Chapter 28 Oral health disorders
Introduction
Holistic dentistry is defined as the practice of dental medicine, the core principle of which is: oral health and the body’s health are intimately connected.1 Holistic dentistry focuses on the whole person and supports their physical, social, psychological, emotional and spiritual wellbeing. It is health promoting and emphasises lifestyle advice such as behaviour modification, dietary changes, stress management, exercise, appropriate sunshine exposure, environmental advice, and integrates appropriate complementary therapies where necessary to improve dental and oral health. Good communication and providing clear education guidelines to dental patients to promote good dental care, especially healthy behaviour patterns (e.g. regular teeth brushing and flossing) to encourage adherence to oral hygiene, is an important aspect of dental treatment.
Lifestyle factors, other than dietary factors, can play an important role in the prevention and management of oral health and dentists and health practitioners are in a unique position to reinforce lifestyle advice to complement dental care. Many dentists worldwide are already reinforcing the importance of maintaining good oral health through a healthy diet, regular brushing, teeth flossing and mouth washes. A small growing number of dentists in Australia and worldwide are practising holistic dentistry, however very few integrate complementary therapies that include acupuncture and homeopathy.1–4
Communication with dental practitioners
Communication plays an important role in the practitioner-patient relationship and contributes to positive health outcomes. Dentists who communicate with patients about their dental fears can help to significantly alleviate dental anxiety. A trial of anxious patients (n = 119) who completed Spielberger’s State Anxiety Inventory (STAI-S) pre- and post-treatment, were randomly allocated to intervention (dentist informed of anxiety score) and control (dentist not informed) groups. Communication was able to reduce the patients‘ level of anxiety and significantly reduce anxiety scores during dental procedure.5 The study concluded that providing the dentist with information regarding the high level of a patient’s dental anxiety prior to treatment and involving the patient with this, helped reduce the patient’s overall anxiety with dental treatment.
Relationship between oral and general health
What is less well recognised is that dental health may actually impact and affect physical health and medical diseases that can then go on to affect the rest of the body. A new growing body of evidence now suggests that oral health can potentially impact on general health.6 For example, periodontal disease appears to be associated with increased risk of cardiovascular disease (CVD).
Periodontal disease (PD) and risk of diseases
PD and CVD
Recent research suggests that PD may have a systemic effect and increase the risk of CVD, including coronary heart disease, myocardial infarction, peripheral vascular disease and stroke.6–11 The exact mechanism to explain this correlation is not clear, but may be linked to bacterial infections associated with PD and atherosclerotic plaque formation.12
PD and diabetes mellitus
There is also an association of PD with poorly controlled diabetes in patients with Type 2 diabetes mellitus (T2DM). Improved periodontal health may benefit diabetes and metabolic control in type 2 diabetes mellitus.13, 14, 15 For example, in a study, non-surgical periodontal treatment involving a full-mouth scaling and root planing led to a statistically significant improvement in plaque index, gingival index, probing pocket depth, clinical attachment levels and bleeding on probing, and improved HbA1c levels and glycaemic control in type 2 diabetic patients compared with the control group that received no periodontal treatment. Similarly, a recent randomised control trial (RCT) also demonstrated improved glycaemic control with periodontal treatment in type 2 diabetic patients.16 However, the difference in improved glycaemic control for type 1 diabetics was not as significant compared with control group following PD treatment.17 The benefits appear to be confined to type 2 diabetics.
Prevention
Tooth brushing
According to a Cochrane review of 42 trials involving 3855 participants, compared to manual toothbrushes, powered toothbrushes with a rotation oscillation action provides better protection against gum inflammation (gingivitis) and plaque removal.20
Chewing gum
Mastic or chewing gum can play an important role in prevention of caries. The benefits of chewing gum are based on its ability to stimulate salivary flow which helps promote food clearance from the mouth and reduce bacterial growth in saliva, such as Streptococcus mutans, and consequent plaque formation on teeth.21, 22 A study found the total number of bacterial colonies was significantly reduced during the 4 hours of chewing mastic gum compared to the placebo gum and significantly reduced plaque index and gingival index compared to the placebo group.23
Chewing gums containing sugar substitute products such as xylitol are very effective for preventing dental caries by stimulating the salivary flow, although an antimicrobial effect cannot be excluded. Xylitol appears to be superior to the other sugar alcohols such as sorbitol, mannitol, maltitol, lactitol and the products Lycasin and Palatinit for caries control. Xylitol is a polyol sugar alcohol and is naturally sourced from plums, strawberries, raspberries and rowan berries. A number of trials suggest xylitol is more effective than those sweetened with sorbitol for reduction of caries24, 25, 26 however, another review of all clinical trials concluded that chewing sugar-free gum 3 or more times daily for prolonged periods of time may reduce caries incidence irrespective of the type of sugar alcohol used. 27 Xylitol is not fermented by bacteria and causes a fall in plaque pH by having an inhibitory effect on mutans streptococci.
One study found a significant statistical reduction in the number of bacteria mutans streptococci found in saliva and plaque with a chewing program using xylitol-only chewing gums compared with xylitol-sorbitol chewing gum or control in 8-year old children over a 39-month period.28 Another study suggested a different mechanism for xylitol chewing gum benefit in preventing caries in children.29 This study demonstrated 5 grams of xylitol-containing chewing gums in children over a 14-day period reduced the glucose-initiated lactic acid formation in supragingival plaque by up to 22% compared with baseline and the control group. The authors concluded reducing lactic acid formation may play a more important role in reducing plaque formation, as the numbers of salivary mutans streptococci were unaffected in this study.29
Chewing gum combined with vitamin C
An RCT of chewing gum containing vitamin C (60mg) and gum containing vitamin C and carbamide (30mg + 30mg) were compared to placebo.30 A significant reduction in the total calculus score was observed after the use of vitamin C (33%) and vitamin C + carbamide (12%) gums compared with no gum use, especially in the heavy calculus formers. Vitamin C also reduced the number of bleeding sites (37%) and a reduced amount of visible plaque was observed after use of vitamin C and non-vitamin C gum. Carbamide added to vitamin C in chewing gum did not offer more benefit.30
Halitosis
Mouth rinses for halitosis
A Cochrane review of 5 RCTs found mouth rinses containing antibacterial agents such as chlorhexidine and cetylpyridinium chloride may play an important role in reducing the levels of halitosis-producing bacteria on the tongue, and mouth rinses containing chlorine dioxide or zinc can be effective in neutralisation of odoriferous sulfur compounds.31 However, there is sufficient evidence to suggest that alcohol-based mouth rinses should be avoided due to their association with oral and pharyngeal cancer, and should not be recommended.32–36
Sunlight
Sunshine is the main source of vitamin D produced by the body in response to direct skin exposure to ultraviolet B (UVB). Consequently, nil or minimal exposure to sun can contribute to vitamin D deficiency as seen in many community groups with dress codes (e.g. wearing veils), those living in geographically prone areas, especially over winter (southern or northern latitudes), those working indoors (e.g. office work), institutionalisation, prolonged hospitalisation and the bed-bound (e.g. stroke, elderly), and particularly in dark-skinned people who need longer sun exposure.38, 39 Vitamin D plays an important role in dental health (see the section on vitamin D below).
Environment
Mercury exposure
Dental amalgam is the most commonly used material in restorative dentistry. Dental amalgam is an alloy of silver, tin, copper and mercury.40 Mercury vapour absorption through the lungs (by dental practitioners or patients) or accidental oral ingestion of mercury from cracked teeth containing amalgams (by patients) are the main sources of mercury exposure in dentistry. The safety of mercury exposure from amalgam fillings has been explored in Australia.41 The findings of the National Health and Medical Research Council (NHMRC) working party concluded there was a lack of evidence to confirm dangers associated with mercury toxicity from amalgams and no studies exploring health outcomes amongst dental patients with and without amalgams and neurotoxicity. Based on current evidence, the NHMRC working party also concluded there is no role for removal protocols and concomitant therapeutic regimens, such as chelation therapy, for patients attributing symptoms to mercury from dental amalgam restorations. The NHMRC working party claim that the release of mercury is at a slow rate from dental amalgams, generally of a few micrograms per person per day among adults dependent upon variables such as number and shape of fillings, eating habits, and bruxism. For the current mean numbers of amalgam fillings in Australian children and adults (0.5 and 8, respectively), daily mercury absorption per person is about 0.3μg and 3.5μg, respectively, which is tenfold and twofold (respectively) higher than dietary sources of mercury retained in the body.19
However, there have since been more studies exploring effects of amalgam in children. A study of 507 children (aged 8–10 years) with at least 1 carious lesion on a permanent tooth and no previous exposure to amalgam were randomised to receive routine dental care during the 7-year trial period, 1 group receiving amalgam restorations (n = 253) and the other group receiving resin composite restorations (n = 254). Baseline mean creatinine-adjusted urinary mercury levels were 1.0–1.5mg/g higher in the amalgam group than in the composite group (P<0.001) at the 7-year follow-up. This study found no significant differences in measures of memory, attention, visuomotor function or nerve-conduction velocities for the groups over the 7 years of follow-up.42
A recent RCT of 534 children (aged 6–10 years) involving 5 community health settings compared neuropsychological and renal function of children whose dental caries were restored using amalgam or mercury-free materials.43 Children with no prior amalgam restorations and ≥2 posterior teeth with caries were randomised to receive dental restoration during a 5-year follow-up period using either amalgam or resin composite (non-amalgam) materials. The researchers found higher mean urinary mercury levels in children with amalgams at 5- and 7-year follow-up respectively but no significant differences in IQ, memory, attention/concentration and motor and visuomotor performance in either group.
