Chapter 22 Osteoarthritis
DEFINITION AND AETIOLOGY
Osteoarthritis, also called degenerative joint disease, is primarily a disease of ageing, as 90% of all people have radiographic features of it in the weight-bearing joints by the age of 40 years.1,2,3 It is defined by degeneration of the cartilage and subsequent hypertrophy of the bone surrounding the articulations. There are no systemic signs of disease with this condition. Typically the pain is localised to joints in a non-symmetrical pattern, and is usually relieved by rest and gentle motion. There are hereditary and mechanical risk factors involved in this condition, with obesity and repetitive mechanical loading especially provocative in the lower limb articulations. Degeneration in a joint can be primary by ‘wear and tear’ or secondary to an articular injury, for example a fracture, or metabolic diseases like hyperparathyroidism.2,3
Incidence and cost
In Australia, arthritis and musculoskeletal conditions are large contributors to illness, pain and disability.4 Accounting for more than 4% of the overall disease burden, measured in terms of disability-adjusted life years (DALY), these conditions account for a significant proportion of healthy years of life lost. More than 6.1 million Australians are reported to have arthritis or a musculoskeletal condition. Most commonly reported conditions are back pain and various forms of arthritis. Further, over 1 million Australians are reported to have disability associated with arthritis and related disorders, with mobility limitation the major feature. These conditions are the second most common reason for presentation to a general practitioner,3,5 and the third leading cause of health expenditure.6 In view of this large disease burden—the number of people affected and the high disability impact—arthritis and musculoskeletal conditions were declared a National Health Priority Area (NHPA) in July 2002. 1.3 million Australians (almost 7% of the population) have diagnosed osteoarthritis and females (8%) are more likely than males (5%) to have the disease.3
RISK FACTORS
The risk factors for osteoarthritis have been categorised into a succinct list of modifiable and unmodifiable factors.7
Modifiable
The first and most obvious factor is injury to a joint complex, and this is especially true in men. Trauma to the meniscus and cruciate ligament tears are particularly provocative in the development of osteoarthritis, and this relationship remains despite surgical repair.2,3,7
Obesity is a major risk factor in both the development of weight-bearing joint osteoarthritis and its severity and subsequent disability.3,6–8 Women are particularly susceptible in this factor, and obesity appears to be predisposing to osteoarthritis, and not just secondary to becoming sedentary because of it.
The link between occupational overuse and the development of osteoarthritis has been shown in many different work activities, particularly when the knee is involved in repeated bending, kneeling, squatting or climbing,3,7 and this effect is exacerbated by the addition of heavy-load lifting.9
Unmodifiable
The prevalence of osteoarthritis has an interesting age and gender relationship. Men more commonly have the radiological signs of the condition before the age of 50 years, and conversely women have it after that age. Women are more likely to have bilateral knee osteoarthritis as well as hand osteoarthritis. The disease increases in incidence and severity with age.7
In terms of family history, there is an inherited tendency towards osteoarthritis, with the heritability component estimated in twin studies at 60–65% for hip and hand osteoarthritis, and 40–50% for knee osteoarthritis, although there has been no single gene defect identified.10 There is also evidence that race is involved as a risk factor. For example, there is evidence that Chinese subjects have a lower incidence of hand and hip osteoarthritis.11 These risk factors can compound, as in obesity with occupational bending and twisting. There is some evidence that recreational overuse is a risk, specifically in elite sports.7
DIAGNOSIS
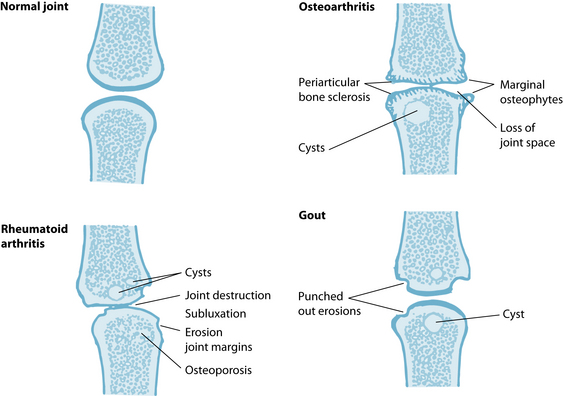
A joint can be defined or diagnosed to have osteoarthritis by symptoms, structural changes, or both (see Figure 22.1). The symptoms of osteoarthritis include:
Radiological changes on X-ray are useful to identify some of the key signs of the osteoarthritic joint: the narrowing of the joint space, marginal osteophyte formation, subchondral sclerosis and the hypertrophy of the periarticular bones. However, radiological changes are not always observed in people with joint symptoms, and people with radiological changes do not always have symptoms. Cartilage is not seen on X-ray as it is not radio-opaque, so ultrasound and magnetic resonance imaging (MRI) and visualisation under arthroscopy might be necessary to clarify the joint changes.2,3
