32 Ophthalmology
Many ophthalmologic diagnoses can be confirmed only by the ophthalmologist examining a cooperative infant or child. An examination under anesthesia (EUA) often is essential for an accurate diagnosis and evaluation of many processes, including trauma, tumors, infiltrative diseases, coloboma, glaucoma and other vascular diseases of the retina, Coats disease, and incontinentia pigmenti. Inpatient preterm infants often require serial EUAs to monitor the development and progress of retinopathy of prematurity (ROP) and the response of the disease to surgery. These examinations may be performed in the neonatal intensive care unit or operating room and may require sedation or general anesthesia.1 Other inpatient trauma victims may require serial EUAs to monitor the development of glaucoma or retinal injury. Serial EUAs are also necessary to monitor progress during outpatient retinoblastoma radiation treatments. The anesthesiologist is essential to the provision of pediatric ophthalmologic diagnostic and therapeutic techniques.
The child in need of ophthalmologic surgery typically requires general anesthesia or deep sedation rather than exclusive use of local or regional anesthesia. Many infants and children cannot cooperate for anything beyond a brief eye examination. Although the ophthalmologist may be able to tolerate small movements by the child during an EUA, unnecessary head or eye globe movement during an ophthalmologic procedure should be prevented. Retrobulbar block is sometimes performed for postoperative analgesia in children before emergence from general anesthesia2; all complications associated with a retrobulbar block identified in adults may also occur in children.3,4
Preoperative Evaluation
The perioperative environment should be welcoming to the child and family.5,6 All team members should be comfortable with the anesthesia considerations for infants and children for ophthalmologic procedures.6 Anticipation and prevention of postoperative nausea and vomiting (PONV) and understanding of anesthesia emergence and postoperative analgesia are essential.
Before an elective ophthalmologic procedure, the patient undergoes a thorough physical examination, including a review of all systems, and a complete medical history is obtained, including a surgical and anesthesia history, list of current medications, known allergies, and family history.7 Because many ophthalmologic diagnoses are commonly associated with systemic conditions, all implications of the systemic illness are a concern for the anesthesiologist.
Common Ophthalmologic Diagnoses Requiring Surgery
Common pediatric diagnoses and surgical procedures are listed in Table 32-1. A diagnosis may exist in isolation or be one aspect of a more complex group of diagnoses. Many involve systemic illnesses, and the anesthesiologist should be familiar with the implications of ophthalmologic disease in these settings.
TABLE 32-1 Common Ophthalmologic Procedures in Children
Communication and flexibility are mandatory in anesthesia planning. Anesthesia may start with a plan for a brief EUA using an inhalational anesthetic with a facemask and no intravenous catheter. However, if corrective surgery becomes necessary, airway control may require placement of a laryngeal mask airway (LMA) or tracheal tube. Intravenous access is required if the airway is instrumented to administer medications, including those to prevent or treat the oculocardiac reflex (OCR), postoperative pain, and nausea and vomiting.8
For procedures of brief or intermediate duration, the use of an LMA allows excellent access to all periorbital structures and provides good airway control in most circumstances. Compared with mask anesthesia, the LMA has the advantage of decreasing environmental contamination by inhalational anesthetics. It is relatively easy to insert and remains secure while avoiding the need for the anesthesiologist to hold a facemask near the surgical site. Compared with the tracheal tube, the LMA does not increase the heart rate, blood pressure, and intraocular pressure (IOP) to the same degree.9
Some of the more common ophthalmologic presentations and associated systemic illnesses or syndromes are listed in Table 32-2. Some systemic conditions have significant cardiorespiratory or central nervous system implications for perioperative management, and they should be fully evaluated before anesthesia (see Chapter 4). Many procedures are performed on preterm infants or formerly preterm infants, and prematurity and its complications have some of the most clinically important implications for anesthesia management.
TABLE 32-2 Ophthalmologic Conditions Associated with Systemic Syndromes and Illnesses
| Syndrome or Illness | Ophthalmologic Conditions |
|---|---|
| Fetal alcohol syndrome | Strabismus, optic nerve hypoplasia |
| Galactosemia | Neonatal cataracts |
| Mucopolysaccharidoses | Corneal involvement; may require transplantation |
| Retinitis pigmentosa | Heart block |
| Sturge-Weber syndrome | Glaucoma |
| Prematurity | Retinopathy of prematurity, strabismus |
| Fabry disease | Whorled corneal opacities |
| Tay-Sachs disease | Cherry-red macular spot |
| Osteogenesis imperfecta | Blue sclerae |
| Craniofacial syndromes (e.g., Crouzon, Apert, Pfeiffer) | Proptosis, strabismus, glaucoma |
Ophthalmologic Conditions Associated with Systemic Disorders
Prematurity
The preterm infant may present for many surgical procedures early in life. ROP, congenital cataracts, and glaucoma may require surgery even when the infant weighs less than 1000 g. The preterm infant may have significant systemic illnesses. Common complications of prematurity include acute and chronic pulmonary disease,10 respiratory failure and pulmonary hypertension, congenital heart disease (unrepaired or with a limited palliative repair), and intraventricular hemorrhage,11 with or without obstructive hydrocephalus.
Acutely ill preterm infants and those younger than 1 year of age are at greater risk than older children and adults for perioperative complications.12 Careful attention to airway management, assisted ventilation, and titration of oxygen therapy with specified goals are essential for success.13 In preterm infants whose airways are already intubated and whose lungs are ventilated mechanically, the anesthesiologist should confirm the position of the tube, transport the infant safely to the operating room, and limit the exposure to high concentrations of oxygen. Although institutional goals are not uniform regarding supplemental oxygen therapy,14 communication with the neonatal team is often helpful in gauging the infant’s previous oxygen requirement and current targeted goals (e.g., hemoglobin-oxygen saturation levels of 90% to 95%). Because most inhalational anesthetics impair hypoxic pulmonary vasoconstriction, a greater fraction of inspired oxygen (Fio2) may be necessary to maintain the targeted hemoglobin saturation.
Infants with extremely low birth weight require many weeks to grow and develop to a weight of approximately 1800 g and to maintain normothermia without special environmental control. These infants commonly have a history of short-term or intermediate-term assisted ventilation and may not require supplemental oxygen before an EUA or planned operative procedure for ROP.15 Many of these infants undergo ophthalmologic examinations while their lungs are ventilated in the neonatal intensive care unit.16 During surgical therapy (i.e., laser or cryosurgical stabilization) for ROP, these infants require anesthesia to provide optimal conditions (Fig. 32-1). Perioperative apnea may preclude tracheal extubation or require close postoperative monitoring after anesthesia.
Perioperative apnea in the preterm infant is widely described.17,18 Whether the child is still hospitalized or presenting for elective surgery as an outpatient, the preoperative assessment should determine the pattern and frequency of apnea before the planned surgical procedure and anesthesia. If the child has been discharged, the current use of respiratory stimulants (i.e., caffeine or theophylline) and oxygen should be determined. Is an apnea monitor being used, or has it been discontinued? Guidelines have not been developed to manage some of the scenarios, but infants who continue to require supplemental oxygen, who are younger than 60 weeks postconceptional age, or who are monitored for apnea or bradycardia should have continuous cardiorespiratory and oxygen saturation monitoring postoperatively for at least 12 hours or until they are apnea free (see Chapter 4). The risk of apnea after general anesthesia and sedation decreases with advancing gestational age at birth and with advancing postnatal-postconceptual age. The risk of apnea is independent of opioid use; its multifactorial origins include the presence of general and neuraxial anesthetics and the immature central nervous system and respiratory center in the preterm infant. Flexible planning for possible postoperative ventilatory support is essential, and families should be informed of this possibility preoperatively.
Although chronic lung disease due to prematurity is prevalent, its intensity has been significantly reduced with the routine use of surfactant and advances in ventilator management strategies. Long-lasting respiratory effects from prematurity may include reactive airway disease, subglottic stenosis from prolonged intubation, and alveolar or interstitial disease with an oxygen requirement lasting weeks to years.10 Because many anesthetics impair hypoxic pulmonary vasoconstriction, an increased oxygen requirement in the perioperative period should be anticipated. Tracheal intubation, a light level of anesthesia, or topical use of β-adrenergic antagonists may exacerbate reactive airway disease, requiring further treatment to reduce air trapping and hypercarbia.
Along with assessing for airway and alveolar diseases, the anesthesiologist should determine whether pulmonary hypertension or right ventricular dysfunction is or was present.19 Some infants may be receiving continuing oxygen therapy as treatment for pulmonary hypertension and to reduce intermittent episodes of hypoxemia due to crying or while sleeping. If pulmonary hypertension was diagnosed previously, an updated evaluation is warranted. Because pulmonary hypertension is exacerbated by hypoxia and hypercarbia, tracheal intubation should be considered to ensure control of ventilation and oxygenation. At emergence, pulmonary hypertension may be exacerbated as hypercapnia develops, causing physiologic or anatomic shunting with systemic hemoglobin-oxygen desaturation. Immediate and continued evaluation of the airway is imperative to rule out an independent respiratory contribution to the systemic hypoxia.
