Chapter 39 Operative Options for Extensor Mechanism Malalignment and Patellar Dislocation
INDICATIONS
Many surgical procedures have been described for realignment of the patellofemoral mechanism including proximal realignment procedures, distal realignment procedures, or a combination of both. Proximal realignment procedures alter the medial-lateral position of the patella through balancing of soft tissue restraints proximal to its inferior pole. Included in this category are lateral retinacular release, medial retinacular capsular and medial patellomeniscal ligament (MPML) plication, vastus medialis obliquus (VMO) advancement, and medial patellofemoral ligament (MPFL) repair or reconstruction. Distal realignment procedures modify the medial-lateral, anterior-posterior, and proximal-distal positions of the patella by transfer of the tibial tubercle. Included in this category are anterior (Maquet55), medial (Elmslie-Trillat93), and anteromedial (Fulkerson33) transfer of the tibial tubercle. Literally hundreds of articles have been written on these operative procedures regarding their indications, technique, and clinical outcome and the reader is referred to recent review articles for further information.2–4,27,31,32,36,61
 Modification of Elmslie-Trillat procedure: arthroscopic lateral release if a contracted lateral retinaculum is present, a modified VMO advancement, and a modified medial transfer of the tibial tubercle.
Modification of Elmslie-Trillat procedure: arthroscopic lateral release if a contracted lateral retinaculum is present, a modified VMO advancement, and a modified medial transfer of the tibial tubercle. Anatomic abnormalities, congenital laxity MPFL, increased Q-angle, trochlear dysplasia: treated conservatively, candidates for surgery if symptomatic.
Anatomic abnormalities, congenital laxity MPFL, increased Q-angle, trochlear dysplasia: treated conservatively, candidates for surgery if symptomatic.The second operative procedure described in detail is an MPFL reconstruction using the quadriceps tendon (QT) based on the proximal patella. This procedure is performed through a limited cosmetic incision and avoids harvesting the semitendinosus tendon or bony fixation at the patella or medial femur. This procedure has proved to be useful in revision cases that failed after a prior proximal realignment in which an inadequate MPFL was not addressed. The operative procedure is combined with a distal realignment when indicated, as is discussed. Other operative procedures the senior author has found useful for correcting patella alta and ITB lengthening are also described. Surgical procedures for rotational malalignment of excessive femoral anteversion and external tibial torsion are discussed in Chapter 40, Patellofemoral Disorders: Correction of Rotational Malalignment of the Lower Extremity, and not repeated here.
The majority of patients with patellofemoral malalignment respond favorably to conservative treatment programs, covered in detail in other publications.17,18,96 In general, three types of extensor mechanism problems present for treatment. The first group of patients have diffuse anterior knee pain without distinct anatomic abnormalities in alignment, subluxation, or joint damage that can be identified. Commonly, these are younger athletes who are involved in multiple sports, and frequently, the only finding is the presence of tenderness of peripatellar soft tissues and the anterior fat pad tissues. On occasion, a mild joint effusion may be present. Authors have stressed that these symptoms frequently represent an overuse condition from excessive participation in athletics.45,91 Treatment consists of allowing the soft tissues time to heal and inflammation to recede by modification of athletics, followed by appropriate strengthening and stretching of the muscle groups. Specific factors that may contribute to the symptomatic state include excessive conditioning, type of sport, and duration and frequency of athletic participation. Recurrence of symptoms is not an indication for surgical treatment, because distinct anatomic abnormalities of the extensor mechanism are not present. A cartilage-sensitive magnetic resonance imaging (MRI) may be helpful in recurrent symptomatic knees to determine the status of the patellofemoral joint and the presence of early articular cartilage damage that is aggravated by vigorous athletic activities.
Nonoperative treatment of first-time patella dislocations has a re-dislocation rate that ranges from 14% to 57% in adult populations15,30,40,50,52,53,92 and from 36% to 71% in pediatric populations.12,72 Stefancin and Parker87 conducted a systematic review of 70 articles that focused on the management of traumatic first-time patellar dislocations and concluded that conservative treatment was indicated initially except in specific circumstances. These included the finding of an osteochondral fracture, a substantial disruption of the MPFL, a laterally subluxated patella with normal alignment of the contralateral knee, or after failed conservative treatment or recurrent dislocations.
