Chapter 13 Optic Nerve Diseases
Diseases of the optic nerve are difficult to assess, as tissue diagnosis is usually unavailable. Therefore, various subjective functional tests, such as visual acuity, visual fields and color testing; and objective tests, such as afferent pupillary defect or electrophysiologic evaluation are relied upon to achieve a differential diagnosis. Imaging modalities such as magnetic resonance (MR) imaging and computerized tomography (CT) are utilized to further clarify the diagnosis. Ultrasonographic imaging provides a readily accessible and inexpensive means for diagnosing and monitoring optic nerve disorders.1
Technique
The details of ophthalmic ultrasonographic technique were described elsewhere (Chapter 3). The basic B-scan imaging technique used for the globe is the same for the optic disc and retrobulbar optic nerve.2,3 Specialized A- scan techniques, are used for measuring and evaluating the retrobulbar optic nerve.
Normal retrobulbar optic nerve measurements
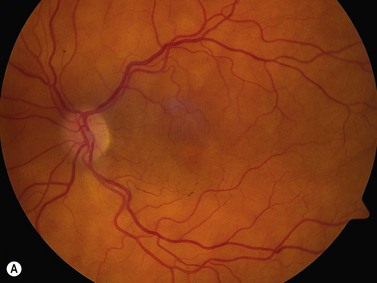
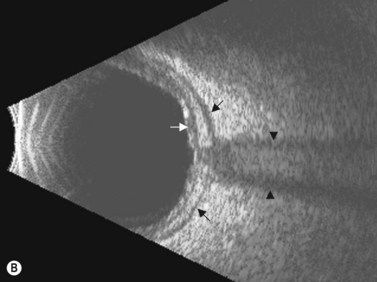
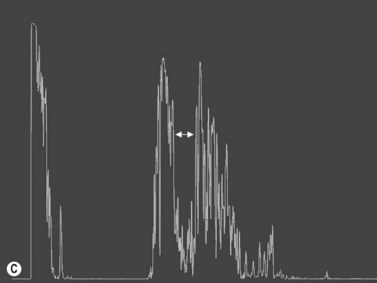
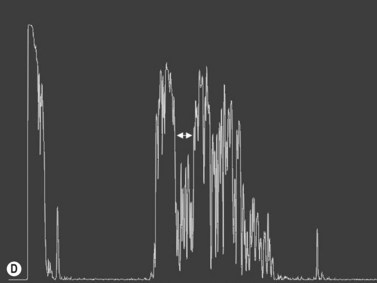
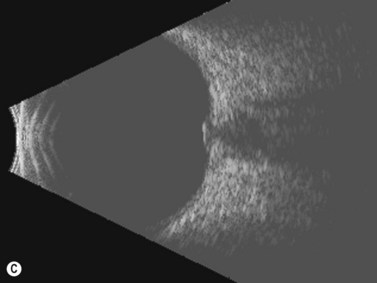
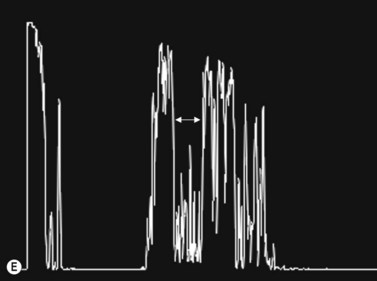
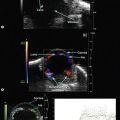
The optic nerves are usually symmetric and measure the same thickness both anteriorly and posteriorly.4 Utilizing ultrasound the optic nerve sheath diameter is measured in two locations, 3 mm posterior to the optic nerve head and as close as possible to the orbital apex (Figure 13.1). Normal retrobulbar optic nerves in an adult measured at the optic nerve sheath are 2.2 to 3.3 mm in diameter.5 Significant variation, can occur in the general population, and it is advised to compare the diameters in both eyes. A difference of 0.5 mm between eyes frequently indicates an abnormal thickness in one eye and should raise suspicion for optic nerve pathology (Figure 13.2).6 There are no significant changes in optic nerve sheath diameter when measured in the Trendelenburg or reverse Trendelenburg position as compared with the supine position in healthy adults.7
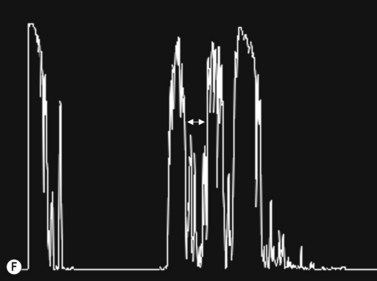
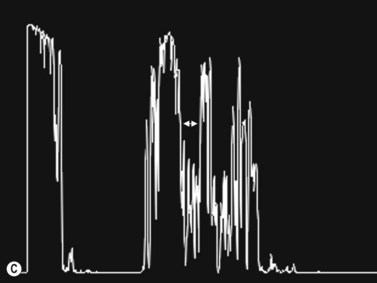
30° test
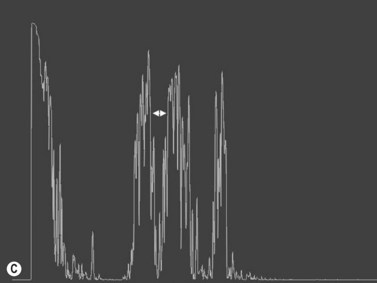
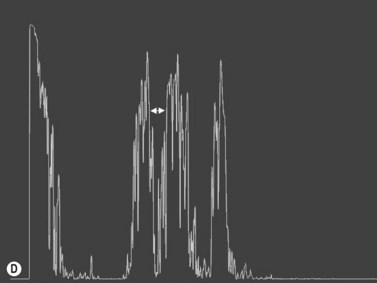
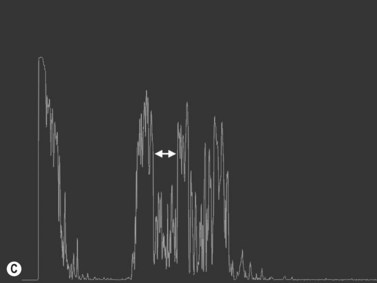
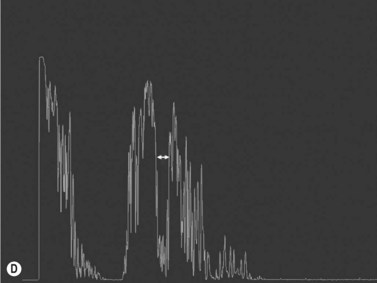