Congenital cardiac disease may be diagnosed in the preterm neonate, infant, or child who presents for ophthalmologic procedures. A patent ductus arteriosus may not close spontaneously or after administration of cyclooxygenase inhibitors. This may lead to persistent congestive failure, reduced pulmonary compliance, and complications of fluid management. Congenital cardiac anomalies require assessment before elective surgery. The various complex congenital cardiac lesions have a wide spectrum of interactions with multiple anesthetic agents.20 Many cardiac conditions require surgery or palliation (e.g., systemic-to-pulmonary shunts) before elective ophthalmologic procedures. Correction of congenital heart disease is limited by the difficulty of using cardiopulmonary bypass in infants weighing less than 2 kg. There may be an urgent need for ophthalmologic evaluation (e.g., congenital tumor, cataract, glaucoma) before repair of the congenital cardiac condition. Preoperative consultation with the infant’s pediatric cardiologist can provide useful information on the infant’s current ventricular function and the risk of dysrhythmias associated with cardiac defects. Medical management must be optimized before undertaking anesthesia and surgery.
Intraventricular hemorrhage is a major source of morbidity and mortality for preterm infants.11 Obstructed hydrocephalus may occur and require cerebrospinal fluid diversion procedures to decompress the obstructed ventricular system and treat the associated increased intracranial pressure. Many of these infants require ophthalmologic surgery for repair of strabismus due to a neurologic insult. If a ventriculoperitoneal shunt is in place, its proper function should be determined by direct evaluation. The anesthesiologist should assess whether there is inappropriate macrocephaly or a bulging or tense fontanelle. Obstructed hydrocephalus may occur after the infant’s discharge from hospital, even though a ventriculoperitoneal shunt was not required previously. The preoperative assessment should include the child’s developmental and neurologic status at the time of surgery. Because intraventricular hemorrhage is associated with long-term morbidity, any history of seizures should be elicited, and the antiepileptic drugs being used should be documented.
Preterm and small infants rapidly lose heat when anesthetized. Prevention of hypothermia is essential in the perioperative environment. Hypothermia can decrease metabolism of most drugs and depresses respiratory drive in preterm infants (see Chapters 35 and 36).
Down Syndrome
Children with Down syndrome (i.e., trisomy 21) frequently present for ophthalmologic surgery because of associated pathologic processes such as neonatal cataracts, significant refractive errors (e.g., hypermetropia, astigmatism), strabismus, glaucoma, keratoconus, nasolacrimal duct obstruction, and nystagmus.21–23 Infants with trisomy 21 should have an ophthalmologic evaluation in the neonatal period. If this requires an EUA, the anesthesiologist should be prepared for the extensive medical implications associated with trisomy 21.24,25 Almost half of these infants are born with congenital heart disease, including septal defects, complete or partial atrioventricular canal, tetralogy of Fallot, transposition of the great arteries, and valvular insufficiency or stenosis. Any child with a left-to-right shunt may develop pulmonary hypertension, and children with trisomy 21 develop irreversible pulmonary hypertension at an earlier age. Bradycardia (25% to 60%) and hypotension (12% to 73%) have been reported during sevoflurane anesthesia in these children.26,27 Complete understanding of the child’s cardiac defects is essential to planning anesthesia (see Chapters 14, 16, and 21).
Airway abnormalities such as narrowed nasopharyngeal passages, macroglossia, pharyngeal hypotonia, and subglottic stenosis are frequently observed in children with trisomy 21. These abnormalities may contribute to development of chronic intermittent hypoxia, further exacerbating pulmonary hypertension, and these children should be expected to demonstrate exacerbations of airway obstruction and hypoxia after general anesthesia.28
Children with trisomy 21 have a wide spectrum of developmental delays. Cervical spine instability occurs, and occiput-C1 and C1-2 instabilities have been described.29,30 Subluxation of the cervical spine has rarely been reported in these children during anesthesia. Nonetheless, the anesthesiologist and the surgeon should avoid extremes of neck flexion and extension and lateral rotation during head positioning for laryngoscopy and surgery. While the child is awake, the range of motion of the neck in flexion and extension should be assessed, along with complaints of numbness or tingling in the hands or feet in a particular position. Previous spine and neck investigations or operations should be reviewed. These infants and children may have ligamentous laxity of the cervical spine and other locations. No consensus exists for the radiologic workup of children who are asymptomatic for cervical disease, although many children have been evaluated radiologically before about 5 years of age.
Children with trisomy 21 may be born with congenital hypothyroidism, or it may develop at any time during their life span. They may develop junctional bradycardia during sevoflurane anesthesia.31 The bradycardia may be associated with or result from occult hypothyroidism. If the child is found to have a goiter on examination or has symptoms consistent with hypothyroidism (i.e., prolonged jaundice, hypothermia, constipation, dry skin, macroglossia, or relative bradycardia), thyroid function studies should be obtained before anesthesia for an elective procedure.
Alport Syndrome
Alport syndrome (i.e., progressive hereditary nephritis) is one of the disorders in a group of familial oculorenal syndromes. This classification includes Lowe (oculocerebral) syndrome and familial renal-retinal dystrophy. Alport syndrome involves sensorineural hearing loss, progressive renal disease, and multiple ophthalmologic disorders, including cataracts, retinal detachment, and keratoconus.32,33 Development of myopathy and renal failure constitutes the major anesthesia concern. If the patient has myopathy, it is prudent to avoid succinylcholine and the risk of a hyperkalemic response. Renal insufficiency may alter the choice of pharmacologic agents to very-short-acting agents or agents that are not renally excreted.
Marfan Syndrome, Ehlers-Danlos Syndrome, and Homocystinuria
Marfan syndrome is caused by a defect in the fibrillin 1 gene (FBN1), which affects elastic and nonelastic connective tissues. These children have an increased risk of retinal detachment, lens dislocation, glaucoma, and cataract formation (E-Fig. 32-1).34 They may have significant pulmonary (scoliosis) and cardiovascular problems,35 which may include aortic, mitral, or pulmonic valve insufficiency. Preoperative cardiovascular evaluation is indicated to determine the progression of cardiovascular abnormalities that inevitably occur. Blood pressure control is essential to prevent aortic dissection.
Homocystinuria has at least three forms and different inborn errors. Enzymatic deficiency of the metabolism of sulfur-containing amino acids causes the intermediate metabolite, homocysteine, to accumulate. These children suffer from cataracts, retinal degeneration, optic atrophy, glaucoma, and lens dislocation. The cardiovascular pathology includes coronary artery disease at a young age. Thromboembolic phenomena occur more frequently because the children may be hypercoagulable.36 Nitrous oxide should be avoided because it inhibits methionine synthase, further limiting the conversion of homocysteine to methionine.
At least 10 forms of Ehlers-Danlos syndrome have been described. Not all forms express significant ocular pathology. From the anesthesiologist’s perspective, positioning is important to avoid trauma to the skin because these children develop hemorrhages with minor trauma and experience delayed wound healing. A thorough preoperative assessment should be performed for cardiac lesions. Hypertension should be avoided to reduce the risk of rupturing an aneurysm. The duration of effect of local anesthetics in patients with class III Ehlers-Danlos syndrome may be less than that in normal patients, and contingency plans should be in place to address these possibilities, including retrobulbar block or general anesthesia.37
Mucopolysaccharidoses
The systemic complications of these disorders are sufficiently severe that even well-planned anesthesia management for ophthalmologic procedures may cause death.38 Airway management can be extremely difficult with poor mask fit, dynamic airway obstruction with narrowed passages, a floppy epiglottis, and difficulty visualizing the larynx.39 Infiltrative material may be deposited in the laryngeal inlet and pretracheal tissues; an LMA is particularly useful in maintaining a patent airway. Tracheal intubation may require the use of advanced airway management techniques, and the anesthesiologist should have several plans for airway management (see Chapter 12). Cardiac evaluation should be considered before an elective procedure to assess ventricular function and arrhythmias. Intravenous access may be difficult due to subcutaneous deposition of mucopolysaccharides.
Craniofacial Syndromes
Craniofacial syndromes may manifest as craniosynostosis or have only middle and lower facial structure involvement.40 Apert and Crouzon syndromes are both disorders of craniofacial development, but syndactyly occurs only in the former (E-Fig. 32-2). They share the potential for many ocular disorders, including severe proptosis, and mask airway management may be difficult.41 Other mutations of the fibroblast growth factor receptor 2 gene (FGFR2) cause Antley-Bixler and Pfeiffer syndromes. These children may develop chronic airway obstruction, and some have complete tracheal rings. Tracheal narrowing should be anticipated, and smaller tracheal tube sizes should be used.
Children with asymmetry of facial and mandibular bone growth may present with limited mouth opening. Children with Goldenhar syndrome (i.e., hemifacial microsomia), Treacher Collins syndrome, and Pierre Robin sequence can be expected to present a challenge to airway management (see Chapters 12 and 33).42 Children with craniosynostosis have an increased risk of congenital heart disease, and a cardiac evaluation should be performed before anesthesia.43 Neurologic morbidity and seizure disorders occur more frequently in this group of patients.
