CHAPTER 42 Ossification of the Posterior Longitudinal Ligament
History
Despite reports of ossification of the posterior longitudinal ligament (OPLL) by Key in 18381 and by Oppenheimer in 1942,2 it was not recognized as a distinct clinical entity until 1960. The first report in Japan of cervical compressive myelopathy caused by OPLL was based on autopsy findings obtained by Tsukimoto in 1960.3 Onji and colleagues4 reviewed clinical symptoms of 18 cases of OPLL in 1967. Since then, many reports of OPLL have been published, and it has been recognized as a common clinical entity that causes compression myelopathy, especially in Japan. The Investigation Committee on Ossification of the Spinal Ligaments, organized by the Japanese Ministry of Public Health and Welfare, has conducted studies of the etiology of OPLL and patient care for OPLL since 1975. Their studies of the pathogenesis, diagnosis, and treatment of OPLL have contributed to improvement in management of OPLL and other spinal disorders in Japan.
OPLL is a common cause of cervical myelopathy in middle-aged and older Japanese adults. Although OPLL has been thought to be rare among whites and to be a “Japanese disease,” several reports of OPLL in whites and in natives of other Asian countries have been published.5 Several studies of growth factors, cytokines, and other molecular and genetic factors in OPLL have been reported, but the etiology of OPLL has not yet been fully elucidated.
Etiology
OPLL has been found in 26% of the parents of probands and 29% of the siblings of probands.6 Familial surveys and human leukocyte antigen (HLA) haplotype studies reveal that genetic background plays an important role in the occurrence of OPLL. A genetic locus for OPLL is thought to be located close to the HLA region, on chromosome 6p. Although a candidate gene in the region, the gene for collagen 11A2, has been analyzed for the presence of molecular variants in affected probands, the pathogenesis of OPLL does not seem to be entirely the result of defects in this gene.7
Pathology
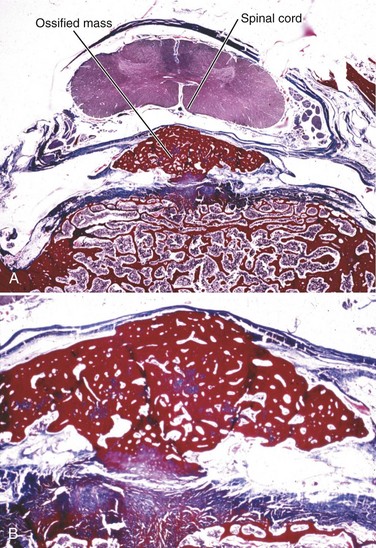
Although OPLL was previously thought to involve calcification of the posterior longitudinal ligament, studies have shown that it actually involves ossification of the ligament.8 OPLL involves ectopic bone formation within the spinal ligaments. The mature form of the ossification consists of lamellar bone with well-developed haversian systems. Immature lesions are often accompanied by woven bone with fibrocartilaginous cell proliferation in the marginal area (Fig. 42–1). The main characteristics of OPLL are as follows9,10: (1) ossification accompanied by ligamentous tissue hyperplasia and cell proliferation; (2) sequential occurrence of fibrocartilaginous cell proliferation, calcification, and tissue resorption with vascular ingrowth; and (3) ossification of the ligament that occurs at specific sites of predilection and often in combination with diffuse idiopathic skeletal hyperostosis.11 The ossification process of the ligament is not always endochondral ossification but sometimes is membranous ossification.10
The posterior longitudinal ligament is initially replaced with bony tissue. Hyperplasia of the fibrocartilaginous tissue may occur in the posterior aspect of the intervertebral disc, extending into the ossified posterior longitudinal ligament.9 Progression of ossification follows an evolutionary process, beginning as endochondral or membranous ossification. There may be no correlation between disc degeneration and occurrence of OPLL.8 Generally, in cases of dense OPLL, normal disc thickness is maintained in the cervical spine, and there are no signs of inflammation or bleeding in these areas of ossification.9
Myelopathy caused by OPLL is characterized by chronic compression of the spinal cord. Autopsy studies of OPLL show the following pathologic characteristics12,13: (1) Demyelination and loss of axon, which is more dominant in the posterolateral than in the anterior column, is observed in the white matter; (2) pathologic changes are restricted to the gray matter, and the white matter is preserved in cases with a boomerang shape in which the transverse area of the spinal cord is greater than 60% of the normal; (3) a triangular shape in which the transverse area of the spinal cord is reduced to less than 60% of the normal is associated with severe and irreversible pathologic changes showing white matter and gray matter are severely involved, and only the anterior column is preserved.
