212 Organ Donation After Cardiac Death
 Historical Perspective
Historical Perspective
The increasing gap between the number of organs available for transplantation and the number of patients listed for transplantation has become the rate-limiting step in reducing both wait times and wait-list deaths in patients with end-organ disease awaiting transplantation. Prior to the passage of the first U.S. brain death law in the state of Kansas in 1970,1 donation after cardiac death (DCD, or non-heartbeating donation) was the primary mode of organ donation in this country. Death in DCD donors was determined according to traditional cardiopulmonary criteria. Early organ procurement strategies were somewhat crude and variable, and consequently, warm ischemia time (time from donor circulatory arrest to cold perfusion) in the DCD donor was often prolonged and outcomes were poor.2 The impact of the type of graft on DCD outcomes was not apparent until experience with organs from donors declared brain dead (DBD) grew. Transplant centers in several states such as Nebraska, Ohio, North Carolina, and Illinois flourished after adoption of DBD in their respective states, and transplant volumes grew.3
The DBD phenomenon was a culmination of critical care physicians’ growing ability to maintain physiologic organ function in patients with little or no hope of neurologic recovery from severe insults to the central nervous system. A new debate was sparked over the precise definition and timing of death and the concept of futile care. This concept was introduced at a CIBA Foundation meeting in England in 1965 and subsequently endorsed with formal diagnostic criteria by Harvard Medical School in 1968.1,4 Acceptance of this medically, philosophically, and legally novel concept radically changed the face of transplantation. The revolutionary ability to certify death while perfusing the donor with oxygenated blood guaranteed procurement with minimal warm ischemia and graft damage and better recipient outcomes. As early experience with the DBD organs demonstrated superior outcomes, the use of DCD organs declined and was subsequently abandoned.5
As a result of the success seen with DBD organ donation, the number of U.S. transplants performed annually increased exponentially. Based on Organ Procurement and Transplantation Network (OPTN) data, in 1988, the first year for which reliable national data were available, 10,794 deceased-donor transplants were performed.6 Just 6 years later, annual volumes increased by nearly 50% to 15,210 total transplants. Most dramatically, the number of lung grafts from deceased donors increased from 33 to 708.6 Moreover, intestinal transplantation gained clinical success with the introduction of DBD donors (in addition to refined medical and surgical techniques). The first case was performed in 1990; by 1994, 96 patients with intestinal failure had received intestinal transplants.6 Concomitantly, advances in critical care resulted in reduced mortality in patients with end-stage organ disease, thereby resulting in increasing additions to and decreased attrition from the wait list, often referred to as the growing “gap” between supply and demand in transplantation. For example, despite a burgeoning number of transplant centers, rapid increase in transplants performed, and increased utilization of living donors, in 1995, only 33% of listed registrants waiting for kidney transplant (33,167) were transplanted (11,081).6 Unfortunately, the rate of transplantation fell to 10% of the list in the subsequent era of 1998 to 2002.7
Exacerbating the impact of this trend, numbers of young, previously healthy DBD donors stagnated due to several statutory changes in the areas of gun control, automobile safety (air bags, seat belts, lowering of legal blood alcohol limits), and cyclist helmet use, thereby reducing traumatic fatalities and consequently changing the face of DBD organ donors in the process.8 The demographics and mode of death of the typical DBD donor transitioned from a young, healthy person rendered brain dead as a result of a devastating head trauma toward an older person rendered brain dead from a neurovascular insult. The change in median donor age and mode of death ultimately eroded some of the benefit of utilization of the DBD donor and prompted a search for additional options.