Increased subarachnoid fluid can be differentiated from thickening of the parenchyma or perineural sheaths with a 30° test.8 The patient fixates in primary gaze (straight ahead position), and the optic nerve perineural sheaths are measured anteriorly and posteriorly. The patient’s gaze is then directed 30° laterally, and the perineural sheaths are measured again. The test is based on the premise that when the eye is fixated laterally, the optic nerve sheaths are stretched and the subarachnoid fluid is spread over a larger area. A decrease in sheath diameter of greater than 10% in lateral gaze, as compared with primary gaze, is considered a positive 30° test and thus indicative of increased subarachnoid fluid.
Papilledema
Adults
Blaivas5 performed a prospective observational study on emergency department patients suspected of having elevated intracranial pressure from various intracranial disorders.6 He correlated ultrasonographic measurements of optic nerve sheath diameter with CT scan findings. All cases exhibiting a midline shift of 3 mm or more, collapse of the third ventricle, hydrocephalus, abnormal mesencephalic cisterns, or sulcal effacement were considered to have raised intracranial pressure.6 Such patients were predicted correctly by optic nerve sheath diameters of greater than 5 mm. The ultrasonographic measurements were correlated more closely to the intracranial pathology than the clinical examination. Mean optic nerve sheath diameter for those not meeting CT criteria for raised intracranial pressure was 4.4 mm. Several studies have also shown the efficacy of optic nerve diameter for following hospitalized patients with increased intracranial pressure, regardless of the underlying etiology.6,9–11
Kimberly correlated optic nerve sheath diameters (measured by operators blinded to the measurements) with directly measured intracranial pressure in patients who had invasive intracranial monitors placed as part of their treatment.12 In an adult, optic nerve sheath diameters greater than 5 mm correlated with intracranial pressure greater than 20 cmH2O, with sensitivity of 88% and a specificity of 93%.
Trauma
CT scan is the gold standard for evaluating acute head trauma. This, however, is not always available or feasible in the immediate evaluation of trauma. Ophthalmic ultrasonography, combining B-scan and use of the 30° test, can provide a means to triage those who may need surgical intervention for acute increased intracranial pressure (Figure 13.3).