Patients with an acute lateral first-time dislocation are separated into three categories:
CONTRAINDICATIONS
The primary contraindication for extensor mechanism malalignment surgery is the absence of a distinct anatomic defect, because the goal of surgery is to restore normal anatomy of the extensor mechanism. The presence of chronic recurrent anterior knee pain is not an indication for surgery when the specific etiology of the pain symptoms cannot be determined. This point is worthy of emphasis, because many patients present with anterior knee pain in which the specific diagnosis is not apparent despite a thorough and careful examination and evaluation. It is probable that there are pain generation factors related to early patellofemoral cartilage deterioration that is still early in the disease course. Alternatively, peripatellar and intra-articular soft tissues may be an explainable source of pain.24,32 A common cause of unexplained pain is a subtle infrapatellar neuroma or neuritis of the sensory nerves about the knee joint, discussed in Chapters 42, Knee Pain of Neural Origin, and 43, Diagnosis and Treatment of Complex Regional Pain Syndrome.
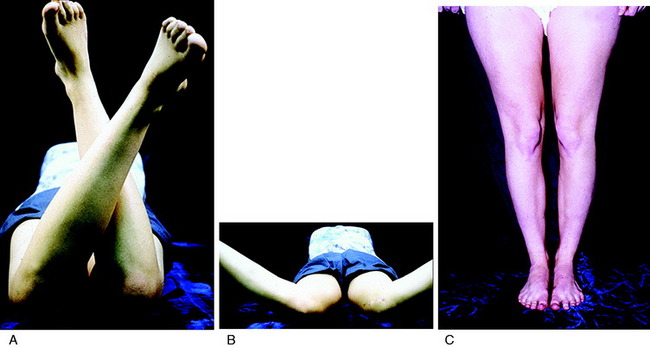
A specific contraindication to extensor mechanism surgery is the presence of excessive hip anteversion or abnormal external tibial torsion as discussed in Chapter 40, Patellofemoral Disorders: Correction of Rotational Malalignment of the Lower Extremity. An MRI or computed tomography (CT) scan for axial rotation of the lower extremity from the hip to the ankle is required. The measurements obtained include femoral anteversion, trochlear groove–tibial tubercle offset, and external tibial torsion as are described. The anterior knee pain is due to excessive lateral patellofemoral forces induced by external tibial torsion or increased femoral anteversion (Fig. 39-1). In these patients, a femoral or tibial derotation osteotomy may be indicated and not a proximal or distal patellofemoral procedure.58,89
A relative contraindication is the presence of patellofemoral arthritis; however, in select knees, a realignment procedure may be performed with a cartilage restoration procedure.9,10,38,43,54,64,74,76
BIOMECHANICS OF MEDIAL AND LATERAL PATELLOFEMORAL RESTRAINTS
Three factors influence patellar stability: articular geometry, dynamic muscle actions, and passive soft tissue restraints.2,27 The geometry of the trochlear groove,2 the height and slope of the lateral femoral condyle, and the angle of knee flexion all affect lateral patellar translation. Amis2 described objective patellar stability in terms of two factors: the amount of force required to displace the patella a given linear distance from its equilibrium position (translation) and the turning moment required to induce a rotation, such as a lateral tilt. Methods used to determine patellar mobility include manual measurement, instrumented quantitative measurement, and radiographic measurement. Kolowich and coworkers47 measured patellar glide by dividing the patella into four quadrants and describing medial and lateral mobility as a fraction of patellar width. Normal subjects had less than two quadrants of translation with the knee flexed 30°. Teitge and associates90 developed a stress radiographic method to measure patellar medial-lateral stability. Knees of normal subjects and symptomatic patients were tested between 30° and 40° of flexion with 71 N of force applied. Wide variation was noted in the normal knees, because lateral displacement ranged from 1 to 32 mm, and medial displacement ranged from 2 to 22 mm. The mean difference in lateral displacement between right and left knees was 1.3 ± 1.1 mm, and the mean difference in medial displacement was 1.2 ± 1.08 mm. The investigators determined that a difference between knees in lateral displacement of 3.7 mm, or a difference in medial displacement of 3.46 mm, was abnormal.
Critical Points BIOMECHANICS OF MEDIAL AND LATERAL PATELLOFEMORAL RESTRAINTS
Hautamaa and colleagues39 developed an instrumented measurement device to determine patella medial-lateral translation in the coronal plane. In 17 cadaver knees, with the knee flexed 30 ± 5°, 5 pounds of laterally directed force produced an average of 9.3 ± 0.9 mm of lateral translation. Instrumented measurement of medial-lateral patellar translation was also conducted by Fithian and coworkers29 in normal and symptomatic patients. Right versus left knee comparisons revealed small mean differences in the control subjects for medial and lateral patellar translation (30° flexion, 2.5-lb force, 0.1 ± 1.9 mm and 0.2 ± 1.0 mm, respectively). Significant differences were found between the control and the symptomatic subjects in the mean differences in lateral translation (P < .01), with the patients demonstrating 1.6 ± 2.5 mm difference under a 2.5-pound force at 30° of flexion.