Amalgam use in children and renal concerns
A study of 403 children in Shanghai found minimal differences in mean urinary mercury concentration for children with and without amalgam fillings, and no differences for either renal function biomarker, or on neurobehavioural, neuropsychological, or intelligence tests.44
However, in the New England Children’s Amalgam Trial there was a significantly increased prevalence of microalbuminuria among children in the amalgam group in years 3–5 (adjusted odds ratio [OR] 1.8; 95% confidence interval [CI], 1.1–2.9) compared with the composite group (p = 0.04) with no differences in levels of renal tubular markers — alpha1-microglobulin (A1M), gamma-glutamyl transpeptidase (gamma-GT) and for N-acetyl-beta-D-glucosaminidase (NAG).45 A sub-study of the New England Children’s Amalgam Trial collected 82 pairs of urine samples from children aged 10–16 years and found the creatinine-corrected excretions of albumin, gamma-GT, and NAG were significantly higher in daytime samples than in overnight samples, with a non-significant trend for A1M.46 Another study also found significantly higher levels of mercury in blood and urine and higher urinary excretion of NAG, gamma-GT and albumin in persons with dental amalgams than those without.47 Albuminuria and urinary excretion of NAG significantly correlated with the number of fillings. The authors concluded from:
… the nephrotoxicity point of view, dental amalgam is an unsuitable filling material, as it may give rise to Hg toxicity‘ and based on the mercury levels in blood and urine ‘renal damage is possible.47
There is considerable international controversy over the safety of mercury amalgam (fillings). In 2008, Norway, Denmark and Sweden totally banned the use of mercury products, including fillings, in their countries.48, 49, 50
Mercury in food
It is well recognised that mercury enters the food chain from naturally occurring mercury (as sulfides) or from air pollutants (e.g. industrial or vehicle exhaust pollution) deposited in sea, rivers and lakes and is transformed into methyl mercury by bacteria in the water. The methyl mercury is ingested by small fish and algae and consequently accumulates and builds up in larger and older predatory fish that are at the top of the food chain. Foods Standards Australia New Zealand (FSANZ) advises pregnant women, women planning pregnancy and young children should limit their intake of shark (flake), broadbill, marlin and swordfish to no more than 1 serve per fortnight with no other fish to be consumed during that fortnight due to risks of mercury toxicity.51 Those at risk of mercury toxicity are children, the unborn infant of pregnant women and newborns who are breastfeeding (exposure via the breast milk) from mothers who frequently (more than once a week) eat large predator species of fish. The developing nervous system of a fetus and children are particularly susceptible to the ill effects of high methyl mercury levels that can cause a multitude of symptoms such as nausea, vomiting, lack of appetite, weight loss, kidney failure, skin irritations, respiratory distress, swollen gums, mouth sores, neurological symptoms such as paraesthesia, numbness, seizures, tremors and incoordination, and mental retardation.52
Conclusion on mercury
Concerns associated with direct mercury toxicity via accidental inhalation and ingestion are real. Amalgams may be best avoided in pregnant women and young children, especially those with kidney disease or renal impairment, although there are very few studies exploring long-term health concerns. Dentists and dental health practitioners may also be at risk, although there are strict guidelines for discarding solid waste from lost or extracted teeth with amalgam fillings and amalgam-contaminated waste, which are carefully contained and in most instances incinerated. Proper collection of mercury-contaminated solid waste using high-frequency suction (while wearing a mask) prevents the release of mercury vapour during combustion. The use of rubber dams stops contaminated saliva secretions and amalgam debris from entering the oral cavity of the patient, minimising mercury exposure.53
Cadmium (Cd) exposure
Periodontal disease
Environmental cadmium (Cd) exposure is known to adversely affect bone remodelling and is associated with higher odds of periodontal disease. Analysis of data from the 3rd National Health and Nutrition Examination Survey (NHANES III) of 11 412 participants included in the study found 15.4% of participants had periodontal disease with a mean urine Cd concentration significantly higher among participants with periodontal disease [0.50; 95% CI, 0.45–0.56] compared with participants without periodontal disease [0.30; 95% CI, 0.28–0.31].54 After adjusting for tobacco exposure, there was a further significant threefold increase in creatinine-corrected urinary Cd concentrations from the 25th (0.18 mug/g) to the 75th (0.63 mug/g) percentile associated with a significant 54% greater risk of prevalent periodontal disease (OR = 1.54; 95% CI, 1.26–1.87).
Smoking
Cigarette smoking, cigar and pipe use in smokers significantly increases the risk of developing a range of dental health problems, including increased risk of caries, gingivitis, tooth loss, ulcer formation, oral cancers and PD.55 In general, smokers are 4–20 times more likely to develop periodontitis compared with persons who never smoked and, amongst current smokers, 74.8% of their periodontitis was attributable to smoking.56, 57 Smoking is a major preventable risk factor for PD and chronic destructive PD.
Smoking can adversely affect dental health by various mechanisms, such as defects in neutrophil function and host defences from nicotine leading to bacterial invasion and plaque formation, impairment of inflammatory and immune responses, increased alveolar bone loss, pocket formation and attachment loss, including adverse local and systemic effects (e.g. heart and lung disease).58, 59 Smoking also reduces wound healing following local trauma and dental treatment.
Smokers are also exposed to various chemicals and heavy metals, such as cadmium and lead from cigarette smoking, which are associated with PD and may aggravate existing renal impairment.60
Smoking cessation can significantly reduce the risk of PD and increase the likelihood of tooth retention but it may take decades for this to happen.61
Sleep and dental pain
Oral pain that impacts on sleep is a common presentation to dentists. Other than oral pain, dentists may also be confronted with pain from orofacial and temperomandibular junction (TMJ) disorders. Pain is well known to disrupt numerous aspects of normal physical and psychological life, including work, social activities and sleep.62, 63, 64
Mind–body therapy
Dental anxiety
Dental anxiety is a common problem. High dental anxiety can be overwhelming for an individual and is strongly associated with poor oral health, particularly due to treatment avoidance. Studies of 272 adult private dental practice patients were assessed for anxiety, frequency of oral health care visits in the last 10 years and their preferred method to alleviate anxiety.65
Mind–body techniques such as cognitive behaviour therapy (CBT), self-help techniques, relaxation and music therapy and exercise can be useful and effective for the management of panic and fear [see Chapter 4, anxiety].66, 67, 68 Many of these techniques can also help address psychological symptoms associated with dental anxiety and fear.
Psychological interventions — general
A Cochrane systematic review identified 4 studies that assessed psychological interventions aimed at increasing compliance of oral hygiene in adult patients with PD.69
Brief relaxation and music distraction
A prospective RCT compared a brief relaxation method with music distraction and a control group.70 Ninety patients with dental anxiety were randomised to either of the 3 groups. Both brief relaxation and music distraction reduced dental anxiety significantly. Brief relaxation was significantly superior to music distraction over the control group. Patients in the control group demonstrated no significant change in anxiety level.
A trial of 44 adult subjects requiring surgery for root canal treatment procedure were randomised to a music group, which listened to selected sedative music using headphones throughout the root canal treatment procedure, or a control group where subjects wore headphones but without the music.71 Heart rate, blood pressure and finger temperature were measured before the study and every 10 minutes until the end of the root canal treatment procedure. The level of anxiety experienced by the patient was measured before the study and at the end of the treatment procedure. The results revealed that there were no significant differences between the 2 groups for baseline data and procedure-related characteristics, except for gender. The music group showed a significant increase in finger temperature and a reduction in anxiety score over time compared with the control group. The authors concluded the findings:
Virtual image audio-visual eyeglass system
Virtual image, audio-visual (AV) eyeglasses may play a role in dental anxiety and pain. In a study of 27 patients, the participants completed a Dental Fear Survey and the Fear of Pain Questionnaire-III before dental treatment.72 They were randomised to either watch and listen to a standard video using AV eyeglasses or not (control group). The AV eyeglass group demonstrated rescued anxiety and discomfort compared with control group. Patients showed a preference to use AV equipment compared to the traditional approach of no glasses and there was a significant reduction in treatment time in the first half of the dental procedure. Clinicians experienced no significant technical interference with their dental work by using the AV eyeglasses. The authors concluded AV virtual image eyeglasses may be beneficial in the reduction of fear, pain and procedure time for most dental patients.72
Hypnosis and dental anxiety
Pre-existing anxiety and cognitions about dental procedures can predict dental anxiety. Hypnosis may be helpful for some patients, but not all. A study found that patient characteristics such as hypnotisability, trait anxiety and negative cognitions can predict which people develop dental anxiety and who will be more responsive to hypnosis.73 Rapid hypnotic induction techniques by oral health practitioners (dentists or dental nurses) can be useful for treating anxious patients prior to procedures.74 Children may be more receptive to hypnosis. A double-blind, control trial of 29 children (ages 4–13) were randomised to both hypnosis group and a no hypnosis group before a local anaesthetic injection for a dental procedure.75 Each subject was evaluated during utilisation of hypnosis before injection, and once without. Subjects were videotaped during the procedure and behaviour rated independently by 2 paediatric dentists, using the North Carolina Behaviour Rating Scale (NBRS). They found hypnosis significantly reduced pulse rate and crying, especially in the 4–6 year-old children.
Behavioural therapy for dental fear
Behaviour therapy may offer more benefit in reducing dental fear compared with hypnosis. In a study with 22 women (mean age of 31.8 years) who suffered dental fear and avoided dental care (median time of 9.5 years), participated in a clinical study and were randomly assigned to 1 of 2 groups; hypnotherapy (HT) versus behavioural treatment based on psychophysiological principles (PP).76 Both therapies involved 8 sessions followed by standardised conventional dental test treatments. Pre- and post-treatment measures were dental fear, general fear, mood, and patient behaviour. PP group statistically significantly reduced dental fear and improved mood during dental situations compared with HT group. General fear levels insignificantly reduced. Overall 11 of the 22 patients completed the conventional dental treatment without complications, indicating that they were more relaxed during the treatment.
Relaxation oriented therapy (ROT) versus cognitively oriented therapy (COT)
A study of 112 adults that were found to be fearful of dental treatments reported that those who received COT by a trained psychologist prior to specialist care were more likely to complete the treatment program, but those who received ROT were more likely to experience significantly less generalised anxiety and fear, and dental fear during the procedure.77 Dropouts were related to lower motivation (willingness to engage in dental treatment), and failed treatment was related to higher levels of general fear and anxiety. This study concluded that both COT and ROT interventions were effective in helping to reduce dental phobic reactions and to complete dental care, and that motivation was a significant predictor of treatment outcome.