Phakomatoses
The phakomatoses are neurocutaneous syndromes with multiple ocular pathologic processes. These syndromes include neurofibromatosis,44,45 encephalotrigeminal angiomatosis (i.e., Sturge-Weber syndrome),46 tuberous sclerosis,47,48 incontinentia pigmenti, and ataxia telangiectasia. Central nervous system involvement varies with each of these diseases. Patients may have developmental delay, seizures, and significant neurologic morbidity. Anticonvulsant drugs should be continued in the perioperative period. Electrolyte and hepatic function studies should be done, and plasma levels of antiepileptic agents should be evaluated preoperatively.
Ophthalmologic Physiology
Intraocular Pressure
IOP is the pressure exerted by the internal components of the globe on the covering (i.e., sclera and conjunctiva). The normal IOP is 12 to 15 mm Hg. An IOP greater than 20 mm Hg is considered abnormal. Aqueous humor is a clear fluid that is secreted by the ciliary body and released into the anterior chamber of the globe. It traverses the anterior chamber and bathes the iris. It flows through the canal of Schlemm into the pores of Fontana and then drains into the episcleral veins (Fig. 32-2). The posterior chamber, which is larger than the anterior chamber, is composed of a gelatinous mix known as vitreous humor. The sclera and globe that encase the intraocular constituents are relatively noncompliant and are protected by the bony orbit. However, intraorbital masses may impinge on the globe and increase the relative IOP or alter the flow of aqueous humor, resulting in increased IOP. Any obstruction to the drainage of aqueous humor causes fluid to build up within the anterior chamber and increases IOP.49 Increased central venous pressure (e.g., Trendelenburg position, coughing, Valsalva maneuver, straining, increased intrathoracic pressure) attenuates the drainage of aqueous humor from the eye. Arterial pressure does not directly effect changes in IOP. However, as arterial blood pressure increases beyond the normal range, approximately 30% of the increase in systolic blood pressure is reflected in IOP increases. Aqueous humor formation is described in the following equation:
K is the coefficient of outflow, OPaq is the osmotic pressure of aqueous humor, OPpl is the osmotic pressure of plasma, and Pc is the capillary pressure. These variables allow calculation to intervene by increasing the plasma osmolality acutely with mannitol to lower the IOP. This increases the gradient of osmolality and draws water out of the aqueous humor, thereby reducing the IOP.
The effect of succinylcholine on IOP is well documented.50,51 Succinylcholine increases IOP 6 to 10 mm Hg, an effect that begins within 1 minute after administration and continues for up to 10 minutes, at which time the IOP returns to normal. This effect has been attributed to four possible mechanisms:
 Cycloplegia induced by succinylcholine, which obstructs the outflow of aqueous humor
Cycloplegia induced by succinylcholine, which obstructs the outflow of aqueous humor
 Tonic contraction of extraocular muscles
Tonic contraction of extraocular muscles
 Increased choroidal blood volume
Increased choroidal blood volume
 Relaxation of orbital muscles, which increases external pressure on the globe
Relaxation of orbital muscles, which increases external pressure on the globe
Specific muscles develop a sustained tonic tension after succinylcholine that may in the presence of a ruptured globe cause extrusion of intraocular contents.52 Extrusion in response to increased IOP depends on the diameter of the orifice; a smaller laceration (<2 mm) is less likely to facilitate extrusion of intraocular contents than a larger laceration (>4 mm). Pretreatment with a nondepolarizing agent (one-tenth the usual intubating dose) and paralysis with succinylcholine are still advocated by some anesthesiologists to minimize the risk of aspiration when dealing with an open globe injury. Alternatively, rocuronium (1.2 mg/kg given intravenously) can provide optimal intubating conditions in 30 seconds for the child with an open globe injury while minimizing the risk for aspiration or succinylcholine-associated increases in IOP.
Most general anesthetics decrease IOP,53 although ketamine has been shown to increase IOP in some studies and decrease it in others.54–56 When intravenous access is unavailable or the cardiovascular status of the patient warrants the use of ketamine, the child’s overall safety takes precedence over the possible ramifications of ketamine on IOP.
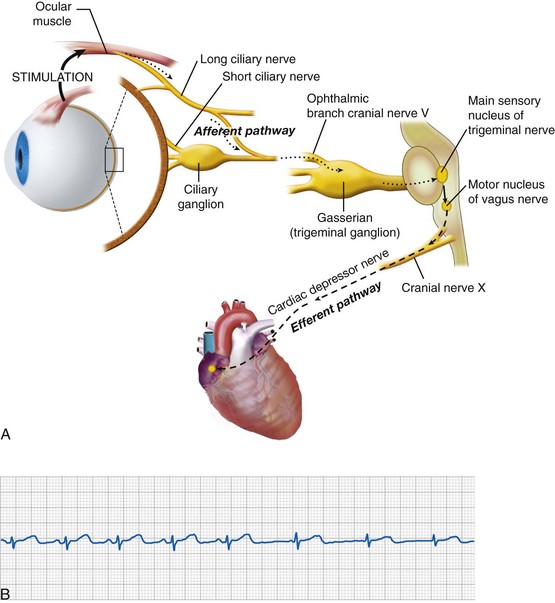
Oculocardiac Reflex
First described in 1908, the OCR is as a consequence of ophthalmologic surgery and other conditions.49 Traction on the extraocular muscles and levator (eyelid elevator) or external pressure applied to the globe triggers an afferent signal through the trigeminal nerve that activates parasympathetic output through the vagus nerve, resulting in many types of dysrhythmias (Fig. 32-3, A), which include sinus or junctional bradycardia, atrioventricular block, ventricular ectopy, and asystole (see Fig. 32-3, B). Retrobulbar block with local anesthetic may precipitate the trigeminovagal (oculocardiac) reflex due to external pressure sensed on the globe, and the local anesthetic may not completely prevent the OCR response to further surgical stimulation or manipulation. For this reason, an anticholinergic medication, such as atropine (20 µg/kg) or glycopyrrolate (10 to 20 µg/kg), is routinely given intravenously at induction of anesthesia or early thereafter. Although these medications do not preclude the occurrence of bradycardia, both decrease its intensity and duration. Because anticholinergics cause pupillary dilatation, they do not present a problem for the ophthalmologist, but they may slightly increase the IOP.
Other strategies to attenuate the OCR include topically applied lidocaine57 or intravenous ketamine.58 In a four-armed study, a ketamine-based anesthetic (10 to 12 mg/kg/hr) was associated with a decreased incidence of OCR compared with the three other anesthetic regimens: propofol/alfentanil, sevoflurane/nitrous oxide, or halothane/nitrous oxide.59 The prevalence of the OCR during sevoflurane and desflurane anesthesia was similar.60
Ophthalmologic Pharmacotherapeutics and Systemic Implications
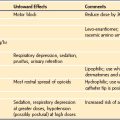
Topical ophthalmologic medications are usually placed directly on the cornea or in the inferior cul-de-sac. Most of these agents need many minutes to 1 hour for maximal effectiveness. Medications delivered topically to the eye are absorbed through the conjunctiva and nasal mucosa into the systemic circulation. When systemically absorbed, their pharmacologic mechanisms of action predict the systemic consequences. Some medications are diluted for use in children to reduce possible systemic toxicity. Anticholinergics, sympathomimetics, and antihistamines can cause pupillary dilation and decrease the movement of aqueous humor, increasing the IOP. Table 32-3 lists the more commonly used topical agents in pediatric ophthalmology.
| Drug | Indication | Side Effect Profile |
|---|---|---|
| Cholinergic Agonists | ||
| Carbachol | Induce miosis | Corneal edema, retinal detachment |
| Pilocarpine | Glaucoma | Corneal edema, retinal detachment |
| Cholinesterase Inhibitors | ||
| Physostigmine | Glaucoma | Retinal detachment, miosis |
| Echothiophate | Glaucoma | Retinal detachment, miosis |
| Muscarinic Antagonists | ||
| Atropine | Cycloplegic retinoscopy | Photosensitivity, blurred vision, increased heart rate, dry mouth |
| Scopolamine | Cycloplegic retinoscopy | Photosensitivity, blurred vision, increased heart rate, dry mouth |
| Homatropine | Cycloplegic retinoscopy | Photosensitivity, blurred vision, increased heart rate, dry mouth |
| Cyclopentolate | Cycloplegic retinoscopy | Photosensitivity, blurred vision, increased heart rate, dry mouth |
| Tropicamide | Cycloplegic retinoscopy | Photosensitivity, blurred vision, increased heart rate, dry mouth |
| Sympathomimetic Agents | ||
| Dipivefrin | Glaucoma | Photosensitivity, hypersensitivity |
| Epinephrine | Glaucoma | Photosensitivity, hypersensitivity |
| Phenylephrine | Mydriasis | Photosensitivity, hypersensitivity |
| Apraclonidine | Glaucoma | Photosensitivity, hypersensitivity |
| Brimonidine | Glaucoma | Photosensitivity, hypersensitivity |
| Cocaine | Local anesthetic | Anisocoria, corneal injury, photosensitivity, hypersensitivity |
| Hydroxyamphetamine | Glaucoma | Anisocoria, photosensitivity, hypersensitivity |
| Naphazoline | Decongestant | Photosensitivity, hypersensitivity |
| Tetrahydrozoline | Decongestant | Photosensitivity, hypersensitivity |
| α- and β-Adrenergic Antagonists | ||
| Dapiprazole (α) | Reverse mydriasis | Conjunctival hyperemia |
| Betaxolol (β1-selective) | Glaucoma | Bradycardia, hypotension |
| Carteolol (β) | Glaucoma | Decreased heart rate, blood pressure, bronchospasm |
| Levobunolol (β) | Glaucoma | Decreased heart rate, blood pressure, bronchospasm |
| Metipranolol (β) | Glaucoma | Decreased heart rate, blood pressure, bronchospasm |
| Timolol (β) | Glaucoma | Decreased heart rate, blood pressure, bronchospasm |
Modified from Brunton LL, Lazo, JS, Parker KL, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill; 2006.
