Chapter 13 Ossicular Reconstruction
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
HISTORICAL PERSPECTIVE
Since Matte’s1 report of a myringostapediopexy in 1901, numerous methods have been attempted to bridge the gap between the tympanic membrane and the inner ear fluids. The modern era of reconstructive middle ear surgery began with reports by Zollner in 19552 and Wullstein in 1956.3 These early attempts focused on creating a sound pressure differential between the oval and round window by adapting the operation to the ossicular problem encountered. If the incus was missing, the graft was placed on the stapes capitulum (type III or columellar tympanoplasty). If the incus and the stapes crura were missing, the graft was laid on the promontory, leaving a mobile footplate exposed (type IV, oval window, or cavum minor tympanoplasty), producing sound protection fort the round window. These techniques usually altered the volume of the middle ear by creating an open mastoid cavity. In 1957, Hall and Rytzner4 described the use of an autogenous incus or malleus to reconnect the mobilized footplate to the tympanic membrane in patients with otosclerosis. Hearing results with this technique were very promising, and the importance of a closed mastoid cavity with a normal middle ear space was quickly realized. Soon thereafter, the search for the ideal material to reconstruct the sound conduction mechanism was begun. In the late 1950s and early 1960s, much attention was focused on the use of autogenous and alloplastic materials.
The first reported use of an artificial material to reconstruct the ossicular chain was by Wullstein in 1952,3 when he used a vinyl-acryl plastic known as palavit to connect the tympanic membrane to the stapes footplate. In 1958, Shea5 described the use of polyethylene tubing placed on the capitulum of the stapes and wedged under the tympanic membrane. His efforts were soon followed by others using various polyethylene prostheses and other inert materials, such as polytef (Teflon) and silicone elastomer (Silastic). Despite many excellent short-term and long-term hearing results, these early alloplastic materials often resulted in extrusion, significant middle ear reactivity, or, worse, penetration of the inner ear. As a result, many surgeons turned to autogenous prostheses that would be more compatible with the middle ear.
Following the early work of Hall and Rytzner,4 several otologists, including Farrior,6 Sheehy,7 and Guilford,8 began reporting on the success of using autografts for ossicular reconstruction. The most commonly used autograft material was the body of the incus; however, cartilage and cortical bone were also used. These natural materials were well tolerated in the middle ear and provided reliable hearing results. The disadvantages that soon became apparent were the prolonged time required to sculpt the prosthesis and the lack of availability in chronically diseased ears. In an effort to circumvent some of these issues, in 1966, House and colleagues9 first reported the use of homografts in middle ear reconstruction. Other reports soon followed describing the use of irradiated ossicles, cartilage, and even homograft tympanic membranes with en bloc ossicles.10,11 Homografts had hearing results and biocompatibility similar to autografts; however, concerns regarding the risk of transmission of human immunodeficiency virus and prions (i.e., Creutzfeldt-Jakob disease) ultimately led to their decline in use.
In a continued effort to find a safe, reliable, and easily available prosthesis, Shea in 197612 reported on the use of high-density polyethylene sponge (Plastipore) for middle ear reconstruction. Made of porous polyethylene, this alloplast had nonreactive properties and sufficient porosity to allow ingrowth of tissue. It was readily available commercially and could be easily trimmed with a knife. A similar porous polyethylene that is thermal-fused (Polycel) was developed later and allowed the prosthesis to be coupled to other materials, such as stainless steel. This capability allowed it to be modified to various prosthesis designs. Early reports of porous polyethylene implants revealed a high incidence of extrusion when placed in direct contact with the tympanic membrane. This problem was significantly reduced by placing a disc of cartilage between the head of the prosthesis and the tympanic membrane, as advocated by Coyle Shea and reported by Brackmann and Sheehy.13 As a result, Plastipore and Polycel total ossicular replacement prostheses (TORPs) and partial ossicular replacement prostheses (PORPs) continue to be used with good long-term success today.