In a year-long, prospective, blinded observational study, Goel evaluated 100 consecutive head trauma patients who presented at an emergency department of a tertiary care institution.13 Their median Glasgow Coma Scale score was 11. Optic nerve sheath diameter using a 7.5 MHz probe was compared with CT scan of the head for evaluation of acute intracranial pressure elevation. Optic nerve sheath diameter of greater than 5 mm was considered to be indicative of increased intracranial pressure. The mean optic nerve sheath diameter in those without increased intracranial pressure was 3.5 ± 0.75 mm. Of 74 patients who had an optic nerve sheath diameter of greater than 5 mm, 59 patients had intracranial hematoma requiring urgent surgical evacuation. Overall, ultrasonography yielded a sensitivity of 98.6% and a specificity of 92.8% for detection of elevated intracranial pressure in this situation.
In another study, Karakitsos observed that optic nerve diameter greater than 5.9 mm at presentation and an increase in nerve diameter of 2.5 mm between repeated measurements was associated with a poor clinical prognosis. Optic nerve monitoring, had a low predictive value for brain death.14
Children
The normal range of optic nerve diameter has also been established for infants and children up to 15 years of age.2,3,10,15,16 In a prospective study of nerve measurements in 102 children admitted to the hospital for diagnoses related to orbital or intracranial disease, but not increased intracranial pressure, range of measured optic nerve sheath diameter was 2.2–4.3 mm (mean 3.08 mm).1 Analysis revealed that the most rapid change in nerve sheath diameter occurred over the first 2 months of life. There was no significant difference between right and left eyes or between the sexes. Analysis of the results found that a sheath diameter of greater than 4 mm in infants under 1 year of age and of greater than 4.5 mm in children age 1 to 15 years should be considered abnormal. Similar normative data have been observed by others.15,16 In those over 15 years of age, mean diameter of greater than 5 mm is considered abnormal and correlates with an intracranial pressure of greater than 20 cmH2O.
