30 Orthopedic and Spinal Surgery
ANESTHESIA FOR ORTHOPEDIC AND SPINAL SURGERY provides a multitude of challenges. Children often present with concomitant diseases that affect cardiovascular and respiratory function. The ability to maintain a clear airway during anesthesia is not straightforward for some children, such as those with arthrogryposis multiplex congenita.1 Operating times can be protracted. Significant blood loss can occur that requires strategies for blood product management and transfusion reduction (see Chapter 10). Major trauma causing orthopedic injuries invariably involves other organ systems that may adversely interact with or compromise anesthesia management (see Chapter 38). The risks of aspiration of gastric contents into the lungs and the requisite fasting times, after even minor trauma involving an isolated forearm fracture, continue to be debated. Fat embolus is uncommon in children with long-bone fractures but should be considered in any child with hypoxia and altered consciousness in the perioperative period.2 Tumor surgery may be complicated by chemotherapy, altered drug disposition, or bone grafting considerations akin to those for plastic and reconstructive surgery (see Chapter 33) and complex postoperative pain management may be required (e.g., phantom pain, reflex sympathetic dystrophy) (see Chapter 44).
Children with chronic illnesses present repeatedly for surgical or diagnostic procedures. A single bad experience can blight attitudes about anesthesia for a long time. Positioning children on the operating table involves care, especially for those with limb deformities and contractures (Video 30-1)![]() . Padding, pillows, and special frames are required to protect against damage from inadvertent pressure ischemia while achieving the best posture for surgery. Plaster application, particularly around the hip, should allow for bowel and bladder function, avoid skin breakdown due to pressure or friction, and allow access to epidural catheters. Postoperative management of casts on peripheral limbs must account for the possibility of compartment syndromes attributable to restrictive casts or compartment pathology. Major plexus blocks may mask pressure effects under plaster casts or compartment syndrome, but epidural blocks using low-dose amide anesthetics are ineffective against the discomfort of pressure.3,4 Intraoperative temperature regulation may be affected by tourniquet application or disease (e.g., osteogenesis imperfecta, arthrogryposis multiplex congenita). The use of radiology is common during orthopedic surgery, and precautions against excessive radiation exposure should not be neglected by the anesthesiologist.
. Padding, pillows, and special frames are required to protect against damage from inadvertent pressure ischemia while achieving the best posture for surgery. Plaster application, particularly around the hip, should allow for bowel and bladder function, avoid skin breakdown due to pressure or friction, and allow access to epidural catheters. Postoperative management of casts on peripheral limbs must account for the possibility of compartment syndromes attributable to restrictive casts or compartment pathology. Major plexus blocks may mask pressure effects under plaster casts or compartment syndrome, but epidural blocks using low-dose amide anesthetics are ineffective against the discomfort of pressure.3,4 Intraoperative temperature regulation may be affected by tourniquet application or disease (e.g., osteogenesis imperfecta, arthrogryposis multiplex congenita). The use of radiology is common during orthopedic surgery, and precautions against excessive radiation exposure should not be neglected by the anesthesiologist.
Regional anesthesia (see Chapter 41) reduces anesthesia requirements intraoperatively and provides analgesia postoperatively. The use of ultrasound techniques to locate neural tissue improves success and reduces local anesthetic doses (see Chapter 42).5,6 This has heralded increasing use of peripheral nerve blockade rather than central blockade for unilateral lower limb surgery. Acetaminophen (paracetamol) and nonsteroidal antiinflammatory drugs (NSAIDs) are the most common analgesics prescribed for moderate pain. Regular administration of acetaminophen and NSAIDs decreases the amount of systemic opioids administered,7 but NSAIDs decrease osteogenic activity and may increase the incidence of nonunion after spinal fusion.8,9 Intravenous acetaminophen improves the early effectiveness of this drug before the child is able to tolerate oral intake, but this formulation is not available in all countries.10 Long-term pain associated with limb-lengthening techniques (e.g., Ilizarov frame) may require oral opioids after hospital discharge.
Scoliosis Surgery
Children presenting for scoliosis surgery represent a spectrum, ranging from uncomplicated adolescents to severely compromised patients with neuromuscular disease, respiratory failure, and cardiac problems. The age range at presentation varies from infancy to young adulthood. Anesthesia techniques for scoliosis surgery vary with individual patient requirements.11,12 Approaches aimed at minimizing blood loss and transfusion requirements have progressed from extremes of hypotension and hemodilution to a more balanced approach involving moderate degrees of both, use of antifibrinolytic agents, predonation programs, and intraoperative cell salvage. The impact of anesthetic agents on complex physiologic signals has become increasingly important as more sophisticated measurements of neural transmission using somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs) have become the standard of care.
Terminology, History, and Surgical Development
Early Hindu literature (3500 to 1800 bc) describes Lord Krishna curing a woman whose back was “deformed in three places.”13 The terms scoliosis (i.e., crooked), kyphosis (i.e., humpbacked), and lordosis (i.e., bent backward) originated with the Greek physician Galen. Scoliosis is a lateral deviation of the normal vertical line of the spine, which is greater than 10 degrees when measured by radiographs. Scoliosis consists of a lateral curvature of the spine with rotation of the vertebrae within the curve. Lordosis refers to an anterior angulation of the spine in the sagittal plane, and kyphosis refers to a posterior angulation of the spine as evaluated on a side view of the spine. Curves may be simple or complex, flexible or rigid, and structural or nonstructural. Primary curves are the earliest to appear and occur most frequently in the thoracic and lumbar regions. Secondary (or compensatory) curves can develop above or below the primary curve and evolve to maintain normal body alignment. Various combinations of curve types have different pathophysiologic consequences.
The magnitude of the scoliosis curve is most commonly measured using the Cobb method.14 Measurement is made from an anteroposterior radiograph and requires accurate identification of the upper and lower end vertebrae involved with the curve. These vertebrae tilt most severely toward the concavity of the curve. The Cobb method of angle measurement is shown in Figure 30-1.
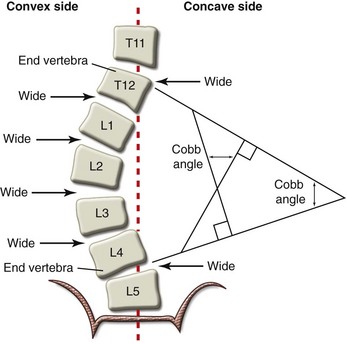
FIGURE 30-1 Diagram of an anteroposterior spinal radiograph shows the Cobb method of scoliosis curve measurement.
Hippocrates (circa 400 bc) developed treatments that relied primarily on manipulation and traction, using an elaborate traction table called a scamnum.15 Nonsurgical treatments for spinal deformities persisted until 1839, when a surgical treatment in the form of a subcutaneous tenotomy and myotomy was described by a French surgeon, Jules Guerin.16
Posterior spinal fusion was first described by Russell Hibbs for tuberculous spinal deformity in 1911.17 The original spinal instrumentation system was the Harrington rod system.18 Modification of this technique that allowed segmental fixation of the rods and early mobilization followed.19 These systems treated the lateral curve but did not allow for correction of the axial rotation. Subsequent developments allowed both corrections by cantilever maneuvers using Cotrel-Dubousset instrumentation.20
Pedicle screws rather than hooks were the next advance. They were initially used as a distal anchor for lumber curves and were found to enhance correction and stabilization, even when used with hooks for the more proximal curves (i.e., hybrid constructs).21 Pedicle screw instrumentation techniques for total curve correction are a recent development that offer better curve correction than hook techniques 22 and the hybrid pedicle screw and hook technique.23
Classification
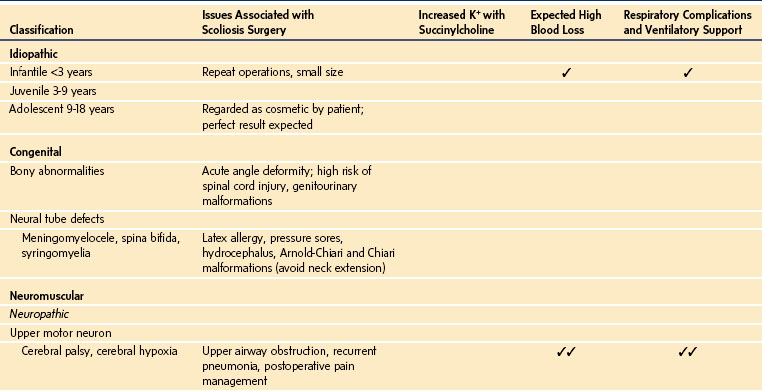
Classification of scoliosis deformities is imperfect because the systems used are clinically rather than etiologically based. Most classifications are surgically based and used for surgical decision making. Curves can be described on the basis of age at onset, associated pathology, and anatomic configurations of the curve, such as single, double, or triple curves; amount of pelvic tilt; curve flexibility; and three-dimensional analysis of the curve.24 A classification that could indicate the risk of an adverse outcome of anesthesia, particularly respiratory failure, would be of clinical benefit. Children younger than 5 years of age with early-onset scoliosis or with independent cardiac or pulmonary disease appear to be at increased risk for respiratory failure, whereas those with idiopathic scoliosis in whom the curve develops at adolescence appear to have minimal risk.25 A classification adapted from that proposed by the Scoliosis Research Society in 1973 remains relevant for anesthesiologists (Table 30-1).26
The Lenke classification system, developed in 2001 for idiopathic scoliosis, provides a means to categorize curves and guide surgical treatment.27 It is increasingly used by the surgeons as an integral part of their decision making.28 Major and structural minor curves included in the instrumentation and fusion are used for the classification, and the nonstructural minor curves are excluded. The system has three components: curve type, a lumbar spine modifier, and a sagittal thoracic modifier. The resulting six curve types have specific radiographic characteristics that differentiate structural and nonstructural curves as proximal thoracic, main thoracic, and thoracolumbar/lumbar regions such that the number, curve type, and main structural curves are related as follows:
Type 1, main thoracic: single; main thoracic structural curve
Type 2, double thoracic: double; proximal, and main thoracic structural curves
Type 3, double major: double; main thoracic (major curve) and thoracolumbar/lumbar structural curves
Type 4, triple major: triple; all three structural curves
Type 5, thoracolumbar/lumbar: single; thoracolumbar/lumbar structural curve
Type 6, thoracolumbar/lumbar main thoracic: double; thoracolumbar/lumbar (major curve) and main thoracic structural curves
In types 1 through 4, the main thoracic curve is the major curve, and in types 5 and 6, the thoracolumbar/lumbar curve is the major curve.
Pathophysiology and Natural History
Vertebral rotation and rib cage deformity usually accompany any lateral curvature. With progression of the curve, the vertebral bodies in the area of the primary curve rotate the convex aspect of the curve and the spinous process to the concave side. This vertebral rotation can be determined by measurement of the position of the pedicles from the midline (i.e., Moe method).29 The vertebral bodies and the discs develop a wedge-shaped appearance, with the apex of the wedge toward the concave side. On the convex side of the curve, the ribs are pushed posteriorly, which narrows the thoracic cavity and causes the characteristic hump. On the concave side, the same rotation forces the ribs laterally, with consequent crowding toward their lateral margins (Fig. 30-2). These changes result in an increasingly restrictive lung defect. Exactly when this becomes a problem depends on the child’s accompanying pathology. The thoracic and lumbar regions are the most common sites of the primary curve. In children in whom the primary curve is in the lumbar region, the rotation of the vertebral bodies and spinous processes should be taken into consideration when spinal or epidural insertion is attempted because the spinal canal is relatively displaced toward the convex aspect of the curve.
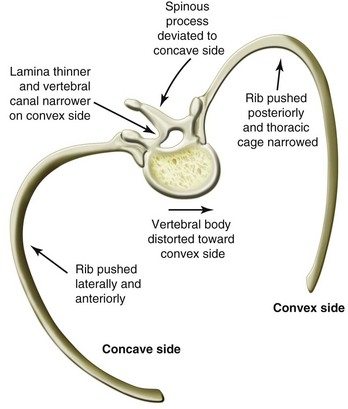
FIGURE 30-2 Characteristic distortion of the vertebra and ribs in thoracic scoliosis.
(Modified from Kleim HA. Scoliosis. Ciba foundation symposium, Vol. 1. Summit, N.J.: Ciba; 1978, p. 609; Gregory GA, editor. Anesthesia for orthopaedic surgery. In: Paediatric anesthesia. 3rd ed. Edinburgh: Churchill Livingstone; 1994.)
The physical distortion in the thorax results in restriction of lung volumes and function. Ventilation depends on the mobility of the thoracic cage, the volume of each hemithorax, and the muscle power and elastic forces required to move the thorax. Children with idiopathic scoliosis with a mild decrease in vital capacity also have reduced forced expired volume at 1 second (FEV1), gas transfer factor, and maximal static expiratory airway pressures (Pemax) (see Fig. 11-4). The predominant deformity of lateral flexion and vertebral rotation results in the lung on the concave side being able to achieve a near-normal end-expiratory position but not end-inspiratory position, whereas the lung on the convex side achieves a normal end-inspiratory position but cannot reach a normal end-expiratory position. The concave side contributes less than normal at total lung capacity, resulting in a decrease in Pemax. Similarly, because the convex side does not reach a normal end-expiratory position, the intercostal muscles and hemidiaphragm will be less efficient, resulting in a reduced maximum static inspiratory airway pressure (Pimax), although this reduction may not be quite so marked.30 The main effect of scoliosis on respiratory function is mechanical, and the anatomic changes in the chest wall cause impaired movement and reduced compliance. Potential long-term respiratory problems when these defects are left untreated include hypoxemia, hypercarbia, recurrent lung infections, and pulmonary hypertension.