As noted by DeVita, in 1993, the University of Pittsburgh Medical Center (UPMC) introduced the nation’s first institutional policy to permit and regulate DCD donation.9 The need for such a policy arose when several patients and their families asked to participate in organ donation after previously electing withdrawal of life-sustaining treatment. This was a request that fell outside the parameters of donation policies and guidelines then in effect. The UPMC policy became the first concrete model for the use of cardiopulmonary criteria to determine death for the purposes of organ procurement,10 and it highlighted a milestone in the evolution of the practice of transplantation in this country. Since, DCD utilization has been adopted by many organ procurement organizations (OPOs) and hospitals nationwide. By December 2006, OPTN bylaws required that all OPTN members have a DCD donor protocol in place.10 Moreover, the Joint Commission now requires that all accredited institutions develop and implement standardized DCD policies.11
 Current Status of DCD Donation
Current Status of DCD Donation
Volume
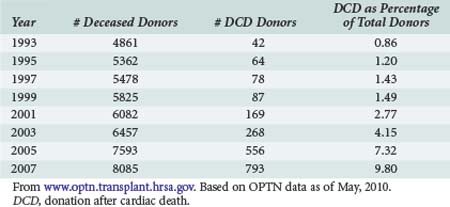
United Network for Organ Sharing (UNOS), the national nonprofit entity charged with disseminating both education and data pertaining to transplantation in the United States, has reported data on organs procured via DCD donation since 1994.6 Data are available via the OPTN website, www.optn.transplant.hrsa.gov/ and in OPTN annual reports. Table 212-1 demonstrates that the annual number of DCD organ donors increased steadily for the better part the mid 1990s to the early 21st century. The 188 DCD donor recoveries performed in 2002 represented 3% of total donors that year. In 2009, DCD recoveries represented 12% of all procurements, a fourfold increase from 2002.6
As a result of the OPTN and Joint Commission mandates mentioned earlier, the number of OPOs facilitating DCD recoveries in a given year has also risen overall, although not as sharply as the number of procurements performed: from 13 in 1993 to 33 in 2001. For the last year reported, 43 of the 59 OPOs facilitated at least one DCD procurement.7 The next logical question is whether the increased volume of DCD procurements has accordingly impacted transplant outcome metrics.
Outcomes
Though fraught with ethical controversy over the years, the real barrier to widespread acceptance of DCD graft utilization is based primarily upon the poor outcomes seen in the early DCD experience. Suboptimal organ function characterized by primary nonfunction (PNF), delayed graft function (DGF),12 and/or abbreviated graft survival have traditionally been a threat to success with DCD donors organs because of the warm ischemic insult associated with cardiopulmonary arrest. Although these observations were valid at the time, they were accumulated during the early experiences with transplantation and are thus inherently confounded by era bias.
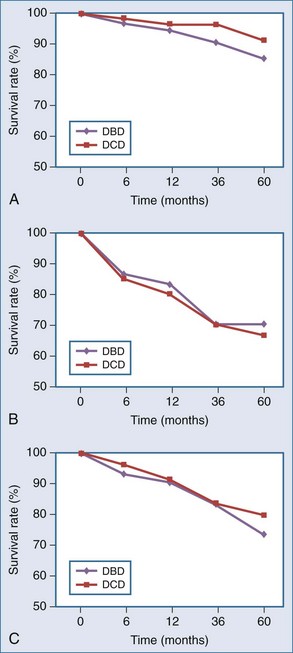
The primary lesson from the early DCD era was that the metabolically active renal cortex, biliary epithelium, pulmonary alveoli/central airways, and islets are sensitive to ischemia, with warm ischemic injury manifesting as acute tubular necrosis (ATN), ischemic-type biliary strictures (ITBS), bronchial dehiscence, and impaired beta cell function. These complications have been postulated to translate into and account for both poor initial graft function and long-term complications, seen particularly in the early era.12,13,14 Droupy and Abt, however, in separate studies, report that outcomes have improved; that intermediate and long-term patient/graft survival in recipients of controlled DCD kidney and liver grafts, respectively, are equivalent to or approach that of DBD.15,16 Per Droupy, DCD and DBD renal grafts followed for 10 years demonstrate equivalent survival despite a higher initial incidence of DGF for the DCD cohort. Salvaggio and colleagues, in an analysis of UNOS/OPTN data, concur17 (Figure 212-1, A).
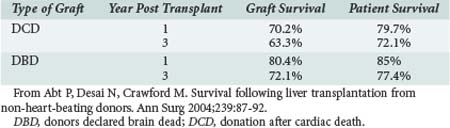
DCD liver outcomes have improved, though less dramatically. Some results of liver transplants using DCD donors were discouraging; both graft and patient survival rates were thought to be significantly lower when compared with DBD donors.18 Morbidity rates were higher as well.19,20 However, reviews isolating recent data—as, for example, data outlined by Abt—demonstrate that 1- and 3-year patient survival rates for liver DCD are now similar to those from DBD, although lower graft survival rates for DCD liver grafts persist16 (Table 212-2).
Much of the available outcome data for pancreatic DCD organs are derived from cases of simultaneous pancreas-kidney (SPK) transplants, demonstrating pancreatic graft and patient survival rates similar to those for DBD17 (see Figure 212-1, B). Utilization of pancreas-alone DCD grafts under the same protocols as SPK DCD grafts, while less frequent over the last decade, have been favorable17 (see Figure 212-1, C). Ongoing clinical experience will determine whether outcomes will reach those of SPK DCD grafts.
