156 Organ Toxicity of Cancer Chemotherapy
Substantial improvements in survival rates among cancer patients admitted to the intensive care unit (ICU) have been achieved over the last decade.1 Three factors have contributed to these advances: (1) better patient selection, following reports in the 1980s of dismal outcomes2,3 and ensuing recommendations that ICU admission be denied in many situations involving cancer patients4–6; (2) improved overall survival of cancer patients,7 owing to therapeutic innovations and measures to prevent infections and drug toxicity; and (3) recent advances in ICU management of acute respiratory failure1,8 and septic shock.9
Today, hospital mortality among cancer patients admitted to the ICU is approximately 50%, which is not higher than in other patient groups (e.g., chronic obstructive pulmonary disease, chronic heart failure, pancreatitis, extensive burns, etc.). In addition, characteristics such as neutropenia, autologous bone marrow transplantation, or progression of malignancy no longer predict mortality.10–12 New treatments such as granulocyte colony-stimulating factor (G-CSF) shorten the duration of bone marrow failure,13,14 thereby diminishing the risk of treatment-related infection, and medications that have limited toxicity can achieve remissions in patients initially considered as having relentlessly progressive disease.15–17 As a result of these major therapeutic advances, the number of cancer patients referred for ICU admission is increasing steadily, with infection and treatment-related toxicity being the most common reasons.18
 Pulmonary Toxicity
Pulmonary Toxicity
Bleomycin-Induced Lung Toxicity
Bleomycin is a glycopeptide antibiotic that has been used since the 1970s in a wide range of solid tumors (lung cancer, esophageal cancer, head and neck cancer, germ-cell tumors of the ovary and testis, Kaposi sarcoma) as well as Hodgkin’s disease and non-Hodgkin’s lymphoma. Bleomycin lung toxicity occurs in 2% to 46% of patients.19,20 Pneumonitis with diffuse infiltrates and fibrosis is the most typical manifestation, and it has a fatal outcome in 1% to 3% of cases.19,20 Mean time to onset is 4 months after bleomycin administration, but some cases can develop up to 10 years after completion of bleomycin treatment.21,22 Earlier lung toxicity is less common and is responsible for clinical and radiographic manifestations reminiscent of bronchiolitis obliterans or hypersensitivity pneumonitis.20
Available knowledge of the pathophysiology of bleomycin-induced lung toxicity stems mainly from animal models. Skin and lungs are main targets because of the lack of bleomycin-inactivating hydrolase in these organs.20 By increasing free radicals, bleomycin induces endothelial damage, nuclear factor-kappa B (NF-κB) stimulation, then proinflammatory and profibrosis cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-1β and IL-18, and transforming growth factor β (TGF-β).23,24 Subsequently, there is an influx of inflammatory cells and fibroblasts, and progression to lung fibrosis can occur.20
Established risk factors include the cumulative dose of bleomycin, although the toxic amount varies across patients, without any consensual threshold for toxicity but rather a linear relation between the bleomycin dose and the incidence of lung toxicity.20 Renal failure seems to be the most important risk factor for predicting lung toxicity, with a significant association between diffusing capacity of the lung for carbon monoxide (DLCO) and creatinine clearance.19 Other risk factors for bleomycin-induced lung fibrosis include age older than 70 years, tobacco use, concomitant radiation therapy to the chest, bolus administration, oxygen exposure (often during or after surgery), and concomitant use of G-CSF or other cancer chemotherapy agents exhibiting lung toxicity.19,20,25
A dry cough, dyspnea on exertion and then at rest, tachypnea, fever, and cyanosis are the earliest symptoms.20 Fine, crackling rales are heard over both lung bases, and later in the course, rhonchi or a friction rub may be found. Infiltrates in both lung bases are typically seen on the chest radiograph, and progression to diffuse interstitial fibrosis may occur.20 However, asymmetric or more focal images are seen. Computed tomography shows earlier changes consisting of subpleural linear and nodular opacities in the lung bases that may suggest lung metastases.26 Blood gas measurements show hypoxemia and hypocapnia, and lung function testing discloses a restrictive defect with decreases in vital capacity and in the DLCO.20 The diagnosis is often one of exclusion when lung metastases and infections are eliminated. No real pathognomonic histologic finding exists. The most characteristic lesions are interstitial inflammatory cell infiltration and fibrosis and squamous metaplasia of bronchiolar epithelium.19,20
To decrease the risk of bleomycin-induced lung toxicity, the total dose should be determined according to the patient’s risk-factor profile, the objective being to find the best compromise between minimizing toxicity and optimizing the anticancer effect. Suggested prophylactic agents include anti-TNF-α and anti-TGF-β antibodies, IL-1 receptor antagonists, and antioxidants such as dexrazoxane, pentoxifylline, amifostine, and diallyl sulfide.20,24 Curative treatment starts with discontinuation of all chemotherapy agents known to cause lung toxicity and with respiratory function support. Infection must be ruled out. Glucocorticoid therapy in a dose of 60 to 100 mg/d of methylprednisolone is usually given, although compelling proof of efficacy is lacking. This practice is warranted given the possibility of bronchiolitis obliterans–organizing pneumonia or hypersensitivity pneumonitis, both of which respond to glucocorticoid therapy.20 In survivors, the symptoms resolve completely, and respiratory function returns to normal.20
Methotrexate Pneumonitis
Methotrexate (MTX) is a cytotoxic agent belonging to the antimetabolite class. It blocks purine synthesis by inhibiting dihydrofolate reductase. Methotrexate is not only used in various solid tumors and hematologic malignancies but also in nonmalignant diseases such as rheumatoid arthritis and severe psoriasis. Acute or subacute pneumonitis simulating an infection, usually with interstitial involvement, occurs in 1% to 7% of patients receiving MTX.27 It may occur even at low doses.28 Toxicity mechanisms include up-regulation of the p38 MAPK pathway and inflammatory cytokines such as IL-1β and IL-8.29 The symptoms may develop gradually over several weeks or months and include dyspnea, dry cough, crackling rales, and less often, fever and headaches. Extrapulmonary manifestations may include erosive mucositis, rash, and hepatic cytolysis.27 Peripheral blood eosinophil counts are moderately and transiently elevated. Hypoxemia, a restrictive defect, and a decrease in DLCO are typically found. BAL fluid contains an abundance of cells, with a predominance of lymphocytes; the CD4/CD8 ratio varies, most notably with the time from MTX administration to respiratory symptom onset.30 Lung biopsy, use of which is declining, shows lymphocytic infiltration of the interstitial tissue and, rarely but distinctively, granulomas in areas of type II pneumocyte hyperplasia27 with a variable degree of lung fibrosis.