In an effort to improve extrusion rates associated with porous polyethylene, in 1979 various ceramics were recommended for use in ossicular reconstruction. These alloplastic materials were termed either bioinert or bioactive. Bioinert implants, such as dense aluminum oxide ceramic, did not react with surrounding tissues and were popular in Germany and Japan. Bioactive implants, such as glass ceramic (Ceravital), were biocompatible and reacted with surrounding soft tissue and adjacent bone allowing a coupling between the implant and the ossicle in contact.14 The advantage of ceramic implants was that they could be placed directly under the tympanic membrane without interposing cartilage; however, they were difficult to handle and shape because of their glass nature.
In 1984, Grote15 introduced the use of the calcium phosphate ceramic, hydroxyapatite, for tympanoplasty surgery. Subsequently, great interest developed in this material for middle ear prostheses. Hydroxyapatite, which is the mineral matrix of living bone, was known to be a bioactive material achieving integration with surrounding bone and tissue. In 1985, Wehrs16 developed an incus prosthesis and an incus/stapes prosthesis made of hydroxyapatite and reported successful hearing results with a low extrusion rate 4 years later. Since that time, this material has been adapted to various uses and prosthesis designs. The advantage of this material is that it is quite rigid and has a good sound transfer function.
In an attempt to find a prosthesis that had the rigidity and biocompatibility of hydroxyapatite, but not the mass, titanium prostheses were developed. The specific density of titanium is low, less than 57% that of stainless steel, yet it is extremely rigid. In addition, it is nonmagnetic, has excellent biocompatibility, and lends itself to being manufactured into various shapes and sizes. Most of the titanium prostheses possess an open head, allowing better visualization during placement. First used for ossicular reconstruction in 1993 in Germany,17 the popularity of titanium prostheses has grown rapidly. Cartilage must still be used, however, between the platform and tympanic membrane to prevent extrusion. Several authors to date have published favorable hearing results with titanium prostheses, and compared with hydroxyapatite, titanium may provide improved hearing responses at higher frequencies because of its low mass.18–21
Despite the improvements in middle ear prostheses, there remained a need for an adhesive or bone cement to stabilize the prostheses or, in some cases, replace them altogether. In the 1980s, ionomeric cements were used for cranioplasties and ossicular chain reconstruction. Although effective in the middle ear,22 aluminum toxicity issues associated with cranioplasties resulted in ionomeric cements being largely supplanted by hydroxyapatite phosphate cements, which are free of aluminum. Two of these cements currently are being produced for otologic applications—Hydroset (Stryker) and OtoMimix (Gyrus). The cements seem to be well tolerated, and have been particularly useful in reconstructing the long process of the incus and stabilizing prostheses.
PATIENT SELECTION AND EVALUATION
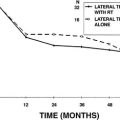
When discussing outcomes with patients, it is important to provide a realistic expectation of hearing results. Successful hearing results in ossicular reconstruction are based on the postoperative air-bone gap and stratified as excellent (<10 dB), good (11 to 20 dB), and fair (21 to 30 dB). This success also depends on several factors including the presence or absence of a mobile stapes superstructure, intact canal wall with normal middle ear volume, and adequate eustachian tube function. Although outcomes vary slightly depending on the type of prosthesis used, successful improvement in hearing is generally broken down according to the use of a PORP versus a TORP. In patients undergoing ossicular reconstruction with a PORP, two thirds of patients should achieve hearing outcomes within 15 dB of their bone scores, whereas two thirds of patients with a TORP should achieve hearing outcomes within 25 dB of their bone scores. The disparity in outcomes between PORPs and TORPs is apparent in Brackmann and Sheehy’s review of 1042 cases in which successful hearing with an air-bone gap less than 15 dB was achieved in 63% of PORPs and only 42% of TORPs.23 Regardless of the technique or type of prosthesis, surgeons should use what they are most comfortable with and provides consistent good hearing results.