Metabolic Background
A high incidence of diabetes mellitus (non–insulin-dependent) and impaired glucose tolerance has been reported among patients with OPLL.14,15 Glucose tolerance tests of 535 patients with OPLL showed that 28% were diabetic, and 18% were borderline diabetic.16 There have also been reports of a relatively high incidence of OPLL and hormonal disorders such as hypoparathyroidism and hypophosphatemic rickets among diabetic patients.16 This finding seems to implicate abnormal calcium metabolism in ossification of the ligaments, although conventional blood chemistry tests have shown no abnormalities in patients with OPLL.16
Epidemiology
Incidence
The incidence of OPLL in Japanese older than 30 years of age has been reported to range from 2% to 4%. In Taiwan, Korea, Hong Kong, and Singapore, the incidence of OPLL reportedly ranges from 0.8% to 3%.5 In the United States and Europe, the incidence of OPLL reportedly ranges from 0.1% to 1.7%.5 In a survey of 599 patients in Utah University Hospital, 8 patients (1.3%) were found to have OPLL in the cervical spine.5,8
Natural History of Myelopathy
A cohort study showed that all patients with more than 60% spinal canal stenosis by OPLL exhibited cervical myelopathy.17Although static compression of the spinal cord is the main cause of myelopathy in OPLL, radiologic findings do not always correlate with clinical severity of neurologic manifestations. In a study of the natural history of 207 patients with OPLL over an average period of 10 years, clinical symptoms did not change in 66% of patients, and myelopathy developed in 16% of patients.18 Kaplan-Meier analysis of 323 patients who did not have myelopathy at their initial evaluation showed a myelopathy-free rate of 71% after 30 years.19
Despite spinal stenosis (6 mm < space available for the spinal cord < 14 mm), myelopathy may not develop in patients with the severe limitation of range of motion of the cervical spine.19,20 This finding indicates that not only static factors, but also dynamic factors such as listhesis or hypermobility at discontinuity of the ossified lesion play important roles in the development of myelopathy, especially in mixed or segmental OPLL.
Presentation
Location
Although OPLL has been observed at all levels of the spine, it occurs most frequently in the cervical spine. Thoracic OPLL occurs at the upper and middle thoracic levels.16 In contrast, ossification of the ligamentum flavum (OLF) is frequently observed at the lower thoracic level and the thoracolumbar junction. Lumbar OPLL occurs relatively infrequently, and it does not usually cause severe disabilities.
Symptoms
Many patients with subclinical or latent OPLL are asymptomatic, and many patients with large ossified lesions experience no disability. Symptoms most typically include myelopathy or myeloradiculopathy rather than radiculopathy alone. The symptoms develop secondary to spinal cord compression caused by the space-occupying lesion of the ossified ligament. These neurologic symptoms develop insidiously without any obvious causes in 80% to 85% of patients, but acute onset or aggravation of the symptoms is often related to a minor trauma or hyperextension of the neck.16
Patients can be divided into three groups according to their neurologic symptoms: (1) patients with spinal cord signs presenting with motor and sensory disturbances predominantly in the lower extremities; (2) patients with segmental signs presenting with motor and sensory disturbances predominantly in the upper extremities; and (3) patients presenting with pain in the neck, shoulder, and arm region without obvious neurologic deficits (axial symptoms alone).9
Diagnosis
Physical Examination
The most common complaint at onset of OPLL is paresthesia or numbness in the hands. Clumsiness of the hands is another symptom. The complaints gradually extend to the lower extremities, leading to gait disturbance. Physical examination generally reveals spasticity of the lower extremities with exaggerated deep tendon reflexes and sensory disturbance in upper and lower extremities. Patients sometimes complain of neck pain or discomfort around the neck. Myelopathy affecting the hand, as indicated by the tests (finger escape sign and 10-second grasp and release test) proposed by Ono and colleagues,21 is a sensitive and specific sign of pyramidal tract involvement in the cervical spine. It is important to differentiate OPLL from other skeletal and neurologic disorders, including cervical spondylosis, spinal cord tumor, periarthritis of the shoulder, entrapment neuropathy in the upper extremities, and motor neuron disease.8
Plain Radiography
Narrow disc spaces or spondylotic changes are occasionally observed at the lower cervical region of OPLL patients, but disc spaces located in the ossified area are usually well preserved.9 There is no involvement of sacroiliac joints or apophyseal joints, which is usually observed in cases of ankylosing spondylitis.
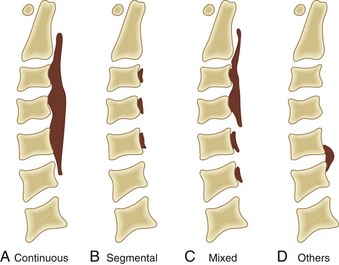
Based on radiographic findings, cervical OPLL is classified into four types (Fig. 42–2): (1) continuous type, in which ossification extends over several contiguous vertebrae; (2) segmental type, in which ossification is fragmented and located immediately behind each vertebral body with interruption at the intervertebral disc levels; (3) mixed type, which is a combination of continuous and segmental types of ossification; (4) others type (circumscribed or localized), in which ossification is confined to the intervertebral disc space.16 In addition to this conventional typing, sagittal shape of the ossified lesion is classified into plateau-shaped or hill-shaped (see Fig. 42–6).22 Plateau-shaped ossification, which is found in segmental-type OPLL and most continuous-type and mixed-type OPLL, is characterized by a narrow spinal canal without massive localized ossification. Hill-shaped ossification, which is found in circumscribed-type OPLL and some cases of continuous-type or mixed-type OPLL, shows massive beak-shaped ossification localized to certain levels.
