5 Optimising safety
Chapter contents
Introduction
Medical opinion generally has been that it is impossible for any medicine to have effects without side effects, that if herbs are claimed to be free from side effects they are probably not effective either. This is a rational view within its own terms. Any intervention at one site is always likely to lead to reactions at other sites, either because of functional or structural connection or because of similarity in sensitivity.
Nonetheless, for all concerned, what is needed is more information on safety. This chapter is not intended to be a comprehensive treatise on the issue of herbal safety. There are already a number of textbooks devoted solely to this topic and the monographs in this book provide detailed safety information for each of the 50 herbs covered. The information below is intended more as an introduction to the topic. Texts such as The Essential Guide to Herbal Safety1 and the Botanical Safety Handbook2 are particularly recommended for more information, as they represent a balanced view based on both clinical experience and the published data.
The case for concern
Adverse case reports
There have been a number of cases reported in the medical literature indicating a link between herbal consumption and adverse effects. Many of these are reviewed in the monographs in this book. Most are minor and infrequent in nature. Where serious adverse events are reported, the information is often of poor quality, making a credible link between cause and effect difficult to establish. However, it is worthwhile to note some defining examples of serious adverse events that have occurred in the past few decades. Many of these have resulted from contamination or adulteration, or relate to known toxic herbs.
During the early 1990s, several patients with renal failure were admitted to hospitals in Brussels with progressive interstitial fibrosis and tubular atrophy that was linked to a herbal slimming preparation. At least 30 individuals were found to have sustained end-stage renal failure from the incident, making it perhaps the single most serious adverse event linked to herbal consumption in modern times. The Chinese herb Aristolochia fangchi was found to be an ingredient of the formulation instead of the intended Stephania tetrandra. The consequent presence of aristolochic acid, a known toxin, was put forward as a hypothesis for the aetiology of the nephropathies in the literature and the phenomenon became known (perhaps incorrectly) as Chinese herb nephropathy (CHN). However, the case prompted the Association Pharmaceutique Belge at the Service du Contrôle des Médicaments to probe the matter further.3 They pointed to the idiosyncratic nature of the reactions and the presence of other powerful synthetic drugs in the mixture as suggesting a more complex story. They considered that the cocktail of sometimes powerful preparations adopted by some observing slimming regimes might have significantly lowered the threshold for renal damage. However, aristolochic acid has been known as a nephrotoxin for decades and cases not linked to the concurrent use of drugs have been reported.4
In 1997, it was reported that two of the women subsequently developed urothelial cancer caused by the genotoxicity of aristolochic acid.5 An article published in June 1999 reported further cases of urothelial cancer. Cosyns and co-workers tested 10 patients with CHN.6 Four (40%) were found to have urothelial carcinoma and abnormal cells were found in all of the 10 patients. Nortier and co-workers7 (June 2000) concluded that the incidence of urothelial cancer among patients with CHN is high and that the risk was related to the cumulative dose of the herb. They reported treating 105 patients with CHN of whom 43 had been admitted with end-stage renal failure. Thirty-nine of these patients were tested for urothelial carcinoma. Eighteen cases were found, and mild-to-moderate dysplasia was found in a further 19 patients.
Cases of CHN have also been reported in France, Spain, Japan, the UK and Taiwan, where cases of urothelial carcinoma have also been detected.7Aristolochia spp. can also be used as substitutes for several other Chinese herbs,8 and Chinese herbal products found to contain aristolochic acid have been recalled in several countries (including Australia9 and the USA10).
During the early 1990s, liver units in France began to report a number of cases of liver disease possibly linked to the consumption of a slimming herb. The hepatotoxicity of germander (Teucrium chamaedrys) was confirmed in isolated rat hepatocytes, particularly a crude fraction containing the diverse furanoditerpenoids. It was concluded that they are activated by cytochrome P450 3 A into electrophilic metabolites that deplete glutathione and protein thiols and form plasma membrane blebs.11 However, these cases were more likely to have been idiosyncratic drug reactions (IDRs) to the germander. Germander was subsequently banned from use in many countries, although germander-induced hepatotoxicity is probably still occurring, mainly from the fact that certain species of Teucrium are commonly used as a substitute for American skullcap (Scutellaria lateriflora).
In fact, so widespread was this adulteration, the macroscopic and microscopic description of skullcap cut herb in the British Herbal Pharmacopoeia 1983 was probably for a species of Teucrium. Adulteration of commercial skullcap continued to be an issue in Europe, the UK and the USA into the late 20th century.12 Idiosyncratic hepatotoxicity was reported for tablets containing skullcap and valerian in 198913 and for tablets containing skullcap, mistletoe, kelp, wild lettuce and motherwort in 198114 (although the presence of mistletoe was later questioned).15 The observed hepatotoxicity of these herbal products is probably attributable to a germander species, rather than the herbs mentioned, including skullcap. Case reports of hepatotoxicity caused by germander are not limited to T. chamaedrys,16 other Teucrium spp. have caused hepatic failure.17 One of the reported UK cases of skullcap-related hepatotoxicity was confirmed as being due to T. canadense rather than skullcap.16
From January 1991 to December 1993, the Medical Toxicology Unit (formerly Poisons Unit) at Guy’s and St Thomas’s Hospital in London received reports of 11 cases of liver damage following the use of Chinese herbal medicine for skin conditions. There was strong evidence of an association in two cases, as recovery after withdrawal and recurrence of hepatitis after rechallenge were observed. The time-course relationship, recovery after ceasing Chinese herbal medicine and absence of alternative causes of liver damage suggested an association in two further symptomatic cases following a single period of exposure. Herbal material was available for analysis in seven cases. The plant mixtures varied, so no single ingredient could account for liver injury. Effects did not appear dose related and it was concluded they were probably idiosyncratic.18 Two patients were additionally described who suffered an acute hepatic illness related to taking traditional Chinese herbs for skin disease. Both recovered fully. The mixtures they took included two herbs that were also present in the mixture taken by a previously reported patient who suffered fatal hepatic necrosis.19 Sporadic cases of IDRs to Chinese herbal formulations resulting in hepatotoxicity have been reported in the literature ever since. A 2009 study from a gastroenterological department in a Chinese hospital concluded that Chinese herbs were a significant factor in idiosyncratic hepatotoxicity, although the liver injury was not severe in most cases.20 Other studies reviewing patients attending single clinics or hospitals in the UK, Germany and Japan have found a much lower incidence of idiosyncratic hepatitis from the use of Chinese herbal formulations.21
Case reports of idiosyncratic hepatotoxicity to Western herbs extend beyond germander. A review of 18 reports of adverse events associated with the ingestion of chaparral reported to the Food and Drug Administration (FDA) between 1992 and 1994 found evidence of hepatotoxicity in 13 cases (causal (10), not followed up (1), probable (2), insufficient data (5)). Of the 13 cases, 10 had ingested chaparral only, with the remainder taking products containing chaparral and other herbs and/or ingredients. Adverse events occurred 3 to 52 weeks after the ingestion of chaparral and resolved 1 to 17 weeks after ceasing intake. The predominant pattern of liver injury was characterised as toxic or drug-induced cholestatic hepatitis. In four individuals there was progression to cirrhosis and in two individuals there was acute fulminant liver failure that required a liver transplant. Of the patients requiring a liver transplant, chaparral was probably not the only factor in one case.22
In 2002 a group of Australian doctors reported suspected hepatotoxicity, presumably an IDR, associated with the ingestion of black cohosh (Actaea racemosa).23 At least 68 more cases have been reported since then, but the association remains a contentious issue. One analysis of the reported cases using the updated Council for International Organisations of Medical Sciences (CIOMS) causality assessment asserted that there was little, if any, evidence for a causal relationship between use of black cohosh and liver damage.24 For more information on this topic see the black cohosh monograph.