SURGICAL CONSIDERATIONS
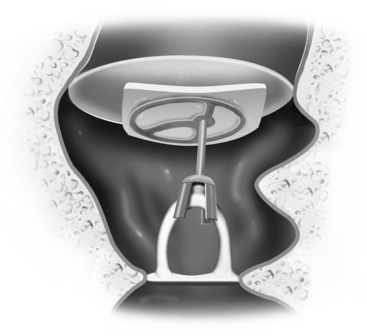
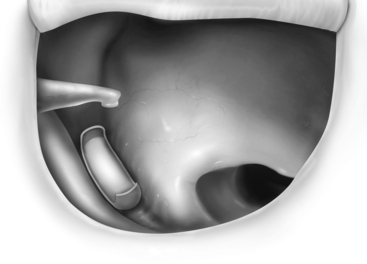
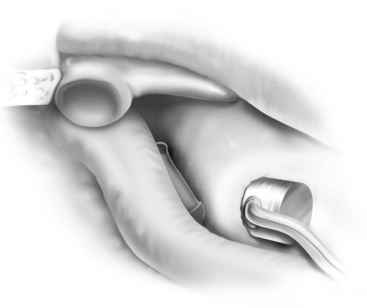
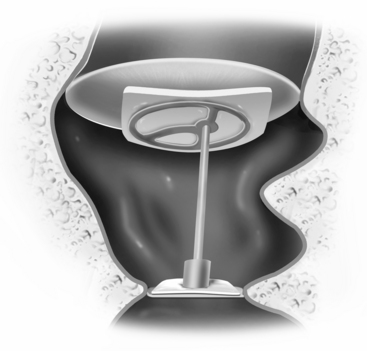
FIGURE 13-1 Partial ossicular replacement prosthesis bridging the gap between the mobile stapes and mobile malleus.
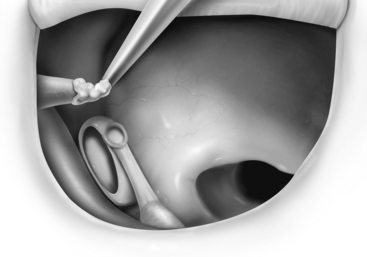
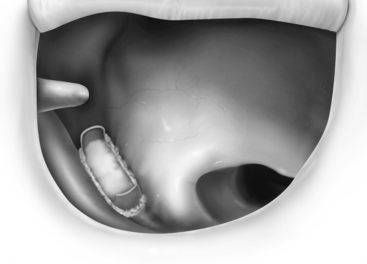
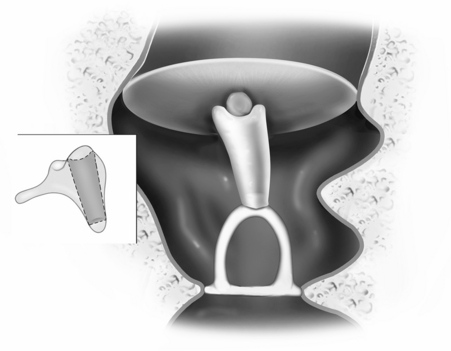
FIGURE 13-2 Reconstruction of the ossicular chain using bone cement to augment the foreshortened incus.
PROSTHESIS SELECTION
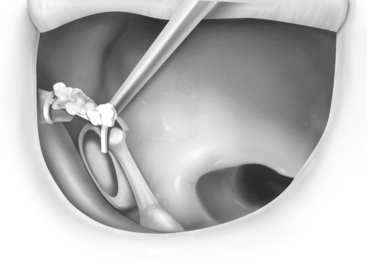
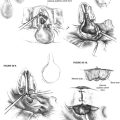
Autografts, which have been used since the earliest reconstruction efforts, continue to be used by many surgeons with good results. These are usually autologous incus grafts that can be shaped intraoperatively with a notch for the malleus handle and a cup for the stapes capitulum when present (Fig. 13-8), or a straight shaft to be positioned on the footplate when the stapes is absent. Studies have shown good long-term viability of autologous ossicular grafts, although some concern remains about eventual reabsorption if blood supply is not maintained.24 The time required to sculpt these grafts and the unavailability in some cases has prompted many surgeons to turn to alloplastic materials.
