Orbit Infection and Inflammation
Periorbital and Orbital Cellulitis
Etiologies, Pathophysiology, and Clinical Presentation: The orbital septum, the anterior reflection of the periosteum of the orbital wall onto the tarsal plate of the eyelid, divides the orbit into preseptal and postseptal compartments.
Periorbital cellulitis refers to infection anterior to the orbital septum involving the eyelid and adnexa. Orbital cellulitis refers to infection posterior to the orbital septum. This distinction is important because orbital cellulitis carries the risks of abscess, blindness, venous thrombosis, intracranial extension, and death. Defects in the orbital septum, direct extension from sinus infection, and valveless veins provide infection with access to the postseptal orbit. Sinusitis is the most common cause (60% to 85%), with stye, dacryoadenitis/cystitis, dental abscess, skin breaks, and hematogenous seeding being less common.1,2 Staphylococcus aureus, S. epidermidis, and S. pyogenes account for ~75% of infections; rates of Hemophilus influenzae and streptococcal pneumonia are declining as a result of immunization.1 Patients present with erythema, swelling, warmth, and tenderness of the eyelid. Although ophthalmoplegia and proptosis predict postseptal involvement and abscess, approximately 50% of patients with an abscess do not have these symptoms. As a result, the guidelines for imaging are unclear and vary: edema preventing a complete examination, signs of central nervous system involvement, deteriorating vision, proptosis, ophthalmoplegia, and/or deterioration after 24 to 48 hours of treatment.3–5
Imaging: Periorbital cellulitis presents with eyelid swelling and thickening of the preseptal soft tissues on computed tomography (CT), with T2 hyperintensity on magnetic resonance imaging (MRI) (Fig. 6-1, A).2,6,7 In orbital cellulitis, similar inflammatory changes of the extraconal and/or intraconal orbital fat are present. The most common complication of orbital cellulitis is subperiosteal abscess, frequently involving the lamina papyracea, and directly extending from ethmoid sinus disease. A subperiosteal abscess presents as a broad-based, peripherally enhancing fluid collection along the medial wall of the orbit that displaces the medial rectus muscle laterally (Fig. 6-2, A). Less commonly, an abscess can form in the extraconal or intraconal orbit separate from the bone, which also is seen as a peripherally enhancing fluid collection. MRI can be helpful in identifying an abscess by demonstrating restricted diffusion.8
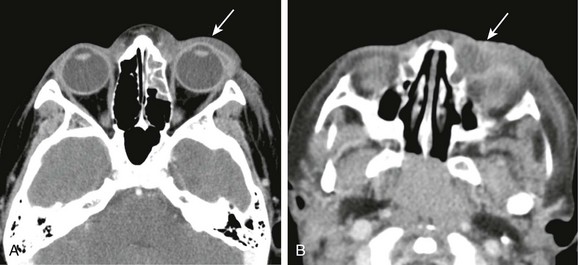
Figure 6-1 Periorbital cellulitis.
A, A computed tomography (CT) image of periorbital cellulitis from adjacent sinusitis demonstrates preseptal soft tissue swelling (arrow). B, A CT image of dacryocystitis causing periorbital cellulitis demonstrates a cystic medial canthus mass (arrow) with adjacent inflammatory changes.
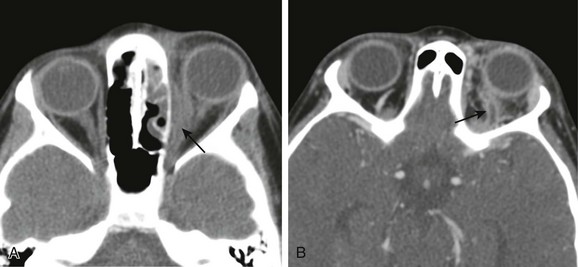
Figure 6-2 Orbital cellulitis.
A, A computed tomography (CT) scan of orbital cellulitis with a subperiosteal abscess demonstrates inflammatory changes of the fat of the postseptal orbit with a broad-based, peripherally enhancing fluid collection adjacent to the lamina papyracea (arrow). B, A CT scan of superior ophthalmic vein (SOV) thrombosis demonstrates an enlarged S-shaped SOV with a filling defect and peripheral enhancement (arrow).
Treatment: Treatment consists of oral antibiotics covering staphylococcus and streptococcus for periorbital cellulitis and admission to the hospital with administration of intravenous (IV) antibiotics for orbital cellulitis. Surgical intervention for drainage of an abscess is required in only 12% of admitted patients,3 and an orbital abscess can be treated with IV antibiotics if it is small or appears in a young child.9,10
Dacryocystitis
Dacryocystitis is the result of bacterial overgrowth of stagnant fluid in the nasolacrimal sac presenting with epiphora, erythema, and edema at the medial canthus. In neonates, dacryocystitis can complicate 33% to 65% of cases of congenital dacryocystocele caused by incomplete canalization of the distal nasolacrimal duct.11,12 In older children, dacryocystitis can result from other causes of nasolacrimal duct obstruction, including rhinitis/sinusitis, tumor, or trauma and fracture. CT or MRI demonstrates a cystic medial canthus mass with adjacent inflammatory changes (Fig. 6-1, B).13–15 Although infection is most commonly associated with periorbital cellulitis, it rarely can extend into the postseptal orbit with abscess formation.16 Treatment typically consists of antibiotics and dacryocystorhinostomy.