Congenital, Infantile, and Juvenile Scoliosis
Congenital spinal anomalies are caused by failures of formation and segmentation that result in scoliosis and kyphosis. The hemivertebra, caused by failure of formation, is the most common anomaly. Fully segmented hemivertebrae contribute to progressive deformity during periods of rapid spinal growth (e.g., first 5 years of life). The most severe deformities are seen in the thoracolumbar spine. Congenital spinal anomalies may be associated with malformations of the ribs, chest wall, and hemifacial microsomia.31 Children with congenital scoliosis have a 25% chance of urologic abnormalities and 10% chance of a cardiac abnormality. Bracing or casting techniques are not effective for this form of scoliosis. Surgical options for these patients include fusion in situ, convex hemiepiphysiodesis, hemivertebra excision, growing rods, and vertical expandable prosthetic titanium rib (VEPTR) treatment.32 Although short-term correction is easily achievable, a short thoracic or even thoracic insufficiency syndrome can result.33 Approximately one half of the children who have extensive thoracic fusions and those whose fusions involve the proximal thoracic spine develop restrictive pulmonary disease (FEV1 <50%).34 Expansion thoracoplasty and stabilization using a VEPTR may be used.35
Infantile and juvenile scoliosis are part of the spectrum of idiopathic scoliosis but are considered here because they manifest and require treatment at an early age. Infantile scoliosis accounts for less than 1% of idiopathic scoliosis and is defined as scoliosis appearing between birth and 3 years of age.32 It usually occurs in the thoracic spine, and the curve is convex to the left. Bracing and serial casting techniques are used for infantile scoliosis. Improvement and resolution in some cases have been achieved at 9-year follow-up.36
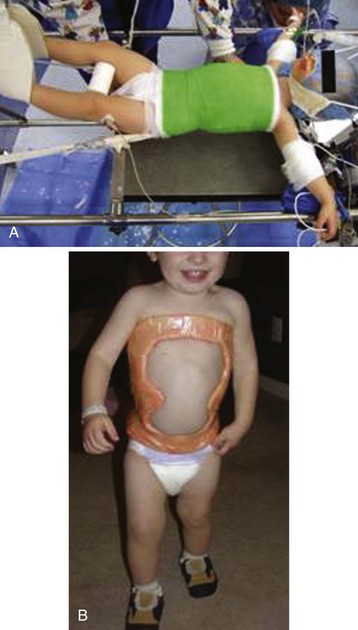
Treatment of infantile scoliosis may begin as early as 4 to 5 months of age or as soon as the diagnosis of scoliosis has been made. Body casting appears useful in selected children, such as those with smaller, flexible spinal curves, but curve progression and the need for secondary treatments affect a significant proportion of these children.37 Bracing is considered when the curve reaches 30 degrees.32 Success has also been reported for more severe curves (60 degrees) when casting was started before 20 months.38 After induction of anesthesia, the child is positioned on the frame (first described by Cottrell and Morel), securing the pelvis to the caudal end of the frame and tethering the head by a chin strap to the rostral end. The spine is mildly distracted, but the main maneuver derotates the spine through the ribs (Fig. 30-3, A). General anesthesia with tracheal intubation is required to facilitate positioning the child, stretching the spine, and molding the body cast. Hemoglobin desaturation frequently occurs when the cast is molded to correct the spinal deformity. An oral airway is also needed to prevent compression of the tracheal tube after the chin strap is applied and tightened. After the cast has hardened, it is cut back and trimmed to maintain the correction to the spine while facilitating breathing, gastrointestinal function, and day-to-day living (see Fig. 30-3, B). Halo traction may be used to stretch and improve the curves. Pin infections occur in almost half of the children.39
Juvenile idiopathic scoliosis comprises 10% to 15% of idiopathic scoliosis and is defined as scoliosis that is first diagnosed between the ages of 4 and 10 years. Approximately 20% of these children and those with infantile scoliosis with a curve greater than 20% have an underlying spinal condition, particularly Arnold-Chiari malformation and syringomyelia.40 Although bracing is used to manage these curves, almost all children in this group with curves greater than 30% require surgical intervention.41
Growing rods may be used for congenital, infantile, or juvenile scoliosis to maintain the correction obtained at initial surgery while allowing spinal growth to continue. Several procedures are required before a definitive fusion.42 All the systems (i.e., growing rods and VEPTR) have a moderate complication rate (i.e., rod breakage and hook displacement).VEPTR systems are being used to correct large-magnitude curves in this group of children when conservative treatment is inadequate.43
Idiopathic Scoliosis
Although adolescent idiopathic scoliosis is relatively common, severe morbidity is seen only in children with early-onset (infantile or juvenile) idiopathic scoliosis.44 Respiratory deterioration alone is seldom the reason for surgery in those who develop scoliosis after the age of 5 years.25 This is explained by the fact that the respiratory alveoli are mature by this age.45,46
Scoliosis evolves during growth spurts. The earlier the age of onset and the more immature the bone growth at the time the process begins, the more severe the outcome. The relentless progression of infantile-onset idiopathic scoliosis with rapidly deteriorating curves and lung function is often not amenable to surgery. Treatment involving spinal instrumentation and anterior epiphysiodesis does not prevent the reappearance of the deformity or the decrease in pulmonary function.47
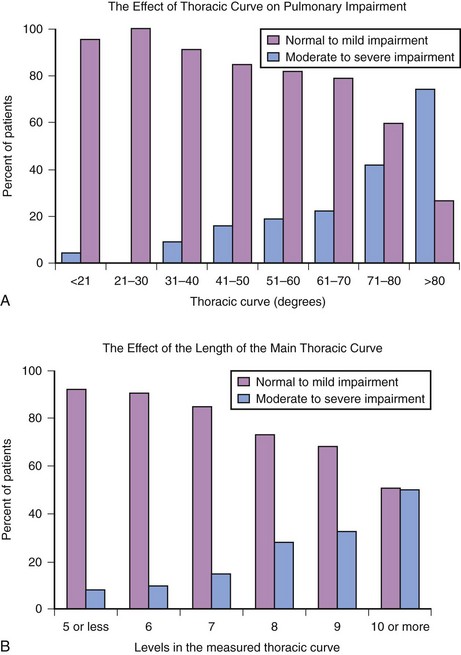
Pulmonary impairment correlates directly with the magnitude of the thoracic curve. Severity of the scoliosis is the most accurate predictor of impaired lung function.48 The morphology of the thoracic curve, the number of vertebrae in the major curve, and the rigidity of the curve also are associated with deteriorating pulmonary function.49 Conventional wisdom has held that there is minimal impact on the vital capacity until the curve exceeds 60 degrees, with clinically relevant decreases in respiratory function occurring only after the thoracic scoliosis has progressed beyond 100 degrees.34 However, it has been shown that children with adolescent idiopathic scoliosis may have pulmonary impairment that is disproportionate to the severity of the scoliosis and that it occurs well before the curve reaches 100 degrees. Forced vital capacity (FVC) may decrease below the normal threshold (<80% of predicted) after the magnitude of the thoracic curve exceeds 70 degrees; FEV1 decreases to less than the normal threshold after the main thoracic curve exceeds 60 degrees.35 Twenty percent of children with a thoracic curve of 50 to 70 degrees have moderate or severe pulmonary impairment (i.e., less than 65% of predicted) (Fig. 30-4, A).50 Those with thoracic hypokyphosis are more likely to have moderate or severe pulmonary impairment; complex curves have a greater prevalence of moderate or severe pulmonary impairment, and the number of vertebrae in the thoracic curve is the most significant predictor of impaired respiratory function (see Fig. 30-4, B).51 These data indicate that children with a structural cephalad thoracic curve, a major thoracic curve spanning eight or more vertebral levels, or thoracic hypokyphosis are at increased risk for moderate to severe pulmonary impairment.
Neuromuscular Scoliosis
Children with neuromuscular scoliosis have the burden of deteriorating muscle function in addition to mechanical distortion. Crowding of the ribs on the concave side of the curve limits chest wall expansion, and the sitting posture restricts diaphragmatic excursion. This inevitably leads to more rapid deterioration in the curve and respiratory function. These children also have the potential for rapid and unpredictable deterioration of the curve.36 It is important to consider the natural history of the specific neuromuscular disease when trying to balance the risks of surgery against conservative management.
Children with Duchenne muscular dystrophy (DMD) suffer from progressive muscular weakness and increasing disability until death occurs, usually by the beginning of the third decade.52 These children tend to become wheelchair bound by 8 to 10 years of age because of increasing motor muscle weakness. Scoliosis then progresses with an acute deterioration during the growth spurt between the ages of 13 and 15 years, such that it becomes difficult or impossible to sit unaided. After the lumbar curve exceeds 35 degrees, further progression becomes inevitable.53 A normal cough requires an inspiratory effort of more than 60% of total lung capacity and effective glottic closure to produce an effective peak flow (more than 160 L/min in adults). Forced expiratory flows are typically reduced proportionally to the decrease in lung volume. As muscle weakness progresses, patients hypoventilate, initially at night. If nocturnal ventilatory support is not provided at this stage, diurnal hypercapnia will result.54
There have been two significant changes in the overall management of children with DMD: the use of steroids and the earlier use of nocturnal noninvasive positive-pressure ventilation (NPPV). Steroid treatment in the early phase of the disease appears to slow disease progression for a few years; treatment with prednisone can stabilize strength and function for 6 months to 2 years.55,56 This may delay the presentation of children for corrective surgery. Earlier adoption of nocturnal NPPV for nocturnal hypoventilation improves survival and quality of life. Clinically unsuspected nocturnal hypoventilation occurs in about 15% of patients with DMD and can be predicted by moderate impairment according to pulmonary function tests (FVC <70% and FEV1 <65% of predicted) and scoliosis. Those with nocturnal hypoventilation have increased gas trapping, decline of muscle strength, and worse perception of health status despite NPPV.57
A 2007 multidisciplinary consensus statement on the “Respiratory and Related Management of Patients with Duchenne Muscular Dystrophy undergoing Anesthesia or Sedation” provided recommendations to standardize the approach to these patients58 and others with flaccid neuromuscular diseases undergoing anesthesia; the most important of these are as follows: An FVC less than 50% of predicted indicates an increase in postoperative respiratory complications, and an FVC less than 30% suggests a further increase in that risk. Respiratory function tests should be part of the preoperative evaluation whenever possible and should include FVC, maximal inspiratory pressure, maximal expiratory pressure, peak cough flow, Spo2 on room air, and Paco2 if the Spo2 value is less than 95%. Consider preoperative training and postoperative use of NPPV if FVC is less than 50% of predicted, and strongly consider NPPV if FVC is less than 30%. Consider preoperative training and postoperative use of manual and mechanically assisted cough in those with impaired cough. In older children, this can be predicted by a peak cough flow less than 270 L/min or maximal expiratory pressure less than 60 cm H2O. Strongly consider planning to extubate the trachea directly to NPPV when the FVC is less than 30%.
Dilated cardiomyopathy occurs in up to 90% of DMD individuals older than 18 years of age. The severity of the physical disability in boys with DMD in their late teens often masks the clinical symptoms of cardiac failure. Cardiomyopathy has been considered responsible for death of up to 20% of individuals with DMD, but this proportion may increase in the future for individuals in whom NPPV prevents respiratory-related mortality.56 (See also Chapter 22.)
Risk Minimization and Improving Outcome from Surgical Intervention
Respiratory Function and Complications in the Early Postoperative Period
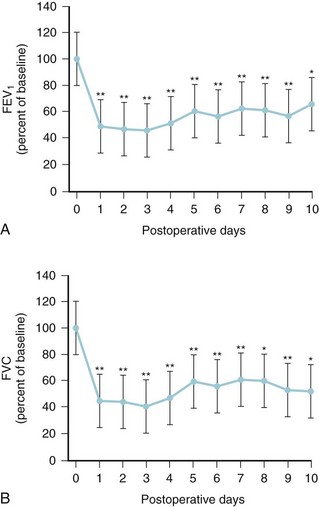
Decreases in lung volumes and flow rates similar to thoracic and upper abdominal surgery occur after scoliosis surgery. The FVC and FEV1 decrease with a nadir at 3 days and are about 60% of preoperative values 7 to 10 days after surgery (Fig. 30-5). It is not until 1 to 2 months after surgery that pulmonary function test results again approach baseline values. The magnitude of this decrease is not affected by the type of surgery performed or whether the scoliosis has an idiopathic or neuromuscular cause.59
Children with neuromuscular disease are more likely to require prolonged mechanical ventilation after spinal surgery because of more severe preoperative respiratory impairment.60 The marked decrease in vital capacity and peak flows is undoubtedly related to the risk of postoperative complications, but determining when it is no longer safe to anesthetize those with a restrictive lung defect remains an imperfect science.
Equipment is available to assist the postoperative management of children with impaired respiratory function. The routine use of NPPV and cough augmentation therapy should be planned if the preoperative FVC is less than 30%. Cough augmentation can be provided manually by hyperinflation and forced expiration, alone or together, and by mechanical insufflation-exsufflation (MIE) therapy.61 The effectiveness of MIE may be limited in children with a weak or enlarged tongue if it blocks exsufflation flow.
Less extensive surgery with the newer pedicles screw systems decreases the need for pelvic fixation to correct pelvic obliquity. The procedures require less extensive surgery and shorter operating times, which may benefit children with impaired respiratory function.62–65
Respiratory complications after surgery for idiopathic scoliosis are relatively uncommon. In contrast, respiratory complications after surgery in children with nonidiopathic scoliosis is reported to be fivefold greater.66 The risk increases as the degree of curvature increases and the respiratory function decreases.67 Anterior spinal procedures are associated with a greater incidence of complications than posterior spinal fusion, such that some consider this to be the main risk factor for postoperative respiratory complications.67 Modern pedicle screw systems may decrease the need for anterior procedures, thereby decreasing the complication rate.68
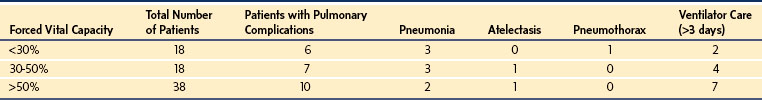
Atelectasis, infiltrates, hemothoraces, pneumothoraces, pleural effusions, and prolonged intubation have the greatest incidence, whereas pneumonia, pulmonary edema, and upper airway obstruction occur less frequently. These problems are more common when the scoliosis is associated with mental retardation and developmental delay. The greatest complication rate occurs for those with cerebral palsy and flaccid neuromuscular scoliosis.66–69 Although respiratory complications increase as the severity of scoliosis and the degree of respiratory impairment increase, reported complication rates vary considerably. Studies of children with neuromuscular scoliosis suggest an overall respiratory complication rate of 15% to 30%69–73 and minimal mortality. In one of these studies, which identified three groups by respiratory impairment (FVC <30%, FVC = 30% to 50%, FVC >50%), an overall complication rate of 31% occurred independent of the degree of respiratory impairment,69 perhaps reflecting improvement with modern management techniques (Table 30-2).