It is well appreciated that the patella is most unstable in the range of 0° to 30° of flexion. When the knee is near full extension, the Q-angle is maximized owing to the external rotation of the tibia. In addition, with the quadriceps muscle relaxed, the patella is not engaged in the trochlear groove and thus is easily mobilized in a medial-lateral direction. As the knee is flexed, patellar stability is increased owing to the combined tensions of the quadriceps muscles and the patellar tendon that pull the patella into the trochlear groove. A shallow or dysplastic trochlear groove allows the patella to displace more easily.81 A patella alta condition results in a loss of the trochlea geometric restraint until 30° to 40° of flexion when patellotrochlear contact finally occurs.
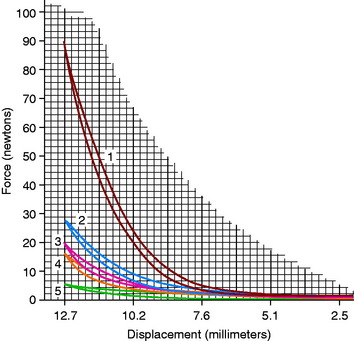
In regard to passive soft tissue restraints, the MPFL is the primary restraint to lateral patellar translation, providing 53% to 67% restraint up to 30° of flexion.4,11,16,23,39,73 Desio and associates23 examined nine cadaver knees (mean age, 57 yr; range, 43–70 yr) at 20° of flexion and reported that the MPFL provided a mean of 60% of the restraining force to lateral patellar translation. The MPML provided a mean of 13% of the restraining force; the lateral retinaculum, 10%, and the medial retinaculum and medial patellotibial ligament, 3% each. Conlan and colleagues16 reported similar findings from 25 cadaver specimens (Fig. 39-2); the MPFL provided an average of 53% of the total restraining force, followed by the MPML (22% contribution), the medial retinaculum (11% contribution), and the medial patellotibial ligament (5% contribution). Sectioning of the MPFL decreased the restraining force significantly; the average stiffness decreased from 225 N/cm in the intact specimens to 104.6 N/cm (P = .001).
Nomura and coworkers68 measured the lateral patellar translation in 10 cadaver knees (aged 45–60 yr) with the quadriceps tensed to 10 N and a lateral force of 10 N applied before and after sectioning of the MPFL. Isolated sectioning of the MPFL significantly increased the lateral patellar translation from 20° to 90° of flexion (P < .05).
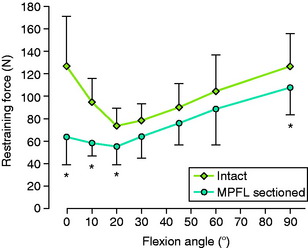
Amis and associates3 reported a mean failure load of the MPFL of 208 N from 10 cadaveric specimens (mean age, 70 yr) and interpreted these findings as “surprisingly strong for such an insubstantial appearance.” These investigators also reported that the contribution of the MPFL in restraining lateral patellar translation was greatest with the knee at 0° extension. Significant increases in lateral translation occurred from 0° to 20° after the MPFL was sectioned (P value not provided; Fig. 39-3).
PREOPERATIVE PLANNING
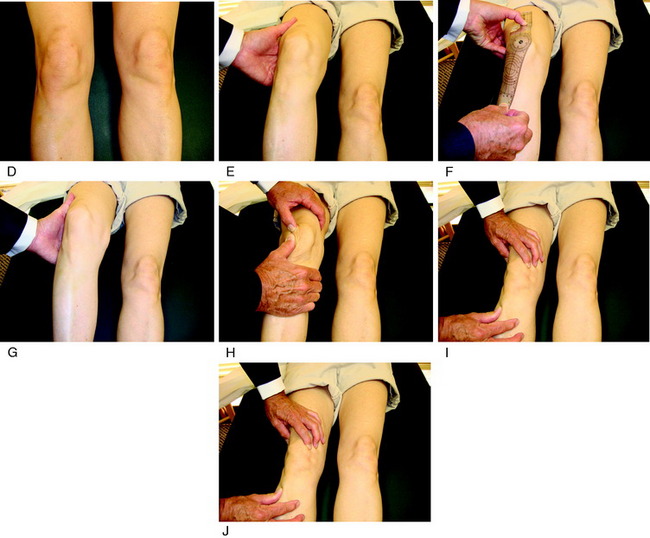
The physical examination is crucial in preoperative planning for patellar realignment surgery. The examination should be performed with the patient in the standing, sitting, and supine positions (see Fig. 39-1D-J). Palpation of parapatellar soft tissues and the fat pad is performed for swelling and elicitation of pain. The examiner should look for evidence of lateralization of the extensor mechanism and tilt. The patellar compression test should be performed with flexion and extension of the knee to evaluate for articular crepitus or pain. Passive patellar tilt and tightness of the lateral retinaculum should be noted. Patellar subluxation tests (patellar glide at 0° and 30° flexion) should be performed in medial and lateral directions, and patellar mobility noted. Other sources of pain such as neuroma, patellar tendinitis, synovial plica, synovitis, meniscus tears, osteochondritis dissecans, complex regional pain syndrome, and advanced tibiofemoral arthritis should be excluded.