Superior Ophthalmic Vein Thrombosis
Superior ophthalmic vein (SOV) thrombosis is a complication of orbital cellulitis that results from inflammatory thrombophlebitis or direct venous invasion by infection; 33% to 75% of isolated SOV thrombosis leads to cavernous sinus thrombosis, which carries a mortality rate of 20%.17,18 Imaging with CT or MRI demonstrates an enlarged S-shaped SOV below the superior rectus muscle with a filling defect and peripherally enhancing vasa vasorum on postcontrast images (Fig. 6-2, B).19 Restricted diffusion of the SOV also has been reported, facilitating identification.20 Treatment consists of aggressive use of antibiotics with or without corticosteroids and anticoagulation, which are not proven therapies.17,18
Ocular Toxocariasis
Ocular toxocariasis refers to infection of the globe by the nematodes Toxocara canis or Toxocara cati. It is one of several ocular infections caused by parasitic worms, including onchocerciasis (Onchocerca volvulus), cysticercosis (Taenia solium), and diffuse unilateral subacute neuroretinitis. Ocular toxocariasis is most common in the southeastern United States in children 6 to 12 years of age as a result of ingestion of food or soil contaminated by the feces of dogs or cats. It presents with painless unilateral vision loss, strabismus, and leukocoria.21
CT and MRI demonstrate an intravitreal enhancing mass with or without adjacent uveoscleral thickening and retinal detachment. A normal-sized globe containing a mass without calcification differentiates toxocariasis from other common causes of leukocoria (e.g., retinoblastoma, persistent hyperplastic primary vitreous, Coats disease, and retinopathy of prematurity).22,23
Orbital Pseudotumor (Idiopathic Orbital Inflammation)
Etiologies, Pathophysiology, and Clinical Presentation: Orbital pseudotumor (OP) is a noninfectious inflammatory condition of the orbit of unclear etiology. In adults it is the most common painful orbital mass, accounting for ~10% of orbital masses, and is the third most common orbital disease. Pediatric OP is rare, with only 68 cases reported in the medical literature as of 2008, accounting for only 7% to 16% of cases of OP.24,25 Children present similarly to adults with pain, proptosis, a mass, swelling, and motility restriction; however, children more frequently demonstrate ptosis and bilateral or intraocular involvement.
Imaging: Both CT and MRI are useful in evaluating OP.26–28 Lacrimal gland involvement is most common, with enlargement and adjacent inflammatory change. Myositis also occurs frequently, typically with unilateral tubular thickening of extraocular muscles and tendons (compared with Graves orbitopathy, which tends to be bilateral with tendon sparing). OP may involve the uvea and sclera with thickening and enhancement (Fig. 6-3, A). Perineuritis, which involves the optic nerve sheath, demonstrates “tramline” inflammatory changes and enhancement surrounding the optic nerve (Fig. 6-3, B-D). Inflammation can extend through the orbital fissures and optic canal into the cavernous sinus and middle cranial fossa. The differential diagnosis includes infection, lymphoma, Wegener granulomatosis, sarcoidosis, and Graves orbitopathy. Diffusion-weighted imaging may be helpful in the diagnosis with the intensity of lymphoid lesions > OP > cellulitis on b-value = 1000 images.29
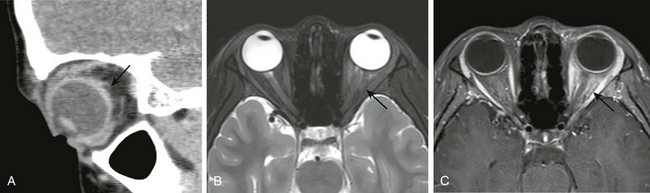
Figure 6-3 Orbital pseudotumor.
A, A sagittal computed tomography image of scleral involvement by a pseudotumor causing posterior scleritis with subtle thickening of the posterior sclera (arrow). B, A T2-weighted fat-saturated magnetic resonance image in a different patient with a pseudotumor demonstrating ill-defined T2 hyperintensity of the intraconal fat (arrow). C, A T1-weighted fat-saturated postcontrast image demonstrating corresponding “tramline” enhancement of the optic nerve sheath and ill-defined enhancement of the intraconal fat (arrow).
Treatment: Administration of oral corticosteroids often results in a rapid response (within 1 to 2 days); radiation is used in refractory cases.25,30 Recurrence after withdrawal of steroids occurs frequently in adults (~50%) but has been reported in only one child.24,31 Biopsy is reserved for atypical symptoms or poor response. Recently, cases of OP have been identified as part of IgG4-related disease, a systemic inflammatory disease demonstrating excellent response to rituximab and corticosteroids.32,33
Graves Orbitopathy (Thyroid Orbitopathy)
Etiologies, Pathophysiology, and Clinical Presentation: Graves orbitopathy is an orbital inflammatory process seen in persons with Graves disease, which is an autoimmune thyroid disease from thyroid-stimulating hormone receptor autoantibodies. Pediatric Graves disease is uncommon, yet children who have the disease experience Graves orbitopathy at similar rates as do adults, in one third to two thirds of cases.34–36 Graves orbitopathy is milder in children than in adults, with mild proptosis and mild eyelid retraction or lag. In children, no cases of compressive optic neuropathy have been reported, and strabismus is rare.
Imaging: Imaging demonstrates fusiform enlargement of extraocular muscles (involving muscle bellies and sparing tendons), which is bilateral in 90% of cases and frequently involves the inferior and medial recti. Increased volume of orbital fat and lacrimal gland enlargement also can be seen.37 Active disease can be differentiated from fibrotic disease by evaluating the T2 signal intensity and dynamic contrast enhancement of the muscles (T2 hyperintensity, shorter time to peak, and greater enhancement and washout ratios are found in persons with active disease). This distinction is important, because medical therapy is not effective in persons with fibrotic disease.38–40
Sarcoidosis
Sarcoidosis is a multisystem disease of unclear etiology characterized by noncaseating granulomas. Sarcoidosis is rare in children; however, a unique form appears in children younger than 5 years and presents with rash, uveitis, and arthritis.41 In older children and adults, ocular involvement is seen in ~25% of cases, most commonly with uveitis.41–44 Additional orbital structures involved can include the lacrimal gland and sac, eyelid, orbital soft tissues, optic nerve and sheath, and extraocular muscles, with enlargement and enhancement of these structures.45,46 Involvement can be well circumscribed (in 85% of cases) or diffuse (in 15% of cases). The mainstay of treatment is oral steroids, which generally results in a good response; methotrexate or surgery is used for refractory cases. In patients with isolated orbital involvement (63%), systemic disease develops in 8% within 5 years.47
Wegener Granulomatosis
Wegener granulomatosis is a necrotizing granulomatous vasculitis of small and medium-sized vessels associated with antineutrophil cytoplasmic antibodies. Pediatric Wegener granulomatosis is rare; it presents in adolescence with a female predominance.48,49 Orbital involvement occurs in approximately 50% of adults (and is the presenting feature in 15%), but it is less common in children.50,51 Orbital disease may be primary or extend from the sinuses with osseous erosion. The globe (conjunctivitis and scleritis), lacrimal gland, retrobulbar space, optic nerve, and extraocular muscles all can be involved. Imaging demonstrates granulomatous masses with variable enhancement and characteristic T2-weighted hypointensity on MRI, presumably related to fibrocollagenous tissue (Fig. 6-4).52,53 Visual impairment related to optic nerve compression or vasculitis is seen in up to 20% to 50% of adults. Nasolacrimal duct obstruction from sinus disease can result in dacryocystitis. Treatment in children can vary but typically consists of administration of steroids and cyclophosphamide.