Children with cerebral palsy have additional problems associated with their lack of muscular control (e.g., swallowing incoordination, excessive salivation, gastroesophageal reflux) and sometimes have developmental delay that contribute to a postoperative complication rate of 30%.70,71,74 Nonambulatory patients and those with curves greater than 60 degrees are at increased risk for major complications, with nonambulatory patients almost four times more likely to have a major complication.74 Gastrointestinal dysmotility in cerebral palsy patients can be exacerbated after scoliosis surgery and cause persistent vomiting and bloating.75 Pancreatitis may occur in up to 30% of cerebral palsy patients after surgery, with a greater incidence among those with documented gastroesophageal reflux and reactive airway disease.76
Long-Term Changes
Idiopathic Scoliosis
Improvements in pulmonary function are not impressive after correction of idiopathic scoliosis. Early studies suggested that spinal fusion stabilized the respiratory dysfunction that existed preoperatively, but failed to offer any improvement.77 Improvements may be possible in certain subgroups of patients with some surgical techniques, but it takes months to years for pulmonary function to improve. Children with a preoperative curve less than 90 degrees undergoing a posterior procedure only had a greater than 10% increase in vital capacity, maximum voluntary ventilation, and maximum respiratory mid-flow rate after 2 years; this improvement did not occur in those who underwent anterior surgery.78 Harrington rod instrumentation in children with idiopathic scoliosis resulted in only a small improvement in vital capacity.79
The newer instrumentation systems, such as the Cotrel-Dubousset instrumentation that allows segmental realignment and approximation, result in further improvements in pulmonary volumes.80 Pulmonary function returns to preoperative values within 3 months after the posterior approach using the newer instrumentation systems, with additional improvements occurring and being sustained for 2 years.81 Pedicle screws provide greater curve correction in adolescent idiopathic scoliosis, with a trend toward improved pulmonary function after 2 years compared with other instrumentation techniques.23 A 10-year follow-up analysis demonstrated an absolute increase in the FVC (3.25 versus 3.66 L) and FEV1 (2.77 versus 3.10 L) but no changes in percent of predicted values in children who underwent a posterior fusion and instrumentation only. In the same analysis, those with chest wall disruption experienced no change in FVC and FEV1 over 10 years, but the assessment demonstrated a significant decrease in percent of predicted FVC (85% versus 79%,) and FEV1 values (80% versus 76%).82
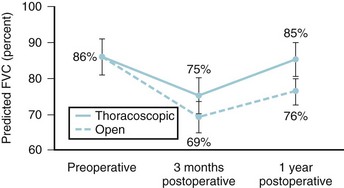
Chest cage disruption (i.e., thoracoplasty or anterior thoracotomy) is associated with reduced pulmonary function at 3 months and a 10% to 20% decrease in total lung capacity and FVC. These values do not return to baseline until 1 to 2 years after surgery. Improvements in lung function with this approach are seldom seen.44,45 Video-assisted thoracoscopic surgery (VATS) for anterior release and instrumentation appears to result in less pulmonary morbidity and a smaller decrease in pulmonary function at 3 months. One year after surgery, values for children treated thoracoscopically had returned to baseline, but this did not occur for those undergoing open thoracotomy (Fig. 30-6).83,84 Two- and 5-year follow-up evaluations of those undergoing VATS showed no significant changes with regard to the correction of the major Cobb angle (56% ± 11% and 52% ± 14%, respectively) or average total lung capacity as a percent of the predicted value (95% ± 14% and 91% ± 10%) over the study period.85
Changing surgical techniques may challenge these findings in the future because some surgeons think that anterior fusions with modern systems offer short-term benefits of reduced blood loss and transfusion and long-term benefits due to shorter fusions, better maintenance of thoracic kyphosis, and improved spontaneous lumbar curve correction. A 2-year postoperative study concluded that VATS for thoracic curves and open procedures for thoracolumbar curves resulted in minimal to no permanent pulmonary impairment 6 months after the procedure compared with posterior spinal fusion, despite a short-term decrease observed after VATS.86
Neuromuscular Scoliosis
Improvements in the scoliosis angle and the degree of pelvic obliquity are achieved after spinal instrumentation in children with neuromuscular disease. Significant improvement can usually be shown in the ability to sit unaided, particularly if children are unable to do so beforehand.87–91 Some investigators have reported an increase in the quality of life perceived by the child or caregiver.89,91
There is little evidence for any improvement in respiratory function in this group of children. There may be a period of delay or even stabilization of the inevitable deterioration of respiratory function.52,88,92 Other investigators have shown no difference in respiratory function after 5 years compared with patients managed conservatively90,93 or an early loss in vital capacity after surgery with a progressive decrease of 25% over 4 years, with 66% of patients requiring mechanical respiratory assistance by that time.89 A Cochrane review was unable to find any data evaluating the effectiveness of scoliosis surgery in patients with DMD and even suggested, “Patients should also be informed about the uncertainty of benefits on long-term survival and respiratory function after scoliosis surgery.”94
Some retrospective analyses, however, deserve consideration. A review of the long-term survival of children with DMD after spinal surgery and nocturnal ventilation demonstrated that those having spinal surgery and ventilation had a median survival of 30 years, whereas those receiving nocturnal ventilation only survived to 22.2 years. This result occurred despite a decrease in mean vital capacity from 1.4 to 1.13 L in the first postoperative year.95 Posterior spinal fusion for scoliosis in DMD was associated with a significant slowing in the rate of decrease in respiratory function; the rate of 4% per year before surgery decreased to 1.75% per year (over 8 years) after surgery.96 In a study of 14 children with DMD and an FVC of less than 30%, the mean rate of decrease in percent of FVC after surgery was 3.6% per year. Most children and parents thought scoliosis surgery improved their function, sitting balance, and quality of life and gave it high satisfaction scores.97
Less outcome information is available for children with cerebral palsy. Surgery is perceived as having a positive impact on patients’ quality of life, overall function, and ease of care by parents and other caregivers,98 despite the high complication rates described earlier. A 3-year follow-up after a pedicle screw construct for scoliosis, which reduced the mean Cobb angle to 31%, demonstrated improved functional ability in 42% of children. Most children had improved sitting balance and nursing care requirements. A 32% complication rate occurred; most were pulmonary in origin but ultimately reversible. There were two perioperative deaths and one transient neurologic deficit due to screw impingement among 56 patients.68
Less morbidity has been claimed for the same-day (one-stage) surgery compared with the two-staged approach in children with neuromuscular disease requiring anterior and posterior spinal surgery.99,100 However, it seems reasonable to avoid anterior thoracotomy in neuromuscular patients in view of the poor respiratory function after chest cage disruption.81,83 Currently, pedicle screw systems in children with neuromuscular scoliosis produce outcomes similar to those of earlier systems but with quicker operating times and less blood loss.63
Spinal Cord Injury during Surgery
Etiology
Spinal cord injury can occur by four main mechanisms: direct contusion of the cord during surgical exposure; contusion by hooks, wires, or pedicle screws; distraction by rods or halo traction; and reduction in spinal cord blood flow.101 Epidural hematoma should be included in the differential diagnosis of deficits occurring postoperatively. The areas of the spinal cord most vulnerable to ischemic injury are the motor pathways, which are supplied by a single anterior spinal artery. This is fed in a segmental manner by the radicular arteries that arise from the vertebral, cervical, intercostals, lumbar, and iliolumbar arteries. The largest radicular artery is the artery of Adamkiewicz, which arises between T8 and L4. A watershed area between T4 and T9 is prone to ischemia because the blood supply in this region of the cord is poorest.102 Paraplegia is the most feared neurologic complication, but partial spinal cord injury resulting in areas of localized weakness and numbness as well as bladder and bowel disturbances also have been reported.
The increasing use of pedicle screws in spinal surgery raises the possibility of increased risk to individual nerve roots. A systematic review of pedicle screw complications that involved a total of 4570 pedicle screws in 1666 patients reported an overall 4% malposition rate that increased to 16% in studies that systematically examined their patients postoperatively.103 Eleven patients required revision surgery for the malpositioned screws, and there was one temporary neurologic complication (i.e., epidural hematoma). No vascular injuries were reported, although six cases of aortic abutment were described.
Risk of Spinal Cord Injury and Spinal Cord Monitoring
Combined surveys undertaken by the Scoliosis Research Society and the European Society for Deformities of the Spine investigating idiopathic scoliosis suggested an incidence of neurologic impairment of 0.72% in 1975.104 At the end of the last millennium, that incidence had decreased to less than half (0.3%), and all were partial cord lesions.101 Patients with curves greater than 100 degrees, congenital scoliosis, kyphosis, and postirradiation deformity appear to be at greatest risk for complications. The use of pedicle screws may have increased the immediate neurologic complication rate. Nine neural complications were reported among 1301 patients, for an incidence of 0.69% in 2007. Three thecal penetrations occurred, two as a result of pedicle screws, all without sequelae. There were two nerve root injuries and four spinal cord injuries, all of which resolved within 3 months.105
A retrospective review of 19,360 cases of pediatric scoliosis showed significantly different overall complication rates among idiopathic (6.3%), congenital (10.6%), and neuromuscular (17.9%) scoliosis. Neurologic deficits had a different distribution, with the greatest rate among congenital cases (2%), and lower rates with neuromuscular (1.1%) and idiopathic scoliosis (0.8%).106 Mortality rates of 0.3% were observed for neuromuscular and congenital scoliosis, with an idiopathic scoliosis rate of 0.02%. Rates of new neurologic deficits were greater with anterior screw–only constructs (2%) or wire constructs (1.7%) than with pedicle screw constructs (0.7%).
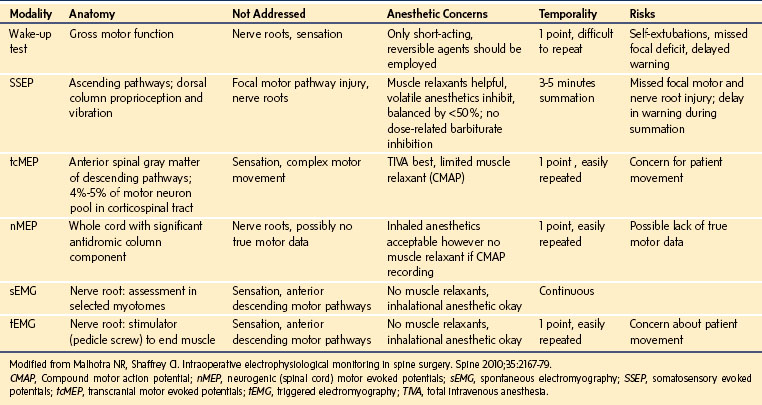
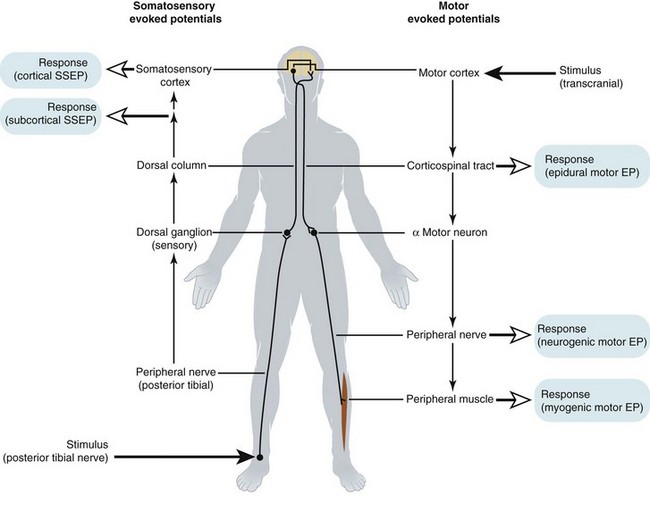
Spinal cord function is monitored to ensure that the complication rate is as small as possible. The Scoliosis Research Society issued a position statement concluding that neurophysiologic monitoring can assist in the early detection of complications and can possibly prevent postoperative morbidity in patients undergoing operations on the spine. For any monitoring technique to be effective, it needs to have a sensitivity and specificity that allows true changes to be recognized with a very low occurrence of false-negative and false-positive results. The test or technique must also produce its results in a time frame that allows the problem to be reversed or prevented. Recognition of the limitation of individual techniques has seen the development of increasingly sophisticated monitoring systems to identify and minimize this risk. Older tests, such as the wake-up test and ankle clonus test, have largely been superceded by monitoring of SSEPs, MEPs, and triggered electromyographic techniques (EMGs). The importance of using a multimodal approach is increasingly recognized and is well addressed in the literature.107–110 The capabilities and limitations of the various techniques are summarized in E-Table 30-1.
Methods of Monitoring Spinal Cord Function
Wake-up Test
The wake-up test measures gross motor function of the upper and lower extremities. It has been used widely since it was first described.111 The test consists of decreasing the depth of anesthesia almost to the point of wakefulness and asking the patient to respond to verbal commands. Failure to move the feet and toes while being able to squeeze a hand suggests a problem with the spinal cord. The test requires limiting or reversing muscle relaxation and reducing the depth of anesthesia sufficiently to enable the patient to follow commands. When the test was initially described, 3 of 124 patients had a positive result (i.e., no movement) and were saved from paraplegia.111 A major concern is that the test is conducted after maximal spinal correction, which may occur after any neurologic insult has occurred; however, removal or modification of the spinal instrumentation within 3 hours of the onset of the neurologic deficit has been reported to prevent the risk of permanent neurologic sequelae.112 The wake-up test is unlikely to detect isolated nerve root injury or sensory changes. It is limited to neurologically normal patients with an appropriate developmental age who can follow instructions.
With the clinical application of SSEP and MEP monitoring (Fig. 30-7) well established and in the absence of intraoperative changes, there is no justification to perform the wake-up test.113 Nonetheless, some surgeons still regard the wake-up test to be the gold standard, and it may be used to confirm changes demonstrated by SSEP or MEP monitoring.114 Risks include lack of nerve root and sensory information, accidental extubation, dislodgement of the instrumentation, intraoperative recall with subsequent psychological trauma, air embolism, and cardiac ischemia. If a wake-up test is planned, it is prudent to fill the wound with saline to reduce the risk of an air embolism.
Ankle Clonus Test
The ankle clonus test uses the clonus that occurs just before consciousness is regained during wakening from anesthesia. Rhythmic muscle contractions are thought to result from spinal reflexes returning while the higher neurologic centers remain inhibited by anesthesia, and the oscillations demonstrate an intact spinal cord. Inability to demonstrate clonus suggests spinal cord injury.115 Like the wake-up test, it is a post hoc test rather than real-time monitoring. However, in a review of more than 1000 patients undergoing spinal procedures in which six postoperative neurologic deficits occurred, this test identified all the deficits but produced three false-positive findings, giving a sensitivity of 100% and a specificity of 99.7%. In comparison, the wake-up test produced false-negative results for four of the five patients who developed deficits.115
Somatosensory Evoked Potentials
SSEPs involve stimulating a peripheral nerve and measuring the response to that stimulation using scalp electrodes (i.e., cortical SSEPs).116,117 Alternatively, the response can be measured subcortically near the spinal cord by electrodes placed in the epidural space, interspinous ligament, or spinous processes of the vertebrae.118 An intranasally placed pharyngeal electrode can act as a surrogate for these. The advantage of the subcortical evoked potential is that the responses are more stable, reproducible, and resistant to the effects of anesthesia.