In clinical research studies, the diagnosis and severity of osteoarthritis is commonly measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).The WOMAC evaluates three clinical domains including pain, stiffness and physical function in people with osteoarthritis of the hip and knee, and assesses change in symptoms of patients who have received therapeutic intervention. Ordinal Likert scales are used to grade the severity of each domain, and the instrument has been extensively evaluated for validity.12,13
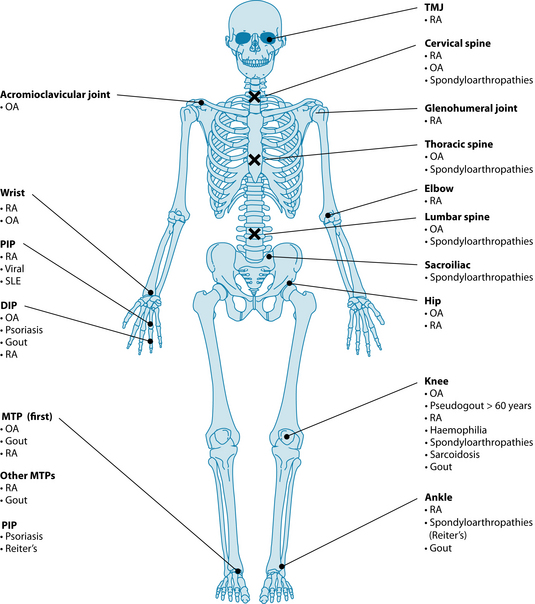
The differential diagnosis of osteoarthritis from other forms of arthritide, like rheumatoid arthritis and gout, should include a consideration of the pattern of joint involvement and whether signs of inflammation are present (see Table 22.1 and Figure 22.2). If the patient has a recent history of infection or fever, is less than 40 years old or presents with abnormal routine blood tests, other forms of arthritis (such as rheumatoid or septic) should be considered (see Chapter 28 on autoimmunity). Laboratory tests (for example, ESR, rheumatoid factor and synovial fluid analysis) may be used to rule out alternative diagnoses.2,3,14
| CHARACTERISTIC | STATUS | DISEASE |
|---|---|---|
| Inflammation | Present | Rheumatoid arthritis, systemic lupus erythematosus, gout |
| Absent | Osteoarthritis | |
| Number of involved joints | Monoarticular | Gout, trauma, septic arthritis, Lyme disease, osteoarthritis |
| Oligoarticular (2–4 joints) | Reiter’s disease, psoriatic arthritis, inflammatory bowel disease | |
| Polyarticular (5 or more) | Rheumatoid arthritis, systemic lupus erythematosus | |
| Site of joint involvement | Distal interphalangeal | Osteoarthritis, psoriatic arthritis (not rheumatoid arthritis) |
| Metacarpophalangeal, wrists | Rheumatoid arthritis, systemic lupus erythematosus (not osteoarthritis) | |
| First metatarsophalangeal | Gout, osteoarthritis |
Source: Adapted from 2009 Current Medical Diagnosis and Treatment.2
CONVENTIONAL TREATMENT
The foundations of conventional treatment can be summarised as:2,3,15,16
The exercise program and weight-loss strategies are elements of the self-help management plan that all health practitioners would support—see the discussion below regarding the evidence.
Prescription of NSAIDs is included as the first choice for pain and inflammation control, with the addition of analgesics where necessary. The prescription should be accompanied by an assessment of the presence of risk factors for NSAIDs including age, hypertension, upper gastrointestinal events and cardiovascular, renal or liver disease. Other issues to be considered are aspirin allergy and polypharmacy (for example, concurrent use of diuretics, ACEI and/or anticoagulants).2,3,15,16
In Australia, the percentage of people with osteoarthritis using the common medications are: 8.0% use celecoxib (NSAID), 6.6% paracetamol, 5.3% meloxicam (NSAID), 3.9% diclofenac sodium (NSAID).3
KEY TREATMENT PROTOCOLS
The goals in the naturopathic treatment of people with osteoarthritis are to:
This schedule of therapeutic aims has a large number of individualised permutations based on the many pieces of information that are gleaned from the personal history and lifestyle analysis of each person with osteoarthritis. In naturopathic medicine, this is a vital component of the management of a multifactorial condition such as osteoarthritis. As in any naturopathic approach to case reasoning, the therapeutic order is useful to prioritise management, and to ensure the naturopathic principles are followed.19 The following is a summary of modalities that may be used within naturopathic medicine that have been investigated for this condition.
The naturopathic approach to the management of this condition reflects the evidence-based guidelines of a number of mainstream groups. The Osteoarthritis Research Society International (OARSI) has published practice guidelines for managing hip and knee osteoarthritis.20 The guidelines state that the optimal management requires
TREAT THE WHOLE PATIENT
Qualitative studies of patients with rheumatoid arthritis suggested that fatigue, not pain, was the factor associated with their condition that affected their life the most.17 Similar findings have since been observed in osteoarthritis patients as well.18 Fatigue may be associated with pain, pain medication, poor sleeping patterns or any number of factors. Arthritis may also affect the patient’s ability to perform daily tasks and interact socially or with their partner, and has other psychosocial or emotional ramifications, all of which need to be appropriately addressed in the naturopathic treatment of arthritis.