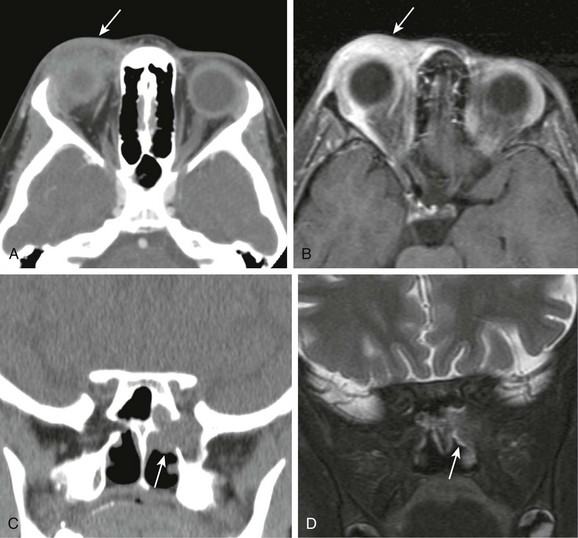
Figure 6-4 Wegener granulomatosis.
A and B, Computed tomography (CT) and T1-weighted fat-saturated postcontrast magnetic resonance (MR) images of the orbit demonstrating inflammatory changes and enhancement adjacent to the lacrimal gland in the preseptal orbit, similar in appearance to periorbital cellulitis (arrow). C and D, Coronal CT and T2-weighted fat-saturated MR images demonstrating a mass centered on the sphenopalatine foramen with osseous erosion and extension to the orbital apex with characteristic T2 hypointensity, making it difficult to visualize on MR imaging (arrows).
Optic Neuritis
Etiologies, Pathophysiology, and Clinical Presentation: Optic neuritis (ON) is an inflammatory disease of the optic nerve with acute onset of vision loss and periocular pain (in >90% of adults). ON may be idiopathic or related to multiple sclerosis (MS), neuromyelitis optica, or acute disseminated encephalomyelitis. ON behaves differently in children than in adults, likely reflecting different etiologies, with parainfectious ON being common in children (in one third to two thirds of cases).54–58 Compared with adults, ON in children is more often bilateral (in 37% to 66% of cases), less often painful (in 37% of cases), and more often demonstrates disk swelling (in 46% to 85% of cases) and profound vision loss. In children it tends to have better visual recovery and is less often associated with MS.
Imaging: MRI demonstrates contrast enhancement (>90%), T2-weighted hyperintensity, and mild enlargement of the optic nerves acutely, with mild volume loss developing chronically (Fig. 6-5).59,60 If frank enlargement of the optic nerve is seen, an optic pathway glioma should be considered. Intraorbital involvement is most common. Intracanalicular and long segment involvement and persistent signal change over time correlate to worse visual outcomes. Retinal nerve fiber layer thinning on optical coherence tomography also correlates to vision loss.61,62 Diffusion tensor imaging of optic nerves is difficult perform clinically, yet demonstrates decreased axial diffusivity acutely and increasing radial diffusivity and apparent diffusion coefficient values during recovery, correlating to changes in visual acuity and retinal nerve fiber layer thinning.63–65 Decreased fractional anisotrophy values also can be seen in the optic tracts and radiations, possibly as a result of Wallerian and transsynaptic degeneration.66–68 Magnetization transfer ratios (MTRs), which are thought to decrease with demyelination, are more sensitive than T2 spin echo imaging. They progressively decrease in the optic nerve, with a nadir at 240 days, and then mildly increase (possibly from remyelination), also correlating to changes in visual acuity.69,70
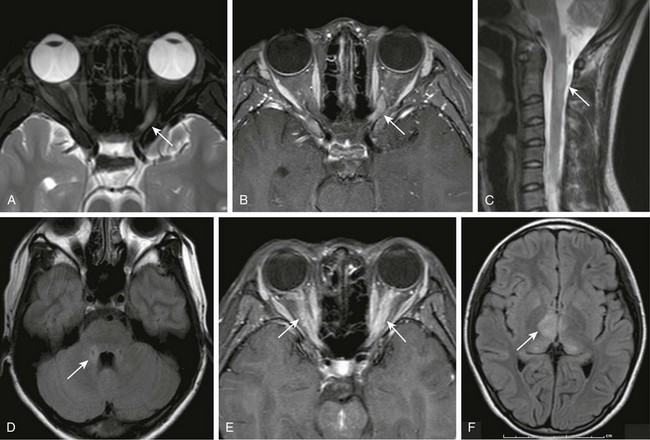
Figure 6-5 Optic neuritis.
T2- (A) and T1-weighted fat-saturated postcontrast (B) magnetic resonance (MR) images of the orbits demonstrating abnormal T2 hyperintensity and enhancement of the left optic nerve (arrows). C, A sagittal T2-weighted MR image of the cervical spine demonstrates a characteristic central long segment cord lesion in a patient with neuromyelitis optica (NMO) (arrow). D, An axial T2-weighted fluid attenuated inversion recovery (FLAIR) MR image in the same patient with NMO demonstrating subtle hyperintensity adjacent to the fourth ventricle in regions of high aquaporin 4 concentration (arrow). E, A T1-weighted fat-saturated postcontrast image of the orbits demonstrating bilateral optic nerve enhancement (arrows) in a patient with acute disseminated encephalomyelitis (ADEM). F, A T2-weighted FLAIR image of the brain in the same patient with ADEM showing lesions in the thalami (arrow).
