Organ Transplantation
Abdominal Organ Donation
The goals of the donor operation are to isolate the necessary anatomic structures and to prevent any damage to the structures to allow successful transplantation. This procedure involves the preservation of arterial and venous supplies to the relatively solid organs. Also, the cannulas must be placed for organ flush and perfusion with preservative solution. Typically, retrograde flush of the organs is accomplished through the distal aorta, with proximal clamping above the celiac axis. The venous effluent is released through an incision in the right atrium or cannulation of the distal cava (Fig. 18-1, A).
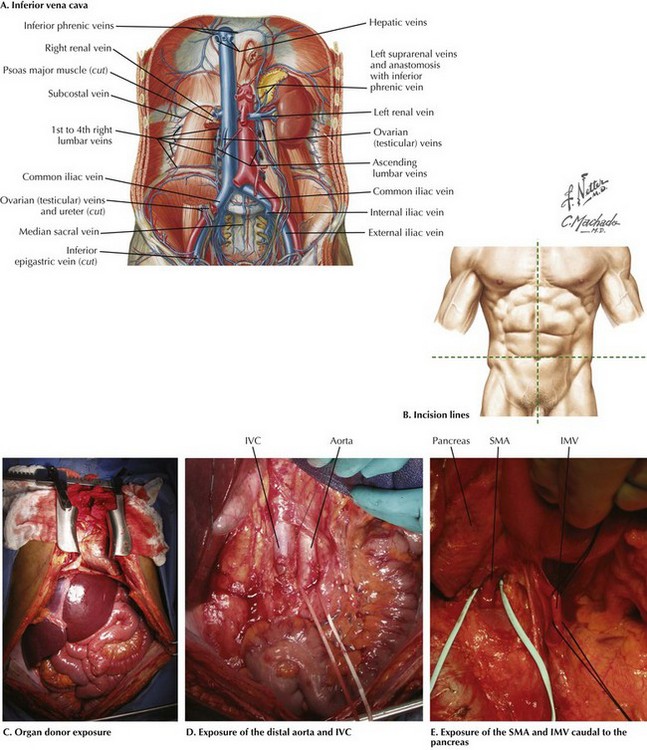
FIGURE 18–1 Abdominal anatomy and organ procurement exposures.
IVC, Inferior vena cava; SMA, superior mesenteric artery; IMV, inferior mesenteric vein.
Abdominal Surgical Approach
The incision for the donor surgery extends from the sternal notch to the pubis of the donor (Fig. 18-1, B and C). A sternotomy is performed, and most often, a sternal retractor and large Balfour retractor are used to provide exposure. When more abdominal exposure is needed, an abdominal cruciate incision can be utilized.
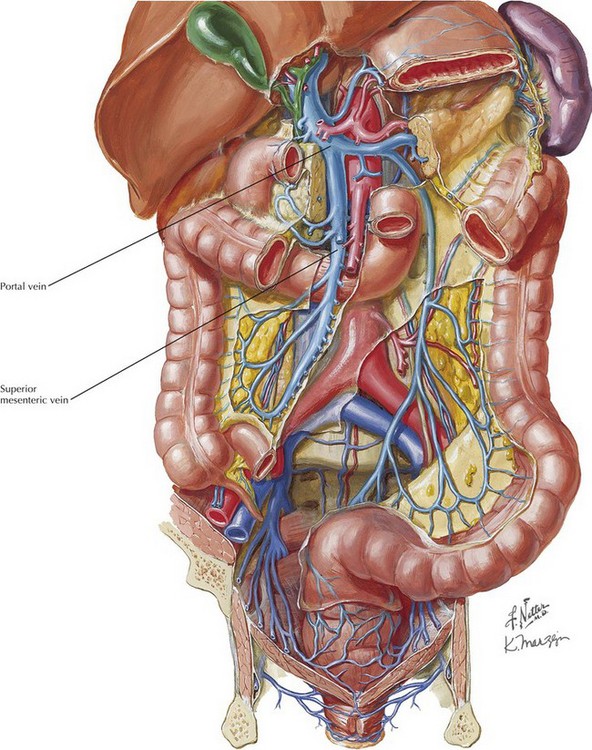
Mobilization of the right hemicolon with a right medial visceral rotation (Cattell-Braasch maneuver) is performed to expose the distal abdominal aorta and inferior vena cava (IVC) (Fig 18-1, D). The left renal vein can be exposed through this maneuver, and further dissection cephalad to the left renal vein will allow the surgeon to control the superior mesenteric artery (SMA). The distal aorta is encircled and cannulated, and the aorta is ligated just proximal to the iliac bifurcation after full heparinization before cross-clamping. Additionally, the inferior mesenteric vein (IMV) also can be isolated and encircled, which can provide access for portal system flush, if elected (Fig. 18-1, E).