a combination of non-pharmacological and pharmacological approaches. The first priority is education of the patient about the condition and the importance of changes in lifestyle, exercise and weight reduction in order to unload the damaged joints. The initial focus should be on self-help and patient-driven treatments rather than on passive therapies delivered by health professionals.21 The focus on self-help in obesity and exercise management is also a key element of the guidelines from both the Royal Australian College of General Practitioners22 and the National Arthritis and Musculoskeletal Conditions Advisory Group.14
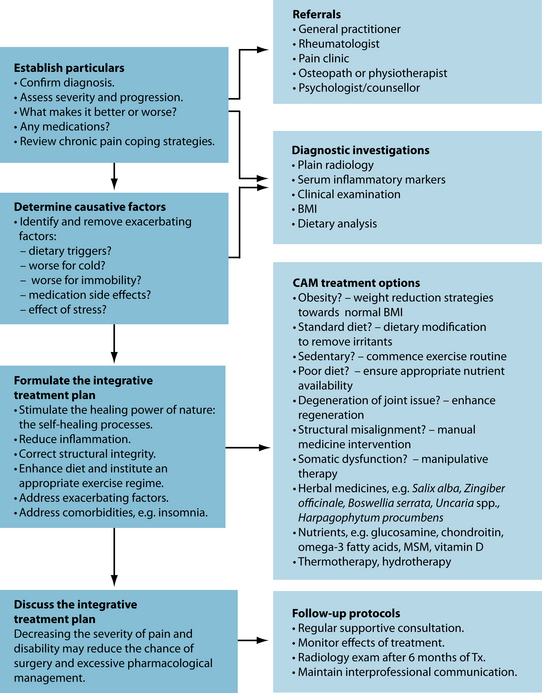
The approach in naturopathic medicine to ‘address weakened or dysfunctioning systems or organs’ coincides with the clinical approach of looking for the existence of comorbidities that may be linked to the primary problem (see Figure 22.3). This is clear in the management of osteoarthritis, where assessment and treatment may be necessary in the (among others):
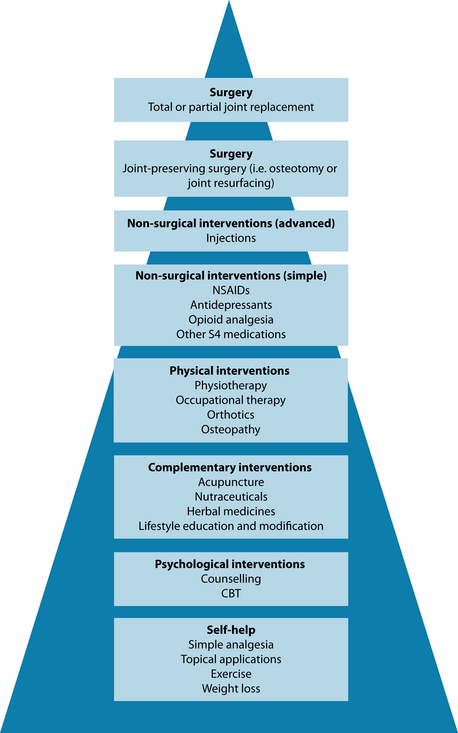
Figure 22.3 Integrative treatment of osteoarthritis
Source: Adapted from Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365(9463):965–973.
Attenuate inflammation
The process of inflammation plays a central role in many disorders, especially those in the musculoskeletal system. Like many processes in the body, there can be positive outcomes from a resolution or there can be uncontrolled and damaging results. The activators of inflammation can include injury, radiation, infection, oxidative stress and certain foods. Tissue injury stimulates the release of inflammatory signalling molecules such as bradykinin, and the release of inflammatory cytokines such as IL-1, TNF and IL-6. Cells that respond to infection or injury include macrophages and mast cells.24 Macrophages and other immune cells secrete chemokines that recruit leukocytes from the circulation to the site of inflammation. Mast cells release histamine, prostaglandins and leukotrienes that act as chemokines, increase vascular permeability and act on the vascular endothelium to increase tissue recruitment of leukocytes.24 Cyclooxygenase (COX) is an enzyme that is responsible for the formation of pro-inflammatory prostaglandins, prostacyclins and thromboxanes from the omega-6 arachidonic acid (see Chapter 28 on autoimmunity for further detail).
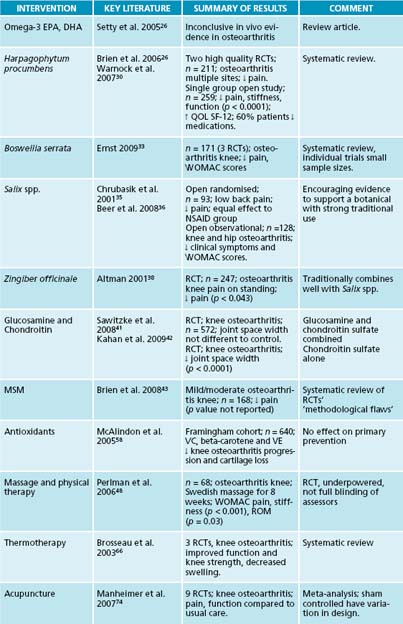
Omega-3 essential fatty acids compete with the omega-6, thereby moderating the inflammatory effect. There is evidence that omega-3 oils containing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have anti-inflammatory actions,25,26 but clinical data are lacking with regard to the treatment of osteoarthritis.25,27 The most widely available sources of EPA and DHA are cold water oily fish such as salmon, herring, mackerel, anchovies and sardines.