Optic Neuritis and Multiple Sclerosis
Most ON research has been conducted with adult subjects, and it has been found that MS develops in 50% of adults with ON within 15 years.72 The greatest predictor is an abnormal MRI scan of the brain, and the risk of MS increases with the number of lesions: MS develops in 25% of adults with a normal MRI at baseline, whereas it develops in 75% of adults with one or more lesions in the brain at baseline.73 In children, the largest series with the longest follow-up demonstrated a 19% conversion to MS (13% in 10 years).57 As with adults, almost all children in whom MS developed had an abnormal MRI scan at baseline, and MS developed in almost no children who had a normal MRI scan.54,55 Furthermore, optic disk swelling is associated with a decreased risk of MS in adults and is a common feature of pediatric ON, likely reflecting the often parainfectious nature of ON in children.54–58
Optic Neuritis and Neuromyelitis Optica (Devic Disease)
Etiologies, Pathophysiology, and Clinical Presentation: Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the optic nerves and spinal cord related to NMO–immunoglobulin G (IgG), a serum autoantibody targeting aquaporin 4, a water channel on astrocytes at the blood-brain barrier.74,75 The distinction of NMO from MS is important because NMO has a worse prognosis and requires different treatment. Diagnostic criteria for children are similar to those for adults: clinical diagnosis of ON and transverse myelitis with either a cord lesion on MRI or NMO-IgG seropositivity.76,77 The prognosis is poor: >50% of patients have vision loss or lose the ability to ambulate within 5 years. Death as a result of respiratory failure also is seen.78 NMO-IgG seropositivity may indicate a worse prognosis with a relapsing course.79–81 Parainfectious NMO is common in children and typically is NMO-IgG negative and monophasic.82,83 Accordingly, pediatric NMO has a better prognosis and a longer time before the onset of disability.84,85
Imaging: Characteristic cord lesions are centrally located and extend three or more segments in length (Fig. 6-5, C). Up to 60% of patients also demonstrate brain lesions, which are part of the new diagnostic criteria.86 Findings include nonspecific signal changes, MS-like lesions (10%), and T2-weighted hyperintensity in areas of high aquaporin 4 concentration adjacent to the third and fourth ventricles (Fig. 6-5, D). Signal changes in the distribution of aquaporin 4 may be specific for NMO and are seen more frequently in children.86,87
Treatment: Treatment of persons with NMO consists of immunosuppression (IV methylprednisolone ± plasmapheresis acutely, with oral prednisone and azathioprine for maintenance), compared with immunomodulation for persons with MS.74,84 NMO-IgG positivity in persons with isolated ON or transverse myelitis may represent a limited form of NMO, requiring more aggressive treatment.81 Similarly, NMO-IgG negativity in children may predict a parainfectious etiology with a monophasic course not requiring immunosuppressive therapy.79,80
Optic Neuritis and Acute Disseminated Encephalomyelitis
Acute disseminated encephalomyelitis (ADEM) is an autoimmune inflammatory and typically monophasic demyelinating disease of the central nervous system that occurs days to weeks after a viral illness or vaccination. Visual loss is seen in up to one fourth of patients with ADEM, and ON in persons with ADEM tends to be bilateral with swollen optic disks (Fig. 6-5).88–90 Although no clear guidelines exist, treatment usually consists of IV steroids with favorable outcomes.91 The high frequency of parainfectious ON in children (in one third to two thirds of cases) likely contributes to the different presentation and more favorable prognosis of ON in children compared with adults.
Papilledema
Papilledema is another cause of optic disk swelling in children. It is the result of elevated intracranial pressure as a result of intracranial masses/inflammation, hydrocephalus, venous sinus thrombosis, or pseudotumor cerebri. Visual loss may result.92 MRI demonstrates optic disc elevation, dilated perioptic subarachnoid spaces, and optic nerve tortuosity.93,94 Enhancement and restricted diffusion of the optic disk have been described, perhaps as a result of venous congestion and ischemia (e-Fig. 6-6).95,96
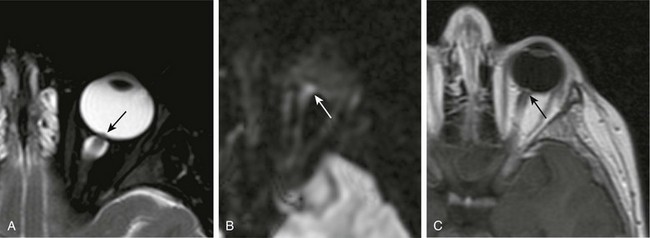
e-Figure 6-6 Papilledema.
A, A T2-weighted fat-saturated image of the orbit demonstrating a dilated optic nerve sheath and a raised optic disk (arrow). B and C, Diffusion-weighted imaging (b-value = 1000) and T1-weighted postcontrast magnetic resonance images demonstrating mild restricted diffusion (white arrow) and enhancement (black arrow) of the optic disk.
Sickle Cell Disease
Etiologies, Pathophysiology, and Clinical Presentation: Sickle cell disease (SCD) is an inherited autosomal-recessive disease of the sickle beta globin gene that results in chronic hemolytic anemia and recurrent vaso-occlusive crises and infection. A vaso-occlusive crisis can affect any bone with active bone marrow. Orbital wall involvement is seen almost exclusively in children because of a greater volume of marrow space in this location during childhood. Patients present with acute periorbital pain, swelling, proptosis, and restricted extraocular movements. Subperiosteal hemorrhage can be a complication in 36% of cases. Resultant orbital compression syndrome can cause optic nerve compression and vision loss.97–100
Imaging: MRI demonstrates bone marrow edema (T2-weighted hyperintensity) and hemorrhage with or without subperiosteal hemorrhage (Fig. 6-7, A). It is important to differentiate bone marrow infarction from osteomyelitis given that both can occur in persons with SCD. On MRI, infarction demonstrates thin peripheral contrast enhancement, whereas infection demonstrates thick peripheral, geographic, or irregular contrast enhancement with or without cortical defects.101 Areas of high signal intensity on T1-weighted fat-saturated noncontrast images due to sequestration of red blood cells also has been found to be helpful in detecting areas of infarction and distinguishing them from infection (Fig. 6-7, B).102
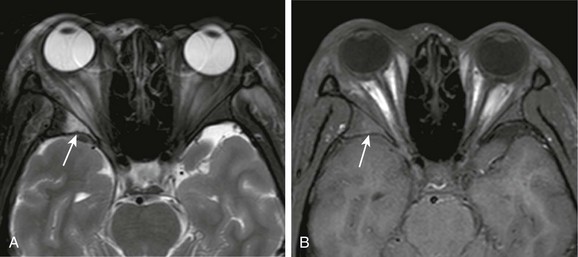
Figure 6-7 Sickle cell disease.
A, A T2-weighted fat-saturated magnetic resonance (MR) image of the orbits demonstrating bone marrow infarction in a patient with sickle cell disease, with T2 hyperintensity of the bone marrow of the lateral wall of the right orbit and adjacent inflammatory changes of the extraconal fat (arrow). B, A T1-weighted fat-saturated noncontrast MR image of the orbit in the same patient demonstrating subtle T1 hyperintensity of the bone marrow from sequestration of red blood cells (arrow).