Other Anticancer Agents With Lung Toxicity
Gemcitabine is an antimetabolite used to treat solid tumors and hematologic malignancies. Although the bone marrow is the main target of gemcitabine toxicity (with at times profound myelosuppression), pulmonary toxicity occurs in 10% to 42% of patients.31 Age older than 65 years, previous lung disease, chest radiation, and concomitant treatment with another agent (especially bleomycin in Hodgkin’s disease) are risk factors. There are two clinical variants: (1) infusion-related reactions, usually mild, characterized by dyspnea or bronchospasm within hours of infusion and by favorable outcome with corticosteroids32; and (2) gemcitabine-induced pneumonitis characterized by pulmonary edema at the time of a capillary leak syndrome, diffuse alveolar damage, or alveolar hemorrhage.32 Dyspnea, fever, pulmonary infiltrates, and cough are the main symptoms.31 Although the mortality can reach 37%, great improvement can be obtained with corticosteroids.31,32
Cytarabine, an agent similar to gemcitabine, has a longer history of use in acute myelogenous leukemia in combination with anthracyclines. Respiratory failure of variable severity develops in 12% to 20% of patients within 2 weeks of cytarabine initiation.33 Noncardiogenic pulmonary edema and organized pneumonia are described, with favorable outcome under corticosteroids. However, differential diagnosis with infection, leukemic infiltrates, or heart failure is often difficult.33,34
Tyrosine kinase inhibitors are generally well tolerated. Imatinib, the main treatment for chronic myeloid leukemia (CML), is also effective in gastrointestinal stromal tumors. It frequently induces edema and weight gain. Dyspnea and cough, observed in up to 14% of treated patients, are often attributed to pulmonary edema and pleural effusion.32 However, interstitial pneumonitis, alveolar hemorrhage, or pulmonary fibrosis can also occur (0.2% and 1.3% of grade 3 and 4 in the chronic phase of CML). Inhibition of platelet-derived growth factor (PDGF) is one of the mechanisms. Corticosteroid therapy can be effective.35 This lung toxicity is also described with some epidermal growth factor (EGF) inhibitors used in solid neoplasm.32
Prognosis of acute promyelocytic leukemia (APL) dramatically improved until introduction of all-trans-retinoic acid (ATRA). However, differentiation syndrome (DS), also known as retinoic acid syndrome, can be a life-threatening complication of this molecule. In the most recent study, it occurs in 25% of patients, with a severe form in 50% of them.36 Unexplained fever, weight gain greater than 5 kg, edema, dyspnea, interstitial pulmonary infiltrates, pleuropericardial effusion, unexplained hypotension, and renal failure are the main diagnosis criteria. High white blood cell count greater than 5 × 109/L and abnormal creatinine level are risk factors. Dexamethasone is used to prevent and treat this syndrome. Mortality in severe forms is 11%.36
Finally, a few cases of interstitial pneumonitis have been reported with carmustine, cyclophosphamide, melphalan, procarbazine, chlorambucil, mitomycin, vinblastine, etoposide, hydroxyurea, taxanes, alkylating agents, platin derivatives, rapamycin analogs, and monoclonal antibodies to EGFR.32 An exhaustive list of drugs potentially responsible for lung toxicity and the corresponding clinical presentations can be found online at http://www.pneumotox.com.
 Cardiac Toxicity
Cardiac Toxicity
Anthracyclines are the main culprits of cardiac toxicity. Evidence of cardiac toxicity for other agents (taxanes, antimetabolites, alkylating agents, and spindle poisons) is limited to anecdotal case reports.37
Anthracycline-Induced Cardiac Toxicity
The anthracycline class—which includes doxorubicin, daunorubicin, epirubicin, idarubicin, and mitoxantrone—plays a major role in the treatment of many solid tumors (breast cancer, esophageal cancer, osteosarcomas) and hematologic malignancies (Hodgkin’s disease, non-Hodgkin’s lymphoma, acute leukemia). Anthracycline-induced myocardial toxicity can be life threatening or dose limiting, thereby affecting the prognosis of the disease by precluding optimal anticancer treatment.38
Anthracyclines induce cell death of dividing cells via inhibition of topoisomerase-2, intercalation to nucleus DNA, and production of free radicals.39 The myocardium is vulnerable to free radicals because antioxidant enzyme activity is weaker in myocytes than in other tissues (e.g., liver, kidney). The cumulative anthracycline dose is the main risk factor for cardiac toxicity (1%-5%, up to 550 mg/m2; 30% at 600 mg/m2; 50% at 1g/m2) with individual variation.40 Other risk factors include female gender, age at either end of the lifespan, black race, and Down’s syndrome.38 Opinions are divided regarding the roles of prior radiation therapy to the chest, lymphoma, preexisting heart disease, and a preexisting decrease in the left ventricular ejection fraction.38,41
Acute cardiotoxicity manifests as an acute myocarditis, namely a rapid deterioration in cardiac function during or within 1 week after the administration of anthracycline therapy, usually with reversal of the abnormalities after discontinuation of the drug.38 Ventricular or supraventricular rhythm disorders are common. Congestive heart failure with or without cardiogenic shock is the most common clinical presentation, although myocarditis or pericarditis may also occur.41 Adjustments in chemotherapy regimens have noticeably reduced the rate of acute cardiac toxicity, which now occurs in fewer than 1% of patients.41
Chronic cardiotoxicity is far more common. The subacute form is characterized by irreversible dilated cardiomyopathy within 1 year after anthracycline discontinuation.42–44 The delayed form develops insidiously after more than 1 year and runs a slowly progressive course.42–44 Long-term follow-up studies allow better evaluation of the prevalence of subclinical cardiotoxicity after anthracycline doses between 450 and 550 mg/m2. It can reach 27.6%, with a median follow-up of 8 years, and the risk of cardiac failure clearly increases over time.40,45