Nonetheless, the RAND Report undertook a comprehensive analysis of adverse consequences from both clinical trials and case reports submitted to the FDA.25 Using data from clinical trials, the report concluded that there is sufficient evidence that the use of ephedrine and/or Ephedra or ephedrine plus caffeine is associated with two to three times the risk of nausea, vomiting, psychiatric symptoms such as anxiety and change in mood, autonomic hyperactivity and palpitations. It was not possible to determine the contribution of caffeine to these events. There were no reports of serious adverse events in the controlled trials.25
From the adverse events reported by one manufacturer of Ephedra-containing dietary supplements and to the FDA, the RAND Report identified what it termed to be ‘sentinel events’. Classification of a sentinel event does not mean to imply a proven cause and effect relationship. These included two deaths, three myocardial infarctions, nine strokes, three seizures and five psychiatric cases associated with prior Ephedra consumption. The report also identified 43 additional cases as possible sentinel events and noted that about half of all the sentinel events occurred in people aged 30 years or younger.25 Hepatotoxicity has also been linked to the use of Ephedra both in traditional Chinese formulations and in weight loss supplements.26–28
Animal exposure to pyrrolizidine alkaloids (PAs), found in several medicinal plants, has led to a dose-dependent swelling of hepatocytes and haemorrhagic necrosis of perivenular cells of the liver, with concomitant loss of sinusoidal lining cells, with sinusoids filled with cellular debris, hepatocyte organelles and red blood cells. These are all features of veno-occlusive disease.29 These effects do not represent an IDR, but rather follow from the direct hepatotoxic activity of these alkaloids. The LD50 for a pyrrolizidine-rich extract of Senecio was found to be 160 mg/kg.30 Despite their similarity in structure, PAs differ in their individual LD50 values and in the organs in which toxicity is expressed. In one study of four PAs, the proportion of the PA removed by liver cultures varied considerably due to differences in the production of reactive metabolites (dehydroalkaloids), which appear to be largely responsible for the toxicity of PAs, and in their conversion to a safer form (GSDHP).31 Among pyrrolizidine-containing plants, heliotrope32 and Senecio33 have been found to be responsible for veno-occlusive disease in humans. Clinical manifestations of poisoning in humans include abdominal pain, ascites, hepatomegaly and raised serum transaminase levels. Prognosis is often poor with death rates of 20% to 30% being reported.34 In vivo studies of coltsfoot, containing senkirkine, have shown some evidence of toxicity.35 However, the key reported case linked to coltsfoot consumption turned out to be a substitution problem. Tea containing peppermint, and what the mother thought was coltsfoot (Tussilago farfara), was associated with veno-occlusive liver disease in an 18-month-old boy. Pharmacological analysis of the tea compounds revealed high amounts of PAs, mainly seneciphylline and the corresponding N-oxide. It was calculated that the child had consumed at least 60 mg/kg body weight per day of the toxic pyrrolizidine alkaloid mixture over 15 months. Macroscopic and microscopic analysis of the leaf material indicated that Adenostyles allariae had been erroneously gathered by the parents in place of coltsfoot. The child was given conservative treatment only and recovered completely within 2 months.36
A review of 18 case reports of pennyroyal ingestion documented moderate to severe toxicity in patients who had been exposed to at least 10 mL of pennyroyal oil. In one fatal case, postmortem examination of a serum sample obtained 72 h after the acute ingestion identified 18 ng/mL of pulegone and 1 ng/mL of menthofuran.37 In another case of ingestion of 30 mL of pennyroyal oil by a pregnant woman, symptoms included abdominal spasm, nausea, vomiting, alternating lethargy and agitated behaviour. Kidney failure and a solid liver necrosis developed subsequently and death occurred 7 days later. In two similar cases where doses used were 10 mL and 15 mL of oil, vomiting, agitation, fainting, flank pain and dermatitis occurred, but with no lasting toxic symptoms.38
Maternal ingestion of blue cohosh (Caulophyllum thalictroides) in late pregnancy has been associated with four documented cases of perinatal adverse events. The first case occurred after a normal labour, where a female infant was not able to breathe spontaneously and sustained central nervous system (CNS) hypoxic-ischaemic damage. A midwife had attempted induction of labour using a combination of blue cohosh and black cohosh given orally (dosage undefined) at around 42 weeks’ gestation.39
In the second case, severe congestive heart failure and myocardial infarction in a newborn male were attributed to maternal consumption of blue cohosh tablets. The woman had been advised to take 1 tablet per day (herb quantity not specified) but she took 3 tablets per day for 3 weeks prior to delivery. Cardiomegaly and mildly reduced left ventricular function were still evident at 2 years of age.40 The tablets were not analysed for their content. Stroke in an infant was reported as a possible association with a blue cohosh-containing dietary supplement in the FDA’s Special Nutritionals Adverse Event Monitoring System database (which listed adverse events but was not subject to preconditions, analysis or peer review).41 The level of documentation of this case is poor.