Leber Hereditary Optic Neuropathy
Etiologies, Pathophysiology, and Clinical Presentation: Leber hereditary optic neuropathy (LHON) is the most common genetic mitochondrial disease, affecting 1 in 25,000 persons.103 Deficient adenosine triphosphate production leads to degeneration of retinal ganglion cells. Three mitochondrial deoxyribonucleic acid mutations (G3460A, G11778A, and T14484C) account for >90% of cases with incomplete penetrance: 50% in males and 10% in females. Accordingly, LHON is most common in young males, with a median age of 24 years. Painless bilateral clouding of vision, centrocecal scotoma, and impairment of color vision progresses to vision loss and optic nerve atrophy in 6 months. Most patients show no functional improvement, remaining legally blind.
Imaging: Most patients demonstrate no imaging findings, yet acutely, T2 hyperintensity, enlargement, and enhancement of the optic nerves, chiasm, and tracts can be seen. Chronically, T2 hyperintensity, volume loss, and decreased MTR values of these structures have been described.104–109 A small portion of patients, particularly females, demonstrate MS-like lesions in the brain preceding or more commonly following optic nerve involvement after an average of 4.3 years.110,111 These MS-like lesions may progress with a relapsing course; difficulties with ambulation develop in 75% of patients. Even brain that appears normal in patients with LHON demonstrates abnormal MTR and mean diffusivity values, suggesting more extensive subclinical neurologic disease.105
Autosomal-Dominant Optic Atrophy
Autosomal-dominant optic atrophy (ADOA) is the most common hereditary optic neuropathy related to mutations in the optic atrophy 1 (OPA1) gene (encoding a mitochondrial guanosine triphosphatase).114 Selective retinal ganglion cell loss results in isolated, progressive, and permanent vision loss, typically in the first two decades of life. Extraocular neurologic involvement is seen in up to one sixth of patients, with sensorineural hearing loss being the most common manifestation, likely as a result of OPA1 expression in the inner ear.115 Few studies describe the imaging appearance of ADOA, although a decrease in the size of the optic nerves throughout their length can be seen.116
Krabbe Disease (Globoid Cell Leukodystrophy)
Krabbe disease is an autosomal-recessive neurodegenerative disorder with a deficiency in galactosylceramide β-galactosidase, a lysosomal enzyme responsible for myelin breakdown and turnover. This deficiency leads to accumulation of neurotoxic psychosine and galactosylceramide, which form globoid cells. Early, late, and adult-onset forms are described. Presentation is most common at 3 to 6 months of age, with death by 2 to 3 years of age. Optic nerve enlargement is an uncommon manifestation, perhaps as a result of globoid cell accumulation in optic nerves, as described at autopsy (e-Fig. 6-8).117–120 The major differential considerations in children with enlarged optic nerves and signal changes in both the white matter and deep grey nuclei are Krabbe disease and neurofibromatosis type 1 with optic pathway gliomas.
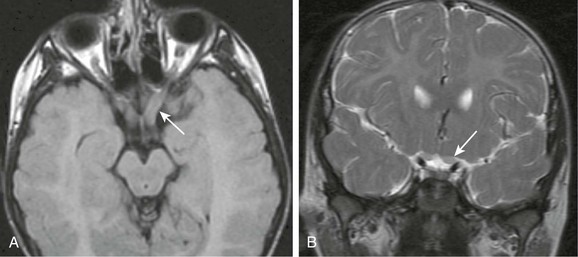
e-Figure 6-8 Metabolic disease.
A and B, Axial T2-weighted (A) fluid attenuated inversion recovery and coronal T2-weighted (B) images of an unknown metabolic disease process, possibly late-onset Krabbe disease, demonstrating mild enlargement and hyperintensity of the optic nerves with associated diffuse white matter signal changes (arrows).
Gorospe, L, Royo, A, Berrocal, T, et al. Imaging of orbital disorders in pediatric patients. Eur Radiol. 2003;13:2012–2026.
Hopper, KD, Sherman, JL, Boal, DK, et al. CT and MR imaging of the pediatric orbit. Radiographics. 1992;12:485–503.
Narla, LD, Newman, B, Spottswood, SS, et al. Inflammatory pseudotumor. Radiographics. 2003;23:719–729.
Saito, N, Nadgir, RN, Flower, EN, et al. Clinical and radiologic manifestations of sickle cell disease in the head and neck. Radiographics. 2010;30:1021–1035.
Smirniotopoulos, JG, Bargallo, N, Mafee, MF. Differential diagnosis of leukokoria: radiologic-pathologic correlation. Radiographics. 1994;14:1059–1079.
References
1. Hauser, A, Fogarasi, S. Periorbital and orbital cellulitis. Pediatr Rev. 2010;31:242–249.
2. Reid, J. Complications of pediatric paranasal sinusitis. Pediatr Radiol. 2004;34:933–942.
3. Mahalingam-Dhingra, A, Lander, L, Preciado, DA, et al. Orbital and periorbital infections: a national perspective. Arch Otolaryngol Head Neck Surg. 2011;137(8):769–773.
4. Sobol, SE, Marchand, J, Tewfik, TL, et al. Orbital complications of sinusitis in children. J Otolaryngol. 2002;31(3):131–136.
5. Rudloe, TF, Harper, MB, Prabhu, SP, et al. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125:e719–e726.
6. Towbin, R, Han, BK, Kaufman, RA, et al. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158:735–737.
7. Gorospe, L, Royo, A, Berrocal, T, et al. Imaging of orbital disorders in pediatric patients. Eur Radiol. 2003;13:2012–2026.
8. Sepahdari, AR, Aakalu, VK, Kapur, R, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193:w244–w250.
9. Greenberg, MF, Pollard, ZF. Medical treatment of pediatric subperiosteal orbital abscess secondary to sinusitis. J AAPOS. 1998;2(6):351–355.
10. Yang, M, Quah, BL, Seah, LL, et al. Orbital cellulitis in children-medical treatment versus surgical management. Orbit. 2009;28(2-3):124–136.
11. Wong, RK, VanderVeen, DK. Presentation and management of congenital dacryocystocele. Pediatrics. 2008;122(5):e1108–e1112.
12. Shekunov, J, Griepentrog, GJ, Diehl, NN, et al. Prevalence and clinical characteristics of congenital dacryocystocele. J AAPOS. 2010;14(5):417–420.
13. Rand, PK, Ball, WS, Jr., Kulwin, DR. Congenital nasolacrimal mucoceles: CT evaluation. Radiology. 1989;173:691–694.
14. Farrer, RS, Mohammed, TL, Hahn, FJ. MRI of childhood dacryocystocele. Neuroradiology. 2003;45:259–261.
15. Asheim, J, Spickler, E. CT demonstration of dacryolithiasis complicated by dacryocystitis. AJNR Am J Neuroradiol. 2005;26:2640–2641.