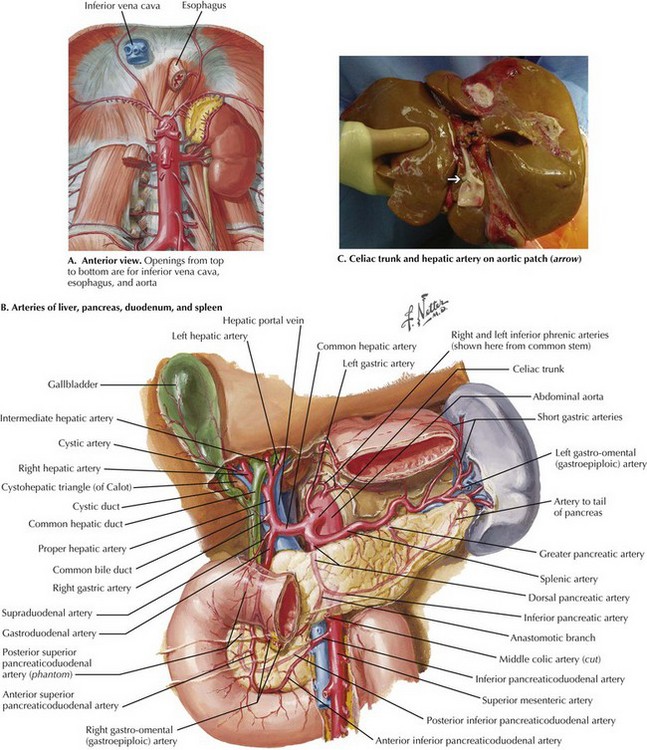
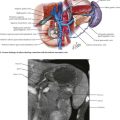
To obtain proximal intraabdominal aortic control for retrograde flush, the surgeon exposes the supraceliac aorta by dividing the crus of the diaphragm while retracting the left lobe of the liver (Fig. 18-2, A). The supraceliac aorta is encircled at this time with a vessel loop or umbilical tape. The gastrohepatic ligament is inspected for accessory left hepatic artery, which arises from the left gastric artery (see Chapter 16, Fig. 16-3). If present, the accessory left hepatic artery is preserved by careful dissection along the lesser curvature of the stomach. Also, the lateral portion of the portal triad is palpated for the presence of a replaced right hepatic artery; if present, it is preserved down to its origin arising from the SMA.