Malyeri performed a case-controlled study of 456 hospitalized children between the ages of 1 and 13 years.15 Half of the subjects had normal intracranial pressure, while the other half had a confirmed increased intracranial pressure. In the group with increased intracranial pressure, mean optic nerve diameters were 5.6 ± 0.6 mm (range, 4.4–7.6 mm). In another study of optic nerve sheath diameters and children who had shunted hydrocephalus, those who had functioning ventriculoperitoneal shunts had a mean optic nerve sheath diameter of 2.9 ± 0.5 mm, compared with diameters of 5.6 ± 0.6 mm in those who had shunt malfunction and increased intracranial pressure.
Optic disc drusen
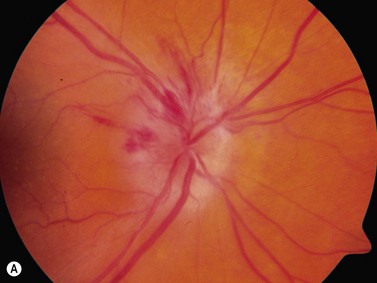
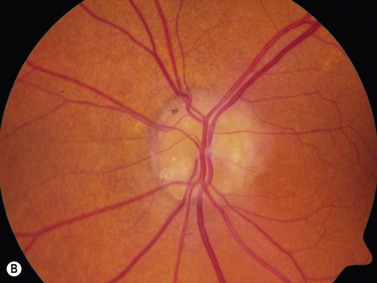
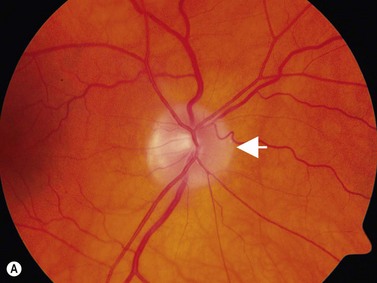
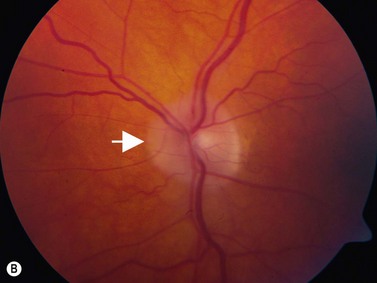
The most common cause of pseudopapilledema is optic disc drusen. Drusen comes from the German word describing small crystals found in spaces in rock substrates. In Caucasian populations, they occur with an incidence of between 0.34% and 2%.17,18 Bilateral optic nerve drusen are observed in 75–86% of patients.19,20 No reliable prevalence data is available for those of non-Caucasian heritage where drusen are observed infrequently. The predisposing factors for drusen formation are unclear. Theories of drusen formation include mechanical obstruction to axonal transport in eyes with small scleral canals, abnormal axonal metabolism, and leakage from abnormal vasculature at the disk head.19,20 Triggers for calcification or the length of time required for its initiation are also poorly understood.21
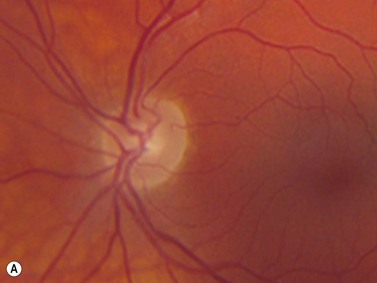
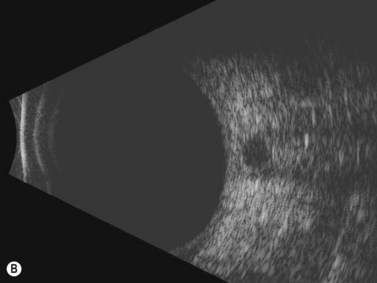
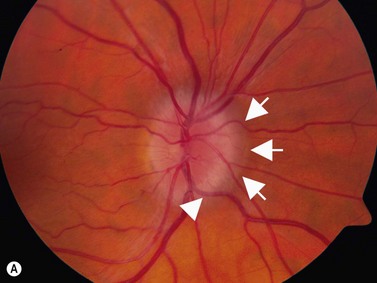
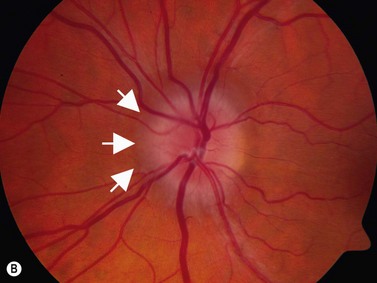
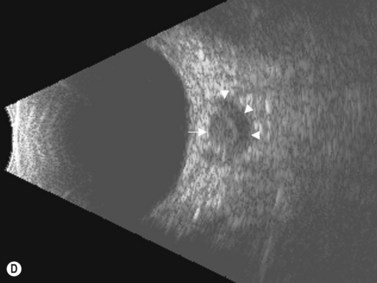
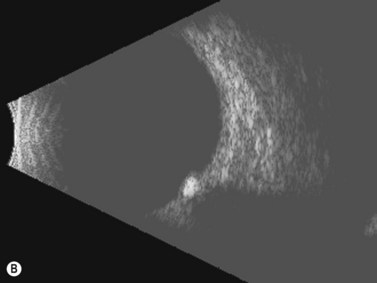
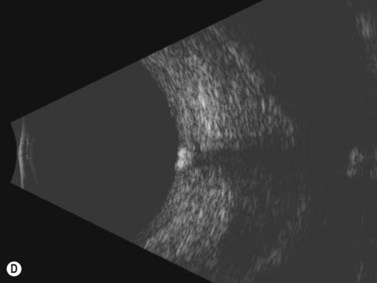
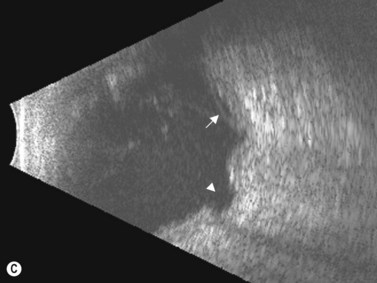
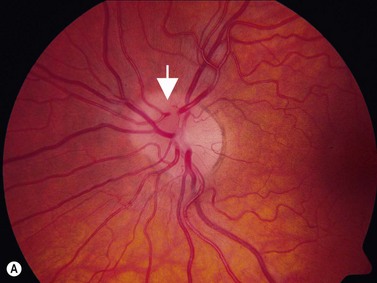
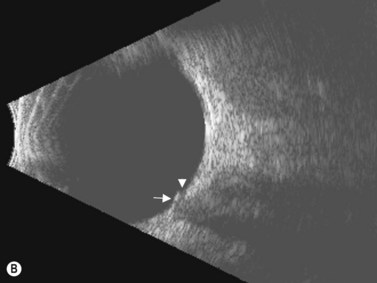
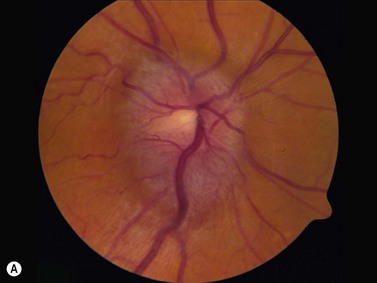
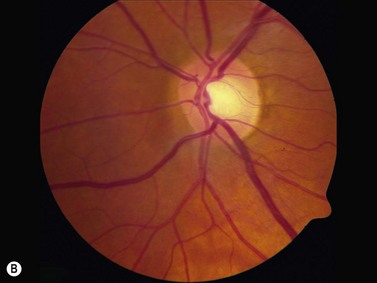
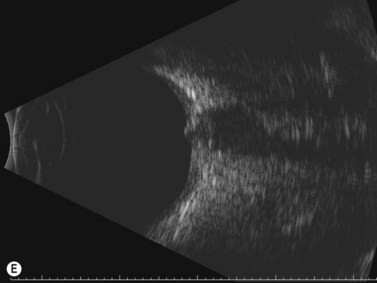
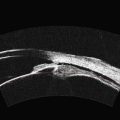
In an adult patient, ophthalmoscopy usually can identify optic disc drusen either by the characteristic scalloped appearance of the nerve edge or the presence of refractile calcified particles on the nerve. These tend to predominate in the nasal portion of the nerve. Red free fundus photography highlights these refractile particles. Sometimes the optic nerve head drusen are buried, and cannot be seen with ophthalmoscopy. The optic disc can appear very similar to elevated disks due to papilledema. In these cases, B-scan ultrasound of the nerve head can be used to clearly identify calcified drusen, making CT scan an unnecessary expense (Figure 13.4). Calcified drusen appear as acoustically bright well-demarcated areas even in low gain settings. Less calcified drusen may be visible as well-demarcated shadows on high gain settings.
