Chapter 4. Orthopaedic medicine treatment techniques
CHAPTER CONTENTS
SUMMARY
The selection of techniques depends on several factors that include the stage the lesion has reached in the healing cycle, with particular attention to the overall irritability. An accurate assessment and clinical diagnosis allows the effective application of the selected treatment techniques and the development of a carefully rationalized treatment programme.
The treatment techniques used in orthopaedic medicine fall into two broad categories of mobilization and injection, and within this chapter the techniques will be considered in turn, on the basis of the theory presented in the preceding chapters, with notes on their application.
Treatment techniques used in orthopaedic medicine may be categorized as follows:
| Mobilization | Injection |
|---|---|
|
• Transverse frictions
• Grade A mobilization
• Grade B mobilization
• Grade C manipulation
• Traction
|
• Corticosteroid
• Local anaesthetic
• Sclerosant therapy
|
MOBILIZATION
Connective tissue structures are more responsive to a decrease in mechanical demand than they are to progressive increases (Tipton et al 1986). Therefore, depriving the healing soft tissues of motion and stress can lead to a number of structural changes within the articular and periarticular connective tissues that may be difficult to reverse. These changes may include all or some of the following:
• Disorganized fibre orientation
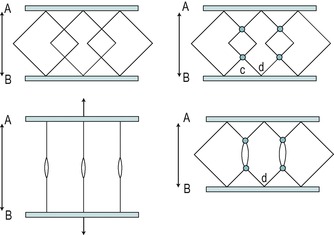
• Adhesion formation at the fibre–fibre interface (Fig. 4.1)
 |
| Figure 4.1
Schematic diagram of the weave pattern of collagen fibres. A and B represent individual collagen fibres with cross-linkage in place to stabilize the structure. In normal tissue, represented by the left-hand diagrams, some separation is allowed between the fibres and the fibres can move apart. Adhesion formation at nodal intercept points at the fibre–fibre interface is represented in the right-hand diagrams at c and d; this adhesion formation will interfere with the free gliding of fibres and inhibit normal tissue function.
From Woo S L, Mathews J V, Akeson W H et al 1975 Connective tissue response to immobility. Arthritis and Rheumatism 18: 257–264. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley and Sons, Inc.
|
• Adhesion formation between ligaments, tendons and their surrounding connective tissue
• Reduced tensile strength of ligaments, tendons and muscles
• Loss of the gliding capacity of connective tissue, especially tendons
• Weakening of ligament and tendon insertion points
• Inhibition of muscle fibre regeneration by scar tissue
• Proliferation of fibrofatty tissue into the joint space and adherence to cartilage surfaces
• Decrease in the volume of synovial fluid with adhesion formation between synovial folds
• Cartilage erosion and osteophyte formation
(Burke-Evans et al 1960, Enneking & Horowitz 1972, Akeson et al., (1973), Akeson et al., (1980) and Akeson et al., (1986), Videman 1986, Hardy 1989, Järvinen & Lehto 1993).
It is noticeable that these connective tissue changes, associated with immobilization, are similar to the changes seen with the degenerative ageing process.
The length of connective tissue structures tends to adapt to the shortest distance between origin and insertion, which produces the consequences of immobilization that can lead to pain and long-term loss of function (Videman 1986).
Several authors have discussed the effects of stress and motion deprivation on healing connective tissues in animal experiments (Akeson et al 1967, 1973, 1986, Woo et al., (1975) and Woo et al., (1990), Arem & Madden 1976, Woo 1982, Akeson 1990). During a 9-week period of immobilization, dysfunction was not simply due to connective tissue atrophy, as there was no statistical difference in the quantity of collagen fibres, but due to an increase in collagen turnover and to other changes within the connective tissue matrix (Amiel et al 1982). Loitz et al (1988) noticed similar changes within a 3-week period of immobilization. The changes noted were:
• Development of anomalous cross-linking of existing and new collagen fibres
• Alteration of the dynamics of collagen turnover (synthesis/lysis)
• Random deposition of new collagen fibres within the existing collagen weave.
In explanation of the changes a difference was noted in the quantity and quality of the amorphous ground substance, consistent in all connective tissue structures, which amounted to a reduced concentration of water and glycos-aminoglycans (GAGs). As a result, the critical distance and separating effects between adjacent collagen fibres were reduced and the interfibrillar lubrication was lost. Friction developed at the fibre–fibre interface leading to the development of anomalous cross-links which altered the gliding function of the collagen fibres. This formation of cross-links was observed to be time-dependent and occurred when fibres remained stationary for a period of time, i.e. assumed a stationary attitude.
The incorporation of new, disorganized collagen fibres into the existing collagen weave physically restrains mobility. Overall this has an effect of altering the elasticity, plasticity and pliability of the connective tissue structures (Eliasson et al 2007). It is important to note that adhesion formation is a normal part of the repair process and it cannot be prevented entirely. However, it may be possible to prevent excessive or unwanted adhesions, or to mobilize them if they develop unwittingly. Treatment aims to maintain or regain the anatomy and biomechanical function of collagen fibres, enabling them to deal with tension, compression, shear and glide.
Collagen turnover (synthesis/lysis) is a normal, dynamic process that is influenced by stress and motion. When deprived of these physical forces through immobilization, the balance of collagen turnover is lost and a greater ratio of immature collagen fibres is present with a potential for the formation of increased anomalous, reducible cross-links. Pliable young scar tissue is ripe for early mobilization techniques that will reduce the formation of anomalous cross-links.
Stress and motion deprivation are also responsible for the random, disorganized deposition of new collagen fibres within the existing weave that results in an overall reduction in tensile strength. Collagen tissue has elastic properties, mainly by virtue of the weave of its collagen subunits, and it will become relatively inelastic if that weave is altered (Peacock 1966). The careful, controlled application of normal stress and motion will stimulate new collagen to be laid down in parallel with the existing weave and the collagen tissue will maintain its elasticity, plasticity and tensile strength. Wolff’s law, relating to laying down of trabeculae in cancellous bone along the lines of stress, could be broadened to encompass the response of the musculoskeletal system to stress as a whole (Akeson 1990).
Immobilization affects the gliding function of tendons and ligaments by virtue of the restricting adhesions failing to elongate to permit pain-free function (Weiner & Peacock 1971). In orthopaedic medicine the principles of mobilization, including transverse frictions, can be applied to healing tendons in tendinopathy, and tenosynovitis. The application of manipulation to rupture adhesions to permit normal gliding function may be necessary in lesions of tennis elbow and some chronic ligamentous strains.
In muscle lesions, collagen or scar tissue formation provides a necessary framework for muscle fibre regeneration. However, excessive scar tissue may form a physical barrier and hinder the progress of the regenerating muscle fibres. The connective tissue component of muscles is subject to the usual deleterious changes of immobilization, as described in Chapter 3. An adequate period of relative rest, depending on the injury and irritability of the lesion, is required to allow the muscle to regenerate sufficiently to combat the mechanical forces of mobilization (Lehto et al 1985, Järvinen & Lehto 1993). The benefit of early mobilization to the regeneration of muscle was explored by Faria et al (2008) who observed muscle fibre regeneration in the rat at 1 h and 3 days after injury, with daily swimming for 15 min and 45 min duration. They found that the earlier mobilization for 45 min showed improved regeneration. This would appear to support early mobilization for injured muscle tissue and the suggestion that early application of graded mobilization, including transverse frictions, will maintain muscle function and stimulate structural orientation without the excessive formation of scar tissue.
Articular changes during periods of immobilization depend on the restriction of the movement, the length of the immobilization period and the amount of contact, pressure and friction between the joint surfaces. The amount of synovial fluid is reduced, which renders the articular cartilage more vulnerable to injury by friction and pressure. Longer periods of immobilization of 45–60 days have shown cartilage erosion, subchondral cyst and osteophyte formation, consistent with the changes observed clinically in joints affected by osteo-arthrosis and age-related changes. Studies involving the immobilization of rat knee joints showed that if immobilization did not exceed 30 days the changes due to immobilization were reversible, but the longer the joints were immobilized, the longer it took to remobilize them, irrespective of the method used (Burke-Evans et al 1960). Degenerative changes occurring during immobilization can be partly inhibited by traction and continuous passive motion (Videman 1986).
The beneficial effects of intermittent and continuous passive motion have been well documented (Loitz et al 1988, Takai et al 1991). The original concept of continuous passive motion was developed by Salter in 1970 to reduce the harmful effects of immobilization (Salter 1989). Joints were moved continuously through a predetermined range of movement. This application of cyclical tensile loading facilitates the orientation of collagen fibres, providing tensile strength and a stronger functional and structural repair of connective tissue, including articular cartilage.
General principles of orthopaedic medicine treatment techniques
Soft tissue injury involves all tissues in the injured area to varying degrees and can be classified into three degrees of severity (Chartered Society of Physiotherapy 1998):
• Grade I or mild injury involves overstretching of the structures with minimal swelling and bruising, mild pain, no joint instability, minimal muscle spasm and minimal loss of function.
• Grade II or moderate injury involves some tearing of fibres with moderate swelling and bruising, moderate pain on movement, moderate muscle spasm, some loss of function and possible joint instability.
• Grade III or severe injury involves a complete tear of the injured structure with significant swelling and bruising, severe pain at rest and severe disturbance of function.
Injury also reduces the tissue’s ability to accept tensile stress, the extent of which is proportional to the degree of tissue damage. Rather than consider an injury acute in terms of days since onset, it is wiser to consider acuteness in terms of the irritability of the lesion. In approaching the application of treatment in this way, the clinician makes a judgment on the amount of intervention and the appropriate time to apply it. In 1998 the Chartered Society of Physiotherapy (CSP), the professional body of physiotherapy in the UK, endorsed the Guidelines for the Management of Soft Tissue (Musculoskeletal) Injury with Protection, Rest, Ice, Compression and Elevation (PRICE) During the First 72 Hours that were prepared by the clinical interest group, the Association of Chartered Physiotherapists in Sports Medicine (ACPSM) (CSP 1998). The principles of PRICE are applicable to any acute injury.
Protection is applied according to the severity of the injury and pain. Based on animal studies, the ACPSM Guidelines (CSP 1998) suggest that a moderate, second degree injury requires 3–5 days’ protection, while a mild or first degree injury requires a shorter period and a severe or third degree injury requires longer. Rest is required following acute injury to reduce metabolic demand and blood flow. However, a balance must be achieved with sufficient controlled movement of the injured and surrounding structures while avoiding any undue stress of the healing breach. Once it has been judged, based on irritability, that the newly formed fibrous tissue can withstand some controlled stress, increased movement is encouraged to stimulate the alignment of fibres. Healing within the presence of movement ensures the development of a strong mobile scar. Ice, compression and elevation can be applied in conjunction with the suggested treatment regimes given below.
Until recently, it has generally been assumed that there is an inflammatory component involved in chronic overuse tendon lesions, hence the term ‘tendinitis’. The work of Khan & Cook (2000) and Cook et al (2000) challenged this thinking based on the evidence of numerous studies which showed few or no inflammatory cells associated with such lesions. Instead changes consistent with the degenerative process (tendinosis) have been noted together with a poor healing response. Hence the term tendinopathy has been adopted to describe chronic overuse tendon lesions where the pathology has not been histologically confirmed.
This raises several issues, not least, how can corticosteroid injection, a known anti-inflammatory treatment, resolve pain in tendinopathy if there is no inflammatory component to the lesion?Khan & Cook (2000) highlight clinical experience together with reference to a number of studies which show corticosteroid injection to provide at least short-term pain relief, but the mechanism of that pain relief remains unknown.
Another issue involves the patient’s prognosis. Based on an inflammatory model, it is commonly suggested that the tennis elbow patient or the Achilles tendon patient will recover with treatment such as those suggested below delivered 2–3 times per week for 2–3 weeks. However, clinical experience shows such lesions to be generally resistant to treatment and the following offers a plausible explanation. If the pathological process of such lesions is degeneration rather than inflammation, this may alter the view on prognosis, with the patient being more likely to take many months to recover fully. The application of deep transverse frictions to chronic lesions clinically produces tenderness in the region, which takes time to settle. The work of Gregory et al (2003), discussed below, suggests that ultrastructural changes as a result of transverse frictions may take up to 6 days to resolve (although the study mentioned is an animal model) and this may have implications for considering the application of the technique on a weekly basis, for example.
In summary, the aim of mobilization treatment techniques applied in orthopaedic medicine, including transverse frictions, is to prevent or to reverse the connective tissue changes associated with a period of immobilization. It is important to recognize that stress deprivation causes rapid structural changes and recovery is much slower, and this must be taken into account when preparing treatment programmes.
Careful consideration should be given to the application of other treatment modalities, either simultaneously or consecutively with the application of appropriate mobilization. Abusive use of movement can produce mechanical forces sufficient to stretch or disrupt the healing breach, producing excessive scar tissue formation. Additional soft tissue trauma leads to secondary inflammation and the vicious circle of chronic inflammation (Noyes 1977). The correct amount of movement applied at the appropriate time is the key.
Mobilization should be used at optimum levels, with appropriate grade, range, force, direction, speed and duration to achieve the specific treatment aims (Arem & Madden 1976). Hunter (1994), however, correctly points out that, although soft tissue mobilization techniques are known to be clinically effective, no research has been conducted to establish the grade of mobilization required for each stage of the healing process. Orthopaedic medicine mobilization techniques (transverse frictions and the specific mobilization techniques, Grades A, B and C) are graded on the basis of patient feedback and observation against the under-pinning knowledge of the different phases of healing and the experience of the clinician. The appropriate depth and grade of mobilization technique is applied according to the severity of the lesion and this is determined in part by assessment of irritability, rather than in terms of length of time from the onset.
TRANSVERSE FRICTIONS
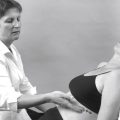
Massage is the manipulation of the soft tissues of the body with the hands using varying degrees of force (Carreck 1994) and a dominant theme in the literature shows massage to be a therapeutic art lacking scientific support. It involves the laying-on of hands and mimics natural gestures such as rubbing a painful area or soothing a child’s injury by kissing it better. As one of the oldest forms of analgesia, it conveys feelings of caring, touch and relaxation which indicate that both physical and psychological factors are relevant (Huebscher 1998, Braverman & Schulman 1999). Hemmings et al (2000) demonstrated a high patient satisfaction rate associated with massage. Techniques include effleurage (gliding movements), pétrissage (kneading) and tapotement (striking), all of which are generally accepted to have their effects in the superficial tissues such as the skin.
Mennell (cited in Chamberlain 1982) first introduced specific massage movements called ‘friction’ in the early 1900s. Cyriax further developed this technique, adding movement for the treatment of pain due to inflammation caused by trauma. He described ‘deep’ friction massage which reaches deep structures of the body such as ligaments, muscles and tendons, to distinguish it from the general massage described above (Cyriax 1984). The technique of transverse frictions has been further refined in recent years to allow the grading of the technique according to the irritability of the lesion and patient feedback.
Transverse frictions, therefore, are a specific type of connective tissue massage applied precisely to move the target tissue – of tendons, muscles and ligaments – for a specific purpose. They are applied prior to and in conjunction with specific mobilization techniques to gain their effects.
The term ‘deep’ transverse frictions has been deliberately avoided as the blanket term to prevent the abuse of this technique. Unfortunately, deep transverse friction has been taught to many therapists as just that, and the technique has not been adapted or graded to suit the lesion to gain specific purpose. Consequently, it has developed a reputation for being very painful for the patient and tiring for the therapist, often being abandoned for these reasons (Ingham 1981, Woodman & Pare 1982, de Bruijn 1984, Cyriax & Cyriax 1993). It is an underrated modality at our fingertips (pun intended) and, when applied correctly, it is an extremely useful technique.
Transverse frictions can be graded in depth and duration of application for acute and chronic lesions. If correctly applied, an analgesic effect is achieved and it does not have to be a painful experience for the patient. In this text, the terms gentle transverse frictions (for acute irritable lesions) and deep transverse frictions (for chronic non-irritable lesions) will be used to give an indication of the grade required for specific treatments. However, no attempts have been made to quantify this grade and it should be developed in response to patient feedback and according to the experience of the therapist.
The evidence base to support the use of transverse frictions needs urgent development, but a number of studies exist which suggest plausible reasons for the effects of the technique, paving the way for future research. With one exception, most studies use a small sample, making it difficult to generalize results to a wider population, and several involve animal models. No one treatment modality has been shown to be significantly better than another, and some studies use subjective terms such as ‘traditional’ and ‘standard physiotherapy’ to indicate the use of combined modalities. There is no consensus on a standardized method of application, with techniques varying from three 1-min applications to a 10-min continuous application in one session. J. Kerr (unpublished work 2006) conducted a survey in Scotland and analysed 86 questionnaires to establish musculoskeletal physiotherapists’ current practice in the use of frictions for treating soft tissue lesions. Frictions were used by 83.7% of those surveyed and treatment times ranged from 3 to 5 min for acute lesions and were consistent at just below 10 min for chronic lesions.
Stratford et al (1989) evaluated the use of phonophoresis (the delivery of drugs via the skin using ultrasound) and transverse frictions for tennis elbow but the lack of statistical power was deemed to be due to the small sample size. Vasseljen (1992) demonstrated that ‘traditional physiotherapy’ methods of transverse frictions and ultrasound performed better than laser in tennis elbow under subjective, but not objective, testing. However, the subjective interpretation in this study may have been more reliably evaluated using qualitative research methods.