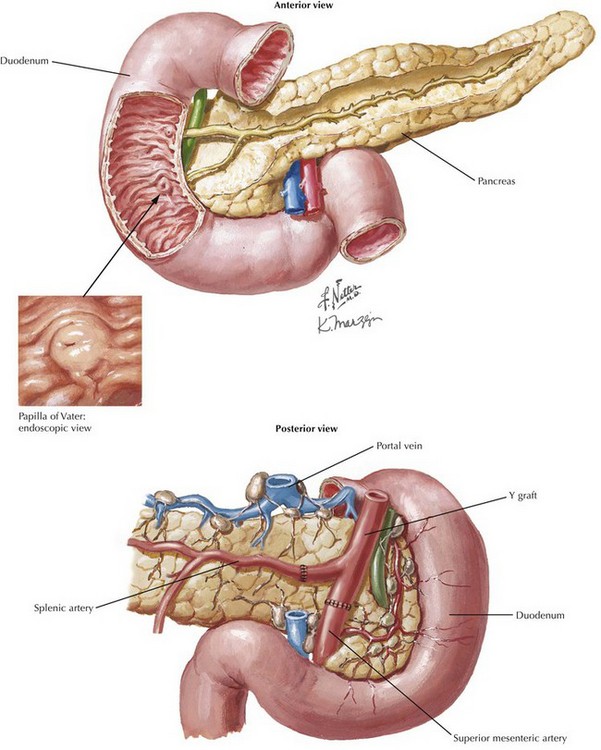
The portal triad structures are dissected to expose more of the hepatic artery, portal vein, and common bile duct (CBD) (Fig. 18-2, B). The peritoneum is divided at the cephalad border of the duodenum to expose the CBD laterally, and the gastroduodenal artery and common hepatic artery (CHA) medially. A full Kocher maneuver can also be performed at this time to trace a replaced right hepatic artery to the SMA origin. This is not necessary if there is no replaced right hepatic artery, or if the pancreas will not be procured. The gastroduodenal artery is identified and followed up to the CHA, which is dissected out to determine whether there are any other anatomic variations. Further dissection to the origin of the splenic artery is often performed “in the warm” (before cross-clamp and perfusion), whereas the rest of the dissection down to the celiac trunk is usually performed “in the cold” (after cross-clamp and perfusion). The donor liver must have a celiac trunk in continuity with the CHA for the intended recipient (Fig. 18-2, C).
When the pancreas is also being recovered for transplant, the entire pancreaticoduodenal allograft is taken in continuity. The spleen is then mobilized in continuity with the tail of the pancreas. The maneuvers are identical to performing a distal pancreatectomy (see Chapter 15). Once completed, the jejunum just distal to the ligament of Treitz is divided. Some surgeons perform gut decontamination of the stomach and duodenum by flushing iodine solution down the nasogastric tube. The proximal duodenum is then divided distal to the pylorus. The vascular supply of the whole pancreaticoduodenal allograft consists of the portal vein, the SMA stump, and the splenic artery. These structures are left intact for engraftment (see Pancreas Transplantation). The reconstruction of the arterial system is performed at the backtable (backbench) by anastomosing the iliac artery conduit (from the same donor) limbs to the SMA and the splenic artery by the recipient surgeon.
After cross-clamping the aorta and retrograde flushing of the organs, the surgeon completes dissection of the vessels for all the allografts being retrieved. The IVC is mobilized from the intrapericardial IVC to the infrahepatic IVC proximal to the renal veins. The CHA is dissected to the celiac trunk with a Carrel patch from the aorta. A stump of splenic artery is left with the pancreas, and the gastroduodenal artery is divided. The portal vein can be mobilized to the confluence of the superior mesenteric vein (SMV) and IMV. and splenic vein if the pancreas is not being taken (Fig. 18-3, A). In cases of pancreas recovery, approximately 1 cm of portal vein is left with the pancreas allograft.
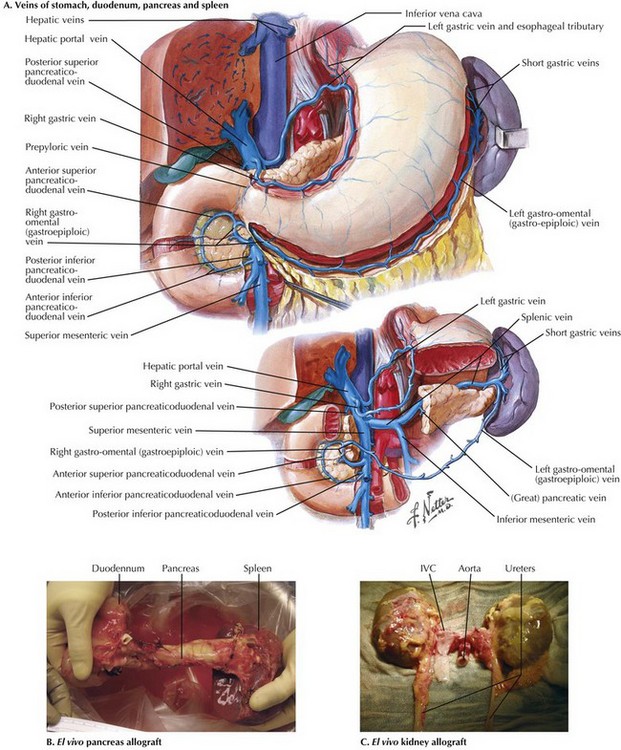
FIGURE 18–3 Pancreas and kidney procurement.