Harpagophytum procumbens, a South African herb, has been reviewed for efficacy and safety in the treatment of osteoarthritis with favourable results, especially in the reduction of pain.28,26 The mechanism of action has not been established, but is thought to be the anti-inflammatory activity of harpagoside.29 An 8-week, single-group, open clinical trial demonstrated statistically significant improvements in patient assessment of global pain, stiffness and function. There were also statistically significant reductions in mean pain scores for hand, wrist, elbow, shoulder, hip, knee and back pain. Quality of life measurements (using the SF-12 health survey) were significantly increased from baseline and 60% of patients either reduced or stopped concomitant pain medication. Adverse effects have been described as no different to placebo.27,30
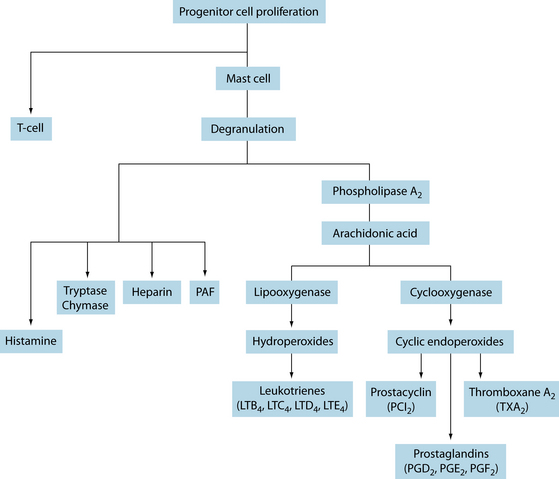
Figure 22.4 The inflammatory cascade
Source: Pearlman DS. Pathophysiology of the inflammatory response. J Allergy Clin Immunol 1999;104:S132–S137.
in arthritis via a variety of mechanisms. While most of the 43 studies justifying the formulation’s use have focused on pain measures, many have also uncovered various anti-inflammatory mechanisms, with efficacy often comparable to conventional anti-inflammatory medication.31,32
The gum resin extracted from the herb Boswellia serrata has some evidence as a potent anti-inflammatory, anti-arthritic and analgesic agent.33 A recent double-blind, randomised placebo-controlled trial of 75 subjects with osteoarthritis demonstrated that an extract of B. serrata (5-Loxin®) conferred clinically and statistically significant improvements in pain scores and physical function scores, and these changes were recorded in the treatment group as early as 7 days after the start of treatment.34
Salix spp. through the active constituent salicin, has anti-inflammatory and analgesic activity. Standardised preparations of the bark have shown positive effects in a number of trials in musculoskeletal conditions, with an analgesic effect dominant in low back pain.26,35 In a recent non-blinded trial of 131 subjects with arthrosis of the hip and knee, willow bark had equivalent effect to NSAIDs, with fewer side effects.36 A recent review found that, for treating mild or fairly severe cases of gonarthrosis and coxarthrosis, the effect of willow bark extract was comparable to that of standard therapies.36 Uncaria spp. is known to have anti-inflammatory activity in vitro, possibly by inhibiting the production of the pro-inflammatory cytokine, TNF-α,26 but clinical trials have been inconclusive.27 Zingiber officinale has been found to have anti-inflammatory actions.26,37 In a double-blind, randomised controlled trial of 261 subjects with knee osteoarthritis, ginger was found to significantly reduce symptoms and had mild adverse effects.38
An interesting pilot study using a real practice model showed that herbal medicine formulas prescribed for the individual by a herbal practitioner resulted in improvement of symptoms of osteoarthritis of the knee.39 Twenty adults, previously diagnosed with osteoarthritis of the knee, were recruited into this randomised, double-blind, placebo-controlled, pilot study carried out in a primary-care setting. All subjects were seen in consultation three times by a herbal practitioner who was blinded to the randomisation coding. Each subject was prescribed treatment and given lifestyle advice according to usual practice: continuation of conventional medication where applicable, healthy-eating advice and nutrient supplementation. Individualised herbal medicine was prescribed for each patient, but only dispensed for those randomised to active treatment—the remainder were supplied with a placebo. At baseline and outcome (after 10 weeks of treatment), subjects completed a food frequency questionnaire and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) knee health and Measure Yourself Outcome Profile (MYMOP) wellbeing questionnaires. There was significant improvement in the active group (n = 9) for the mean WOMAC stiffness subscore at week 5 and week 10, but not in the placebo group (n = 5). Also the mean WOMAC total and subscores all showed clinically significant improvement (20%) in knee symptoms at weeks 5 and 10 compared with baseline. Moreover, the mean MYMOP symptom 2 subscore, mostly relating to osteoarthritis, showed significant improvement at week 5 and week 10 compared with baseline for the active, but not for the placebo, group. This pilot study showed that herbal medicine prescribed for the individual by a herbal practitioner resulted in improvement of symptoms of osteoarthritis of the knee. This methodology mirrors normal clinical procedures, and should encourage similar larger clinical trials that have more relevance to naturopathic practice.