Patients develop systolic or diastolic dysfunction indistinguishable from heart failure due to other causes. Coronary artery disease is rare,45 so electrocardiographic (ECG) changes are nonspecific and include sinus tachycardia, flat T waves, QT prolongation, and low amplitudes. Ventricular tachycardia and supraventricular rhythm disorders have been reported in patients with acute cardiac toxicity.41 B-type natriuretic peptide (BNP) is under study but not validated.40 Echocardiography with tissue Doppler studies is the most widely used noninvasive and sensitive tool for monitoring and early detection of anthracycline cardiomyopathy.38,40,41 Diastolic dysfunction is often the earlier sign. However, myocardial scintigraphy with technetium-99m may be more informative than transthoracic echocardiography, notably in obese patients. Dobutamine stress echocardiography has also been suggested as a diagnostic tool.46 Finally, myocardial biopsy is an invasive diagnostic method whose sensitivity and specificity are controversial. Histologic analysis shows myofibril loss, dilation of the sarcoplasmic reticulum, and intracytoplasmic vacuoles in myocytes.41
Preventive Treatment
Epirubicin and idarubicin may be less likely to induce cardiotoxicity than the other anthracyclines. Continuous administration over several hours also seems to reduce the cardiotoxicity of anthracyclines. Available cardioprotective agents include dexrazoxane, an antioxidant that chelates iron.47 Finally, liposomal encapsulation of anthracyclines reduces their cardiotoxicity without altering their anticancer effects.48
 Hematologic Toxicity
Hematologic Toxicity
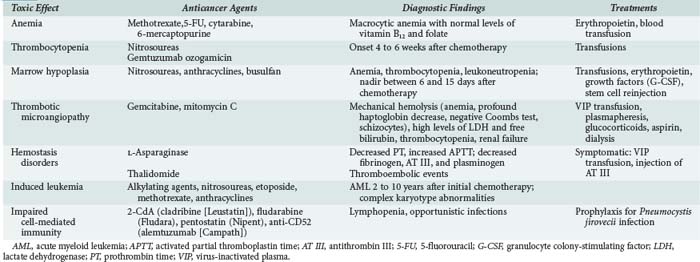
In addition to the myelosuppressive effects expected with all anticancer agents, alterations in hemostasis, impairments in cell-mediated immunity, and second leukemia or myelodysplasia can occur in patients with a history of chemotherapy for cancer (Table 156-1).
Myelosuppression
Myelosuppression is virtually unavoidable but usually reversible. The mechanism of action of the anticancer agent (i.e., the cell cycle phase affected by the drug) determines which cell lines are affected and governs the severity of marrow toxicity. For instance, nitrosoureas and mitomycin selectively destroy stem cells, causing severe and in some cases irreversible myelosuppression. In contrast, myelotoxicity is less marked with drugs that act more selectively on a specific cell-cycle phase, such as vincristine, bleomycin, and cisplatin. Table 156-2 recapitulates the severity of myelosuppression seen with various agents.
TABLE 156-2 Severity of Myelosuppression Seen with Various Chemotherapy Agents
| Mild | Moderate | Severe |
|---|---|---|
| Cisplatin | Antipurine | Anthracycline |
| Bleomycin | Podophyllin | Nitrogen mustard |
| Vinca alkaloids | Alkylating agents | Antifolates |
| Hydroxyurea | Antipyrimidines | |
| Mitomycin Procarbazine |
Nitrosoureas (carmustine, lomustine) | |
| Busulfan | ||
| Dacarbazine |
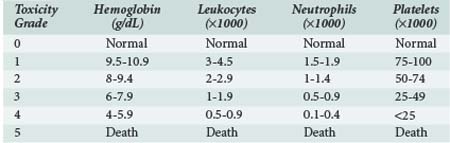
The severity of myelosuppression varies also with patient-dependent factors such as age, extent of bone marrow invasion by tumor, prior treatments (radiation therapy and/or chemotherapy associated with myelofibrosis), and nutritional status. The World Health Organization has suggested a scheme for classifying the severity of myelosuppression based on peripheral blood cell counts, as shown in Table 156-3.
Infection, anemia, and bleeding are the main complications of myelosuppression. Whereas febrile neutropenia has been associated with 90% mortality in the absence of antimicrobial therapy,50 mortality among neutropenic inpatients is now less than 10% in hematology wards and 50% in ICUs.11 This improved survival can be ascribed to the development of recommendations for the diagnosis, prophylaxis, and treatment of infections in neutropenic patients,51,52 improved knowledge of the pharmacokinetics and toxicity of anticancer agents, and introduction of medications with greater efficacy in fungal infections.53,54 Shortened duration of neutropenia may be obtained with injection of G-CSF13,14 or injection of mobilized peripheral stem cells (autologous or allogeneic). Bone marrow transplantation is followed by approximately 3 weeks of myelosuppression.
Anemia induced by chemotherapy requires transfusion of packed red blood cells but sometimes can be minimized by regular injections of erythropoietin.55 Finally, careful attention should be given at all times to correcting nutritional deficiencies, particularly deficiencies of folic acid, iron, and vitamin B12.
No consensus exists about the amount of platelet transfusion necessary to avoid bleeding. Dose above 1.1 × 1011 platelets per square meter of body-surface area when platelet count is 10,000/mm3 or lower does not decrease the incidence of bleeding.56 In our practice, situations such as diffuse alveolar hemorrhage require a platelet count above 50,000/mm3.
Hemostasis Disorders
L-Asparaginase is widely used to treat acute lymphoblastic leukemia (ALL). Produced from strains of Escherichia coli, L-asparaginase hydrolyzes asparagine, an amino acid required by cells for protein synthesis. However, L-asparaginase causes a global decrease in protein synthesis, notably in clotting factors. This leads to low levels of fibrinogen, prothrombin, antithrombin (AT), plasminogen, and factors IX and X, so activated partial thromboplastin time (APTT) is increased.57 If the alterations in hemostasis are severe, thromboembolic events (pulmonary embolism, stroke, and cerebral vein thrombosis) are more frequent than bleeding episodes and are reported in up to 15% of adults.58
Prevention includes detection of inherited thrombophilia, monitoring of fibrinogen and AT levels, and AT substitution without consensual scheme. Otherwise, the administration of a pegylated form of asparaginase is preferred at present because it has fewer thromboembolic complications.58 Thromboembolism treatment should consider risks and benefits of anticoagulation.57
Thalidomide, used in multiple myeloma treatment, has immunomodulatory and anti-angiogenic effects. When combined with dexamethasone or doxorubicin, but not as monotherapy, it increases the risk of venous thromboembolic events (VTE). Incidence is usually around 15% but can reach 58% with doxorubicin.59 Pulmonary embolisms are not specifically reported in all studies but occur in about 7% of patients treated with thalidomide and dexamethasone, versus less than 2% in those treated with dexamethasone only.60 These complications are usually observed during the 2 months after beginning therapy.