IVC, Inferior vena cava; SMA, superior mesenteric artery; IMV, inferior mesenteric vein.
For pancreas recovery, the entire pancreaticoduodenal allograft containing the duodenal segment, the entire pancreas, and the spleen is removed, often en bloc with the liver (Fig. 18-3, B). The small bowel mesentery is divided and oversewn or stapled. The SMA is kept in continuity with the pancreas allograft, and the IMV is ligated.
The kidneys are removed after the other abdominal organs. Dissection of the ureters in the retroperitoneum is performed as distal as possible. The IVC is divided proximal to the bifurcation, and the aorta and IVC are dissected en bloc, protecting the renal arteries and veins. The kidneys are dissected and removed en bloc with the adrenal glands (Fig. 18-3, C).
Kidney Transplantation
Kidney Surgical Approach
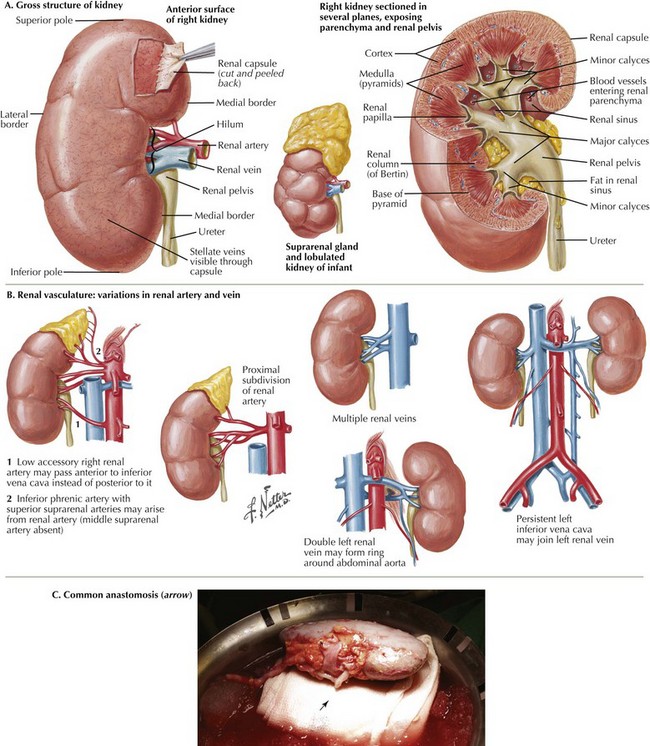
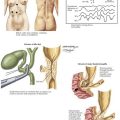
Classic renal anatomy includes single renal artery, renal vein, and ureter (Fig. 18-4, A), although multiple blood vessels can be present, and kidneys with duplicate ureters can still be used for transplant (18-4, B). If present, multiple arteries can be implanted on a common patch, joined together for a common anastomosis (Fig. 18-4, C), or implanted separately, depending on the distance between the vessels. Smaller accessory renal veins can be ligated because the venous system is collateralized within the kidney. For right kidneys, the donor IVC can be used as an extension, because the right renal vein can be very short. These reconstructions are often performed on the backbench.