An additional study compared ROT and COT with nitrous oxide sedation (NOS). Sixty-five patients with severe dental fear were randomly assigned to COT, ROT or NOS over 10 weekly sessions of individual therapy.78 Overall there were low dropout rates. All patients who completed the therapy sessions completed dental treatment and anxiety scores on dental fear tests significantly reduced in all treatment groups. There were no major differences between each group or with the advent of any serious adverse events. Furthermore, on following up these participants at 1 year after initiating the trial with 62 patients, it was reported that 95% of the participants had attended dental treatment with continued favourable effects with respect to dental fear and general distress. The relaxation group established the greatest reductions on the dental fear measures.79 All patients judged the 3 interventions namely, COT, ROT and NOS as beneficial for dental fear. Of the participants, 80% judged the treatment given in the year after the dental fear treatment as successful and all 3 treatment groups scored in the normative range for general distress both at the end of treatment and at follow-up.
In a further 5-year follow-up study of 43 patients, it was observed that the majority (81%) of the participants assessed the dental fear treatment they received 5 years previously to have been useful for dental fear and general psychological distress for participants in all 3 treatment groups.80
Diet
It is well established that nutritional intake plays a vital and significant role in oral and bone health. A number of reports have demonstrated that poor nutrition significantly affects teeth during development, exacerbates PD and dental diseases, craniofacial development (from in utero), risk of oral cancer and oral infections.81, 82
Dairy intake — calcium and vitamin D
Low dietary calcium intake is associated with reduced bone mass and increased risk of osteoporosis. Best sources of calcium foods include dairy products (milk, yoghurts and cheese), fish (sardines with bones), green leafy vegetables and fruits. The recommended dietary allowance for calcium is 800mg/day.83 Vitamin D deficiency is associated with rickets in children, osteoporosis; osteomalacia (reduced mineralisation of bone) and is common in the elderly and dark skinned people. The best source of vitamin D is from sun exposure. (Refer to Chapter 30 on osteoporosis for more information on calcium and vitamin D.)
Early childhood caries (ECC)
Data from the Third National Health and Nutrition Examination Survey (NHANES III) of 2- to 5-year-old children, found those consuming best dietary practices (uppermost tertile of the Healthy Eating Index [HEI]) were 44% less likely to exhibit severe ECC compared with children with the worst dietary practices (lowest tertile of the HEI).84
Sugars
Dental caries are more prevalent if sugars are consumed more frequently, particularly if retained in the mouth for long periods of time.85 Sucrose forms glucan in the mouth that facilitates firm bacterial adhesion to teeth and limits diffusion of acid and buffers in the plaque.
Soft drinks
Five to 6 servings per week of soft drinks, particularly cola, are recognised as a risk factor and linked to low bone mineral density and osteoporosis according to a population study of over 2500 adults.86 The harmful effects of soft drinks on general, dental and oral health are well confirmed.
Acidic diet and caries
It is well recognised that an acidic diet promotes caries. A study of 309 children’s diets found that large consumption of high-acidic beverages (e.g. fruit juices), vinegar, fruit, vitamin C supplements with low consumption of water and milk is associated with increased risk in caries and erosion.87 A recent Australian study investigated the erosive potential of beverages in schools.88 This study found that the majority of the tested beverages sold from school canteens exhibited erosive potential.
Table 28.1 lists the acidity of common drinks. A pH less than 5.0 should generally be reduced or avoided. Water and milk are preferred drinks to maintain good oral health.
| Beverages | Acidity pH |
|---|---|
| Water | 7.0 |
| Milk | 6.8 |
| Flat mineral water | 5.3 |
| Soda water | 5.1 |
| Apricot yoghurt | 5.1 |
| Beer and/or wine | 4.0 |
| Sparkling mineral water | 3.9 |
| Orange juice | 3.6 |
| Apple juice | 3.4 |
| Diet coke | 3.0 |
| Ribena | 2.8 |
| Lemonade and Fanta® | 2.7 |
| Powerade | 2.7 |
| Coca cola® and Pepsi® | 2.3 |
| Vinegar | 2.2 |
| Lemon juice | 2.0 |
Nutritional supplements
Vitamins
Vitamin D
Vitamin D has a pivotal role in bone metabolism, it controls intestinal calcium absorption plus its deposition into bone.89 The main source of vitamin D is sunlight exposure (see Figure 28.1). Ninety percent of vitamin D is produced in the skin from sunshine exposure (UVB) with only 10% from dietary sources. Dietary sources include fatty fish (e.g. mackerel), cod liver oil, sun-exposed mushrooms and liver. The majority of women with osteoporosis have vitamin D deficiency and resulting bone loss.90 The Geelong Vitamin D Study on postmenopausal women found that the majority of the participants were vitamin D deficient during winter.91
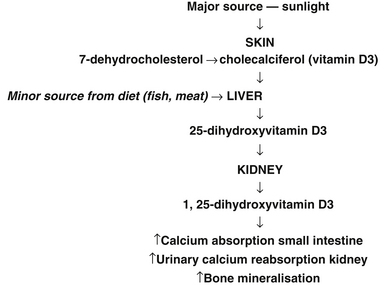
Figure 28.1 Major sources of sunlight
(Source: adapted from Nowson CA, Diamond TH, Psco JA, et. al. Australian Family Physician. 2004;33(3):133–8)
Vitamin D deficiency is likely to be the commonest nutritional deficiency in many countries, and may well be the most important one.92–97 Risk factors for vitamin D deficiency include dark skin colour, dress codes (e.g. wearing veils), migrants, infants of migrant families, living in geographical prone areas, especially over winter (southern or northern latitude), institutionalisation, bed-bound, intellectual disability, prolonged and exclusive breastfeeding, restricted sun exposure, and certain medical conditions. Vitamin D deficiency has re-emerged as a public health issue particularly affecting infants and young children, potentially causing hypocalcemia, seizures, rickets, limb pain, tooth loss and poor dentition and risk of fracture.98–104 Breastfed infants of mothers who were vitamin D deficient during pregnancy were at high risk of vitamin D deficiency.105
A position statement released recently by the Australian and New Zealand Bone and Mineral Society and Osteoporosis Australia and the Endocrine Society of Australia is summarised in Figure 28.2.106

Figure 28.2 Position statement: calcium and bone health
(Source: Australian and New Zealand Bone and Mineral Society and Osteoporosis Australia and the Endocrine Society of Australia, 2009)106
Improved bone mineral density (BMD)
A large RCT of elderly people over a 5-year period demonstrated vitamin D combined with calcium supplementation has long-term benefit on BMD compared with calcium alone or control group, particularly in subjects with sub-optimal vitamin D levels.107 Moreover, it is well documented that throughout the lifecycle the skeleton requires optimum development and maintenance of its integrity to prevent fracture. The data is promising for the use of combined calcium with vitamin D and the use of vitamin K.108
Periodontal disease (PD)
Numerous articles indicate vitamin D and calcium deficiency are associated with bone loss and increased inflammation, which are well recognised symptoms of PD. Whilst studies suggest calcium and vitamin D may benefit periodontal health and calcium deficiency may be a risk factor for periodontal disease, there are no RCTs done to date to test this hypothesis.109
Vitamin D and calcium enhances tooth retention
A placebo-controlled trial of 145 healthy subjects aged over 65 years were randomised over a 3-year period to calcium or vitamin D supplementation to assess bone loss from the hip. Furthermore, participants were further followed up for 2-years after discontinuation of study supplements and teeth were counted at 18 months and 5 years.110 During the trial, subjects were less likely to lose 1 or more teeth (13%) whilst taking calcium and vitamin D supplements compared with a larger proportion of subjects (27%) whilst taking placebo. At the 2-year follow-up period, of those with total calcium intake >1000mg/day, 31 of 77 subjects (40%) lost 1 or more teeth compared with a larger proportion of 40 of 68 subjects (59%) who consumed less calcium.
Recommended dosage is calcium citrate 800mg/day or calcium carbonate 1000mg/day.
Vitamin B complex
Vitamin B may help wound healing post-surgery for PD. A randomised, double-blind, placebo-controlled trial of 30 patients with moderate to severe chronic periodontitis found that vitamin B complex at a dose of 50mg/day supplementation, in combination with surgery for 30 days following access flap surgery for PD, resulted in significantly better wound healing and clinical attachment levels up to 180 days compared with placebo and surgery.111
During the decades of the 1980s and 1990s several case reports of peripheral neuropathy associated with high-dose pyridoxine appeared in the scientific literature, where the consumption in most of the reported cases was of dosages that ranged from 1–5g per day.112 Vitamin B6 is usually safe at intakes up to a maximum of 200mg/day in adults for short-term supplementation. However, vitamin B6 can cause neurological disorders, such as loss of sensation in legs and imbalance, when taken in high doses (50 mg or more per day) over a long period of time. Vitamin B6 toxicity can damage sensory nerves, leading to numbness in the hands and feet as well as difficulty walking. Symptoms of a pyridoxine overdose may include poor coordination, peripheral neuropathy, staggering, numbness, decreased sensation to touch, temperature, and vibration and tiredness for up to 6 months. It is important to note though that there is a requisite to avoid overuse of single vitamin products (e.g. oral and injectable forms of vitamin B6) or concomitant use of multivitamin products that could lead to some patients routinely exceeding the upper limit for vitamins and hence risk severe toxicity.113
Vitamin C (ascorbate)
Low dietary intake of vitamin C is associated with increased risk of PD, particularly in past and present smokers.114 Those with lowest vitamin C levels demonstrated greater clinical effects of PD. The effect appeared to be dose-responsive. For those taking 0–29mg/day of vitamin C the odds ratio (OR) for PD was 1.30 and for those taking 100–179mg/day the OR was 1.16, compared to those taking 180mg or more of vitamin C per day.
Multivitamins
Wound healing
The importance of nutritional intake for wound healing is well established.115, 116 For example, the elderly are particularly prone to nutritional deficiencies due to various factors such as poor dentition, relying on dentures, difficulty chewing food, and being on various medications, and are therefore more likely to suffer chronic diseases. (See Chapter 2 for more information on nutritional assessment.) This makes the elderly more prone to poor wound healing with dental treatment.