 Neurologic Toxicity
Neurologic Toxicity
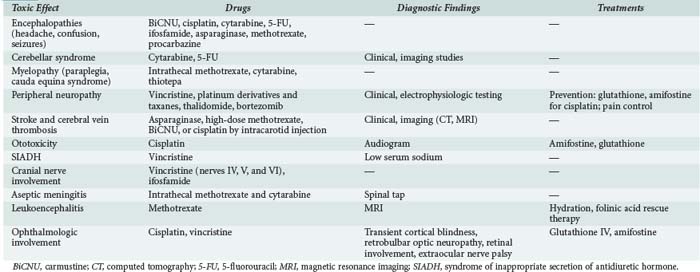
Neurologic adverse effects of anticancer agents are both common and severe (Table 156-4). They may preclude administration of optimal chemotherapy, thereby compromising the chances for recovery. Both peripheral and central components of the nervous system may be affected. The diagnosis is one of exclusion; infections, trauma, and infiltration by malignant cells should be ruled out first.61
Consequences of Intrathecal Injections
Other complications are less common but usually severe. Spinal cord lesions manifested by motor deficit and cauda equina syndrome develop some weeks after injection, with a median of 10 days. Both MTX and aracytine are incriminated. Risk factors for MTX toxicity are cumulative dose, high MTX levels in the cerebrospinal fluid, and concomitant radiation therapy.62 High dose and the liposomal form of aracytine lead to more toxicity by prolonging release of aracytine, but neurotoxicity occurs above all when both drugs are associated.63 Magnetic resonance imaging eliminates epidural compression or cord infiltration and shows multiple foci of atrophy and demyelination selectively affecting the periventricular white matter and the centrum semiovale, ventricular dilation, and calcifications. Recovery is poor, often with bowel and urinary incontinence. No treatment exists. Folate supplementation may decrease MTX toxicity when patients receive concomitant systemic MTX therapy or display renal failure.63
Peripheral Neuropathies
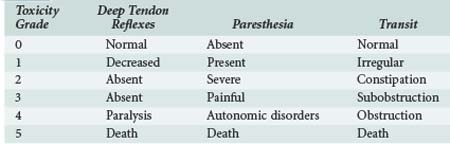
Chemotherapy-induced peripheral neuropathy (CIPN) is a major dose-limiting side effect of many drugs including taxanes, vinca alkaloids, platinum compounds, and newer agents such as thalidomide and bortezomib. Incidence is variable and can be influenced by age, alcohol abuse, diabetes mellitus, liver or renal dysfunction, dose intensity, cumulative dose, and concomitant administration of several neurotoxic agents. Distal sensory peripheral neuropathy with neuropathic pain and paresthesias is the most common presentation but is not specific. Some motor losses are also described (Table 156-5). CIPN may appear from the first chemotherapy infusion or later. Symptoms can resolve totally or partially with interruption of treatment or dose reduction, but severe impairment can persist. Treatment continuation should consider the relative prognosis of CIPN and cancer, drug importance for cancer control, and quality of life impairments due to neuropathy.64
More precisely, paclitaxel is the main taxane associated with CIPN (significant neuropathy in up to 10% of cases), especially when used with a platinum compound. Vincristine, the most neurotoxic among vinca alkaloids, can produce CIPN in up to 75% of patients with a cumulative dose over 10 mg/m2. It leads to severe autonomic dysfunction associated with peripheral sensorimotor loss. Cisplatin and oxaliplatin, two platinum derivatives, are more toxic than carboplatin, but cisplatin is also a better antitumor agent. The incidence of CIPN is close to 50%, whatever the grade. Except this typical form, acute reversible neuropathy is observed in 80% of patients who received oxaliplatin. Distal paresthesia and pain develop within a few hours to days of infusion and resolve spontaneously.64
CIPN is observed in over half of patients treated with thalidomide, and the risk increases greatly after 6 months on therapy. A recent trial reported a grade 3 and 4 neurotoxicity in 3.4% of patients.60 Lenalidomide, an analog of thalidomide, has less neurotoxicity. Finally, bortezomib, a proteasome inhibitor, can lead to 35% of CIPN, with some degree of motor loss in a third of cases.64
No specific treatment exists. Calcium and magnesium infusion can attenuate the development of CIPN after oxaliplatin treatment, but there is some controversy about consequences on antitumor effect. Many other drugs are promising but need larger studies.64,65
Central Nervous System Toxicity
Most anticancer agents are high-molecular-weight or water-soluble compounds that do not cross the blood-brain barrier. Central nervous system toxicity is therefore uncommon with intravenous chemotherapy but may occur with high doses of some compounds (MTX, cytarabine). Cerebellar syndrome has been reported with cytarabine (cytosine arabinoside) and 5-FU (particularly in patients with dihydropyrimidine dehydrogenase deficiency). High-dose cisplatin can cause encephalopathy (headache, behavioral or personality disorders, confusion, drowsiness, seizures, and coma); concomitant optic nerve involvement may occur (Table 156-6). Ifosfamide is responsible for reversible non–dose-dependent neuropsychiatric disorders including visual or auditory hallucinations, a dreamlike state, confusion, personality disorders, and anxiety. Seizures or coma may occur. Extrapyramidal manifestations with myoclonus and spasticity are classic manifestations of ifosfamide neurotoxicity. Among patients treated with MTX in doses greater than 1 g/m2, 15% experience spontaneously reversible encephalopathy, which must be differentiated from leukoencephalopathy with irreversible chronic pseudo dementia. L-Asparaginase treatment causes encephalopathy in 15% to 60% of patients. Fludarabine is associated with neurotoxicity in 15% of patients. It is dose dependent and can be prevented by using low doses (25 mg/m3, 5 days per month).66
TABLE 156-6 Classification of Encephalopathy According to Severity
| Severity Grade | Description |
|---|---|
| 0 | No symptoms |
| 1 | Agitation, drowsiness |
| 2 | Bedridden |
| 3 | Requires treatment |
| 4 | Coma, manic episode, suicidal behavior |
| 5 | Death |
 Urologic and Renal Toxicity
Urologic and Renal Toxicity
Many factors can cause renal dysfunction in patients receiving anticancer chemotherapy,67 including radiation-induced nephritis, tumor lysis syndrome, hyperuricemia, hyperphosphatemia, hypercalcemia, lysozymuria, thrombotic microangiopathy, disseminated intravascular coagulation, infiltration by cancer cells, amyloidosis, and renal consequences of obstructive uropathy. Exacerbation of renal dysfunction can occur with nephrotoxic agents (e.g., aminoglycosides, antifungal agents, antiviral agents, iodine).