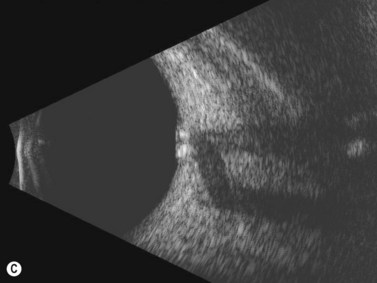
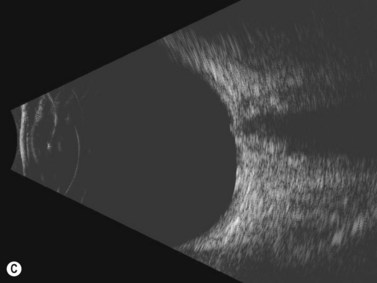
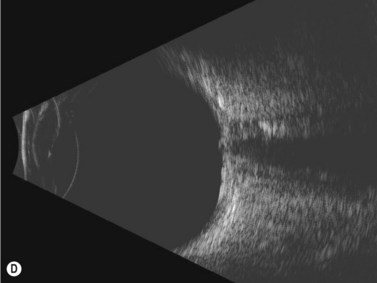
Disk drusen create a congested optic nerve, increasing the risk of anterior ischemic optic neuropathy (AION). In the acute setting of this disease, not only does the patient present with a typical history of vision loss, but the ultrasound findings clearly show disk edema distinguishable from the underlying calcified drusen (Figure 13.5).20
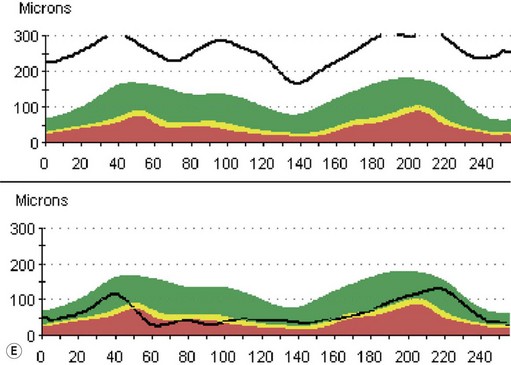
On optical coherent tomography, eyes with buried drusen can have a decreased retinal nerve fiber layer thickness (Figure 13.5).22 It has been noted in young adults that buried drusen are less likely to be associated with visual field defects than is papilledema.23,24 Absence of the clinical findings listed, normal ultrasonographic appearance of the optic nerve head, and a negative 30° test for nerve sheath distention go against the diagnosis of true papilledema.25
Congenital disk anomalies
Optic disc coloboma
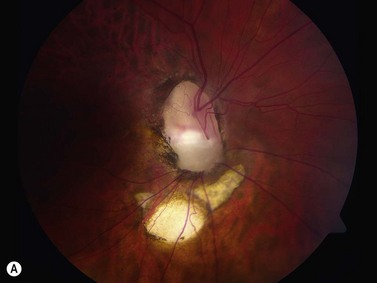
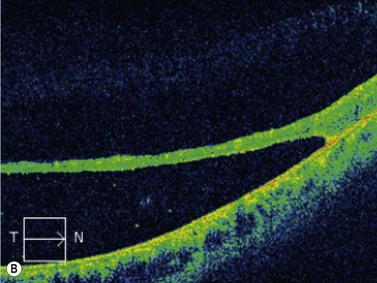
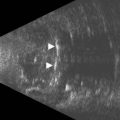
Coloboma of the optic disc appear as a white bowl-shaped excavation that is decentered inferiorly in an enlarged optic disc. They result from incomplete or abnormal closure of the embryonic optic fissure (Chapter 14). Minimal peripapillary pigmentation is present. Occurrence is split equally between unilateral and bilateral cases. Visual function can be decreased mildly or severely depending on involvement of the papillomacular bundle. Optic disc coloboma are seen in conjunction with other systemic anomalies such as Aicardi’s or Goldenhars’ syndromes, and have been linked to a mutation of PAX6.2 B-scan clearly delineates the inferior location of the excavations and also may demonstrate the small diameter of the associated optic nerve (Figure 13.6).
Morning glory disk anomaly