The signal produced with SSEP monitoring travels from the peripheral nerve through the nerve root and up the ipsilateral dorsal column. The impulses then cross over at the level of the brainstem and progress rostrally through the thalamus to the primary sensory cortex. The rationale for using SSEP to monitor motor deficits is based on the fact that the sensory tracts are in proximity to the motor tracts of the spinal cord. Injury to the motor tracts indirectly affects the sensory tracts and causes changes in the SSEP. When spinal cord function is significantly impaired, there is usually an increase in latency and a decrease in amplitude in the SSEP, with eventual loss of signal. A 10% increase in latency of the first cortical peak (P1) or 50% decreases in the peak-to-peak amplitude (P1N1) constitute an indication for intervention.119,120 Although SSEP signals primarily monitor transmission through the sensory dorsal columns, they are effective,120 and SSEP monitoring is associated with a 50% decrease in the incidence of neurologic deficits.
It is unusual for motor tract injury to occur when SSEPs remain unchanged, but false-positive and false-negative results have been reported.120,121 Seventy percent of the postoperative complications were detected by the monitor, but 30% (false negatives) were not detected. Pedicle screw misplacement leading to radiculopathy may not be detected by SSEP monitoring.122 Several case reports of paraparesis also attest to the limitations of SSEP monitoring. These problems are caused by injury occurring outside the monitored domain of SSEP rather than a failure of this modality. These concerns encouraged development of methods to monitor the motor tracts of the spinal cord. SSEP monitoring is possible in patients with cerebral palsy, whereas MEPs may not be.123
Motor Evoked Potentials
The motor pathways can be activated by transcranial stimulation of the motor cortex or by spinal cord stimulation. Transcranial stimulation is achieved using electrical or magnetic stimulation applied to the scalp. Electrical stimulators are most commonly used in spinal surgery and operate by applying high-voltage pulses to the scalp using corkscrew, needle, or surface electrodes. The stimulation pulses can be applied as single stimuli or brief pulse trains with intervals between the pulse trains. Multiple stimuli result in a stronger signal with less variability due to temporal summation of the excitatory postsynaptic potential.124 Epilepsy and proconvulsant medicines are considered relative contraindications to MEP monitoring because of concerns about brain injury from prolonged seizure activity due to the electrical current required for stimulation.125,126 Not surprisingly, MEPs may be difficult to record and interpret in patients with cerebral palsy and should not be attempted if the child has seizures.
MEP monitoring may be a problem in younger children, particularly those younger than 6 or 7 years of age.125,127 Use of a spatial summation technique in addition to temporal summation increased the success rate from 78% to 98% over all ages. Using this technique along with ketamine anesthesia in children younger than 6 years of age, reliable MEPs were documented in 98% (111 of 113) of children older than 6 years of age and in 86% (18 of 21) in children younger than 6 years of age.127 There is also evidence that younger children require a greater stimulating voltage and pulse train frequency for MEP monitoring, probably because of immaturity of the central nervous system, specifically the descending corticospinal tracts.128
Spinal cord stimulation is achieved electrically and can be applied using electrodes placed outside or inside the spinal cord rostral to the area of interest. Single stimuli rather than brief pulse trains typically are used for spinal cord stimulation.129 This approach is not commonly used in scoliosis surgery.
Responses can be recorded anywhere distal to the area of interest. They have included the lower lumbar epidural space (i.e., epidural MEP), peripheral nerve (i.e., neurogenic MEP), and peripheral muscles using compound muscle action potential (CMAP) (see Fig. 30-7).130 Each recording site has its limitations regarding the accuracy of the information displayed and the susceptibility to anesthetic drug interference. Epidural MEPs are the least affected by neuromuscular blocking drugs (NMBDs), but they only monitor conduction in the corticospinal tract and provide no information about the anterior horn gray matter.131 They have a much slower response to acute spinal cord ischemia when compared with myogenic responses (i.e., CMAPs).132 Neurogenic MEPs are also resistant to anesthetic interference but appear not to accurately measure motor conduction. Most of the spinally elicited peripheral nerve responses seen with neurogenic MEPs occur through the dorsal columns in a retrograde fashion and are sensory rather than motor.133 Anterior spinal cord injury has been demonstrated with normal neurogenic MEPs.134 CMAPs after transcranial stimulation are thought to be exclusively generated by motor tract conduction, and unlike epidural MEPs, they include the ischemia-sensitive anterior horn alpha motor neurons.130 These responses are very sensitive to anesthetic agents. The responses obtained with CMAPs after spinal cord stimulation also appear to contain signals that include transmission through the dorsal columns and may represent a mixed response.135
One outstanding problem with MEP monitoring is deciding when and how much change in the signal is significant and indicative of spinal cord ischemia. Some centers use the same criteria they have adopted for SSEP monitoring, whereas others require a greater degree of change, such as a 75% decrease in amplitude.136 An amplitude decrease of 80% at one of six sites using transcranial myogenic MEP monitoring was demonstrated to have a sensitivity of 1.0 and a specificity of 0.91 when used as the sole monitor during spinal surgery.137 A 65% decrease in amplitude identified all postoperative motor deficits (SSEP changes identified only 43%) in children with idiopathic scoliosis.138 An alternative technique of measuring MEP has been described in which a minimum threshold for producing a response is established, and a significant increase in that threshold is used to signal a problem.139 Supportive data for this technique are lacking.
The dorsal columns may be injured without involvement of the motor tract.140 Occasionally, adverse changes in SSEPs occur without changes in MEPs.129,141 Because of these reports, MEP monitoring should be used in addition to SSEP monitoring rather than as a replacement.107–110142
Triggered Electromyographic Techniques
The increasing use of pedicle screws allows greater curve and rotational correction than earlier techniques but has an additional risk of direct nerve root trauma. Triggered EMGs using a monopolar needle or bipolar handheld stimulator have been described, with a threshold stimulation level of more than 8 mA considered to be normal, 5 to 8 mA to be critical, and less than 5 mA to be pathologic, indicating that there was not enough distance between the screws and the neural tissue.142 This technique requires monitoring rectus abdominis or intercostal muscles when used for thoracic curves.143,144
Preoperative Assessment and Postoperative Planning
Respiratory Assessment and Planning FOR Postoperative Ventilatory Support
The preoperative pulmonary assessment should identify patients at increased risk for postoperative respiratory compromise. Patients with idiopathic scoliosis tend to have less decrease in pulmonary function with correspondingly reduced complication rates, so most studies have focused on nonidiopathic patients.66 The rate of postoperative pulmonary complications correlates broadly with the decrease in vital capacity.60,145,146 Vital capacity less than 30% to 35% of predicted values indicates marginal respiratory reserve and a level at which complications and a need for postoperative respiratory support are likely. Many patients with these low vital capacities are unable to cough effectively, rendering them prone to postoperative atelectasis, pneumonia, and respiratory failure.
Studies involving a mixed population with a vital capacity less than 40% (but few with neuromuscular disorders) suggest that although short-term and middle-term pulmonary complications occur, these patients can be successfully managed to achieve discharge to home, although some require prolonged postoperative ventilation.147,148 Modest numbers of patients with a vital capacity of less than 25% of the predicted value are described in these studies and do not appear to have greater complication rates than those with greater vital capacity values. Anterior or combined approaches increase the likelihood of respiratory complications, particularly due to pleural effusion.147,148
Children with neuromuscular scoliosis are likely to need postoperative ventilation that is often prolonged.60,146 These patients may also have abnormalities in the central control of breathing and impaired airway defense mechanisms. Impaired coordination of laryngeal and pharyngeal muscles may result in impaired swallowing and inadequate cough with increased risk of aspiration. Initial work suggested that as the vital capacity decreased to less than 35% of predicted, most patients would need a short period of postoperative ventilation.84 The earlier use of nocturnal NPPV and use of NPPV in the postoperative period may alter our perception of risk by decreasing the impact or severity of postoperative respiratory complications while allowing children with increasingly severe respiratory impairment to be considered for surgery. Scoliosis surgery can be successfully undertaken in patients with a vital capacity of less than 35% of predicted, often with no more than 24 hours of planned ventilation followed by a period of noninvasive ventilation (e.g., bi-level positive airway pressure [BiPAP]).60,69,149,150 In one study (n = 30), the overall complication rate was similar whether the FVC was more than or less than 30%, and the average hospital stay was approximately 3 weeks (see Table 30-2). Tracheostomy was required in two children, and the overall pulmonary complication rate was 30%.150 Similar results are reported by others.69 It seems reasonable to anticipate using noninvasive ventilator support for several days after spine stabilization surgery in children with a vital capacity of less than 25% of predicted values. Children with a mean FVC of 20% of predicted have been successfully managed with a brief period of postoperative ventilation and transitioned to BiPAP within 48 hours.151
Whether a child should be denied surgery requires consideration of individual patient factors. The successful management of children with a vital capacity of 15% to 20% of predicted has been described, although the numbers were small.147–150 Although the risk of an unsuccessful outcome can increase at this level, individual circumstances may justify the risk.
Cardiovascular Assessment
Muscle disorders may affect the myocardium and the skeletal system. Children with DMD develop a cardiomyopathy that may be difficult to evaluate because the child is wheelchair bound. Sinus tachycardia is an early manifestation, and in terms of frequency and severity, cardiac function declines from early adolescence.152 More than 90% of adolescents with DMD have subclinical or clinical cardiac involvement.153 Echocardiography is an essential part of the preoperative evaluation of any wheelchair-bound patient presenting for scoliosis surgery. (See also Chapter 14.)
Postoperative Pain Management
Scoliosis surgery is associated with severe pain that lasts for at least 3 days.154 Effective analgesia minimizes postoperative respiratory complications by allowing deep breathing, chest physiotherapy, early ambulation, and rehabilitation. Postoperative pain may be managed with systemic or epidural analgesics. A multimodal approach is likely to be most effective.
Intraoperative Intrathecal and Intravenous Opioids
Intraoperative intrathecal morphine (2 to 5 µg/kg) has provided potent analgesia during the first 24 hours after spinal fusion in children.155,156 Intrathecal morphine also decreases the amount of remifentanil required intraoperatively, contributing to less pain on cessation of remifentanil.157,158 However, perioperative administration of intravenous morphine, when using remifentanil as part of the anesthesia technique, does not result in any measurable benefit.159
Methadone (0.2 mg/kg) decreases pain scores and opioid requirements for 36 hours in children undergoing surgery, but it seems to have been ignored in modern practice.160 This drug is used in adult spinal surgery, but reports of its use in children are few.161
Nonsteroidal Antiinflammatory Drugs
NSAIDs, but not acetaminophen, impair fracture healing in animal models.162 Cyclooxygenase-2 (COX-2) activity plays an important role in bone healing, and the use of NSAIDs decreases osteogenic activity that may increase the incidence of nonunion after spinal fusion.8,9 The effect on osteogenic activity is dose dependent and reversible.163 Similar effects have not been demonstrated in humans. Nonetheless, based on animal evidence, NSAIDs should be used with caution and in consultation with the surgeon during the first 3 to 5 days after scoliosis surgery.164
Systemic Analgesics
Morphine remains the mainstay of systemic analgesic regimens. Morphine infusions of 20 to 40 µg/kg/hr are required during the first 48 hours after surgery. Achieving a balance of effective analgesia while avoiding sedation can be difficult in children with neurodevelopmental delay. Regular evaluation of these children is important if complications are to be avoided. Patient-controlled analgesia (PCA) is appropriate for children older than 6 to 7 years of age. It can be used with a typical bolus dose of 20 µg/kg and a lockout interval of 5 to 10 minutes. The use of a background morphine infusion may be effective in some patients, although its inclusion is controversial.165,166 Our preference is to use a nighttime background infusion at 5 to 10 µg/kg/hr but to use PCA alone during the day (see Chapter 43). Nurse- and parent-controlled analgesia are effective if the child is too young or unable to use PCA.167 Intrathecal morphine plus PCA appears to offer the optimal combination of effective analgesia and minimal adverse effects in idiopathic scoliosis patients compared with PCA morphine alone or epidural morphine.168
Low-dose ketamine infusion (0.05 to 0.2 mg/kg/hr) has been used as an adjunct to morphine infusions or PCA, although its role is debated.169–174 Ketamine may be initiated intraoperatively (initial infusion of 5 µg/kg/min, decreasing to 2 µg/kg/min at the end of surgery) as part of the anesthetic technique to minimize the hyperalgesia reported after high-dose remifentanil infusions.175,176 The literature continues to be confused with conflicting reports showing no effect177 or a modest decrease in pain scores and morphine consumption.178 Ketamine added to morphine PCA has produced mixed results, with no clear beneficial effect in orthopedic surgery, despite such evidence being apparent for thoracic surgery.179 If added to PCA, the optimal combination of morphine: ketamine is a 1 : 1 ratio.172 Despite scoliosis causing severe pain, it is probably best to reserve the use of ketamine for those with significant preoperative pain or morphine-resistant pain.
Gabapentin and pregabalin provide some benefit with an opioid-sparing effect,180 although postoperative nausea and vomiting benefits are limited.181 Gabapentin (15 mg/kg followed by 5 mg/kg three times daily for 3 days) reduced morphine consumption by about 30% over the study period but without any improvement in morphine’s adverse effects. Improved pain relief was observed only until the morning after surgery.182
Epidural Analgesia
Continuous epidural analgesia using single- and double-catheter techniques may provide effective analgesia after spinal surgery. The single-catheter technique using bupivacaine-fentanyl and sited at T6-7 for patients undergoing a mean 12-level scoliosis surgery resulted in analgesia similar to that of PCA but with more postoperative nausea and vomiting and pruritus. Bowel sounds returned earlier in the epidural group, but liquid intake and hospitalization were similar.183 Similar results were reported with a bupivacaine-morphine combination in patients undergoing 10-level spinal fusions. Full diet and discharge from hospital were achieved one-half day earlier with the epidural technique than with PCA.184 A retrospective review of more than 600 patients treated with an epidural or PCA for analgesia after scoliosis surgery, in which the average number of segments fused was 8.5, confirmed the effectiveness of epidural analgesia.185 In that study, a bupivacaine-hydromorphone epidural combination was used, and although pain management was effective, more complications occurred in the epidural group. Respiratory depression and transient neurologic changes were the most common complications observed. Thirteen percent of patients with an epidural catheter required discontinuation of the epidural, most commonly for inadequate pain relief.185 Effective analgesia and a large incidence of postoperative nausea and vomiting and pruritus have been features of studies that combined bupivacaine and morphine.186,187
Patient-controlled epidural analgesia (PCEA) has been successfully used in children older than 5 years of age for orthopedic surgery and thoracotomies,188 In scoliosis surgery, PCEA with bupivacaine and hydromorphone compared with PCA resulted in a slightly improved pain score but a 37% failure rate.189 PCEA with a single- or double-catheter technique, depending on the number of spinal segments involved, and using a bupivacaine, fentanyl, and clonidine solution has been successful and is associated with a relatively small incidence of complications.190
Improved pain control and bowel function with decreased adverse effects may be possible by using a double-epidural technique using moderate amounts of fentanyl and clonidine with local anaesthetics.191 Double-epidural techniques comprise an upper catheter positioned in the upper to middle thoracic segments and a lower catheter at the upper to middle lumbar level.192,193 This modality resulted in improved pain control compared with morphine infusion and had fewer gastrointestinal adverse effects.