Oliveira et al. have, in the largest single-center series to date, demonstrated that lung grafts from DCD donors can also confer graft and patient survival rates equivalent to those from DBD donors.13 Of interest, these outcomes have been achieved in many settings in recipients who have been disproportionately more ill prior to transplant but deemed reasonable potential DCD recipients because of the long potential wait for DBD grafts. Consequently, clinicians now consider use of grafts that were previously routinely declined.21
 Identification and Categorization of the Potential DCD Donor
Identification and Categorization of the Potential DCD Donor
An important initial step in the process of DCD organ transplantation is recognizing the potential suitable donor. A significant consideration is the need to minimize organ ischemia in the presence of an unanticipated uncontrolled cardiac arrest; thus while organ procurement from DCD donors under uncontrolled conditions is technically feasible, it remains rare in contemporary practice.22,23 Similarly, graft quality is compromised in situations in which a patient’s wishes regarding organ donation are unknown. Organ suitability declines while attempts are made to locate family members to obtain consent. The once popular practice by some institutions to manage potential DCD donors brought to the emergency department by placement of vascular and/or intraperitoneal catheters in order to infuse cold organ preservation solution before consent for procurement became available24 has been largely abandoned. The practice stimulated contentious debate from opponents in both the medical and lay communities; unlike several European countries, no U.S. state at the time of the writing of this chapter has adopted presumed consent into law.
Remaining potential DCD donors are patients consented for donation with impending cardiopulmonary death, the timing of which is either unpredictable or predictable based upon patient/family-requested withdrawal of care, or unpredictable with premature arrest before withdrawal. Understandably, each type of DCD confers a varying risk of ischemic injury. A discussion of the management of DCD donors is facilitated by use of a classification scheme developed at a donor conference convened in 1994 by Maastricht, Netherlands, investigators.25 The Maastricht Categories define potential donors by the circumstances under which their cardiovascular death occurs. A distinction is made between those donors whose cardiopulmonary failure is uncontrolled or emergent (categories 1, 2, and 4) and those donors whose death by cardiopulmonary criteria occurs in a controlled, planned fashion by withdrawal of futile life-sustaining support (category 3). Maastricht Categories are outlined in Table 212-3.
| Category | Description | Condition |
|---|---|---|
| 1 | Cardiac arrest outside the hospital, no resuscitation attempted | Uncontrolled |
| 2 | Cardiac arrest followed by unsuccessful resuscitation, either inside or outside a hospital | Uncontrolled |
| 3 | Cardiac arrest after planned withdrawal of life-support technology | Controlled |
| 4 | Cardiac arrest in a brain-dead patient awaiting organ procurement | Uncontrolled |
From Koostra G, Daemen JHC, Oomen APA. Categories of non-heartbeating donors. Transplant Proc 1995;27:2893-4.
Of note, recent initiatives in the northeast United States involve training prehospital personnel in the rapid conversion of preconsented victims of unsuccessful resuscitation after cardiopulmonary arrest (category 2) to potential DCD donors.26 For the sake of uniformity, the remainder of this chapter will be devoted to discussion of category 3 donors.
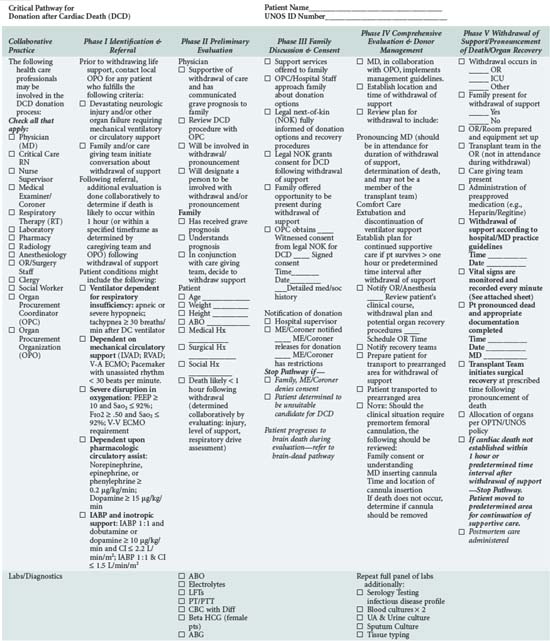
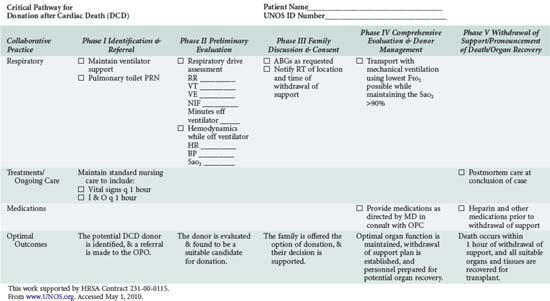
In addition to understanding the classification scheme and expected outcomes based upon absence or presence of controlled ischemia, the intensivist and OPO personnel must be familiar with the diagnoses and clinical circumstances qualifying a patient as a potential DCD donor. Again, candidates are patients in whom withdrawal of futile life-sustaining treatment is being planned. As shown in Table 212-4, the UNOS Critical Pathway for DCD,6 typical patients may have the following characteristics: absent or hyperactive respiratory drive, lack of adequate respiratory muscle strength, severe hypoxemia, or inadequate circulation in the absence of inotropic or vasopressor drugs. Such patients are usually supported by ventilators or mechanical circulatory assistance such as ventricular-assist devices (VAD) or intraaortic balloon pumps. They are often patients who have also suffered a severe neurologic insult. Conscious patients are usually suffering from degenerative neuromuscular diseases or end-stage cardiopulmonary disease and are often ventilator or VAD dependent. These patients or their families may decide to discontinue their support devices and request that their organs subsequently be donated.