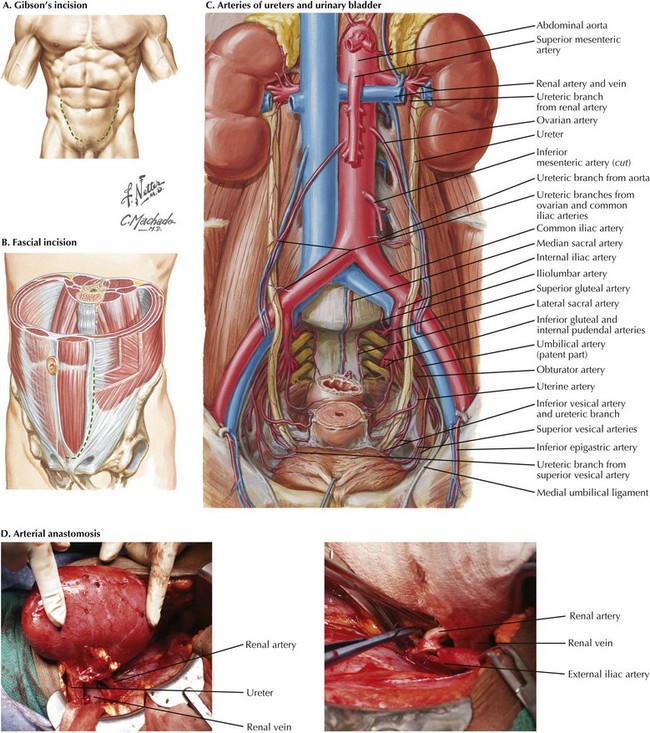
The preperitoneal space is entered on the right or left side. Gibson’s incision starts at the midline just over the pubis and is extended laterally to the edge of the rectus abdominis muscle sheath, at which point it follows the muscle’s edge (Fig. 18-5, A). The fascial incision is made along the edge of the rectus abdominis muscle (18-5, B). Without entering the peritoneum, the surgeon develops plane between the peritoneum and the lateral abdominal wall, from the area above the bladder laterally and along the psoas muscle superiorly, exposing the external iliac artery and vein, as well as the spermatic cord in men (Fig. 18-5, C). The rectus abdominis muscle usually does not need to be divided and is retracted medially along with the spermatic cord. The vessels are exposed.
The venous anastomosis is usually performed first, followed by the arterial anastomosis (Fig. 18-5, D). Clamps are taken off the vein first. The renal artery is temporarily occluded, and the clamps are taken off the iliac artery. The renal artery occlusion is released, reperfusing the kidney.
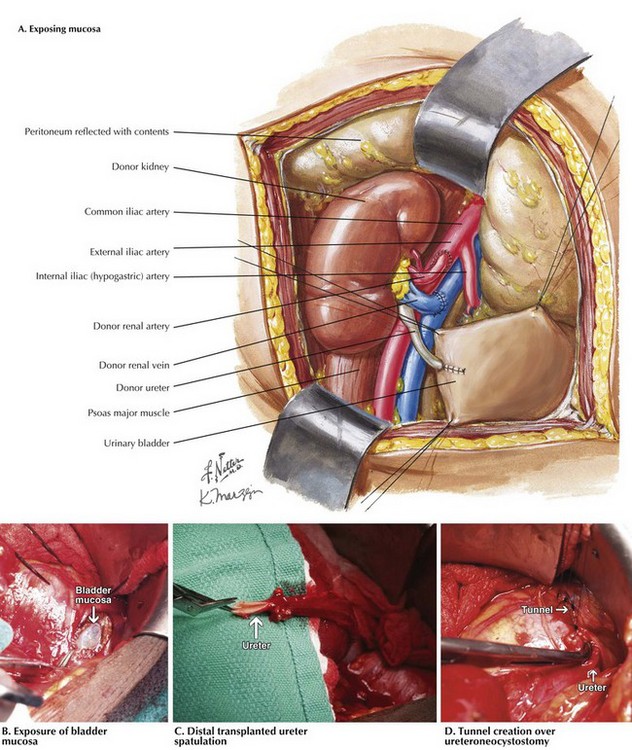
Once hemostasis is obtained, the bladder is distended in retrograde fashion and a 2-cm incision is made through the muscle layers of the bladder, exposing mucosa (Fig. 18-6, A and B). The ureter is cut to length and spatulated, and the surgeon performs a modified Lich-Gregoir ureteroneocystostomy with absorbable suture after creating a hole in the mucosa. A tunnel is then made over a portion of the anastomosis (Fig. 18-6, C and D).
Pancreas Transplantation
Pancreas Surgical Approach
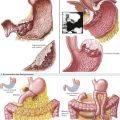
Proper backbench preparation of the pancreas is critical. During the procurement, the donor duodenum remains attached (see Fig. 18-2, B). The proximal end should be examined to ensure the pylorus is not present. The duodenum can be shortened to a reasonable length and the staple lines oversewn. The spleen is removed, with the splenic arteries and veins ligated at the tip of the tale of the pancreas. The bile duct should have been ligated during the donor procedure, which should be confirmed on the backbench. The blood vessels are mobilized. The portal vein should be long enough to allow visualization during anastomosis in the recipient. Infrequently, venous extension grafts may be needed to lengthen the vein. A Y graft, utilizing donor iliac artery bifurcation, is constructed, bringing the splenic artery and SMA onto the common trunk of the donor common iliac artery (Fig. 18-7).