Hydroxyapatite has become popular because of its excellent biocompatibility and multiple applications in prosthesis design. Hydroxyapatite is a calcium ceramic that chemically attaches to bone and is osteoconductive. With time, the implant gradually becomes covered with an epithelial layer resembling normal middle ear mucosa. Cartilage does not have to be used with hydroxyapatite, although some authors still recommend it. Early prostheses were made entirely of dense hydroxyapatite and noted to be brittle. More recent innovations have involved attaching a hydroxyapatite-reinforced polyethylene composite (HAPEX) shaft or cuff to a dense hydroxyapatite body. This allows the cuff or shaft to be easily trimmed to length with a knife. Various HAPEX prostheses have been produced and usually possess one or two notches in the hydroxyapatite body for placement under the malleus handle (Fig. 13-9).

FIGURE 13-9 Partial and total ossicular replacement prostheses currently available for implantation.
More recently, titanium has been favored as an alloplastic material because of its excellent biocompatibility, rigid strength, and light weight. Titanium prostheses are designed as PORPs or TORPs, and require interposition of cartilage between the prosthesis and tympanic membrane to prevent extrusion. The platform of the prosthesis has an open-top design facilitating placement (see Fig. 13-9). The prostheses come as presized implants of variable length that can be sized and adjusted intraoperatively. Hearing results to date with titanium implants are comparable to hydroxyapatite and polyethylene sponge, and may improve hearing at higher frequencies.17–21
In addition to conventional prostheses, bone cements have been used with success in ossicular reconstruction. These cements are currently hydroxyapatite-based cements that are mixed intraoperatively and harden within 4 to 6 minutes. They are useful in the setting of incus necrosis, when the gap is not too large, to span the incus and stapes. When a significant amount of incus necrosis is found, a titanium incus/bridge prosthesis can be used to set up a framework or carrier for the bone cement to be applied between the incus and stapes (see Fig. 13-3). Care must be taken to place Gelfoam around the oval window before application to decrease the risk of bone cement getting around the footplate and causing fixation. More recent reports of using bone cement in ossicular reconstruction have shown good hearing results (air-bone gap ≤20 dB) in 90% of patients.22,25,26
Occasionally, one may encounter patients with hearing loss not amenable to standard reconstruction of the ossicular chain or who have failed multiple attempts at ossicular reconstruction. More recent attempts at improving hearing in such situations have focused on introducing sound through the round window using a vibratory transducer. The Vibrant Soundbridge (Med-El, Innsbruck, Austria) comprises an implanted receiver module placed behind the ear and is connected via a small cable to a floating mass transducer that is placed in the round window niche. Electric current from the receiver module drives the magnet within the transducer, transmitting vibratory stimulation to the inner ear. Early hearing results with this novel technique are promising.27
SURGICAL TECHNIQUE
Exposure and Assessment
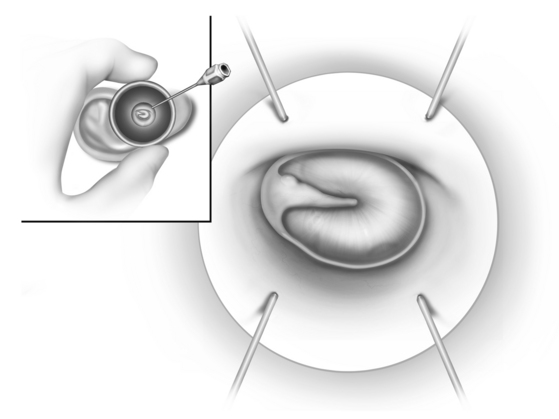
Regardless of the type of anesthesia, if one is performing an ossicular chain reconstruction without a concomitant mastoidectomy, the procedure usually is performed with a transcanal approach. If the canal is quite narrow, an endaural or postauricular approach can be employed. When using a transcanal approach, the ear canal is injected with 1% lidocaine (Xylocaine) and 1:40,000 epinephrine. If the patient is older than 65 years, a 1:60,000 solution is used. Using a 3 mL glass syringe, the solution is injected just inside the meatus, and not at the bony-cartilaginous junction where the skin is more adherent (Fig. 13-10). The ear canal is dilated with specula gradually increasing in size; this forces the local anesthetic solution medially. The vascular strip is injected last (Fig. 13-11); this is probably the most important injection.