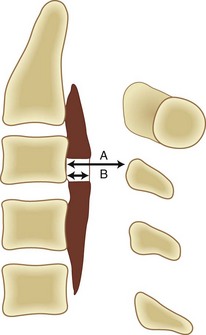
The occupying ratio of OPLL is calculated as the ratio of the maximum anteroposterior thickness of OPLL to the anteroposterior diameter of the spinal canal at the corresponding level on a lateral radiograph or tomogram (Fig. 42–3).16 An occupying ratio greater than 60% indicates high risk of the development of myelopathy.17,23 Matsunaga and colleagues19,20 reported that with a constant tube-to-film distance of 150 cm, all of the patients with a value of space available for the spinal cord (SAC) of less than 6 mm had myelopathy, whereas none of the patients with SAC of 14 mm or more developed myelopathy. They also reported that in patients with myelopathy whose minimal SAC diameter ranged from 6 mm to less than 14 mm, the range of motion of the cervical spine was significantly greater.18,20 These results suggest that the primary factor in the development of myelopathy is reduced SAC diameter resulting from static compression by the ossified ligament, although below the critical point (SAC >6 mm or maximum spinal canal stenosis ≤60%), dynamic effects seem to be the main factors in the development of myelopathy.
Computed Tomography
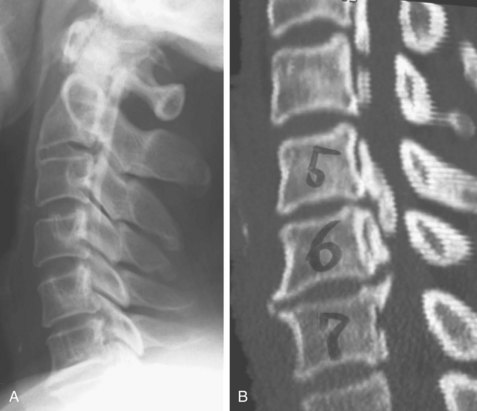
Computed tomography (CT) is particularly useful for imaging of the lower cervical spine and visualizing ossification that is difficult to detect with plain radiographs (Fig. 42–4). Because of the high frequency of association of cervical OPLL with thoracolumbar OPLL and OLF, a preoperative survey should include imaging of the entire spinal column. Because it is difficult to detect ossified lesions at the cervicothoracic and thoracolumbar junctions on plain radiographs, careful preoperative and postoperative CT or magnetic resonance imaging (MRI) or both should be performed to assess spinal stenosis throughout the entire spine. Myelography is useful to assess spinal canal stenosis by OPLL or OLF or both from the cervical spine to the lumbar spine, and combined myelography and CT is mandatory for planning of anterior removal or floating of the ossified lesion.
Magnetic Resonance Imaging
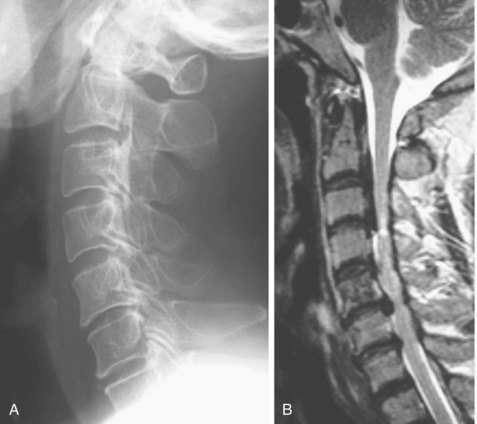
MRI is not effective for detection of OPLL because OPLL lacks signal intensity on T1-weighted and T2-weighted images (Fig. 42–5). Bone marrow within the ossified lesion appears as an isointense or hyperintense area, however. MRI can illustrate pathology of the spinal cord. Kameyama and colleagues12 reported that in cases of OPLL, a triangular spinal cord with a transverse area of less than 60% of normal in more than one segment seemed to be associated with severe and irreversible pathologic changes. MRI is also useful for detection of cervical disc herniation. In one study, disc protrusion was detected in 81% of patients with segmental OPLL, and intramedullary hyperintensity was observed on T2-weighted images in 43% of the patients whose neurologic deficits were significantly more severe than average (see Fig. 42–5B).24
Treatment of Ossification of the Posterior Longitudinal Ligament of the Cervical Spine
Conservative Treatment
A cervical orthosis and skull traction are used in conservative treatment of OPLL. Such conservative treatment is indicated to eliminate dynamic factors of the cervical spine for patients whose predominant complaint is neck, shoulder, and arm pain (local pain, radicular pain, or both) without any symptoms of myelopathy or patients with mild ossification in whom myelopathy is subclinical and not predominant.25 It is important to advise patients with OPLL not to hyperextend the neck and to be vigilant regarding trauma and falls secondary to motor vehicle accidents, sports activities, or excessive alcohol intake.25
Surgical Treatment
Surgical decompression of the spinal cord is necessary for patients with apparent myelopathy because long-term compression of the spinal cord may cause irreversible degeneration. For patients with symptoms and signs of moderate or progressive myelopathy, the authors recommend early surgical decompression, especially for younger patients with a narrow spinal canal, because reports indicate that better neurologic recovery is associated with younger age at operation and milder myelopathy.26 Even if the myelopathy is mild, surgery may be indicated for patients with severe spinal stenosis (SAC ≤6 mm or occupying ratio ≥60%). There is no evidence indicating the effectiveness of prophylactic surgery for patients who have no symptoms or signs of myelopathy.23
Authors’ Preferred Choice of Surgical Procedure
There is some controversy over the appropriate method of surgery for myelopathy caused by cervical OPLL. In Japan, most surgeons use an anterior approach with extirpation or floating of the ossified lesion or a posterior approach including various types of expansive laminoplasty. The choice between two approaches should be based on the following considerations: skill of the surgeon, age and general condition of the patient, extent of ossification, OPLL type and sagittal shape of the ossified lesion (plateau-shaped [Fig. 42–6A] or hill-shaped [Fig. 42–6B]), OPLL occupying ratio, sagittal curvature of the cervical spine and spinal cord (kyphotic or lordotic), and intervertebral mobility at the maximum compression level (dynamic factors).22,27–30