If a kidney transplant will be performed at the same time, the kidney is prepared as well.
A midline incision is made and the retractors are placed. The surgeon mobilizes the cecum and right colon medially, taking care to avoid damaging the recipient ureter or duodenum. If needed, the pancreas can be placed on the left as well. The iliac vein and distal IVC are exposed, as well as the common iliac artery. If portal drainage is planned instead, the SMV is exposed at this time (Fig. 18-8).
Liver Transplantation
One of the mainstays of treatment of end-stage liver disease and hepatocellular carcinoma is liver transplantation (Fig. 18-9). Other common indications for liver transplant include viral hepatitis, alcoholic cirrhosis, autoimmune liver diseases (primary biliary cirrhosis, primary sclerosing cholangitis), and nonalcoholic fatty liver disease. Adult and pediatric liver transplant is considered treatment for appropriate liver tumors and end-stage liver disease of varied etiology. Overall patient survival rate is greater than 85% at 1 year, with a 5-year survival rate of 60% to 70%.
Liver Surgical Approach
Orthotopic liver transplant (OLT) dissection focuses on the IVC and portal triad. The liver transplant surgeon must be familiar with many anatomic variations. There are two exposure goals for the liver transplant incision (Fig. 18-10, A). First, the most cephalad extent of the incision must allow the surgeon to visualize and gain access to the suprahepatic IVC. Second, the right, most lateral extent of the incision must allow the surgeon to visualize and gain access to the infrahepatic IVC. The left subcostal extension can often be omitted, especially in patients with large ascites or in small donor liver allograft implantation.
Mobilization of the left and right lobes of the liver is performed (Fig. 18-10, B). The left and right triangular ligaments, ligamentum teres, and falciform ligaments are all divided. The falciform can contain significant collateral vessels (i.e., recanalized umbilical vein) and should be controlled before division. At this time, a retractor (Omni, Thompson, or Bookwalter) can be applied to maintain cephalad subcostal retraction.
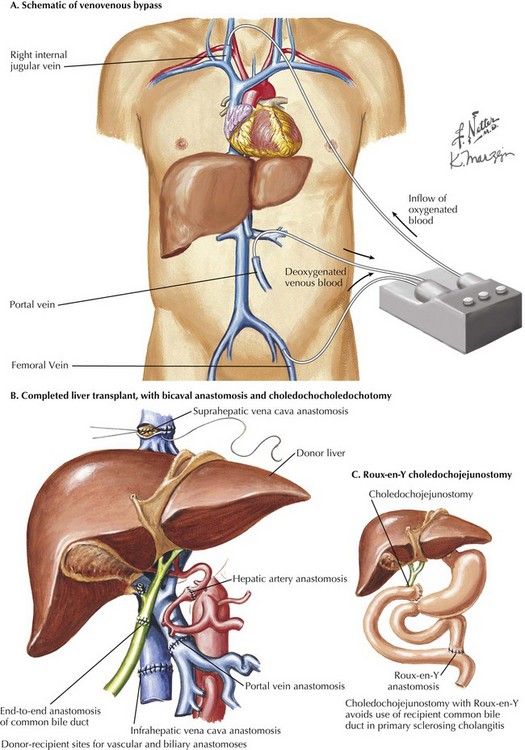
The portal triad is dissected out carefully (see Fig. 18-2, B). There are multiple small collateral vessels that form as a result of portal hypertension, especially with portal vein thrombosis. The main structures (hepatic arteries, common hepatic or common bile duct, portal vein) are dissected out and divided at the most cephalad position possible, to preserve length of these structures in the recipient. Similar to the donor surgery, a left accessory or right replaced hepatic artery must be identified (see Fig. 16-3). At this point, venovenous bypass is an option (Fig. 18-11, A).