Finally, a case report linked stroke in a baby with blue cohosh consumption by the mother.42 A female infant weighing 3.86 kg was born at just over 40 weeks’ gestation to a healthy 24-year-old woman. The obstetrician reportedly had advised the woman to drink a tea made from blue cohosh because induction of labour was a recognised effect of this herb. A caesarean section was performed after a failed attempt at vaginal delivery. The infant had focal motor seizures of the right arm, which began at 26 h of age, and were controlled with phenobarbital and phenytoin. A computed tomographic (CT) scan obtained when the infant was 2 days of age showed an evolving infarct in the distribution of the left middle cerebral artery. There were no other apparent causes for the baby’s condition.
In a curious development with this fourth case, urine and meconium were positive for the cocaine metabolite benzoylecgonine, and testing of the mother’s bottle of blue cohosh and another brand of the same herb were also positive for this metabolite. Maternal cocaine is a well-known cause of perinatal stroke. Later the authors commented that the finding of a cocaine metabolite in blue cohosh should be interpreted with caution.43 The finding is most likely due to a false positive reading from the analytical tests used (which did not have a high degree of specificity). In other words, blue cohosh most likely contains a phytochemical which reacts like the cocaine metabolite in terms of the test used, but is not related in any way to cocaine.
Adverse effects have also been documented for a pregnant woman ingesting blue cohosh. Nicotinic toxicity was reported following the attempted use of blue cohosh as an abortifacient.44 A 21-year-old woman developed tachycardia, sweating, abdominal pain, vomiting and muscle weakness following the ingestion of a blue cohosh tincture. The authors suggested that these symptoms were consistent with nicotinic toxicity and probably resulted from N-methylcytisine, a phytochemical component of blue cohosh. Symptoms resolved over 24 h.
Plants as poisons
In general, plants have considerably less acute toxicity than many other agents in our modern environment, such as chemicals and drugs. This could be expected since the chemicals in plants are diluted by a large percentage of inert plant material. This assertion is borne out by statistics, for example data from the American Association of Poison Control Centres (AAPCC). In a recent publication, information from the 1983 to 2009 annual reports of the AAPCC was analysed, together with queries of the 2000 to 2009 AAPCC Toxic Exposure Surveillance System and the National Poison Data System databases.45 During 2000 to 2009, 668 111 plant ingestion exposures were reported, with around 90% of these involving single plants. There was a steady decline in the number of plant exposures, falling from 8.9% of all reported exposures in 1983, to 2.4% in 2009. Young children accounted for more than 80% of plant ingestion exposures. Only 45 fatalities were recorded between 1983 and 2009, with Datura and Cicuta species accounting for about one-third of these. The authors concluded that, while plant ingestion remains a common call for poison information centres, morbidity and mortality associated with these were very low relative to the total number of reported plant exposures.
Given the existence of toxic plants, it is accepted by all involved that not all plants are safe to use as remedies. The incidence of these clearly varies from country to country. Appendix B provides a list of several potentially toxic medicinal herbs. With a few exceptions, their use is best avoided, especially during pregnancy and lactation. The individual herb monographs in this book contain detailed toxicity information, where available.
Adverse reactions
Although the term ‘benefit-risk ratio’ is convenient and often used, such an assessment is qualitative not quantitative (in other words, the division of a number for risk by a number for efficacy is rarely actually performed) and hence always involves some element of subjectivity. For a drug that is lifesaving, a greater safety risk is acceptable when it is used in that context. This is the reason dangerous therapeutic drugs are tolerated by the regulators.
A number of milder adverse reactions are predictable on the basis of the known phytochemistry of the herb. More details of such side effects are provided in Chapter 2, but a few key examples follow:
• Tannin-containing herbs, such as cranesbill (Geranium maculatum) and oak bark (Quercus robur), can inhibit trace element and B vitamin absorption. They should therefore not be used in high doses for long periods, or alternatively given away from food and other medications
• Saponins are gastric irritants. Hence, doses of herbs which contain saponins, such as horsechestnut (Aesculus hippocastanum) and Gymnema (Gymnema sylvestre), can cause reflux and/or vomiting in sensitive individuals. The alternative is to prescribe them in enteric-coated tablets or with meals
• Licorice root (Glycyrrhiza spp) can cause sodium and fluid retention and potassium loss. This effect only occurs with extended use at high doses and can be minimised by a high potassium diet. An adult dose equivalent to 3 g/day should not cause this problem
• Korean ginseng (Panax ginseng) can cause overstimulation, usually only at higher doses (in excess of 1 g/day)
• Pungent herbs such as capsicum (Cayenne spp) and ginger (Zingiber officinale) create a burning sensation which patients may find uncomfortable. In the case of herbs that contain mustard oils, such as horseradish (Armoracia rusticana), the burning sensation is real and can cause considerable gastric discomfort. High doses of ginger can cause heartburn
• Bitter herbs in high doses may cause some patients to vomit when given in liquid form
• Echinacea and prickly ash (Zanthoxylum clava-herculis) in liquid form cause tingling in the mouth and promotion of saliva, which in a few patients can give them a choking sensation and rarely a panic reaction
• Thujone can cause CNS stimulation and possibly epilepsy. Care should be exercised when giving thujone-containing herbs in high doses to epileptics. These herbs include Thuja (Thuja occidentalis), sage (Salvia officinalis), tansy (Tanacetum vulgare), wormwood (Artemisia absinthium) and some types of yarrow (Achillea millefolium). Thujone-containing herbs can also cause headaches in high doses
• Garlic can inhibit thyroid function
• Blood root (Sanguinaria canadensis) and bryony (Bryonia alba) are potent irritants and should only be used in low doses
• Bladderwrack (Fucus vesiculosus) and kelp (Laminaria) may aggravate or induce hyperthyroidism when given in high doses for prolonged periods
• Laxative herbs can cause abdominal pain. Abuse can lead to electrolyte loss, especially potassium. Chronic use leads to a characteristic pigmentation of the colonic mucosa known as melanosis coli. This is harmless and reversible
• Use of kava (Piper methysticum) for insomnia can cause a mild lethargy the next morning and chronic use is associated with skin changes
• It is unlikely that St John’s wort (Hypericum perforatum) causes photosensitivity with normal usage. It may cause an allergic skin rash in some cases, which has been misinterpreted as photosensitivity.
Idiosyncratic reactions
Idiosyncratic reactions are reactions that are peculiar to a single individual or a very small group of people. They are by nature unexpected and unpredictable.