Anesthetic and Intraoperative Management
Positioning and Related Issues
The patient must be positioned so that extreme pressure points are avoided, the limb positions are adjusted to prevent nerve injury, and the abdomen is free to minimize venous congestion. This is usually achieved by the use of the Relton-Hall frame or a variant.194 The frame comprises four well-padded supports arranged into V-shaped pairs, with the upper pads supporting the thoracic cage and the lower pair supporting the anterolateral aspects of the pelvic girdle at the anterior iliac crests. The arms must not be abducted or extended greater than 90 degrees from their natural position. The weight of the arms is evenly distributed across the forearm to avoid pressure on the ulnar nerve at the elbow. The range of motion of the shoulders should be assessed preoperatively for optimal positioning during anesthesia. This can present quite a challenge in children with severe deformities, and creative positioning may be required. In some centers, the nipples are covered with Tegaderm (3M, St. Paul, Minn.) and positioned free of direct pressure. It is also essential that the head is maintained in a neutral position and that pressure is evenly distributed between the forehead and face, avoiding direct pressure on the eyeballs. Care must be taken to avoid any direct pressure on the knees, and the patient’s weight should be distributed throughout the lower limb (Fig. 30-8). Reston self-adhering foam (3M, St. Paul, Minn.) may be used to pad the pelvic brim and knees.
Not all spinal tables and frames affect cardiac function in the same way. There is some evidence that the Jackson spine table or longitudinal bolsters have minimal effects on cardiac function, whereas Wilson, Siemens, and Andrews frames may negatively impact cardiac function.195
Postoperative visual loss is an uncommon, unpredictable, and devastating complication associated with spinal surgery in the prone position. It may occur in up to 0.2% of cases, and although most of the reports involve adult patients, older children are not immune.196,197 The most common cause is ischemic optic neuropathy, but the cause remains obscure. Prolonged operating time (>6 hours) and increased or uncontrolled blood loss are features of most of the reports.198–201 The phenomenon is unrelated to pressure on the globe and usually occurs without evidence of any other ischemia-related complications.201 There is nothing to support controlled hypotension or hemodilution as contributory factors despite occasional expert opinion to the contrary.198,201,202
Temperature Regulation
The long preparation time and exposure of an undraped patient on the spinal frame render them susceptible to hypothermia. Hypothermia is associated with hemodynamic instability and increased blood loss.203 A threefold increase in surgical wound infection occurs with a 2° C decrease in core temperature.204 Efforts should be made to increase the ambient temperature in the operating room while the patient is prepared for surgery. Subsequent hypothermia can be minimized if the room temperature is maintained at 24° C during this period rather than at 18° to 21° C, as is often encountered during surgery.205 After the patient has cooled during preparation and positioning, it may take several hours before the core temperature begins to return toward normal. Even with forced air warming systems, it is often difficult to restore normothermia because only a small amount of the patient’s body is exposed to these devices. It may be possible to position a warming blanket underneath the frame so that warming from below and above occurs (see Fig. 30-8 and Video![]() 30-1).
30-1).
Patient Monitoring
Patient monitoring needs to be tailored to the individual case, but at a minimum, arterial oxygen saturation, end-tidal carbon dioxide (CO2), electrocardiographic (ECG) patterns, core temperature, and urine output should be recorded. In most cases, invasive arterial and central venous pressures are monitored because of large blood losses, fluid shifts, and the risk of cardiovascular instability. Direct pressure by the surgeon during dissection or curve correction may compromise cardiac function or filling. Central venous pressure is an accurate and valid measurement in the prone position, providing the zero is adjusted for the patient’s position on the spinal frame. Patients with a significant kyphotic component are at increased risk for venous air embolism and should be monitored for this possibility. Depth of anesthesia monitoring should be considered, particularly when MEP monitoring limits the concentrations of anesthetic drugs. Care should be taken when positioning the head because pressure on the forehead by the sensor while the patient is in the prone position for many hours may cause erythema, localized swelling, and tissue necrosis. Contact dermatitis from the adhesive has also been reported.206 Mixed venous oxygen tension trends may be helpful for children with myocardial compromise. Transesophageal echocardiography can be useful for determining ventricular filling and function when hemodynamic compromise is identified or suspected preoperatively.
Minimizing Blood Loss and Decreasing Transfusion Requirements
Scoliosis surgery involves exposure of a large wound over a considerable period. Positioning the patient with the abdomen free to avoid venous compression is important to control and minimize blood loss. Increased intraabdominal pressure attributable to positioning can double intraoperative blood loss.207
Posterior spinal fusion procedures tend to lose more blood than anterior procedures. This loss probably corresponds to the greater number of vertebral levels fused with the posterior approach. Blood loss increases as the number of vertebrae included in the fusion increases. The estimated blood loss (EBL) is approximately 750 to 1500 mL in patients with idiopathic scoliosis, or 60 to 150 mL per vertebral segment fused. The blood loss of 1300 to 2200 mL (100 to 190 mL per vertebral segment) is significantly greater in patients with cerebral palsy. Children with DMD experience the largest EBL—2500 to 4000 mL (200 to 280 mL per vertebral level).208
Children with neuromuscular scoliosis demonstrate a prolonged prothrombin time and a decrease in factor VII activity intraoperatively, suggesting that consumption of clotting factors and dilution of clotting factors enhance the blood loss.209 It has been postulated that children with DMD lack dystrophin in all muscle types and that the poor vascular smooth muscle vasoconstrictor response may be a factor in the increased blood loss.210 Hypothermia exacerbates blood loss by decreasing platelet function, decreasing coagulation factor activity, and slowing vasoconstriction.203
Adverse reactions to blood appear to be more common in children than adults, with human error as the most common cause.211 Several techniques have been used to decrease blood loss and minimize exposure to blood products.
Hypotensive Techniques
Controlled hypotension has been used to minimize blood loss during scoliosis surgery since it was first described more than 30 years ago. A greater than 50% decrease in blood loss with a decreased need for blood replacement and a reduced operating time was demonstrated in early studies. Ganglion-blocking agents (i.e., pentolinium and trimethaphan) have been superseded by β-blockers, direct arterial vasodilators, calcium channel blockers, and α2-agonists. It remains uncertain whether reduced blood loss results from lowered blood pressure212 or lower cardiac output.213 A target mean arterial pressure (MAP) of 50 to 65 mm Hg has been recommended. Although this appears to be safe, concerns that the margin of safety for cerebral and spinal cord ischemia is reduced by controlled hypotension apply to operations of prolonged duration, and there is the potential for periods of hypovolemic hypotension in addition to drug-induced (controlled) hypotension.214,215 The incidence of these feared complications is fortunately very small with or without hypotension. Renal function appears well preserved even when hypotensive anesthesia is used during scoliosis surgery.216,217 Because of these concerns and the concomitant use of hemodilution, less extreme degrees of hypotension are usually employed. Robust data supporting the beneficial effects of controlled hypotension in scoliosis surgery is minimal, although clear benefits have been demonstrated for orthognathic and orthopedic surgery.218,219
In modern practice, moderate hypotension with good control of the heart rate can often be achieved without the use of specific vasoactive drugs by using a remifentanil infusion titrated to the desired blood pressure.220 Although not considered a hypotensive agent, intrathecal morphine decreases blood loss and may facilitate blood pressure control, particularly with a remifentanil infusion. At an analgesic dose of 5 µg/kg, a decrease in EBL from 41 to 14 mL/kg occurred.156 Using this technique, blood pressure control can frequently be achieved without any additional agents. We have found that using an anesthetic combination of less than 1 minimal alveolar concentration (MAC) of inhalational agent plus remifentanil with clonidine (2 µg/kg)221 provides controlled hypotension in most patients without the need for additional agents. Dexmedetomidine may be used as part of a technique for controlling blood pressure.222,223
Short-acting calcium channel blockers have been used for controlled hypotension for scoliosis surgery, and although they are effective, experience is limited. Nicardipine is associated with less blood loss at the same MAP compared with sodium nitroprusside, although a slower return to baseline blood pressure (27 versus 7 minutes) is seen.224,225 Clevidipine has been used without evidence of clear benefit, but it may be associated with an increase in heart rate.226 If a hypotensive technique is to be used, invasive arterial monitoring is essential, and central venous pressure catheters are useful for the safe conduct of anesthesia (see Chapter 10).
Hemodilution
Decreasing the hemoglobin concentration by removing red cells and replacing the volume with a combination of crystalloid and colloid means that for a given volume loss, there is less red cell loss (see Chapter 10). The decreased metabolic rate during anesthesia suggests that oxygen delivery can be maintained with a reduced hemoglobin concentration if normovolemia is maintained.
It has been estimated that more than 2 to 3 units of blood must removed during hemodilution to significantly reduce transfusion requirements. Hemodilution modeling in adult patients has suggested that as many as 5 units of blood must be removed before there is a decrease in transfusion requirements.227 Deciding on the degree of hemodilution and establishing a threshold for transfusion may be difficult. In scoliosis surgery, reduction to an initial hematocrit of 30% has been effective for reducing and minimizing transfusion requirements.228 Some posit that only modest benefits are gained from the technique.229 Hypotensive anesthesia, hemodilution, and a cell saver used as part of a “bloodless surgery” program with erythropoietin and supplemental iron resulted in an average EBL of 855 mL (blood returned by the cell saver averaged 341 mL), with an average drop in hemoglobin after surgery of 3.1 g/dL.230
Tachycardia and hemodynamic instability are common at hemoglobin concentrations less than 7 g/dL. Myocardial ischemia becomes a risk as the hemoglobin concentration decreases below 5 g/dL.231 At this level of anemia, cyanosis cannot develop because 5 g/dL of desaturated hemoglobin are required for cyanosis to be detected. Extreme hemodilution techniques such as these are reserved for patients who oppose blood transfusion. One report detailed patients who had hemodilution during scoliosis surgery that produced a hemoglobin concentration of 3 g/dL in the absence of preexisting cardiac disease.232 Cardiac output increased by more than 30%, with only a modest increase in heart rate and decrease in blood pressure.232 Although no cerebral sequelae were reported, this degree of extreme hemodilution is not recommended.