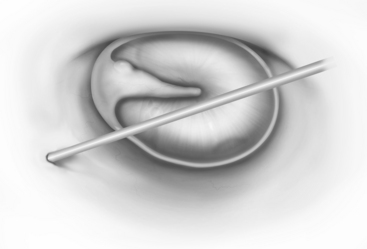
Vertical incisions are made at 6 o’clock and 12 o’clock positions, beginning 1 mm lateral to the annulus, and connected by a horizontal incision 10 to 12 mm lateral to the annulus (Fig. 13-12). Although adequate surgical exposure is important, less exposure may facilitate obtaining the proper tension on the prosthesis because the tympanic membrane is stabilized at the bony annulus.
When adequate exposure is achieved, the status of the middle ear is assessed. In performing a second look procedure, it is essential to check carefully for any residual or recurrent cholesteatoma before considering ossicular chain reconstruction. In addition, the presence of serous fluid may necessitate concomitant placement of a ventilation tube, whereas a dehiscent facial nerve obscuring the oval window may preclude ossicular chain reconstruction using conventional techniques. Vibroplasty techniques may be useful in such cases and in cases with mixed hearing loss, allowing the surgeon to address the conductive and the sensorineural components.27 Although the surgeon’s options may be limited in most situations to a PORP or a TORP, with the advent of bone cements the surgeon must consider alternative techniques.
Placement of Prosthesis
Despite perfect alignment of the PORP or TORP intraoperatively, the prosthesis can shift during the healing process. Adequate middle ear packing may minimize this; a small amount of bone cement placed at the junction of the PORP with the capitulum of the stapes gives the best stability. Bone cement cannot be used on the footplate, however. Instead, a cartilage punch (Kurz) is used to create a “cartilage shoe” that stabilizes the shaft of the TORP and minimizes slippage on the flat footplate surface (Fig. 13-13). A small piece of perichondrium placed on the footplate may limit further slippage on the footplate.
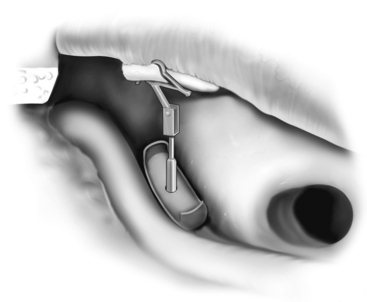
The most challenging ossicular chain reconstruction is when the incus is absent, and the footplate is fixed. One can use a TORP, but great care is needed to ensure that the prosthesis does not sublux into the vestibule. Several prostheses attach to the malleus handle to limit the incursion of the prosthesis into the vestibule. A slit is made in the periosteum of the malleus, and the prosthesis is secured laterally; the shaft of the prosthesis is then rotated into the fenestra created in the footplate (see Fig. 13-6).
If performing a vibroplasty, the floating mass transducer can be placed in various locations. It is most frequently placed on the footplate or on the round window. When placed in the round window niche, the transducer should be reinforced with a piece of cartilage to prevent displacement of the transducer away from the round window during activation (see Fig. 13-7).
1. Matte. Uber Versuche mit Ainheilung des Trommelfells an das Kopfchen des Steigbugels nach operative Behandlung chronischer Mittelohreiter-ungen. Arch Ohren Nasen Kehlkopfheilkd. 1901;53:96.