16. Kikkawa, DO, Heinz, GW, Martin, RT, et al. Orbital cellulitis and abscess secondary to dacryocystitis. Arch Ophthalmol. 2002;120:1096–1099.
17. Schmitt, NJ, Beatty, RL, Kennerdell, JS. Superior ophthalmic vein thrombosis in a patient with dacryocystitis-induced orbital cellulitis. Ophthal Plast Reconstr Surg. 2005;21(5):387–389.
18. Berenholz, L, Kessler, A, Shlomkovitz, N, et al. Superior ophthalmic vein thrombosis: complication of ethmoidal rhinosinusitis. Arch Otolaryngol Head Neck Surg. 1998;124:95–97.
19. Walker, JC, Sandhu, A, Pietris, G. Septic superior ophthalmic vein thrombosis. Clin Experiment Ophthalmol. 2002;30:144–146.
20. Parmar, H, Gandhi, D, Mukherji, SK, et al. Restricted diffusion in the superior ophthalmic vein and cavernous sinus in a case of cavernous sinus thrombosis. J Neuroophthalmol. 2009;29(1):16–20.
21. Ament, CS, Young, LH. Ocular manifestations of helminthic infections: onchocerciasis, cysticercosis, toxocariasis, and diffuse unilateral subacute neuroretinitis. Int Ophthalmol Clin. 2006;46(2):1–10.
22. Smirniotopoulos, JG, Bargallo, N, Mafee, MF. From the archives of the AFIP: differential diagnosis of leukokoria: radiologic-pathologic correlation. Radiographics. 1994;14:1059–1079.
23. Kadom, N, Sze, RW. Radiological reasoning: leukocoria in a child. AJR Am J Roentgenol. 2008;191:s40–s44.
24. Kitei, D, DiMario, FJ, Jr. Childhood orbital pseudotumor: case report and literature review. J Child Neurol. 2008;23(4):425–430.
25. Yan, J, Qui, H, Wu, Z, et al. Idiopathic orbital inflammatory pseudotumor in Chinese children. Orbit. 2006;25:1–4.
26. Ding, ZX, Lip, G, Chong, V. Idiopathic orbital pseudotumour. Clin Radiol. 2011;66:886–892.
27. Narla, LD, Newman, B, Spottswood, SS, et al. Inflammatory pseudotumor. Radiographics. 2003;23:719–729.
28. Park, SB, Lee, JH, Weon, YC, et al. Imaging findings of head and neck inflammatory pseudotumor. AJR Am J Roentgenol. 2009;193:1180–1186.
29. Kapur, R, Sepahdari, AR, Mafee, MF, et al. MR imaging of orbital inflammatory syndrome, orbital cellulitis, and orbital lymphoid lesions: the role of diffusion-weighted imaging. AJNR Am J Neuroradiol. 2009;30:64–70.
30. Guerriero, S, Leo, ED, Piscitelli, D, et al. Orbital pseudotumor in a child: diagnostic implications and treatment strategies. Clin Exp Med. 2011;11(1):61–63.
31. Mendenhall, WM, Lessner, AM. Orbital pseudotumor. Am J Clin Oncol. 2010;33:304–306.
32. Carruthers, MN, Stone, JH, Khosroshahi, A. The latest on IgG4-RD: a rapidly emerging disease. Curr Opin Rheumatol. 2012;24:60–69.
33. Wallace, ZS, Khosroshahi, A, Jakobiec, FA, et al. IgG4-related systemic disease as a cause of “idiopathic” orbital inflammation, including orbital myositis, and trigeminal nerve involvement. Surv Ophthalmol. 2012;57(1):26–33.
34. Eha, J, Pitz, S, Pohlenz, J. Clinical features of pediatric Graves’ orbitopathy. Int Ophthalmol. 2010;30:717–721.
35. Goldstein, SM, Katowitz, WR, Moshang, T, et al. Pediatric thyroid–associated orbitopathy: the Children’s Hospital of Philadelphia experience and literature review. Thyroid. 2008;18(9):997–999.
36. Durairaj, VD, Bartley, GB, Garrity, JA. Clinical features and treatment of Graves ophthalmopathy in pediatric patients. Ophthal Plast Reconstr Surg. 2006;22(1):7–12.
37. Harnsberger, HR. Diagnostic imaging: head and neck, ed 2. Salt Lake City, UT: Amirsys Inc; 2011.
38. Yokoyama, N, Nagataki, S, Uetani, M, et al. Role of magnetic resonance imaging in the assessment of disease activity in thyroid-associated ophthalmopathy. Thyroid. 2002;12(3):223–227.
39. Jiang, H, Wang, Z, Xian, J, et al. Evaluation of rectus extraocular muscles using dynamic contrast-enhanced MR imaging in patients with Graves’ ophthalmopathy for assessment of disease activity. Acta Radiol. 2012;53(1):87–94.
40. Kirsch, EC, Kaim, AH, DeOliveira, MG, et al. Correlation of signal intensity ratio on orbital MRI-TIRM and clinical activity score as a possible predictor of therapy response in Graves’ orbitopathy—a pilot study at 1.5 T. Neuroradiology. 2010;52:91–97.
41. Shetty, AK, Gedalia, A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol. 2008;6:16.
42. El Hansali, Z, Oukabli, M, Laktaoui, A, et al. Childhood sarcoidosis: ophthalmological manifestations and diagnostic difficulties in two cases. J Fr Ophtalmol. 2012;35(4):290.e1–290.e5.
43. Miller, AK, Khademian, Z, Pearl, PL. Uveitis and white matter abnormalities in pediatric sarcoidosis. Arch Ophthalmol. 2010;67(7):890–891.
44. Prabhakaran, VC, Saeed, P, Esmaeli, B, et al. Orbital and adnexal sarcoidosis. Arch Ophthalmol. 2007;125(12):1657–1662.
45. Carmody, RF, Mafee, MF, Goodwin, JA, et al. Orbital and optic pathway sarcoidosis: MR findings. AJNR Am J Neuroradiol. 1994;15(4):775–783.
46. Mafee, MF, Dorodi, S, Pai, E. Sarcoidosis of the eye, orbit, and central nervous system. Role of MR imaging. Radiol Clin North Am. 1999;37(1):73–87.