Autologous Predonation
Preoperative strategies, including predonation of blood and preoperative red cell augmentation, may be used alone or in addition to intraoperative techniques to minimize exposure to blood (see Chapter 10). In idiopathic scoliosis surgery, preautologous blood donation plus intraoperative red cell salvage and controlled hypotension resulted in an average EBL of 1055 mL, avoided transfusion, and the hematocrit only decreased by 10% (35.6 to 32.4) at discharge.233 The advantages of preoperative erythropoietin in addition to preautologous blood donation include a greater preoperative hemoglobin concentration and fewer donated units. Its effect on blood use depends on the total blood loss.234,235 In children with neuromuscular scoliosis, erythropoietin alone did not affect the amount of blood transfused, although the preoperative and discharge hematocrits were greater in treated patients.236
Antifibrinolytic Agents
The use of synthetic antifibrinolytic agents to decrease perioperative blood loss after scoliosis surgery has produced mixed results. To be most effective, an effective plasma concentration of the antifibrinolytics should be established before skin incision. ε-Aminocaproic acid (Amicar) decreases the EBL by 25% during the perioperative period,237 mainly attributable to decreasing the postoperative suction drainage.238 In contrast, an initial dose of tranexamic acid (10 mg/kg) followed by an infusion of 1 mg/kg/hr failed to significantly decrease blood loss in a small sample.239 High-dose tranexamic acid (100 mg/kg loading dose, followed by an infusion of 10 mg/kg/hr) did decrease blood loss by 40% but did not affect transfusion requirements. Post hoc analysis in patients with secondary (neuromuscular) scoliosis showed significant reduction in blood loss and transfusion requirements.240 The correct dose of tranexamic acid remains elusive, but it may be one half of the high dose reported previously.241
A meta-analysis of aprotinin, tranexamic acid, and ε-aminocaproic acid on blood loss and use of blood products in children undergoing scoliosis surgery showed that all antifibrinolytic drugs decreased the amount of blood transfused and that aprotinin, tranexamic acid, and ε-aminocaproic acid were equally effective.242 A similar meta-analysis of major pediatric surgery showed that in the scoliosis studies, aprotinin and tranexamic acid reduced blood loss compared with placebo (385 mL, 95% confidence interval [CI] 42 to 727 mL, versus 682 mL, 95% CI 214 to 1149 mL).243 In all operations, both drugs also decreased red cell transfusion. Demonstration that tranexamic acid is as effective as aprotinin is fortunate, because aprotinin has been withdrawn in many countries after reports of increased morbidity and mortality in adults after cardiac surgery.244 However, after a review of the evidence, aprotinin has been reintroduced in Canada, citing off-label use of the drug as the cause of complications. Studies are being conducted in the United States. Use of ε-aminocaproic acid reduces mean intraoperative blood loss (1125 mL versus 2194 mL), total perioperative blood loss (1805 mL versus 3055 mL), and transfusion requirements (660 mL versus 1548 mL) compared with placebo.245 Reduced blood loss has significant cost savings from decreased operating room time and blood products use.246 Desmopressin is ineffective in decreasing blood loss associated with spinal surgery. Initial beneficial results with desmopressin247 have not been reproduced in patients with idiopathic scoliosis248,249 or in those with neuromuscular scoliosis.250,251
Intraoperative Salvage of Shed Blood
Decisions concerning the use of intraoperative salvage of shed blood (e.g., cell saver) depend on the anticipated blood loss, size of the patient, and use of other methods to minimize blood transfusion, such as predonation and hemodilution (see Chapter 10). It is important to have some idea of the blood loss associated with idiopathic scoliosis in your institution when deciding to use cell saver techniques. For example, the cell saver was found to be beneficial in less than 5% of adolescents with idiopathic scoliosis involved with an autologous predonation program or modest intraoperative hemodilution in one institution.228 At another institution, however, allogeneic transfusion rates were reduced from 55% to 18% when the cell saver was used. The allogeneic transfusion relative risk was 2.04 for patients undergoing surgery lasting more than 6 hours and 5.87 for patients not receiving cell saver blood.252
Pediatric systems are available with small spinning bowls (55 to 75 mL). These systems benefit children with smaller body weights and greater than anticipated blood loss such as patients with neuromuscular scoliosis undergoing extensive spinal fusion.253,254 Among children undergoing anterior instrumentation for thoracolumbar curves, cell saver use decreased the number requiring allogenic blood transfusion from 39.4% to 6.7% and with similar mean postoperative hemoglobin values (10.2 versus 9.6 g/dL).255
Managing Blood Loss
Autologous blood before donation requires an organized schedule of donation with or without the administration of erythropoietin.256,257 This may be the safest and most effective method of avoiding or minimizing the use of allogenic blood products in this group of patients.258 A predonation program was effective in minimizing blood exposure in idiopathic adolescents undergoing surgical correction for their scoliosis. A mean of 3.7 units of blood was donated by each patient before surgery, and 97% of adolescents avoided the use of allogeneic blood during and after surgery.256
The decision about when to administer blood component therapy (i.e., non–red cell blood components) is often based on clinical judgment. Dilutional thrombocytopenia is expected only after several blood volumes have been lost and depends on the preoperative platelet count (see Chapter 10). Platelet concentrations should be measured after loss of one blood volume and at periodic intervals after this. Dilution of coagulation factors may also lead to surgical bleeding when only packed red blood cells are used to replace blood loss. Prolongation of prothrombin time and activated partial thromboplastin time may occur when the blood loss exceeds one blood volume, and they should be checked for at this time. These coagulation tests are not usually associated with increased bleeding until values are greater than 1.5 times mean control values, at which time increased surgical bleeding can be effectively treated with fresh frozen plasma.259 Platelet counts after one blood volume loss, whether associated with normal or abnormal clotting, were within the normal range.259 Blood component therapy should probably be based on abnormal clotting test results, uncontrolled bleeding, or the absence of normal clotting in the surgical field. It is preferable to intervene with blood component therapy before uncontrolled bleeding develops. If pooled blood in a dependent part of the operative field fails to show evidence of clotting, it is time to transfuse with blood components starting with fresh frozen plasma and administering platelets only if this approach is not effective.259
Massive transfusion protocols, in which predefined ratios of red blood cells, plasma factors, and platelets (usually in a 1 : 1 : 1 ratio) are administered early in the resuscitation phase of massive trauma, are being increasingly used in all situations of uncontrolled blood loss.260,261 Evidence that these protocols decrease morbidity and mortality in the trauma setting has resulted in their implementation in surgery, in which significant and uncontrolled bleeding may be expected.262,263 This approach results in proportionally greater administration of factors and platelets than with conventional approaches to severe hemorrhage, but it is associated with increased survival.264
The thromboelastogram is also useful in refining blood product administration if multiple blood volumes are required for resuscitation.262,264 Scoliosis surgery in patients with neuromuscular scoliosis or cerebral palsy, particularly in those with severe complex curves in whom pelvic stabilization and iliac crest grafts are considered, fulfill these criteria. In this group of patients, early administration of blood and factors using a massive transfusion protocol may be beneficial.265
Recombinant factor VIIa may be a useful therapy for patients with a dilutional coagulopathy who are unresponsive to blood component replacement therapy. Successful use with doses as small as 20 µg/kg, has been described in spinal surgery.266–269
Effects of Anesthetics on Somatosensory Evoked and Motor Evoked Potentials
Anesthetic agents act by directly inhibiting synaptic pathways or by indirectly changing the balance of inhibitory and excitatory influences.270,271 The greater the number of synapses and the more complex the neuronal pathway being monitored, the greater the potential impact of anesthetic agents on the evoked potentials. Most anesthetic agents depress the amplitude and increase the latency of SSEPs and MEPs. For this reason, cortical SSEPs are more sensitive than spinal cord or brainstem measured SSEPs. MEPs are susceptible to anesthetic agents at three sites: the motor cortex, the anterior horn cell, and the neuromuscular junction. Consequently, transcranial stimulation with peripheral muscle detection (using CMAPs) is most susceptible to anesthetic interference. Although inhalational anesthetics and most intravenous anesthetics markedly depress SSEPs and MEPs, ketamine and etomidate appear to enhance the amplitudes of both, possibly by attenuating inhibition.271
Inhalational Anesthetics
Inhalational anesthetics cause dose-dependant depression of the SSEP and myogenic MEP. At equipotent concentrations, the MEP is affected to a greater degree than the SSEP. This means that while inhalation agents can be used during SSEP monitoring, they often need to be administered in subanesthetic doses during MEP monitoring. Adequate cortical SSEPs and subcortical SSEPs can be measured with up to 1 MAC of isoflurane, sevoflurane, and desflurane, although some increase in latency and decrease in amplitude may be detected.272,273 It is important to maintain constant end-tidal concentrations throughout anesthesia after baseline measurements have been established. Concentrations of these anesthetics that allow adequate monitoring are significantly less than was possible with halothane.274
Myogenic MEPs (i.e., CMAPs) are recordable only at low concentrations of inhalational anesthetics. The exact concentration depends on the system being used and is greatly influenced by the number of pulses in the stimulus. Single-pulse transcranial stimuli may be inhibited by end-tidal concentrations as small as 0.2 MAC and abolished by end-tidal concentrations as small as 0.5 MAC.275–277 This suppression can be partially overcome by using greater intensity stimuli with multipulse stimulation of up to 6 pulses per stimulus. An increasing number of patients lose recordable myogenic MEPs, even when multipulse stimuli are used, as the concentration of inhalational anesthetic exceeds 0.5 MAC. At end-tidal concentrations in excess of 0.75% isoflurane, monitoring conditions become unacceptable.278–282 Stimulus intensity and pulse train frequency probably are factors in determining successful myogenic MEPs with inhalational anesthetics. Using direct stimulation of the cortex during craniotomy, CMAP was easily recordable at 1 MAC of isoflurane and sevoflurane.283 Similar results have been demonstrated with sevoflurane using transcranial stimulation.284 Information regarding desflurane is limited, and although it causes a dose-dependent depression, myogenic MEPs have been successfully recorded at 0.5 MAC.282,285 Using a multipulse stimulation technique, intraoperative recording of MEPs was equally successful during desflurane or propofol anesthesia.286 In contrast to its effects on SSEPs, halothane depresses myogenic MEPs to a lesser extent than the newer inhalational anesthetics.284
Nitrous Oxide
Nitrous oxide reduces the amplitude of the cortical SSEP, but comparisons with other inhalational anesthetics are limited. Nitrous oxide (0.5 MAC) depresses SSEPs to a greater extent than isoflurane at a similar MAC.287 Similarly, 66% nitrous oxide depressed SSEPs to a greater extent than propofol (6 mg/kg/hr; 100 µg/kg/min).288 Nitrous oxide depresses myogenic MEPs.273 The effect relative to other inhalational anesthetics is difficult to determine. Nitrous oxide appears to affect CMAP amplitude to a lesser extent than isoflurane.289 Multipulse stimulus techniques can partially reverse nitrous oxide–induced depression of amplitude. Compared with a propofol infusion designed to maintain a target concentration of 3 µg/mL, 50% nitrous oxide decreases CMAPs with single or paired stimuli to a lesser extent.290 When 60% nitrous was added to low-dose propofol infusion at a target concentration of 1 µg/mL, adequate CMAPs were obtained using multipulse transcranial stimulation.291 Conversely, the addition of nitrous oxide to a variety of different total intravenous techniques significantly depressed the CMAP such that some were not recordable.292 With the widespread availability of remifentanil and the variable but mostly negative effects of nitrous oxide on SSEP and MEP signals, it seems that nitrous oxide is best avoided when spinal cord monitoring is used.
Propofol
Propofol decreases the amplitude of the cortical SSEP, but adequate signals can be recorded, even in the presence of nitrous oxide, at doses used for anesthesia (6 mg/kg/hr; 100 µg/kg/min).293 Propofol better preserves cortical SSEP amplitude and provides a deeper level of hypnosis as measured by processed electroencephalographic values than combinations of low-dose isoflurane and nitrous oxide or low-dose isoflurane or sevoflurane alone.294–296
Propofol depresses the amplitude of myogenic MEPs. In addition to its cortical effect, it suppresses activation of the alpha motor neuron at the level of the spinal gray matter.297,298 Low-dose propofol infusions have become popular as part of the anesthetic technique used with MEP monitoring due to the rapid improvement of signals when the drug is terminated and because multipulse stimulation techniques can improve the response amplitude.279,299 Propofol, even in combination with nitrous oxide, depresses multipulse transcranial CMAPs less than isoflurane.279 Propofol (5 mg/kg/hr; 83 µg/kg/min) combined with 66% nitrous oxide produced satisfactory CMAP recordings in 75% of patients when a four-pulse stimulation sequence was used. In contrast, no recordings were possible with 1 MAC of isoflurane.280 The infusion rates or target concentrations that allow acceptable myogenic MEP recordings vary considerably and reflect different adjuvants (e.g., opioids, ketamine, nitrous oxide), degrees of neuromuscular blockade, and transcranial pulse rates. Propofol at a target of 4 µg/mL or at an infusion rate of 6 mg/kg/hr (100 µg/kg/min) produces acceptable signals with multipulse stimuli.299–301
α2-Adrenoreceptor Agonists: Clonidine and Dexmedetomidine
The cerebral effects of the α2-agonists appear to act mainly at the locus ceruleus, rather than by the more generalized inhibition of synaptic pathways, as in the case of general anesthetics.302 Clonidine at doses of 2 to 5 µg/kg given intravenously had minimal effects on cortical SSEPs when added to isoflurane.303–305 In view of its lack of effect on SSEPs and its anesthetic-sparing properties with inhalational agents and propofol,305–307 it seems reasonable to consider clonidine at a dose of 2 to 4 µg/kg as part of an anesthetic technique. Dexmedetomidine has similar beneficial properties on SSEPs.308,309
There are no published studies on the effects of clonidine on MEPs, but a few publications have examined dexmedetomidine and MEPs with variable results.310–313 The effects of dexmedetomidine, like other anesthetic agents, produce a dose-dependent depression of MEPs, and the ability to interpret these signals may depend on the depth of anesthesia, which suggests that depth of anesthesia monitoring should be used when recording MEPs to maintain a plane of anesthesia that is adequate to prevent recall but still enable good MEP signals to be recorded.
Opioids
Alfentanil, fentanyl, sufentanil, and remifentanil minimally depress SSEP and MEP signals.314,315 Dose-dependent depression of the CMAP does occur at doses of opioids that far exceed those used in clinical anesthesia.316,317 Comparison of alfentanil, fentanyl, and sufentanil at doses sufficient to suppress noxious stimuli suggested that sufentanil exerted the least effect.316 A similar study that included remifentanil showed that this drug had the least depressive effects, with CMAPs measurable at infusion rates of 0.6 µg/kg/min.317 It is likely that greater doses can be used if clinically indicated.
Ketamine and Etomidate
Ketamine enhances the cortical SSEP amplitude and has a minimal effect on subcortical and peripheral SSEP responses.318 It also produces minimal effects on the myogenic MEP responses as a bolus of 0.5 mg/kg 319 or when used in moderate doses (1 to 4 mg/kg/hr; 17 to 83 µg/kg/min) as a supplement to a nitrous oxide–opioid anesthesia.319,320 Experimental evidence suggests S(+)-ketamine modulates the CMAP by a peripheral mechanism at or distal to the spinal alpha motor neuron.321 Ketamine (4 µg/kg/min) has been successfully used with MEP monitoring during propofol-remifentanil anesthesia for scoliosis correction.127,322
Etomidate, although capable of inducing general anesthesia, behaves more like ketamine in its effect on evoked potentials. It improves the quality of SSEPs and enhances the amplitude of MEPs.323 It produces minimal changes in MEPs compared with barbiturates or propofol.297 Etomidate infusions (10 to 35 µg/kg/min) produce adequate MEP monitoring signals.319,324 Concerns regarding adrenocortical depression with etomidate infusions remain and limit its widespread use.325 Bolus doses of etomidate, however, can transiently depress MEPs.319
Midazolam
Intravenous midazolam (0.2 mg/kg) decreases the SSEP amplitude by 60%.326 This does not occur with subcortical SSEPs, for which a slight increase in latency but no change in amplitude has been demonstrated.327 Although midazolam (0.5 mg/kg) caused marked depression of MEPs in monkeys that persisted during awakening,328 this finding does not hold true in human studies. MEP amplitude was unaffected by a midazolam-ketamine infusion technique compared with propofol-ketamine or propofol-alfentanil techniques.292 Midazolam did not suppress myogenic MEPs, even at doses sufficient to produce anesthesia.317 Effects were similar to those with etomidate.317
Neuromuscular Blockade
Two methods have been used to assess the degree of neuromuscular blockade for MEP monitoring. One is measurement of the amplitude of the CMAP produced by single supramaximal stimulation (T1) before an NMBD is administered. When T1 is maintained between 20% and 50% of the baseline level, reproducible CMAP responses can be obtained with a degree of muscular blockade that allows surgery.324,329 The other technique is adjustment of the neuromuscular blockade based on the train-of-four responses. Comparison of the fourth twitch (T4) with that of first twitch (T1) suggests acceptable CMAP monitoring is possible when two of the four twitches remain.329–331 Neuromuscular blockade should be evaluated in the specific muscle groups that are used for electrophysiologic monitoring because different muscle groups have different sensitivities to the NMBDs. Patients with preoperative neuromuscular dysfunction tend to demonstrate greater effect after partial neuromuscular blockade than those with normal preoperative motor function. It is appropriate to avoid neuromuscular blockade in most of these patients.324
Choosing Anesthetic Drugs and Techniques
Ketamine as the main component of anesthesia may improve MEP monitoring because it better preserves the MEP signals and allows reduced doses of other hypnotic agents to be used, but low-dose ketamine used as an adjunct to a conventional anesthetic does not. If processed electroencephalographic monitoring is used to determine anesthetic depth, the addition of ketamine may confound the reading by increasing it.332,333 This occurs despite a deepening level of hypnosis.332 An NMBD improves the SSEP monitoring and may be used in conjunction with MEP monitoring within the confines described earlier. However, even in patients with idiopathic scoliosis, adequate operating conditions after the initial muscle dissection can be produced in the absence of neuromuscular blockade. In the absence of muscle relaxation, muscle contractions, including those of the masseter muscles, occur during stimulation. In this situation, it is prudent to insert a bite block to prevent obstructing the tracheal tube or to intubate the patient nasally.