Pregnancy and lactation
It is a general principle that one should refrain from giving medicines to a pregnant woman unless clearly necessary. Although some herbs have been used safely by women when pregnant and may thus be seen to have a degree of positive vetting, they should be prescribed particularly carefully in the crucial first trimester when fetal organ development is underway. Although there are very few accounts that link any pregnancy problems to herb consumption, too little is known for any sweeping recommendation. Particular caution should be exercised for plants with alkaloidal principles, strong volatile constituents (notably including pure essential oils and plants with high levels of thujone) and in cases where there is a history of miscarriage or where low back or abdominal pains occur. Toxic herbs should be avoided; see Appendix B for such a list.
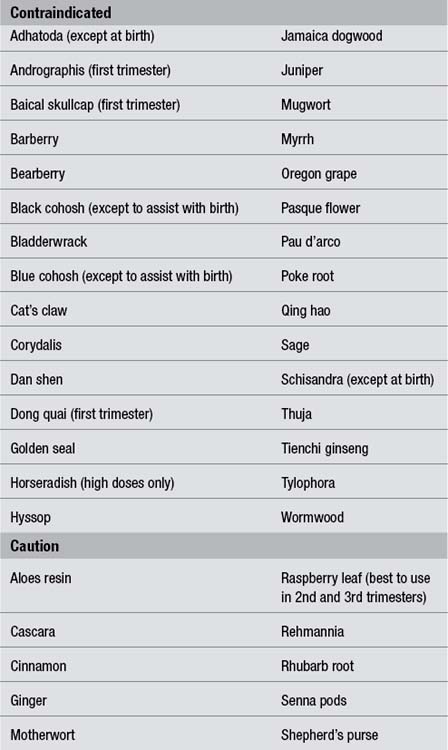
The pregnancy safety ratings advocated in The Essential Guide to Herbal Safety1 have been adopted for the monographs in this text. This categorisation is designed to remove the subjectivity from assessing the safety information for herbs during pregnancy. The ratings are self-explanatory, but a further discussion is provided in Chapter 10 (How to use the monographs). However, some herbal clinicians might prefer a simpler approach of a list of contraindications and cautions, and this is provided in Table 5.1 for more than just the 50 herbs detailed in this book.
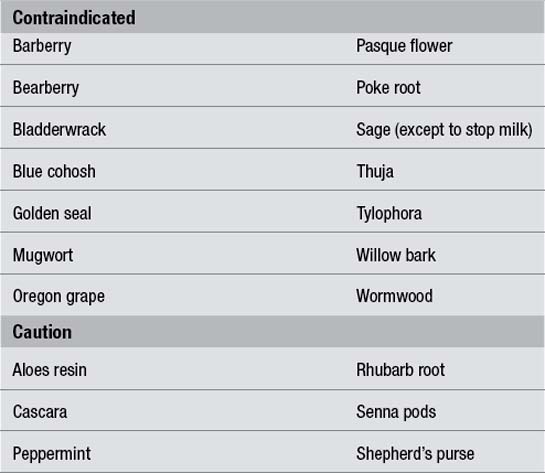
In regulatory terms, the caution referred to above in the case of pregnancy is also extended to the stage of lactation. Although critical organ development is not threatened, there remains some doubt in many cases about how secondary plant metabolites, many of which pass easily and even preferentially into breast milk, affect the baby. Practitioners should therefore maintain a degree of caution in attending to clinical problems affecting mother and suckling baby. However, it should be pointed out that documented adverse effects for herbs used during lactation are minimal, other than milk reduction or reduced infant feeding. Table 5.2 provides some basic guidelines for commonly used herbs.
Adverse herb-drug interactions
The issue of adverse herb-drug interactions (HDIs) is probably the most contentious problem in the understanding of the safe use of medicinal plants. Many texts carry pages and pages of theoretically possible interactions for each herb, often based on unsupported extrapolations from pharmacological data (typically in vitro studies). Such information is not only needlessly cautious it is potentially alarmist. The reader might experience such difficulty managing the complexity of the provided information that he or she might conclude that the only safe option is never to recommend any herbs in conjunction with conventional drugs. In addition to these hypercautions, there is also considerable misinformation in the field. For a full discussion of these issues, the reader is referred to the relevant chapter in The Essential Guide to Herbal Safety.1
Appendix C provides a reference table for HDIs, together with explanatory information. The table is designed to be accurate and responsible and is based on a critical assessment of the clinical relevance of the available information. Entries are mainly drawn from case reports and clinical studies. In addition, the various relevant monographs typically contain a more complete discussion of some of the issues summarised in the appendix.
Cases of contention
False alarms?
In 1999 a team of scientists at Loma Linda University School of Medicine in California undertook studies to analyse the effects of some popular herbs on the fertilisation process and on sperm DNA.46 They used hamster eggs with their outer coating (zona pellucida) removed, exposed them first to the herbs for 1 h and then to human sperm. In a separate test, human sperm cells were incubated with the herbs for 7 days and then tested for viability and the intactness of their DNA. The coding sequence for an important gene in the sperm was also evaluated as a test for mutagenic activity.
But there is an equally important consideration that the authors failed to take into account. Many pharmacological screening programmes using in vitro tests on herbs, such as the programme of the National Cancer Institute in the USA, first remove interfering compounds like tannins. Tannins, which are relatively common in herbs, can bind non-specifically to proteins such as enzymes, effectively producing false positive results. In addition, tannins have very low bioavailability, so any observed non-specific activity on intracellular enzymes is likely to be little more than a scientific curiosity. This consideration was well demonstrated by an elegant but perhaps ultimately futile study published in the Journal of Ethnopharmacology.47 Owen and Johns found that the inhibitory activity of 26 plants on the enzyme xanthine oxidase was positively correlated with their tannin content (p<0.001).
Perhaps the most bizarre episode of a herbal false alarm was the reported occurrence of the toxic alkaloid colchicine in Ginkgo biloba. A group of US scientists investigating natural anti-inflammatory substances in pooled placental blood found a compound present that they identified as colchicine.48 Naturally the scientists were curious as to the origins of this plant chemical, so they decided to examine individual blood samples from 24 pregnant women. Only five of these contained colchicine and the group reported that all of the colchicine-containing blood samples came from women who used herbal supplements. For some undisclosed reason they next tested Ginkgo and Echinacea products from local retail outlets and found that only Ginkgo contained significant levels of colchicine (26 µg per tablet). The authors concluded that due to its potential harmful effects, it would appear that Ginkgo biloba should be avoided by women who are pregnant or are trying to conceive.