Assessing the effect of the addition of transverse frictions to a ‘standard’ physiotherapy programme, Schwellnus et al (1992) showed no significant difference between comparison groups in the treatment of iliotibial band friction syndrome. This study was identified for the Cochrane Library by Brosseau et al (2002) as the only randomized controlled trial to meet investigation criteria. In providing a rationale for their research, Schwellnus et al suggest that the effect of transverse frictions is to realign fibres without detaching them from their origins. However, this is inconsistent with current physiological evidence which shows fibre alignment to occur along the lines of the applied stress. Therefore it is the applied mobilization technique following the application of transverse frictions which is important for realignment of fibres.
Verhaar et al (1996) conducted the only study to investigate the use of transverse frictions combined with mobilization, in this case a manipulation for tennis elbow. A prospective randomized controlled trial was conducted with 106 patients divided into two groups, one receiving transverse frictions and Mill’s manipulation (see Ch. 6) and the other corticosteroid injection. Short-term follow-up showed significant improvement in both groups, with the injection group faring better than the transverse frictions group, while long-term follow-up showed no statistical difference between the two groups. An honest evaluation of the limits of this study is included by the authors, citing reasons such as failing to eliminate observer bias together with blinding and organizational difficulties. They suggest that as the conclusion of the study identified all methods of treatment as effective, then the least invasive, least expensive and most time-efficient should be used, in this case injection. However, injection is an invasive technique and the number of patients requiring surgery in the injection group in the long term was not addressed in the study.
Pellecchia et al (1994) demonstrate some support for ‘combined modalities’ including transverse frictions when compared with iontophoresis (delivery of drugs via the skin using electrical energy), but conclude that iontophoresis may be more effective and efficient. The study is limited by its small sample size of 26 subjects, scant discussion on how randomization and blinding occurred and a lack of analysis of the six subjects who dropped out. The authors discuss the limitations of their study, explaining that ethical considerations resulted in decisions which favoured the interests of the patients over a stronger experimental design. This study clearly illustrates the difficulties experienced in developing good experimental evidence.
Drechsler et al (1997) provide a clear rationale for an experiment to compare two treatment regimes for tennis elbow, while also adding to the current debate on the deficiencies of treatment regimes which address inflammatory rather than degenerative causes of tendinopathy. A ‘standard’ treatment group was exposed to several interventions including transverse frictions while a neural tension group was exposed to mobilization of the radial nerve. Results showed no significant difference for any variable tested; nevertheless, it is concluded that neural and joint mobilizations together were superior to the ‘standard’ treatment techniques.
In contrast to the above studies, Davidson et al (1997) and Gehlsen et al (1998) used animal studies to provide support for the application of augmented soft tissue mobilizations, applied parallel to the fibre direction, to promote the healing process in tendinopathy. These soft tissue pressures could be considered to be similar to the pressures delivered by deep transverse frictions. Controlled application of microtrauma through pressure in this way promoted fibroblastic proliferation and activation, dependent upon the depth of the pressure applied, leading to repair in the absence of inflammation.
Parallels could be drawn with a study conducted by Gregory et al (2003) who used an animal model to demonstrate the ultrastructural changes occurring in previously normal muscle tissue following 10 min deep transverse frictions. Obvious reddening of the skin was present immediately after deep transverse frictions, with inflammation and changes in myofibre morphology still apparent 24 h later. However, the ultrastructural changes described after 6 days were not those routinely associated with regeneration of muscle tissue after injury, such as haemorrhage, inflammation and macrophage activity, but more consistent with the repair process. It was also clear that the ultrastructural changes occurred locally under the area to which treatment had been directed. What the study was unable to clarify was whether or not these changes were potentially beneficial and would facilitate the healing process, or would aggravate the injury if present.
Kelly (1997) conducted a study specifically to test transverse frictions as a single modality, although this involved normal volunteers rather than symptomatic patients. The gastrocnemius muscle in 46 subjects received 5 min deep transverse frictions. An increase in the range of movement (dorsiflexion) and force of isometric muscle contraction (plantarflexion) occurred, indicating that transverse frictions may promote improved muscle function. Credit in this study was also given to motivational and psychological factors arising from the hands-on treatment; this has further implications for external validity.
Iwatsuki et al (2001) appear to corroborate the work of Kelly in an investigation into the biting force of the masticatory muscles in subjects diagnosed with cerebrovascular accident before and after transverse frictions. Improved function due to an increase in the biting force of these muscles was demonstrated. The reason for the result was deemed to be due to relaxation of muscle spasm, although the sample size was small.
Fernández-de-las-Peñas et al (2005) set out to prove that trigger pointing (ischaemic pressure technique) was better than frictions for treating myofascial trigger points. However, in their pilot study (with a total of 40 subjects split into two groups), both techniques were equally effective in reducing tenderness. The frictions were applied for less time than the 10 min currently advocated on the basis of clinical experience but the study may have implications in widening out the range of situations where the application of frictions could be beneficial.
Despite the paucity of evidence, good clinical results have been observed following transverse frictions and, until proven ineffective, its use is advocated according to certain principles discussed below. The above studies suggest that possible beneficial effects include improved muscle function, stimulation of an inflammatory reaction and repair due to proliferation and activation of fibroblasts in the absence of inflammation. If ultrastructural changes occur local to the applied pressure and take time to subside, this has implications for ensuring that the technique is delivered to the target tissue and for the timing of subsequent applications of the technique.
A snapshot of current practice is provided in Table 4.1 that lists the ‘top ten’ lesions treated with frictions drawn from a survey of 86 physiotherapists’ practice in the mid-Lothian area of Scotland (J. Kerr, unpublished work 2006).
| POSITION | LESION |
|---|---|
| 1 | Lateral collateral ligament of the ankle |
| 2 | Rotator cuff tendons |
| 3 | Tennis elbow |
| 4 | Medial collateral ligament of the knee |
| 5 | Achilles tendinopathy |
| 6 | Muscle strain |
| 7 | Patella tendinopathy |
| 8 | Tendinopathy |
| 9 | Golfer’s elbow |
| 10 | De Quervain’s tenosynovitis |
Aims of transverse frictions
To induce pain relief
An in-depth discussion of the pain-relieving effects of manual therapy techniques is outside the scope of this book and the reader is referred to the work of Vicenzino & Wright (2002), who provide an account of the current evidence. Pain relief following the application of transverse frictions has been clinically observed and a number of working hypotheses are proposed to substantiate this. Farasyn (2007), for example, suggests that frictions applied to muscle releases stimulation of sensitized neurons and reduces referred pain.
The reduction in pain is experienced as a numbing or analgesic effect, i.e. a decrease in pain perceived by the patient who will often acknowledge this by saying you have ‘gone off the spot’. However, with faith in diagnosis and accurate knowledge of anatomy, this should be the signal to grade the friction more deeply to apply beneficial treatment appropriate to the lesion.
Following treatment the comparable signs can be reassessed and a reduction in pain and increase in strength is usually noted. This induced analgesia is utilized to allow the application of graded mobilization techniques in acute lesions. In chronic lesions, an initial analgesic effect is required through the application of a gentler grade of transverse frictions; the technique is then applied with increasing depth to provide a traumatic hyperaemia and pain relief to allow graded mobilization to be applied.
The traumatic hyperaemia induced by the application of deeper graded transverse frictions over a longer period of time in chronic lesions may produce analgesia through changes in the local microenvironment of the tissues via an increased blow flow. These changes may include removal of the chemical irritants that sensitize or excite local nociceptors, a decrease in local oedema and pressure, the production of local heat and facilitation of the repair process.
Pain modulation may be induced through several complex phenomena, including spinal and central nervous system mechanisms acting individually or in combination, to produce desired effects. The gate control theory proposed by Melzack & Wall in 1965 is a theory of pain modulation that affects the passage of sensory information at spinal cord level, especially nociceptive impulses. Within the spinal cord there are mechanisms which ‘open the gate’ to impulses provoked by noxious stimuli, and mechanisms which ‘close the gate’, thus reducing the awareness of noxious stimuli.
Pressure stimulates low-threshold mechanoreceptors, the A-beta fibres, that reduce the excitability of the nociceptor terminals by presynaptic inhibition, effectively ‘closing the gate’ on the pain. The A-beta fibres are myelinated, fast conducting fibres which basically ‘overtake’ the slow conducting fibres to reach the gating mechanism, closing it to incoming painful stimuli. The greater the mechanoreceptor stimulation, the greater the level of pain suppression (Bowsher 1994, Wells 1994). Put simply, rubbing a painful spot reduces pain, enabling the transverse frictions to be graded in depth, specific to individual lesions, and thus to produce their beneficial effects.
The reduction in pain achieved seems to be fairly long-lasting, possibly longer than expected. Transverse frictions, like rubbing or scratching the skin, are considered to be a form of noxious counterirritation that leads to a desired analgesic effect (de Bruijn 1984). Inhibition, which produces lasting pain relief, is believed to be through the descending inhibitory control systems via the periaqueductal grey area – the key centre for endogenous opioid analgesic mechanisms. Endogenous opioids are inhibitory neurotransmitters which diminish the intensity of the pain transmitted to higher centres (de Bruijn 1984, Melzack & Wall 1988, Goats 1994).
De Bruijn (1984) treated 13 patients with deep transverse frictions to various soft tissue lesions. The time required to produce analgesia during the application of deep transverse frictions was noted to be 0.4–5.1 min (mean 2.1 min) while the post-massage analgesic effect lasted 0.3 min–48 h (mean 26 h).
Carreck (1994) evaluated the effect of a 15-min general lower limb massage (effleurage and pétrissage) on pain perception threshold in 20 healthy volunteers and showed that the massage significantly increased the pain perception threshold for experimentally induced pain. Conversely, Kelly (1997), using deep transverse frictions on normal volunteers, reported no significant change in the pain pressure threshold, although there was an improvement in function that will be discussed below.
Massage in its various forms is considered to be a placebo, having a therapeutic effect on the psychological aspects of pain. There is no doubt that a professional attitude, including a thorough patient assessment and diagnosis, an explanation of the problem and the beneficial effects of the proposed treatment intervention, together with involvement of patients in their own recovery, has great effect. Transverse frictions as a form of massage may also have a placebo effect.
To produce therapeutic movement
Transverse friction aims to achieve a transverse sweeping movement of the target tissues – muscles, tendons and ligaments – to prevent or eliminate adhesions (Wieting Cugalj 2004). This transverse movement, together with graded mobilization, discourages the stationary attitude of fibres that promotes anomalous cross-link formation. By combining transverse friction and mobilization in this way, the technique attempts to reduce abnormal fibrous adhesions and improve scar tissue mobility by encouraging normal alignment of soft tissue fibres (Brosseau et al 2002, Wieting Cugalj 2004, Lorenzo 2008). The therapeutic movement may facilitate the shear and gliding properties of collagen, allowing subsequent longitudinally applied stresses, through graded mobilization techniques, to stimulate fibre orientation towards enhanced tensile strength. In this way adhesion formation is prevented in acute situations and mobilized in chronic situations.
Friction potentially exists whenever two objects come into contact with each other, which is why it is important that the depth of application of transverse frictions should ensure that the therapist’s finger makes contact with the target tissue, albeit via the skin and superficial tissues. A shear force is one that attempts to move one object over or against another and is applied parallel to the contacting surfaces in the direction of the attempted movement. The greater the contact force via the therapist’s finger on the target tissue and/or the rougher the contacting surfaces, the greater the force of friction. Imagine rubbing your hands together; the harder you press, the greater the contact force and the greater the friction generated (Levangie & Norkin 2001). Friction generates heat and may therefore promote movement of the tissues by reducing pain and viscosity.
A therapeutic to-and-fro movement aims to mimic the function of the target tissue to prevent adhesive scar tissue formation in acute lesions and to mobilize established scarring in chronic lesions. For this reason, muscle bellies are placed into a shortened position to facilitate broadening of the muscle fibres and ligaments are moved across the underlying bone. In the case of tendons, transverse frictions aim to move the structures passively, either within the surrounding connective tissue or between a tendon and its sheath. Tendons in sheaths are therefore put on the stretch to allow mobilization of the sheath around the solid base of the underlying tendon.
When applied in the early inflammatory phase, gentle transverse frictions (approximately six sweeps) cause an agitation of tissue fluid that may increase the rate of phagocytosis by chance contact with the macrophages (Evans 1980). It is a useful treatment to apply in the acute inflammatory stage following injury, depending upon the irritability of the condition. It is applied in this ‘gentle’ manner before scar tissue formation has truly begun, but should be graded appropriately to avoid increased bleeding or disruption of the healing breach.
In an experiment to evaluate pain and range of movement in acute lateral ligament sprain of the ankle, the effect of transverse frictions together with Grade A mobilization was compared to Grade A mobilization alone (Kesson & Rees, unpublished work 2002). The experimental group received transverse frictions and Grade A mobilization, while the comparison group received Grade A mobilization alone. The sample size of 16 was too small to allow statistical significance or generalization but the experimental group showed a greater overall increase in range of movement and a greater overall decrease in pain on single leg standing than the comparison group. However, it was noted that two subjects experienced a decreased range of movement and four experienced increased pain on single leg standing in the experimental group. The mean number of days post-injury for the experimental group was 4.4 and if this trend were upheld in a larger trial it could have implications for assessing the appropriate time to apply transverse frictions in terms of assessment of irritability. The suggestion is that the appropriate time for applying transverse frictions and Grade A mobilization cannot be determined in terms of number of days post-injury, but should be assessed in terms of the injury sustained and the irritability of the lesion.
In the chronic inflammatory phase, deep transverse frictions produce therapeutic movement, increasing frictional forces and generating heat to soften and mobilize adhesive scar tissue. This prepares the structure for other graded mobilizations that aim to apply longitudinal stress.
To produce a traumatic hyperaemia in chronic lesions
Increasing the depth of transverse frictions, i.e. the contact between the therapist’s finger and the target tissue, together with an increase in time of the application of the technique to approximately 10 min produces a controlled traumatic hyperaemia and is used exclusively for chronic lesions. The hyperaemia is caused by the mechanical action of the technique (Brosseau et al 2002).
A superficial erythema (redness) develops in the skin through dilatation of the arterioles and it is assumed that the same reaction is occurring in the deeper tissues where the effect is required (Winter 1968). The area of redness that develops under the finger may also be slightly raised and warm (a wheal), indicating increased permeability of the capillary walls that allows tissue fluid to pass into the surrounding area (Norris 2004). Massage, which increases blood flow in this way, changes the microenvironment of the tissues, producing local heat and decreasing pain by facilitating the removal of chemical irritants which sensitize or excite local nociceptors. This may also produce a decrease in pain by facilitating the transportation of endogenous opiates.
The work of Gregory et al (2003), described above, indicated that transverse frictions applied for 10 min produced ultrastructural changes in skeletal muscle initially consistent with the inflammatory process and ultimately with the reparative process. The observation of the redness and the wheal following the application of 10 min transverse frictions may be sufficient to stimulate a controlled inflammatory reaction which could be responsible for boosting the repair process in chronic lesions. Applying appropriate graded mobilizations after the friction technique aims to ensure healing within the presence of movement and the production of a strong mobile scar.
Although no clinical evidence exists to support the exact timing of 10 min after achieving an analgesic effect, it is the approximate time taken to induce and observe the skin changes described. However, tenderness in the target and overlying tissues may be a product of the induced inflammatory response; therefore at least 48 h (possibly 6 days, see the work by Gregory et al described above) is recommended between the treatment sessions in chronic lesions, in order for the tenderness to subside. The work of Davidson et al (1997) and Gehlsen et al (1998) suggests that in chronic tendinopathy (i.e. a degenerative process) repair may occur in the absence of inflammation by increasing the number and activity of the fibroblasts. Their work suggests that greater fibroblastic proliferation and activity occur with deeper pressures, indicating the appropriateness of deeper graded transverse frictions in chronic lesions.
Although the traumatic hyperaemia may be desirable in chronic lesions, this is not the case in acute lesions where the response is already excessive. Gentle transverse frictions applied to acute lesions for approximately six sweeps after achieving an analgesic effect are performed with reduced depth and time to avoid producing this traumatic hyperaemia. Such patients are treated ideally on a daily basis, or as often as possible, to ensure healing within the presence of movement and to encourage the formation of strong mobile scar tissue.
To improve function
The therapeutic movement achieved and pain relief induced by transverse frictions produce an immediate clinical improvement in function of the structure treated. This can be demonstrated by reassessment of the comparable signs immediately after treatment and provides an optimum situation for the application of other graded mobilizing techniques.
As discussed above, Kelly (1997) and Iwatsuki et al (2001) conducted studies which demonstrated improved muscle function in terms of strength of contraction, with Kelly also demonstrating increased range of movement.
Principles of application of transverse frictions
Transverse frictions should be applied to the exact site of the lesion. This relies on clinical diagnosis and palpation of the lesion, based on anatomical knowledge and the structural organization of the tissue. Tenderness is not necessarily an accurate localizing sign, therefore palpation must reproduce the patient’s pain and be different in comparison with the other limb.