Periodontal disease (PD)
In a 60 day, 2-cell, randomised, parallel clinical trial for PD patients, 63 patients were randomised to a vitamin tablet or placebo.117 Clinical parameters assessed included gingival index (GI), bleeding index (BI), periodontal pocket depth (PD), and attachment levels (AL), all of which were recorded at baseline and at 60 days. After 60 days, the vitamin group demonstrated reduced clinical GI, BI, PD (P < .0001) but no significant changes for AL between both groups. However, further analysis of patients with PD > or = 4mm showed the vitamin group demonstrated clinically significant improvements in GI and PD compared with baseline, with no significant differences in BI and AL. The authors concluded ‘multi-vitamin nutritional supplement might be a beneficial adjunct to the required established periodontal treatment‘.117
A review of the literature to assess the effects of nutritional supplements (e.g. vitamin B-complex, vitamin C and dietary calcium) on general wound healing, periodontal disease status and response to periodontal therapy found that multivitamins may have a possible influencing factor on periodontal status and wound healing but more studies are required.118
Minerals
Magnesium
A cross-sectional epidemiological study of 4290 subjects (aged 20–80 years) recorded periodontal risk factors, serum magnesium and calcium levels, relating them to periodontal parameters.119 Magnesium deficiency was found to be associated with PD. In a matched-pair study, 60 subjects using oral magnesium-containing drugs and 120 without were compared. Subjects aged over 40 years with higher serum magnesium and calcium was significantly associated with reduced probing depth (p<0.001), less attachment loss (p = 0.006), and an increased number of remaining teeth (p = 0.005). Subjects taking magnesium supplementation showed less attachment loss (p<0.01) and more remaining teeth than the control group. The authors concluded that these results suggest ‘nutritional Mg supplementation may improve periodontal health‘.119
Calcium
Whilst the association of low dietary calcium intake with periodontal disease is well recognised, a statistically significant association occurred for low total serum calcium and periodontal disease found in young females (20–39 years) with OR of 6.11 (95% CI: 2.36 to 15.84), after adjusting for tobacco use, gingival bleeding, and dietary calcium intake.120 However, further studies are warranted to better define the role of calcium supplementation effect on risk of PD and tooth loss.
Fluoride
A Cochrane review of 25 studies involving 7747 children, found the effect of fluoride gel applied topically once or several times yearly by dental health practitioners led to, on average, a 21% reduction in dental caries, ‘decayed, missing and filled tooth surfaces‘ compared with placebo.121
Similarly, a Cochrane review of 9 studies involving 2709 children found fluoride varnishes applied professionally 2–4 times a year substantially reduced tooth decay in children compared with no treatment controls.122
However, whilst Cochrane reviews found topical fluorides from supervised mouth rinses, varnishes and gels are helpful, they are not any more effective at reducing tooth decay in children and adolescents than using fluoride toothpaste alone.123–126
A number of systematic reviews have demonstrated that water fluoridation is associated with reduced dental caries and the number of teeth affected by caries in children.127
Fluoride added to the water supply, salt or milk as a public health measure has remained a controversial issue. There is evidence to suggest that there is a possible association between fluoride in drinking water during growth and development (5–10 years) and the incidence of osteosarcoma in boys aged 10–19 years.128–134 A literature review (1970–2008) of all papers covering possible aetiological factors involved in the development of bone tumours in children and young adults found several associations have been reported with some consistency for osteosarcoma: the presence of hernias and Ewing sarcoma; high fluoride exposure and osteosarcoma; and parental farming and residence on a farm, younger age at puberty and family history of cancer for all bone tumours, especially osteosarcoma.135 One study found higher levels of serum fluoride levels in 25 case patients with osteosarcoma compared with patients with other bone tumours, suggesting a role of fluoride in the disease.136 One study also suggested a possible link between fluoride drinking and other tumours, such as brain tumours, T-cell system Hodgkin’s disease, Non-Hodgkin lymphoma, multiple myeloma, melanoma of the skin and monocytic leukaemia, and concluded that the likelihood of fluoride being causal for cancer requires further assessment.137
However, an association with osteosarcoma risk has been disputed by authorities who have found, based on the research, that fluoridation of drinking water remains the most effective and socially equitable means of achieving community-wide exposure to the caries prevention, when consumed at a safe target range of 0.6–1.1mg/l, depending on the climate.138–142
A Cochrane review found that there is insufficient evidence to show that fluoride added to milk can prevent tooth decay.143 Fluoride use for the prevention of osteoporosis and fractures remains controversial with a meta-analysis demonstrating no improvement, and a more recent study showing improvement in BMD in post-menopausal osteoporosis.144, 145
Potassium containing toothpastes — reduces tooth sensitivity
Dentine hypersensitivity is a common problem that causes sudden, sharp teeth pain and discomfort when exposed to touch, hot or cold foods. A Cochrane review of 6 studies found statistically significant effect of 6–8 weeks use of potassium nitrate toothpaste in improving dentine hypersensitivity although trials involved a small number of individuals for a conclusion to be generated.146
Zinc combined with herb blood root (Sanguinaria candensis extract)
Toothpaste and oral rinse
Sixty subjects with moderate levels of plaque and gingivitis were randomly assigned to a treatment group (combined use of toothpaste and oral rinse containing blood root extract and zinc chloride) and compared to placebo products in a 28-week clinical trial.147 The treatment group significantly reduced non-invasive measures of plaque (21%), gingivitis (25%), bleeding on probing (43%) compared with placebo group. The product was well tolerated with 3 subjects of the 30 in the treatment group exhibiting minor soft-tissue irritations that resolved spontaneously without discontinuation of treatment.
Other nutritional supplements
Coenzyme Q10 (CoQ10)-PD
Topical application of CoQ10 to the periodontal pocket in 10 male patients suffering periodontitis led to significant improvements in symptoms and signs such as GI and bleeding on probing as a sole treatment or in combination with traditional non-surgical periodontal therapy, warranting more research.148, 149
Alpha-lipoic acid (burning mouth syndrome)
Burning mouth syndrome describes a group of symptoms of unknown cause that may include burning sensation of the lips, tongue, mouth, dryness and altered taste. A Cochrane review explored a number of therapies and found there was some evidence alpha-lipoic acid, coping strategies and anti-convulsants may help, but not analgesia, hormone therapies or antidepressants.150
Herbal medicines
Green tea extract (PD, caries prevention)
Green tea extract mouth rinses may play a role in the treatment of periodontal disease and prevention of dental caries as the green tea catechins can inhibit acid production in dental plaque bacteria.156
Blood root (Sanguinaria candensis)
When used in a toothpaste and oral rinse, blood root may reduce plaque, gingival inflammation and bleeding parameters for up to 6 months with no adverse effects.157 However, blood root has produced mixed results in other studies, showing no benefit when used as a dentrifice.158
When combined with zinc as an oral rinse, there was demonstrable improvement in reducing plaque (21%), gingivitis (25%), and bleeding on probing (43%) compared with placebo group.159 It is possible that the zinc component of the dentrifice plays the more important role here in view of the mixed results found in studies with the herb blood root.
Clove oil — analgesia
Clove oil has a long tradition of use in dental care, especially as a topical anaesthetic. In 1 study of 73 adult volunteers, topical agents applied to maxillary canine buccal mucosa were compared in efficacy for dental pain.160 The 4 substances tested included homemade clove gel, benzocaine 20% gel, placebo that resembled clove, and a placebo resembling benzocaine.
After 5 minutes of application, each participant received 2 needle sticks and pain response was assessed using a 100mm visual analogue pain scale. Both clove and benzocaine gels equally significantly reduced mean pain scores compared with both placebos (p = 0.005). The authors concluded clove gel ‘might possess a potential to replace benzocaine as a topical agent before needle insertion‘.160
Eucalyptus extract chewing gum — gingivitis
A randomised placebo-controlled trial of healthy subjects with gingivitis found eucalyptus extract chewing gum could exhibit antibacterial properties and improve periodontal health over 12 weeks.161 A high-concentration group (n = 32) using 0.6% eucalyptus extract chewing gum (90mg/day) was compared to a lower concentration group (n = 32) using 0.4% eucalyptus extract chewing gum (60mg/day) and to placebo group (n = 33) that used chewing gum without eucalyptus extract. Both eucalyptus chewing gum groups statistically reduced/improved plaque accumulation, improved gingival index, bleeding on probing, periodontal probing depth (PD) but not for clinical attachment level compared with placebo chewing gum.161
Table 28.2 summarises potential interactions and adverse effects that can occur during dental surgery if patients are taking complementary medicines and herbal medicines. 161, 163, 164
Table 28.2 Potential adverse herbal or complementary medicine effects on dental surgery
| Effect during dental surgery | Herb or complementary medicine |
|---|---|
| ↑ bleeding time | garlic, ginseng, gingko, glucosamine, high doses of fish oils |
| ↑ sedating effect with anaesthetic | kava, hops, valerian, passionflower |
| Nutrient–herb–drug interaction(s) |
– bromelain, cayenne, chamomile, feverfew, dong quai, eleuthro/Seberian ginseng, garlic, ginkgo, ginger, ginseng and licorice interacting with aspirin;
|
Adverse effects of some herbal supplements that may cause rare oral manifestations are summarised in Table 28.3.
Table 28.3 Adverse oral manifestations of some herbs
| Herb | Adverse manifestation |
|---|---|
| Feverfew | Aphthous ulcers, lip and tongue irritation and swelling |
| Feverfew and ginkgo | Gingival bleeding |
| Echinacea | Tongue numbness |
| St John’s wort | Xerostomia |
| Yohimbe | Salivation |
| Kava | Oral and lingual dyskinesia |
Physical therapies
Temperomandibular junction (TMJ) disorders
Pain and difficulty opening the oral cavity are features of TMJ disorder.