Enhance joint integrity and repair damage
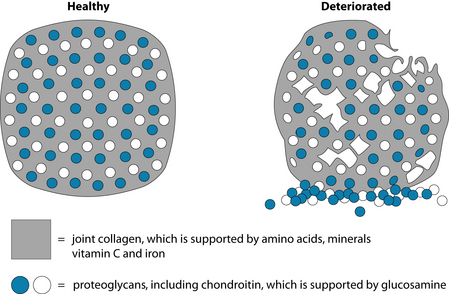
Primary and secondary prevention are the main foci of naturopathic medicine, and maintaining and repairing articular and periarticular tissue is a foundation for the management of osteoarthritis. Developing, maintaining and repairing collagen-based connective tissue requires optimal tissue levels of the essential amino acids, as well as vitamin C and iron as cofactors. Bone and cartilage are built on a matrix of minerals, mainly calcium and phosphorus, and an extracellular ground substance of proteoglycans (see Figure 22.5). Proteoglycans are glycoproteins that have a core protein with glycosaminoglycan (GAG) chains.
The large Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT) trials40,41 have found varying positive and negative results in both domains of clinical effect, and one study27puts forward a list of concerns that may explain why the body of evidence remains equivocal, including:
A recent randomised, double-blind, placebo-controlled trial of chondroitin sulfate given to 622 patients with knee osteoarthritis for 2 years found that the structure-modifying and symptom-modifying effects of chondroitin sulfate over the long-term suggest that it could be a disease-modifying agent.42
Methylsulfonylmethane (MSM) has been used as a sulfur-based nutritional supplement in conditions with joint pain, but a recent systematic review of clinical trials was
unable to reach a firm conclusion on its usefulness, because of methodological flaws and despite a number of positive trial findings.43–45
Improving joint mobility
The result of inflammation and fibrosis in osteoarthritis is limited joint motion, and this interferes with activities of daily living and has the global effect of reducing fitness and compliance to exercise therapy.
Massage and physical therapy provide viable options for managing osteoarthritis. There have been some trials using massage for osteoarthritis, but methodological quality is lacking, particularly with regard to the difficulties of blinding and control. A randomised controlled trial of 68 adults with radiologically confirmed osteoarthritis of the knee demonstrated significant improvements from standard Swedish massage in the WOMAC global scores, pain, stiffness and physical function domains.48 The subjects also had improvement in the visual analogue scale of pain assessment, range of motion in degrees and time to walk 50 feet (15.24 metres).
Evidence of efficacy in treating osteoarthritis may exist for the addition of physical therapy modalities. The term ‘multimodal therapy’ generally includes range of motion exercise, soft tissue mobilisation and muscle strengthening and stretching—all within the scope of the naturopath inclined to physical medicine.49 Studies suggest that patients with osteoarthritis receive moderate short-term (up to 8 weeks) clinical impact measured on WOMAC global and pain scores.
Adding therapeutic oils to the massage appears to have potential as an alternative method for short-term knee pain relief. In a double-blind, placebo-controlled trial of 59 older people, the intervention group had ginger and orange essential oils added to the massage oil, and the improvements of WOMAC-measured physical function and pain were superior in the intervention group compared with both the placebo and the control group at post 1-week time but not sustained at post 4 weeks.50
Reduce pain
Pain management is a vital component of chronic disease therapy in general, and non-pharmacological approaches should be considered in patients with osteoarthritis (see Chapter 37 on polypharmacy and pain management for more detail of pain management in osteoarthritis).
QUALITY MATTERS
As with many complementary medicines, quality and dosing variances can affect the therapeutic application and efficacy of treatment in trials. Glucosamine comes in a variety of forms which, though they theoretically can be hydrolysed in the stomach into equivalent forms, may have effects on therapeutic dosing requirements. Additionally, quality issues may also arise. Some formulations are demonstrated to be consistently more effective than placebo or other formulations in clinical trials.46,1 Although concerns have been raised that these positive results may be related to industry sponsorship, it has been deemed equally likely that these results may also be due to the stricter quality controls of some formulations.47 In clinical practice recommending any generic complementary medicine can be fraught; it is recommended that practitioners make specific recommendations for products they know to have high standards of quality and efficacy.
A Cochrane systematic review51 found that it is most effective in an acute stage of osteoarthritis when minor joint inflammation is present and is administered through the application of an ice pack wrapped in a towel for 20 minutes, 5 days a week for 2 weeks.
Vitamin D deficiency appears to be prevalent in people with chronic musculoskeletal pain.52–54 The mechanism appears to be related to a decrease in calcium absorption leading to dysfunctional collagen deposition in the periosteum, which generates painful stimuli. Oral supplementation with vitamin D in deficient subjects leads to a reduction in pain.55
The Framingham study data56 link vitamin D deficiency to osteoarthritis. In a study of 556 subjects, dietary and supplement intake of vitamin D and serum levels of 25-hydroxyvitaminD were evaluated. Osteoarthritis was measured using knee radiographs. The authors concluded that low intake and low serum levels of vitamin D each appeared to be associated with an increased risk for progression of osteoarthritis of the knee.56 These conclusions have been supported in a more recent review.57
Address oxidation
The Framingham study demonstrated that in an open trial of 640 subjects vitamin C 150–500 mg/day and beta-carotene 6000–10,000 IU/day in the diet was found to reduce risk of cartilage loss and knee pain over 8 years, with possible effects from vitamin E 8–10 mg/day in patients with knee osteoarthritis.58
This early promise has not been supported in trials since, with two systematic reviews finding very limited evidence for vitamin C or E.45,59
Remove metabolic waste products
A traditional naturopathic protocol used to treat osteoarthritis involves stimulation of the body’s detoxification pathways to clear metabolic waste. While this philosophy has not yet been scientifically tested via rigorous studies, traditional evidence does support this practice.60,61 The traditional use of herbal ‘alteratives’ and ‘diuretics’ such as celery seed or dandelion leaf, or nettles to treat rheumatism, appears to be predicated upon these herbs’ abilities to stimulate the removal of metabolic waste by increasing diuresis and potentially reducing blood composition of metabolic byproducts. This protocol may be potentially beneficial in treating gout (see the box on gout below).