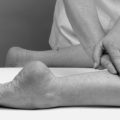
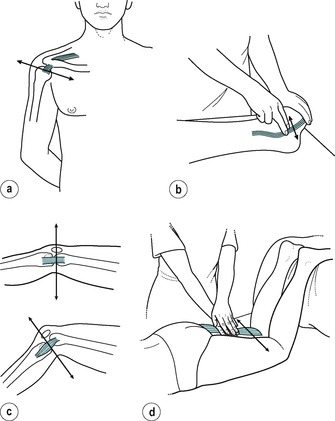
Transverse frictions are always applied transversely across the longitudinal fibre orientation of the structure (Fig. 4.2). This aims to prevent or mobilize adhesion formation between individual fibres and between fibres and the surrounding connective tissues. Application of the technique in this manner deters a stationary attitude of fibres and aims to prevent or mobilize anomalous cross-links. The longitudinal stress applied in accompanying graded mobilization techniques stimulates fibre orientation within the collagen weave and increases tensile strength.
 |
| Figure 4.2
Transverse frictions of: (a) a tendon (supraspinatus); (b) a tendon in sheath (peronei); (c) a ligament (medial collateral) in flexion and extension; (d) muscle belly (hamstring).
|
Transverse frictions are applied with sufficient sweep to cover the full width of the structure, theoretically aiming to move the connective tissue fibres transversely across the whole affected site. The therapist must be positioned to ensure that this sweep is maintained through the application of body weight.
With the transverse friction techniques demonstrated in the photographs within this book, the direction of friction usually follows the direction in which the fingers or thumbs are pointing. Arrows have been included where the direction of movement differs from that ‘rule’ or to emphasize the direction where it may not be clear.
Transverse frictions are applied with effective depth, aiming to reach and benefit the target tissue. Knowledge of anatomy is paramount. The depth is maintained by applying the technique slowly and deliberately, but it is the shear force between the superficial tissues and the underlying target tissue which generates friction and produces heat, as mentioned above. While this latter effect is desirable, care must be taken to maintain the depth of the technique, as increasing speed tends to lift the effect towards the more superficial tissues.
The grading and duration of application of transverse frictions is intended as a clinical guide, the depth of technique being dependent upon the irritability of the lesion and feedback from the patient. The depth of the initial sweeps should always be gentle to gain an analgesic effect before proceeding with the effective frictions. Application in this way will dispel the myth that transverse frictions are a painful treatment technique.
The patient is positioned taking into account the situation in which the structure is most accessible and the degree of stretch or relaxation appropriate:
• Tendons in a sheath are placed on the stretch to allow the transverse frictions to roll the sheath around the firm base of the tendon, facilitating functional movement between the tendon and its sheath.
• Tendons are placed into a position of accessibility, with the lesion commonly lying at the teno-osseous junction. Consideration is given to anatomy.
• Musculotendinous junctions usually respond well to transverse frictions and need to be placed in a position of accessibility.
• Muscle bellies are placed in a shortened, relaxed position to facilitate broadening of the muscle fibres, imitating function.
• Ligaments are put under a small degree of tension to tighten the overlying soft tissues, allowing the target tissue to be reached.
The therapist should adopt a position that utilizes body weight optimally, ensuring sufficient sweep and depth. Transverse frictions can place considerable strain on the hands (Sevier & Wilson 1999). Snodgrass et al (2003) looked at factors relating to thumb pain in physiotherapists and manual techniques in general were highlighted as the cause of pain for all symptomatic subjects studied. Whilst not ideal, handheld devices have been developed to help to prevent occupational injury but strain on the digits can be reduced by using body weight to perform the technique. Care should be taken to adopt a position that places the operator at a mechanical advantage. Sitting is possible, but the most effective position to apply body weight is with the patient situated on a plane lower than the therapist.
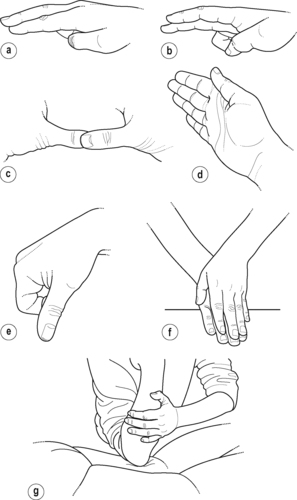
Consideration should also be given to the variety of ways the hands can be used to avoid fatigue and overuse (Fig. 4.3). The therapist’s finger and the patient’s skin and superficial tissues should move simultaneously to avoid raising a blister and to ensure that the superficial tissues are moving over the underlying target tissue, generating a friction force between them. The most efficient way of achieving this is to apply the technique in both directions. Pressure is first directed downwards onto or against the structure and then maintained while the transverse sweep is applied.
 |
| Figure 4.3
Hand positions for transverse frictions. (a) Index finger reinforced by middle finger; (b) middle finger reinforced by index finger; (c) thumbs; (d) ring finger; (e) pinch grip; (f) hands; (g) hand guiding the flat of the elbow.
|
As a general guideline, transverse frictions are applied for an appropriate time to achieve the desired effects. For chronic lesions, 10 min after the analgesic effect has been achieved is suggested. However, due to the induced traumatic hyperaemia or local inflammatory response, treatment is repeated at a suggested minimum interval of 48 h to allow the tenderness generated in the target and overlying tissues to subside.
It is suggested that gentle transverse frictions are applied to acute lesions for approximately six effective sweeps once some analgesia is achieved and the target tissue reached. It may be necessary to work through soft tissue swelling associated with the injury, to be able to reach the target site. Treatment can be on a daily basis, if practically possible, to promote pain relief and therapeutic movement.
These guidelines are largely based on expert clinical opinion and, as discussed above, no clinical evidence has been found to support them. From the practical aspect, the duration of application usually fits in with appointment times and allows for both reassessment and the application of other modalities of treatment judged to be appropriate. This was not borne out by a survey by J. Kerr (unpublished work 2006) however, where respondents did not claim appointment times as a factor in the number of minutes they selected for the application of frictions.
Absolute contraindications to transverse frictions are few (see Box below). The technique is never applied to active conditions, e.g. areas of active infection such as a boil or pimple or active inflammatory arthritis in which the connective tissue structures are too irritable. Care is required if there is fragile skin or the patient is currently undergoing anticoagulant therapy. Bursitis may be aggravated.
GRADED MOBILIZATION TECHNIQUES
Grade A, B and C mobilizations (Saunders 2000) are specific mobilization techniques applied to achieve a particular purpose. Each mobilization described below is an individual technique aiming to achieve specific effects and one is not intended to be a progression of the other. The terms are used in different ways when referring to peripheral and spinal joints and the similarities and differences will be highlighted throughout this section. Peripheral graded mobilization techniques will be explained first, followed by a definition of the spinal graded mobilization techniques.
PERIPHERAL GRADED MOBILIZATION TECHNIQUES
Peripheral Grade A mobilization
Peripheral Grade A mobilization is a passive, active or active/assisted mobilization performed within the patient’s pain-free range of movement, aiming to provide or maintain mobility. It is applied within the elastic range. In peripheral lesions it is usually applied to irritable, acutely inflamed or painful lesions and it may be facilitated by the previous application of transverse frictions.
Aims of peripheral Grade A mobilization
To promote tissue fluid agitation
In peripheral lesions, Grade A mobilization, either active or passive, produces a gentle soft tissue movement agitating tissue fluid in the acute inflammatory phase and facilitating the phagocytic action of the macrophages (Evans 1980). The movements are of small amplitude, slow, pain-free, without force and repeated often.
To prevent a stationary attitude of fibres
Peripheral Grade A mobilization will promote movement within connective tissue structures to prevent a stationary attitude of fibres. In the acute phase of inflammation, the movement should occur without stress or disruption of the healing breach. The patient is instructed to exercise up to the point of discomfort, but not into or through pain, and must understand the importance of this controlled, precise movement. In this way a critical amount of movement is applied to encourage function but not to delay healing.
The function of a muscle belly is maintained by performing active inner-range, isometric, pain-free contractions aiming to broaden muscle fibres. The function of a ligament is promoted by performing active pain-free movement, maintaining the ligament’s ability to glide over the underlying bone. In overuse lesions, e.g. tendinopathy, the patient maintains normal function by performing movement within the pain-free range and avoiding the painful movements.
To apply a longitudinal stress to connective tissue structures
Peripheral Grade A mobilization aims to impart sufficient longitudinal stress to promote orientation of collagen fibres within the existing parallel collagen weave. As acute lesions subside, and once it is judged that there is sufficient tensile strength in the wound, the patient may begin to ‘nudge’ pain to provide longitudinal stress but without disrupting the healing breach. Grade A mobilizations performed in this way are of short duration, are not forceful and are repeated often. This form of mobilization is appropriate for the repair phase of connective tissue injuries, and as progress is made an increasing range of movement is encouraged. In this way the developing scar tissue is stressed, not stretched (to avoid over-stressing the healing tissues), aiming to restore full functional range. Tissues which heal within the presence of movement in this way should not require ‘traditional’ stretching into the painful range.
To promote normal function
Peripheral Grade A mobilization is applied early in the inflammatory phase to ensure that function is rapidly regained. Healing within the presence of movement promotes a return to function, i.e. form follows function. The range of movement is gradually increased, applying sufficient stress to the wound to promote orientation of fibres. Controlled mobilization of acute lesions should prevent the need for stretching tissue.
In chronic injuries, once the lesion is rendered pain-free by transverse frictions or corticosteroid injection, normal movement will promote remodelling and alignment of fibres and restoration of function. However, until signs and symptoms subside, it is suggested that overuse activity should be avoided in lesions aggravated by repetitive movements, and resisted exercise and stretching postponed until the scar tissue is fully mobilized and the selective tension tests pain-free.
To reduce a loose body or bony subluxation in a peripheral joint
To reduce a peripheral joint loose body (e.g. in the hip, knee or elbow) or a bony subluxation (e.g. carpal bone), a Grade A mobilization is performed under strong traction, the principal component of this technique, giving the fragment space to move.
Peripheral Grade B mobilization
A peripheral Grade B mobilization is a mobilization performed at the end of available range into the plastic range. It is a specific sustained stretching technique that aims to facilitate permanent elongation of connective tissue.
Aims of peripheral Grade B mobilization
To stretch capsular adhesions
In the early stages of arthritis, pain causes the joint to be held in a position of ease, restricting movement of some parts of the joint capsule. This promotes a disordered deposition of collagen fibres and the normal flexibility of the joint capsule is impeded by anomalous cross-link formation. The changes in the capsule contribute to contracture and the capsular adhesions severely restrict the range of movement, causing pain and altered function. The aim of treatment by Grade B mobilization is to lengthen the capsular contracture permanently, to restore normal movement.
Adhesions that develop within the capsule of a synovial joint tend to be tough and unyielding. The shoulder and hip joints commonly demonstrate this gross loss of function and respond well to Grade B mobilization, providing the joints are in a non-irritable state. However, the technique could be applied to any non-irritable capsulitis in which pain and loss of movement are clinical features. Characteristically the joint is limited in the capsular pattern, with the restricted movements demonstrating a ‘hard’ end-feel (see Ch. 1). Peripheral Grade B mobilization is aimed at restoring the movements limited in the capsular pattern.
Peripheral Grade B mobilization is applied at the end of available range, appreciating the end-feel and the patient’s pain response. To be successful, the end-feel should still have some elastic quality, but the sensation of muscle spasm may also be apparent as the technique is applied within a painful range. The technique is a slow, sustained and repeated stretch, performed at the end of available range for the duration of the patient’s pain tolerance. As soon as the end range position is released, an immediate relief of pain should be felt. If pain lingers at this stage, the joint may be too irritable and the technique inappropriate.
Successful treatment is dependent upon patient compliance as the management of the condition is long-term. Theoretically the tissue to be stretched needs to be taken into the plastic range to achieve permanent lengthening, with sufficient force applied to strain and break some of the collagen cross-links in the adhesive scar tissue, producing microfailure. However, as the capsular tissue possesses viscoelastic properties, this can be utilized to gain increased range of movement. The following factors relating to the behaviour of connective tissue under mechanical stress (see Ch. 2) can be applied to this mobilization, which is suggested for tough, unyielding capsular adhesions.
Prolonged immobilization produces joint contracture, with more involvement of the joint capsule than the soft tissues surrounding the joint (Usuba et al 2007). A static stretch of slow, sustained, low force, applied towards the end of the elastic limit of the scar tissue, utilizes the viscous flow phenomenon which enables the contracted scar tissue to creep or lengthen gradually (Amis 1985, Hardy & Woodall 1998, Usuba et al 2007).
Grade B mobilization is a static stretch applied to the patient’s pain threshold point. The increased length achieved is apparently due to cross-link disruption, fibre slippage and eventual structural changes (Hardy & Woodall 1998). Lengthening, under these circumstances, is inversely proportional to the velocity of the applied stretch with a slowly applied force meeting with less resistance or stiffness (it is easier to move slowly through a viscous medium). Hysteresis (the slower rate at which the structure recovers from the elongating force) may also play a role and can be linked to cyclical loading where subsequent applications of Grade B mobilization achieve a modest increment of increased length with each mobilization applied.
The structural changes occurring in the mobilized tissue may produce a low-grade inflammatory response and the patient should be warned about post-treatment soreness. The importance of continued movement to maintain the elongation achieved cannot be stressed enough, and the patient must be given an appropriate exercise programme to maintain the range achieved.
The optimum duration and frequency of the static stretch has yet to be determined, but the work of Brandy & Irion (1994) and Brandy et al (1997) suggests that in order to achieve an increase in range of knee flexion, a static stretch of 30 s applied to the hamstring muscles is sufficient. However, their studies fall short of determining how long the increased flexibility in the muscles lasted.
The application of heat relieves pain and lowers the viscosity of collagen tissue, allowing a greater elongation of collagen tissues for less force. Warren et al (1971) considered the effect of temperature and load on elongation of the collagen fibre structure of rat tail tendon. A range of loads was applied at selected temperatures and the greatest elongation with least microdamage was achieved with lower loads at the higher therapeutic temperatures. The mechanism of a combined application of temperature and load affected the viscous flow properties of collagen and increasing the temperature of a structure allowed lower sustained loads to achieve greater elongation (Warren et al 1971, Usuba et al 2007).
Raising local temperature to between 40 and 45°C (40°C is the temperature of a very hot bath) achieves this useful therapeutic effect. Ultrasound can be applied to produce this thermal effect as it has a preference for heating collagen tissue (Low & Reed 1994). Usuba et al (2007) found from their previous studies that heat combined with stretching was more effective than stretching alone and, to achieve its best effects, heat should be applied concurrently with the Grade B mobilization. However, for practical purposes it is usually applied beforehand.
To reduce pain
In arthritis, the inflammatory changes involve both the synovial lining of the joint and the fibrous capsule. The capsule develops small lesions that undergo scar tissue formation, producing adhesions or capsular thickenings. The adhesions are affected by joint movement which, through abnormal tension, stimulates the free nerve endings lying in the capsule, producing mechanical pain. The nerve endings also respond to the chemical products of inflammation – histamine, kinins and prostaglandins. Impulses are transported in the non-myelinated C fibres and a ‘slow’, aching, throbbing pain is produced which is poorly defined, i.e. chemical pain (Norris 2004). The pain and associated involuntary muscle spasm reduce movement, allowing healing to occur in a shortened position and resulting in reduced function.
Sustained or repetitive passive joint movement may modify the firing responses in large-diameter mechanoreceptor joint afferents, particularly when the capsular stretch is maintained at or near the end of range. This presents an explanation that accounts for the pain-relieving effects of end-range mobilization, including passive Grade B stretching techniques as well as manipulation.
The permanent lengthening achieved by Grade B mobilization reduces the mechanical stress and therefore inflammation, which in turn relieves pain. Normal movement patterns are restored. This provides a stimulus to fibre alignment and orientation within the collagen weave.
To improve function
The increase in joint range and pain relief achieved by peripheral Grade B mobilization improves overall function. However, the gradual onset of loss of movement through capsular contraction can considerably alter movement patterns. Careful assessment of the patient is necessary and a treatment programme planned which considers all components of the dysfunction, including altered biomechanics, muscle imbalance and neural tension.
Peripheral Grade B mobilization is not used exclusively for capsular contractures. In chronic muscle, tendon and ligament lesions, adaptive shortening may be part of an overall dysfunction. The shortened tissues can be stretched by applying the principles of Grade B mobilization, both by the therapist and as a home treatment regime. Grade B mobilization is applied once the structure has been rendered functionally pain-free by deep transverse frictions and negative findings are established on examination by selective tension. Contracted tissue has an influence on neural structures; however, neural tension techniques fall outside the scope of this text.
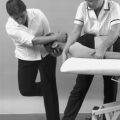
Peripheral Grade C manipulation
Peripheral Grade C manipulation is a passive movement performed at the end of available range and is a minimal amplitude, high velocity thrust. For clarity, it is not sustained, and should be applied with a small, quick overpressure, once all the slack has been taken up.
Manipulative rupture of shortened scar tissue may be appropriate in peripheral lesions. The aim of the technique is manipulatively to rupture unwanted adhesions, producing permanent elongation and restoring full painless function. Threlkeld (1992) expands on the viscous properties of connective tissue and describes how when the tissue is loaded more quickly it behaves more stiffly than the same tissues loaded more slowly. Thus adhesions may be ruptured more readily by stress applied quickly, as in manipulation. The short toe-phase of the adhesions ensures that the slack is taken up more rapidly than in the surrounding normal tissue and the minimal amplitude, high velocity thrust causes macrofailure, or rupture, of the adhesions while the normal tissue remains intact.