A systematic review of 12 studies that met inclusion criteria included 4 studies of therapeutic exercise interventions, 2 on acupuncture and 6 on electro-physical modalities to treat TMJ disorders.165
In general, postural exercises reduced pain and improved function and oral opening, manual therapy in combination with active exercises reduced pain and improved oral opening, and acupuncture reduced pain compared with no treatment. Also, muscular awareness relaxation therapy, biofeedback training and low-level laser therapy treatment significantly improved oral opening. In another study identified by the review, there were no significant differences in pain outcomes between acupuncture and sham.165
In another systematic review of 30 studies, active exercises and manual mobilisations, postural training in combination with other interventions, and mid-laser therapy may be more effective for TMJ disorder.163 Relaxation techniques, biofeedback, electromyography training, and proprioceptive re-education were more effective than placebo treatment or occlusal splints. Combinations of active exercises, manual therapy, postural correction, and relaxation techniques were also effective for TMJ disorder. 166
Splints and TMJ disorders
A Cochrane review of 12 RCTs demonstrated that there was no evidence of a statistically significant difference in the effectiveness of stabilisation splint (SS) therapy in reducing symptoms in patients with pain dysfunction syndrome compared with other active treatments such as acupuncture, bite plates, biofeedback, stress management, visual feedback, relaxation, jaw exercises and with no treatment.167
Occlusal adjustment for treating and preventing TMJ Disorders
Occlusal adjustment involves adjusting the teeth’s biting surfaces with the aim to relieve symptoms of TMJ disorder. A Cochrane review of RCTs found no evidence for the use of occlusal adjustment in preventing or treating TMJ disorders.168
Acupuncture
TMJ dysfunction
A study of 60 case reports (50 females, 10 males; mean age of 40.6 years [range 14–68]) with TMJ dysfunction with an average pain score of 3.2 out of 5 explored the benefit of acupuncture for TMJ pain relief.169 Each patient received acupuncture (mean of 3.4 treatments), lasting on average 12 minutes, by dentists over the TMJ and in the masticatory muscles, points on the neck, and additional relaxing points. There were significant reductions in mean pain scores with beneficial effects observed in 85% of cases and an average reduction in pain intensity by 75%.169
A review of the literature identified 6 RCTs that explored the efficacy of acupuncture in the treatment of craniomandibular dysfunction.170 Whilst methodological flaws were found in most studies, the study concluded that acupuncture appeared to be suitable as a treatment for the management of TMJ dysfunction.
Xerosterma
A case report of 7 patients suffering xerostomia found that acupuncture was able to increase salivary flow and the ability to eat and speak, and improved sleep in all patients.171
In another study of 21 patients with severe xerostomia, 11 were treated with acupuncture and 10 received placebo acupuncture.172 True acupuncture treatment significantly increased salivary flow rates during and after the acupuncture treatment over the observation year. Placebo acupuncture demonstrated some improvement of salivary flow rates only during treatment.
Gagging
Some people suffer pronounced gag reflex that severely limits their ability to accept dental care and clinicians‘ ability to provide the care. It compromises all aspects of dentistry, from diagnostic procedures to active treatment and can be distressing for all concerned.173 Acupuncture may help reduce the gag reflex.
One study involved 21 dentists and 37 case reports (20 females and 17 males; mean age of 46.8 years) of patients who were unable to accept the impression-taking during dental care.174 After acupuncture of the point CV-24, there was improvement up to 51–55% (mean 53%) for the 3 stages of impression-taking and up to 30 patients (81%) were able to accept the impression-taking. The authors concluded ‘that acupuncture of point CV-24 is an effective method of controlling severe gag reflex during dental treatment including impression taking‘.174
Homeopathy and dentistry
A pilot study of 14 homeopathic dentists collected clinical and outcome data over a 6-month period of 726 individual patients (mean age of patients was 46.2 years) who received homeopathic treatment.175 Of the followed-up 496 individual cases, positive outcome was demonstrated in 90.1% (negative in 1.8%; no change in 7.9%; outcome not recorded in 0.2%)
Strongly positive outcomes (scores of +2 or +3) were achieved most notably in the frequently treated conditions of pericoronitis, periodontal abscess, periodontal infection, reversible pulpitis, sensitive cementum, and toothache with decay.178
Conclusion
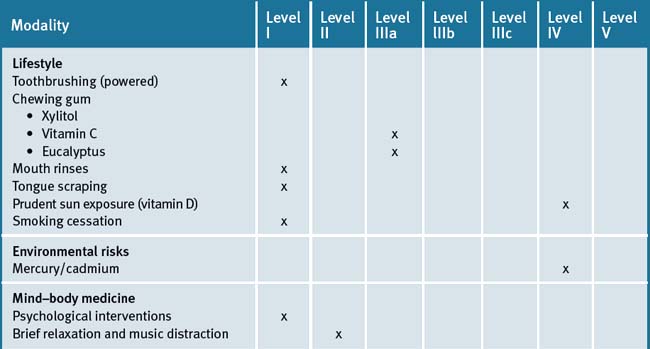
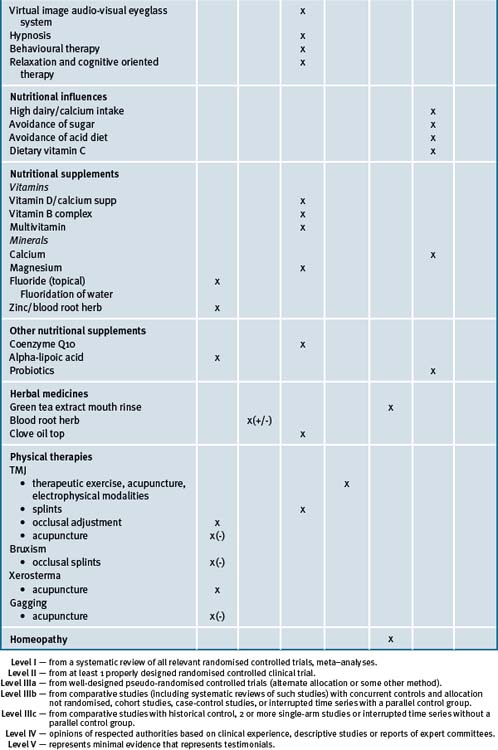
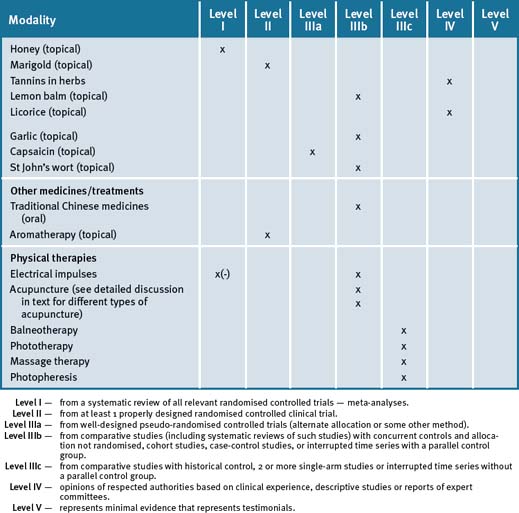
Table 28.4 summarises the level of evidence for lifestyle factors and complementary medicines that may be useful in dental health care.
Clinical tips handout for patients — oral health
Refer to this link for details for prevention of dental caries:
http://www.dentalhealthweek.com.au/2008/bootcamp.htm
1 Lifestyle advice
Sunshine
2 Physical activity/exercise
3 Mind–body medicine
Rest and stress management
5 Dietary changes
6 Physical therapies
7 Supplements
Vitamins
Warning: Vitamin A — excessive intake of vitamin A, above 3000IU/day, may contribute to bone loss.
Vitamin D3
Vitamin B group
Vitamin B12
Multivitamin
Minerals
Magnesium and calcium (best provided together)
Calcium citrate — 800mg/day or calcium carbonate 1000mg/day in adults. Children 30–40mg/day.
Zinc
Prevention of dental caries — additional tips
Refer to this link for details for prevention of dental caries:
http://www.dentalhealthweek.com.au/2008/bootcamp.htm
Other specific conditions
Burning mouth syndrome — try alpha-lipoic acid supplement.
Dental pain — try acupuncture; clove oil applied onto the painful gum may help.
Dry mouth — fish oil supplements; increase oils in diet; acupuncture may help.
Excess gagging — try acupuncture before dental treatment.
Warning: avoid the following herbs or supplements before any dental surgery:
↑ bleeding time — garlic, ginseng, gingko, glucosamine, high doses of fish oils
↑ sedating effect with anaesthetic: kava, hops, valerian, passionflower
herb–drug interactions: ↑ metabolism of drugs used in the perioperative period (e.g. St John’s wort).
1 Kron J. Holistic Dentistry. Journal of Complementary Medicine. 2007;6(1):42-47.
2 Australian Research Centre for Population Oral Health. Australian dentist labour force 2003. Aust Dent J. 2006;51(2):191-194.
3 Darby P. Dentists practising CAM. British Dental Journal. 2002;193(5):244-245.
4 Watts T.L.P. Dentists practising CAM. British Dental Journal. 2002;193(9):487.
5 Dailey Y.M., Humphris G.M., Lennon M.A. Reducing patients‘ state anxiety in general dental practice: a randomized controlled trial. J Dent Res. 2002 May;81(5):319-322.
6 Beltran-Aguilar E.D., Beltran-Neira R.J. Oral diseases and conditions throughout the lifespan. II. Systemic diseases. Gen Dent. 2004;52(2):107-114.
7 Kinane D.F., Marshall G.J. Periodontal manifestations of systemic disease. Aust Dent J. 2001;46(1):2-12.
8 Beck J.D., Offenbacher S. Systemic effects of periodontitis; epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76(11 Suppl):2089-2100.
9 Blaizot A., Vergnes J.N., Nuwwareh S., et al. Periodontal diseases and cardiovascular events: meta-analysis of observational studies. Int Dent J. 2009;59(4):197-209.
10 Meurman J.H., Sanz M., Janket S.J. Oral health, atherosclerosis and cardiovascular disease. Crit Rev Oral Biol Med. 2004;15(6):403-413.
11 Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(9499):1809-1820.
12 Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138:534-536.
13 Herring M.E., Shah S.K. Periodontal Disease and Control of Diabetes Mellitus. J Am Osteop Assoc. 2006;106(7):416-421.
14 Kiran M., Arpak N., Unsal E., et al. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005 Mar;32(3):266-272.
15 Westfelt E., Rylander H., Blohmé G., et al. The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol. 1996 Feb;23(2):92-100.