Critical Points PREOPERATIVE PLANNING
CT, computed tomography; MRI, magnetic resonance imaging; TT/TG, tibial tubercle/trochlear groove.
CT and MRI have been used to assess lower limb rotational alignment and are important tests for a complete diagnosis of the anatomic abnormalities that may be present. These studies obtain axial images of the patellofemoral joint at or near full extension to evaluate trochlear dysplasia and the tibial tubercle/trochlear groove (TT/TG) distance. Various investigators have studied this distance and found that it is increased in patients with patellofemoral pain and instability.21,46,82
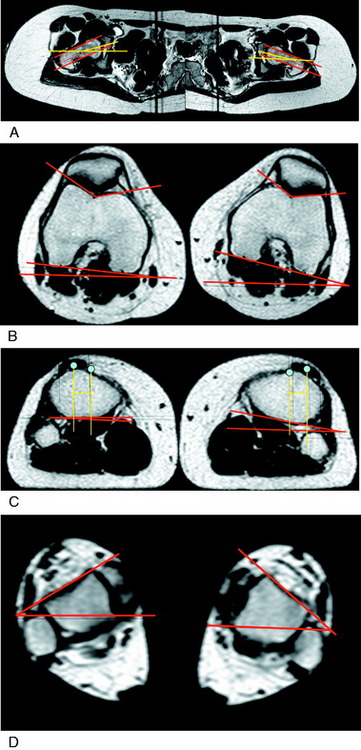
Femoral anteversion has been measured by a variety of techniques by many authors. Yoshioka and Cooke97 measured anteversion from the bone in 32 femora and reported a mean of 13.1° ± 8° (range, –11°–+22°) in both males and females when the distal measurement was the tangent across the femoral condyles, and a mean of 7.4° when measured across the epicondyles. These authors conducted a review of the literature on this measurement and found reported normal values were between 8° and 16° in twelve different investigations. Teitge and associates90 arbitrarily selected 13° as the goal for correction (see Chapter 40, Patellofemoral Disorders: Correction of Rotational Malalignment of the Lower Extremity).
The technique for measuring tibial torsion by CT has not been standardized and wide variation exists in the literature regarding normal values. Yoshioka and colleagues98 reported a mean lateral tibial torsion of 21° ± 4.9° in males and 27° ± 11.0° in females, a significant difference (P < .05). Eckhoff and coworkers25 reported mean tibial “version” of 37.0° ± 1.7° in control knees and 32.8° ± 1.7° in a group of symptomatic knees. Turner94 measured a mean of 19° ± 4.8° in a control group compared with a mean of 24.5° ± 6.3° in a group with unstable patella. Sayli and associates79 reported an average of tibial torsion in females of 31.07° for the right side and 30.02° for the left. For males, the averages were 32.7° and 35.26° for the right and left sides, respectively. Tamari and colleagues88 conducted a reliability study that examined different methods of measuring tibial torsion. The results showed that clinical methods currently available do not accurately measure true torsion of the femur and tibia. The authors stressed that even so, these methods may be useful for screening and descriptive purposes as indices of true torsion and use of different reference axes could improve their reliability.