It is important that the elongation achieved should be maintained by the patient. In orthopaedic medicine, manipulative rupture of unwanted adhesions is applied in two situations:
Aims of peripheral Grade C manipulation
Manipulative rupture of unwanted adhesions between a ligament and bone
Peripheral Grade C manipulation is applied to a chronic ligamentous sprain of two ligaments in the lower limb, the medial collateral ligament at the knee and the lateral collateral ligament at the ankle. Unwanted adhesions may have developed between the ligament and the underlying bone that disrupt the normal functional movement of the joint and on examination a non-capsular pattern of pain and limitation will be found. The ligament is prepared by deep transverse frictions to achieve an analgesic effect and the manipulation follows immediately. Effective manipulation should achieve instant results and vigorous exercise is required to maintain the lengthening achieved. Rehabilitation must address all components contributing to the dysfunction.
To rupture adherent scar tissue at the teno-osseous junction of the common extensor tendon (tennis elbow)
A special manipulation (Mills’ manipulation) is applied at the teno-osseous junction of the common extensor tendon as a treatment for tennis elbow (Ch. 6). The manipulation aims to rupture adhesions interfering with the mobility of the tendon at its insertion and within the adjacent tissues. The tendon is first prepared by deep transverse frictions to gain some pain relief, followed by the manipulation. The elongation gained must be maintained through exercise.
Further trials are needed to substantiate the application of frictions in tendinopathies and the effectiveness of applying the Mills’ manipulation for the common extensor teno-osseous junction at the elbow. As mentioned above, the Mills’ manipulation has traditionally been a ‘special’ manipulation and this is the only example of a teno-osseous junction being manipulated in current orthopaedic medicine practice. This is challenged by Stasinopoulos & Johnson (2007), however, and other sites suitable for manipulation may emerge through continuing reflection on practice; possibly spurred on by the emergence of evidence to support the use of the technique.
SPINAL GRADED MOBILIZATION TECHNIQUES
Spinal Grade A mobilization
At the spinal joints, and specifically at the cervical spine, the term ‘Grade A’, is used to indicate that the mobilization technique is applied to mid-range, i.e. the middle of the range available.
As when applied to peripheral joints, it should be a pain-free mobilization that is used to reduce the patient’s pain and to facilitate movement. It is particularly indicated in the treatment of an acute, irritable spinal lesion.
Spinal Grade B mobilization
At the spinal joints, the term ‘Grade B mobilization’ is used to indicate that the mobilization technique is performed to the end of the available range.
As with peripheral joints, it aims to reduce pain and facilitate movement.
The important difference is that, whereas in peripheral joints the Grade B mobilization is normally performed into a painful range, with likely provocation of involuntary muscle spasm, the Grade B mobilization at the spinal joints should never be applied into pain or muscle spasm and the technique should be stopped immediately if either is provoked.
Spinal Grade C manipulation
The definition of a Grade C manipulation is shared by both peripheral and spinal joints: Grade C manipulation is a passive movement performed at the end of available range and is a minimal amplitude, high velocity thrust. It is worth repeating that, as for peripheral joints, spinal Grade C manipulation is not sustained, and should be applied with a small, quick overpressure, only when all the slack has been taken up.
Within the orthopaedic medicine approach, spinal Grade C manipulation can be applied to the sacroiliac joint as well as to the cervical, thoracic and lumbar spine.
Aims of spinal and sacroiliac joint manipulation
To restore movement
In the spinal or sacroiliac joints in which a non-capsular pattern exists, indicating internal derangement limiting the range of movement in some, but not all directions, manipulation aims to ‘unlock’ the joint, possibly reducing the internal derangement and relieving compression to restore full, painless function. As suggested above, the effects of mobilization and manipulation may not be that simple and are probably multifactorial, involving all adjacent tissues.
To relieve pain
Manipulation-induced hypoalgesia (pain relief) is well documented (Wright 1995, Vernon 2000). Restoration of functional movement may in itself lead to reduction in pain, but relief of pain may also be required first to achieve this. Pain relief may also occur through stimulation of the mechanoreceptors effecting the pain-gate mechanism or through stimulation of the descending inhibitory controls.
Mechanoreceptors within the joint capsule and spinal ligaments are stimulated by the tension created by spinal manipulation. This inhibits the small-diameter nociceptor afferent input to the ascending pathways at spinal cord level via the gating mechanism described above. The relief of pain achieved reduces the reflex muscle spasm and an increase in the range of movement occurs.
The periaqueductal grey (PAG) area of the brain, as a control centre for endogenous analgesia, is considered to play an important role in the control of pain. Two different forms of analgesia may be produced: an opioid form that seems to be associated with sympathetic inhibition and takes a period of time to develop, and a non-opioid form that is associated with sympathetic excitation that has a more rapid onset. Spinal manipulation has an immediate effect, producing pain relief within seconds or minutes due to the non-opioid form, associated with sympathetic excitation that is related to mechanical nociception. Over a period of about 20–45 min the analgesia changes to the opioid form associated with sympathetic inhibition. Spinal manipulation provides an appropriate stimulus to activate the descending pain inhibitory systems from the PAG to the spinal cord (Wright 1995).
Haavik-Taylor & Murphy (2007) looked at 12 subjects with a history of recurrent neck stiffness and neck pain, to establish the effect of manipulation on sensorimotor integration. The subjects had no acute symptoms at the time of the study and all were screened for contraindications, including vertebal artery insuffiency, before being treated with manipulation. The single session resulted in cortical evoked responses for up to 30 min post-manipulation before returning to baseline levels. These transient cortical changes may help to explain the mechanisms responsible for the pain relief and restoration of function following manipulation.
It must not be forgotten that spinal manipulation can also relieve pain through the placebo effect of skilful handling and care. Indeed, Ernst (2000) offers the opinion that ‘the success of manipulation is largely due to a placebo effect’. Further discussion on this topic is outside the scope of this book except to acknowledge the role of the placebo effect as one of the myriad of factors that can affect treatment outcomes. It is not possible to quantify this effect, although many trials try to eliminate its effect by establishing controls.
A discussion follows on manipulation, its proposed effects and how they might be achieved.
Discussion on the effects of spinal manipulation
By definition, to manipulate is defined as ‘to work with one’s hands, to treat by manipulation’ (Chambers Dictionary 2006). Historically the term was applied to describe any technique that was applied using the hands. The term manipulation has now come to mean ‘a minimal amplitude, high-velocity movement performed at the end of range’. The essence of this definition has been adopted by all practitioners of manipulation, and may also commonly be termed ‘Grade C’, ‘high velocity thrust’ or ‘Grade V’ technique, etc.
Spinal manipulation can produce an immediate reduction in pain and a dramatic restoration of range of movement, but the mechanism by which manipulation achieves this is not well understood. Clinicians must work to the current best models in attempting to provide an explanation for their manual treatment interventions to both professional colleagues and patients alike. The benefit of manipulation may come from many interrelated factors rather than a single mechanism. A major factor towards successful manipulation is in the selection of appropriate patients with uncomplicated back pain of recent onset.
A key point of an applied manipulation is that it is passive, i.e. out of the patient’s control, and, as with spinal Grade B mobilization, it should never be performed through pain or spasm. It aims to move beyond the end of the passive range of the joint into the ‘paraphysiological space’, described by Sandoz (1976) as existing after the end of the passive limit but before the limit of anatomical integrity is reached where damage can occur. This section will briefly discuss some theories that relate to the proposed effects of manipulation. The evidence to support the application of manipulation as part of the management of back pain will be discussed within the relevant chapters covering the cervical, thoracic and lumbar regions of the spine and the sacroiliac joint.
The effects of manipulation on the soft tissues are still to be fully established but moves have been made towards developing a ‘theory of manipulation’ to explain treatment outcomes. Drawn from the consensus of opinion of many authors, the broad effects of manipulation are the relief of pain, relief of muscle spasm and the restoration of movement and function.
Twomey (1992) drew together, then generally dismissed, the traditional theories that manipulation reduces subluxations, corrects vertebral alignment, adjusts nuclear prolapse or tears joint adhesions. Shekelle (1994) modified these theories, however, suggesting that manipulation releases entrapped synovial folds or plicae, ‘unbuckles’ displaced motion segments, relaxes hypertonic muscle by sudden stretching, or disrupts articular or periarticular adhesions.
With respect to the first hypothesis listed above, synovial fringes are described within the facet joint (Bogduk 2005) but theories suggesting that they may present as a primary or secondary source of pain through entrapment are unsubstantiated. Twomey (1992) has already described the synovial fringes as ‘slippery’ structures that are unlikely to be trapped.
Earlier, Taylor & Twomey (1986) presented the hypothesis that, rather than implicating the synovial fringes, the ‘acute locked back’ arises from impaction of a tag of articular cartilage within the apophyseal space, rather as a torn meniscus in the knee that, being connected to a well innervated capsule, can be a sudden cause of pain. ‘Gapping’ of the joint surfaces could allow the tag to return to its normal position so relieving the load on the capsule, allowing normal movement to return and a subsequent reduction in pain. This model depends on the demonstration that ‘gapping’ of the apophyseal joint does indeed occur with manipulation, which will now be explored.
The relative movements between the target and other vertebrae are difficult to demonstrate and several studies rely only on mathematical predictions based on the forces applied. Dolan & Adams (2001) support that moderately flexed postures tend to unload the apophyseal joints. This is also supported by Punjabi et al cited in Lee & Evans (2001), who note that flexion increases the size of the intervertebral foramina. Gal et al (1995) performed a series of lower thoracic manipulative thrusts on two unenbalmed post-rigor mortis human cadavers and recorded significant relative movements during thrusts, that were primarily between the targeted vertebra and immediately adjacent vertebrae. Vertebral pairs remained slightly hyperextended after the thrusts, even when the posteroanterior forces had returned to preload levels. The link to the in vivo situation is probably unreliable but Gal et al concluded that their findings might be a useful step towards understanding the deformation behaviour of the vertebral column during manipulation.
Solinger (1996) set out to develop an analytical theory to describe the dynamics of small movement impulses applied to vertebrae in manipulation. He noted an initial passive oscillatory response to the thrust, governed by ligamentous and discogenic forces, and a subsequent less regular motion that he postulated was caused by muscular reflex contractions. He also measured relative separations of the L2, L3 vertebrae in vivo following high-speed manipulative thrusts and demonstrated that both force and speed or duration of the thrust are important to optimize the efficacy of manipulation.
Zusman (1994) corroborates the suggestion that manipulation moves or frees the impediment, permitting movement and halting nociceptive input and associated muscle spasm. He also highlights the need for this to be demonstrated and suggests that magnetic resonance imaging (MRI) will hold the key to demonstrating what is happening at spinal level as manipulation is applied.
Cramer et al (2000) performed a small randomized trial on 16 healthy subjects comparing the effects of side-posture with rotation positioning and side-posture with rotation adjusting (manipulation) on the lumbar zygapophyseal joints, as evaluated by before and after measurements of MRI scans. Two other small groups in the study were measured in the neutral position, both before and after manipulation. The side-posture with rotation position itself produced some separation that was apparently increased following manipulation. This comparative increase was less than 1 mm. Both groups measured in neutral remained unchanged. The authors of the study acknowledge that a larger clinical trial is needed to define their results further.
The technology for producing moving MRI images (interventional MRI) has now been developed and McGregor et al (2001) used this method to observe the effects of Grade I and Grade IV posteroanterior mobilization in the cervical spine to assess for any movement at individual spinal levels. The scans demonstrated clear deformation of the overlying soft tissues and some angulation of the spine as a whole but minimal, if any, intervertebral movement (Fig. 4.4).
 |
| Figure 4.4
Sagittal images obtained during (a) Grade 1 PA mobilization of 6th cervical vertebra; (b) Grade 4 PA mobilization of 6th cervical vertebra.
From McGregor A, Wragg P, Gedroyc W M W (2001) Can interventional MRI provide an insight into the mechanics of a posterior-anterior mobilisation? Clinical Biomechanics 16:926–929. Reprinted by permission of Elsevier Ltd.
|
Twomey (1992) noted the common belief at the time that the disc was not the primary cause of back pain and that the zygapophyseal joint was more likely to be the source of the problem. Adams & Hutton (1981) and Adams et al (2002) have examined the biomechanical behaviour of the disc under different stresses, supporting the shift of evidence towards internal derangements within the outer annulus of the disc as the primary cause of back pain.
Bogduk (2005) describes common theories of discal pathology that he terms ‘internal disc disruption’ where the internal architecture of the disc is disrupted with degradation of the nucleus pulposus and the presence of radial fissures in the lumbar spine that may reach the outer third of the annulus. The outer annulus is known to have a nerve supply and may therefore present as a primary cause of back pain. In internal disc disruption, the external surface remains essentially normal but the nucleus may bulge, as a bulge or protrusion, into the outer layers of the disc, where it may also produce pain.
Mixter & Barr (1934) were the first to suggest that rupture of the intervertebral disc could be a causal factor in sciatica, and Cyriax was one of the first clinicians to propound that in nuclear prolapse discal material ruptures through the outer annulus into the vertebral canal, where it can have a secondary effect on pain-sensitive structures. Cyriax (1982), Twomey (1992) and Zusman (1994) all suggest that manipulation is unlikely to change or benefit a nuclear disc prolapse and there is little support from the literature that manipulation is – or is not – effective with this underlying pathology. The model of spontaneous recovery (Bush et al 1992) may provide a more plausible explanation for resolution of disc prolapse (see Ch. 13).
Several earlier and more recent studies have aimed to examine the effect of forces on the spine to explain disc degeneration (Farfan et al 1970, Adams & Hutton 1981, Noren et al 1991, Krismer et al 1996, Adams et al 2002) that may provide the groundwork towards the development of a hypothesis for the mechanism of treatment effects, particularly on the application of rotational manipulation techniques. Bogduk (2005) describes torsion injuries that can strain the collagen fibres of the annulus fibrosus, comparable to a ligament sprain. He warns that the condition has yet to be demonstrated in vivo but offers a feasible model (discussed below) for the pathology acquired on injury and for the effect of manipulation in relieving symptoms.
The reflex response to manipulation has invited study towards clarification of the therapeutic benefits of manipulation. Herzog et al (1999) demonstrated consistent EMG reflex responses following manipulation. Colloca & Keller (2001) and Keller & Colloca (2000) studied the EMG reflex responses to manipulation and found that patients with frequent to constant low back pain tended to have more marked responses than patients with occasional or intermittent back pain.
Evans (2002) discusses mechanisms for the relaxation of hypertonic muscle, describing both reduction in paraspinal spontaneous electromyography activity and reduction in hyperalgesia of paraspinal myofascial trigger points. Since hypertonic muscle, i.e. muscle spasm, would tend to be protective, he suggests that the emphasis should be on the creation of post-manipulative hypoalgesia that may lead in itself to relaxation of hypertonic muscle, the mechanisms of which are still being investigated.
Lehman et al (2001) applied a painful mechanical stimulus above the spinous process in 17 subjects with chronic low back pain and found that manipulation weakened the ensuing electromyography response. Although there was no statistical significance in the results and the effects were short term, the possibility of these responses causing some of the clinically observed beneficial effects, including reduction of pain and decrease in hypertonicity of muscles, has been put forward.
Wyke’s work on the neurology of joints was pivotal in providing a model to explain the reflex relaxation of periarticular muscles following sudden stretching of the capsule, periarticular ligaments and tendons (Wyke (1967), Wyke (1979) and Wyke (1980)). Wyke (1979) and Zusman (1994) suggested that passive movements may activate the mechanoreceptors within capsular and other tissues.
In addition, Katavich (1998) concludes that the deformation of joint capsule can modify reflex motor responses, leading to changes in muscle tone. The author notes the work of Clarke where sustained or repetitive passive joint movement, or indeed pressure over the joint, has been shown to modify firing responses in large-diameter mechanoreceptor joint afferents, particularly when the capsular stretch is maintained at or near the end of range. This presents an explanation that accounts for the pain-relieving effects of end-range mobilization, including passive Grade B stretching techniques (see above) as well as manipulation.
If these observations are put together, mobilization and manipulation may stimulate mechanoreceptors to modify neural activity within the central nervous system, altering reflex pathways that affect the voluntary and involuntary activation of muscles and leading to relief of muscle spasm, increased movement and reduction in pain (Wright 1995).
The general rule is that manipulation should not be applied through spasm and that other techniques or modalities, such as graded mobilizations, heat or electrotherapy, may need to be applied to achieve reflex relaxation of this protective mechanism. It was the opinion of Brodeur (1995), however, that the high velocity thrust of manipulation had a split second’s chance to affect the capsule and the Golgi organs within the periarticular contractile structures before any reflex spasm could resist the movement.
Cyriax, in 1984, and for at least 50 years before, did not shirk from his opinion that ‘when minor ligamentous adhesions limit movement and cause pain they should be manipulatively ruptured’. His statement referred mainly to peripheral joints, and in spinal lesions he tended to refer to the effects of manipulation on what he termed ‘small cartilaginous displacements’. He put forward these disruptions of the annulus fibrosus as a primary cause of low back pain of sudden onset, a theory that is supported by Bogduk (2005) (see below).
Lamb (1994) suggested that manipulation ‘releases minor adhesions’ and Zusman (1994) acknowledges the formation of fragile collagen fibrils in the early stages of repair, suggesting that graduated or ‘abrupt’ alteration in the length or location of undesirable and restrictive scar tissue is the most logical means by which ranges of movement might be improved with manually delivered passive movements: the ‘abrupt’ presumably describing manipulation.