2. Zollner F. Principles of plastic surgery of the sound conduction apparatus. J Laryngol Otol. 1955;69:637.
3. Wullstein H. Theory and practice of tympanoplasty. Laryngoscope. 1956;66:1076.
4. Hall A., Rytzner C. Stapedectomy and autotransplantation of ossicles. Acta Otolaryngol. 1957;47:318.
5. Shea J.J. Fenestration of the oval window. Ann Otol Rhinol Laryngol. 1958;67:932.
6. Farrior J.B. Ossicular repositioning and ossicular prosthesis in tympanoplasty. Arch Otolaryngol. 1960;71:443-449.
7. Sheehy J.L. Ossicular problems in tympanoplasty. Arch Otolaryngol. 1965;81:115-122.
8. Guilford F. Repositioning of the incus. Laryngoscope. 1965;75:236-242.
9. House W.F., Patterson M.E., Linthicum F.H. Incus homografts in chronic ear surgery. Arch Otolaryngol. 1966;84:148-153.
10. Wehrs R.E. The borrowed incus in tympanoplasty. Arch Otolaryngol. 1967;85:371-379.
11. House W.F., Glasscock M.E., Sheehy J.L. Homograft transplants of the middle ear. Trans Am Acad Ophthalmol Otolaryngol. 1969;873:836-841.
12. Shea J.J. Plastipore total ossicular replacement prosthesis. Laryngoscope. 1976;86:239.
13. Brackmann D.E., Sheehy J.L. Tympanoplasty: TORPs and PORPs. Laryngoscope. 1979;89:108-114.
14. Niparko J.K., Kemink J.L., Graham M.D., et al. Bioactive glass ceramic in ossicular reconstruction: A preliminary report. Laryngoscope. 1985;95:249-258.
15. Grote J.J. Tympanoplasty with calcium phosphate. Arch Otolaryngol. 1984;110:197-199.
16. Wehrs R.E. Incus replacement prosthesis of hydroxyapatite in middle ear reconstruction. Am J Otol. 1989;10:181-182.
17. Dalchow C.V., Grun D., Stupp H.F. Reconstruction of the ossicular chain with titanium implants. Otolaryngol Head Neck Surg. 2001;125:628-630.
18. Krueger W.O., Feghali J.G., Shelton C. Preliminary ossiculoplasty results using the kurz titanium prostheses. Otol Neurotol. 2002;24:836-839.
19. Gardner E.K., Jackson C.G., Kaylie D.M. Results with titanium ossicular reconstruction prostheses. Laryngoscope. 2004;114:65-70.
20. Martin A.D., Harner S.G. Ossicular reconstruction with titanium prosthesis. Laryngoscope. 2004;114:61-64.
21. Zenner H.P., Stegmaier A., Lehner R., et al. Open tubingen titanium prostheses for ossiculoplasty: A prospective clinical trial. Otol Neurotol. 2001;22:582-589.
22. Feghali J., Barrs D.M., Beatty C., et al. Bone cement reconstruction of the ossicular chain: A preliminary report. Laryngoscope. 1998;108:829-836.
23. Brackmann D.E., Sheehy J.L. TORPs and PORPs in tympanoplasty: A review of 1042 operations. Otolaryngol Head Neck Surg. 1984;92:32-37.
24. Merchant S.N., Nadol J.B.Jr. Histopathology of ossicular implants. Otolaryngol Clin North Am. 1994;27:813-833.
25. Babu S., Seidman M. Ossicular reconstruction using bone cement. Otol Neurotol. 2004;25:98-101.
26. Goebel J.A., Jacob A. Use of Mimix hydroxyapatite bone cement for difficult ossicular reconstruction. Otolaryngol Head Neck Surg. 2005;132:127-134.
27. Colletti V., Soli S.D., Carner M., et al. Treatment of mixed hearing losses via implantation of vibratory transducer on the round window. Int J Audiol. 2006;45:600-608.
28. Gardner E.K., Jackson C.G., Kaylie D.M. Results with titanium ossicular reconstruction prostheses. Laryngoscope. 2004;114:65-70.