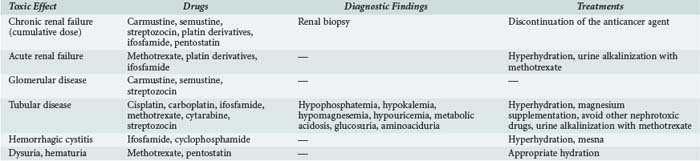
A number of anticancer agents can cause renal failure (Table 156-7). This effect is dose limiting and therefore compromises the chances of recovery from the malignancy. In addition to renal failure, tubular disease or, more rarely, glomerular disease or thrombotic microangiopathy may occur with some agents. The distal urinary tract may be affected by ifosfamide or cyclophosphamide.
The World Health Organization has developed a grading system for chemotherapy-related renal failure, based on urine output and serum creatinine levels, as shown in Table 156-8.
TABLE 156-8 World Health Organization Grading System for Chemotherapy-Related Renal Failure
| Grade | Urine Output | Creatinine (µmol/L, mg/dL) |
|---|---|---|
| 0 | Normal | Normal |
| 1 | Transient decrease | 115-180, 13-20 |
| 2 | Diuretic agents | 181-354, 20-40 |
| 3 | High-dose diuretic agents | 355-530, 40-60 |
| 4 | Dialysis | 531-800, 60-90 |
| 5 | Death | — |
Renal Failure
Methotrexate nephrotoxicity occurs with high doses (>1 g/m2).68 Whereas several toxicities are due to its antimetabolite action, the underlying mechanism of nephrotoxicity involves precipitation of MTX and its even less soluble metabolite, 7-hydroxymethotrexate, within the renal tubules.68,69 Acute renal failure enhances other toxic effects by delaying MTX elimination. Tubule precipitation is more likely to occur at acid pH, so it is important to maintain high urine output and alkaline urine. Folinic acid is given to antagonize the other side effects of MTX. With optimal management, the incidence of renal failure has decreased from 10% to 2%, with less than 1% grade 3 or 4 and a mortality close to 4%.68,70 Coadministration of several drugs, notably antibiotics, promotes this toxicity.71 When renal failure occurs, hydration and folinic acid administration are increased based on plasma MTX concentration, so daily monitoring is essential. Hemodialysis decreases plasma MTX concentration, but a marked rebound can occur after the procedure, so it is not recommended. The carboxypeptidase G can metabolize MTX to its inactive metabolite and seems to be effective but is not routinely used.68,70,72
With platin derivatives, nephrotoxicity is common, dose dependent, and potentially irreversible. In non–small-cell lung cancer, platinum-based chemotherapy triples the risk of nephrotoxicity.73 Cisplatin nephrotoxicity is reported in up to 40% of patients.74 This toxicity is the main dose-limiting adverse effect of these agents.75 Cisplatin, the main platinum salt, induces tubular injury with minimal proteinuria, polyuria, and potentially severe hypomagnesemia. Secondary hypokaliemia, hypocalcemia, and tubular acidosis are observed. If a renal biopsy is performed, histologic analysis shows tubular dilation, epithelial cell necrosis, interstitial edema and fibrosis, and thinning of the tubular basement membrane. Prevention of tubular injury and acute renal failure includes generous hydration. It is also essential to adjust the doses of cisplatin according this renal function. Diuretics are not advised. Magnesium supplementation is often necessary.74 Carboplatin and oxaliplatin are less toxic, moreover, since high doses can be adjusted for renal function.76 However, in some cases, they are less effective against cancer than cisplatin.
Prolonged ifosfamide therapy can rarely result in progressive renal failure and damage the proximal tubules. The suspected mechanism is inhibition of the Na+/H+ pump and impairment of the sodium-dependent transporters of glucose, phosphate, and L-alanine by two ifosfamide metabolites, chloroacetaldehyde and 4-OH-ifosfamide. Risk factors for ifosfamide-induced tubulopathy include a cumulative dose greater than 45 g/m2, age younger than 5 years, a history of cisplatin therapy, and a preexisting renal dysfunction of any cause.67,77,78
Thrombotic Microangiopathy
Chemotherapy-induced thrombotic microangiopathy is manifested by atypical hemolytic uremic syndrome (aHUS), usually with thrombocytopenia, mechanical hemolytic anemia (with schizocytes and negative Coombs test), elevated blood pressure, proteinuria, hematuria, and acute renal failure. Peripheral edema and neurologic signs can occur. It is often difficult to separate the respective roles of chemotherapy and underlying cancer. The incidence ranges from 3% to 13% for either malignancy-induced or chemotherapy-induced aHUS.79 The first cases were described after mitomycin C. A few cases have been reported in patients treated with gemcitabine, CCNU (lomustine), and platin derivatives; combinations such as daunorubicin/cytarabine or bleomycin/cisplatin have also been reported to cause thrombotic microangiopathy.80
Chemotherapy-induced thrombotic microangiopathy occurs after several months of treatment (up to 24 months with gemcitabine). Pathogenesis is unclear, but endothelial injury seems to be a central feature. Direct endothelial damage is described.79 ADAMTS 13 activity is not always tested but is usually not decreased. Management of these aHUS is not well established. Discontinuation of the treatment involved and blood pressure control are the first step. Then virus-inactivated plasma transfusions, plasmapheresis, or glucocorticoid therapy can be proposed, but no study is able to determine their efficacy.81 Prognosis is poor, with a mortality rate of 40% to 90% despite discontinuation of treatment. This prognosis is determined as much by underlying disease as by aHUS.79
Hemorrhagic Cystitis
Hemorrhagic cystitis is a common adverse effect with the alkylating agents, cyclophosphamide and ifosfamide.82,83 Degradation of oxazaphosphorine in the kidneys produces acrolein, which has direct toxic effects on the bladder mucosa.82,83 Prevention relies on appropriate saline hydration and administration of mesna,84,85 which binds to acrolein, producing a stable, water-soluble thioester that is promptly eliminated. Mesna has no curative effects.85 If cystitis occurs despite preventive measures, a double-lumen urinary catheter should be inserted for continuous bladder irrigation until the bleeding stops completely.83
 Digestive Toxicity
Digestive Toxicity
Liver Toxicity
Many antineoplastic drugs have potential liver toxicity. Alcoholism, malnutrition, hepatic neoplasm or metastases, infection, or other toxic medicines are risk factors for this toxicity, above all when chemotherapy doses are not adjusted according to baseline liver function. Several injuries are described. Hepatitis, characterized by cytolysis and sometimes by liver failure, can be observed with many antineoplastic drugs such as alkylating agents, nitrosoureas, platinum derivatives, antimetabolites. Cholestasis is also nonspecific and can occur with many drugs. Fibrosis and chronic hepatitis is rare and mainly described with methotrexate.86 For all these toxicities, drug discontinuation is the best treatment.