Cramer et al (2000) present a theoretical model of manipulation breaking adhesions within the zygapophyseal joints and Bogduk (2005) describes torsion injuries that can strain the collagen fibres of the annulus fibrosus, comparable to a ligament sprain. If the fibres can be injured by torsion, the implication is that they can be affected by manipulative techniques, particularly those applied with rotation.
Dolan & Adams (2001) comment on the mathematical models that are often used to quantify the overall forces and moments acting on the lumbar spine, and explain that in order to describe how spinal tissues can be injured, it is necessary to distribute these forces between and within different spinal structures. They emphasize that it is the concentration of the force which causes injury and elicits pain. It may be reasonable, therefore, to extrapolate that opinion to propose that it is also the concentration of force on the application of manipulation that can lead to the relief of pain.
Scientific evidence is perhaps lacking in terms of knowing exactly what does happen to adverse adhesions, if they are implicated in joint hypomobility, but biomechanical models are useful towards the development of hypothesis.
As referred to above, Threlkeld (1992) describes the viscous properties of connective tissue and explains that when a tissue is loaded more quickly it behaves more stiffly than the same tissue loaded more slowly. Thus adhesions may be ruptured more readily by stress applied quickly, as in manipulation. If the concentration of forces applied in manipulation tends to fall selectively on the resisting scar tissue within the annulus, before the normal tissue is affected (Threlkeld 1992) (see Ch. 2), this can provide a sound model for the achievement of increased movement and pain relief with particular presentations of back pain.
Manipulation is often associated with an audible ‘crack’, said to be due to cavitation of the joints; a small diversion onto the subject of cavitation is pertinent at this stage. The forces applied on manipulation increase the joint volume, with a corresponding decrease in fluid partial pressure, creating a gas bubble as the intra-articular gases are drawn out of the solution. As the fluid rushes into the area of low pressure volume, the gas bubble collapses, producing the ‘crack’ (Herzog et al 1993). Some practitioners argue that the ‘crack occurs’ as the bubble forms but any significance in the distinction is unclear.
Gibbons & Tehan (2001) propose that it is the cavitation that distinguishes manipulative techniques from other manual modalities and many clinicians judge the success of a manipulation by whether or not cavitation occurs (Conway et al 1993). Gibbons & Tehan focus on appropriate positioning to be able to localize forces to achieve cavitation, with the implication that it is a necessary component, although they do not specify why.
Ross et al (2004) state that cavitation is not an accurate guide to being specific in manipulation since it can occur at multiple joints and is only accurate 50% of the time in manipulation. In general, the manipulative techniques used in the orthopaedic medicine approach do not require specificity, although some are applied ‘at the painful level’, as will be seen in the relevant spinal chapters.
Brodeur (1995) provided a comprehensive review of the ‘audible release’ associated with joint manipulation. He cited an early study by Roston & Haines which demonstrated with serial X-rays of the third metacarpal joint that if the distraction force was high enough in the third metacarpophalangeal joint to produce a crack, there was a dramatic increase in joint space of up to 3 mm. Cavitation was not achieved in lax or overly tight articular capsules but an increase in the joint space was still apparent, albeit that it was achieved more steadily.
Gibbons & Collins (2001) aimed to determine whether high velocity thrust manipulation of the atlanto-axial joint improved pre-existing atlanto-axial rotation asymmetry in asymptomatic subjects, and then to compare this with the effects of a sham thrust without cavitation. The results indicated that while there was a trend towards improvement of the more restricted rotation in both the cavitation and non-cavitation groups, neither reached significance and no evidence was provided that the noiseless manipulation is or is not as effective as the manipulation with cavitation.
The placebo effect of the patient hearing or feeling a ‘click’ can be powerful; it can be perceived that ‘something has been done’ to remove the cause of their pain. More nervous or suspicious patients might perceive that the fact that ‘something has been done’ could have made them worse, however.
Sweetman (2009) updated the review on ‘clicks and clunks’ and concluded that there was no correlation between cavitation and the typical electromyographic changes produced at manipulation, nor with therapeutic benefit. The orthopaedic medicine approach to manipulation does not focus on the requirement to achieve cavitation.
Evans (2002) provides an interesting discussion of the effects of manipulation but appears to misrepresent that Cyriax suggested that manipulation could benefit nuclear herniation. As discussed in Chapter 13, Cyriax (1982, 1984) did attribute presentations of low back pain to discal pathology but produced models of ‘cartilaginous’ and ‘nuclear’ displacements, devising associated sets of signs and symptoms to guide treatment choice. The cartilaginous presentation was linked to disruption of the annulus of the disc, often, perhaps erroneously, called ‘fragments’ of cartilage, and was suggested as being suitable for manipulation where changes could be effected within the collagen structure of the annulus, with a consequent sudden reduction in pain. The ‘nuclear’ presentation was designated by Cyriax as being more suitably treated with traction, in line with the more progressive or prolonged positions more appropriate for inducing movement in the viscoelastic nucleus, as Evans suggests.
Evans’ theories relating to structures within the zygapophyseal joint as a possible cause of the ‘locked back’, and as discussed above, are indeed feasible but they are no more proven than theories relating to symptoms associated with torn fibres within the collagen structure of the annulus, as put forward by Bogduk (2005) and less explicitly by Cyriax himself. Thus, in the meantime, the model of disruption of fibres within the outer annular layers can provide a possible effect of manipulation in breaking down adhesions in the outer annulus.
While the debate on the pathology of back pain and the effects of manipulation will no doubt continue, the reader of this text will be encouraged to devise treatment programmes based on models of presentation and patient selection, with careful consideration of patients’ history and presenting signs and symptoms, and thorough screening for possible contraindications to treatment.
As a final consideration, Lee et al (1996) draw attention to the many variations in technique that may affect the movements at the spine. They can be summarized as: the magnitude of the applied force, the rate of increase of the force, the duration of loading and the accuracy and specificity of the application of the force. These, along with other variations in the patient, the clinician and the treatment couch, all need to be considered within the clinical reasoning process and can confound efforts to develop an ultimate theory of spinal manipulation.
Principles of application of spinal and sacroiliac joint manipulation
Certain principles need to be considered in order to apply the technique effectively.
A short- or long-lever arm is used and a torque or twisting force is usually applied, taking up the slack in the surrounding connective tissue to induce a passive movement at one or more spinal joints (Hadler et al 1987). The minimal amplitude, high velocity thrust is of great importance since tissues loaded quickly behave more stiffly and have a higher ultimate strength (see Ch. 2). Thus, as the spinal manipulative technique is applied, the force needed to affect the target tissue does not cause damage to the surrounding structures (Threlkeld 1992), i.e. the weak link in the chain effect.
Manipulative reduction is more likely to succeed if the joint space is increased. Therefore an element of distraction is usually included. Spinal manipulation, in particular, requires skilful handling that takes time and practice to acquire. The hands must be continuously sensitive to the end-feel of the joints and any abnormal end-feel, e.g. bony resistance or muscle spasm, should alert the manipulator to abandon the procedure.
Successful manipulation requires accurate clinical diagnosis and the choice of the right patient with know-ledge of the effects, contraindications and limits of the technique. Thought, care and expert skill are essentials, together with an explanation to the patient of the diagnosis, treatment and possible complications, in order to gain informed consent. Safety is essential and the precautions necessary will be discussed in the appropriate chapters.
TRACTION
The terms traction and distraction have the same meaning in describing a force applied to produce separation of joint surfaces and widening of the joint space. There is a convention that traction is applied to spinal joints and distraction to peripheral joints, but this is not a hard and fast rule.
This section considers the theory underpinning the traction and distraction techniques used in orthopaedic medicine for both peripheral and spinal joints. Emphasis will be placed on the use of traction as a treatment for back and neck pain. Many other treatment modalities employ distraction in the treatment of peripheral joint lesions and the aims and effects will be described briefly here.
The general indications for traction and distraction will be mentioned, but these, together with the contraindications for treatment, will be discussed in greater detail in the following chapters.
The aims of traction were originally devised from Cyriax’s hypotheses of the effects on the spinal joints. They have been developed subsequently to encompass the peripheral joints, where appropriate, and may be stated as follows:
To relieve pain
Pain relief through the use of traction is gained by relieving pressure. Traction reduces the compression force, muscles relax and the pressure on the joint and pain-sensitive structures is reduced.
To create space
The application of distraction/traction creates a degree of space within the joint, allowing a displaced bulge or fragment, a loose body or a displaced carpal bone room to move.
To produce negative pressure within a joint
Creating space within the joint creates a suction effect that helps to move a displaced intra-articular fragment.
To tighten the ligaments around a joint
Tightening up of the ligaments around the joint produces a centripetal force. A disc displacement will tend to be pushed towards the centre as the traction is applied.
To reduce a loose body in a peripheral joint
Distraction is the main element of the manoeuvre, with a Grade A mobilization applied during traction. The aim is to move the displaced fragment to another area of the joint where it does not block joint movement.
Indications for traction at peripheral joints
Stiff osteoarthritic joints respond well to distraction, as do joints demonstrating the capsular pattern following adaptive changes in response to traumatic arthritis. The distraction is applied to increase mobility, using longitudinal or lateral distraction techniques, which may produce separation of joint surfaces and assist mobilization by stretching the joint capsule and capsular ligaments.
It may be applied manually as a treatment in itself or in conjunction with mobilization and manipulation techniques.
Where signs and symptoms indicate the presence of a loose body in a joint, or a carpal bone needs to be relocated, distraction before Grade A mobilization is applied. The distraction of joint surfaces allows space for movement of the fragment, assisted by the accompanying suction effect which distraction tends to produce.
Indications for spinal traction
Cyriax (1982) identified factors from the patient’s history, together with certain symptoms and signs, which he believed were characteristic of a nuclear disc protrusion. Treatment with sustained traction was therefore indicated to reduce the protrusion.
He stated that, with a nuclear protrusion, the pain is usually of gradual onset, often with no recollection of the mode or time of onset. There is usually little central pain, but referred pain is usually present in the arm or leg. The pattern of spinal movement is non-capsular and the movements provoke the limb pain.
Cyriax ascribed the pathology to posterolateral movement of the protrusion, compressing the dural nerve root sleeve and producing unilateral pain. Smyth & Wright (1958) had also proposed this mechanism but when, in the light of investigation, opinion moved away from the notion of a ‘jelly-like’ nucleus, thus refuting the possibility of a nuclear ooze, Cyriax’s hypothesis was largely rejected. The empirical findings nonetheless remained that traction could afford relief with that particular onset and pattern of pain.
Mathews & Hickling (1975) suggested that traction should be applied to a defined syndrome rather than a specific condition. This would appear to be a more satisfactory premise against which clinicians from the myriad of musculoskeletal backgrounds can apply the basic principles, while the debate on pathology continues as fresh evidence emerges.
Apart from the application of mechanical traction, in the cervical spine most of the mobilization and manipulation techniques are performed under manual traction.
When applying the techniques of traction or distraction to the cervical spine or peripheral joints, the following principles should be employed:
• Body weight should be used.
• The application of body weight should be achieved by leaning out with straight arms.
• The distraction/traction should be sustained for a moment or two to become established before proceeding with the manoeuvre.
Discussion on the evidence for the aims and effects of traction
To provide some support for the aims, the following paragraphs set out to review the evidence for the effects and effectiveness of traction, particularly as a treatment for back and neck pain. Since the discussion covers all regions of the spine it is more appropriate to keep it together within this section than to attempt to divide it into the appropriate spinal chapters.
‘Traction is generally considered to be an empirical treatment’, stated Colachis & Strohm (1965), and, in spite of several well organized trials to demonstrate the contrary, this statement unfortunately continues to be true.
In these days of evidence-based practice, demands have been made for traction, as an expensive, time-consuming modality, to be stopped as a treatment until evidence for its effectiveness has been produced. Mathews & Hickling’s presentation of their paper (1975) was criticized for not honestly stating that no evidence had been found and an editorial in the British Medical Journal (‘Trial by traction’, BMJ 1976) urgently called for thorough research into traction to avoid further waste of resources.
Swezey (1983) represented the views of the many who use traction when he acknowledged that ‘there is a consensus among thoughtful clinicians that traction is useful, and until further study argues to the contrary, it deserves a place in our therapeutic armamentarium’. Sharing that view, Saunders (1998) set out to evaluate the reviews that were considered for the Clinical Guidelines for the Management of Low Back Pain (Waddell et al 1996) that concluded that ‘traction does not appear to be effective for low back pain with radiculopathy’.
Saunders provides an interesting discussion which addresses the methodological weaknesses of the studies selected for the review, suggesting that the randomized controlled trial on this single modality, aiming for statistical significance, is clinically inappropriate and that a multi-modal approach to treatment, including the use of specific and general exercise programmes, is likely to be more realistic in the clinical setting. He compares the trials in the reviews to a separate series of articles which, while they may not have achieved statistical significance, have suggested that traction may be effective for a specific population.
Van der Heijden et al (1995a), in one of the reviews considered by Waddell et al (1996), acknowledge that the studies they included ‘do not allow clear conclusions due to methodological flaws in their design and conduct’. Van der Heijden et al (1995b) also designed a pilot study to compare the effect of high-dose continuous lumbar traction and low-dose continuous lumbar traction on the magnitude and rate of recovery for patients with low back pain. They tentatively concluded that traction seemed to be more effective than sham traction in the short term but acknowledged that the small numbers involved prevented the achievement of statistical significance. Following evaluation of the pilot study, it was extended by Beurskens et al (1997) to include a total of 151 subjects. Beurskens et al were meticulous in their methodology, including patient selection, but nonetheless the study failed to demonstrate a significant difference between the two groups.
A literature search conducted by van Tulder & Koes (2002) for Clinical Evidence Concise, the self-acclaimed ‘international source of the best available evidence for effective health care’, concluded that randomized controlled trials found conflicting evidence and no support for the effects of traction for both acute and chronic low back pain. The question of patient selection and the parameters of the studies reviewed are left unstated, however, and Saunders (1998) warns that we should avoid making judgments about traction based on reports drawn from reviews without critically reviewing the articles ourselves. There was no mention in the same issue of Clinical Evidence Concise of the effects of traction on cervical pain.
A study has emerged that supports the inclusion of traction in the treatment of confirmed symptomatic disc herniation and therefore implies the importance of patient selection in evaluating the technique. Ozturk et al (2006) set out to assess the effect of continuous lumbar traction on the size of herniated disc material in lumbar disc herniation. The size of the herniation was measured by computed tomography. Forty-six patients with lumbar disc herniation were included in this randomized controlled trial; 24 patients were assigned to the traction group and 22 patients to the control group. Both groups were given physical therapy modalities including hot packs, ultrasound and diadynamic currents, and the experimental group was also given traction, continuously for 15 min. All patients were given 15 sessions of daily (weekdays) treatment by the same physical therapist. The size of the herniated disc material decreased significantly only in the traction group. Data collected for the clinical signs and symptoms also showed increased improvement in the traction group.
Traction has been documented as a treatment for back pain at least since the time of Hippocrates (Hume Kendall 1955). Cyriax himself developed his first traction bed in 1949 and collaborated with his physiotherapists on how it could be used to relieve the symptoms produced by nuclear disc protrusion. His belief was that pain of gradual onset, usually associated with leg pain, was due to nerve root compression from herniated nuclear material through a tear in the annulus, i.e. a disc protrusion (see Ch. 13).
Cyriax’s hypothesis was that effective traction could produce a suction effect within the disc to draw the nuclear material centrally and away from the nerve root. At the same time a mechanical ‘push’ would be given to the herniated material by tightening the ligaments spanning the bulge – the posterior longitudinal ligament in particular. Andersson et al, cited in Lee & Evans (2001), studied intradiscal pressure and showed Cyriax’s hypothesis of a suction effect to be unfounded. However, Cyriax had claimed that in order to achieve these effects traction would need to produce separation of the intervertebral and zygapophyseal joints between the vertebrae accompanied by tensioning of the intervertebral ligaments, and there is considerable evidence that this separation occurs.
To demonstrate the separation of the vertebrae, Cyriax (1982) compared two X-rays of the cervical spine taken before and after the application of ‘fairly’ strong manual traction for several seconds. He measured an increase of 2.5 mm at each of the joints between the fourth cervical and first thoracic vertebrae. His maximum manual traction had previously (in an experiment in 1955) been estimated to be 140 kg, but his use of the term ‘fairly’ implies something less than that. Similarly, before and after X-rays of the lumbar spine were superimposed upon each other after 50 kg of traction had been applied for 10 min, and a widening of the disc space was observed.
Judovitch (1952) took a series of X-rays as progressive poundages were applied to the cervical spine of seven patients. He observed that with 45 lb (20.5 kg) of static, vertical traction there was separation of the interbody joints of C2–C7 and a small widening of the zygapophyseal joints. A similar serial study was performed by Colachis & Strohm (1966), but using 30 lb (14 kg) of intermittent cervical traction at a 24° angle of pull. In all 10 subjects the mean separation of the vertebrae increased proportionally, both anteriorly and posteriorly, to a maximum at 25 min.
On the assumption that increased disc space with traction would be manifest in an increase in height, Worden & Humphrey (1964) applied sustained traction of up to 132 lb (60 kg) to normal fit males. The traction was divided between the head and thorax and pelvis and ankles for 1 h on each of several consecutive days. They demonstrated an average increase in height of 8 mm.
They continued to observe two subjects and noted that they lost their increased height at a rate of 4 mm/h. In the Colachis & Strohm study mentioned above, no increase in posterior separation remained at 20 min after the cessation of traction but the residual anterior separation was statistically significant (Colachis & Strohm 1966).