Morning glory disk is a congenital anomaly characterized by a funnel-shaped excavation of the optic nerve surrounded by a wide annulus of choriopapillary depigmentation. It is associated with intracranial abnormalities including agenesis of the corpus callosum and basal encephalocele. There is an associated increased frequency of retinal detachment adjacent to the optic disc. Ultrasonography B-scan is useful to confirm the centralized location of the depression in morning glory disk as compared with the asymmetric inferiorly located depression found in coloboma.26,27
Tilted optic disc
High myopia often is associated with tilted optic discs, which may appear to have an elevated nasal rim. Cup-to-disk ratio cannot be evaluated adequately because of the oblique view of the nerve head on ophthalmoscopy. This may raise suspicion of the presence of optic disc edema. In an asymptomatic patient without dyschromatopsia or an afferent pupillary defect, ultrasonographic studies should be obtained. Ultrasonography and OCT of the disk reveal a normal nerve fiber layer thickness (Figure 13.7). In conjunction with a negative 30° test the patients can be followed clinically without the need for further imaging or lumbar puncture.
Pseudodoubling of the optic disc
Pseudodoubling of the optic disc is a rare clinical occurrence associated with chorioretinal coloboma. In this entity, there is the appearance of two optic nerves because of the presence of focal and anomalous vascular anastomoses between the choroidal and retinal circulations adjacent to the coloboma (Figure 13.8). B-scan ultrasound can image the coloboma and rule out other retrobulbar optic nerve pathology. Vascular channels can be delineated further using color Doppler imaging.28
Retrobulbar optic nerve lesions
Unilateral optic nerve elevation in a patient with gradually progressive painless visual loss or blur can be initially evaluated with ultrasound. Asymmetric optic nerve sheath diameters should arouse suspicion of retrobulbar lesions of the optic nerve. Glioma, meningioma, circumpapillary choroidal melanoma,29 and demyelinating optic neuritis30 have been examined ultrasonographically (Figure 13.9). Asymmetry of retrobulbar optic nerve diameter greater than 0.5 mm should raise suspicion of pathology.
Ultrasound can aid in assessing the need for and type of further diagnostic testing indicated. Measurements can provide a rapid, inexpensive adjunct to neuroimaging in following response to treatment. Sudan presented optic nerve cysticercosis in which a cystic lesion was present just posterior to the globe an ultrasonography B-scan.31 Following treatment, involution of the cyst could be observed ultrasonographically.