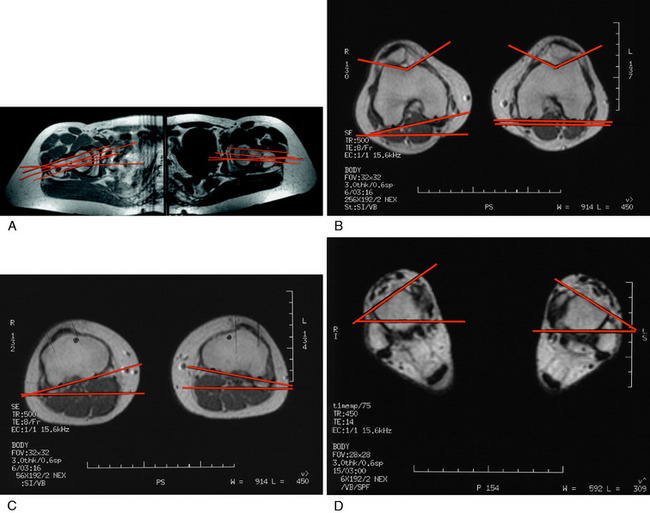
MRI has some advantages over CT, including improved ability to image cartilaginous structures, better anatomic approximation of the trochlear articular cartilage groove, and avoidance of radiation exposure. MRI also provides meaningful information about all of the soft tissue structures about the knee joint. The MRI is obtained in standard fashion, with images obtained of the hip parallel to the femoral neck and axial images obtained of the knee and ankle (Fig. 39-4). These individual images are then measured based on the techniques described by Murphy and coworkers65 and Guenther and associates37 (Figs. 39-5 and 39-6).
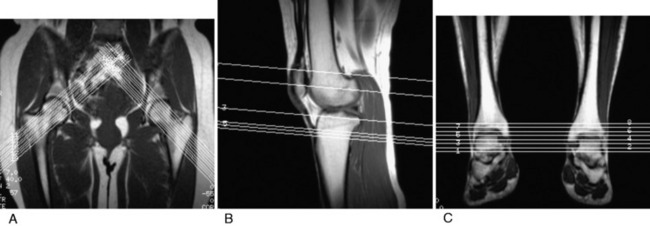
FIGURE 39-5 MRI T1 images are obtained at the hip (A), knee (B), and ankle (C). This allows selection of the proper images for the measurements shown in Figure 39-6.
(A–C, From Parikh, S. N.; Noyes, F. R.; Albright, J.: Proximal and distal extensor mechanism realignment: the surgical technique. Tech Knee Surg 5:27–38, 2006.)
TT/TG is measured using the trochlear groove–patellar tendon insertion distance. TT/TG is measured in millimeters and is the difference from the center of the deepest point of the femoral groove to the center of the patellar tendon attachment to the tibial tubercle. This is done by superimposing the line perpendicular to these points and measuring the distance between the two lines, adjusting for magnification. Dejour and Walch22 reported a mean TT/TG distance of 12.7 ± 3.4 mm in a group of asymptomatic knees. In a group of knees with patellar instability, the mean TT/TG distance significantly increased to 19.8 ± 1.6 mm (P < .001). The authors concluded that the pathologic threshold is 20 mm, which has been agreed upon by others.89 In some knees with trochlear dysplasia, it is difficult to determine the true center point for the measurement, rendering the TT/TG offset to be inaccurate. In these knees, this measurement cannot be used as an evaluation measurement of the desired operative correction.
SURGICAL TECHNIQUES
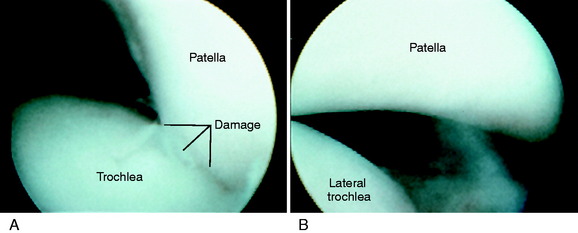
The patient is positioned supine on the operating table. Under anesthesia, the peripatellar retinaculum, patellofemoral crepitation, patellar tilt, passive medial-lateral patellar glide (0°, 30° flexion), and Q-angle are assessed and compared with preoperative measurements. A sterile thigh tourniquet is applied as proximal as possible, so that an intraoperative assessment of the Q-angle can be made. Routine diagnostic arthroscopy is performed, and any intra-articular pathology is evaluated and treated. Particular attention is paid to patellar position, mobility, tracking, and articular surface. Approximately one fourth of the patella should establish trochlear contact at 0° to 5° of knee flexion. The medial and lateral translation of the patella is assessed at 0° and 30° of knee flexion (Fig. 39-7). This is an important test, because a lateral translation of the patella greater than 50% of its width indicates incompetency of the MPFL. The medial translation of the patella tests for lateral retinacular tightness, and a normal lateral translation of 10 to 12 mm should be obtained with the knee at 30° of flexion. The patella and femoral groove are assessed for articular cartilage pathology or dysplasia. Articular cartilage lesions are evaluated for nature, location, and depth. The finding of loose flaps of articular cartilage requires a careful débridement; otherwise, fibrillated cartilage is left alone because it is possible to produce further damage by the débridement procedure. Radiofrequency cartilage débridement devices are never used, because excessive cartilage damage may occur. Most important at the time of arthroscopy is the critical assessment of whether the realignment procedure will transfer the loading from soft or fragmented articular surface to an intact or less involved cartilage surface. The articular cartilage is graded by the classification system described in Chapter 47, Articular Cartilage Rating Systems.70
Lateral Retinacular Release
Critical Points SURGICAL TECHNIQUE
 Performed only if patellar medial subluxation test is abnormal (patellar medial subluxation ≤ 5 mm on the manual medial translation test, knee at 30° flexion).