Tourniquets
Indications and Design
The tourniquet was used by the Romans to control bleeding during amputation.334 The arterial tourniquet is used during orthopedic procedures to reduce blood loss and provide good operating conditions, for intravenous regional blockade and sympathectomy, and for isolated limb perfusion in the management of localized malignancy.335
The word tourniquet is derived from the French verb tourner, meaning “to turn,” referring to the twisting or screwing action applied to the constricting bandage to tighten it. In 1873, von Esmarch introduced the use of a flat rubber bandage wrapped repeatedly around a limb.334 Although this rubber bandage is still used to render a limb bloodless, the pneumatic tourniquet, introduced by Cushing in 1904, has replaced the rubber bandage to maintain ischemia. Compressed nitrogen or air is used for inflation. The target pressure is preset, and compensatory feedback mechanisms maintain that pressure during inflation. Curved and wider tourniquet cuffs, which are designed to fit conical limbs, are associated with lower arterial occlusion pressures than standard cuffs.336 A soft dressing applied to the limb before tourniquet application helps to prevent wrinkles and blisters that may occur when the skin is pinched.337 Adequate exsanguination can also be achieved by elevation of the arm at 90 degrees or the leg at 45 degrees for 5 minutes.338,339
Physiology
Reperfusion
Systemic effects after deflation of the tourniquet include a shift of blood volume back into the limb with a transient decrease in blood pressure that is exacerbated by a postischemic reactive hyperemia in the limb. CO2 release transiently increases the minute volume. The rapid increase in CO2 is also associated with a transient (8 to 10 minutes) increase in cerebral blood volume that may affect patients with raised intracranial pressure.335
Ischemic Conditioning
Short periods of ischemia followed by reperfusion render muscle more resistant to subsequent ischemia. Ischemic preconditioning improves skeletal muscle force, contractility, and performance and decreases fatigue of skeletal muscle. This preconditioning may enable prolongation of orthopedic and reconstructive procedures.340
Complications
Local Complications
Muscle Damage
Histologic changes in the muscle beneath the tourniquet occur after 2 hours of tourniquet time (at 200 mm Hg [26.7 kPa]), but similar changes can occur in the distal ischemic muscle after 4 hours of tourniquet use. Direct pressure and mechanical deformation contribute to increased severity of muscle damage under the cuff.335 These changes include an increase in the number of inflammatory cells in the perivascular space, focal fiber necrosis, and signs of hyaline degeneration.
The combination of muscle ischemia, edema, and microvascular congestion contributes to posttourniquet syndrome: edema, stiffness, pallor, weakness without paralysis, and subjective numbness of the extremity without objective anesthesia. The common use of postoperative casts may conceal the true incidence of this syndrome. Recovery usually occurs over 7 days.341
Nerve Damage
The cause of nerve injuries after tourniquet use probably is direct compression under the cuff rather than ischemia. Sheer forces that are maximal at the upper and lower edges of the tourniquet cause the most damage. These forces are greater with the Esmarch bandage than with the pneumatic tourniquet. The incidence of nerve injuries is greater in the upper limb (1 case per 11,000 patients) than in the lower limb (1 case per 250,000 patients); the radial nerve is the most vulnerable nerve in the upper extremity, and the sciatic nerve is the most vulnerable in the lower extremity.342
Vascular Damage
Arterial injury is uncommon in children. It is an injury of adults with atheromatous vessels, and the tourniquet should be avoided in patients with absent distal pulses, poor capillary return, a calcified femoropopliteal system, or a history of vascular surgery on the involved limb.343
Skin Safety
Pressure necrosis and friction burns may occur with poorly applied tourniquets, and some form of skin protection should be used routinely.344 Chemical burns may result from antiseptic skin preparations that seep beneath the tourniquet and are then retained and compressed against the skin.
Tourniquet Pain
The tourniquet causes a vague, dull ache that becomes intolerable after approximately 30 minutes.345 This pain is associated with an increase in heart rate and blood pressure that is not ameliorated by general anesthesia and neuraxial blockade.345 The pain is transmitted by unmyelinated C fibers, which are normally inhibited by fast pain impulses transmitted by myelinated A-delta fibers, but in this case, mechanical compression reduces transmission through the larger A-delta fibers.346
Systemic Complications
Temperature Regulation
The combination of decreased heat loss from the ischemic limb and reduced heat transfer from the central to ischemic peripheral compartment increases core body temperature.347,348 Bilateral tourniquets increase the temperature more than use of a unilateral tourniquet.348 Children who require intraoperative tourniquets should not be aggressively warmed during surgery.348 Redistribution of body heat and the efflux of hypothermic venous blood from the ischemic area into the systemic circulation after deflation of the tourniquet decreases the core body temperature, which may switch off thermoregulatory vasodilation and decrease the skin-surface temperature.349
Deep Vein Thrombosis and Emboli
The incidence of emboli after release of the tourniquet in children is unclear. The tourniquet appears to have no influence on deep vein thrombosis, but release of the tourniquet may be associated with an increased risk of embolism in adults. Some clinicians have suggested that heparin be used during total joint arthroplasty in adults to prevent emboli formation,350 although this practice is not routine in children. Some surgeons use such therapy in adolescents.
Sickle Cell Disease
Hypoxia, acidosis, and circulatory stasis contribute to the sickling of sickle cells in susceptible individuals. However, several institutions routinely use tourniquets in children with sickle cell disease while maintaining acid-base status and oxygenation throughout the procedure.351,352 Each case must be assessed individually for the balance between the advantages of a bloodless field and the risks of precipitating sickling crises (see Chapter 9).
Drug Effects
Antibiotics given after the tourniquet is inflated do not produce effective concentrations in the blood and tissue of the ischemic limb. Inflation of the tourniquet should be delayed at least 5 minutes after administration of the antibiotics.353,354 Medications administered before inflation of the tourniquet may be sequestered in the ischemic limb and then re-released into the systemic circulation when the tourniquet is deflated. The antibiotic effect depends on the amount of antibiotic sequestered, the tissue binding, and the concentration-response relationship for the antibiotic, although the impact is minimal for most medications used in anesthesia. Volume of distribution may be reduced if the drug is administered after tourniquet inflation, but the plasma clearance remains unaffected.
Recommended Cuff Pressures
Tourniquets usually should remain inflated for less than 2 hours, and most clinicians suggest a maximal time of 1.5 to 2 hours. Techniques such as hourly release of the tourniquet for 10 minutes, cooling of the affected limb, and alternating dual cuffs may reduce the risk of injury.355 Nerve and muscle injuries that occur beneath the tourniquet cuff are related to the pneumatic pressure. Consequently, the lowest possible pressure that maintains ischemic conditions should be sought. Hypotensive anesthetic techniques have been used in adults to reduce the need for high cuff inflation pressures,356 but there seems to be little need for this in children. Lieberman and colleagues suggested that pediatric occlusion pressures should be measured by Doppler and the tourniquet pressure set at 50 mm Hg above this value.357 Maximum mean pressures recommended for the upper and lower extremities are 173.4 ± 11.6 mm Hg (range, 155 to 190 mm Hg) and 176.7 ± 28.7 mm Hg (range, 140 to 250 mm Hg), respectively.357 Wider cuffs exert less force per unit area and reduce the risk of local sequelae. Recommendations for adults suggest that the cuff should exceed the circumference of the extremity by 7 to 15 cm. This is difficult to achieve in infants, in whom the proximal limb length is proportionally shorter than in adults and the wide cuffs impinge on the surgical field.
Acute Bone and Joint Infections
The mainstay of management for osteomyelitis and septic arthritis are antibiotics and surgical drainage. The incidence of these infections is increasing, particularly in immunocompromised children with human immunodeficiency virus (HIV) infection. Tuberculosis remains a scourge in many developing countries. Mortality rates for hospital-acquired staphylococcal disease in compromised children358 and community-acquired disease in healthy children359,360 range from 8% to 47% for those presenting with severe sepsis.361 Mycobacterium and Staphylococcus species resistant to conventional antibiotics increase morbidity and mortality rates.
Pathophysiology
Staphylococcus aureus is the most common pathogen. Osteomyelitis develops after bacteremia and occurs mostly in prepubertal children. Normal bone is highly resistant to infection, but S. aureus adheres to bone by expressing receptors for components of bone matrix, and the expression of collagen-binding adhesin permits the attachment to cartilage.362 After the microorganisms adhere to bone, they express phenotypic resistance to antimicrobial treatment.362
Clinical Presentation
Most children with staphylococcal disease present with musculoskeletal symptoms and fever, but those with disseminated disease may be critically ill (4% to 10%) with severe sepsis and lung disease.359,360 There is often a history of trauma.359,360 It can be difficult to diagnose extracutaneous foci. One report found that 50% of extracutaneous foci of staphylococcal infection were not detected on hospital admission, and one third of these lesions were observed for the first time at autopsy.358 An absolute polymorphonuclear cell count of greater than 10,000/mm3 or an absolute band-form count of greater than 500/mm3, or both, correlates with the presence of one or more inadequately treated sites of staphylococcal infection.358 Tuberculosis is the great mimic and must always be suspected in endemic areas.
Treatment Options
Antibiotic therapy is the mainstay of treatment. Initial antibiotic choice is dictated by age and by local pathogen and sensitivity profiles. Antibiotic treatment should be extended to cover gram-negative enterococci in neonates and Streptococcus in older children. Haemophilus influenzae remains a pathogen in unvaccinated regions. Surgical decompression of acute osteomyelitis that is responding poorly to antimicrobial therapy may release intramedullary or subperiosteal pus and lead to clinical improvement. Pus within fascial planes also requires release. Venous thrombosis attributable to pus in soft tissue planes around major joints was associated with a high mortality rate in one series.359 Determining and eradicating the primary focus improves the mortality and reduces recurrence rates.363 An aggressive search for foci and surgical drainage of infective foci is required.
Highly active antiretroviral therapy (HAART) has positively altered the mortality rates for HIV-infected children. However, acute bone and joint infections still occur,364 and these drugs can cause significant morbidity due to changes in fat distribution, lipid profiles, glucose concentrations, homeostasis, and bone turnover.365 Infarction may replace infection as the major cause of morbidity and mortality from HIV.365 It is uncertain whether HAART should be continued during acute osteomyelitis. Worsening cell-mediated immune function may occur during tuberculosis treatment if HAART is continued.366 The combination of HIV infection and tuberculosis can be lethal in children, and antituberculosis treatment is continued for 12 to 18 months.
Anesthesia Considerations
Children with disseminated staphylococcal disease may be critically ill with multisystem disease and require fluid volume augmentation, inotrope support, positive-pressure ventilation, extracorporeal renal support, and coagulation factor replacement. Others may appear clinically stable before induction of anesthesia; the assessment of hypovolemia in children is subject to moderate to poor interrater agreement.367 Intravenous access and rehydration are required before beginning anesthesia to avoid a precipitous blood pressure drop immediately after induction. Bacteremic showering during manipulation and drainage of pus causes further decompensation. Excessive bleeding due to altered coagulation status should also be anticipated.
The presence of a septic arthritis in the shoulder or neck may cause cervical ligamentous laxity predisposing to C1-2 subluxation during intubation.368 Pneumatoceles from staphylococcal pneumonia can rupture during positive-pressure ventilation. A spontaneous breathing mode, however, may be difficult to achieve because of laryngospasm, breath holding, increased secretions, and bronchospasm. The use of NMBDs and positive-pressure ventilation in these patients with a low threshold for introducing inotropes to support the cardiovascular system is an easier option. Vigilance is required for acute pneumothorax.
Myocarditis, pericarditis, and pericardial effusions compromise myocardial function. A 12% prevalence of infective endocarditis among children with hospital-acquired Staphylococcus aureus bacteremia has been reported.369 This prevalence of infective endocarditis was frequently associated with congenital heart disease and the necessity for multiple blood cultures.369 The incidence of infective endocarditis among children with community-acquired disease without preexisting cardiac abnormalities was low,359 suggesting that echocardiography could be reserved for children with preexisting cardiac disease, those with suspicious clinical findings, those whose temperature fails to stabilize, or those who have prolonged bacteremia without an obvious source of infection.
Pain Management
Morphine and acetaminophen are the analgesics commonly used for postoperative pain management. The use of tramadol in children is increasing as our understanding of the pharmacokinetics of this medication increases.370,371 The low incidence of respiratory depression and constipation, fewer controls on use, and similar frequency of nausea and vomiting (10% to 40%) compared with opioids make tramadol an attractive alternative.372–374 NSAIDs are relatively contraindicated in the presence of coagulation disorders, altered renal function, and COX-2–mediated osteogenesis.
Common Syndromes
Cerebral Palsy
Clinical Features
Cerebral palsy is an umbrella term that describes a group of nonprogressive, but often changing motor impairment syndromes caused by lesions or anomalies in the brain that occur during the early stages of its development.375 It is the leading cause of motor disability during childhood, with a prevalence of approximately 2 cases per 1000 live births in developed countries.376
Disorders include cognitive impairment, sensory loss (i.e., vision and hearing), seizures, and communication and behavioral disturbances. Systemic disorders resulting from cerebral palsy affect the gastrointestinal, respiratory, urinary tract, and orthopedic systems. Cerebral palsy is divided into three broad categories: spastic (70%), dyskinetic (10%), and ataxic (10%). Children with spastic cerebral palsy commonly present for orthopedic procedures because of contractures at major peripheral joints.377,378 Functional improvement after surgery in children with spastic diplegia and spastic hemiplegia is better than in those suffering spastic quadriplegia.377
Orthopedic Considerations
Orthopedic manipulations form only part of the treatments designed to improve performance or improve the ease of care. Management, including orthopedic surgery, physical and occupational therapy, recreational therapy, orthotics, and assistive devices, improve functional outcomes. Medical modalities such as intramuscular injections of botulinum toxin and intrathecal administration of baclofen by means of an implanted pump may also be of benefit.379 Selective dorsal rhizotomy has been used to control spasticity.380,381
The indications for and timing of surgical interventions vary. Gait analysis increases the age of the patient at the first orthopedic surgical procedure, and botulinum toxin type A treatment delays and reduces the frequency of surgical procedures on the lower extremities.382,383 Bone and soft tissue surgical procedures are designed to lengthen or weaken spastic muscles to give opposing muscles a chance to attain muscle balance.