Topical cycloplegic agents dilate the pupil, and these drugs eliminate lens accommodation by paralyzing the ciliary muscle. This effect aids in retinal evaluation with the indirect ophthalmoscope and scleral depression and in evaluating refractive errors for which the child is using accommodation for compensation. These agents are muscarinic cholinergic antagonists (i.e., antimuscarinic agents). Cyclopentolate hydrochloride (0.5%) can cause central nervous system toxicity with disorientation, seizures, and blurred vision. Atropine (0.5% to 2.0%) and scopolamine (0.25%) solutions are used for cycloplegia and pupil dilatation. Tropicamide (0.5% and 1.0%) is less commonly used in children. When systemically absorbed, these agents may result in antimuscarinic, anticholinergic toxicity, including tachycardia, dry mouth, pupillary dilation, flushing of the skin, heat intolerance or fever, and disorientation (E-Fig. 32-3).
A novel agent being studied in the treatment of retinal angiogenic diseases is bevacizumab (Avastin), which inhibits the formation of new blood vessels.61 In the doses prescribed, it should not be an anesthesia concern, but the visual outcome of any infant must be monitored for all perioperative care provided.
Emergent, Urgent, and Elective Procedures
One of the greatest controversies in pediatric anesthesia is defining the optimal technique to induce anesthesia in a child with an open globe injury and a full stomach.52 The issue of aspiration while securing the airway versus the possible extravasation of intraocular contents due to an increase in IOP is a difficult risk–benefit assessment. The debate is likely to continue because the risks are real and the incidence of either phenomenon is rare, difficult to study, and probably underreported.
Among adults, aspiration is an uncommon event with a small mortality rate. The incidence is less than 1 case of aspiration per 200,000 children, precluding an estimate of the mortality rate.62–65 The rapid-acting, depolarizing muscle relaxant succinylcholine, when used for a rapid-sequence induction in the treatment of a perforated globe, has been challenged because of its known propensity to increase IOP. Development of rapid-acting, nondepolarizing agents such as rocuronium has provided the anesthesiologist with alternatives to succinylcholine. High-dose rocuronium provides excellent intubating conditions66 and has the advantage of reducing IOP by blocking the neuromuscular junction of extraocular muscles. However, if the child is not adequately anesthetized and fully paralyzed during instrumentation of the airway, any episode of coughing, retching, or increased systolic blood pressure may increase IOP. Lidocaine (1 to 2 mg/kg given intravenously) usually attenuates the hemodynamic responses to laryngoscopy and tracheal intubation, but this is not a consistent experience.67,68 Pretreatment with opioids (0.05 to 0.15 µg/kg of sufentanil, 0.03 mg/kg of morphine, and 0.1 µg/kg of remifentanil) has been proposed to achieve a similar effect, although they are not consistently recommended. Although reported to blunt the IOP response to intubation, opioids may also induce vomiting, increasing the IOP.
 Intramuscular ketamine (and succinylcholine or rocuronium)
Intramuscular ketamine (and succinylcholine or rocuronium)
 Mask induction with sevoflurane or halothane
Mask induction with sevoflurane or halothane
An argument can be made for placement of an intraosseous needle for induction when intravenous access has not already been established. Even with local anesthesia, placement of an intraosseous needle is poorly tolerated by a conscious child. Mask induction with sevoflurane may facilitate induction of anesthesia, but if only a light plane of anesthesia is reached, coughing and vomiting may occur. The overpressure technique should be used to achieve a rapid and deep plane of anesthesia. Intravenous access should then be established. If this is unsuccessful, an intramuscular dose of muscle relaxant may be administered.69–71 In this circumstance, IOP may increase, and regurgitation remains a possibility. Rectal methohexital (30 mg/kg) is used only in infants; and if the child evacuates part of the dose, induction is incomplete.72 Because there is no reliable absorption of neuromuscular relaxant rectally, intramuscular or intravenous administration is necessary.
Induction and Maintenance of Anesthesia
Intravenous propofol, etomidate, or ketamine produce a smooth induction, and the anesthesiologist can then support and secure the airway as planned. Inhalational induction with oxygen, nitrous oxide, and sevoflurane or halothane also produces smooth induction states. If laryngospasm develops during induction, an intravenous or intramuscular neuromuscular relaxant or intravenous propofol (1 to 2 mg/kg given intravenously) should be administered promptly. Atropine (20 µg/kg) should be given intravenously or intramuscularly before bradycardia occurs. For planned tracheal intubation, an intravenous nondepolarizing muscle relaxant is preferred because succinylcholine may raise the IOP. Intramuscular administration of succinylcholine or rocuronium may also break laryngospasm if an intravenous catheter has not been placed.64–71,73–75
Alternative induction methods include intramuscular administration of ketamine (4 to 10 mg/kg) and, in infants, rectal methohexital (25 to 30 mg/kg). Ketamine may increase IOP, but it has been successfully used in ophthalmologic procedures in children.58 Rectal methohexital has a dependable latency to induction of general anesthesia of 7 to 8 minutes, but its elimination can vary widely, and its respiratory depressant effects may linger. Currently, rectal methohexital is infrequently used.
Anesthesia can be maintained with a variety of techniques. Inhalational sevoflurane, isoflurane, or desflurane may provide excellent conditions for maintenance of anesthesia60 and rapid emergence. Emergence from halothane anesthesia is slower. We communicate with the ophthalmologist and reinforce the need for an absolutely immobile field. When neuromuscular blockade is necessary, we routinely use nondepolarizing neuromuscular relaxants and train-of-four monitoring to ensure the adequacy of surgical conditions. Carefully titrated doses of opioids are used as an anesthetic adjunct if postoperative pain is anticipated.
The anesthetic and type of surgery affect the incidence of PONV. Strabismus surgery in children is associated with the greatest incidence (45% to 85%) of PONV.76 The type of ventilation does not attenuate the incidence of PONV.77 Fluid replacement has a significant effect on the risk of PONV. Avoiding opioids during strabismus surgery may also be effective.78,79 Nonopioid analgesics such as acetaminophen,80 diclofenac,81 and ketorolac78,79 may be used. If opioids are needed, short-acting medications are preferred: remifentanil, alfentanil, or fentanyl. Some evidence suggests that the more extraocular muscles that require surgery and specific muscles that require more traction (e.g., inferior oblique), the greater the incidence of PONV, although these data have not been firmly established.
A host of medications can significantly affect the risk of PONV.82 Preoperative use of benzodiazepines,83 avoidance of nitrous oxide,84 and superhydration with balanced salt solutions85; using of propofol,84,86 clonidine,87 5-HT3 receptor antagonists,88–92 dimenhydrinate,93,94 metoclopramide,92,95 or dexamethasone96–100; and delaying oral fluid ingestions after surgery101 attenuate the incidence of PONV. With clonidine and 5-HT3 receptor blockers, but not dexamethasone, there is a dose-response relationship with PONV.87,88,96 Older antiemetics such as droperidol that have been very effective in attenuating the incidence of PONV80,102–105 have fallen into disfavor because of their sedative side effects and concerns associated with prolonged QT intervals,106 although the latter has not been a common problem in children.107
Anesthesiologists have adopted a multimodal approach to PONV in the case of strabismus surgery. A commonly used regimen includes premedication with oral midazolam, choice of anesthesia, superhydration with intravenous fluid, and the combination of 5-HT3 receptor antagonists and dexamethasone. There is no evidence in children undergoing strabismus surgery that dosing 5-HT3 receptor antagonists at the end of surgery provides better protection against PONV than earlier in the surgery.108 However, dosing 5-HT3 receptor antagonists during emergence in children with congenital prolonged QT interval increases the risk of adverse events.109 There is a dichotomy of practice with respect to maintenance of anesthesia. Some avoid nitrous oxide, maintaining anesthesia by propofol infusion and including the previously mentioned supplementary medications. Others use nitrous oxide and an inhalational anesthetic for maintenance but also include the remainder of the preoperative and intraoperative supplementary medications.