Performed only if patellar medial subluxation test is abnormal (patellar medial subluxation ≤ 5 mm on the manual medial translation test, knee at 30° flexion). Arthroscopic retinacular release performed with commercial radiofrequency wand through anterolateral portal.
Arthroscopic retinacular release performed with commercial radiofrequency wand through anterolateral portal. Extensor mechanism reconstructed with medial plication of VMO and MPFL so that the VMO advancement is in line with its fibers and prior insertion.
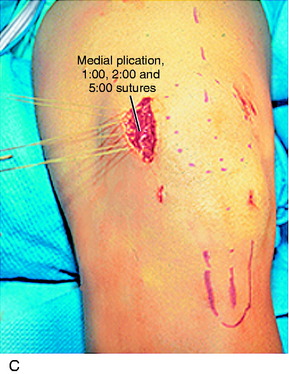
Extensor mechanism reconstructed with medial plication of VMO and MPFL so that the VMO advancement is in line with its fibers and prior insertion. Suture placed at 1 o’clock position (right knee) so that the distal part of the VMO is brought laterally in the line of its attachment to restore normal tension at 30° flexion.
Suture placed at 1 o’clock position (right knee) so that the distal part of the VMO is brought laterally in the line of its attachment to restore normal tension at 30° flexion. Indicated symptomatic medial patellar subluxation, manual medial glide at 30° of flexion grossly positive.
Indicated symptomatic medial patellar subluxation, manual medial glide at 30° of flexion grossly positive. Goal is to restore lateral muscle function by reattachment of the VLO if possible and reconstruct lateral soft tissue restraints.
Goal is to restore lateral muscle function by reattachment of the VLO if possible and reconstruct lateral soft tissue restraints. Dissect distal VLO tendon proximally, reattach VLO as distally as possible to restore normal anatomy.
Dissect distal VLO tendon proximally, reattach VLO as distally as possible to restore normal anatomy. Tendon placed through a lateral patellar tunnel (10 mm), which enters and exits at the one third and two third junction points along the vertical height of the patella.
Tendon placed through a lateral patellar tunnel (10 mm), which enters and exits at the one third and two third junction points along the vertical height of the patella.In select cases in which there is extensive contracture of the lateral retinacular tissues and the VLO with associated arthrofibrosis, an open Z-plasty release is indicated as presented in Chapter 41, Prevention and Treatment of Knee Arthrofibrosis.
MPFL Reconstruction
MPFL deficiency is diagnosed when the patella translates greater than 50% of its width from the center of the sulcus. Symptomatic patients with chronic dislocations or subluxations will frequently demonstrate patellar translation of 75% to 100% (of its width) under manual pressure at both 0° and 30° of flexion (Fig. 39-8). An MPFL reconstruction is almost always indicated in revision surgery when a prior proximal realignment procedure has failed and a residual lateral patellar subluxation exists. The intraoperative examination includes the lateral subluxation test at 0° and 30° of flexion. The balancing of the medial soft tissue restraints requires that tension be restored at both knee positions to guide the patella into the trochlea and resist lateral patellar subluxation at full extension and with knee flexion. The operative procedure includes a balancing of three structures, namely, the MPFL, VMO, and medial retinacular fibers, including the MPML. Preoperative radiographs and MRI define whether a patella alta or a lateral tibial tendon offset exists, requiring distal correction.