Mathews (1968) used epidurography, involving the injection of radio-opaque contrast medium into the epidural space for subsequent radiological investigation, and applied 120 lb (55 kg) of lumbar traction to three patients, two with suspected disc protrusions and one control. In one patient the bulge of the protrusion began to decrease in depth from 4 min and was further decreased at 20 min, with partial recurrence on release.
Evidence was provided for Cyriax’s proposed suction effect on observing a second patient where not only was there a reduction in the depth of the multiple protrusions, but also contrast medium appeared to have been drawn into the disc spaces, implying the production of negative pressure within the disc.
The apparent suction effect may be a sequel to the reduction of load on the disc when traction is applied. Nachemson (1980), with the use of a miniature intra-discal pressure transducer, measured the approximate load on the L3 disc in a 70 kg individual, in different postures and exercises. Minimal load of 30 kg was recorded on the disc with the subject in supine lying and this was reduced to 10 kg on the application of 30 kg of traction.
This finding provided foundation for Cyriax’s suggestion that the same effect on a disc prolapse could be achieved in a short time with sustained traction as that brought about by weeks or months of bed rest. Bed rest had disadvantages in terms of expense, if prolonged hospitalization was involved, as well as incapacitating the patient. Lumbar traction, on the other hand, would allow the patient to continue with daily activities and commitments.
Lee & Evans (2001) have examined loads in the lumbar spine during traction therapy and propose that traction does not simply produce axial distraction of the spine. They suggest that traction also produces a flexion moment leading to an increase in the posterior height of the discs and a flattening of the lumbar lordosis. This effect is enhanced by the use of the Fowler’s position (hips and knees flexed to 90°) and may have clinical significance in tightening the posterior annulus and the overlying posterior longitudinal ligament, theoretically stimulating mechanoreceptors and reducing a confined disc prolapse towards the relief of pain.
Lumbar flexion has been demonstrated to increase the size of the intervertebral foramina (Punjabi et al cited in Lee & Evans 2001) and this would tend to support the use of traction as a treatment tool to enlarge a pathologically narrowed foramen, i.e. with symptoms of spinal stenosis.
Since the ambulant position subjects the disc to compressive forces (Nachemson 1980), certain considerations need to be applied to prevent the loss of the benefits of traction between treatments. Cyriax (1982) claimed that traction needs to be:
• As strong as possible
• Applied for as long as is both practicable and tolerable
• Applied daily, or at least five times a week
• Applied continuously
When Cyriax proposed that the physiological and biomechanical effects of static traction were more effective at reducing a nuclear protrusion, the debate of continuous (static) versus intermittent traction had little evidence to support either side.
Wyke, referred to in Cyriax (1982), observed that motor activity in the sacrospinalis muscles increased as the distracting force was increased, until the mechanoreceptors in the tendons were stimulated, producing an inhibitory effect after which the stress was allowed to fall on the spinal joints. Cyriax claimed that electromyographic (EMG) silence in the sacrospinalis was not achieved until 3 min into the application of traction. For this reason he proposed that intermittent traction would not be as suitable to produce nuclear movement, since repeated pulls of shorter duration would continually elicit the stretch reflex, producing muscular contraction and preventing joint distraction.
Cyriax’s argument for wanting to avoid muscular contraction is supported by the study of Goldie & Reichmann (1977) to examine the influence of traction on the cervical spine. They observed that the force of muscle contraction in the cervical spine could overcome a traction force in excess of 30 kg (66 lb).
However, Hood & Chrisman (1968) used EMG recordings of lumbar sacrospinalis activity to compare the effect of static and intermittent traction and found no difference. Moreover, Klaber Moffett et al (1990) investigated the effects of sustained cervical traction on neck musculature using EMG recordings. They applied 6–12 lb (3–5 kg) of traction for 20 min in the recumbent position, but found that relaxation was not induced.
Support for sustained, as opposed to intermittent, traction may lie in the hydrostatic behaviour of the disc (Nachemson 1980). Sustained traction would be expected to be more effective in producing creep in the tissues gradually to reduce the herniation by the combined effect of both suction from within the disc and the ‘push’ produced by the tightening of the tissues spanning the joint.
Onel et al (1989) performed computed tomographic investigation of 30 patients diagnosed as having disc prolapse to observe the effect of traction on lumbar disc herniations. While establishing that 45 kg of traction was effective in reducing disc herniations, it was also apparent that intervertebral joint and zygapophyseal joint space was increased, with associated stretching of all anatomical structures of the spine. Grieve (1981) had already acknowledged the much wider range of application of traction, irrespective of the disc. The effect of traction on other tissues would suggest that it should be seen as a useful and adaptable method of mobilization of the motion segment, or segments, as a whole. This being so, intermittent traction would also have a place in mobilizing stiffness in the motion segments, relieving pain through the stimulation of the mechanoreceptors and by increasing circulation, as well as by reducing the compressive effects.
ORTHOPAEDIC MEDICINE INJECTION TECHNIQUES
This text will provide an outline of the principles and application of injection therapy. The interested reader is referred to other texts that cover injections if further detail is required. In orthopaedic medicine, injection techniques are often an alternative treatment to transverse frictions and mobilization techniques. Transverse frictions and mobilization appear to be most efficacious when used together or with other physiotherapy modalities. This also applies to the use of injection techniques that also benefit from attention to biomechanical and causative factors to prevent recurrence. However, injection techniques may initially stand alone to allow for a period of relative rest and protection to gain maximum benefit from the injection. The drugs administered often have effects for some time after the application of treatment and they must be given time to achieve these beneficial effects.
In many instances the orthopaedic medicine clinician has a choice of treatments to apply: either injection or transverse frictions and mobilization (e.g. tendinopathy). In some cases an injection is more appropriate (e.g. bursitis), and in others transverse frictions may be more appropriate (e.g. musculotendinous lesions). Injections tend to be more cost-effective when compared to manual therapy (e.g. capsulitis). The need for collaboration between the patient, physician and physiotherapist is emphasized to ascertain the best mode of treatment for the patient.
Corticosteroid injections
Corticosteroids have a potent anti-inflammatory effect, much more dramatic than the anti-inflammatory effect of non-steroidal anti-inflammatory drugs (NSAIDs), and are therefore used therapeutically to achieve this effect in orthopaedic medicine. They are applied intra-articularly or intralesionally for chronic inflammatory lesions, including inflammatory arthritis. The intense anti-inflammatory effect is also beneficial in some acute lesions (e.g. acute bursitis or an acute flare in chronic arthritis) but is unwanted in other conditions (e.g. acute muscle belly lesion and acute ligamentous lesions) because of its detrimental effects on collagen production. Corticosteroids are also indicated in many chronic conditions, including tendinopathy, where inflammation may not be a feature and their exact mode of action is not yet fully understood in this situation.
As well as their anti-inflammatory effect, corticosteroids have other potent effects, including immunosuppression and a delay in the normal physiological process of healing. These effects cannot be separated from the beneficial effects of corticosteroids. Much of the reported unwanted effects of corticosteroids involve systemic, high-dose applications of the drugs for long periods of time.
A locally applied injection of corticosteroid in orthopaedic medicine aims to achieve a rapid and intense local anti-inflammatory effect. The local effect will depend on the dose applied, the relative potency of the corticosteroid and its solubility, which determines the length of time it stays in the tissues. In orthopaedic medicine the aim is to give the smallest dose that will achieve the desired effects, although there is increasing evidence that larger doses may give better results in some conditions, e.g. osteoarthritis of the knee and hip and capsulitis at the shoulder.
Intra-articular injections are most appropriate if there is an inflammatory component; therefore they are indicated whenever a joint capsule is inflamed, e.g. in rheumatoid arthritis, traumatic arthritis and acute episodes of osteoarthrosis. Intralesional injections are applied to chronic tendinopathy, tenosynovitis, bursitis and ligamentous lesions, usually associated with inflammatory arthritis.
The use of corticosteroids would seem to affect all stages of the inflammatory process, the initial acute phase of heat, redness, swelling and pain, as well as the proliferative phases of repair and remodelling.
Corticosteroids also produce a potent immunosuppressive effect, which is why it is so important to pay attention to aseptic technique.
In this text the corticosteroid referred to is triamcinolone acetonide. Two different concentrations of triamcinolone acetonide, Adcortyl (10 mg/mL) and Kenalog (40 mg/mL), provide versatility for different applications. In spite of the past traditional use of Adcortyl, as noted in older orthopaedic medicine texts, Kenalog is now in much wider use and recommended dosages of triamcinolone acetonide are usually obtained by drawing up the appropriate volume of Kenalog. In light of this, Adcortyl will not be referred to from here on.
Corticosteroid injection technique
The appropriate dose (corticosteroid alone, or mixed with local anaesthetic) will be determined by the size of the joint or lesion to be injected and the previous response to injection, if any. The size of the patient is another basic factor to take into account when deciding on the dose. For many of the injections in this book a range is provided for the dose of corticosteroid and clinical judgment will be needed to guide selection.
With regard to mixing the drugs, at the time of writing it is illegal under the Medicines for Human Use Regulations (1994) for physiotherapists to mix cortico-steroid and local anaesthetic in the same syringe as part of a patient group direction (PGD), since that would be considered to create a new product which is not licensed for use. The Chartered Society of Physiotherapy presented a position paper in March 2008 (CSP 2008) that was prepared in collaboration with the Medicines and Healthcare products Regulatory Agency (MHRA) and the Department of Health and PGD Website Board members. It was made clear that to comply with the law, when working under a PGD, CSP members would need to modify their injection practice which would involve choosing to use a pre-mixed commercially available preparation; giving two separate injections; not using local anaesthetic or varying administration techniques so that products do not mix in the syringe, e.g. using separate syringes for each drug.
A patient specific direction (PSD) is a written prescription from a doctor, dentist or other independent prescriber where the patient is identified by name. Physiotherapists can mix the drugs under a PSD, in accordance with the doctor’s directions, and in these situations the mixing of drugs is the doctor’s direct responsibility. Physiotherapists trained in injection therapy are advised to contact the CSP for information and updating.
Triamcinolone acetonide
• Kenalog 40 mg/mL, usually contained in 1 mL vials (Fig. 4.5).
 |
| Figure 4.5
Containers of drugs in common use for musculoskeletal injections.
|
Accurate needle placement is essential. This depends on clinical diagnosis. Knowledge of anatomy will allow accurate location of the lesion and avoid unnecessary complications. Points worth considering are:
• The tissue in which the lesion lies
• How deep the lesion lies
• The extent of the lesion
• The position of the patient to make the site of the lesion accessible
• The direction in which the needle should be inserted
• The length of needle and the size of syringe required.
Unwanted side-effects of corticosteroid therapy
Most of the unpleasant side-effects of corticosteroid therapy relate to large, long-term doses of the drugs taken systemically. In looking at the systemic effects of cortico-steroid injection in the knee following injection with 40 mg triamcinolone hexacetonide, Wittkowski et al (2007) concluded that the corticosteroid was restricted to the site of injection and had very little systemic effect. The study supports the clinical observation that it is rare to produce systemic side-effects from a single local cortico-steroid injection, although it is acknowledged that it can happen with larger doses.
Post-injection flare is a self-limiting increase in pain occurring 6–12 h after injection once the local anaesthetic effect has subsided. Symptoms usually resolve within 48 h, but may mimic infection, which should be suspected if symptoms do not settle in the expected time.
Local soft tissue atrophy and pigment changes. These may occur with inaccurate needle placement and/or leakage of fluid, particularly of long-acting corticosteroids, into subcutaneous tissue, and have been documented to promote fat necrosis, dermal atrophy, senile purpura, depigmentation and subcutaneous atrophy (Ponec et al 1977, Marks et al 1983, Grillet & Dequeker 1990). These subcutaneous changes may be reversible, although the patient may be left with long-lasting evidence of injection. The best way to minimize the risk of this side-effect is accurate needle placement and good needle proprioception. Some injectors also advise using hydrocortisone for superficial injections as, being more soluble, it is less likely to cause these problems.
Connective tissue weakening is believed to occur due to a loss in tensile strength of the collagen tissue, possibly leading to tendon rupture in particular. The balance of collagen synthesis is altered to produce degradation of fibres and new immature scar tissue is laid down. Appropriate mobilization is required at the right time to encourage alignment of fibres and increased tensile strength.
The corticosteroid will affect the normal tissue as well as the damaged tissue. During its time of action, corticosteroid potentially causes a weakening of collagen fibres, damaged and normal, and this should be recognized as a potential hazard. For this reason it is especially important that steroid is not injected into the body of a tendon, only into its sheath or at the teno-osseous junction. In all cases, the patient is instructed to rest from aggravating activities while normal, pain-free function is encouraged, to provide mechanical stimulus for the new fibres. Since triamcinolone acetonide is absorbed within approximately a 2-week period, it would seem appropriate to advise the patient to rest for up to 2 weeks if practical.
Steroid arthropathy, a Charcot-type arthropathy, has been reported in association with corticosteroid injections; however, reports have refuted this (Grillet & Dequeker 1990, Cameron 1995). It has been considered that the deterioration in the cartilage and joint may be more likely to be due to the underlying inflammatory or degenerative condition rather than the treatment with corticosteroids. It is appropriate to instruct the patient to rest following intra-articular injection. Cameron (1995) reviewed the evidence on steroid arthropathy and found that the greatest risk of its development is from prolonged, high-dose oral steroids in the presence of associated underlying disease.
There is a general consensus that repeated corticosteroid injections into weight-bearing joints should be limited to a maximum of two, or perhaps three, per year because of the risk of steroid arthropathy. However, Raynauld et al (2003) conducted a randomized, double blind, placebo-controlled trial to determine the efficacy of long-term intra-articular corticosteroid injection (40 mg triamcinolone acetonide every 3 months) in osteoarthritis of the knee. The 1- and 2-year follow-up showed no difference in the loss of joint space over time between the corticosteroid injection group and the saline injected group. There was a trend in the corticosteroid group to show greater symptomatic relief with pain and stiffness significantly improved. The authors concluded that there was no significant deleterious effect on the anatomical joint structure in either group, indicating that repeated intra-articular injections are safe.
Iatrogenic (clinician induced) septic arthritis is a rare but avoidable side-effect. It is difficult to establish the exact incidence for septic arthritis and Hughes (1996) suggests figures that range widely between 1:15 000–50 000.). D. Knott (personal communication 2009) pulls the range in closer to between 1:20 000–30 000. The ‘no touch’ technique described below is advised.
Systemic effects include: hyperglycaemia in a diabetic patient; facial flushing in the first 2–3 days and lasting a day or two; menstrual disturbance, including postmenopausal bleeding.
Local anaesthetic
The earliest local anaesthetic, cocaine, first used in 1860, was employed as a local anaesthetic for surgery. A synthetic substitute, procaine, was developed in 1905 and many other compounds have since been produced.
Local anaesthetics work by penetrating the nerve sheath and axon membrane. They produce a reversible blockade to impulse conduction and do this more readily in small-diameter, myelinated and unmyelinated nerve fibres. Nociceptive and sympathetic impulses are therefore blocked more readily (Rang et al 2003).
This pain-relieving effect of local anaesthetic is utilized in orthopaedic medicine for its therapeutic and diagnostic effects. Given together with corticosteroid, local anaesthetic allows immediate reassessment of the patient to confirm diagnosis and to ensure that the lesion has been completely infiltrated. It gives the patient initial pain relief and may reduce the effects of post-injection flare from corticosteroid injection. It is also useful to distribute the corticosteroid throughout the extent of the lesion and can provide an element of distension which may be useful in joints and bursae.
In this text, lidocaine (lignocaine) hydrochloride will be used. It has a rapid rate of onset and moderate duration. It may be used as 0.5%, 1% or 2% solution and the maximum dose is 200 mg (equal to 20 mL of a 1% solution, for example). This dose is within safe limits to give by infiltration to a fit adult of average weight; it would be unsafe to give this dose intravenously.
Unwanted side-effects of local anaesthetic
It is important to recognize the maximum dose of local anaesthetic in order to avoid the unwanted side-effects (Rang et al 2003) and patients should always be questioned about sensitivity to previous injections. Steps should always be taken to ensure that the needle does not lie in a blood vessel, by performing a safety aspiration before injection, to avoid giving the dose intravenously.
Central nervous system effects may initially be due to stimulation and may include feelings of inebriation and light-headedness, restlessness and tremor, confusion progressing to extreme agitation. Further increase in drug levels leads to depression of the central nervous system which may present as sedation, twitching, convulsion or respiratory depression which is potentially life-threatening.
Cardiovascular effects may present as a combination of myocardial depression and vasodilatation producing sudden hypotension which may be life-threatening. Myocardial depression occurs as a result of inhibition of sodium current in cardiac muscle. Vasodilatation is due to the local anaesthetic effect on the smooth muscle of the arterioles and sympathetic inhibition.
Allergic reactions include rashes and anaphylaxis which, although rare, can be life threatening. It is therefore a prerequisite that any practitioners using local anaesthetic must be trained in how to recognize and manage this complication.
Contraindications to corticosteroid or local anaesthetic injection
Absolute
• Absence or withdrawal of patient consent
• Allergy to corticosteroid or local anaesthetic
• Sepsis
■ Systemic – significant/febrile (e.g. active TB)
■ Local
■ Suspected
Relative
• Poorly controlled diabetes or hypertension
• Anticoagulants or blood clotting disorders
• Fracture
• Psychosis
• Regular doses of systemic steroids
• Immunosuppressed patient
• Tuberculosis (non-active)
• Pregnancy
‘No-touch’ technique
A ‘no-touch’ technique is essential to minimize the risk of infection. It is essential to avoid injecting in the presence of infection because the corticosteroid also has an immunosuppressive effect.