The next important step in identification of the potential DCD donor is predicting when rapid physiologic deterioration and death are expected occur in a period of less than 30 to 60 minutes (depending on the organ to be procured) after withdrawal of life-sustaining treatment.27 Failure of a potential donor to progress to cardiac death within the prescribed time disqualifies the potential donor owing to the extent of warm ischemic injury sustained by the organs. Factors such as age, comorbidities, and preterminal pressor requirement have been shown to have predictive value, but no strict criteria have been universally adopted.28 Kaufman et al. have proposed four readily obtainable clinical criteria: (1) requirement for vasopressors to support arterial blood pressure, (2) absence of primary brain injury, (3) history of 6 or more days on mechanical ventilation, and (4) respiratory rate less than 20 breaths/min (in the absence of mechanical ventilatory support).29 They noted that the presence of two or more of the indicators accurately predicted death within 60 minutes after life-supporting treatments were withdrawn, with a sensitivity and specificity of 81% and 78%, respectively.
 Principles of DCD Donor Management
Principles of DCD Donor Management
Appropriate management of the DCD organ donor requires integration of several fundamental principles exercised to protect the rights and interests of the donor and simultaneously prevent the care of the organ from superseding the care of the dying patient. The debate arises in the paradox that can emerge from attempts to protect those interests while preserving suitability of the potential grafts. Hence, the role of the intensivist and/or palliative care physician (terminal care of the patient, pronouncement of death) and the managing OPO (facilitating organ procurement) must be rigidly defined; the two factions must travel distinct paths to achieve their goals. As Ozark states, “as a general rule two discussions—whether to forego life-sustaining therapy and whether to donate organs—must be made separately and on their own individual merit.”30 Ideally, discussion regarding withdrawal of life-sustaining treatment should come first so as not to be biased by the issue of transplantation.
The push for optimal palliative care has been a hallmark of recent critical care management initiatives.31 The dying patient who also wishes to be a DCD organ donor presents a special challenge, requiring care that is not only comparable to that afforded to all dying patients but also sensitive to the concerns already described. The Society of Critical Care Medicine (SCCM) has offered recommendations specific to DCD donation.32,33 These guidelines, supplemented by individual transplant center reports and the UNOS pathway (see Table 212-4), provide direction for intensivists caring for patients who wish to become DCD donors. It is vital that all healthcare providers involved in this process be comfortable with, and knowledgeable about, their specific role such that the patient’s wishes can be respected.
In 2008, in the first criminal case brought against a transplant surgeon for the death of a donor, the defendant allegedly administered high doses of analgesic and anxiolytic to a potential donor to hasten his death prior to procurement.11 Although the surgeon was acquitted, the case highlighted the potential legal ramifications of the recovery team’s involvement in the care of a dying patient. There is a consensus that “medications given to provide comfort are reasonable, even if they might hasten death” but “no medication whose purpose is to hasten death should be given to the patient.”34 Failure to attend to potential DCD donors’ comfort in contemporary practice is considered suboptimal end-of-life care but is not in any circumstance managed by anyone other than the physician(s) caring for the patient.