Extensive laminoplasty generally is effective and safer for most patients with the following characteristics: (1) OPLL occupying ratio of less than 60%, (2) plateau-shaped ossification (see Figs. 42-6A and 42-11A), and (3) lordotic alignment of the cervical spine or the spinal cord.22,26,28,29 The authors’ clinical experience indicates that laminoplasty is not contraindicated if kyphosis of the cervical spine is mild. The anterior floating method using autogenous fibular graft is recommended for patients with the following characteristics: (1) OPLL occupying ratio of less than 60%; (2) hill-shaped ossification (Fig. 42–7A; see Figs. 42-4B and 42-6B); (3) local kyphotic alignment of the cervical spine or the spinal cord; and (4) intervertebral hypermobility at the maximum compression level or between the interrupted ossified lesion.22,28–30
Internal rigid fixation is rarely necessary for surgical treatment of OPLL. Although the authors do not recommend the use of spinal instrumentation for laminoplasty, which has the advantage of allowing some movement of the cervical spine, laminoplasty with posterior instrumented fusion may have the advantage for patients with a flexible kyphotic alignment of the cervical spine or evident intervertebral mobility at the level of maximum spinal cord compression.30 An anterior plate and screws are rarely necessary for the anterior approach if a halo vest is worn for 6 to 10 weeks. Posterior instrumentation is often used, however, during salvage surgery for dislodgment or pseudarthrosis of bone graft (see Fig. 42–8D).
Anterior Approaches
The results of anterior interbody fusion without decompression have been reported.31,32 One report described anterior resection of an ossified lesion by drilling into the anterolateral part of the vertebral body without bone grafting.33 The indications of these anterior approaches have not been clearly identified, however, and the results of such surgery have been inconsistent.
Several surgeons have attempted to remove ossified lesions using an anterior approach. The results of these procedures varied owing to insufficient decompression resulting from ossification of the dura (see Fig. 42–9A) or massive bleeding from the epidural space and intraoperative injury to the spinal cord or nerve roots. Anterior removal is also associated with a high incidence of complications. The anterior floating method devised by Yamaura and colleagues,34 in which the spinal cord is decompressed without resection of the ossified lesion, has made anterior decompression surgery for OPLL safer and more reliable. The advantages of this procedure include gradual decompression without extirpation but with an anterior shift of the OPLL to avoid dural tears caused by ossification of the dura and lower risk of injury to neural tissues. The anterior floating method is indicated for ossified lesions localized from C2 to T3. Locally prominent ossified masses and highly occupied lesions can be treated effectively using this method (see Fig. 42–7).
Surgical Technique
The vertebral bodies are resected according to the orientation obtained by total discectomy. Based on CT and reconstruction images, resection is extended laterally. Transverse decompression should extend at least 20 to 25 mm to ensure sufficient decompression.27,34 When the posterior cortex of the vertebral body is exposed, the cortex and the ossified ligament should be shaved with a diamond bur to make their thickness as uniform as possible. Care should be taken not to perforate the lateral part of the posterior cortex or injure the venous plexus. When the ossified ligament and the posterior cortex have been thinned, the ossified lesion and vertebral edge are cut at the cranial, caudal, and lateral margins by continuing to shave the cortex and the ossified ligament with a diamond bur. When these margins are released completely, the released ossified lesion begins to rise slightly. After release of the ossified lesion, the remnant of the ossified mass should not be shaved but should be allowed to move ventralward spontaneously.
If the lesion can be released easily, the ossified lesion can be removed, but this is not always necessary if the lesion adheres strongly to the dura or the dura itself is ossified (see Fig. 42–9A). The possible causes of insufficient floating include the following: narrow width of decompression, insufficient release, disorientation of the lateral border of the vertebral body, insufficient thinning of the ossified ligament, and eccentric location of the ossified segment. A bone graft harvested from the fibula is inserted in the space. Immobilization of the spine with a halo vest is maintained for 6 to 10 weeks postoperatively. Postoperatively, released ossification gradually shifts anteriorly under cerebrospinal fluid pressure. The period required for anterior shifting of the ossification is 4 to 8 weeks (average 6 weeks) (see Fig. 42–7E-G).27,34
Complications