Veno-occlusive disease, also called sinusoidal obstruction syndrome, is a rare complication that can lead to organ dysfunction. Risk factors are treatment by cyclophosphamide, busulfan, gemtuzumab ozogamicin, sirolimus, and total body irradiation, so patients with stem cell transplantation ongoing are particularly exposed. Incidence is around 10% to 15% after stem cell transplantation within 70% of severe cases. Patients develop painful hepatomegaly, ascites, weight gain, and increase in bilirubin levels. Mortality can reach 100% when multiple organ failure appears.87 Management consists in supportive care including renal replacement therapy. Few effective treatment or prevention options exist apart from defibrotide.88
Pancreatitis
Acute pancreatitis occurs in up to 18% of patients treated for acute lymphoblastic leukemia (ALL). The main drug involved is L-asparaginase, followed by cytarabine and corticosteroids. Mortality is higher in these patients, among others by impairment of ALL management.89
Gut Toxicity
Neutropenic enterocolitis or typhlitis is the consequence of direct toxicity to bowel mucosa and microbial invasion of this mucosa during immunosuppression. It can be secondary to high-dose chemotherapy for acute leukemia or lymphoma, especially in cases of cytarabine administration. The incidence is unknown but may reach 46% (in an autopsy study) and can be fatal.90 Diagnosis and management are difficult. Surgery is limited by the presence of cytopenia.91
 Metabolic Toxicity
Metabolic Toxicity
Metabolic disorders in patients receiving cancer chemotherapy fall into two groups: disorders related directly to the tumor (e.g., urinary tract compression, spontaneous lysis, syndrome of inappropriate secretion of antidiuretic hormone [SIADH]) and disorders related to anticancer agents (e.g., drug-induced tumor lysis, electrolyte disturbances).92 Hyponatremia (related chiefly to SIADH) is the main source of clinical symptoms.93 SIADH can be observed with spindle poisons (vincristine, vinblastine, and more rarely, vinorelbine),94,95 alkylating agents such as cyclophosphamide melphalan, and more rarely, chlorambucil and thiotepa.92,93 Cisplatin is associated with hyponatremia related to SIADH or tubular wasting in 4% to 10% of patients. However, a diagnosis of chemotherapy-induced SIADH requires the exclusion of other causes including paraneoplastic syndromes, central nervous system disorders, lung infections, and SIADH induced by other drugs.
Key Points
Kintzel PE. Anticancer drug-induced kidney disorders. Drug Saf. 2001;24:19-38.
Renal toxicity of chemotherapy and physiopathologic explanations.
Lewis C. A review of the use of chemoprotectants in cancer chemotherapy. Drug Saf. 1994;11:153-162.
The interest of chemoprotectants in oncohematology.
Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900-905.
Diagnostic procedures of doxorubicin-induced cardiomyopathy.
Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120:617-624.
Clinical features, pathogenesis, risk factors, and treatment of bleomycin-induced pneumonitis.
Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63:1549-1563.
1 Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344(7):481-487.
2 Carlon GC. Admitting cancer patients to the intensive care unit. Crit Care Clin. 1988;4(1):183-191.
3 Schuster DP. Everything that should be done—not everything that can be done. Am Rev Respir Dis. 1992;145(3):508-509.
4 Consensus statement on the triage of critically ill patients. Society of Critical Care Medicine Ethics Committee. JAMA. 1994;271(15):1200-1203.
5 Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27(3):633-638.
6 Azoulay E, Pochard F, Chevret S, et al. Compliance with triage to intensive care recommendations. Crit Care Med. 2001;29(11):2132-2136.
7 Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360(9340):1131-1135.
8 Azoulay E, Alberti C, Bornstain C, et al. Improved survival in cancer patients requiring mechanical ventilatory support: impact of noninvasive mechanical ventilatory support. Crit Care Med. 2001;29(3):519-525.
9 Larche J, Azoulay E, Fieux F, et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29(10):1688-1695.
10 Azoulay E, Moreau D, Alberti C, et al. Predictors of short-term mortality in critically ill patients with solid malignancies. Intensive Care Med. 2000;26(12):1817-1823.
11 Darmon M, Azoulay E, Alberti C, et al. Impact of neutropenia duration on short-term mortality in neutropenic critically ill cancer patients. Intensive Care Med. 2002;28(12):1775-1780.
12 Massion PB, Dive AM, Doyen C, et al. Prognosis of hematologic malignancies does not predict intensive care unit mortality. Crit Care Med. 2002;30(10):2260-2270.
13 Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42(15):2433-2453.
14 Klastersky J, Awada A, Aoun M, et al. Should the indications for the use of myeloid growth factors for the prevention of febrile neutropenia in cancer patients be extended? Curr Opin Oncol. 2009;21(4):297-302.