Intravenous fluid replacement can reduce PONV.110 Children who present for elective surgery drink clear fluids up to 2 hours before surgery, reducing the fasting period. However, it is important to identify those who have fasted for a prolonged period and to administer sufficient intravenous fluids to reestablish euvolemia, avoiding the activation of antidiuretic hormone and aldosterone that may cause fluid retention. When using balanced salt solutions, children should receive 10 mL/kg/hr of the solution intravenously during the first 4 hours of surgery and recovery to reestablish euvolemia and to avoid activation of the antidiuretic hormone and aldosterone pathways. This should be followed postoperatively by a maintenance intravenous fluid infusion rate at one half of the previously considered rate, using 2 mL/kg/hr for the first 10 kg, 1 mL/kg/hr for the next 10 kg, and 0.5 mL/kg/hr for every kilogram greater than 20 kg until the child takes fluids by mouth.111–113 The incidence of PONV is reduced when large volumes of balanced salt solution (20 to 30 mL/kg) are administered compared with smaller volumes (10 mL/kg).85 When the surgical procedure is not likely to involve an increased IOP, we routinely replace almost 100% of the calculated deficit. We have not observed children to experience urinary retention or hypertension from this strategy. However, if the duration of surgery exceeds 3 hours, then we catheterize the bladder to reduce the risk of urinary retention and overdistention of the bladder.
At the completion of surgery, neuromuscular relaxation is antagonized, maintenance agents are discontinued, and the child is allowed to awaken. Deep extubation is preferred by some to reduce coughing and increases in IOP at the time of extubation.114 Others prefer extubating the trachea when the child is fully awake with intact airway reflexes, although coughing and increases in IOP may occur. For most procedures in children, a short interval of increased IOP does not damage a surgical correction, such as strabismus, ptosis, or ROP treatment. Appropriate postoperative analgesics may include local anesthetics, acetaminophen, and opioids. Nonsteroidal agents usually are not given to these children due to concerns about perioperative bleeding at the surgical site because of their mild anticoagulant profile, but ketorolac has been useful.78 Lorazepam may also have some utility.83 Postoperatively, withholding oral fluids until the child expresses a desire to drink reduces the incidence of PONV.101
Specific Ophthalmologic Procedures
Strabismus repair is common in children.91 Corrective surgery realigns the divergent visual axes of the eyes by detaching and reattaching extraocular muscles to the globes (Fig. 32-4). The procedures may be brief if only one or two muscles are involved. In infants, inhalational or intravenous induction is performed, and neuromuscular blockade is provided with nondepolarizing neuromuscular relaxants. Strabismus may be an isolated finding in a child or a manifestation of other systemic diseases or syndromes.115 The anesthesiologist should carefully review the birth history, history of prematurity, central nervous system disorders, syndrome identification, possible coexistent myopathy, and cardiovascular and respiratory history. He or she should also anticipate the OCR and pretreat with atropine or glycopyrrolate. PONV is common and may be reduced by multiple strategies (Table 32-4).
TABLE 32-4 Antiemetic Strategies for Prophylaxis and Treatment of Postoperative Nausea and Vomiting
| Strategy | Drug and Dose |
|---|---|
| Butyrophenone (dopamine antagonist) | Droperidol (10-70 µg/kg) |
| Serotonin (5HT3 receptor antagonists) | Ondansetron (0.1 mg/kg) Granisetron (10-40 µg/kg) Dolasetron (0.35 mg/kg) |
| Propofol-based total intravenous anesthesia | Propofol (100-175 µg/kg/min) |
| Local anesthetic | Lidocaine local-topical and systemic (1-1.5 mg/kg) |
| Opioid-sparing analgesics or anesthetics | Retrobulbar block with bupivacaine |
| Other pharmacology | Dexamethasone (10-500 µg/kg); maximum, 8 mg |
| Dimenhydrinate (0.5-1 mg/kg) | |
| Metoclopramide (0.15-0.25 mg/kg) | |
| Benzodiazepines (e.g., lorazepam, midazolam) (10-100 µg/kg) | |
| Avoid nitrous oxide (N2O) | |
| Avoid opioids Use ketorolac (Toradol) (0.5 mg/kg PO, IV, IM), acetaminophen (30-40 mg/kg PR or 15 mg/kg IV), or diclofenac (1 mg/kg PR), or short-acting opioids (e.g., remifentanil, alfentanil, fentanyl) |
|
| Nonpharmacologic adjuvants | Intravenous hydrationGastric decompression |
IV, Intravenous; IM, intramuscular; PO, per os (oral); PR, per rectum (suppository).
We avoid succinylcholine for routine use in children in general, and in this circumstance, if succinylcholine is used, the surgeon should be informed because it may alter the forced duction testing and affect the planned repair.116 After induction of anesthesia and tracheal intubation, the operating room table is rotated to permit complete access to the orbits. It is common to use a preformed tube that lies flat against the mandible.117 The tracheal tube is positioned away from the surgical field. Ventilation may be spontaneous or controlled if the surgery is extraocular. If the surgery is intraocular or medical conditions dictate, controlled ventilation should be considered. Some anesthesiologists are willing to perform strabismus repair using an LMA with spontaneous ventilation.118 Although regional block is performed in some adults, strabismus surgery in children is routinely performed with general anesthesia.
Anterior Chamber Paracentesis
An anterior chamber paracentesis may be performed in children for evaluation of uveitis, infection,119 or leukemia120 or for removal of fluid to decrease IOP. Because the field needs to be sterilized and the needle must enter a small target area of the anterior chamber, the child should be placed under general anesthesia to make the field immobile for the surgeon.
Dacryocystorhinostomy
Infants may be born with nasolacrimal duct stenosis (i.e., congenital dacryostenosis) (Fig. 32-5 and E-Fig 32-4). If conservative measures do not demonstrate improvement, the ophthalmologist may need to dilate the duct or pierce a hole in the intact membrane using a metal probe.121,122 These infants are induced with general anesthesia, and when the level of anesthesia is sufficient, the ophthalmologist completes the procedure in a few minutes. To confirm that the duct is patent, the ophthalmologist can touch the metal probe within the duct with a second probe that is inserted into the nostril. Alternatively, the ophthalmologist may inject fluorescein into the nasolacrimal duct and detect the fluorescein on a pipe cleaner in the nasal airway. In both instances, fluorescein or blood may reach the larynx and trigger breath holding or laryngospasm. To avoid this, it is prudent to tilt the operating table to a 5- to 10-degree Trendelenburg position and place a small roll under the child’s shoulders to pool the fluids away from the larynx. These secretions are suctioned out of the oropharynx and nasopharynx before emergence. Anesthesia may be maintained by facemask inhalation or with intravenous agents. The airway and respirations are carefully monitored visually and by capnography. The infant is awakened, and postoperative analgesia may be offered with acetaminophen or opioids, or both, as necessary.
Alternatively, an endonasal endoscopic approach may be used for complicated or recurrent dacryorhinocystotomy.123,124 This makes the procedure significantly longer, and it requires airway control to facilitate the surgeon’s approach and to protect the child from aspirating blood during the procedure.
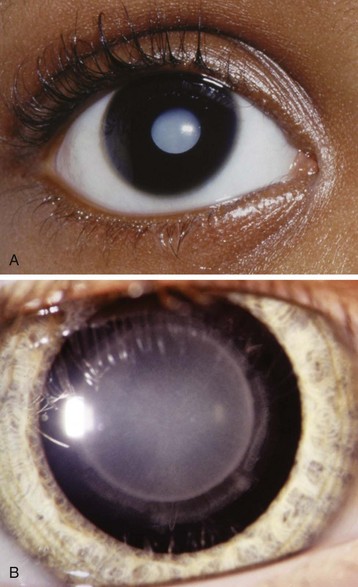
Cataract Surgery
Cataracts are opacifications of the lens of the eye (Fig. 32-6). Cataracts in children may be congenital, posttraumatic, or metabolic in origin.125 Congenital cataracts require surgery very early in life to permit photostimulation of the retina.126 Although the surgery can be performed as an outpatient, the formerly premature and young infant may require monitoring for postoperative anesthetic-induced respiratory depression or apnea. Cataracts are associated with some systemic diseases, and intraocular lens implants are often offered to improve the long-term visual prognosis.127 The child should be examined carefully for dysmorphology, with close attention to issues of airway management and the cardiorespiratory system.
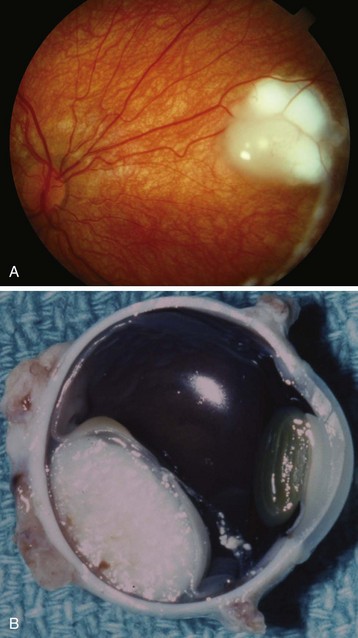
Enucleation
Enucleation of the eye may be necessary when a child has an intraocular tumor (e.g., retinoblastoma) (Fig. 32-7),128 a ruptured globe or ocular trauma,129 recurrent or chronic infections, or a blind, painful eye. Leukocoria is the term meaning “white pupil.” The differential diagnosis is extensive and includes many intraocular tumors. Other disorders causing leukocoria include Coats disease, cataract, coloboma, and Toxocara canis infection.