Anesthetic Considerations
Children with cerebral palsy who present for orthopedic surgery often have previous experience of operating rooms. They should be handled with sensitivity because communication disorders and sensory deficits may mask mildly impaired or normal intellect. They may be accompanied by a parent or caregiver or be premedicated for induction of anesthesia or in the recovery room. If there is a communication problem, the parent or caregiver should be present before and after anesthesia.377,384
Medical conditions (e.g., seizure control, respiratory function, gastroesophageal reflux) should be optimized preoperatively. Contracture deformities, spinal deformities, decubitus ulceration, and skin infection must be considered when positioning the child for anesthesia and surgery. Poor nutritional status affects postoperative wound healing and the risk of infection. Concurrent medications may influence anesthesia; cisapride for gastroesophageal reflux is associated with prolonged QT interval, sodium valproate can cause platelet dysfunction and affect drug metabolism, and anticonvulsant use increases resistance to NMBDs.385 A history of latex allergy should be sought because of exposure to latex allergens from an early age.386
Intravenous access may be difficult. Drooling, a decreased ability to swallow secretions, and gastroesophageal reflux may dissuade some against an inhalational induction, but there is no evidence that rapid-sequence induction is safer. Succinylcholine may be used because it does not cause hyperkalemia in these children, whose muscles have never become denervated. Noncommunicative or nonverbal children with cerebral palsy require less propofol to obtain the same BIS values (i.e., 35 to 45) than do otherwise healthy children.387 The minimum alveolar concentration of halothane is 20% less in children with cerebral palsy, whether they took anticonvulsant drugs or not (MAC of 0.62 and 0.71, respectively).388
Pain and spasm are regular features postoperatively. Epidural analgesia is particularly valuable when major orthopedic procedures are performed. Occasionally, two epidurals at different spinal sites may be required for multilevel surgery. Systemic benzodiazepines, baclofen, dantrolene, and clonidine have been used to reduce muscle spasms. Selective dorsal rhizotomy is associated with severe pain, muscle spasms, and dysesthesia. Epidural and intrathecal forms of morphine have been used to control this pain. Intravenous morphine and midazolam also have been successfully used.389 Oral benzodiazepines may be required to reduce the incidence and severity of muscle spasms but should be used with caution if combined with opioid analgesia.
Pain assessment is difficult in these children, but several scoring systems are available.390,391 (See also Chapter 43.) The opinions of parents and caregivers are extremely valuable in the assessment of pain and discrimination from other factors such as irritability on anesthetic emergence, poor positioning, a full bladder, or nausea.
Spina Bifida
Anesthetic Considerations
The potential for infection of the central nervous system dictates closure of the sac within the first few days of life. Subsequent surgical procedures and urinary catheterizations set the stage for a sensitivity to latex.392 Primary prophylaxis (i.e., avoiding all latex materials and using a latex-free operating room) is recommended for prevention of latex allergy and anaphylaxis.393
Preoperative assessment should include motor and sensory deficits, respiratory and renal function, and functioning of a ventriculoperitoneal shunt. Positioning on the operating table may require additional pillows for support of limbs with contractures. As a result of hypesthesia in the lower extremities, intravenous cannulae can be inserted painlessly. However, venous access is usually poor in the lower extremities because of limited use. The risk of endobronchial intubation is increased because of a short trachea (36% of patients).394 Kyphoscoliosis may distort tracheal anatomy. Renal dysfunction may dictate NMBD choice and avoidance of NSAIDs. Succinylcholine may be used because it does not cause hyperkalemia in these children.395 A reduced hypercapnic ventilation response means that these children should be closely observed in the recovery period.
Osteogenesis Imperfecta
Clinical Features
OI is a genetically determined disorder of connective tissue that is characterized by bone fragility. The disease state encompasses a phenotypically and genotypically heterogeneous group of inherited disorders that result from mutations in the genes that code for type I collagen.396 The disorder manifests in tissues in which the principal matrix protein is type I collagen—mainly bone, dentin, sclerae, and ligaments. Musculoskeletal manifestations vary in severity along a continuum ranging from perinatal lethal forms with crumpled bones to moderate forms with deformity and propensity for fracture to clinically silent forms with subtle osteopenia and no deformity.396
Classification (types I through IV) is based on the timing of fractures or on multiple clinical, genetic, and radiologic features. Type I is the most common (1 case per 30,000 live births), and type I and type IV have autosomal dominant inheritance patterns. These children have the classic triad of blue sclera, multiple fractures, and conductive hearing loss in adolescence. Bowing of the lower limbs, genu valgum, flat feet, and scoliosis develop with age. Type IV is characterized by osteoporosis, leading to bone fragility without many of the other features of type I. Types II and III are more severe forms of OI and have autosomal recessive inheritance patterns. Molecular genetic studies have identified more than 150 mutations of the COL1A1 and COL1A2 genes, which encode for type I procollagen.396
Orthopedic Considerations
The goals of treatment of OI are to maximize function, minimize deformity and disability, maintain comfort, achieve relative independence in activities of daily living, and enhance social integration. Physiotherapy, rehabilitation, and orthopedic surgery are the mainstays of treatment for moderate and severe forms of OI. Medical treatment with the antiresorptive bisphosphonates (e.g., pamidronate) can decrease pain, lower the fracture incidence, and improve mobility. Initial investigations have demonstrated an acceptable safety profile for pamidronate. Long-term follow-up data are lacking and will be necessary for the development of responsible therapeutic guidelines.397 Medical therapies other than bisphosphonates, such as growth hormone and parathyroid hormone, have only minor roles. Gene-based therapy remains in the early stages of preclinical research.398,399
Operative intervention is indicated for recurrent fractures or deformity that impairs function.396 Fractures in mild to moderately severe cases of OI type I are treated using the same methods as for patients without OI. Deformed bones that are fracturing are realigned, frequently followed by providing external or internal support. In selecting various modes of treatment, it is important to consider the natural history of the particular type of OI and to set realistic goals.400–404
Anesthetic Considerations
Abnormal temperature homeostasis may result in intraoperative hyperthermia that may be severe and accompanied by tachycardia and metabolic acidosis. This response is different from that of malignant hyperthermia in that there is an absence of respiratory acidosis and muscle rigidity.405 Surface cooling is usually effective in restoring thermal homeostasis.
Duchenne Muscular Dystrophy
Clinical Features
DMD is the most common of the progressive muscular dystrophies. It is an X-linked recessive disorder with an incidence of 3 cases per 10,000 births (see Chapter 22). The DMD gene (located at Xp21.2) codes for a large sarcolemmal membrane protein, dystrophin, which is associated muscle cell membrane integrity and signal transduction. Dystrophin is missing or nonfunctional in DMD patients. Both sexes can carry the DMD mutation, but girls rarely exhibit signs of the disease.
Children usually present before school age with a waddling gait and later develop a lumbar lordosis and difficulty climbing stairs. Children use their arms to assist standing up (i.e., Growers sign) due to proximal weakness of the hip girdle. Distal muscles, such as the calves, appear hypertrophied. The disease process is progressive, with increasing muscle weakness occurring with age. These boys often become wheelchair bound by 10 to 11 years of age. Respiratory weakness, often exacerbated by scoliosis and by difficulty swallowing secretions due to pharyngeal involvement, can progress to a terminal pneumonia in late teenage years.406 By late adolescence, most children with DMD have cardiac disease, whether it is an abnormal electrocardiogram, arrhythmias, or a cardiomyopathy. Death from cardiorespiratory failure usually occurs before age 30, although respiratory support is extending life expectancy.
DMD should be suspected in male preschool children with delayed walking ability, and measurement of high serum creatinine phosphokinase concentrations provides a screening tool. Steroids are increasingly used for the management of DMD because they appear to increase muscle mass by decreasing protein breakdown.407
Becker muscular dystrophy (BMD) is a milder form of DMD with an onset at puberty or later in adolescence. Clinical expression varies, but even adolescents presenting with mild or subclinical weakness can develop cardiomyopathy with age progression. Death from cardiac or respiratory failure does not usually occur until the fourth or fifth decade. Because of improvements in respiratory care, dilated cardiomyopathy has become the major cause of death.408
BMD is an autosomal recessive myopathy that also results from mutations of dystrophin caused by a deletion of the exons 11 to 13 in the DMD gene (located at Xp21.2).409 Dystrophin exerts its effect at the voltage-gated chloride channel 1 (CLCN1). Genetic analysis is an essential step in confirming the diagnosis. Additional EMG procedures may be of diagnostic value even when muscle biopsy may reveal no evidence of dystrophy.
Of importance to anesthesiologists, two thirds of patients with mild or subclinical BMD have evidence of right ventricular dilation, and one third have evidence of left ventricular dysfunction.410 A thorough cardiac evaluation (similar to that for DMD) is recommended before scoliosis surgery.408 Hyperthermia and heart failure, mimicking malignant hyperthermia and hyperkalemia with rhabdomyolysis after inhalational agents, have been reported in patients with BMD.411,412 Despite these reports, the relationship between BMD and malignant hyperthermia remains unclear.
Orthopedic Considerations
Orthopedic surgery is indicated to improve or maintain ambulation and standing. Early treatment of contractures of the hips and the lower limbs prevents severe contractures and delays the progression of scoliosis.413 Techniques designed to improve deformities and permit early postoperative mobilization include subcutaneous release of contracted tendons and percutaneous removal of cancellous bone with corrective manipulation of the feet. Maintenance of upright posture extends the ability of patients to attend to the tasks of daily living.414 Spinal deformities attributable to muscle imbalance or a collapsing spine are corrected to improve or maintain sitting posture. Spinal fusion may also decrease the rate of deterioration of respiratory function, although this has been questioned.415
Anesthetic Considerations
Respiratory and cardiovascular compromise dominates preoperative assessment. Deformities and contractures of limb joints hinder vascular access, regional anesthetic techniques, and positioning on the operating table. Hypertrophy of the tongue may cause difficulty during intubation. Gastric motility is delayed, and gastric emptying times are prolonged.416 Tracheobronchial tree compression has been described in a child positioned prone for spinal instrumentation.417 These children tend to have greater blood loss during surgery. The precise cause remains unclear, but it may be because fat and connective tissue have replaced muscle or because of abnormalities in the blood vessels.210
Nondepolarizing NMBDs have a slow onset and prolonged duration of action.418–420 All NMBDs should be monitored with a peripheral nerve stimulator.421 Succinylcholine is contraindicated in these children because of the risk of hyperkalemia, muscle rigidity, rhabdomyolysis, myoglobinuria, arrhythmias, and cardiac arrest. There is no clear link between DMD and malignant hyperthermia.422,423 The predominant candidate gene (RYR1) for malignant hyperthermia is located on the long arm of chromosome 19, whereas the DMD gene is located on the short arm of the X chromosome.424 Although volatile anesthetic agents continue to be used in young children with DMD, rhabdomyolysis and hyperkalemia have been reported in the recovery room after halothane, isoflurane, desflurane, and sevoflurane anesthesia.425–429 Potent inhalational anesthetics are best avoided in young children with DMD; instead, alternative anesthetics that do not trigger rhabdomyolysis and hyperkalemia, such as propofol, ketamine, opioids, and benzodiazepines, are preferred.430
Regional techniques such as epidurals may be technically more difficult due to kyphoscoliosis and obesity. The use of ultrasound-guided peripheral nerve blockade can improve the quality and reduce complications of neuronal blockade.6 Opioids are not contraindicated in the postoperative period but should be used with caution in children with respiratory compromise. Tramadol is an effective alternative. Noninvasive ventilation support using BiPAP or continuous positive airway pressure is sometimes required after major surgery or in those already receiving this treatment overnight.
Arthrogryposis Multiplex Congenita
Clinical Features
Arthrogryposis multiplex congenita is a spectrum syndrome of multiple, persistent limb contractures often accompanied by associated anomalies, including cleft palate, genitourinary defects, gastroschisis, and cardiac defects.431 The incidence is 1 case per 3000 births. Joint contractures are present at birth and are a result of immobility in utero, commonly related to a neurogenic abnormality or myopathy.432 These children have been likened to a “thin, wooden doll,” because muscles connected to affected joints are atrophic and replaced by fibrous tissue and fat.433 The temporomandibular joint may also be involved, causing restricted jaw opening and micrognathia. Scoliosis commonly develops. Restrictive lung disease, rib cage deformities, and pulmonary hypoplasia predispose to recurrent chest infections.
Orthopedic Considerations
The aim of surgery is to improve function. Most operations involve the soft tissues, tendons, and osteotomies of the lower limbs and hips.434 Upper limb surgery is less common. Extension contracture of the elbow joint makes it impossible to reach the mouth or to perform hygienic necessities. Improvement in passive elbow flexion by capsulotomy or in active flexion by triceps transfer can increase independence and personal hygiene. When both arms are involved, consideration may be given to maintaining one arm in flexion for reaching the head and mouth passively or even actively and one arm in extension for basic hygiene cares.435
Anesthetic Considerations
Arthrogryposis multiplex congenita is commonly associated with other syndromes that may complicate anesthesia.431,436 Venous cannulation is difficult because veins tend to be small and fragile. The concavity of joints is difficult to access. Care must be taken in positioning the patient on the operating table and protecting skin overlying bony joints to prevent pathologic fractures.
These children should be evaluated for a difficult airway because of temporomandibular joint limitation and micrognathia.437–439 Fusion or underdevelopment of the first and second cervical vertebrae may further complicate laryngoscopy and tracheal intubation with a severe reduction in neck mobility. Tracheal intubation may become progressively more difficult with age. During infancy, however, evaluating mouth opening may be difficult; it may be necessary to insert a tongue blade into the mouth to determine whether the mandible can be distracted from the maxilla.
Hyperthermia and persistent tachycardia have been reported during general anesthesia.431,440–442 These signs occur irrespective of the anesthetic agent and are not associated with malignant hyperthermia. In this case, hyperthermia responds to simple cooling techniques.
Pulmonary dysfunction and an increased sensitivity to opioids dictate suitable monitoring postoperatively. Regional techniques may be difficult in the presence of contractures, but if successful, they offer intraoperative and postoperative analgesia.433 Success can be improved by using ultrasound-guided techniques.