The following procedure describes the full injection procedure and incorporates the ‘no-touch’ technique:
• Having decided that injection is appropriate for your patient and after screening for contraindications, discuss the treatment options, ensure that the patient understands the proposed treatment and gain informed consent.
• Position the patient making sure that the lesion and injection site are accessible.
• Assemble equipment – needles, syringes, alcohol swabs, cotton wool, sharps bin, plaster; check and record drugs.
• Wash hands – soap and water/surgical scrub.
• Draw up the corticosteroid and local anaesthetic – change needle for delivery.
• Mark skin.
• Apply gloves or wash hands.
• Clean the skin – alcohol swabs, etc.
• Deliver the injection – without touching the needle or the injection site. Perform a safety aspiration prior to infiltration, use appropriate peppering or bolus technique (see below), withdraw and dispose of sharps; apply plaster/dressing, ensuring there are no allergies to these.
• Reassess after 5 min keep the patient in the department for 30 min to check that there is no reaction. Advise relative rest for approximately 2 weeks; reassess the outcome.
Needle size (Fig. 4.6)
Syringes (Fig. 4.7)
Two techniques are generally used to deliver the injection. A bolus technique is used for joint spaces and bursae, where no resistance is felt on pressing the plunger. A peppering technique is used for tendon insertions and ligaments where a series of small droplets is delivered throughout the substance of the structure to cover the extent and depth of the lesion.
 |
| Figure 4.6
Needles in common use for musculoskeletal injections.
|
 |
| Figure 4.7
Syringes in common use for musculoskeletal injections.
|
Lack of response to corticosteroid injection may require a review of the diagnosis and the technique. It is fairly common to have a relapse or recurrence of symptoms in soft tissue lesions. This is particularly likely if the patient returns to the overuse activity too soon or the cause of the problem is not properly investigated and remedied.
Non-steroidal drugs also aim to reduce inflammation and relieve pain associated with musculoskeletal lesions and although their mechanism of action is different, a note of their effects might be useful at this point.
Non-steroidal anti-inflammatory drugs (NSAIDS)
These are some of the most widely used drugs for musculo-skeletal lesions as well as the symptoms of headaches, flu, colds and other minor aches and pains. However, nearly all NSAIDs have side-effects, the most notorious being gastrointestinal problems, which caution against their long-term use. They can also cause harm to the kidneys and the cardiovascular system if taken for prolonged periods in susceptible individuals; NSAIDs seem to have their action within the injured tissue itself.
NSAIDs produce the following three main effects:
• Anti-inflammatory: they modify the inflammatory reaction by reducing vasodilatation, oedema and permeability of the postcapillary venules.
• Analgesic: they reduce pain by inhibiting prostaglandin formation. They are therefore most effective in treating the chemical pain produced by inflammation in which the prostaglandins sensitize the nociceptors. They have little effect in mechanical pain, which is why they are not particularly effective in treating the acute pain associated with recent disc protrusion.
• Antipyretic: they reduce a raised temperature, but have no effect on the normal body temperature.
NSAIDs commonly prescribed include ibuprofen, naproxen, indometacin, piroxicam, diclofenac and aspirin.
In acute inflammatory musculoskeletal lesions (e.g. acute muscle belly lesions, acute ligament sprains and acute tenosynovitis) it is probably better to allow the first 3–5 days of the inflammatory phase to pass before using anti-inflammatory drugs such as the corticosteroids (Boruta et al 1990, Watson 2009). If the condition is very painful, NSAIDs may be administered, or other analgesics such as paracetamol, together with the physical treatments of controlled and precise mobilization coupled with protection, relative rest, ice, compression and elevation (PRICE). In chronic inflammatory states, however, the use of corticosteroid injection is strongly indicated and most beneficial, but the patient should be instructed to rest following injection.
Epidural injections
The use of epidural injections may be appropriate in the management of low back pain, particularly if sciatica is also present (see Ch. 13). The orthopaedic medicine approach is injection via the caudal route with the aim of bathing the dura mater and sensitized nerve roots in an anti-inflammatory solution with or without local anaesthetic to relieve pain.
Bush & Hillier (1991) discuss three possible mechanisms of pain relief, citing Bhatia & Parikh and Gupta et al, who join Cyriax (1984) in the suggestion that the introduction of the fluid local anaesthetic into the epidural space is enough in itself to influence the relationship between the disc and nerve root. Bush & Hillier (1991) also cite Wall & Melzack in describing how, in spite of its short action, the local anaesthetic may nonetheless break a pain cycle. Corticosteroid is usually added to the solution, however, with the logic of reducing both the swelling and inflammation at the disc–nerve root interface as an additional benefit of the large-volume injection (Bush & Hillier 1991). Studies have shown that although local anaesthetic in the epidural mixture gives short-term pain relief (a few hours) it makes no difference to the outcome in the medium to long term. Accuracy of needle placement is improved considerably if the injection is given with the aid of X-ray imaging.
It should be stressed that, although epidural injection via the caudal route can be performed as an outpatient procedure, the injection should only be carried out by an experienced medical practitioner who has undergone appropriate training.
The injection typically involves the introduction of 80 mg triamcinolone acetonide in 20–25 mL procaine, lidocaine or bupivacaine introduced via the sacral hiatus, using a no-touch technique. Mixing the steroid with saline is an alternative which is safer and has the same efficacy, but does not give the initial short-term pain relief which may be helpful diagnostically.
Most studies of epidural reveal an overall efficacy rate (removal of pain or worthwhile reduction) of approximately 70%.
Sclerosant therapy (prolotherapy)
The aim of sclerosant therapy is to increase ligament or tendon mass and ligament–bone or tendon–bone strength. Experiments have shown a statistically significant increase in collagen fibril diameter (Liu et al 1983), suggesting that sclerosing solution has an influence on connective tissue at the insertion sites.
Sclerosant solutions include hypertonic dextrose and sodium morrhuate and most practitioners are now using hypertonic dextrose
The following P2G mixture has been commonly used in the past:
• Phenol 2% w/v
• Dextrose 25% w/v
• Glycerol 30% w/v.
The sclerosant causes an intense inflammatory reaction at the site of injection. The intention in producing such an intense inflammatory reaction is to stimulate the formation of scar tissue. The immature scar tissue laid down is encouraged to contract and shorten by avoiding movement and stress during the repair and remodelling phases. The intense reaction causes considerable pain, but it would not be rational to use anti-inflammatory medication following sclerosant therapy.
Unwanted side-effects of sclerosant therapy
Strong phenol penetrates the nerve endings producing a local anaesthetic effect (Goodman & Gilman 1970) which is permanent. It is not known whether the very low concentrations used in sclerosant injections have any effect other than antiseptic.
REFERENCES
Adams, M.A.; Hutton, W.C., The relevance of torsion to the mechanical derangement of the lumbar spine., Spine 6 (1981) 241–248.
Adams, M.; Bogduk, N.; Burton, K.; et al., The Biomechanics of Back Pain. ( 2002)Churchill Livingstone, Edinburgh.
Akeson, W., The response of ligaments to stress modulation and overview of the ligament healing response, In: (Editor: O’Connor, J.J.; et al.) Knee Ligaments: Structure, Function, Injury and Repair ( 1990)Lippincott, Williams & Wilkins, Philadelphia, pp. 315–327.
Akeson, W.; Amiel, D.; La Violette, D., The connective-tissue response to immobility: a study of chondroitin-4 and 6-sulfate and dermatan sulfate changes in periarticular connective tissue of control and immobilized knees of dogs., Clin. Orthop. Relat. Res. 51 (1967) 183–197.
Akeson, W.; Woo, S.L.-Y.; Amiel, D.; et al., The connective tissue response to immobility: biochemical changes in periarticular connective tissue of the immobilized rabbit’s knee., Clin. Orthop. Relat. Res. 93 (1973) 356–362.
Akeson, W.; Amiel, D.; Woo, S.L.-Y., Immobility effects on synovial joints, the pathomechanics of joint contracture., Biorheology 17 (1980) 95–110.
Akeson, W.; Amiel, D.; Abel, M.F.; et al., Effects of immobilization on joints, Clin. Orthop. Relat. Res. 219 (1986) 28–37.
Amiel, D.; Woo, S.L.-Y.; Harwood, F.L.; Akeson, W.H., The effects of immobilization on collagen turnover in connective tissue: a biochemical-biomedical correlation, Acta Orthop. Scand. 53 (1982) 325–332.
Amis, A.A., Biomechanics of ligaments, In: (Editor: Jenkins, D.H.R.) Ligament Injuries and their Treatment ( 1985)Chapman and Hall, London, pp. 3–28.
Arem, A.J.; Madden, J.W., Effects of stress on healing wounds, 1: intermittent noncyclical tension, J. Surg. Res. 20 (1976) 93–102.
Beurskens, S.A.J.; de Vet, H.C.; Köke, A.J.; et al., Efficacy of traction for nonspecific low back pain: 12-week and 6-month results of a randomized clinical trial., Spine 22 (23) ( 1997) 2756–2762.
BMJ, Trial by traction [editorial], Br. Med. J. 1 (6000) ( 1976) 2–3.
Bogduk, N., Clinical Anatomy of the Lumbar Spine and Sacrum. 4th edn. ( 2005)Churchill Livingstone, Edinburgh.
Boruta, P.M.; Bishop, J.O.; Braly, W.G.; et al., Acute lateral ankle ligament injuries: a literature review, Foot Ankle 11 (1990) 107–113.
Bowsher, D., Modulation of nociceptive input, In: (Editors: Wells, P.E.; Frampton, V.; Bowsher, D.) Pain Management by Physiotherapy2nd edn. ( 1994)Butterworth Heinemann, Oxford, pp. 30–31.
Brandy, W.D.; Irion, J.M., The effect of time on static stretch on the flexibility of the hamstring muscles, Phys. Ther. 74 (9) ( 1994) 845–852.
Brandy, W.D.; Irion, J.M.; Briggler, M., The effect of time and frequency of static stretching on the flexibility of the hamstring muscles, Phys. Ther. 77 (10) ( 1997) 1090–1096.
Braverman, D.; Schulman, R., Massage techniques in rehabilitation medicine., Phys. Med. Rehabil. Clin. N. Am. 10 (3) ( 1999) 631–649.
Brodeur, R., The audible release associated with joint manipulation, J. Manipulative Physiol. Ther. 18 (3) ( 1995) 155–164.
Brosseau, L.; Casimiro, L.; Milne, S.; et al., Deep transverse friction massage for treating tendinitis, Cochrane Database Syst. Rev. (4) CD003528 ( 2002); Available: http://www.athensams.net June 2002.
Burke-Evans, E.; Eggers, G.W.N.; Butler, J.K.; et al., Experimental immobilization and remobilization of rat knee joints., J. Bone Joint Surg. 42A (1960) 737–758.
Bush, K.; Hillier, S., A controlled study of caudal epidural injections of triamcinolone plus procaine for the management of intractable sciatica., Spine 16 (5) ( 1991) 572–575.
Bush, K.; Cowan, N.; Katz, D.E.; et al., The natural history of sciatica associated with disc pathology., Spine 17 (1992) 1205–1212.
Cameron, G., Steroid arthropathy: myth or reality? A review of the evidence., J. Orthop. Med. 17 (1995) 51–55.
Carreck, A., The effect of massage on pain perception threshold., Manip. Phys. 26 (1994) 10–16.
Chamberlain, G.J., Cyriax’s friction massage: a review, J. Orthop. Sports Phys. Ther. 4 (1982) 16–22.
Chambers Dictionary. 2006. Chambers Harrap, Edinburgh.
Chartered Society of Physiotherapy, Guidelines for the Management of Soft Tissue (Musculoskeletal) Injury with Protection, Rest, Ice, Compression and Elevation (PRICE) During the First 72 Hours. ( 1998)Association of Chartered Physiotherapists in Sports Medicine, London.
Chartered Society of Physiotherapy, Chartered Society of Physiotherapy (CSP) Position Paper on the Mixing of Medicines in Physiotherapy Practice. ( 2008)CSP, London.
Colachis, S.C.; Strohm, B.R., A study of tractive forces and angle of pull on vertebral interspaces in the cervical spine., Arch. Phys. Med. Rheumatol. 46 (1965) 820–830.
Colachis, S.C.; Strohm, B.R., Effect of duration of intermittent cervical traction on vertebral separation, Arch. Phys. Med. Rehabil. 47 (1966) 353–359.
Colloca, C.J.; Keller, T.S., Electromyographic reflex responses to mechanical force, manually assisted spinal manipulative therapy., Spine 26 (10) ( 2001) 1117–1124.
Conway, P.J.W.; Herzog, W.; Zhang, Y.; et al., Forces required to cause cavitation during spinal manipulation of the thoracic spine, Clin. Biomech. 8 (1993) 210–214.
Cook, J.L.; Khan, K.M.; Maffulli, N.; et al., Overuse tendinosis, not tendinopathy, part 2: applying the new approach to patellar tendinopathy, Phys. Sports Med. 28 (6) ( 2000) 31–41.
Cramer, G.D.; Tuck Jr, N.R.; Knudsen, T.; et al., Effects of side-posture adjusting on the lumbar zygapophyseal joints as evaluated by magnetic resonance imaging: a before and after study with randomization, J. Manipulative Physiol. Ther. 23 (6) ( 2000) 380–394.
Cyriax, J., 8th ednTextbook of Orthopaedic Medicine. vol.1 ( 1982)Baillière Tindall, London.
Cyriax, J., 11th ednTextbook of Orthopaedic Medicine. vol. 2 ( 1984)Baillière Tindall, London.
Cyriax, J.; Cyriax, P., Cyriax’s Illustrated Manual of Orthopaedic Medicine.. ( 1993)Butterworth Heinemann, Oxford.
Davidson, C.; Ganion, L.; Gehlsen, G.; et al., Rat tendon morphologic and functional changes resulting from soft tissue mobilization, Med. Sci. Sports Exerc. 29 (3) ( 1997) 313–319.
de Bruijn, R., Deep transverse friction: its analgesic effect, Int. J. Sports Med. 5 (1984) 35–36.
Dolan, P.; Adams, M.A., Recent advances in lumbar spinal mechanics and their significance for modelling, Clin. Biomech. 16 (Suppl. 1) ( 2001) S8–S16.
Drechsler, W.; Knarr, J.; Snyder-Mackler, L., A comparison of two treatment regimes for lateral epicondylitis: a randomised trial of clinical interventions, J. Sport Rehabil. 6 (1997) 226–234.
Eliasson, P.; Fahlgren, A.; Pasternak, B.; et al., Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity., J. Appl. Physiol. 103 (2007) 459–463.
Enneking, W.F.; Horowitz, M., The intra-articular effects of immobilization of the human knee., J. Bone Joint Surg. 54 (1972) 973–985.
Ernst, E., Does spinal manipulation have specific treatment effects?Fam. Pract. 17 (6) ( 2000) 554–556.
Evans, P., The healing process at cellular level: a review., Physiotherapy 66 (1980) 256–259.
Evans, D., Mechanisms and effects of spinal high velocity, low amplitude thrust manipulation: previous theories, J. Manipulative Physiol. Ther. 25 (4) ( 2002) 251–262.
Farasyn, A., Referred muscle pain is primarily peripheral in origin: the barrier-dam theory., Med. Hypotheses 68 (2007) 144–150.
Farfan, H.F.; Cossette, J.W.; Robertson, G.H.; et al., The effects of torsion on the lumbar intervertebral joints: the role of torsion in the production of disc degeneration., J. Bone Joint Surg. 52A (1970) 468–497.
Faria, F.E.T.; Ferrari, R.J.; Distefano, G.; et al., The onset and duration of mobilization affect the regeneration in the rat muscle., Histol. Histopathol. 23 (2008) 565–571.
Fernández-de-las-Peñas, C.; Alonso-Blanco, C.; Fernández-Carnero, J., The immediate effect of ischaemic compression technique and transverse friction massage on tenderness of active and latent myofascial trigger points: a pilot study., J. Bodyw. Mov. Ther. 10 (2005) 3.
Gal, J.M.; Herzog, W.; Kawchuk, G.N.; et al., Forces and relative vertebral movements during SMT to unembalmed post-rigor human cadavers: peculiarities associated with joint cavitation, J. Manipulative Physiol. Ther. 18 (1) ( 1995) 4–9.
Gehlsen, G.; Ganion, L.; Helfst, R., Fibroblastic responses to variation in soft tissue mobilization pressure, Med. Sci. Sports Exerc. 31 (4) ( 1998) 531–535.
Gibbons, P.; Collins, A., Comparison of high velocity low amplitude manipulation with cavitation verses [ sic] non-cavitation: effect upon atlanto-axial rotation asymmetry in asymptomatic subjects., J. Orthop. Med. 23 (1) ( 2001) 2–8.
Gibbons, P.; Tehan, P., Patient positioning and spinal locking for lumbar spine rotation manipulation, Man. Ther. 6 (3) ( 2001) 130–138.
Goats, G.C., Massage – the scientific basis of an ancient art, part 1: the techniques, Br. J. Sports Med. 28 (1994) 149–152.