15 Gianni AM, Magni M, Martelli M, et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen). Blood. 2003;102(2):749-755.
16 Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21(9):1746-1751.
17 O’brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994-1004.
18 Kress JP, Christenson J, Pohlman As, et al. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med. 1999;160(6):1957-1961.
19 Azambuja E, Fleck JF, Batista RG, et al. Bleomycin lung toxicity: who are the patients with increased risk? Pulm Pharmacol Ther. 2005;18(5):363-366.
20 Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120(2):617-624.
21 Tashiro M, Izumikawa K, Yoshioka D, et al. Lung fibrosis 10 years after cessation of bleomycin therapy. Tohoku J Exp Med. 2008;216(1):77-80.
22 Uzel I, Ozguroglu M, Uzel B, et al. Delayed onset bleomycin-induced pneumonitis. Urology. 2005;66(1):195.
23 Hoshino T, Okamoto M, Sakazaki Y, et al. Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice. Am J Respir Cell Mol Biol. 2009;41(6):661-670.
24 Kalayarasan S, Sriram N, Sudhandiran G. Diallyl sulfide attenuates bleomycin-induced pulmonary fibrosis: critical role of iNOS, NF-kappaB, TNF-alpha and IL-1beta. Life Sci. 2008;82(23-24):1142-1153.
25 Azoulay E, Herigault S, Levame M, et al. Effect of granulocyte colony-stimulating factor on bleomycin-induced acute lung injury and pulmonary fibrosis. Crit Care Med. 2003;31(5):1442-1448.
26 Rossi SE, Erasmus JJ, Mcadams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. 2000;20(5):1245-1259.
27 Imokawa S, Colby TV, Leslie KO, et al. Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J. 2000;15(2):373-381.
28 Salliot C, Van Der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100-1104.
29 Kim YJ, Song M, Ryu JC. Inflammation in methotrexate-induced pulmonary toxicity occurs via the p38 MAPK pathway. Toxicology. 2009;256(3):183-190.
30 Fuhrman C, Parrot A, Wislez M, et al. Spectrum of CD4 to CD8 T-cell ratios in lymphocytic alveolitis associated with methotrexate-induced pneumonitis. Am J Respir Crit Care Med. 2001;164(7):1186-1191.
31 Belknap SM, Kuzel TM, Yarnold PR, et al. Clinical features and correlates of gemcitabine-associated lung injury: findings from the RADAR project. Cancer. 2006;106(9):2051-2057.
32 Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133(2):528-538.
33 Forghieri F, Luppi M, Morselli M, et al. Cytarabine-related lung infiltrates on high resolution computerized tomography: a possible complication with benign outcome in leukemic patients. Haematologica. 2007;92(9):e85-e90.
34 Kopterides P, Lignos M, Mentzelopoulos S, et al. Cytarabine-induced lung injury: case report. Anticancer Drugs. 2005;16(7):743-745.
35 Ohnishi K, Sakai F, Kudoh S, et al. Twenty-seven cases of drug-induced interstitial lung disease associated with imatinib mesylate. Leukemia. 2006;20(6):1162-1164.
36 Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113(4):775-783.
37 Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22(4):263-302.
38 Valero V, Perez E, Dieras V. Doxorubicin and taxane combination regimens for metastatic breast cancer: focus on cardiac effects. Semin Oncol. 2001;28(4 Suppl 12):15-23.
39 Arola OJ, Saraste A, Pulkki K, et al. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60(7):1789-1792.
40 Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991-4008.
41 Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339(13):900-905.
42 Kajihara H, Yokozaki H, Yamahara M, et al. Anthracycline induced myocardial damage. An analysis of 16 autopsy cases. Pathol Res Pract. 1986;181(4):434-441.
43 Mortensen SA, Olsen HS, Baandrup U. Chronic anthracycline cardiotoxicity: haemodynamic and histopathological manifestations suggesting a restrictive endomyocardial disease. Br Heart J. 1986;55(3):274-282.
44 Wojtacki J, Lewicka-Nowak E, Lesniewski-Kmak K. Anthracycline-induced cardiotoxicity: clinical course, risk factors, pathogenesis, detection and prevention—review of the literature. Med Sci Monit. 2000;6(2):411-420.
45 Moser EC, Noordijk EM, Van Leeuwen FE, et al. Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood. 2006;107(7):2912-2919.
46 Cottin Y, L’Huillier I, Casasnovas O, et al. Dobutamine stress echocardiography identifies anthracycline cardiotoxicity. Eur J Echocardiogr. 2000;1(3):180-183.
47 Sparano JA, Speyer J, Gradishar WJ, et al. Phase I trial of escalating doses of paclitaxel plus doxorubicin and dexrazoxane in patients with advanced breast cancer. J Clin Oncol. 1999;17(3):880-886.
48 Wollina U, Dummer R, Brockmeyer NH, et al. Multicenter study of pegylated liposomal doxorubicin in patients with cutaneous T-cell lymphoma. Cancer. 2003;98(5):993-1001.
49 Giordano SH, Booser DJ, Murray JL, et al. A detailed evaluation of cardiac toxicity: a phase II study of doxorubicin and one- or three-hour-infusion paclitaxel in patients with metastatic breast cancer. Clin Cancer Res. 2002;8(11):3360-3368.
50 Bodey GP, Rodriguez V, Chang HY, et al. Fever and infection in leukemic patients: a study of 494 consecutive patients. Cancer. 1978;41(4):1610-1622.
51 Marti FM, Cullen MH, Roila F. Management of febrile neutropenia: ESMO clinical recommendations. Ann Oncol. 2009;20(Suppl. 4):166-169.
52 Maschmeyer G, Beinert T, Buchheidt D, et al. Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients: Guidelines of the infectious diseases working party of the German Society of Haematology and Oncology. Eur J Cancer. 2009;45(14):2462-2472.
53 Goldberg E, Gafter-Gvili A, Robenshtok E, et al. Empirical antifungal therapy for patients with neutropenia and persistent fever: Systematic review and meta-analysis. Eur J Cancer. 2008;44(15):2192-2203.
