless progressive enlargement occurs, they do not warrant operative management. In contrast, Scranton and associates41 suggested that type V cystic lesions have a poorer surgical prognosis, and they suggested that an aggressive surgical approach using osteochondral autografting was more appropriate for patients with large cystic lesions. Concern also exists about the urgency of intervening operatively if nonoperative management fails, but it is generally agreed that delaying operative intervention for a period of up to 12 months does not compromise the results of subsequent surgical treatment.31
For skeletally immature patients, most physicians have traditionally recommended a trial of nonoperative management for stage I, II, or III lesions because they retain the propensity to heal. This was disputed by Letts and colleagues32, who reported a series of 26 lesions followed for a mean of 16 months. They reported good results for only 37.5% of all patients with stage I, II, or III lesions treated with immobilization and protected weight bearing.32 The trend will likely be toward treating skeletally immature patients with the same principles as applied to the adult population, with a trial of nonoperative treatment for stable stage I or II lesions and operative intervention for stages III and IV.
The indications for operative and nonoperative treatment of osteochondral lesions remain controversial because most published series do not provide an accurate description of the size and site of the involved lesions.26 When making treatment decisions, it is important to consider patient characteristics (e.g., age, activity) and lesion characteristics (e.g., size, site, stability, chronicity). Based on these characteristics, a decision can be made to treat with nonoperative regimens or with surgical intervention.
Operative Treatment
Treatment can be accomplished by open or arthroscopic means, depending on the location of the lesion and the expertise of the surgeon. Operative management includes primary fixation or intervention using techniques of nontissue transplantation or tissue transplantation for the treatment of an irreparable or chronic osteochondral lesion (Table 11-2). Many techniques have been described, and they often are based on the patient’s age and the stage and size of the osteochondral lesion. Am in-depth review of all techniques is beyond the scope of this chapter, but an overview of the available techniques follows.
We recommend arthroscopic débridement associated with any technique of subchondral bone penetration (i.e., curettage, drilling, or microfracture) for osteochondral lesion of the talus that is less than 15 mm in diameter (see Fig. 11-1). This approach aims at promoting revascularization of the lesion and creating stability with a healthy rim of attached cartilage and a base of bleeding subchondral bone. Thorough débridement of the degenerative cartilage together with a secondary intervention (e.g., drilling) does make a significant difference in lesion grade over time and is recommended.33 These techniques are simple, are cost effective, and have shown good results, as reported in the literature for primary and revision procedures. However, the base of the lesion is filled with fibrocartilage, and although this is usually sufficient for smaller lesions, the inferior mechanical properties compared with hyaline cartilage may lead to earlier failure in larger lesions. Other techniques are recommended for larger lesions, but the exact size is controversial. Some surgeons suggests that lesions should be no larger than 1.5 cm2 and 7 mm deep and lesions that fail to respond to arthroscopic débridement and subchondral bone penetration.34
For larger lesions and lesions that fail to respond to arthroscopic débridement and subchondral bone penetration, management options include tissue transplantation with autograft tissue (i.e., osteochondral autologous transfer system [OATS] or mosaicplasty) or allograft tissue (i.e., fresh allograft or fresh-frozen allograft). Common guidelines suggest lesions with a diameter of more than 10 mm are best suited to tissue transplantation. OATS and mosaicplasty harvest full-thickness osteochondral plugs from a donor site and implant these plugs into the osteochondral lesion’s site using an interference fit. Donor site cartilage appears to remain as type II collagen with normal articular proteoglycans and microarchitecture. However, the border area of most grafts is filled in with fibrocartilage.35–37 These techniques have shown good or excellent results (mostly level IV evidence) during short-term to intermediate-term follow-up. Results are less promising in older patients with larger lesions, and concern still exists about donor site morbidity and the considerable mechanical differences between knee and ankle cartilage. The use of osteochondral allografts negates the risk of donor site morbidity but comes with a low risk of disease transmission. Short-term and intermediate-term results with fresh and fresh-frozen allograft transplantation are promising, with most grafts incorporating and functioning reasonably well at long-term follow-up.38
Autologous chondrocyte implantation has gained popularity based on its reported success in treating lesions of the knee. Koulalis and colleagues39 have reported good or excellent results with no complications for their small series of patients, and Whittaker and coworkers40 reported a 90% rate of good or excellent results in their series; the defect was filled in all cases. This method can treat larger lesions and, with careful technique, can address shoulder lesions. However, it is costly, requires staged procedures, and is associated with a longer recovery period. Despite these factors, autologous chondrocyte implantation remains a favorable approach for certain osteochondral lesions involving the talus, such as large, noncystic lesions in younger patients. Autologous stem cells have also been implanted on acellular scaffolds or mixed within the implanted matrix. These pluripotential cells are thought to have the ability to organize and recreate structural tissue, including chondral and subchondral anatomy.