DeVita notes that “The initial University of Pittsburgh policy called for the withdrawal of care to occur in the operating room, which offered the advantage of minimizing the need to transport the patient after death and permitting the prepping and draping of the patient before death. This protocol was denounced for subjecting the patient to ‘a desolate, profanely “high tech” death’ surrounded by ‘masked, gowned, and gloved strangers.’35 The initial experience in Pittsburgh found some truth to the proposition that presence of family at the patient’s bedside at the time of death may be more important to patients and families than organ donation or location of death. When three of the first four families approached about non-heartbeating donation agreed to consent only if they could be physically present at the time of death, the Pittsburgh policy was changed to allow families into the operating room or to move the withdrawal of care to an operating room ‘holding’ area. The area selected for withdrawal of support should allow family members to be present, accommodate the necessary monitors and equipment, and be close enough to the operating room to allow rapid transport immediately after death.”7 Other programs followed in kind; according to the 2000 IOM report on DCD, the family’s need to be present and involved in the dying process is generally widely cited and respected in the development of hospital policies on the setting for withdrawal of care.34
Determination of Death, an Exact Scientific Concept?
The 1980 Uniform Determination of Death Act (UDDA) established that death is determined when there is irreversible cessation of circulatory and respiratory function.36 Death is declared most often based on cessation of cardiac and pulmonary function; however, required asystolic time is perhaps the single most contentious issue in the debate surrounding DCD donation.37,38 Simply, “the longer you wait the more uncertainty there is about the organs, and the shorter you wait the more uncertainty [there is about] whether the person is really dead or not.”39
As the limits of life-sustaining practices are expanded, medical professionals are encouraged to maintain focus with reference to the UDDA. The term irreversible can be interpreted as a shifting paradigm, or as Wilner remarks, the concept is subject to “serial displacement by advancing clinical science.” He further notes that “the question [of death] is thus reformulated to explore whether the morally relevant time of death is reached when death is certain despite all possible medical intervention or whether death is assured once all ethically permissible remedies have been utilized.”7 Although consensus has yet to be reached on the question of the time at which death is irreversible, it does seem logical that once a principled decision is made not to correct a loss of function, that loss becomes irreversible.40
No investigator has documented the spontaneous return of circulation after more than 65 seconds of combined circulatory and respiratory arrest.41 However, the standard applied by most U.S. hospitals ranges from a 2- to 10-minute asystolic interval (pulselessness, apnea, and unresponsiveness). This broad standard is addressed by the SCCM’s statement that “there is no ethically or physiologically important distinction between the two minute observation period utilized by the University of Pittsburgh, the five minutes recommended by the IOM, and the ten minutes” (utilized by some institutions).32 Ostensibly, the standard does satisfy proponents of the waning possibility of autoresuscitation beyond 65 seconds of asystole, and furthermore serves to address the ethical concerns raised by those proponents. Because of the paucity of empirical evidence, the IOM continues to encourage investigators to provide additional studies in this matter.
A final logistic issue in the determination of death is the management of patients who progress too slowly to be considered for donation. This occurs in approximately 5% to 10% of potential donors.28 Most programs disqualify patients from donation if there is cardiac activity 60 minutes after discontinuing life support.42 For this reason, contingency plans should be in place so that these patients receive appropriate ongoing end-of-life care.
 DCD Procurement: an Opportunity for Standardization
DCD Procurement: an Opportunity for Standardization
Premortem
Because these premortem measures are not part of standard end-of-life care and have been argued by some to potentially hasten death, their use remains limited.50 The position of the SCCM and IOM is that the use of these medications and devices is acceptable so long as they cause no significant harm to the patient32,34 and family consent is obtained wherever practical. That they are of no direct benefit to the patient is countered by the fact that they improve the likelihood that the patient’s wish of organ donation will ultimately be realized.