54 Segal BH, Almyroudis NG, Battiwalla M, et al. Prevention and early treatment of invasive fungal infection in patients with cancer and neutropenia and in stem cell transplant recipients in the era of newer broad-spectrum antifungal agents and diagnostic adjuncts. Clin Infect Dis. 2007;44(3):402-409.
55 Rizzo JD, Lichtin AE, Woolf SH, et al. Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. J Clin Oncol. 2002;20(19):4083-4107.
56 Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362(7):600-613.
57 Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2009;7(9):600-606.
58 Piatkowska-Jakubas B, Krawczyk-Kulis M, Giebel S, et al. Use of L-asparaginase in acute lymphoblastic leukemia: recommendations of the Polish Adult Leukemia Group. Pol Arch Med Wewn. 2008;118(11):664-669.
59 Musallam KM, Dahdaleh FS, Shamseddine AI, et al. Incidence and prophylaxis of venous thromboembolic events in multiple myeloma patients receiving immunomodulatory therapy. Thromb Res. 2009;123(5):679-686.
60 Rajkumar SV, Rosinol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26(13):2171-2177.
61 Verstappen CC, Heimans JJ, Hoekman K, et al. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63(15):1549-1563.
62 Seidel H, Moe PJ, Nygaard R, et al. Evaluation of serious adverse events in patients treated with protocols including methotrexate infusions. Pediatr Hematol Oncol. 1994;11(2):165-172.
63 Kwong YL, Yeung DY, Chan JC. Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities. Ann Hematol. 2009;88(3):193-201.
64 Kannarkat G, Lasher EE, Schiff D. Neurologic complications of chemotherapy agents. Curr Opin Neurol. 2007;20(6):719-725.
65 Wolf S, Barton D, Kottschade L, et al. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507-1515.
66 Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009;63(5):761-767.
67 Kintzel PE. Anticancer drug-induced kidney disorders. Drug Saf. 2001;24(1):19-38.
68 Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694-703.
69 Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009;146(5):489-503.
70 Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100(10):2222-2232.
71 De Miguel D, Garcia-Suarez J, Martin Y, et al. Severe acute renal failure following high-dose methotrexate therapy in adults with haematological malignancies: a significant number result from unrecognized co-administration of several drugs. Nephrol Dial Transplant. 2008;23(12):3762-3766.
72 Van Den Bongard HJ, Mathjt RA, Boogerd W, et al. Successful rescue with leucovorin and thymidine in a patient with high-dose methotrexate induced acute renal failure. Cancer Chemother Pharmacol. 2001;47(6):537-540.
73 D’addario G, Pintilie M, Leighl NB, et al. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol. 2005;23(13):2926-2936.
74 Launay-Vacher V, Rey JB, Isnard-Bagnis C, et al. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol. 2008;61(6):903-909.
75 De Jongh FE, Van Veen RN, Veltman SJ, et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer. 2003;88(8):1199-1206.
76 Lyttelton MP, Newlands ES, Giles C, et al. High-dose therapy including carboplatin adjusted for renal function in patients with relapsed or refractory germ cell tumour: outcome and prognostic factors. Br J Cancer. 1998;77(10):1672-1676.
77 Beyer J, Rick O, Weinknecht S, et al. Nephrotoxicity after high-dose carboplatin, etoposide and ifosfamide in germ-cell tumors: incidence and implications for hematologic recovery and clinical outcome. Bone Marrow Transplant. 1997;20(10):813-819.
78 Wright JE, Elias A, Tretyakov O, et al. High-dose ifosfamide, carboplatin, and etoposide pharmacokinetics: correlation of plasma drug levels with renal toxicity. Cancer Chemother Pharmacol. 1995;36(4):345-351.
79 Izzedine H, Isnard-Bagnis C, Launay-Vacher V, et al. Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant. 2006;21(11):3038-3045.
80 Pisoni R, Ruggenenti P, Remuzzi G. Drug-induced thrombotic microangiopathy: incidence, prevention and management. Drug Saf. 2001;24(7):491-501.
81 Gore EM, Jones BS, Marques MB. Is therapeutic plasma exchange indicated for patients with gemcitabine-induced hemolytic uremic syndrome? J Clin Apher. 2009;24(5):209-214.
82 Kaijser GP, Korst A, Beijnen JH, et al. The analysis of ifosfamide and its metabolites (review). Anticancer Res. 1993;13(5A):1311-1324.
83 West NJ. Prevention and treatment of hemorrhagic cystitis. Pharmacotherapy. 1997;17(4):696-706.
84 Goren MP. Oral administration of mesna with ifosfamide. Semin Oncol. 1996;23(3 Suppl 6):91-96.
85 Haselberger MB, Schwinghammer TL. Efficacy of mesna for prevention of hemorrhagic cystitis after high-dose cyclophosphamide therapy. Ann Pharmacother. 1995;29(9):918-921.
86 Rodriguez-Frias EA, Lee WM. Cancer chemotherapy I: hepatocellular injury. Clin Liver Dis. 2007;11(3):641-662. viii
87 Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16(2):157-168.
88 Richardson PG, Soiffer RJ, Antin JH, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multi-organ failure post stem cell transplantation: a multi-center, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16:1005-1017.
89 Treepongkaruna S, Thongpak N, Pakakasama S, et al. Acute pancreatitis in children with acute lymphoblastic leukemia after chemotherapy. J Pediatr Hematol Oncol. 2009;31(11):812-815.
90 Cunningham SC, Fakhry K, Bass BL, et al. Neutropenic enterocolitis in adults: case series and review of the literature. Dig Dis Sci. 2005;50(2):215-220.
91 Badgwell BD, Cormier JN, Wray CJ, et al. Challenges in surgical management of abdominal pain in the neutropenic cancer patient. Ann Surg. 2008;248(1):104-109.
92 Berghmans T. Hyponatremia related to medical anticancer treatment. Support Care Cancer. 1996;4(5):341-350.
93 O’regan S, Carson S, Chesney RW, et al. Electrolyte and acid-base disturbances in the management of leukemia. Blood. 1977;49(3):345-353.
94 Moses AM, Miller M. Drug-induced dilutional hyponatremia. N Engl J Med. 1974;291(23):1234-1239.
95 Stuart MJ, Cuaso C, Miller M, et al. Syndrome of recurrent increased secretion of antidiuretic hormone following multiple doses of vincristine. Blood. 1975;45(3):315-320.