Vitrectomy
Vitrectomy may be necessary for retinal injury or detachment induced in circumstances of nonaccidental trauma or ROP.130 Any infant or child presenting acutely with a closed head injury from suspected abuse should have an ophthalmologic consultation to completely evaluate all orbital structures.131 These delicate tissues may demonstrate pathology that requires long-term follow-up or urgent medical or surgical therapy. Glaucoma may occur with hyphema or lens subluxation or dislocation with tearing of the support tissue. Early diagnosis is paramount if surgical intervention is to have the greatest benefit.
Retinopathy of Prematurity Treatment
Preterm infants may present with multiple ocular pathologic conditions, but none is more common than ROP. It has been extensively studied,15 and multiple collaborative trials have reported their results and recommendations for medical and surgical intervention.132–136 The cause is unknown, but oxygen-related theories have been described for more than 40 years. Because of this concern, oxygen range targeting has become common137–139 to reduce the oxidative stress140 on the infant in general and to reduce the effect of oxygen on neovascularization in the eye.
Laser and cryotherapy are common treatments for this condition.132,133 Because of the delicacy and exacting accuracy required in laser procedures, we routinely intubate the trachea and use neuromuscular relaxants. This facilitates the immobile field necessary for the surgeon, and it provides better perioperative physiologic stability for the infant.141 The complications of prematurity determine whether extubation is possible, and many infants require postoperative ventilation, if only for a brief period or overnight. The operative environment should be kept warm to reduce thermal stress on the infant.
Borland LM, Colligan J, Brandom BW. Frequency of anesthesia-related complications in children with Down syndrome under general anesthesia for noncardiac procedures. Pediatr Anaesth. 2004;14:733–738.
Cunningham AJ, Barry P. Intraocular pressure—physiology and implications for anaesthetic management. Can Anaesth Soc J. 1986;33:195–208.
Donahue SP. Clinical practice. Pediatric strabismus. N Engl J Med. 2007;356:1040–1047.
Saugstad OD, Aune D. In search of the optimal oxygen saturation for extremely low birthweight infants: a systematic review and metaanalysis. Neonatology. 2011;100:1–8.
Stephen E, Dickson J, Kindley AD, et al. Surveillance of vision and ocular disorders in children with Down syndrome. Dev Med Child Neurol. 2007;49:513–515.
1 Lyon F, Dabbs T, O’Meara M. Ketamine sedation during the treatment of retinopathy of prematurity. Eye. 2008;22:684–686.
2 Ates Y, Unal N, Cuhruk H, Erkan N. Postoperative analgesia in children using preemptive retrobulbar block and local anesthetic infiltration in strabismus surgery. Reg Anesth Pain Med. 1998;23:569–574.
3 Pragt E, Van Zundert AA, Kumar CM. Delayed convulsions and brief contralateral hemiparesis after retrobulbar block. Reg Anesth Pain Med. 2006;31:275–278.
4 Navaleza JS, Pendse SJ, Blecher MH. Choosing anesthesia for cataract surgery. Ophthalmol Clin North Am. 2006;19:233–237.
5 Hackel A, Badgwell JM, Binding RR, et al. Guidelines for the pediatric perioperative anesthesia environment. American Academy of Pediatrics, Section on Anesthesiology. Pediatrics. 1999;103:512–515.
6 Society for Pediatric Anesthesia, Policy statement on provision of pediatric anesthesia care. Available at http://www.pedsanesthesia.org/policyprovision.iphtml (accessed July 2012)
7 American Society of Anesthesiologists (ASA), Basic standards for preanesthesia care (approved by the ASA House of Delegates on October 14, 1987, and last affirmed on October 20, 2010). Available at http://www.asahq.org/~/media/For%20Members/documents/Standards%20Guidelines%20Stmts/Preanesthesia%20Care%20Basic%20Standards%20for.ashx (accessed July 2012)
8 Van B, Hirons RR, Yolton RL. The oculocardiac reflex: a review. J Am Optom Assoc. 1982;53:407–413.
9 Watcha MF, White PF, Tychsen L, Stevens JL. Comparative effects of laryngeal mask airway and endotracheal tube insertion on intraocular pressure in children. Anesth Analg. 1992;75:355–360.
10 Stocks J, Coates A, Bush A. Lung function in infants and young children with chronic lung disease of infancy: the next steps? Pediatr Pulmonol. 2007;42:3–9.
11 Owens R. Intraventricular hemorrhage in the premature neonate. Neonatal Netw. 2005;24:55–71.
12 Morray JP. Anesthesia-related cardiac arrest in children: an update. Anesthesiol Clin North Am. 2002;20:1–28.
13 Bhananker SM, Ramamoorthy C, Geiduschek JM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest (POCA) Registry. Anesth Analg. 2007;105:344–350.
14 Tin W, Gupta S. Optimum oxygen therapy in preterm babies. Arch Dis Child Fetal Neonatal Ed. 2007;92:F143–F147.
15 Quiram PA, Capone A, Jr. Current understanding and management of retinopathy of prematurity. Curr Opin Ophthalmol. 2007;18:228–234.
16 Quinn GE. The “ideal” management of retinopathy of prematurity. Eye. 2005;19:1044–1049.
17 Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy: a combined analysis. Anesthesiology. 1995;82:809–822.
18 Welborn LG, Greenspun JC. Anesthesia and apnea: perioperative considerations in the former preterm infant. Pediatr Clin North Am. 1994;41:181–198.
19 Kumar VH, Hutchison AA, Lakshminrusimha S, et al. Characteristics of pulmonary hypertension in preterm neonates. J Perinatol. 2007;27:214–219.
20 Higgins RD, Bancalari E, Willinger M, Raju TN. Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics. 2007;119:790–796.
21 Stephen E, Dickson J, Kindley AD, Scott CC, Charleton PM. Surveillance of vision and ocular disorders in children with Down syndrome. Dev Med Child Neurol. 2007;49:513–515.
22 Arora P, Tullu MS, Muranjan MN, et al. Congenital and inherited ophthalmologic abnormalities. Indian J Pediatr. 2003;70:549–552.
23 Creavin AL, Brown RD. Ophthalmic abnormalities in children with Down syndrome. J Pediatr Ophthalmol Strabismus. 2009;46:76–82.
24 Santamaria LB, Di PC, Mafrica F, Fodale V. Preanesthetic evaluation and assessment of children with Down’s syndrome. Sci World J. 2007;7:242–251.
25 Butler MG, Hayes BG, Hathaway MM, Begleiter ML. Specific genetic diseases at risk for sedation/anesthesia complications. Anesth Analg. 2000;91:837–855.
26 Kraemer FW, Stricker PA, Gurnaney HG, et al. Bradycardia during induction of anesthesia with sevoflurane in children with Down syndrome. Anesth Analg. 2010;111:1259–1263.
27 Bai W, Voepel-Lewis T, Malviya S. Hemodynamic changes in children with Down syndrome during and following inhalation induction of anesthesia with sevoflurane. J. Clinical Anesthesia. 2010;22:592–597.
28 Borland LM, Colligan J, Brandom BW. Frequency of anesthesia-related complications in children with Down syndrome under general anesthesia for noncardiac procedures. Paediatr Anaesth. 2004;14:733–738.
29 Hata T, Todd MM. Cervical spine considerations when anesthetizing patients with Down syndrome. Anesthesiology. 2005;102:680–685.
30 Morton RE, Khan MA, Murray-Leslie C, Elliott S. Atlantoaxial instability in Down’s syndrome: a five year follow up study. Arch Dis Child. 1995;72:115–118.
31 Roodman S, Bothwell M, Tobias JD. Bradycardia with sevoflurane induction in patients with trisomy 21. Paediatr Anaesth. 2003;13:538–540.
32 Traboulsi EI. Ocular malformations and developmental genes. J AAPOS. 1998;2:317–323.
33 Colville DJ, Savige J. Alport syndrome: a review of the ocular manifestations. Ophthalmic Genet. 1997;18:161–173.
34 Dean JC. Marfan syndrome: clinical diagnosis and management. Eur J Hum Genet. 2007;15:724–733.
35 von KY, Robinson PN. Marfan syndrome: an update of genetics, medical and surgical management. Heart. 2007;93:755–760.
36 Cattaneo M. Hyperhomocysteinemia and venous thromboembolism. Semin Thromb Hemost. 2006;32:716–723.
37 Johnston BA, Occhipinti KE, Baluch A, Kaye AD. Ehlers-Danlos syndrome: complications and solutions concerning anesthetic management. Middle East J Anesthesiol. 2006;18:1171–1184.
38 Chan YL, Lin SP, Man TT, Cheng CR. Clinical experience in anesthetic management for children with mucopolysaccharidoses: report of ten cases. Acta Paediatr Taiwan. 2001;42:306–308.
39 Man TT, Tsai PS, Rau RH, et al. Children with mucopolysaccharidoses—three cases report. Acta Anaesthesiol Sin. 1999;37:93–96.
40 Cohen AJ. Oculoplastic and orbital surgery. Ophthalmol Clin North Am. 2006;19:257–267.
41 Hasan RA, Nikolis A, Dutta S, Jackson IT. Clinical outcome of perioperative airway and ventilatory management in children undergoing craniofacial surgery. J Craniofac Surg. 2004;15:655–661.