Operative
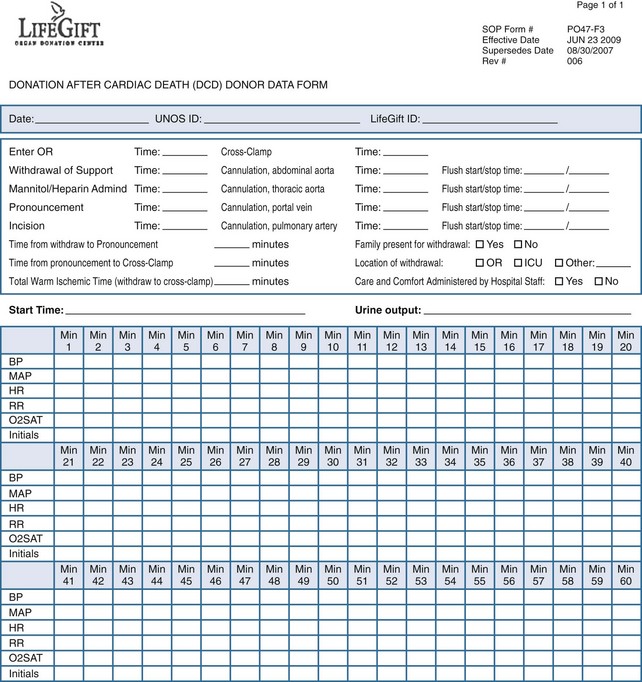
The conduct of the operative procedure is dictated by the tenets already mentioned: the procurement team is not physically present at the time of death, and recovery of organs must be accomplished expeditiously with careful coordination of numerous personnel, equipment, and resources. To do so, the operative team prepares and drapes the patient upon arrival to the operating room. The team outlines the necessary instruments and maneuvers requested of the OPO staff to ensure a seamless procedure. The team, gowned and gloved, is escorted from the operating room and is notified by OPO staff if the patient progresses within the prescribed time frame. Optimally, after withdrawal and prior to incision, the OPO staff will complete a data form (Figure 212-2) allowing for recording of minute-by-minute hemodynamic and oxygenation data. The benefit of compiling data in such a fashion is that as DCD procurements become standardized, data can be collated without the encumbrance of shifting definitions of the initiation of warm ischemic time (and hence acirculatory status) that currently confounds comparison of outcomes between hospitals and OPOs. For instance, the definition of the warm ischemia start time remains variable, with some advocating that a threshold of systolic blood pressure of less than 80 mm Hg be used, others, a mean arterial pressure of 50 mm Hg, others a systolic blood pressure less than 50 mm Hg, and yet others when the arterial oxygen saturation decreases to less than 80%.16 The lack of a universal definition renders comparison of outcomes between centers and organs difficult.
 Future Directions
Future Directions
Owing to unavoidable warm ischemia, heart and intestinal grafts are difficult to utilize when procured from DCD donors. Nevertheless, cardiac DCD transplantation is technically possible. The first human cardiac transplant 40 years ago was made possible using a DCD donor heart, which started and functioned well after a single electric shock.51 It is conceivable, then, with the advent of preconditioning therapy, a new era of DCD cardiac transplantation may begin.
Furthermore, the concern by the lay public is that physicians caring for patients who are potential donors have shifted the focus of care from the dying patient, and that there exists now more than ever, the latitude to violate the “dead donor rule” (comprising two complementary ideas: that the patient must be dead before the initiation of organ procurement; and that organ procurement itself must not be the cause of the donor’s death).40 This concern may translate in the public mind into a fear that their likelihood of receiving aggressive life support will be compromised by consenting to organ donor status. These misperceptions can be particularly damaging when the overriding goal of the transplant community is to maintain and build public support of maximizing organ donation.
 Conclusion
Conclusion
The widening gap between suitable donors and patients in need of transplant continues to be the single issue that keeps solid-organ transplantation from achieving its full potential for relieving suffering and improving survival in patients with end-stage organ disease. As current practice and outcomes have shifted the paradigm of the binary cadaveric donor (DBD versus DCD) to a spectrum of standard criteria to extended criteria, with factors mentioned earlier affecting where on this spectrum a DCD organ may fall, the ultimate impact of the estimated unrealized annual 22,000 DCD donors8 on the actual number of organs available for transplantation will remain unclear until sufficient data obtained under similar protocols are obtained. Whereas ethical questions regarding DCD donation persist, the process, as it is increasingly practiced in a standardized fashion, has proven to accommodate the needs of dying patients, as well as those awaiting transplantation, with improving success.38 A number of organizations including the SCCM,32 UNOS,6 and the IOM35 have endorsed the concept and issued relevant guidelines.
Key Points
Choi E-K, Fredland C, Zachodni C, et al. Brain death revisited: the case for a national standard. J Law Med Ethics. 2008:824-836.
Childress J, Liverman C, editors. Organ donation: opportunities for action. Washington DC: The National Academies Press, 2006.
Reich DJ, Mulligan DC, Abt PL, et al. ASTS Recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004-2011.
Ho KJ, Owens CD, Johnson SR, et al. Donor postextubation hypotension and age correlate with outcome after donation after cardiac death transplantation. Transplantation. 2008;85:1588-1594.
A unique analysis of donor factors which impact graft function after DCD donation.
Salvaggio P, Davies D, Fernandez L, et al. Outcomes of pancreas transplantation in the United States using cardiac-death donors. Am J Transpl. 2006;6:1059-1065.