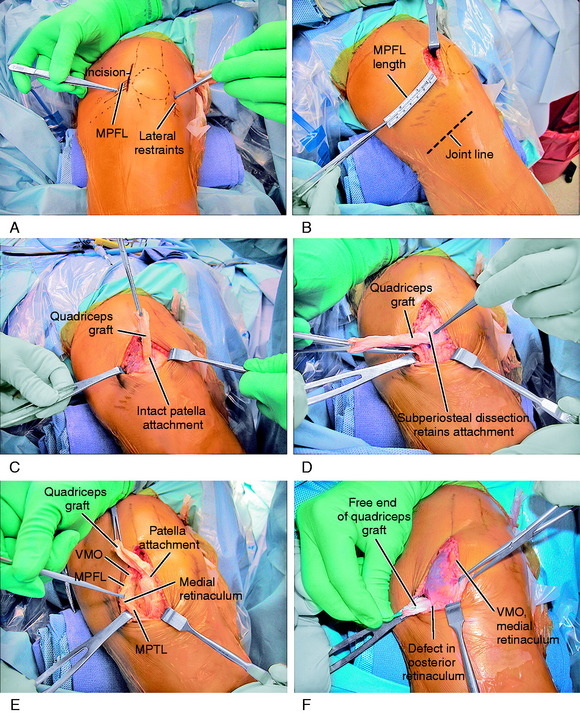
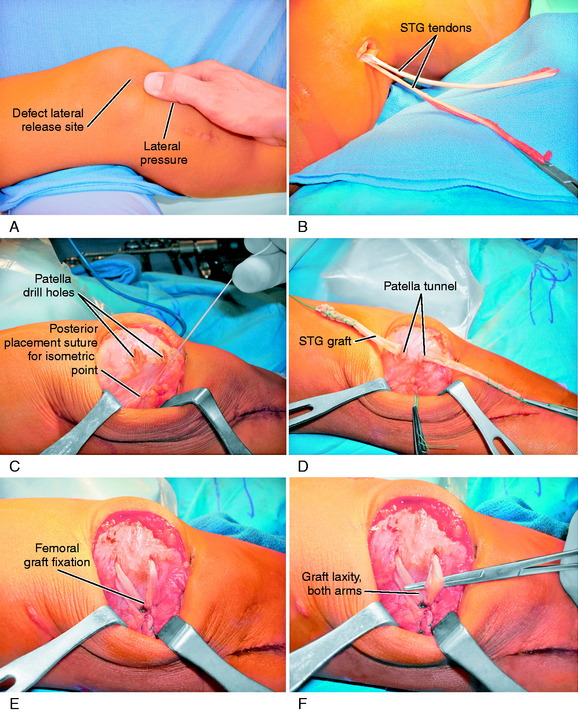
The operative steps of MPFL reconstruction are shown in Figure 39-9 and summarized in Table 39-1. A 5- to 6-cm incision is made at the medial border of the patella and extended 1 to 2 cm proximally (see Fig. 39-9A). A dissection of the skin flaps is carefully performed beneath the fascia layer to preserve the skin blood supply. This allows the incision to be mobilized in a proximal-distal direction, providing a cosmetic incision one half the usual length. The dissection is carried over the medial aspect of the knee and medial epicondyle to the adductor tendon. A head light and careful dissection technique is used at this point to avoid the infrapatellar nerve branches, which can result in injury and a painful neuroma. The medial nerves are highly variable in their course, as shown in Chapter 1, Medial and Anterior Knee Anatomy. The skin flaps are further developed in all directions to be able to reach the proximal aspect of the QT, a distance of 6 to 7 cm from the patellar insertion for the planned turn-down of the medial QT graft.
TABLE 39-1 Key Points to Successful Medial Patellofemoral Ligament Reconstruction
MPFL, medial patellofemoral ligament; MRI, magnetic resonance imaging; VMO, vastus medialis obliquus.
Using an Army/Navy retractor, the insertion of the VMO into the QT is identified. A ruler is used to measure the MPFL attachment length from the medial border of the patella, past the epicondyle to the adductor tubercle and tendon. This provides the length of the QT graft to be harvested (see Fig. 39-9B). A ruler is used to mark the medial QT to harvest a graft 8 mm wide and usually 60 mm in length. In a smaller knee, the graft is 6 mm in thickness. The full-thickness medial QT graft is harvested 5 mm from the medial border of the VMO to allow for VMO tendon-to-tendon suturing after harvest. The proximal graft never extends into the proximal QT-muscle junction, because this would weaken the attachment. Two full-thickness incisions are made into the medial QT at the marked graft site, avoiding penetration into the capsule. The incision connects to the prior medial patella retinaculum incision. The medial QT graft is transected proximally, preserving its attachment to the superomedial border of the patella (see Fig. 39-9C). The three tendon components of the QT (rectus, confluent VMO-VLO, intermedius) are carefully identified and the proximal end of the graft is sutured with a baseball-type stitch (No. 1 nonabsorbable suture).