Gaze-evoked amaurosis
Gaze-evoked amaurosis is a transient blurring of vision associated with movement of the eye. It usually is caused by an underlying posterior orbital mass that compresses the optic nerve and its blood supply in extremes of gaze. Gaze-evoked amaurosis may also be caused by vitreopapillary traction at the optic nerve.23 Ultrasonography has demonstrated elevation of the nerve head associated with partial posterior vitreous detachment in which persistent vitreopapillary attachments were present. The authors postulated that traction upon eye movement was transmitted to the nerve fibers in the papilla, causing phosphenes followed by gaze-evoked amaurosis.
Orbital trauma
Avulsion of the optic nerve on B-scan appears as a hypolucent area in the region of the optic nerve head that may be associated with a defect in the posterior sclera. This can aid in planning potential intervention in instances of orbital trauma associated with poor visualization of the fundus caused by hemorrhage (Chapter 16).32
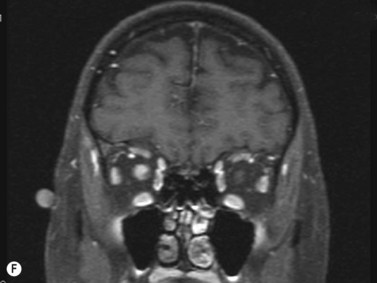
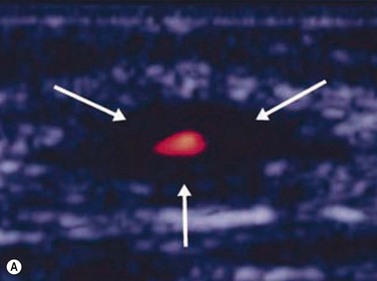
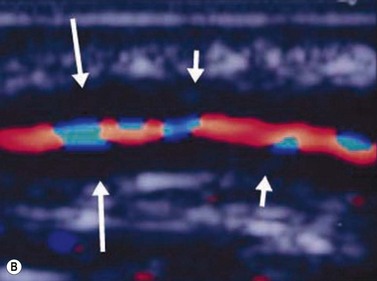
Giant cell arteritis
An ultrasound of the optic nerve is not directly useful in the diagnosis of vision loss because of anterior ischemic optic neuropathy. Ultrasonography may be useful to evaluate inflammation within the superficial temporal artery, associated with this disease.33 Correlation of histologic findings with ultrasonography of the superficial temporal artery; in 36 patients presenting with signs and symptoms suggestive of giant cell arteritis, a dark halo around the lumen of the temporal arteries bilaterally was revealed, corresponding with positive biopsy results (Figure 13.10).34 Equivocal or negative ultrasonography results, however, were found in patients with both positive and negative biopsies and were not associated with ocular disease. Further study in larger numbers of patients is needed to establish definitive guidelines about the need for biopsy. Treatment decisions remain based on clinical symptoms, serology and temporal artery biopsy.
1 Lystad LD, Hayden BC, Singh AD. Optic nerve disorders. Ultrasound Clin. 2008;3(2):257-266.
2 Ballantyne J, Hollman AS, Hamilton R, et al. Transorbital optic nerve sheath ultrasonography in normal children. Clin Radiol. 1999;54(11):740-742.
3 Hewick SA, Fairhead AC, Culy JC, et al. A comparison of 10 MHz and 20 MHz ultrasound probes in imaging the eye and orbit. Br J Ophthalmol. 2004;88(4):551-555.
4 Byrne S, Green R. Ultrasound of the Eye and Orbit, 2nd ed. St Louis: Mosby; 2002.
5 Gans MS, Byrne SF, Glaser JS. Standardized A-scan echography in optic nerve disease. Arch Ophthalmol. 1987;105(9):1232-1236.
6 Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003;10(4):376-381.
7 Romagnuolo L, Tayal V, Tomaszewski C, et al. Optic nerve sheath diameter does not change with patient position. Am J Emerg Med. 2005;23(5):686-688.
8 Ossoinig K, Cennamo G, Byrne S. Echographic differential diagnosis of optic nerve lesions. In: Thijssen JM, Verbeek AM, editors. Ultrasonography in Ophthalmology. Dordrecht: Dr. W Junk, 1981.
9 Girisgin AS, Kalkan E, Kocak S, et al. The role of optic nerve ultrasonography in the diagnosis of elevated intracranial pressure. Emerg Med J. 2007;24(4):251-254.