INTEGRATIVE MEDICAL CONSIDERATIONS
Self-management
Self-management education programs have an important role to play in the management of chronic conditions such as osteoarthritis, and suit an integrative model of shared care between conventional and complementary medicine practitioners. Self-management education programs are interventions designed to educate the patient in self-care activities that promote health and the management of chronic diseases, increasing their motivation and decreasing the negative effects the condition has on their daily function.63 The National Health Priority Action Council states that, by taking the behavioural approach to the management of the psychosocial aspects of chronic disease, patients have outcomes of decreased pain and improved quality of life.63 This approach matches the holistic underpinning of naturopathic medicine.
GOUT62
Protocols
CAM interventions
Weight loss
Obesity is clearly a risk factor for developing osteoarthritis, particularly for women. There is very strong evidence that people who are obese (with a body mass index (BMI) of over 30) are at higher risk of their osteoarthritis being symptomatic and progressing than those with a BMI less than 30. The relationship between obesity and osteoarthritis is thought to be because of the increased stress on articular surfaces in the weight-bearing joints.2,3,8,64 Reducing obesity in the patient with osteoarthritis should be considered as both a primary and a secondary prevention strategy. The condition is multifactorial, and is best managed in a multidisciplinary team including naturopaths.
The Arthritis, Diet, and Activity Promotion Trial (ADAPT) was a randomised, single-blind controlled clinical trial that demonstrated that weight loss was central to the improvement in WOMAC physical function, pain and stiffness scores; these results were enhanced when combined with a moderate exercise program.64
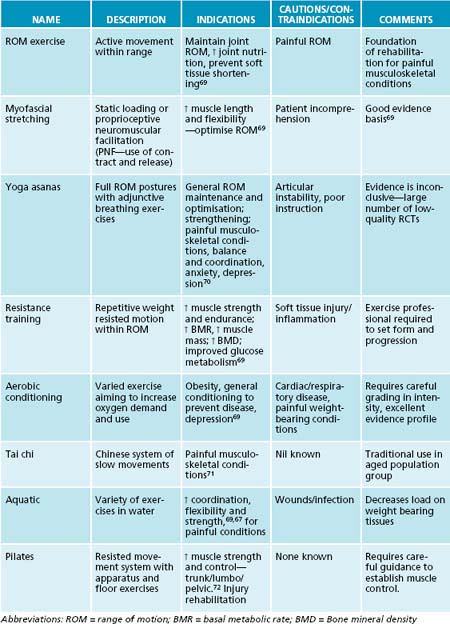
Exercise
Exercise guidance is a foundation of a naturopathic approach to wellness, and in the management of osteoarthritis coincides with the mainstream evidence-based guidelines. Exercise is important both as a treatment of symptoms and to prevent the development of osteoarthritis. Increasing physical activity improves general physical health and assists in the management of obesity, both vital risk factors for osteoarthritis, as mentioned.
Physical exercise of a light-to-moderate intensity increases muscle strength as well as range of motion, aerobic capacity and endurance that contributes to improved physical functioning and pain reduction. Various programs (see Table 22.2) offer different benefits and no specific type of exercise regimen has been shown to be superior.2,3,20,22 A number of Cochrane reviews support the application of therapeutic exercise in the management of osteoarthritis,65 with evidence for moderate intensity66 and aquatic programs.67
An interesting systematic review looked at tai chi, a traditional Chinese martial art style, which has been one of the most researched therapeutic exercises. The authors report that there is evidence suggesting that tai chi may be effective for pain control in patients with knee osteoarthritis, but the evidence is not as convincing for pain reduction or improvement of physical function.68
Acupuncture
Acupuncture is commonly used in an integrated clinical setting by acupuncturists, medical practitioners, physiotherapists and naturopaths, and has been assessed for efficacy in the treatment of osteoarthritis. In a randomised, controlled, single-blind trial of 88 subjects, acupuncture plus diclofenac was more effective than placebo acupuncture plus diclofenac for the symptomatic treatment of osteoarthritis of the knee.73 A recent meta-analysis regarding acupuncture for knee osteoarthritis was inconclusive, concluding that sham-controlled trials show clinically irrelevant short-term benefits of acupuncture for treating knee osteoarthritis.74 This study reveals the difficulties of control with research into therapies like acupuncture, where the placebo is not inert but has some effect, in many cases equal to the active intervention.
Differential diagnosis2
OSTEOARTHRITIS: COMORBID DEPRESSION AND PAIN
Patients with chronic diseases such as osteoarthritis have a higher rate of depression and anxiety than the general population.14 Osteoarthritis also has a feature of chronic pain, which can result in feelings of lowered self-worth, anxiety and depression.23 The practitioner needs to be educated about the effect these psychosocial aspects have on the condition and its progression. Osteoarthritis can also affect relationships and community and social activities,23,14 which are all addressed in the naturopathic assessment of the individual.