Likely complications after anterior decompression include dural tear with cerebrospinal fluid leakage, nerve root palsy, and dislodgment or pseudarthrosis of grafted bone (Fig. 42–8).35,36 Postoperative C5 paresis has reportedly been observed in about 10% of patients who have undergone anterior decompression.27 Cerebrospinal fluid leakage and dislodgment or pseudarthrosis of the strut graft are major complications of the anterior approach, and the rate of these complications reportedly ranges from 3% to 15%.29,36–38 The rate of reoperation after anterior decompression has been reported to be 12% to 26%.29,38
Posterior Approaches
Laminectomy was previously the procedure of choice for cervical OPLL.39 Laminectomy has some disadvantages, however, such as postlaminectomy kyphotic deformity and scar formation (laminectomy membrane). In 1977, Hirabayashi and colleagues40 developed expansive open-door laminoplasty. Currently, laminoplasty (in various forms) is the treatment of choice for not only OPLL but also cervical spondylotic myelopathy.26 Although there is no clear evidence indicating that laminoplasty is clinically superior to laminectomy,41 some reports suggest that laminoplasty has biomechanical and clinical advantages over laminectomy.42–46
Several types of laminoplasty have been devised, and there is no conclusive statistical evidence that any of them is superior to the others.41 Generally, the indications for laminoplasty are (1) multisegmental OPLL of continuous or mixed type and (2) any type of OPLL associated with developmental narrow spinal canal (anteroposterior diameter <13 mm). Rigid or severe kyphosis of the cervical spine is not a good indicator for laminoplasty. Although there are no available data indicating how great a degree of kyphosis is acceptable for laminoplasty, the authors’ clinical experience indicates that mild kyphosis is not a contraindication to laminoplasty (see Fig. 42–6).22,26
Complications
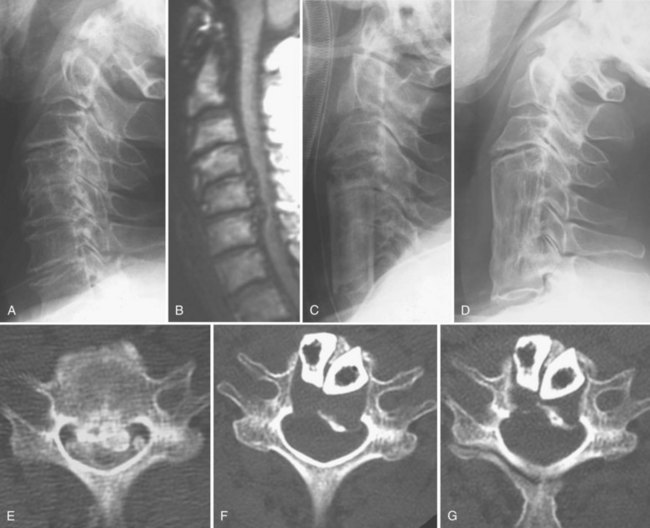
Common complications after laminoplasty include motor palsy of the upper extremity (commonly C5 segment or nerve root), kyphotic deformity of the cervical spine, hematoma, and neck pain.35 Although the mean incidence of postlaminoplasty C5 palsy in patients with OPLL is 5% to 10% (range 3% to 29%),26,47,48 spontaneous recovery can be expected in most cases.26,47,49 There is no way to preclude the possibility of C5 palsy being caused by the tethering effect of the nerve roots, and it is important that the bony gutter of the hinge side not be drilled excessively, to prevent severing of the inner cortex of the lamina (Fig. 42–9).
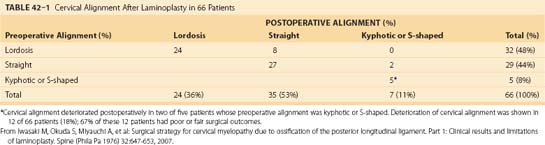
Postoperative deterioration in cervical alignment has been observed in 12 (18%) of 66 patients who have undergone laminoplasty (Table 42–1); 67% of these 12 patients had poor or fair surgical outcomes.22 Kyphotic deformity has been observed in 47% of patients who have undergone laminectomy and 6% to 8% of patients who have undergone laminoplasty, although mild kyphotic deformity of the cervical spine is rarely associated with neurologic deterioration (Fig. 42–10; see Table 42–1).22,26,39
Surgical Results
Neurologic severity of cervical myelopathy is assessed using a scoring system proposed by the Japanese Orthopaedic Association (JOA) and the recovery rate.22,26,27,39,50,51 A full score is 17 points and indicates healthy status. This JOA scoring system is useful and reliable for assessment of cervical myelopathy.51
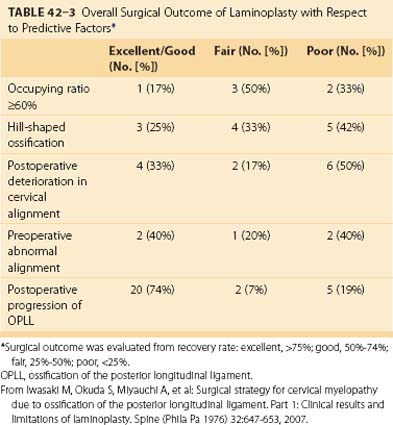
Long-term results of laminectomy,39 laminoplasty,22,26 and anterior floating method27,29 are summarized in Table 42–2. Statistical analysis indicates that predictive factors affecting clinical results of posterior decompression are sagittal shape of ossification (hill-shaped), preoperative severity of myelopathy (low total JOA score), postoperative deterioration in cervical alignment, and age at operation.22,26,39 Overall surgical outcome of laminoplasty is presented in Table 42–3.22
TABLE 42–2 Long-Term (>10 Years) Results of Laminectomy, Laminoplasty, and Anterior Floating Procedure for Cervical Ossification of the Posterior Longitudinal Ligament (OPLL)

Occupying ratio of OPLL or SAC is statistically unrelated to surgical outcome of posterior decompression, although a retrospective study of OPLL with an occupying ratio of greater than 50% to 60% indicates that neurologic outcome of anterior decompression and fusion is better than that of laminoplasty.22,29,50 With the anterior floating method, good outcome is strongly associated with preoperative severity and duration of myelopathy, preoperative cross-sectional area of the spinal cord, and age at last follow-up.27
OPLL generally continues to progress after surgery (Fig. 42–11). In a Japanese nationwide multicenter study, the incidence of OPLL progression was 56.5% at 2 years after posterior decompression and 71% at 5 years (Kaplan-Meier analysis).52 In this multicenter study, younger patients (<60 years old) had a higher risk of progression (Fig. 42–12A).52 Patients with mixed-type OPLL had the highest rate of progression, and patients with segmental-type OPLL had the lowest incidence of progression (Fig. 42–12B).26,52,53 In a long-term (>10 years) follow-up study, postoperative progression of ossification was observed in 70% to 73% of patients who underwent laminectomy or laminoplasty, but few were found to have related neurologic deterioration.26,39,53 The incidence of postoperative progression after anterior decompression and fusion ranges from 36% to 64%, which is lower than the rate for posterior decompression.27,54