Goldie, I.F.; Reichmann, S., The biomechanical influence of traction on the cervical spine, Scand. J. Rehabil. Med. 9 (1977) 31–34.
Goodman, L.S.; Gilman, A., The Pharmacological Basis of Therapeutics. 4th edn. ( 1970)Macmillan, New York.
Gregory, M.A.; Deane, M.N.; Mars, M., Ultrastructural changes in untraumatised rabbit skeletal muscle treated with deep transverse friction., Physiotherapy 89 (7) ( 2003) 408–416.
Grieve, G.P., Common Vertebral Joint Problems. ( 1981)Churchill Livingstone, Edinburgh.
Grillet, B.; Dequeker, J., Intra-articular steroid injection: a risk benefit assessment, Drug Saf. 5 (1990) 205–211.
Haavik-Taylor, H.; Murphy, B., Cervical spine manipulation alters sensorimotor integration: a somatosensory evoked potential study, Clin. Neurophysiol. 118 (2) ( 2007) 391–402.
Hadler, N.M.; Curtis, P.; Gillings, D.B.; et al., A benefit of spinal manipulation as adjunctive therapy for acute low back pain: a stratified controlled trial., Spine 12 (1987) 703–706.
Hardy, M.A., The biology of scar formation, Phys. Ther. 69 (1989) 1014–1023.
Hardy, M.; Woodall, W., Therapeutic effects of heat, cold and stretch on connective tissue, J. Hand Ther. 11 (2) ( 1998) 148–156.
Hemmings, B.; Smith, M.; Graydon, J.; et al., Effects of massage on physiological restoration, perceived recovery and repeated sports performance, Sports Med. 34 (2000) 109–115.
Herzog, W.; Conway, P.J.; Kawchuk, G.N.; et al., Forces exerted during spinal manipulative therapy., Spine 18 (1993) 1206–1212.
Herzog, W.; Scheele, D.; Conway, P.J., Electromyographic responses of back and limb muscles associated with spinal manipulative therapy., Spine 24 (2) ( 1999) 146–152.
Hood, L.B.; Chrisman, D., Intermittent pelvic traction in the treatment of the ruptured intervertebral disk, Phys. Ther. 48 (1968) 21–30.
Huebscher, R., An overview of massage, part 1: history, types of massage, credentialing and literature, Nurse Pract. Forum 9 (4) ( 1998) 197–199.
Hughes, R.A., Septic arthritis., Rep. Rheum. Dis – Series 3, Pract. Probl. 7 (1) ( 1996).
Hume Kendall, P., A history of lumbar traction., Physiotherapy 41 (1955) 177–179.
Hunter, G., Specific soft tissue mobilization in the treatment of soft tissue lesions., Physiotherapy 80 (1994) 15–21.
Ingham, B., Transverse friction massage for the relief of tennis elbow., Physician Sports Med. 9 (1981) 116.
Iwatsuki, H.; Ikuta, Y.; Shinoda, K., Deep friction massage on the masticatory muscles in stroke patients increases biting force, J. Phys. Ther. Sci. 13 (1) ( 2001) 17–20.
Järvinen, M.J.; Lehto, M.U.K., The effects of early mobilization and immobilization on the healing process following muscle injuries, Sports Med. 15 (1993) 78–89.
Judovitch, B., Herniated cervical disc, Am. J. Surg. 84 (1952) 646–656.
Katavich, L., Differential effects of spinal manipulative therapy on acute and chronic muscle spasm: a proposal for mechanisms and efficacy, Man. Ther. 3 (3) ( 1998) 132–139.
Keller, T.S.; Colloca, C.J., Mechanical force spinal manipulation increases trunk muscle strength assessed by electromyography: a comparative clinical trial, J. Manipulative Physiol. Ther. 23 (9) ( 2000) 585–595.
Kelly, E., The effects of deep transverse frictional massage to the gastrocnemius muscle., J. Orthop. Med. 19 (1997) 3–9.
Khan, K.M.; Cook, J.L., Overuse tendon injuries: where does the pain come from?Sports Med. Arthrosc. Rev. 8 (2000) 17–31.
Klaber Moffett, J.A.; Hughes, G.I.; Griffiths, P., An investigation of the effects of cervical traction, part 2: the effects on the neck musculature, Clin. Rehabil. 4 (1990) 287–290.
Krismer, M.; Haid, C.; Rabl, W., The contribution of anulus fibers to torque resistance., Spine 21 (1996) 2551–2557.
Lamb, D.W., A review of manual therapy for spinal pain, In: (Editors: Boyling, J.; Palastanga, N.) Grieve’s Modern Manual Therapy2nd edn. ( 1994)Churchill Livingstone, Edinburgh.
Lee, M.; Steven, G.P.; Crosbie, J.; et al., Towards a theory of lumbar mobilization – the relationship between applied manual force and movements of the spine, Man. Ther. 1 (2) ( 1996) 67–75.
Lee, R.Y.W.; Evans, J.H., Loads in the lumbar spine during traction therapy, Aust. J. Physiother. 47 (2001) 102–108.
Lehman, G.J.; Vernon, H.; McGill, S.M., Effects of a mechanical pain stimulus on erector spinae activity before and after a spinal manipulation in patients with back pain: a preliminary investigation, J. Manipulative Physiol. Ther. 24 (6) ( 2001) 402–406.
Lehto, M.; Duance, V.C.; Restall, D., Collagen and fibronectin in a healing skeletal muscle injury., J. Bone Joint Surg. 67B (1985) 820–828.
Levangie, P.K.; Norkin, C.C., Joint Structure and Function: a Comprehensive Analysis. ( 2001)F A Davis, Philadelphia.
Liu, Y.K.; Tipton, C.M.; Matthes, R.D.; et al., An in situ study of the influence of a sclerosing solution in rabbit medial collateral ligaments and its junction strength, Connect. Tissue Res. 11 (1983) 95–102.
Loitz, B.J.; Zernicke, R.F.; Vailas, A.C.; et al., Effects of short-term immobilization versus continuous passive motion on the biomechanical and biochemical properties of the rabbit tendon, Clin. Orthop. Relat. Res. 244 (1988) 265–271.
Lorenzo, C.T., 2008. Lateral epicondylitis. Online. Available at eMedicine.com 30 Mar 2009
Low, J.; Reed, A., Electrotherapy Explained: Principles and Practice. ( 1994)Butterworth Heinemann, Oxford.
McGregor, A.; Wragg, P.; Gedroyc, W.M.W., Can interventional MRI provide an insight into the mechanics of a posterior-anterior mobilisation?Clin. Biomech. 16 (2001) 926–929.
Marks, J.G.; Cano, C.; Leitzel, K.; et al., Inhibition of wound healing by topical steroids, J. Dermatol. Surg. Oncol. 9 (1983) 819–821.
Mathews, J.A., Dynamic discography: a study of lumbar traction., Ann. Phys. Med. 9 (1968) 275–279.
Mathews, J.A.; Hickling, J., Lumbar traction: a double-blind controlled study for sciatica., Rheumatol. Rehabil. 14 (1975) 222–225.
Medicines for Human Use (Marketing Authorisation etc) Regulations 1994 (SI 1994/3149) HMSO, London.
Melzack, R.; Wall, P.D., The Challenge of Pain. 2nd edn. ( 1988)Penguin, Harmondsworth.
Mixter, W.J.; Barr, J.S., Rupture of the intervertebral disc with involvement of the spinal canal, N. Engl. J. Med. 211 (1934) 210–215.
Nachemson, A., Lumbar intradiscal pressure, In: (Editor: Jayson, M.I.V.) The Lumbar Spine and Back Pain2nd edn. ( 1980)Pitman, London, pp. 341–358.
Noren, R.; Trafimow, J.; Andersson, G.B.J.; et al., The role of facet joint tropism and facet angle in disc degeneration., Spine 16 (1991) 530–532.
Norris, C.M., Sports Injuries: Diagnosis and Management for Physiotherapists. 3rd edn. ( 2004)Butterworth Heinemann, Oxford.
Noyes, F., Functional properties of knee ligaments and alterations induced by immobilization, Clin. Orthop. Relat. Res. 123 (1977) 210–242.
Onel, D.; Tuzlaci, M.; Sari, H.; et al., Computed tomographic investigation of the effect of traction on lumbar disc herniations., Spine 14 (1989) 82–90.
Ozturk, B.; Gunduz, O.H.; Ozoran, K.; et al., Effect of continuous lumbar traction on the size of herniated disc material in lumbar disc herniation, Rheumatol. Int. 26 (7) ( 2006) 622–626.
Peacock, E.E., Some biochemical and biophysical aspects of joint stiffness, Ann. Surg. 164 (1966) 1–12.
Pellecchia, G.L.; Hamel, H.; Behnke, P., Treatment of infrapatellar tendinopathy: a combination of modalities and transverse friction massage versus iontophoresis., J. Sports Rehabil. 3 (1994) 135–145.
Ponec, M.; de Haas, C.; Bachra, B.N.; et al., Effects of glucocorticosteroids on primary human skin fibroblasts., Arch. Dermatol. Res. 259 (2) ( 1977) 117–123.
Rang, H.P.; Dale, M.M.; Ritter, J.M.; et al., Pharmacology. 5th edn. ( 2003)Churchill Livingstone, Edinburgh.
Raynauld, J.-P.; Buckland-White, C.; Ward, R.; et al., Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee, Arthritis Rheum. 48 (2) ( 2003) 370–377.
Ross, J.; Bereznick, D.; McGill, S., Determining cavitation location during lumbar and thoracic spinal manipulation., Spine 29 (13) ( 2004) 1452–1457.
Salter, R.B., The biologic concept of continuous passive motion of synovial joints, Clin. Orthop. Relat. Res. 242 (1989) 12–25.
Sandoz, R., Some physical mechanisms and effects of spinal adjustments., Ann. Swiss Chiropractic Assoc. 6 (1976) 91–141.
Saunders, H.D., The controversy over traction for neck and low back pain., Physiotherapy 84 (6) ( 1998) 285–288.
Saunders, S., Orthopaedic Medicine Course Manual. ( 2000)Saunders, London.
Schwellnus, M.P.; Mackintosh, L.; Mee, J., Deep transverse frictions in the treatment of iliotibial band friction syndrome in athletes: a clinical trial., Physiotherapy 78 (1992) 564–568.
Sevier, T.L.; Wilson, J.K., Treating lateral epicondylitis, Sports Med. 28 (5) ( 1999) 375–380.
Shekelle, P.G., Spine update: spinal manipulation., Spine 19 (1994) 858–861.
Smyth, M.J.; Wright, V., Sciatica and the intervertebral disc., J. Bone Joint Surg. 40A (1958) 1401.
Snodgrass, S.; Rivett, D.; Chiarelli, P.; et al., Factors relating to thumb pain in physiotherapists, Aust. J. Physiother. 49 (2003) 243–250.
Solinger, A.B., Oscillations of the vertebrae in spinal manipulative therapy, J. Manipulative Physiol. Ther. 19 (4) ( 1996) 238–243.
Stasinopoulos, D.; Johnson, M.I., It may be time to modify the Cyriax treatment of lateral epicondylitis., J. Bodyw. Mov. Ther. 11 (2007) 64–67.
Stratford, P.W.; Levy, D.R.; Gauldie, S.; et al., The evaluation of phonophoresis and friction massage as treatments for extensor carpi radialis tendonitis: a randomised controlled trial., Physiother. Can. 41 (1989) 93–99.
Sweetman, B., Click and clunks with low back pain; what do they mean? A literature review., Int. Musculoskelet. Med. 31 (1) ( 2009) 25–28.
Swezey, R.L., The modern thrust of manipulation and traction therapy, Semin. Arthritis Rheum. 12 (1983) 322–331.
Takai, S.; Woo, S.L.-Y.; Horibe, S.; et al., The effects of frequency and duration of controlled passive mobilization on tendon healing, J. Orthop. Res. 9 (1991) 705–713.
Taylor, J.R.; Twomey, L.T., Age changes in lumbar zygapophyseal joints: observations on structure and function., Spine 11 (1986) 739–745.
Threlkeld, A.J., The effects of manual therapy on connective tissue, Phys. Ther. 72 (1992) 893–902.
Tipton, C.M.; Vailas, A.C.; Matthes, R.D., Experimental studies on the influences of physical activity on ligaments, tendons and joints: a brief review., Acta Med. Scand. 711 (Suppl.) ( 1986) 157–168.
Twomey, L., A rationale for the treatment of back pain and joint pain by manual therapy, Phys. Ther. 72 (12) ( 1992) 885–892.
Usuba, M.; Akai, M.; Shirasaki, Y.; et al., Experimental joint contracture correction with low torque – long duration repeated stretching, Clin. Orthop. Relat. Res. 456 (2007) 70–78.
Van der Heijden, G.J.M.G.; Buerskens, A.J.H.M.; Koes, B.W.; et al., The efficacy of traction for back and neck pain: a systematic, blinded review of randomized clinical trial methods, Phys. Ther. 75 (1995) 93–104.
Van der Heijden, G.J.M.G.; Beurskens, A.J.H.M.; Dirx, M.J.M.; et al., Efficacy of lumbar traction., Physiotherapy 81 (1) ( 1995) 29–35.
Van Tulder, M.; Koes, B., Review – Low back pain and sciatica, In: Clinical Evidence Concise ( 2002)BMJ Publishing, London.
Vasseljen, O., Low level laser versus traditional physiotherapy in the treatment of tennis elbow., Physiotherapy 78 (1992) 329–334.
Verhaar, J.A.N.; Walenkamp, G.H.I.M.; van Mameren, H.; et al., Local corticosteroid injection versus Cyriax-type physiotherapy for tennis elbow., J. Bone Joint Surg. 78B (1996) 128–132.
Vernon, H., Qualitative review of studies of manipulation-induced hypoalgesia, J. Manipulative Physiol. Ther. 23 (2) ( 2000) 134–138.
Vicenzino, B.; Wright, A., In: (Editors: Unruh, A.M.; Baxter, G.; et al.) Pain: a Textbook for Therapists ( 2002)Churchill Livingstone, Edinburgh.
Videman, T., Connective tissue and immobilization: key factors in musculoskeletal degeneration?Clin. Orthop. 221 (1986) 26–32.
Waddell, G.; Feder, G.; McIntosh, A.; et al., Low Back Pain Evidence Review. ( 1996)Royal College of General Practitioners, London.
Warren, C.G.; Lehmann, J.F.; Koblanski, J.N., Elongation of the rat tail tendon: effect of load and temperature., Phys. Med. Rehabil. 52 (1971) 465–474.
Watson T 2009 Tissue repair phases and timescale. Online. Available: http://www.electrotherapy.org 30 Mar 2009
Weiner, I.J.; Peacock, E.E., Biologic principles affecting repair of flexor tendons, Adv. Surg. 5 (1971) 145–188.
Wells, P., Manipulative procedures, In: (Editors: Wells, P.E.; Frampton, V.; Bowsher, D.) Pain Management by Physiotherapy2nd edn. ( 1994)Butterworth Heinemann, Oxford, pp. 187–212.
Wieting, J.M.; Cugalj, A.P., Massage, traction and manipulation, J. Pain Symptom Manage. 28 (3) ( 2004) 244–249; Online. Available http://www.eMedicine.com.
Winter, B., Transverse frictions., S. Afr. J. Physiother. 24 (1968) 5–7.
Wittkowski, H.; Foell, D.; af Klint, E.; et al., Effects of intra-articular corticosteroids and anti-TNF therapy on neutrophil activation in rheumatoid arthritis, Ann. Rheum. Dis. 66 (8) ( 2007) 1020–1025.
Woo, S.L.-Y., Mechanical properties of tendons and ligaments, II: the relationship of immobilization and exercise on tissue remodelling, Biorheology 19 (1982) 385–408.
Woo, S.L.-Y.; Matthews, J.; Akeson, W.; et al., Connective tissue response to immobility: correlative study of the biomechanical and biochemical measurements of normal and immobilised rabbit knees, Arthritis Rheum. 18 (1975) 257–264.
Woo, S.L.-Y.; Wang, C.W.; Newton, P.O.; et al., The response of ligaments to stress deprivation and stress enhancement, In: (Editor: O’Connor, J.J.; et al.) Knee Ligaments: Structure, Function, Injury and Repair. ( 1990)Lippincott, Williams & Wilkins, Philadelphia, pp. 337–349.
Woodman, R.M.; Pare, L., Evaluation and treatment of soft tissue lesions of the ankle and forefoot using the Cyriax approach, Phys. Ther. 62 (1982) 1144–1147.
Worden, R.E.; Humphrey, T.L., Effect of spinal traction on the length of the body, Arch. Phys. Med. Rehabil. 44 (1964) 318–320.
Wright, A., Hypoalgesia post-manipulative therapy: a review of a potential neurophysiological mechanism, Man. Ther. 1 (1995) 11–16.
Wyke, B., The neurology of joints., Ann. R. Coll. Surg. Engl. 41 (1) ( 1967) 25–50.
Wyke, B., The neurology of the cervical spine joints., Physiotherapy 65 (1979) 72–76.
Wyke, B., The neurology of back pain, In: (Editor: Jayson, M.) The Lumbar Spine and Back Pain2nd edn. ( 1980)Pitman, London.
Zusman, M., What does manipulation do? The need for basic research, In: (Editors: Boyling, J.D.; Palastanga, N.) Grieve’s Modern Manual Therapy2nd edn. ( 1994)Churchill Livingstone, Edinburgh, pp. 651–672.