A recent study of depression in 1021 patients with osteoarthritis75 revealed that subjects who had concomitant depression had more contacts to general practitioners and to providers of complementary alternative medicine, resulting in increased health-service use. The authors conclude that appropriate treatment of depression would increase quality-of-life measures and also lower costs by decreasing health-service use, and that depression aggravates the burden of osteoarthritis significantly.75 See Chapter 37 on polypharmacy and pain management for further consideration of pain management in naturopathic medicine.
Example treatment
Physical medicine
Chaitow L., editor. Naturopathic physical medicine. Edinburgh: Elsevier, 2008.
Brosseau L.Y., et al. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst Rev. 4, 2003. CD002826
Towheed T., et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2, 2006. CD002946
1. Reginster J., et al. Current role of glucosamine in the treatment of osteoarthritis. Rheumatology (Oxford). 2007;46(5):731-735.
2. 2009 Current medical diagnosis and treatment, 48th edn. New York: McGraw Hill Medical; 2009.
3. Australian Institute of Health and Welfare. A picture of osteoarthritis in Australia. Canberra: Australian Institute of Health and Welfare; 2007.
4. Australian Institute of Health and Welfare. Arthritis and musculoskeletal conditions in Australia, 2005. Canberra: Australian Institute of Health and Welfare; 2005.
5. Australian Institute of Health and Welfare. General practice activity in Australia 2000-01. Canberra: Australian Institute of Health and Welfare; 2001.
6. Australian Institute of Health and Welfare. Health system expenditure on disease and injury in Australia, 2000-01. Canberra: Australian Institute of Health and Welfare; 2005.
7. March L.B., Bagga H. Epidemiology of osteoarthritis in Australia. Med J Aust. 2004;180(5 Suppl):SS6-SS10.
8. Hart D.S., Spector T.D. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford study. J Rheumatol. 1993;20:331-335.
9. Coggon D.C., et al. Occupational activity and osteoarthritis of the knee. Arthritis Rheum. 2000;43:1443-1449.
10. Spector T.C., et al. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312:940-943.
11. Zhang Y.X., et al. Lower prevalence of hand osteoarthritis among Chinese subjects in Beijing compared with white subjects in the United States: the Beijing Osteoarthritis study. Arthritis Rheum. 2003;48:1034-1040.
12. Bellamy N.B., et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-1840.
13. Auw Yang K.R., et al. Validation of the short-form WOMAC function scale for the evaluation of osteoarthritis of the knee. J Bone Joint Surg. 2007;89-B(1):50-56.
14. National Arthritis and Musculoskeletal Conditions Advisory Group. Evidence to support the national action plan for osteoarthritis, rheumatoid arthritis and osteoporosis: opportunities to improve health-related quality of life and reduce the burden of disease and disability. Canberra: Department of Health and Ageing; 2004.
15. American College of Rheumatology. American College of Rheumatology Subcommittee on Osteoarthritis guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905-1915.
16. Jordan K.A., et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
17. Hewlett S., et al. Patients’ perceptions of fatigue in rheumatoid arthritis: overwhelming, uncontrollable, ignored. Arthritis Rheum. 2005;53(5):697-702.
18. Power J., et al. Fatigue in osteoarthritis: a qualitative study. BMC Musculoskelet Disord. 2008;1(9):63.
19. Zeff J., et al. A hierarchy of healing: the therapeutic order. The unifying theory of naturopathic medicine. In Pizzorno J.E., Murray M.T., editors: Textbook of natural medicine, 3rd edn, Edinburgh: Churchill Livingstone, 2006.
20. Zhang W.M., et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137e-162e.
21. Hart J. Osteoarthritis and complementary therapies. Alternative and Complementary Therapies. 2008;14(3):116-120.
22. Royal Australian College of General Practitioners. Osteoarthritis Working Group: Guideline for the non-surgical management of hip and knee osteoarthritis. 2009. Online. Available: http://www.nhmrc.gov.au/PUBLICATIONS/synopses/_files/cp117-hip-knee-osteoarthritis.pdf.
23. Kelly A. Managing osteoarthritis pain. Nursing. 2006;36(11):20-21.
24. Laufer S., et al, editors. Inflammation and rheumatic diseases: the molecular basis of novel therapies. Stuttgart: Thieme, 2008.
25. Calder P. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345-358.
26. Setty A.S., L.H. Herbal medications commonly used in the practice of rheumatology: mechanisms of action, efficacy, and side effects. Semin Arthritis Rheum. 2005;34(6):773-784.
27. Tancred J. Joint care. Journal of Complementary Medicine. 2009;8(2):12-19.
28. Brien S.L., et al. Devil’s claw (Harpagophytum procumbens) as a treatment for osteoarthritis: a review of efficacy and safety. J Altern Complement Med. 2006;12(10):981-993.
29. Braun L., Cohen M. Herbs and natural supplements. An evidence-based guide. Sydney: Elsevier; 2005.
30. Warnock M.M., et al. Effectiveness and safety of devil’s claw tablets in patients with general rheumatic disorders. Phytother Res. 2007;21(12):1228-1233.