The main problem with anterior decompression is restenosis at levels adjacent to the fusion area, caused by postoperative progression of OPLL or spinal instability.27,29 Matsuoka and colleagues27 reported that posterior decompression of the cervical spine was required postoperatively in 8% of patients who underwent the anterior floating procedure. In contrast, additional cervical surgery was required to treat progression of OPLL in only 1 of 64 patients (2%) undergoing laminoplasty.26
Serial radiographic analysis of patients with OPLL, performed more than 10 years after they underwent laminoplasty, revealed spontaneous anterior fusion between vertebral bodies in 64% of patients and spontaneous posterior fusion between facets or laminae in 97% of patients.26 In a study of 30 patients with OPLL who underwent laminoplasty, the mean age at death was 76 years (range 58 to 85 years), and the most frequent cause of death was cancer, followed by heart disease, pneumonia, and cerebrovascular accident.26
Ossification of the Posterior Longitudinal Ligament or Ossification of the Ligamentum Flavum of the Thoracic Spine
OLF can usually be identified on a plain lateral radiograph of the thoracolumbar junction. Plain radiography is insufficient, however, to delineate ossified lesions in the upper thoracic spine, which is a common site of OLF and OPLL. The authors recommend CT or MRI or both to detect ossification in the cervicothoracic and thoracolumbar junctions (Fig. 42–13).
Surgical Treatment
Conservative treatment for thoracic OPLL or OFL is less effective in patients who present with symptoms of myelopathy because the thoracic spine undergoes less motion and has a narrower spinal canal than the cervical spine. The only currently available surgical treatment for OLF of the thoracic spine is posterior decompression. Surgical outcome of posterior decompression for myelopathy caused by thoracic OPLL has generally been poor and quite inferior to outcome of posterior decompression for myelopathy caused by cervical OPLL.55,56 The choice of treatment for thoracic OPLL depends on the spinal level of the ossification, coexistence of OLF, and degree of thoracic kyphosis. The relative importance of these factors is controversial among surgeons.
The surgical method most frequently used for thoracic OPLL at the upper thoracic level is cervicothoracic laminoplastic decompression because the spinal curvature is lordotic or slightly kyphotic at the cervicothoracic junction.56,57 For patients with OLF and OPLL at the middle and lower thoracic level, the common choices of treatment are anterior decompression via a posterior approach, wide laminectomy with posterior instrumentation, lateral rachiotomy, and combined anterior and posterior decompression (Fig. 42–14).55,56,58–62 Postoperative paraplegia is still sometimes associated with each of these procedures, owing to technical difficulties and the vulnerability of the thoracic spinal cord. A retrospective multi-institutional study revealed that neurologic deterioration immediately after surgery was recognized in 11.7%.56
Among the choices of surgical procedure, Yamazaki and colleagues62 reported that none of the patients who underwent posterior decompression and instrumented fusion for thoracic OPLL developed postoperative paralysis. The use of instrumentation is recommended with posterior decompression for thoracic OPLL at the middle and lower thoracic spine because it would enhance and maintain decompression effect with correction of kyphosis or prevention of progression of kyphosis.56,62 Surgical treatment of thoracic OPLL remains one of the most challenging problems for spinal surgeons in Japan.
Key Points
1 Ono K, Ota H, Tada K, et al. Ossified posterior longitudinal ligament: A clinicopathological study. Spine (Phila Pa 1976). 1997;2:126-138.
2 Yonenobu K, Nakamura K, Toyama Y, editors. OPLL: Ossification of the Posterior Longitudinal Ligament, 2nd ed, Tokyo: Springer, 2006.
This is the key textbook that provides overall information about OPLL.
3 Matsunaga S, Sakou T, Taketomi E, et al. Clinical course of patients with ossification of the posterior longitudinal ligament: A minimum 10-year cohort study. J Neurosurg. 2004;100(Spine 3):245-248.
4 Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 1: Clinical results and limitations of laminoplasty. Spine (Phila Pa 1976). 2007;32:647-653.
The authors clarify factors predicting neurologic outcome and limitations of laminoplasty.
5 Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 2: Advantages of anterior decompression and fusion over laminoplasty. Spine (Phila Pa 1976). 2007;32:654-660.
The authors report a retrospective comparative study between the anterior approach and laminoplasty.
1 Key GA. On paraplegia depending on the ligament of the spine. Guy Hosp Rep. 1838;3:17-34.
2 Oppenheimer A. Calcification and ossification of vertebral ligaments (spondylitis ossificans ligamentosa): Roentgen study of pathogenesis and clinical significance. Radiology. 1942;38:160-173.