42 Sculerati N, Gottlieb MD, Zimbler MS, Chibbaro D, McCarthy JG. Airway management in children with major craniofacial anomalies. Laryngoscope. 1998;108:1806–1812.
43 Blum RH, McGowan FX, Jr. Chronic upper airway obstruction and cardiac dysfunction: anatomy, pathophysiology and anesthetic implications [review]. Pediatr Anesth. 2004;14:75–83.
44 Hirsch NP, Murphy A, Radcliffe JJ. Neurofibromatosis: clinical presentations and anaesthetic implications. Br J Anaesth. 2001;86:555–564.
45 Diaz JH. Perioperative management of children with congenital phakomatoses. Pediatr Anesth. 2000;10:121–128.
46 Delvi MB, Takrouri MS. Anesthesia for encephalo-trigeminal angiomatosis (Sturge-Weber syndrome). Middle East J Anesthesiol. 2006;18:785–790.
47 Lee JJ, Imrie M, Taylor V. Anaesthesia and tuberous sclerosis. Br J Anaesth. 1994;73:421–425.
48 Shenkman Z, Rockoff MA, Eldredge EA, et al. Anaesthetic management of children with tuberous sclerosis. Paediatr Anaesth. 2002;12:700–704.
49 Cunningham AJ, Barry P. Intraocular pressure—physiology and implications for anaesthetic management. Can Anaesth Soc J. 1986;33:195–208.
50 Kelly RE, Dinner M, Turner LS, et al. Succinylcholine increases intraocular pressure in the human eye with the extraocular muscles detached. Anesthesiology. 1993;79:948–952.
51 Metz HS, Venkatesh B. Succinylcholine and intraocular pressure. J Pediatr Ophthalmol Strabismus. 1981;18:12–14.
52 Hall SC, Lerman J. Should succinylcholine be used in the child with an open globe injury and a full stomach? Anesth Rev. 1993;20:98-105.
53 Murphy DF. Anesthesia and intraocular pressure. Anesth Analg. 1985;64:520–530.
54 Pandey K, Badola RP, Kumar S. Time course of intraocular hypertension produced by suxamethonium. Br J Anaesth. 1972;44:191–196.
55 Yoshikawa K, Murai Y. The effect of ketamine on intraocular pressure in children. Anesth Analg. 1971;50:199–202.
56 Ausinsch B, Rayburn RL, Munson ES, Levy NS. Ketamine and intraocular pressure in children. Anesth Analg. 1976;55:773–775.
57 Ruta U, Mollhoff T, Markodimitrakis H, Brodner G. Attenuation of the oculocardiac reflex after topically applied lignocaine during surgery for strabismus in children. Eur J Anaesthesiol. 1996;13:11–15.
58 Choi SH, Lee SJ, Kim SH, et al. Single bolus of intravenous ketamine for anesthetic induction decreases oculocardiac reflex in children undergoing strabismus surgery. Acta Anaesthesiol Scand. 2007;51:759–762.
59 Hahnenkamp K, Honemann CW, Fischer LG, et al. Effect of different anaesthetic regimes on the oculocardiac reflex during paediatric strabismus surgery. Paediatr Anaesth. 2000;10:601–608.
60 Oh AY, Yun MJ, Kim HJ, Kim HS. Comparison of desflurane with sevoflurane for the incidence of oculocardiac reflex in children undergoing strabismus surgery. Br J Anaesth. 2007;99:262–265.
61 Quiroz-Mercado H, Ustariz-González O, Martinez-Castellanos MA, et al. Our experience after 1765 intravitreal injections of bevacizumab: the importance of being part of a developing story. Semin Ophthalmol. 2007;22:109–125.
62 Warner MA, Warner ME, Warner DO, et al. Perioperative pulmonary aspiration in infants and children. Anesthesiology. 1999;90:66–71.
63 Ahmed A, Ali M, Khan M, Khan F. Perioperative cardiac arrests in children at a university teaching hospital of a developing country over 15 years. Pediatr Anesth. 2009;19:581–586.
64 Bharti N, Batra YK, Kaur H. Paediatric perioperative cardiac arrest and its mortality: database of a 60-month period from a tertiary care paediatric centre. Eur J Anaesthesiol. 2009;26:490–495.
65 van der Griend BF, Lister NA, McKenzie IM, et al. Postoperative mortality in children after 101,885 anesthetics at a tertiary pediatric hospital. Aneth Analg. 2011;112:1440–1447.
66 Lysakowski C, Suppan L, Czarnetzki C, et al. Impact of the intubation model on the efficacy of rocuronium during rapid sequence intubation: systematic review of randomized trials. Acta Anaesthesiol Scand. 2007;51:848–857.
67 Warner LO, Bremer DL, Davidson PJ, et al. Effects of lidocaine, succinylcholine, and tracheal intubation on intraocular pressure in children anesthetized with halothane-nitrous oxide. Anesth Analg. 1989;69:687–690.
68 Zimmerman AA, Funk KJ, Tidwell JL. Propofol and alfentanil prevent the increase in intraocular pressure caused by succinylcholine and endotracheal intubation during a rapid sequence induction of anesthesia. Anesth Analg. 1996;83:814–817.
69 Kaplan RF, Uejima T, Lobel G, et al. Intramuscular rocuronium in infants and children: a multicenter study to evaluate tracheal intubating conditions, onset, and duration of action. Anesthesiology. 1999;91:633–638.
70 Reynolds LM, Lau M, Brown R, et al. Intramuscular rocuronium in infants and children: dose-ranging and tracheal intubating conditions. Anesthesiology. 1996;85:231–239.
71 Reynolds LM, Lau M, Brown R, et al. Bioavailability of intramuscular rocuronium in infants and children. Anesthesiology. 1997;87:1096–1105.
72 Liu LMP, Goudsouzian NG, Liu P. Rectal methohexital premedication in children: a dose comparison study. Anesthesiology. 1980;53:343–345.
73 Liu LM, DeCook TH, Goudsouzian NG, et al. Dose response to intramuscular succinylcholine in children. Anesthesiology. 1981;55:599–602.
74 Hannallah RS, Oh TH, McGill WA, Epstein BS. Changes in heart rate and rhythm after intramuscular succinylcholine with or without atropine in anesthetized children. Anesth Analg. 1986;65:1329–1332.
75 Sutherland GA, Bevan JC, Bevan DR. Neuromuscular blockade in infants following intramuscular succinylcholine in two or five per cent concentration. Can Anaesth Soc J. 1983;30:342–346.
76 Karanovic N, Carev M, Ujevic A, et al. Association of oculocardiac reflex and postoperative nausea and vomiting in strabismus surgery in children anesthetized with halothane and nitrous oxide. Paediatr Anaesth. 2006;16:948–954.
77 Walsh C, Smith CE, Ryan B, et al. Postoperative vomiting following strabismus surgery in paediatric outpatients: spontaneous versus controlled ventilation. Can J Anaesth. 1988;35:31–35.
78 Munro HM, Riegger LQ, Reynolds PI, et al. Comparison of the analgesic and emetic properties of ketorolac and morphine for paediatric outpatient strabismus surgery. Br J Anaesth. 1994;72:624–628.
79 Shende D, Das K. Comparative effects of intravenous ketorolac and pethidine on perioperative analgesia and postoperative nausea and vomiting (PONV) for paediatric strabismus surgery. Acta Anaesthesiol Scand. 1999;43:265–269.
80 Lerman J, Eustis S, Smith DR. Effect of droperidol pretreatment on postanesthetic vomiting in children undergoing strabismus surgery. Anesthesiology. 1986;65:322–325.
81 Wennstrom B, Reinsfelt B. Rectally administered diclofenac (Voltaren) reduces vomiting compared with opioid (morphine) after strabismus surgery in children. Acta Anaesthesiol Scand. 2002;46:430–434.
82 Rodgers A, Cox RG. Anesthetic management for pediatric strabismus surgery: Continuing professional development. Can J Anaesth. 2010;57:602–617.
83 Khalil SN, Berry JM, Howard G, et al. The antiemetic effect of lorazepam after outpatient strabismus surgery in children. Anesthesiology. 1992;77:915–919.
84 Watcha MF, Simeon RM, White PF, Stevens JL. Effect of propofol on the incidence of postoperative vomiting after strabismus surgery in pediatric outpatients. Anesthesiology. 1991;75:204–209.
85 Goodarzi M, Matar MM, Shafa M, et al. A prospective randomized blinded study of the effect of intravenous fluid therapy on postoperative nausea and vomiting in children undergoing strabismus surgery. Paediatr Anaesth. 2006;16:49–53.
86 Weir PM, Munro HM, Reynolds PI, et al. Propofol infusion and the incidence of emesis in pediatric outpatient strabismus surgery. Anesth Analg. 1993;76:760–764.
87 Mikawa K, Nishina K, Maekawa N, et al. Oral clonidine premedication reduces vomiting in children after strabismus surgery. Can J Anaesth. 1995;42:977–981.
88 Sadhasivam S, Shende D, Madan R. Prophylactic ondansetron in prevention of postoperative nausea and vomiting following pediatric strabismus surgery: a dose-response study. Anesthesiology. 2000;92:1035–1042.