Colchicine is a drug mainly used to treat acute gout and was originally isolated from the autumn crocus (Colchicum autumnale). It is highly toxic and the effective dose is quite close to a toxic dose. Toxic effects include nausea, vomiting and bone marrow suppression.49 Colchicine inhibits normal cell division (mitosis) and is linked to Down’s syndrome. Clearly, it is contraindicated in pregnancy.50
• Were the five women with the colchicine levels taking Ginkgo?
• If not, what herbs were they taking?
• Why would a pregnant woman wish to take Ginkgo?
• Why choose just Echinacea and Ginkgo for analysis?
• How many Ginkgo products were tested?
Questions about the research from a scientific perspective might include:
• Is it known that Ginkgo contains colchicine?
• Can we expect, from a phytochemical perspective, that Ginkgo should contain colchicine?
• How conclusively was colchicine identified in Ginkgo?
• Could the levels of ‘colchicine’ in Ginkgo account for the levels found in placental blood?
• Are these levels of ‘colchicine’ in placental blood toxic? If so, what effects were observed on the mother and child?
In fact colchicine has never before been reported in Ginkgo and a comprehensive literature search by the late Professor Farnsworth at the University of Chicago found no evidence of colchicine in Ginkgo.51 According to Professor Farnsworth: ‘Based on biogenetic considerations, colchicine should never be found outside of the Monocotyledoneae (Araceae, Liliaceae) and the report of its occurrence in Saussurea sacra (Asteraceae) is an anomaly that has not been duplicated by other reports on the chemistry of this species. Thus, colchicine has never been reported as a normal constituent of Ginkgo biloba nor would it be expected or predicted to be present’.
Farnsworth also questioned the scientific validity of the study and the editorial process it underwent prior to publication. ‘Anyone who thinks that colchicine can be found naturally in Ginkgo is not qualified to be a peer reviewer of this paper,’ he said, referring to the editorial process for scientific journals in which papers are reviewed by independent experts to determine their scientific merit and the accuracy of their conclusions prior to publication. Schwabe, the German company that researched and developed Ginkgo extract, tested three separate samples of Ginkgo leaf and failed to find any colchicine. This finding was backed up by industry testing in the USA.51
In response to the study, the Australian Therapeutic Goods Administration tested five Ginkgo biloba products. Colchicine was not found in any (detection limit 1 µg/g). Interestingly, using a method similar to the study in question, a substance was found in the Ginkgo products which had similar analytical properties to colchicine. Although its identity was not determined, further analysis demonstrated conclusively that it was not colchicine.52
Were the levels of colchicine in Ginkgo responsible for those reportedly observed in the pregnant women? The scientists claimed to have found 49 to 763 µg/L of colchicine in the placental blood of the women allegedly taking herbal supplements.48 They also claimed to have found 26 µg of colchicine per Ginkgo tablet. In a multiple dose study of the pharmacokinetics of colchicine, 1 mg/day achieved plasma concentrations in human subjects in the range of 0.3 to 2.5 µg/L.53 Hence to achieve a level of 49 µg/L of colchicine (the lowest value in the reported range) a person would need to consume around 50 mg of colchicine per day. Since each Ginkgo tablet contains only 26 µg, this equates to around 2000 Ginkgo tablet per day. In their defence, the authors claimed that placental tissue is known to concentrate ingredients from the mother’s blood. But even assuming a concentration factor of 50, this is still 40 tablets per day to achieve the lowest reported concentration of colchicine, when the normal dose is typically 3 to 4 tablets per day.
Perhaps most astounding of all, the reported levels of colchicine would have been lethal to the unborn children. Several cases involving suicide by the ingestion of colchicine tablets have been reported in the literature. In one case, the plasma level of colchicine 24 h after ingestion was 4.5 µg/L,54 in another the femoral blood level was 62 µg/L.55Colchicum autumnale is the richest plant source of colchicine. Yet a case report of a man who consumed 17.1 g of flowers found that his maximum colchicine level was just 4.34 µg/L, which occurred 13 h after ingestion of the flowers.56 Nonetheless he was hospitalised with nausea, vomiting and abdominal pain.
But perhaps best of all was the quote attributed in London’s Daily Mail to a UK professor working in the field of complementary medicine who was quoted as saying that the alleged Ginkgo health risk was ‘a disaster waiting to happen’ and likened it to ‘another catastrophe like thalidomide’. Despite criticism from the herbal industry, the scientists and journal editor involved in the publication of the study were reported to be unrepentant.57
On April 10, 2003, an Australian newspaper (the Brisbane Courier-Mail)58 ran a brief article relating concerns raised by recent research that the herb black cohosh may increase the toxicity of two chemotherapy drugs. The article went on to say: ‘Many women diagnosed with breast cancer use the herb as an alternative to hormone replacement therapy’. Similar alarmist reports of this research were also carried in the US media.
The article was in response to a press release of research findings that were to be presented at the American Association for Cancer Research meeting in Toronto, but the event was cancelled due to concerns over SARS in that city.59 Notwithstanding the lack of an appropriate forum for the presentation and discussion of their findings, the authors pressed ahead and released their findings via the media.
Inaccuracies regarding the true risks of HDIs have already been discussed. However, it is worth noting the alarmist issues raised in a recent paper published in the Journal of the American College of Cardiology.60 As one example, concerns over alfalfa and fenugreek interacting with warfarin were expressed; these are not supported by sound science. To phytochemists, the term ‘coumarin’ means plant chemicals based on the coumarin structure. To pharmacists the term ‘coumarin’ means anticoagulant drugs derived from or related to coumarin phytochemicals. There is no solid evidence that normal plant coumarins have anticoagulant activity (see Chapter 2).
The authors claimed green tea interacts with warfarin purely because it is a leafy material (and hence contains vitamin K). Saw palmetto was linked to bleeding and Echinacea to causing arrhythmias, but there is no sound evidence to suggest any such problems with either herb (see the relevant monographs). The irony is that the authors complained about public misinformation about herbs! Despite a detailed and well-credentialled response from the American Botanical Council pointing out the many inaccuracies,61 the journal was unrepentant.