47. Demirci, H, Christianson, MD. Orbital and adnexal involvement in sarcoidosis: analysis of clinical features and systemic disease in 30 cases. Am J Ophthalmol. 2011;151(6):1074–1080.
48. Cabral, DA, Uribe, AG, Benseler, S, et al. Classification, presentation, and initial treatment of Wegener’s granulomatosis in childhood. Arthritis Rheum. 2009;60(11):3413–3424.
49. Akikusa, JD, Schneider, R, Harvey, EA, et al. Clinical features and outcome of pediatric Wegener’s granulomatosis. Arthritis Rheum. 2007;57(5):837–844.
50. Duffy, M. Advances in diagnosis, treatment, and management of orbital and periocular Wegener’s granulomatosis. Curr Opin Ophthalmol. 1999;10:352–357.
51. Vischio, JA, McCrary, CT. Orbital Wegener’s granulomatosis: a case report and review of the literature. Clin Rheumatol. 2008;27:1333–1336.
52. Courcoutsakis, NA, Langford, CA, Sneller, MC, et al. Orbital involvement in Wegener granulomatosis: MR Findings in 12 patients. J Comput Assist Tomogr. 1997;21(3):452–458.
53. Provenzale, JM, Mukherji, S, Allen, NB, et al. Orbital involvement by Wegener’s granulomatosis: imaging findings. AJR Am J Roentgenol. 1996;166:929–934.
54. Bonhomme, GR, Waldman, AT, Balcer, LJ, et al. Pediatric optic neuritis: brain MRI abnormalities and risk of multiple sclerosis. Neurology. 2009;72(10):881–885.
55. Wilejto, M, Shroff, M, Buncic, JR, et al. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006;67(2):258–262.
56. Morales, DS, Siatkowski, RM, Howard, CW, et al. Optic neuritis in children. J Pediatr Ophthalmol Strabismus. 2000;37(5):254–259.
57. Lucchinetti, CF, Kiers, L, O’Duffy, A, et al. Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology. 1997;49(5):1413–1418.
58. Lana-Peixoto, MA, Cardoso de Andrade, G. The clinical profile of childhood optic neuritis. Arq Neuropsiquiatr. 2001;59(2-B):311–317.
59. Kupersmith, MJ, Alban, T, Zeiffer, B, et al. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain. 2002;125:812–822.
60. Hickman, SJ, Toosy, AT, Jones, SJ, et al. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain. 2004;127:2498–2505.
61. Costello, F, Coupland, S, Hodge, W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–969.
62. Trip, SA, Schlottmann, PG, Jones, SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58(3):383–391.
63. Naismith, RT, Xu, J, Tutlam, NT, et al. Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology. 2009;72:589–594.
64. Naismith, RT, Xu, J, Tutlam, NT, et al. Diffusion tensor imaging in acute optic neuropathies. Arch Neurol. 2011;12:e1–e7.
65. Smith, SA, Williams, ZR, Ratchford, JN, et al. Diffusion tensor imaging of the optic nerve in multiple sclerosis: association with retinal damage and visual disability. AJNR Am J Neuroradiol. 2011;32(9):1662–1668.
66. Kolbe, S, Bajraszewski, C, Chapman, C, et al. Diffusion tensor imaging of the optic radiations after optic neuritis. Hum Brain Mapp. 2001;13:e1–e15.
67. Dasenbrock, HH, Smith, SA, Ozturk, A, et al. Diffusion tensor imaging of the optic tracts in multiple sclerosis: association with retinal thinning and visual disability. J Neuroimaging. 2011;21:e41–e49.
68. Reich, DS, Smith, SA, Gordon-Lipkin, EM, et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol. 2009;66(8):998–1006.
69. Hickman, SJ, Toosy, AT, Jones, SJ, et al. Serial magnetization transfer imaging in acute optic neuritis. Brain. 2004;127(3):692–700.
70. Boorstein, JM, Moonis, G, Boorstein, SM, et al. Optic neuritis: imaging with magnetization transfer. AJR Am J Roentgenol. 1997;169:1709–1712.
71. Sun, MH, Wang, HS, Chen, KJ, et al. Clinical characteristics of optic neuritis in Taiwanese children. Eye. 2011;25(11):1527–1528.
72. Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65(6):727–732.
73. Beck, RW, Arrington, J, Murtagh, FR, et al. Brain magnetic resonance imaging in acute optic neuritis. Experience of the optic neuritis study group. Arch Neurol. 1993;50(8):841–846.
74. Lennon, VA, Wingerchuk, DM, Kryzer, TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112.
75. Lennon, VA, Kryzer, TJ, Pittock, SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477.
76. Krupp, LB, Banwell, B, Tenembaum, S, et al. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68:s7–s12.
77. Wingerchuk, DM, Lennon, VA, Pittock, SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–1489.
78. Wingerchuk, DM, Hogancamp, WF, O’Brien, PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53:1107–1114.
79. Huppke, P, Blüthner, M, Bauer, O, et al. Neuromyelitis optica and NMO-IgG in European pediatric patients. Neurology. 2010;75(19):1740–1744.
80. Banwell, B, Tenembaum, S, Lennon, VA, et al. Neuromyelitis optica-IgG in childhood inflammatory demyelinating CNS disorders. Neurology. 2008;70(5):344–352.
81. Weinshenker, BG, Wingerchuk, DM, Vukusic, S, et al. Neuromyelitis optica: IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol. 2006;59:566–569.
82. Nakamura, Y, Ikeda, K, Yoshii, Y, et al. Influenza-associated monophasic neuromyelitis optica. Intern Med. 2011;50:1605–1609.
83. Sellner, J, Hemmer, B, Mühlau, M. The clinical spectrum and immunobiology of parainfectious neuromyelitis optic (Devic) syndromes. J Autoimmunity. 2010;34:371–379.
84. Lotze, TE, Northrop, JL, Hutton, GJ, et al. Spectrum of pediatric neuromyelitis optica. Pediatrics. 2008;122:e1039–e1047.
85. Collongues, N, Marignier, R, Zéphir, H, et al. Long-term follow-up of neuromyelitis optica with a pediatric onset. Neurology. 2010;75(12):1084–1088.
86. Pittock, SJ, Lennon, VA, Krecke, K, et al. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006;63:390–396.
87. Pittock, SJ, Weinshenker, BG, Lucchinetti, CF, et al. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63:964–968.
88. Toker, E, Yenice, O, Yilmaz, Y. Isolated bilateral optic neuropathy in acute disseminated encephalomyelitis. J Pediatr Ophthalmol Strabismus. 2003;40(4):232–235.