3 Tsukimoto H. A case report: Autopsy of syndrome of compression of the spinal cord owing to ossification within the cervical spinal canal. Arch Jpn Chir. 1960;29:1003-1007.
4 Onji Y, Akiyama H, Shimomura Y, et al. Posterior paravertebral ossification causing cervical myelopathy: A report of eighteen cases. J Bone Joint Surg Am. 1967;49:1314-1328.
5 Matsunaga S, Sakou T. OPLL: Disease entity, incidence, literature search, and prognosis. In: Yonenobu K, Nakamura K, Toyama Y, editors. OPLL: Ossification of the Posterior Longitudinal Ligament. 2nd ed. Tokyo: Springer; 2006:11-17.
6 Terayama K. Genetic studies on ossification of the posterior longitudinal ligament of the spine. Spine (Phila Pa 1976). 1989;14:1184-1191.
7 Koga H, Sakou T, Taketomi E, et al. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 1998;62:1460-1467.
8 Sakou T, Matsunaga S, Epstein N. Ossification of the posterior longitudinal ligament (OPLL): Epidemiology, pathology, etiology, diagnosis and treatment. In: Ono K, Dvorak J, Dunn E, editors. Cervical Spondylosis and Similar Disorders. Singapore: World Scientific; 1998:701-753.
9 Ono K, Ota H, Tada K, et al. Ossified posterior longitudinal ligament: A clinicopathological study. Spine (Phila Pa 1976). 1977;2:126-138.
10 Ono K, Yonenobu K, Miyamoto S, et al. Pathology of ossification of the posterior longitudinal ligament and ligamentum flavum. Clin Orthop Relat Res. 1999;359:18-26.
11 Resnick D, Guerra JJr, Robinson CA, et al. Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. AJR Am J Roentgenol. 1978;131:1049-1053.
12 Kameyama T, Hashizume Y, Ando T, et al. Spinal cord morphology and pathology in ossification of the posterior longitudinal ligament. Brain. 1995;118:263-278.
13 Hashizume Y, Kaneyama, Mizuno J, et al. Pathology of spinal cord lesions caused by ossification of the posterior longitudinal ligament. In: Yonenobu K, Nakamura K, Toyama Y, editors. OPLL: Ossification of the Posterior Longitudinal Ligament. 2nd ed. Tokyo: Springer; 2006:65-70.
14 Akune T, Ogata N, Seichi A, et al. Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J Bone Joint Surg Am. 2001;83:1537-1544.
15 Shingyouchi Y, Nagahama A, Niida M. Ligamentous ossification of the cervical spine in the late middle-aged Japanese men: Its relation to body mass index and glucose metabolism. Spine (Phila Pa 1976). 1996;21:2474-2478.
16 Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 1984;184:71-84.
17 Matsunaga S, Nakamura K, Seichi A, et al. Radiographic predictors for the development of myelopathy in patients with ossification of the posterior longitudinal ligament: A multicenter cohort study. Spine (Phila Pa 1976). 2008;33:2648-2650.
18 Matsunaga S, Sakou T, Taketomi E, et al. The natural course of myelopathy caused by ossification of the posterior longitudinal ligament in the cervical spine. Clin Orthop Relat Res. 1994;305:158-177.
19 Matsunaga S, Sakou T, Taketomi E, et al. Clinical course of patients with ossification of the posterior longitudinal ligament: A minimum 10-year cohort study. J Neurosurg. 2004;100(Spine 3):245-248.
20 Matsunaga S, Kukita M, Hayashi K, et al. Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;96(Spine 2):168-172.
21 Ono K, Ebara S, Fuji T, et al. Myelopathy hand: New clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69:215-219.
22 Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 1: Clinical results and limitations of laminoplasty. Spine (Phila Pa 1976). 2007;32:647-653.
23 Matsunaga S, Sakou T, Hayashi K, et al. Trauma-induced myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;97(Spine 2):172-175.
24 Koyanagi I, Iwasaki Y, Hida K, et al. Magnetic resonance imaging findings in ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg. 1998;88:247-254.
25 Iwasaki M. Overview of treatment for ossification of the posterior longitudinal ligament and the ligamentum flavum. In: Yonenobu K, Nakamura K, Toyama Y, editors. OPLL: Ossification of the Posterior Longitudinal Ligament. 2nd ed. Tokyo: Springer; 2006:165-167.
26 Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: More than 10 years follow up. J Neurosurg. 2002;96(Spine 2):180-189.
27 Matsuoka T, Yamaura I, Kurosa Y, et al. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2001;26:241-248.
28 Iwasaki M, Yonenobu K. Choice of surgical procedure. In: Yonenobu K, Nakamura K, Toyama Y, editors. OPLL: Ossification of the Posterior Longitudinal Ligament. 2nd ed. Tokyo: Springer; 2006:181-185.
29 Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 2: Advantages of anterior decompression and fusion over laminoplasty. Spine (Phila Pa 1976). 2007;32:654-660.
30 Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: Anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20:7-13.
31 Onari K, Akiyama N, Kondo S, et al. Long-term follow-up results of anterior interbody fusion applied for cervical myelopathy due to ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2001;26:488-493.
32 Tominaga S. The effects of intervertebral fusion in patients with myelopathy due to ossification of the posterior longitudinal ligament of the cervical spine. Int Orthop. 1980;4:183-191.