It appears there is indeed a media bias in the reporting of herbal studies. In late November 2008, a study investigating the print media coverage of clinical trials was published in the open access journal BMC Medicine.62 Public health researchers from Canada investigated the nature and tone of newspaper reporting of clinical trials of herbal medicines compared with reporting of clinical trials of drugs used to treat the same medical conditions. Databases were searched for newspaper articles in the UK and Ireland, the US, Australia/New Zealand and Canada covering the period from January 1995 to June 2005. The clinical trials were identified and sourced. The study was limited to newspaper articles that were directly related to peer-reviewed clinical trials.
Poisonous food
Many ordinary foods naturally contain poisonous constituents:
• Wheat, rye and barley contain the protein gluten (or one that is gluten-like) that is hydrolysed in the digestive system to yield the peptide alpha-gliadin, a well-established and occasionally dangerous intestinal irritant that has caused many thousands of deaths around the world through coeliac disease and sprue.
• Apple seeds and the kernels of apricots, plums and other stone fruits, as well as bitter almonds, contain significant quantities of glycosides that yield cyanide on hydrolysis in the digestive tract.
• The cabbage family contain glucosinolates that yield toxic nitriles and goitrogenic thiocyanates.
• The oil from rapeseed, widely grown in temperate climates as a cheap vegetable oil, can contain erucic acid, which is known to cause heart damage in experimental animals.63
• Potatoes are members of the deadly nightshade family; when the tuber turns green under the influence of light it produces the same poisonous alkaloids.
• Many common household pulses, including soya bean, red kidney bean and haricot bean (as in baked beans), contain toxic lectins called phytohaemagglutinins as well as trypsin inhibitors, that can only reliably be neutralised by boiling for at least 30 minutes.
The nocebo effect
What is much less debated are negative placebo effects. In reports on most double blind clinical trials there are accounts of adverse effects among the placebo group. The symptoms listed cover a wide range and are not necessarily short-term and transient. In one report of studies of 1228 healthy volunteers the overall incidence of adverse events during placebo administration was 19%. Complaints were more frequent after repeated dosing (28%) and in elderly subjects (26%). Overall, the most frequent adverse events were headache (7%), drowsiness (5%) and asthenia (4%), with some variation depending on study design and populations.64 Pain can certainly be induced by placebo65 and placebo can also interact negatively with other medications.66
Unfortunately, placebo-controlled safety studies are not feasible but it will be helpful in assessing overall herbal safety to have some better understanding of the potential of the nocebo effect to confound the picture. For a more extensive discussion of this phenomenon, the reader is referred to The Essential Guide to Herbal Safety.1
An innocent bystander?
In some cases (perhaps many), an adverse effect attributed to a herb might only represent a coincidental association. One possible example is the hepatotoxic IDRs attributed to black cohosh. Research in several countries has found that one of the most serious causes of liver damage is unknown. Around about one-third of all liver transplants are due to a disorder known as idiopathic or non-A non-B hepatitis.67,68 The demographics of idiopathic hepatitis (female, late 30s to early 50s) and potential black cohosh use strongly overlap. Hence, there is a distinct possibility that some patients who develop idiopathic hepatitis might also coincidentally be taking black cohosh, given that this herb is now so popular. The herb could then be mistakenly attributed as the cause. Once one mistaken case is described in the literature, however poor its quality, it is likely that others will follow in a process akin to a self-fulfilling prophecy.
Poor-quality case reports might not only incorrectly attribute a harmful effect to a herb, but they can also hamper the value of case reports as red flags for genuine adverse events. One group recently developed an instrument for assessing the quality of case reports, based on a point-based rating scale incorporating 21 items yielding a total possible score of 42.69 A review was undertaken of adverse reports for herbal products over three periods, 1986 to 1988, 1996 to 1998 and 2006 to 2008. In total, 137 case reports were included. The percentage of high-quality case reports (scoring 29 or more) rose from 0% in 1986 to 1988, to 27.9% in 1996 to 1998 and 34.2% in 2006 to 2008. This study demonstrates that, while the quality of adverse herbal case reports is improving, there is still a long way to go.
Herbal safety: the arguments
A matter of debate
The doubts
• Herbal remedies are complex mixtures of chemicals, about whose effects on the body little is known even in their isolated state, let alone when mixed in infinitely variable ways.
• Chemical complexity can work in both directions, buffering against and towards potentiation of adverse effects.
• Traditional use is likely to have spotted only acute and relatively frequent adverse reactions; chronic, delayed or infrequent reactions would probably not have been associated with the herb.
• Traditional reputations are in any case highly unreliable in their transmission.
• It is possible to exceed modern standards of risk, say 1:1000, and still statistically mean that very few working practitioners will see the adverse events in their lifetimes (although this argument does not apply to consumer branded products selling in millions of units per annum).
• There are particularly heavy biases in the way of reporting adverse effects of herbal remedies at the present time (see below); therefore the current state of information is almost certainly understating the risk.
The reassurances
• The remedies used in herbal medicine represent only a tiny proportion of available plant species around the world. It is likely that humans through history moved inexorably to using those plants that were effective with a minimum of toxic or other adverse consequences.
• Even allowing for under-reporting of adverse events, levels in databases are remarkably low and certainly do not compare with the levels of iatrogenic problems in conventional medicine.
• Some benign qualities may arise from the very complexity of the plants, for which they are dismissed by conventional pharmacologists. The existence of tannins, mucilages, saponins or other constituents is likely to buffer or modulate the effect of more active constituents, which are often in any case present in only low levels.
• Most of the serious adverse events reported involve problems of product quality and adulteration (see elsewhere in this chapter). Attendance to pharmaceutical standards of quality assurance and quality control (as is the norm for European herbal medicinal products) and insisting on minimal training standards for those who prescribe herbal remedies will reduce risk.
• Most importantly, the thrust of treatment in phytotherapy is often different from that of conventional pharmacology. The herbs may better be understood as promoting healing responses in the body rather than directly targeting symptoms or pathology; this allows for a more elliptical, nutritional tilt at the body with consequent reduced negative impact. Certain herbs may be contraindicated in certain cases, not because they can cause side effects or threaten danger, but because they may simply be inappropriate for the job.
The impact of quality on safety
Pharmaceutical GMP
• validation of equipment and processes
• documented standard operating procedures covering every aspect of manufacture
• documented cleaning and calibration logs for equipment
• control of the manufacturing environment, air and water
• quarantining and unique identification and testing of raw materials, labels and packaging
• discrete batch identification
• comprehensive batch record documentation
• reconciliation of raw materials, product, packaging and labels
• quarantining and testing of finished products
• documented release-for-sale procedures
• testing of stability of finished product
• documentation of customer complaints and recall procedures.