1 Choi E-K, Fredland C, Zachodni C, et al. Brain Death Revisited: The Case for a National Standard. J Law Med Ethics. 2008:824-836.
2 Deshpande R, Heaton N. Can Non-Heart-Beating Donors Replace Cadaveric Heart-Beating Liver Donors? J Hepatol. 2006;45:499-503.
3 Rager E. The Donation of Human Organs and the Evolving Capacity for Transplantation: Exciting Developments and Future Prospects. NC Med J. 2004;65:18-25.
4 Machado C. The First Organ Transplant From a Brain-Dead Donor. Neurology. 2005;66:460-461.
5 DeVita M. History of organ donation by patients with cardiac death. Kennedy Inst Ethics J. 1993;3:113-129.
6 www.optn.transplant.hrsa.gov/. Based on OPTN data as of May, 2010
7 Wilner LS, DeVita MA. Non-heartbeating organ donation (donation after cardiac death. In Fink MP, Abraham E, Vincent J-L, Kochanek PM, editors: Textbook of Critical Care, 5th ed, Philadelphia: Saunders, 2002.
8 Childress J, Liverman C, editors. Organ Donation: Opportunities for Action. Washington, D.C: The National Academies Press, 2006.
9 DeVita MA, Snyder JV. Development of the University of Pittsburgh Medical Center policy for the care of terminally ill patients who may become organ donors after death following the removal of life support. Kennedy Inst Ethics J. 1993;3:131-143.
10 Rady M, Verheijde J, McGregor J. Organ procurement after cardiocirculatory death: a critical analysis. J Intensive Care Med. 2008;23:303-312.
11 Steinbrook R. Organ Donation after Cardiac Death. N Engl J Med. 2007;357:209-213.
12 D’Alessandro AM, Odorico JS, Knechtle SJ, et al. Simultaneous pancreas-kidney (SPK) Transplantation from controlled non-heartbeating donors (NHBDs). Cell Transplant. 2000;9:889-893.
13 Oliviera N, Osaki S, Maloney J. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thor Cardiovasc Surg. 2010;139:1306-1315.
14 D’Alessandro AM, Hoffman RM, Knechtle SJ, et al. Liver transplantation from controlled non-heart-beating donors. Surgery. 2000;128:579-586.
15 Droupy S, Blanchet P, Eschwege P, et al. Long-term results of renal transplantation using kidneys harvested from non-heartbeating donors: A 15-year experience. J Urol. 2003;169:28-31.
16 Abt P, Desai N, Crawford M. Survival following liver transplantation from non-heart-beating donors. Ann Surg.. 2004;239:87-92.
17 Salvaggio P, Davies D, Fernandez L, et al. Outcomes of pancreas transplantation in the United States using cardiac-death donors. Am J Transpl.. 2006;6:1059-1065.
18 de Vera ME, Lopez-Solis R, Dvorchik I, et al. Liver Transplantation Using Donation After Cardiac Death Donors: Long-Term Follow-Up From A Single Center. Am J Transplant. 2009;9(4):773-781.
19 Maheshwari A, Maley W, Thuluvath P. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl.. 2007;13:1645-1653.
20 Mateo R, Cho Y, Singh G, et al. Risk Factors for the Survival After Live Transplantation From Donation After Cardiac Death Donors: An Analysis of OPTN/UNOS Data. Am J Transplant. 2006;6:791-796.
21 Cypel M, Sato M, Yildrim E, et al. Initial Experience With Lung Donation After Cardiocirculatory Death in Canada. J Heart Lung Transplant. 2009;28:753-758.
22 Doig CJ, Zygun DA. Uncontrolled) Donation After Cardiac Determination Of Death: A Note Of Caution. J Law Med Ethics. 2008;36(4):760-765. 610
23 Gagandeep S, Matsuoka L, Mateo R, et al. Expanding The Donor Kidney Pool: Utility Of Renal Allografts Procured In A Setting Of Uncontrolled Cardiac Death. Am J Transplant. 2006;6(7):1682-1688.
24 Veatch RM. Consent for perfusion and other dilemmas with organ procurement from non-heartbeating cadavers. In: Arnold RM, Youngner SJ, Schapiro R, et al, editors. Procuring Organs for Transplant—The Debate over Non-Heart-Beating Cadaver Protocols. Baltimore: The Johns Hopkins University Press, 1995.