PEARLS& PITFALLS
Postoperative Rehabilitation
Postoperative management is based on each patient’s individual intervention. For simple arthroscopic débridement and subchondral bone penetration there is no consensus on whether immediate weight bearing or protected weight bearing is best. For patients undergoing initial primary fixation or intervention other than simple débridement a period of limited or non-weight bearing for six weeks is recommended. The guidelines for transplantation have not been established with the literature suggesting limited weight bearing from two up to twelve weeks. There appears to be more consensuses on range of motion with active and passive range initiated as soon as can be tolerated by the patient. For intervention requiring bone healing, rehabilitation begins after healing is demonstrated with the goal of restoring ankle range of motion with active and passive exercises progressed to strength and proprioception training tailored to the patients need.
1. Barrie HJ. Osteochondritis dissecans 1887-1987. A centennial look at Konig’s memorable phrase. J Bone Joint Surg Br. 1987;69:693-695.
2. Pappas AM. Osteochondrosis dissecans. Clin Orthop Relat Res. 1981;158:59-69.
3. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41A:988-1020.
4. Sugimoto K, Takakura Y, Tohno Y, et al. Cartilage thickness of the talar dome. Arthroscopy. 2005;21:401-404.
5. Athanasiou KA, Niederauer GG, Schenck RCJr. Biomechanical topography of human ankle cartilage. Ann Biomed Eng. 1995;23:697-704.
6. Canale ST, Belding RH. Osteochondral lesions of the talus. J Bone Joint Surg Am. 1980;62:97-102.
7. Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus). review of the literature and new surgical approach for medial dome lesions, Foot Ankle. 51985 165-185.
8. Bauer RS, Ochsner PE. Nosology of osteochondrosis dissecans of the trochlea of the talus. Z Orthop Ihre Grenzgeb. 1987;125:194-200.
9. Anderson DV, Lyne ED. Osteochondritis dissecans of the talus. case report on two family members, J Pediatr Orthop. 41984 356-357.
10. Woods K, Harris I. Osteochondritis dissecans of the talus in identical twins. J Bone Joint Surg Br. 1995;77:331.
11. Coltart WD. Aviator’s astragalus. J Bone Joint Surg Br. 1952;34B:545-566.
12. Bosien WR, Staples OS, Russell SW. Residual disability following acute ankle sprains. J Bone Joint Surg Am. 1955;37A:1237-1243.
13. Leontaritis N, Hinojosa L, Panchbhavi VK. Arthroscopically detected intra-articular lesions associated with acute ankle fractures. J Bone Joint Surg Am. 2009;91:333-339.
14. Hintermann B, Regazzoni P, Lampert C, et al. Arthroscopic findings in acute fractures of the ankle. J Bone Joint Surg Br. 2000;82:345-351.
15. Chew KT, Tay E, Wong YS. Osteochondral lesions of the talus. Ann Acad Med Singapore. 2008;37:63-68.
16. Anderson I.F, Chrichton KJ, Grattan-Smith T, et al. Osteochondral fractures of the dome of the talus. J Bone Joint Surg Am. 1989;71:1143-1152.