16 Garcia R. Periodontal treatment associated with improved glycaemic control in type 2 diabetic patients. Evid Based Dent. 2007;8(1):13.
17 Skaleric U., Schara R., Medvescek M., et al. Periodontal treatment by Arestin and its effects on glycemic control in type 1 diabetes patients. J Int Acad Periodontol. 2004 Oct;6(4 Suppl):160-165.
18 Garcia R.I., Nunn M.E., Vokonas P.S. Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann Periodontol. 2001;6(1):71-77.
19 Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol. 2001;6(1):197-208.
20 Robinson P., Deacon S.A., Deery C., et al. Manual versus powered toothbrushing for oral health. Cochrane Database of Systematic Reviews. (Issue 1):2005. Art. No.: CD002281. doi: 10.1002/14651858.CD002281.pub2
21 Stookey G.K. The effect of saliva on dental caries. J Am Dent Assoc. 2008 May;139:11S-17S.
22 Aksoy A., Duran N., Koksal F. In vitro and in vivo antimicrobial effects of mastic chewing gum against Streptococcus mutans and mutans streptococci. Arch Oral Biol.. 2006 Jun;51(6):476-481. Epub 2005 Dec 15
23 Takahashi K., Fukazawa M., Motohira H., et al. A pilot study on antiplaque effects of mastic chewing gum in the oral cavity. J Periodontol. 2003 Apr;74(4):501-505.
24 Edgar W.M. Sugar substitutes, chewing gum and dental caries-a review. Br Dent J. 1998 Jan 10;184(1):29-32.
25 Gales M.A., Nguyen T.M. Sorbitol compared with xylitol in prevention of dental caries. Ann Pharmacother. 2000 Jan;34(1):98-100.
26 Mäkinen K.K., Bennett C.A., Hujoel P.P., et al. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res. 1995 Dec;74(12):1904-1913.
27 Van Loveren C. Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res. 2004 May-Jun;38(3):286-293.
28 Mäkinen K.K., Alanen P., Isokangas P., et al. Thirty-nine-month xylitol chewing-gum programme in initially 8-year-old school children: a feasibility study focusing on mutans streptococci and lactobacilli. Int Dent J. 2008 Feb;58(1):41-50.
29 Twetman S., Stecksén-Blicks C. Effect of xylitol-containing chewing gums on lactic acid production in dental plaque from caries active pre-school children. Oral Health Prev Dent. 2003;1(3):195-199.
30 Lingstrom P., Fure S., Dinitzen B., et al. The release of vitamin C from chewing gum and its effects on supragingival calculus formation. Eur J Oral Sci. 2005 Feb;113(1):20-27.
31 Fedorowicz Z., Aljufairi H., Nasser M., et al. Mouthrinses for the treatment of halitosis. Cochrane Database of Systematic Reviews. (Issue 4):2008. Art. No.: CD006701. doi: 10.1002/14651858.CD006701.pub2
32 Winn D.N., Blot W.J., McLaughlin J.K., et al. Mouthwash Use and Oral Conditions in the Risk of Oral and Pharyngeal Cancer. Cancer Research. 1991 June 1;51:3044-3047.
33 Cole P., Rodu B., Mathisen A. Alcohol-containing mouthwash and oropharyngeal cancer: A review of the epidemiology. J Am Dent Assoc. 2003 August 1;134(8):1079-1087.
34 McCullough M.J., Farah C.S. A review of the role of alcohol in oral carcinogenesis with particular reference to alcohol containing mouthwashes. Aust Dent J. 2008;53(4):302-305.
35 La Vecchia C. Mouthwash and oral cancer risk: An update. Oral Oncology. 2008 Oct 24. Epub ahead of print
36 Walsh L.J. Are alcohol containing dental mouthrinses safe? A critical look at the evidence. Aust Dental Practice. 2008;Nov/Dec:64-68.
37 Outhouse T.L., Al-Alawi R., Fedorowicz Z., et al. Tongue scraping for treating halitosis. Cochrane Database of Systematic Reviews. (Issue 2):2006. Art. No.: CD005519. doi: 10.1002/14651858.CD005519.pub2
38 Brand C.A., Abi H.Y., Cough D.E., et al. Vitamin D deficiency: a study of community beliefs among dark skinned and veiled people. Intern J Rheumatic Dis. 2008;11:15-23.
39 Diamond T.H., Levy S., Smith A., et al. High bone turnover in Muslim women with vitamin D deficiency. MJA. 2002;177:139-141.
40 Australian Dental Association. Frequently Asked Questions. Amalgum Fillings. Online. Available: http://www.ada.org.au/faqs/faq, documentid, 26712.aspx (accessed 1 Sept 2009).
41 NHMRC. Dental Amalgam and Mercury in Dentistry – Report of an NHMRC Working Party. 1999. Online. Available: http://www.nhmrc.gov.au/publications/synopses/d17syn.htm (accessed 1st September 2009).
42 DeRouen T.A., Martin M.D., Leroux B.G., et al. Neurobehavioural effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295(15):1784-1792.
43 Bellinger D.C., Trachtenberg F., Barregard L., et al. Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006 Apr 19;295(15):1775-1783.
44 Ye X., Qian H., Xu P., et al. Nephrotoxicity, neurotoxicity, and mercury exposure among children with and without dental amalgam fillings. Int J Hyg Environ Health. 2009 Jul;212(4):378-386.
45 Barregard L., Trachtenberg F., McKinlay S. Environ Health Perspect. Renal effects of dental amalgam in children: the New England children’s amalgam trial. 2008 Mar;116(3):394-399.
46 Trachtenberg F., Barregard L., McKinlay S. The influence of urinary flow rate in children on excretion of markers used for assessment of renal damage: albumin, gamma-glutamyl transpeptidase, N-acetyl-beta-D -glucosaminidase, and alpha1-microglobulin. Pediatr Nephrol. 2008;23(3):445-456.
47 Mortada W.L., Sobh M.A., El-Defrawy M.M., et al. Mercury in dental restoration: is there a risk of nephrotoxicity? J Nephrol. 2002 Mar-Apr;15(2):171-176.
48 Dental mercury use banned in Norway, Sweden and Denmark. Reuters, January 3, 2008. Online. Available: http://www.reuters.com/article/pressRelease/idUS108558+03-Jan-2008+PRN20080103 (accessed 1 September 2009)
49 Ministry of the Environment. Press release 21 December 2007. Minister of the Environment and International Development Erik Solheim: Bans mercury in products. http://www.regjeringen.no/en/dep/md/press-centre/Press-releases/2007/Bans-mercury-in-products.html?id = 495138 (accessed 1st September 2009)
50 Amendment of regulations of 1 June 2004 no 922 relating to restrictions on the use of chemicals and other products hazardous to health and the environment (Product regulations). Adopted by the Ministry of the Environment on 14 December 2007 pursuant to section 4 of the Product Control Act of 11 June 1979. Online. Available: http://www.regjeringen.no/Upload/MD/Vedlegg/Forskrifter/product_regulation_amendment_071214.pdf (accessed 1 September 2009)
51 Foods Standards Australia New Zealand (FSANZ). Online. Available: http://www.foodstandards.gov.au/foodmatters/mercuryinfish.cfm (accessed 1 Sept 2009)
52 US Food and Drug Administration; FDA; US Department of Health and Human Services. What You Need to Know About Mercury in Fish and Shellfish, Advice for Women Who Might Become Pregnant, Women Who are Pregnant, Nursing Mothers, and Young Children Online. Available: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm110591.htm (accessed 1 Sept 2009)
53 Chin G., Chong J., Kluczewska A., et al. REVIEW. The environmental effects of dental amalgam. Australian Dental Journal. 2000;45(4):246-249.
54 Arora M., Weuve J., Schwartz J., et al. Association of environmental cadmium exposure with periodontal disease in U.S. Adults. Environ Health Perspect. 2009 May;117(5):739-744.
55 Albandar J.M., Streckfus C.F., Adesanya M.R., et al. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol. 2000 Dec;71(12):1874-1881.
56 Tomar S.L., Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000 May;71(5):743-751.
57 Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004 Sep;92(1):1-8.
58 Obeid P., Bercy P. Effects of smoking on periodontal health: a review. Adv Ther. 2000 Sep-Oct;17(5):230-237.
59 Barbour S.E., Nakashima K., Zhang J.B., et al. Tobacco and smoking: environmental factors that modify the host response (immune system) and have an impact on periodontal health. Crit Rev Oral Biol Med. 1997;8(4):437-460.
60 Mortada W.I., Sobh M.A., El-Defrawy M.M. The exposure to cadmium, lead and mercury from smoking and its impact on renal integrity. Med Sci Monit. 2004 Mar;10(3):CR112-CR116.
61 Krall E.A., Dawson-Hughes B., Garvey A.J., et al. Smoking, smoking cessation, and tooth loss. J Dent Res. 1997 Oct;76(10):1653-1659.
62 Brousseau M., Manzini C., Thie N., et al. Understanding and managing the interaction between sleep and pain: an update for the dentist. J Can Dent Assoc. 2003 Jul-Aug;69(7):437-442.
63 Bailey D.R. Sleep disorders. Overview and relationship to orofacial pain. Dent Clin North Am. 1997 Apr;41(2):189-209.
64 Lavigne G.J., Goulet J.P., Zuconni M., et al. Sleep disorders and the dental patient: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999 Sep;88(3):257-272.
65 Biggs Q.M., Kelly K.S., Toney J.D. The effects of deep diaphragmatic breathing and focused attention on dental anxiety in a private practice setting. J Dent Hyg. 2003 Spring;77(2):105-113.
66 Parslow R., Morgan A.J., Allen N.B., et al. Effectiveness of complementary and self-help treatments for anxiety in children and adolescents. Med J Aust. 2008 Mar 17;188(6):355-359.
67 Royal Australian and New Zealand College of Psychiatrists Clinical Practice. Guidelines Team for Panic Disorder and Agoraphobia. Australian and New Zealand Journal of Psychiatry. 2003;37:641-656.
68 Wipfli B.M., Rethorst C.D., Landers D.M. The anxiolytic effects of exercise: a meta-analysis of randomized trials and dose-response analysis. J Sport Exerc Psychol. 2008 Aug;30(4):392-410.