25 Koostra G, Daemen JHC, Oomen APA. Categories of non-heartbeating donors. Transplant Proc. 1995;27:2893-2894.
26 Wall S. Derivation of the uncontrolled DCD protocol for New York City. American Society of Transplant Surgeons Winter Meeting, Ft. Lauderdale, 2010.
27 Bernat A, D’Alessandro F, Port T, et al. Report of a national conference on donation after cardiac death. Am J Transplant. 2006;6:281-291.
28 Ho KJ, Owens CD, Johnson SR, et al. Donor Postextubation Hypotension And Age Correlate With Outcome After Donation After Cardiac Death Transplantation. Transplantation. 2008;85(11):1588-1594.
29 Kaufman DA, Higgins TL, Nathanson BH. Factors predictive of time to death after withdrawal of life support. Crit Care Med. 2003;30(Suppl):A145.
30 Ozark S, DeVita MA. Non-heartbeating organ donation: Ethical controversies and medical considerations. Int Anesthesiol Clin. 2001;39:103-116.
31 Prendergast TJ, Puntillo KA. Withdrawal of life support—intensive caring at the end of life. JAMA. 2002;288:2732-2740.
32 Ethics Committee, American College of Critical Care Medicine, Society of Critical Care Medicine. Position Paper: Recommendations for non-heartbeating organ donation. Crit Care Med. 2001;29:1826-1831.
33 Truog RD, Cist AF, Brackett SE, The Ethics Committee of the Society of Critical Care Medicine. Recommendations for end-of-life care in the intensive care unit. Crit Care Med. 2001;29:2332-2348.
34 Maximizing benefits, minimizing harms: A national research agenda to assess the impact of non-heart-beating organ donation. Institute of Medicine, Committee on Non-Heart-Beating Transplantation II. In: Solomon MZ, editor. Non-Heart-Beating Organ Transplantation: Practice and Protocols. Washington, DC: National Academy Press, 2000.
35 Fox RC. “An ignoble form of cannibalism”: Reflections on the Pittsburgh protocol for procuring organs from non-heartbeating cadavers. Kennedy Inst Ethics J. 1993;3:231-239.
36 Keely GC, Gorsuch AM, McCabe JM, et al. Uniform Determination of Death Act. Chicago, IL, National Conference of Commissioners on Uniform State Laws, 1980.
37 Suntharalingam C, Sharples L, Dudley C, et al. Time To Cardiac Death After Withdrawal Of Life-Sustaining Treatment In Potential Organ Donors. Am J Transplant. 2009;9:2157-2165.
38 Reich DJ, Mulligan DC, Abt PL, et al. ASTS Recommended Practice Guidelines For Controlled Donation After Cardiac Death Organ Procurement And Transplantation. Am J Transplant. 2009;9(9):2004-2011.
39 Arnold RA, Youngner SJ. Time is of the Essence: The pressing need for comprehensive non-heart-beating cadaveric donation policies. Transplant Proc. 1995;27:2913-2921.
40 Robertson JA. The dead donor rule. Hastings Cent Rep. 1999;29:6-14.
41 DeVita MA. The death watch: Certifying death using cardiac criteria. Prog Transplant. 2001;11:58-66.
42 Sample Policy for Organ Donation After Cardiac Death. American Society of Critical Care Anesthesiologists Committee on Transplant Anesthesia. 2007.
43 Personal communication, John Fung, M.D., FACS, Director, Cleveland Clinic Transplant Center.
44 Gok MA, Shenton BK, Peaston R, et al. Improving the quality of kidneys from non-heart-beating donors, using streptokinase: an animal model. Transplantation. 2002;73:1869-1874.
45 Richter S, Yamauchi J, Minor T, et al. Heparin and phentolamine combined, rather than heparin alone, improves hepatic microvascular procurement in a non-heartbeating donor rat-model. Transplant Int. 2000;13:225-229.
46 Hernandez A, Light JA, Barhyte D, et al. Ablating the ischemia-reperfusion injury in non-heartbeating donor kidneys. Transplantation. 1999;67:200-206.
47 Rehman H, Connor H, Ramshesh V, et al. Ischemic preconditioning prevents free radical production and mitochondrial depolarization in small-for-size rat liver grafts. Transplantation. 2008;85:1322-1331.
48 Murray CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136.
49 Cope J, Mauney M, Banks D, et al. Intravenous phenylephrine preconditioning of cardiac grafts from non-heart-beating donors. Ann Thor Surg. 1997;63:1664-1668.
50 DuBois JM. Is organ procurement causing the death of patients? Issues Law Med. 2002;18:21-41.
51 Barnard CN. The operation: A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. South Afr Med J. 1967;41:1271-1274.