• may be incorrectly identified
• may vary in chemical content and hence efficacy
• carries with it ‘a history’, for example it may be contaminated with unwanted substances
• processing of herbs may enhance or impair their safety and efficacy
As part of GMP, herbal raw materials are subjected to a battery of tests to ensure their quality and purity. These tests are outlined in Box 5.1. A useful guide to the British and European standards in these areas is provided by the British Herbal Pharmacopoeia 1996,70 together with the current British and European Pharmacopoeias and the United States Pharmacopeia – National Formulary.
Thin layer chromatography (TLC) is a particularly useful technique for the identification of plant material. It can also be used to quantify plant constituents. The process of performing TLC is outlined in Box 5.2.
Box 5.2 Thin layer chromatography
• An extract of a herb is spotted at the bottom of a thin layer of silica gel on a glass plate
• The plate is dipped in a solvent mixture so that the level of the solvent is below where the herb was applied
• The solvent draws up the layer and carries the phytochemical components in the herb extract different distances
• Sprays and/or ultraviolet light are used to view the components, giving a characteristic pattern of spots
• Each spot corresponds to a phytochemical in the herb
• Different solvent systems draw out different classes of phytochemicals in the herb
Finished herbal products also need to undergo testing before their release. Boxes 5.3 and 5.4 provide examples of possible testing protocols for finished products.
Microbial contamination
A. Herbal remedies to which boiling water has been added before use:
Substitution problems in herbal medicine
Contamination issues in herbal medicine
• products made in China and India contaminated with heavy metals (sometimes added intentionally) or potentially pathogenic microorganisms
• products made in China contaminated with conventional drugs
• contamination of a safe herb with a toxic herb, for example Digitalis (foxglove leaves) found among Arctium lappa (burdock) leaves.
As mentioned above, one persistent quality problem that can dramatically impact on safety is the adulteration of herbal products with undeclared conventional drugs. Examples of the adulteration of Chinese herbal medicines with synthetic drugs are provided in two reviews.71,72 A wide variety of agents including corticosteroids, non-steroidal anti-inflammatory drugs, analgesics, benzodiazepines, anticonvulsants and hypoglycaemic drugs have been found.
Cases of adrenal suppression have been linked to the intake of Chinese herbal products. A Taiwanese study found that 8 of 13 patients with severe illness and low cortisol levels reported using herbal products.73 Two cases of adrenal suppression were reported in New Zealand and attributed to the intake of the Chinese herbal product Shen Loon.74 The product was later found to contain the corticosteroid betamethasone, although the authors suggested other factors could be involved as well.
Undeclared codeine was detected in a Chinese antiasthmatic proprietary product.75 One phenomenon which has fortunately received a high degree of media attention is the adulteration of Chinese weight loss products with banned weight loss drugs such as fenfluramine. This has led to toxic reactions or fatalities in the UK, Singapore, Japan and China.76–79
This problem is still current, with the US FDA recently issuing a recall notice on a herbal slimming capsule contaminated with sibutramine, a controlled substance that was withdrawn from the US market in October 2010 for safety reasons.80
A particularly cautionary tale is that of PC-SPES. PC-SPES was a herbal formulation specifically targeted at the treatment of prostate cancer (hence PC) developed and patented in the early 1990s by a research chemist.81 It ostensibly contained seven Chinese herbs and one American herb (saw palmetto: Serenoa repens). The product was very successful in the US marketplace and there were consistent anecdotal accounts of its efficacy, especially for controlling PSA (prostate specific antigen) levels. In particular, naturopathic physicians and holistic medical doctors recommended the product to many patients. Being a US-sponsored product they had no reason to believe it was anything other than a herbal product, despite the fact that it was manufactured in China.
PC-SPES soon began to attract the interest of well-respected research scientists including those at Johns Hopkins School of Medicine, Harvard Medical School and the National Center for Complementary and Alternative Medicine.81 In 2002, three reviews of the use of PC-SPES were published which surveyed the major publications on its pharmacology and clinical activity.82–84 By early 2002 there were 116 published clinical and laboratory-based studies of PC-SPES.85
The reviews highlighted the significant in vitro and in vivo activities of PC-SPES in prostate cancer models and noted the small number of positive but preliminary clinical trials.82–84 Oestrogenic activity was confirmed for the product, which was consistent with some of the side effects exhibited by patients taking the formulation such as gynaecomastia, loss of libido, decrease in body hair and superficial thrombosis. However, there was also another worrying and paradoxical side effect of PC-SPES: it was linked to severe bleeding in one case86 and suspected of potentiating the effects of warfarin in another.87
Another perspective on PC-SPES began to emerge in 2002 at around the same time the above reviews were published. In early April a group of scientists presented their findings to the 93rd Meeting of the American Association for Cancer Research that PC-SPES samples from 1996 to 2001 contained the oestrogenic drug diethylstilbestrol as well as warfarin and indomethacin.88 Their comprehensive results were published later that year.89 Earlier in February 2002, the FDA alerted consumers to stop taking PC-SPES because the California Department of Health Services had detected the presence of warfarin. Another product called SPES from the same corporation had been found to contain the anti-anxiety drug alprazolam.90 The manufacturer undertook a voluntary recall and PC-SPES was subsequently withdrawn from the market.81
Resolving the safety issue: pharmacovigilance initiatives
Postmarketing surveillance
Given that so few herbal extracts have ever been submitted to extensive preclinical studies, the toxicological information about herbal remedies is generally sketchy. In European legislation the principle has been accepted that drugs already widely used in the population need not go back to the laboratory for new toxicological studies. Instead, those supplying such remedies are under increasing burden to establish safety on an ongoing basis; postmarketing surveillance or pharmacovigilance are terms generally used to describe this monitoring process. There is an overwhelming mathematical case for systematising observations of adverse reactions. Statisticians have calculated that if, for example, an adverse event occurred in as many as 1 in 1000 cases, one would need to see 3000 cases to have a 95% probability of spotting one.91 This ‘law of three’ makes the individual practitioner of little use in identifying even substantial risks.