69 Renz A., Ide M., Newton T., et al. Psychological interventions to improve adherence to oral hygiene instructions in adults with periodontal diseases. Cochrane Database of Systematic Reviews. (Issue 2):2007. Art. No.: CD005097. doi: 10.1002/14651858.CD005097.pub2
70 Lahmann C., Schoen R., Henningsen P., et al. Brief relaxation versus music distraction in the treatment of dental anxiety: a randomized controlled clinical trial. J Am Dent Assoc. 2008 Mar;139(3):317-324.
71 Lai H.L., Hwang M.J., Chen C.J., et al. Randomised controlled trial of music on state anxiety and physiological indices in patients undergoing root canal treatment. J Clin Nurs. 2008 Oct;17(19):2654-2660.
72 Frere C.L., Crout R., Yorty J., et al. Effects of audiovisual distraction during dental prophylaxis. J Am Dent Assoc. 2001 Jul;132(7):1031-1038.
73 DiClementi J.D., Deffenbaugh J., Jackson D. Hypnotizability, absorption and negative cognitions as predictors of dental anxiety: two pilot studies. J Am Dent Assoc. 2007 Sep;138(9):1242-1250. quiz 1267–8
74 Finkelstein S. Rapid hypnotic inductions and therapeutic suggestions in the dental setting. Int J Clin Exp Hypn. 2003 Jan;51(1):77-85.
75 Gokli M.A., Wood A.J., Mourino A.P., et al. Hypnosis as an adjunct to the administration of local anesthetic in pediatric patients. ASDC J Dent Child. 1994;61(4):272-275.
76 Hammarstrand G., Berggren U., Hakeberg M. Psychophysiological therapy vs. hypnotherapy in the treatment of patients with dental phobia. Eur J Oral Sci. 1995;103(6):399-404.
77 Berggren U., Hakeberg M., Carlsson S.G. Relaxation vs. cognitively oriented therapies for dental fear. J Dent Res. 2000 Sep;79(9):1645-1651.
78 Willumsen T., Vassend O., Hoffart A. A comparison of cognitive therapy, applied relaxation, and nitrous oxide sedation in the treatment of dental fear. Acta Odontol Scand. 2001 Oct;59(5):290-296.
79 Willumsen T., Vassend O., Hoffart A. One-year follow-up of patients treated for dental fear: effects of cognitive therapy, applied relaxation, and nitrous oxide sedation. Acta Odontol Scand. 2001 Dec;59(6):335-340.
80 Willumsen T., Vassend O. Effects of cognitive therapy, applied relaxation and nitrous oxide sedation. A five-year follow-up study of patients treated for dental fear. Acta Odontol Scand. 2003 Apr;61(2):93-99.
81 Moynihan P., Petersen P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004 Feb;7(1A):201-226.
82 Sheiham A. Dietary effects on dental diseases. Public Health Nutr. 2001 Apr;4(2B):569-591.
83 Gennari C. Calcium and vitamin D nutrition and bone disease of the elderly. Public Health Nutr. 2001 Apr;4(2B):547-559.
84 Nunn M.E., Braunstein N.S., Krall Kaye E.A., et al. Healthy eating index is a predictor of early childhood caries. J Dent Res. 2009 Apr;88(4):361-366.
85 Tinanoff N., Palmer C.A. Dietary determinants of dental caries and dietary recommendations for preschool children. J Public Health Dent. 2000 Summer;60(3):197-206. discussion 207–9
86 Tucker K.L., Morita K., Qiao N., et al. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study. AJCN. 2006;84:936-942.
87 Sullivan E.A., Curzon M.E. A comparison of acidic dietary factors in children with and without dental erosion. ASDC J Dent Child. 2000;67(3):186-192.
88 Cochrane N.J., Cai F., Yuan Y., et al. Erosive potential of beverages sold in Australian schools. Aust Dent J. 2009;54(3):238-244.
89 Moyad M.A. Vitamin D: a rapid review. Urol Nurs. 2008;28(5):343-349.
90 Bischoff-Ferrari H.A., Dietrich T., Orav E.J., et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and non-active persons aged > or = 60 y. AJCN. 2004;80:752-758.
91 Nowson C.A., Margerison C. Vitamin D intake and vitamin D status of Australians. MJA. 2002;177:149-152.
92 Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
93 Van der Mei I.A., Ponsonby A.L., Engelsen O., et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007;115(8):1132-1139.
94 Guardia G., Parikh N., Eskridge T., et al. Prevalence of vitamin D depletion among subjects seeking advice on osteoporosis: a five –year cross-sectional study with public health implications. Osteoporosis Int. 2008;19(1):13-19.
95 Kull MartJr, Kallikorm Riina, Tamm Anu, et al. Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European country. BMC Public Health. 9(22), 2009. doi:10.1186/1471-2458-9-22 http://www.biomedcentral.com/1471-2458/9/22
96 John Livesey, et al. Seasonal variation in vitamin D levels in the Canterbury, New Zealand population in relation to available UV radiation. The New Zealand Medical Journal 120(1262):1-13
97 Weisber P., Kelley S.S., Ruowei Li, et al. Nutritional rickets among children in the United States: review of cases reported between 1986-2003. Am J Clin Nutr. 2004;80(suppl):1697S-1705S.
98 Munns C., Zacharin M.R., et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. MJA. 2006;185:268-272.
99 Van der Meer I.M., Karamali N.S., Boeke J.P., et al. High prevalence of vitamin D deficiency in pregnant non-Western women in the Hague, Netherlands. Am J Clin Nutr. 2006;84:350-353.
100 Erbas Bircan, Ebeling Peter R, Couch Dianne, et al. Suburban clustering of vitamin D deficiency in Melbourne, Australia. Asia Pac J Clin Nutr. 2008;17(1):63-67.
101 Diamond Terrence H, Levy Sherel, Smith Angelina, et al. High bone turnover in Muslim women with vitamin D deficiency. MJA. 2002 Aug 5;177:139-141.
102 Grover S.R., Morley M. Vitamin D deficiency in veiled or dark skinned pregnant women. MJA. 2001;175:251-252.
103 Tohill Carmel, Laverty Anne. Sunshine, diet and mobility for healthy bones-an interventional study designed to implement these standards into the daily routine in an at risk population of adults with intellectual disability. Journal of Intellectual and Developmental Disability. 2001;26(3):217-231.
104 Leif Mosekilde. Vitamin D and the elderly. Clinical Endocrinology. 2005;62:265-281.
105 Thompson K., Morley R., Grover S.R., et al. Postnatal evaluation of vitamin D and bone health in women who were vitamin D-deficient in pregnancy, and in their infants. MJA. 2004;181(9):486-488.
106 KerrieSanders M., CarylNowson A., Mark A., Kotowicz, et al. Position Statement. Calcium and bone health: position statement for the Australian and New Zealand Bone and Mineral Society, Osteoporosis Australia and the Endocrine Society of Australia. MJA. 2009;190(6):316-320. Online. Available: http://www.mja.com.au/public/issues/190_06_160309/san10083_fm.html (accessed 1 Sept 2009)
107 Zhu K., Devine A., Dick I.M., et al. Effects of calium and vitamin S supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a 5-year randomized controlled trial. J Clin Endocrino Metabol. 2007;93:743-749.
108 Lanham-New S.A. Importance of calcium, vitamin D and vitamin K for osteoporosis prevention and treatment. Proc Nutr Soc. 2008;67(2):163-176.
109 Hildebolt C.F. Effect of vitamin D and calcium on periodontitis J Periodontol. 2005;76:1576-1587.
110 Krall E.A., Wehler C., Garcia R.I., et al. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001 Oct 15;111(6):452-456.
111 Neiva R.F., Al-Shammari K., Nociti F.H.Jr, et al. Effects of vitamin-B complex supplementation on periodontal wound healing. J Periodontol. 2005;76(7):1084-1091.
112 Head K.A. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev. 2006;11(4):294-329.
113 Scott K., Zeris S., Kothari M.J. Elevated B6 levels and peripheral neuropathies. Electromyogr Clin Neurophysiol. 2008;48(5):219-223.
114 Nishida M., Grossi S.G., Dunford R.G., et al. Dietary vitamin C and the risk for periodontal disease. J Periodontol. 2000 Aug;71(8):1215-1223.
115 Anderson B. Nutrition and wound healing: the necessity of assessment. Br J Nurs. 14(19), 2005 Oct 27-Nov 9. S30, S32, S34 passim
116 Casey G. Nurs Stand. Nutritional support in wound healing. 2003 Feb 19-25;17(23):55-58.
117 Munoz C.A., Kiger R.D., Stephens J.A., et al. Effects of a nutritional supplement on periodontal status. Compend Contin Educ Dent. 2001;22(5):425-428.
118 Neiva R.F., Steigenga J., Al-Shammari K.F., et al. Effects of specific nutrients on periodontal disease onset, progression and treatment. J Clin Periodontol. 2003 Jul;30(7):579-589.
119 Meisel P., Schwahn C., Luedemann J., et al. J Dent Res. Magnesium deficiency is associated with periodontal disease. 2005 Oct;84(10):937-941.
120 Nishida M., Grossi S.G., Dunford R.G., et al. Calcium and the risk for periodontal disease. J Periodontol. 2000 Jul;71(7):1057-1066.
121 Marinho V.C.C., Higgins J.P.T., Logan S., et al. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews. (Issue 2):2002. Art. No.: CD002280. doi: 10.1002/14651858.CD002280
122 Marinho V.C.C., Higgins J.P.T., Logan S., et al. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews. (Issue 3):2002. Art. No.: CD002279. doi: 10.1002/14651858.CD002279
123 Marinho V.C.C., Higgins J.P.T., Sheiham A., et al. One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews. (Issue 1):2004. Art. No.: CD002780. doi: 10.1002/14651858.CD002780.pub2
124 Marinho V.C.C., Higgins J.P.T., Sheiham A., et al. Combinations of topical fluoride (toothpastes, mouthrinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews. (Issue 1):2004. Art. No.: CD002781. doi: 10.1002/14651858.CD002781.pub2
125 Marinho V.C.C., Higgins J.P.T., Logan S., et al. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews. (Issue 3):2003. Art. No.: CD002284. doi: 10.1002/14651858.CD002284
126 Marinho V.C.C., Higgins J.P.T., Logan S., et al. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews. (Issue 4):2003. Art. No.: CD002782. doi: 10.1002/14651858.CD002782
127 McDonagh M.S., Whiting P.F., Wilson P.M., et al. Systematic review of water fluoridation. BMJ. 2000;321(7265):855-859.