89. Bangsgaard, R, Larsen, VA, Milea, D. Isolated bilateral optic neuritis in acute disseminated encephalomyelitis. Acta Ophthalmol Scand. 2006;84:815–817.
90. Tenembaum, S, Chamoles, N, Fejerman, N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–1231.
91. Sonnevillea, R, Kleinb, IF, Wolff, M. Update on investigation and management of postinfectious encephalitis. Curr Opin Neurol. 2010;23:300–304.
92. Schirmer, CM, Hedges, TR. Mechanisms of visual loss in papilledema. Neurosurg Focus. 2007;23(5):E5.
93. Jinkins, JR, Athale, S, Xiong, L, et al. MR of optic papilla protrusion in patients with high intracranial pressure. AJNR Am J Neuroradiol. 1996;17:665–668.
94. Seitz, J, Held, P, Strotzer, M, et al. Magnetic resonance imaging in patients diagnosed with papilledema: a comparison of 6 different high-resolution T1- and T2(*)-weighted 3-dimensional and 2-dimensional Sequences. J Neuroimag. 2002;12(2):164–171.
95. Pakzad-Vaezi, K, Cochrane, D, Sargent, M, et al. Conventional and diffusion-weighted magnetic resonance imaging findings in a pediatric patient with a posterior fossa brain tumor and papilledema. Pediatr Neurosurg. 2009;45:414–418.
96. Brodsky, MC, Glasier, CM. Magnetic resonance visualization of the swollen optic disc in papilledema. J Neuroophtalmol. 1995;15(2):122–124.
97. Ganesh, A, Al-Zuhaibi, S, Pathare, A, et al. Orbital infarction in sickle cell disease. Am J Ophthalmol. 2008;146:595–601.
98. Noble, J, Schendel, S, Weizblit, N, et al. Orbital wall infarction in sickle cell disease. Can J Ophthalmol. 2008;43:603–604.
99. Ozkavukcu, E, Fitoz, S, Yagmurlu, B, et al. Orbital wall infarction mimicking periorbital cellulitis in a patient with sickle cell disease. Pediatr Radiol. 2007;37:388–390.
100. Saito, N, Nadgir, RN, Flower, EN, et al. Clinical and radiologic manifestations of sickle cell disease in the head and neck. Radiographics. 2010;30:1021–1035.
101. Umans, H, Haramati, N, Flusser, G. The diagnostic role of gadolinium enhanced MRI in distinguishing between acute medullary bone infarct and osteomyelitis. Magn Reson Imaging. 2000;18:255–262.
102. Jain, R, Sawhney, S, Rizvi, SG. Acute bone crises in sickle cell disease: the T1 fat-saturated sequence in differentiation of acute bone infarcts from acute osteomyelitis. Clin Radiol. 2008;63:59–70.
103. Yu-Wai-Man, P, Turnbull, TM, Chinnery, PF. Leber hereditary optic neuropathy. J Med Genet. 2002;39:162–169.
104. Van Westen, D, Hammar, B, Bynke, G. Magnetic resonance findings in the pregeniculate visual pathways in Leber hereditary optic neuropathy. J Neuroophthalmol. 2011;31:48–51.
105. Inglese, M, Rovaris, M, Bianchi, S, et al. Magnetic resonance imaging, magnetization transfer imaging, and diffusion weighted imaging correlates of optic nerve, brain, and cervical cord damage in Leber’s hereditary optic neuropathy. J Neurol Neurosurg Psychiatry. 2001;70:444–449.
106. Phillips, P, Vaphiades, M, Glasier, CM, et al. Chiasmal enlargement and optic nerve enhancement on magnetic resonance imaging in Leber hereditary optic neuropathy. Arch Ophthalmol. 2003;121(4):577–579.
107. Batioglu, F, Atilla, H, Eryilmaz, T. Chiasmal high signal on magnetic resonance imaging in the atrophic phase of Leber hereditary optic neuropathy. J Neuroophthalmol. 2003;23:28–30.
108. Kermode, AG, Moseley, IF, Kendall, BE, et al. Magnetic resonance imaging in Leber’s optic neuropathy. J Neurol Neurosurg Psychiatry. 1989;52(5):671–674.
109. Mashima, Y, Oshitari, K, Imamura, Y, et al. Orbital high resolution magnetic resonance imaging with fast spin echo in the acute stage of Leber’s hereditary optic neuropathy. J Neurol Neurosurg Psychiatry. 1998;64:124–127.
110. Perez, F, Anne, O, Debruxelles, S, et al. Leber’s optic neuropathy associated with disseminated white matter disease: a case report and review. Clin Neurol Neurosurg. 2009;11:83–86.
111. Kuker, W, Weir, A, Quaghebeur, G, et al. White matter changes in Leber’s hereditary optic neuropathy: MRI findings. Eur J Neurol. 2007;14:591–593.
112. Newman, NJ. Treatment of Leber hereditary optic neuropathy. Brain. 2011;134:2447–2455.
113. Klopstock, T, Yu-Wai-Man, P, Dimitriadis, K, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134:2677–2686.
114. Milea, D, Amati-Bonneau, P, Reynier, P, et al. Genetically determined optic neuropathies. Curr Opin Neurol. 2010;23:24–28.
115. Yu-Wai-Man, P, Griffiths, PG, Gorman, GS, et al. Multi-system neurological disease is common in patients with OPA1 mutations. Brain. 2010;133:771–786.
116. Votruba, M, Leary, S, Losseff, N, et al. MRI of the intraorbital optic nerve in patients with autosomal dominant optic atrophy. Neuroradiology. 2000;42:180–183.
117. Hussain, SA, Zimmerman, HH, Abdul-Rhaman, OA, et al. Optic nerve enlargement in Krabbe disease: a pathophysiologic and clinical perspective. J Child Neurol. 2011;26(5):642–644.
118. Patel, B, Gimi, B, Vachha, B, et al. Optic nerve and chiasm enlargement in a case of infantile Krabbe disease: quantitative comparison with 26 age-matched controls. Pediatr Radiol. 2008;38:697–699.
119. Jones, BV, Barron, TF, Towfighi, J. Optic nerve enlargement in Krabbe’s disease. AJNR Am J Neuroradiol. 1999;20(7):1228–1231.
120. Bussiere, M, Kotylak, T, Naik, K, et al. Optic nerve enlargement associated with globoid cell leukodystrophy. Can J Neurol Sci. 2006;33:235–236.