In the cases where analysis of the data has been done, reports of adverse effects from human consumption of herbs appear to be relatively infrequent, making up around 1% of spontaneous reporting.92 There is a widespread concern among the regulatory authorities, however, that these cases represent only a small proportion of such incidents. Spontaneous reporting for even conventional prescribed drugs is an inefficient mechanism, with reporting rates among physicians as low as a few per cent of actual cases.93 In the case of herbal remedies, so many of which are self-prescribed or are prescribed by non-doctors, there is much less opportunity for doctors to become aware of this factor in adverse events. Reporting mechanisms for non-doctors are rarely in place and even pharmacists have only variable opportunities or tradition for notifying adverse events. Even where reporting is possible, there are other reasons why spontaneous notification is likely to be particularly ineffective for herbal remedies (Box 5.5).
Box 5.5 Problems affecting the reporting of ADRs involving herbal remedies
There may be fewer adverse drug reactions (ADRs) for herbal remedies due to the following:
• Lethargy (general reporting rates for all drugs are very poor)
• Physicians are usually unaware of natural product consumption
• Physicians do not routinely ask about non-prescribed medications
• Common bias against associating adverse effects with natural medicines
• Ignorance of reporting mechanisms by potential non-physician notifiers
• Users reluctant to talk to professionals if self-prescribed treatment goes wrong
• Potential notifier’s reluctance to get involved in ‘the system’
• Potential notifier’s reluctance to risk losing a favourite remedy
• Uncertainty of connections in multiple ingredient prescriptions
On the other hand the herbal sector can claim that uncontrolled observations of adverse events are likely to be misleading for the case of herbal safety, and even the regulators accept that there are particular problems (Box 5.6).
Box 5.6 Perceived problems in applying ADRs to assessing the safety of herbal remedies
Adverse drug reaction (ADR) reporting is not sensitive to herbal use. It does not easily:
• detect adverse reactions that are:
• pick out causative agents from multiple ingredients
• disentangle multiple/multicausal pathologies most often seen in chronic diseases.
There is a wider lack of context in which to place herbal ADRs, in particular concerning:
In this context it is an encouraging development that most countries now include herbal adverse reaction reporting in their respective databases, often with a formal requirement on manufacturers to report their knowledge of such events to the relevant statutory authority. Such adverse reaction data from government or WHO databases that have occasionally been drawn on for safety information regarding individual herbs and, where available, in published form, are included in the relevant monograph in Section Three.
In addition, studies are published from time to time that examine the totality of case reports on a specific database. One relatively recent example is from Sweden that drew on adverse reactions submitted to the Swedish Medicinal Products Agency between 1987 and 2006.94 Among a total of 64 493 reports, 778 concerned 967 suspected adverse reactions related to 175 complementary medicine products. Of these 967 adverse reactions, the most reported substance was Echinacea purpurea singly (8.1%) and in combination (7.3%), with Ginkgo biloba leaf next at 6.7%. In 221 reports, at least one reaction was categorised as serious, the frequency of these being pulmonary embolism (1.7%), liver reactions (2.8%) and anaphylactic reaction (2%).
For a detailed discussion of the pharmacovigilance of herbal medicines, see Chapter 11 of The Essential Guide to Herbal Safety.1
References
3. Violon C. Belgian (Chinese herb) nephropathy: why? J Pharm Belg. 1997 1993;52(1):7–27.
8. US Food and Drug Administration. Listing of Botanical Ingredients of Concern. US Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Nutritional Products, Labeling, and Dietary Supplements. Revised April 9, 2001. Available online. <http://www.fda.gov/Food/DietarySupplements/Alerts/ucm095283.htm> Accessed 15.06.11.
10. Contaminated Chinese Herbs Recalled. Associated Press; 2001.
12. Mills SY. The A–Z of Modern Herbalism. London: Thorsons, 1989. pp. 190–191
14. Harvey J, Colin-Jones DG. Mistletoe hepatitis. Br Med J (Clin Res Ed). 1981;282(6259):186–187.
21. Shaw D. Toxicological risks of Chinese herbs. Planta Med. 2010;76(17):2012–2018.
34. Mokhobo KP. Herb use and necrodegenerative hepatitis. S Afr Med J. 1976;50(28):1096–1099.
42. Finkel RS, Zarlengo KM. Blue cohosh and perinatal stroke. N Engl J Med. 2004;351(3):302–303.
49. Hood RL. Colchicine poisoning. J Emerg Med. 1994;12(2):171–177.
52. CMEC 29 Complementary Medicines Evaluation Committee. Extracted Ratified Minutes Twenty Ninth Meeting 24 September 2001 Ginkgo and colchicine. <http://www.tga.gov.au/pdf/archive/cmec-minutes-29.pdf>.
57. Borman S. Toxin reported in supplements. Chem Eng News. 2001;79(33):33–34.
58. [No author listed] Health risk. The Courier-Mail Thursday, 10, 2003; 4.
59. Health – Reuters. Black cohosh may make breast cancer drug more toxic. Yahoo! News, April 7, 2003. Available. <http://www.cancerpage.com/news/article.asp?id=5746> Accessed 15.06.11.
77. Koo E. Chinese medicine appeal outweighs diet pill worry. Reuters August 29, 2002. Available online. <http://www.manilatimes.net/national/2002/aug/29/opinion/20020829opi6.html>.
78. Shimbun Y. Chinese diet aids kill 1, sicken 11. Daily Yomiuri Online July 12, 2002. Available online. <http://www.yomirui.co.jp/newse/20020712wo21.htm>.
79. New diet pill blamed for at least 1 death in China. <http://www.voanews.com/english/news/a-13-a-2002-07-15-13-New-67433567.html>. Accessed 15.06.11.
80. US FDA, Public Notification: ‘Slim Xtreme Herbal Slimming Capsule’ Contains Undeclared Drug Ingredient. <http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/MedicationHealthFraud/ucm254905.htm>. Accessed 14.06.11.
81. Ochs R. Going to bat for prostate cancer herbs. Newsday.com October 8, 2002. <http://www.newsday.com/going-to-bat-for-prostate-cancer-herbs-1.344309>. Accessed 14.06.11.
82. Oh WK, Small EJ. PC-SPES and prostate cancer. Urol Clin North Am. 2002;29(1):59–66.
87. Davis NB, Nahlik L, Vogelzang NJ. Does PC-SPES interact with warfarin? J Urol. 2002;167(4):1793.
90. Reuters Health. Prostate herbals contain prescription drugs: FDA. February 8, 2002. Available online. <http://abcnews.go.com/wire/Living/reuters20020208_458.html>. Accessed 14.02.02.
91. De Smet PAGM. An introduction to herbal pharmacoepidemiology. J Ethnopharmacol. 1993;38:197–208.