128 Bassin E.B. Association Between Fluoride in Drinking Water During Growth and Development and the Incidence of Ostosarcoma for Children and Adolescents. Doctoral Thesis, Harvard School of Dental Medicine; 2001.
129 Cohn P.D. A Brief Report On The Association Of Drinking Water Fluoridation And The Incidence of Osteosarcoma Among Young Males. New Jersey Department of Health Environ. Health Service; 1992. 1–17
130 Gelberg K.H., et al. Fluoride Exposure and Childhood Osteosarcoma: A Case-Control Study. Am J Pub Hlth. 1995;85:1678-1683.
131 Hoover R.N., et al. Time trends for bone and joint cancers and osteosarcomas in the Surveillance, Epidemiology and End Results (SEER) Program. National Cancer Institute. In: Review of Fluoride. Benefits and Risks Report of the Ad Hoc Committee on Fluoride of the Committee to Coordinate Environmental Health and Related Programs US Public Health Service; 1991:F1-F7.
132 Moss M.E., Kanarek M.S., Anderson H.A., et al. Osteosarcoma, seasonality, and environmental factors in Wisconsin, 1979-1989. Arch Envir Health. 1995;50:235-241.
133 Operskalski E.A., et al. A case-control study of osteosarcoma in young persons. Am J Epidemiol. 1987;126:118-126.
134 Bassin E.B., Wypij D., Davis R.B., et al. Age-specific fluoride exposure in drinking water and osteosarcoma (United States). Cancer Causes Control. 2006 May;17(4):421-428.
135 Eyre R., Feltbower R.G., Mubwandarikwa E., et al. Epidemiology of bone tumours in children and young adults. Pediatr Blood Cancer. 2009 Jul 17. Epub ahead of print
136 Sandhu R., Lal H., Kundu Z.S., et al. Serum Fluoride and Sialic Acid Levels in Osteosarcoma. Biol Trace Elem Res. 2009 Apr 24. Epub ahead of print
137 Takahashi K., Akiniwa K., Narita K. Regression analysis of cancer incidence rates and water fluoride in the U.S.A. based on IACR/IARC (WHO) data (1978-1992). International Agency for Research on Cancer. J Epidemiol. 2001 Jul;11(4):170-179.
138 Freni S.C., Gaylor D.W. International trends in the incidence of bone cancer are not related to drinking water fluoridation. Cancer. 1992;70(3):611-618.
139 Mcguire S.M., et al. Is there a link between fluoridated water and osteosarcoma? Am Dent Assoc. 1991;122:38-45.
140 Spencer A.J. Water fluoridation in Australia. Community Dental Health. 1996;13(suppl 2):27-37.
141 National Health and Medical Research Centre (NHMRC). Online. Available: www.nhmrc.gov.au/news/media/rel07/_files/fluoride_flyer.pdf (accessed 1 Sept 2009)
142 Yeung C.A. A systematic review of the efficacy and safety of fluoridation. Evid Based Dent. 2008;9(2):39-43.
143 Yeung A., Hitchings J.L., Macfarlane T.V., et al. Fluoridated milk for preventing dental caries. Cochrane Database of Systematic Reviews. (Issue 3):2005. Art. No.: CD003876. doi: 10.1002/14651858.CD003876.pub2
144 Haguenuaer D., Welch V., Shea B., et al. Fluoride for the treatment of postmenopausal osteoporotic fractures: a meta-analysis. Osteoporosis Int. 2000;11:727-738.
145 Pak C.Y., Sakhaee K., Zerwekh J.E., et al. Treatment of postmenopausal osteoporosis with slow-release of sodium fluoride. Final report of randomized controlled trial. Ann Int Med. 1995;123:401-408.
146 Poulsen S., Errboe M., Lescay Mevil Y., et al. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database of Systematic Reviews. (Issue 2):2006. Art. No.: CD001476. doi: 10.1002/14651858.CD001476.pub2
147 Harper D.S., Mueller L.J., Fine J.B., et al. Clinical efficacy of a dentifrice and oral rinse containing sanguinaria extract and zinc chloride during 6 months of use. J Periodontol. 1990;61(6):352-358.
148 Watts T.L. Coenzyme Q10 and periodontal treatment: is there any beneficial effect? Br Dent J. 1995;178(6):209-213.
149 Hanioka T., Tanaka M., Ojima M., et al. Effect of topical application of coenzyme Q10 on adult periodontitis. Mol Aspects Med. 1994;15(Suppl):s241-s248.
150 Zakrzewska J.M., Forssell H., Glenny A- M. Interventions for the treatment of burning mouth syndrome. Cochrane Database of Systematic Reviews. (Issue 4):2004. Art. No.: CD002779. doi: 10.1002/14651858.CD002779.pub2
151 Twetman S., Stecksén-Blicks C. Probiotics and oral health effects in children Int J Paediatr Dent. 2008 Jan;18(1):3-10.
152 Caglar E., Kargul B., Tanboga I. Bacteriotherapy and probiotics‘ role on oral health. Oral Dis. 2005 May;11(3):131-137.
153 de Vrese M., Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1-66.
154 Meurman J.H., Stamatova I. Probiotics: contributions to oral health. Oral Dis. 2007 Sep;13(5):443-451.
155 Meurman J.H. Probiotics: do they have a role in oral medicine and dentistry? Eur J Oral Sci. 2005 Jun;113(3):188-196.
156 Hirasawa M., Takada K., Otake S. Inhibition of acid production in dental plaque bacteria by green tea catechins. Caries Res. 2006;40(3):265-270.
157 Kuftinec MM, Mueller-Joseph LJ, Kopczyk RA. Sanguinaria toothpaste and oral rinse regimen clinical efficacy in short- and long-term trials. J Can Dent Assoc1990;56(7 suppl):31–3.
158 Cullinan M.P., Powell R.N., Faddy M.J., et al. Efficacy of a dentifrice and oral rinse containing sanguinaria extract in conjunction with initial periodontal therapy. Aust Dent J. 1997;42(1):47-51.
159 Harper D.S., Mueller L.J., Fine J.B., et al. Clinical efficacy of a dentifrice and oral rinse containing sanguinaria extract and zinc chloride during 6 months of use. J Periodontol. 1990;61(6):352-358.
160 Alqareer A., Alyahya A., Andersson L. The effect of clove and benzocaine versus placebo as topical anesthetics J Dent. 2006 Nov;34(10):747-750.
161 Nagata H., Inagaki Y., Tanaka M., et al. Effect of eucalyptus extract chewing gum on periodontal health: a double-masked, randomized trial. J Periodontol. 2008 Aug;79(8):1378-1385.
162 Izzo A.A., Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001;61(15):2163-2175.
163 Ang-Lee M.K., Moss J., Yuan C.S. Herbal medicines and perioperative care. JAMA. 2001 Jul 11;286(2):208-216.
164 Abebe W. An overview of herbal supplement utilization with particular emphasis on possible interactions with dental drugs and oral manifestations. J Dent Hyg. 2003 Winter;77(1):37-46.
165 McNeely M.L., Armijo Olivo S., Magee D.J. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys Ther. 2006 May;86(5):710-725.
166 Medlicott M.S., Harris S.R. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys Ther. 2006 Jul;86(7):955-973.
167 Al-Ani M.Z., Davies S.J., Gray R.J.M., et al. Stabilisation splint therapy for temporomandibular pain dysfunction syndrome. Cochrane Database of Systematic Reviews. (Issue 1):2004. Art. No.: CD002778. doi: 10.1002/14651858.CD002778.pub2
168 Koh H., Robinson P. Occlusal adjustment for treating and preventing temporomandibular joint disorders. Cochrane Database of Systematic Reviews. (Issue 1):2003. Art. No.: CD003812. doi: 10.1002/14651858.CD003812
169 Rosted P., Bundgaard M., Pedersen A.M. The use of acupuncture in the treatment of temporomandibular dysfunction–an audit. Acupunct Med. 2006;24(1):16-22.
170 Fink M., Rosted P., Bernateck M., et al. Acupuncture in the treatment of painful dysfunction of the temporomandibular joint – a review of the literature. Forsch Komplementmed. 2006 Apr;13(2):109-115.
171 Morganstein W.M. Acupuncture in the treatment of xerostomia: clinical report. Gen Dent. 2005 May-Jun;53(3):223-226.
172 Blom M., Dawidson I., Angmar-Månsson B. The effect of acupuncture on salivary flow rates in patients with xerostomia. Oral Surg Oral Med Oral Pathol. 1992 Mar;73(3):293-298.
173 Dickinson C.M., Fiske J. A review of gagging problems in dentistry: I. Aetiology and classification. Dent Update. 2005 Jan-Feb;32(1):26-28. 31–2
174 Rosted P., Bundgaard M., Fiske J., et al. The use of acupuncture in controlling the gag reflex in patients requiring an upper alginate impression: an audit. Br Dent J. 2006;201:721-725. 715
175 Lavigne G., Kato T. Usual and unusual orofacial motor activities associated with tooth wear. Int J Prosthodont. 2003;16(Suppl):80-82. discussion 89–90
176 Barbour M.E., Rees G.D. The role of erosion, abrasion and attrition in tooth wear. J Clin Dent. 2006;17(4):88-93.
177 Macedo C.R., Silva A.B., Machado M.A.C., et al. Occlusal splints for treating sleep bruxism (tooth grinding). Cochrane Database of Systematic Reviews. (Issue 4):2007. Art. No.: CD005514. doi: 10.1002/14651858.CD005514.pub2
178 Mathie R.T., Farrer S. Outcomes from homeopathic prescribing in dental practice: a prospective, research-targeted, pilot study. Homeopathy. 2007 Apr;96(2):74-81.