31. Ernst E. The efficacy of Phytodolor® for the treatment of musculoskeletal pain—a systematic review of randomized clinical trials. Nat Med J. 1999;2(1):14-16.
32. Gundermann K., Müller J. Phytodolor—effects and efficacy of a herbal medicine. Wien Med Wochenschr. 2007;157(13–14):343-347.
33. Ernst E. Frankincense: a systematic review. BMJ. 337, 2009. a2813
34. Sengupta K.A., et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10(4):R85.
35. Chrubasik S.K., et al. Treatment of low-back pain with a herbal or synthetic anti-rheumatic: a randomized controlled study. Willow bark extract for low-back pain. Rheumatology (Oxford). 2001;40:1388-1393.
36. Beer A-M T. Willow bark extract (Salicis cortex) for gonarthrosis and coxarthrosis—results of a cohort study with a control group. Phytomedicine. 2008;15:907-913.
37. Tjendraputra E.T., et al. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem. 2001;29(3):156-163.
38. Altman R.M., KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44(11):2531-2538.
39. Hamblin L.L., et al. Improved arthritic knee health in a pilot RCT of phytotherapy. J R Soc Promot Health. 2008;128(5):255-262.
40. Clegg D.R., et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795-808.
41. Sawitzke A.S., et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheu. 2008;58(10):3183-3191.
42. Kahan A.U., et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60(2):524-533.
43. Brien S.P., et al. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2008;16(11):1277-1288.
44. Kim L.A., et al. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial. Osteoarthritis Cartilage. 2006;14(3):286-294.
45. Ameye L.G., Chee W.S. Osteoarthritis and nutrition: from nutraceuticals to functional foods: a systematic review of the scientific evidence. Arthritis Res Ther. 2006;8(4):R127.
46. Towheed T., et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. (2):2006. CD002946
47. Vlad S.L., et al. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum. 2007;56(7):2267-2277.
48. Perlman A.S., et al. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533-2538.
49. Orrock P. Naturopathic physical medicine. In: Chaitow L., editor. Naturopathic physical medicine. Edinburgh: Elsevier, 2008.
50. Yip Y.B., Tam A.C.Y. An experimental study on the effectiveness of massage with aromatic ginger and orange essential oil for moderate-to-severe knee pain among the elderly in Hong Kong. Complement Ther Med. 2008;16(13):131-138.
51. Brosseau L.Y., et al. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst Rev. (4):2003. CD004259
52. Thomas ML-J., et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777-783.
53. Nesby-O’Dell S.S., et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;79:187-192.
54. Plotnikoff G.A., Quigley J.M. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463-1470.
55. Holick M. Vitamin D deficiency: what a pain it is. Mayo Clin Proc. 2003;78(12):1457-1459.
56. McAlindon T.F., et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125(5):353-359.
57. Holick M. Vitamin D deficiency: medical progress. N Engl J Med. 2007;357(3):266.
58. McAlindon T.J., et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 2005;39(4):648-656.
59. Wang Y.P., et al. The effect of nutritional supplements on osteoarthritis. Altern Med Rev. 2004;9(3):275-296.
60. Chaitow L., editor. Naturopathic physical medicine. Edinburgh: Elsevier, 2008.
61. Mills S., Bone K. Principles and practice of phytotherapy. Edinburgh: Churchill Livingstone; 2000.
62. Demio P. Gout. In Rakel D., editor: Integrative medicine, 2nd edn, Philadelphia: Saunders Elsevier, 2008.
63. National Health Priority Action Council. National service improvement framework for ostearthritis, rheumatoid arthritis and osteoporosis. Canberra: Department of Health and Ageing; 2006.
64. Messier S.L., et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2004;50(5):1501-1510.
65. Fransen M.M., et al. Exercise for osteoarthritis of the hip or knee. Cochrane Database Syst Rev. (3):2003. CD004376
66. Brosseau L.M., et al. Intensity of exercise for the treatment of osteoarthritis. Cochrane Database Syst Rev. (3):2003. CD004376
67. Bartels E.L., et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. (4):2007. CD005523
68. Soo Lee M.P., et al. Tai chi for osteoarthritis: a systematic review. Clin Rheumatol. 2008;27(2):211-218.
69. Bandy W.D., Sanders B. Therapeutic exercise: techniques for intervention, 1st edn. Philadelphia: Lippincott Williams and Wilkins; 2001.
70. Lipton L. Using yoga to treat disease: an evidence based review. JAAPA. 2008;21(2):34-41.
71. Lee M., et al. Tai chi for osteoarthritis: a systematic review. Clinical Rheumatology. 2008;27(2):211-218.
72. Bernardo L. The effectiveness of Pilates training in healthy adults: an appraisal of the research literature. J Bodyw Mov Ther. 2007;11:106-110.
73. Vas J.M., et al. Acupuncture as a complementary therapy to the pharmacological treatment of osteoarthritis of the knee: randomised controlled trial. BMJ. 2004;329:1216.
74. Manheimer E.L., et al. Meta-analysis: acupuncture for osteoarthritis of the knee. Ann Intern Med. 2007;146(12):868-877.
75. Rosemann T.G., et al. The impact of concomitant depression on quality of life and health service utilisation in patients with osteoarthritis. Rheumatol Int. 2007;27(9):859-863.