17. Bruns J, Rosenbach B, Kahrs J. Etiopathogenetic aspects of medial osteochondrosis dissecans tali. Sportverletz Sportschaden. 1992;6:43-49.
18. Ferkel RD, Scranton PEJr. Arthroscopy of the ankle and foot. J Bone Joint Surg Am. 1993;75:1233-1242.
19. Hepple S, Winson LG, Glew D. Osteochondral lesions of the talus. a revised classification, Foot Ankle Int. 201999 789-793.
20. Stroud CC, Marks RM. Imaging of osteochondral lesions of the talus. Foot Ankle Clin. 2000;5:119-133.
21. Ragozzino A, Rossi G, Esposito S, et al. Computerized tomography of osteochondral diseases of the talus dome. Radiol Med. 1996;92:682-686.
22. Heare MM, Gillespy T 3rd, Bittar ES. Direct coronal computed tomography arthrography of osteochondritis dissecans of the talus. Skeletal Radiol. 1988;17:187-189.
23. De Smet AA, Fisher DR, Burnstein MI, et al. Value of MR imaging in staging osteochondral lesions of the talus (osteochondritis dissecans). results in 14 patients, AJR Am J Roentgenol. 1541990 555-558.
24. Schimmer RC, Dick W, Hintermann B. The role of ankle arthroscopy in the treatment strategies of osteochondritis dissecans lesions of the talus. Foot Ankle Int. 2001;22:895-900.
25. Verhagen RA, Maas M, Dijkgraaf MG, et al. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br. 2005;87:41-46.
26. Stone JW. Osteochondral lesions of the talar dome. J Am Acad Orthop Surg. 1996;4:63-73.
27. McCullough CJ, Venugopal V. Osteochondritis dissecans of the talus. the natural history, Clin Orthop Relat Res. 1441979 264-268.
28. Bruns J. Osteochondrosis dissecans tali. Results of surgical therapy. Unfallchirurg. 1993;96:75-81.
29. Tol JL, Struijs PAA, Bossuyt PMM, et al. Treatment strategies in osteochondral defects of the talar dome. a systematic review, Foot Ankle Int. 212000 119-126.
30. Shearer C, Loomer R, Clement D. Nonoperatively managed stage 5 osteochondral talar lesions. Foot Ankle Int. 2002;23:651-654.
31. O’Farrell TA, Costello BG. Osteochondritis dissecans of the talus. The late results of surgical treatment. J Bone Joint Surg Br. 1982;64:494-497.
32. Letts M, Davidson D, Ahmer A. Osteochondritis dissecans of the talus in children. J Pediatr Orthop. 2003;23:617-625.
33. Takao M, Uchio Y, Kakimaru H, et al. Arthroscopic drilling with débridement of remaining cartilage for osteochondral lesions of the talar dome in unstable ankles. Am J Sports Med. 2004;32:332-336.
34. Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome. current concepts review, Foot Ankle Int. 252004 168-175.
35. Lee CH, Chao KH, Huang GS, Wu SS. Osteochondral autografts for osteochondritis dissecans of the talus. Foot Ankle Int. 2003;24:815-822.
36. Baltzer AW, Arnold JP. Bone-cartilage transplantation from the ipsilateral knee for chondral lesions of the talus. Arthroscopy. 2005;21:159-166.
37. Hangody L, Kish G, Módis L, et al. Mosaicplasty for the treatment of osteochondritis dissecans of the talus. two to seven year results in 36 patients, Foot Ankle Int. 222001 552-558.
38. Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22:385-391.
39. Koulalis D, Schultz W, Heyden M. Autologous chondrocyte transplantation for osteochondritis dissecans of the talus. Clin Orthop Relat Res. 2002;395:186-192.
40. Whittaker JP, Smith G, Makwana N, et al. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87:179-183.
41. Scranton PE, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88:614-619.