10 Tayal VS, Neulander M, Norton HJ, et al. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49(4):508-514.
11 Tsung JW, Blaivas M, Cooper A, et al. A rapid noninvasive method of detecting elevated intracranial pressure using bedside ocular ultrasound: application to 3 cases of head trauma in the pediatric emergency department. Pediatr Emerg Care. 2005;21(2):94-98.
12 Kimberly HH, Shah S, Marill K, et al. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15(2):2012-2014.
13 Goel RS, Goyal NK, Dharap SB, et al. Utility of optic nerve ultrasonography in head injury. Injury. 2008;39(5):519-524.
14 Karakitsos D, Soldatos T, Gouliamos A, et al. Transorbital sonographic monitoring of optic nerve diameter in patients with severe brain injury. Transplant Proc. 2006;38(10):3700-3706.
15 Malayeri AA, Bavarian S, Mehdizadeh M. Sonographic evaluation of optic nerve diameter in children with raised intracranial pressure. J Ultrasound Med. 2005;24(2):143-147.
16 Newman WD, Hollman AS, Dutton GN, et al. Measurement of optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol. 2002;86(10):1109-1113.
17 Boyce SW, Platia EV, Green WR. Drusen of the optic nerve head. Ann Ophthalmol. 1978;10(6):695-704.
18 Lorentzen SE. Drusen of the optic disc. A clinical and genetic study. Acta Ophthalmol (Copenh). 1966;90(Suppl):1-180.
19 Davis PL, Jay WM. Optic nerve head drusen. Semin Ophthalmol. Dec 2003;18(4):222-242.
20 Brodsky M. Pseudopapilledema. In: Miller N, Newman N, editors. Walsh and Hoyt’s Clinical Neuro-ophthalmology. Philadelphia, Lippincott: Williams & Wilkins, 2005.
21 Mustonen E. Pseudopapilloedema with and without verified optic disc drusen. A clinical analysis II: visual fields. Acta Ophthalmol (Copenh). 1983;61(6):1057-1066.
22 Katz BJ, Pomeranz HD. Visual field defects and retinal nerve fiber layer defects in eyes with buried optic nerve drusen. Am J Ophthalmol. 2006;141(2):248-253.
23 Katz B, Hoyt WF. Gaze-evoked amaurosis from vitreopapillary traction. Am J Ophthalmol. 2005;139(4):631-637.
24 Wilkins JM, Pomeranz HD. Visual manifestations of visible and buried optic disc drusen. J Neuroophthalmol. 2004;24(2):125-129.
25 Friedman AH, Beckerman B, Gold DH, et al. Drusen of the optic disc. Surv Ophthalmol. 1977;21(5):373-390.
26 Harasymowycz P, Chevrette L, Decarie JC, et al. Morning glory syndrome: clinical, computerized tomographic, and ultrasonographic findings. J Pediatr Ophthalmol Strabismus. 2005;42(5):290-295.
27 Deb N, Das R, Roy IS. Bilateral morning glory disc anomaly. Ind J Ophthalmol. 2003;51(2):182-183.
28 Cellini M, Alessandrini A, Bernabini B, et al. Pseudodoubling of the optic disc: a colour Doppler imaging study. Ophthalmologica. 2003;217(5):370-372.
29 Shields CL, Santos MC, Shields JA, et al. Extraocular extension of unrecognized choroidal melanoma simulating a primary optic nerve tumor: report of two cases. Ophthalmology. 1999;106(7):1349-1352.
30 Titlic M, Erceg I, Kovacevic T, et al. The correlation of changes of the optic nerve diameter in the acute retrobulbar neuritis with the brain changes in multiple sclerosis. Coll Antropol. 2005;29(2):633-636.
31 Sudan R, Muralidhar R, Sharma P. Optic nerve cysticercosis: case report and review of current management. Orbit. 2005;24(2):159-162.
32 Simsek T, Simsek E, Ilhan B, et al. Traumatic optic nerve avulsion. J Pediatr Ophthalmol Strabismus. 2006;43(6):367-369.
33 Schmidt D, Hetzel A, Reinhard M, et al. Comparison between color duplex ultrasonography and histology of the temporal artery in cranial arteritis (giant cell arteritis). Eur J Med Res. 2003;8(1):1-7.
34 Reinhard M, Schmidt D, Hetzel A. Color-coded sonography in suspected temporal arteritis-experiences after 83 cases. Rheumatol Int. 2004;24(6):340-346.