89 Litman RS, Wu CL, Lee A, et al. Prevention of emesis after strabismus repair in children: a prospective, double-blinded, randomized comparison of droperidol versus ondansetron. J Clin Anesth. 1995;7:58–62.
90 Fujii Y, Tanaka H, Toyooka H. Granisetron reduces vomiting after strabismus surgery and tonsillectomy in children. Can J Anaesth. 1996;43:35–38.
91 Scuderi PE, Weaver RG, Jr., James RL, et al. A randomized, doubleblind, placebo controlled comparison of droperidol, ondansetron, and metoclopramide for the prevention of vomiting following outpatient strabismus surgery in children. J Clin Anesth. 1997;9:551–558.
92 Fujii Y, Toyooka H, Tanaka H. Antiemetic efficacy of granisetron and metoclopramide in children undergoing ophthalmic or ENT surgery. Can J Anaesth. 1996;43:1095–1099.
93 Vener DF, Carr AS, Sikich N, et al. Dimenhydrinate decreases vomiting after strabismus surgery in children. Anesth Analg. 1996;82:728–731.
94 Welters ID, Menges T, Graf M, et al. Reduction of postoperative nausea and vomiting by dimenhydrinate suppositories after strabismus surgery in children. Anesth Analg. 2000;90:311–314.
95 Broadman LM, Ceruzzi W, Patane PS, et al. Metoclopramide reduces the incidence of vomiting following strabismus surgery in children. Anesthesiology. 1990;72:245–248.
96 Madan R, Bhatia A, Chakithandy S, et al. Prophylactic dexamethasone for postoperative nausea and vomiting in pediatric strabismus surgery: a dose ranging and safety evaluation study. Anesth Analg. 2005;100:1622–1626.
97 Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90:186–194.
98 Riad W, Altaf R, Abdulla A, Oudan H. Effect of midazolam, dexamethasone and their combination on the prevention of nausea and vomiting following strabismus repair in children. Eur J Anaesthesiol. 2007;24:697–701.
99 Splinter WM. Prevention of vomiting after strabismus surgery in children: dexamethasone alone versus dexamethasone plus low-dose ondansetron. Paediatr Anaesth. 2001;11:591–595.
100 Subramaniam B, Madan R, Sadhasivam S, et al. Dexamethasone is a cost-effective alternative to ondansetron in preventing PONV after paediatric strabismus repair. Br J Anaesth. 2001;86:84–89.
101 Schreiner MS, Nicolson SC, Martin T, Whitney L. Should children drink before discharge from day surgery? Anesthesiology. 1992;76:528–533.
102 Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Combination of granisetron and droperidol for the prevention of vomiting after paediatric strabismus surgery. Paediatr Anaesth. 1999;9:329–333.
103 Stead SW, Beatie CD, Keyes MA, Isenberg SJ. Effects of droperidol dosage on postoperative emetic symptoms following pediatric strabismus surgery. J Clin Anesth. 2004;16:34–39.
104 Bharti N, Shende D. Comparison of anti-emetic effects of ondansetron and low-dose droperidol in pediatric strabismus surgery. J Pediatr Ophthalmol Strabismus. 2003;40:23–26.
105 Eustis S, Lerman J, Smith DR. Effect of droperidol pretreatment on post-anesthetic vomiting in children undergoing strabismus surgery: the minimum effective dose. J Pediatr Ophthalmol Strabismus. 1987;24:165–169.
106 Meyer RJ. FDA “black box” labeling. Ann Emerg Med. 2003;41:559–560.
107 Stuth EA, Stucke AG, Cava JR, et al. Droperidol for perioperative sedation causes a transient prolongation of the QTc time in children under volatile anesthesia. Paediatr Anaesth. 2004;14:831–837.
108 Madan R, Perumal T, Subramaniam K, et al. Effect of timing of ondansetron administration on incidence of postoperative vomiting in paediatric strabismus surgery. Anaesth Intensive Care. 2000;28:27–30.
109 Nathan AT, Berkowitz DH, Montenegro LM, et al. Implications of anesthesia in children with long QT syndrome. Anesth Analg. 2011;112:1163–1168.
110 Couture DJ, Maye JP, O’brien D, Beldia Smith A. Therapeutic modalities for the prophylactic management of postoperative nausea and vomiting. J Perianesth Nurs. 2006;21:398–403.
111 Holliday MA, Friedman Al, Segar WE, Chesney R, Finberg L. Acute hospital-induced hyponatremia in children: a physiologic approach. J Pediatr. 2004;145:584–587.
112 Holliday MA. Isotonic saline expands extracellular fluid and is inappropriate for maintenance therapy. Pediatrics. 2005;115:193–194.
113 Holliday MA, Ray PE, Friedman AL. Fluid therapy for children: facts, fashions and questions. Arch Dis Child. 2007;92:546–550.
114 Valley RD, Freid EB, Bailey AG, et al. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of desflurane and sevoflurane. Anesth Analg. 2003;96:1320–1324.
115 Donahue SP. Clinical practice: pediatric strabismus. N Engl J Med. 2007;356:1040–1047.
116 Dell R, Williams B. Anaesthesia for strabismus surgery: a regional survey. Br J Anaesth. 1999;82:761–763.
117 Ring WH, Adair JC, Elwyn RA. A new pediatric endotracheal tube. Anesth Analg. 1975;54:273–274.
118 Eltzschig HK, Darsow R, Schroeder TH, et al. Effect of tracheal intubation or laryngeal mask airway insertion on intraocular pressure using balanced anesthesia with sevoflurane and remifentanil. J Clin Anesth. 2001;13:264–267.
119 Sridhar MS, Sharma S, Gopinathan U, Rao GN. Anterior chamber tap: diagnostic and therapeutic indications in the management of ocular infections. Cornea. 2002;21:718–722.
120 Dadeya S, Malik KP, Guliani BP, et al. Acute lymphocytic leukemia presenting as masquerade syndrome. Ophthalmic Surg Lasers. 2002;33:163–165.
121 Nemet AY, Fung A, Martin PA, et al. Lacrimal drainage obstruction and dacryocystorhinostomy in children. Eye. 2008;22:918–924.
122 Singh BG, Singh BH. Repeated probing results in the treatment of congenital nasolacrimal duct obstruction. Eur J Ophthalmol. 2004;14:185–192.
123 Nussbaumer M, Schreiber S, Yung MW. Concomitant nasal procedures in endoscopic dacryocystorhinostomy. J Laryngol Otol. 2004;118:267–269.
124 Dolman PJ. Comparison of external dacryocystorhinostomy with nonlaser endonasal dacryocystorhinostomy. Ophthalmology. 2003;110:78–84.
125 Guercio JR, Martyn LJ. Congenital malformations of the eye and orbit. Otolaryngol Clin North Am. 2007;40:113–140.
126 Long V, Chen S, Hatt S. Surgical interventions for bilateral congenital cataract. Cochrane Database Syst Rev. 3, 2006. CD003171
127 Fan DS, Yip WW, Yu CB, Rao SK, Lam DS. Updates on the surgical management of paediatric cataract with primary intraocular lens implantation. Ann Acad Med Singapore. 2006;35:564–570.
128 Shields CL, Shields JA. Diagnosis and management of retinoblastoma. Cancer Control. 2004;11:317–327.
129 Chaudhry IA, Al-Sharif AM, Shamsi FA, Elzaridi E, Al-Rashed W. Severe ocular injuries from pointed door handles in children. Ophthalmology. 2005;112:1834–1837.
130 Hutcheson KA. Application of new ophthalmic technology in the pediatric patient. Curr Opin Ophthalmol. 2007;18:384–391.
131 Wang NK, Chen YP, Yeung L, et al. Traumatic pediatric retinal detachment following open globe injury. Ophthalmologica. 2007;221:255–263.
132 Connolly BP, Ng EY, McNamara JA, et al. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: II. Refractive outcome. Ophthalmology. 2002;109:936–941.
133 Dobson V, Quinn GE, Summers CG, Hardy RJ, Tung B, Cryotherapy for Retinopathy of Prematurity Cooperative Group. Visual acuity at 10 years in Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study eyes: effect of retinal residua of retinopathy of prematurity. Arch Ophthalmol. 2006;124:199–202.
134 Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity Randomized Trial. Arch Ophthalmol. 2003;121:1684–1694.
135 Good WV, Hardy RJ, Dobson V, et al. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116:15–23.
136 Ng EY, Connolly BP, McNamara JA, et al. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: I. Visual function and structural outcome. Ophthalmology. 2002;109:928–934.
137 Johnson K, Scott SD, Fraser KD. Oxygen use for preterm infants: factors that mayinfluence clinical decisions surrounding oxygen titration. Adv Neonatal Care. 2011;11:8–14.
138 Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr. 2011;23:173–178.
139 Saugstad OD, Aune D. In search of the optimal oxygen saturation for extremely low birthweight infants: a systematic review and metaanalysis. Neonatology. 2011;100:1–8.
140 Saugstad OD. Oxidative stress in the newborn—a 30-year perspective. Biol Neonate. 2005;88:228–236.
141 Haigh PM, Chiswick ML, O’Donoghue EP. Retinopathy of prematurity: systemic complications associated with different anaesthetic techniques at treatment. Br J Ophthalmol. 1997;81:283–287.