33 Ohara S, Momma F, Ohyama T, et al. Anterolateral partial vertebrectomy for ossification of the posterior longitudinal ligament of the cervical spine. Spinal Surg (Jpn). 1994;8:125-130.
34 Yamaura I, Kurosa Y, Matsuoka T, et al. Anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Clin Orthop Relat Res. 1999;359:27-34.
35 Yonenobu K, Hosono N, Iwasaki M, et al. Neurological complications of surgery for cervical compression myelopathy. Spine (Phila Pa 1976). 1991;16:1277-1282.
36 Epstein NE. Evaluation and treatment of clinical instability associated with pseudarthrosis after anterior cervical surgery for ossification of the posterior longitudinal ligament. Surg Neurol. 1998;49:246-252.
37 Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990-997.
38 Shinomiya K, Okamoto A, Kamikozuru M, et al. An analysis of failures in primary cervical anterior spinal cord decompression and fusion. J Spinal Disord. 1993;6:277-288.
39 Kato Y, Iwasaki M, Fuji T, et al. Long-term follow-up results of laminectomy for cervical myelopathy due to ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217-223.
40 Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976). 1981;6:354-364.
41 Ratliff JK, Cooper PR. Cervical laminoplasty: A critical review. J Neurosurg. 2003;98(Spine 3):230-238.
42 Baisden J, Voo LM, Cusick JF, et al. Evaluation of cervical laminectomy and laminoplasty: A longitudinal study in the goat model. Spine (Phila Pa 1976). 1999;24:1283-1289.
43 Epstein NE. Circumferential surgery for the management of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1998;11:200-207.
44 Fields MJ, Hoshijima K, Feng AH, et al. A biomechanical, radiologic, and clinical comparison of outcome after multilevel cervical laminectomy or laminoplasty in the rabbit. Spine (Phila Pa 1976). 2000;25:2925-2931.
45 Heller JG, Edwards CC2nd, Murakami H, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: An independent matched cohort analysis. Spine (Phila Pa 1976). 2001;26:1330-1336.
46 Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine (Phila Pa 1976). 1988;13:774-780.
47 Hirabayashi K, Toyama Y, Chiba K. Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res. 1999;359:35-48.
48 Sakaura H, Hosono N, Mukai Y, et al. C5 palsy after decompression surgery for cervical myelopathy: Review of the literature. Spine (Phila Pa 1976). 2003;28:2447-2451.
49 Satomi K, Nishu Y, Kohno T, et al. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 1994;19:507-510.
50 Tani T, Ushida T, Ishida K, et al. Relative safety of anterior microsurgical decompression versus laminoplasty for cervical myelopathy with a massive ossified posterior longitudinal ligament. Spine (Phila Pa 1976). 2002;27:2491-2498.
51 Yonenobu K, Abumi K, Nagata K, et al. Inter- and intra-observer reliability of the Japanese Orthopaedic Association Scoring System for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976). 2001;26:1890-1894.
52 Chiba K, Yamamoto I, Hirabayashi H, et al: Multicenter study to investigate postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine using a new computer-assisted measurement. Presented at 31st Annual Meeting of Cervical Spine Research Society, Scottsdale, AZ, December 2003.
53 Kawaguchi Y, Kanamori M, Ishihara H, et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am. 2001;83:1798-1802.
54 Tomita T, Harada M, Ueyama K, et al. Radiological follow-up evaluation of the progression of ossification of posterior longitudinal ligament: The operative influence on the progression of ossification. Rinsho Seikei (Jpn). 1999;34:167-172.
55 Seichi A, Takeshita K, Nakamura K. Choice of surgical procedures for thoracic ossification of the posterior longitudinal ligament. In: Yonenobu K, Nakamura K, Toyama Y, editors. OPLL: Ossification of the Posterior Longitudinal Ligament. 2nd ed. Tokyo: Springer; 2006:225-230.
56 Matsumoto M, Chiba K, Toyama Y, et al. Surgical results and related factors for ossification of posterior longitudinal ligament of the thoracic spine: A multi-institutional retrospective study. Spine (Phila Pa 1976). 2008;33:1034-1041.
57 Tsuzuki N, Hirabayashi S, Abe R, et al. Staged spinal cord decompression through posterior approach for thoracic myelopathy caused by ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2001;26:1623-1630.
58 Ohtsuka K, Terayama K, Tsuchiya T, et al. A surgical procedure of the anterior decompression of the thoracic spinal cord through the posterior approach. Seikei-Saigaigeka (Jpn). 1983;26:1083-1090.
59 Tomita K, Kawahara N, Baba H, et al. Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine (Phila Pa 1976). 1990;15:1114-1120.
60 Yonenobu K, Ebara S, Fujiwara K, et al. Thoracic myelopathy secondary to ossification of the spinal ligament. J Neurosurg. 1987;66:511-518.
61 Yonenobu K, Korkusuz F, Hosono N, et al. Lateral rachiotomy for thoracic spinal lesions. Spine (Phila Pa 1976). 1990;15:1121-1125.
62 Yamazaki M, Mochizuki M, Ikeda Y, et al. Clinical results of surgery for thoracic myelopathy due to ossification of the posterior longitudinal ligament: Operative indication of posterior decompression with instrumented fusion. Spine (Phila Pa 1976). 2006;31:1452-1460.