Once the portal vein is divided, this allows the surgeon to mobilize fully the right and left lobes of the liver. The portal vein can be cannulated and drained into the venovenous bypass circuit, or a temporary end-to-side portocaval shunt can be performed to minimize portal congestion during the implantation of the liver allograft. The retrohepatic dissection is completed to allow placement of clamps for the caval interposition technique. Otherwise, the anterior surface of the retrohepatic IVC (see Fig. 18-1) is cleared by dividing the small vessels between the IVC and the posterior surface of the liver, completing mobilization of the liver up to the level of the hepatic veins.
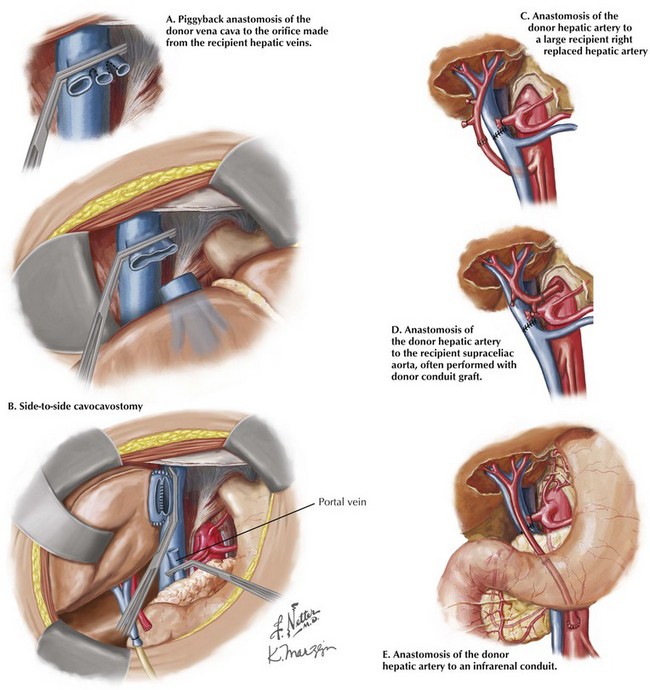
Once completed, the final anastomoses in a liver transplant restore continuity in the vena cava, hepatic artery, portal vein, and biliary drainage. The first vascular anastomosis in the liver transplant is performed to reestablish vena caval outflow. This anastomosis can be accomplished in one of three ways: caval interposition (Fig. 18-11, B), piggyback anastomosis (Fig. 18-12, A), or side-to-side cavocavostomy (Fig. 18-12, B). The portal vein is typically reconstructed after caval reconstruction. The portal vein anastomosis is done in an end-to-end fashion, using a growth knot.
The arterial management can be done in a number of ways, mostly by using the proper or common hepatic artery of the recipient as the inflow source (see Fig. 18-11, B). Because of the number of variations in the hepatic artery, the inflow can sometimes be created by using an alternate artery or even by using an arterial conduit originating from the supraceliac or infrarenal aorta (Fig. 18-12, C-E). The arterial anastomosis can be completed before or after reperfusion of the portal vein.
The duct anastomosis in the liver transplant surgery is usually straightforward. Most cases require a primary end-to-end duct-to-duct anastomosis (choledochocholedochostomy). Primary sclerosing cholangitis patients almost always require a Roux-en-Y choledochojejunostomy (Fig. 18-11, C), because of potential disease in the remnant recipient ducts, which can cause biliary obstruction later. Once hemostasis is obtained, drains can be placed, and the incision is closed in the standard fashion.
Demartines, N, Schiesser, M, Clavien, PA. An evidence-based analysis of simultaneous pancreas-kidney and pancreas transplantation alone. Am J Transplant. 2005;5:2688–2697.
Kelly, WD, Lillehei, RC, Merkel, FK, Idezuk, Y, Goetz, FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61:827–837.
Mazzaferro, V, Regalia, E, Doci, R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699.
Merion, RM, Ashby, VB, Wolfe, RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733.
Moers, C, Smits, JM, Maathuis, MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19.
Murray, JE, Merrill, JP, Harrison, JH. Renal homotransplantation in identical twins. Surg Forum. 1956;6:432–436.
Starzl, TE, Marchioro, TL, von Kaulla, KN, et al. Homotransplantation of the liver in humans. Surgery. 1963;117:659–676.