With care taken not to transect the graft, subperiosteal dissection of the patella attachment of the graft is performed to a point approximately 30% of the proximal height of the patella at the normal MPFL attachment (see Fig. 39-9D). Later in the procedure, appropriate sutures are placed to secure the proximal patella attachment of the QT graft to adjacent tissues. Preserving the medial synovial pouch and avoiding penetration of the joint, the medial retinaculum is dissected off the capsule past the medial epicondyle to the adductor tendon (see Fig. 39-9E). At the junction immediately anterior to the adductor, distal to the insertion of the VMO, and superficial to the medial epicondyle, a hole is punctured in the retinaculum with a right-angle hemostat (see Fig. 39-9F). The graft is turned 90° and folded over on itself at the proximal medial portion of the patella. The free end of the QT graft is passed beneath the retinaculum, adjacent to the VMO attachment, and through the created retinaculum hole to exit and lay over the adductor tubercle and tendon.
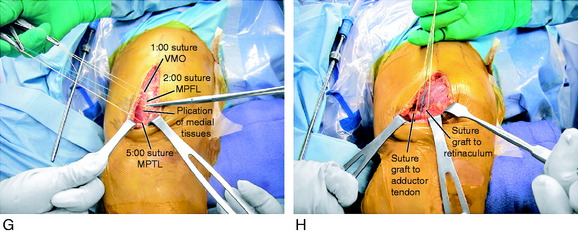
The final proximal realignment tensioning procedure is then performed following the steps previously outlined (and after the distal realignment when added to the overall realignment surgery). With the knee flexed 30° and the patella located within the trochlea, the medial vest-over-pants procedure is performed, including closure of the QT graft harvest site. The entire medial soft tissue and VMO is grasped with an Ellis clamp placed at the 1 o’clock (VMO), 2 o’clock (MPFL), and 4 o’clock (retinaculum, MPTL) areas and gently brought in line of its attachments to determine the amount of plication and overlap for suturing. Three sutures are placed at these respective sites with care taken not to overtension the repair. It is emphasized that there must always be a normal lateral glide of 25% of the width of the patella at 0° and 30° of flexion under manual pressure to avoid limiting normal patellar mobility. The imbrication procedure restores the patella to its reduced position (see Fig. 39-9G).
The final portion of the procedure consists of tensioning the MPFL-QT graft. Two absorbable sutures are passed into the adductor tendon, avoiding penetrating the medial geniculate at the posterior border of the VMO and the medial retinacular nerve (see Chapter 1, Medial and Anterior Knee Anatomy). The sutures are brought up through the free end of the graft that lays on the adductor tendon. No tension is applied to the graft, which simply lies in its created tunnel underneath the retinaculum and over the adductor tendon, because the tension in the medial soft tissue restraints has already been set by the proximal realignment just described. Three to four absorbable sutures are passed through the medial retinaculum into the graft to complete the procedure. Again, the lateral glide test is performed to make sure the graft is not under any resting tension and that the combined medial soft tissue imbrication and MPFL reconstruction is initially lax and allows the patella to be displaced 25% of its width, restoring the normal length of the medial soft tissues. After suturing is complete, the knee should be ranged from 0° to 135° restoring normal patellofemoral tracking. An additional two to three sutures are then placed in a figure-eight fashion, reinforcing the fixation of the graft to the medial retinaculum around the rent created for the graft to pass through the retinaculum (see Fig. 39-9H).
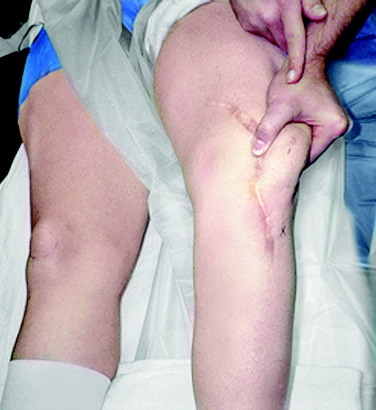
The advantage of this procedure is that no bone tunnels are placed into the patella or medial femoral condyle, as described in other MPFL reconstructions. The graft is sutured along the path of the native MPFL by pliable soft tissue fixation, reproducing patella and femoral attachments. It is not necessary to add a harvest of a medial hamstring tendon. The disadvantage of the procedure is related to the medial QT graft harvest, because this adds additional postoperative pain in comparison with a hamstring autograft. In knees with previous dislocations or revision cases, the restoration of the normal proximal VMO tension is performed with closure of the proximal harvest site. It is important that MPFL reconstruction be performed in the majority of knees with a prior dislocation, because it is usual to find a medial retinaculum that is thin with no identifiable MPFL structure. The resultant cosmetic appearance of the incision and restoration of full knee flexion are demonstrated in Figure 39-10.
Distal Realignment
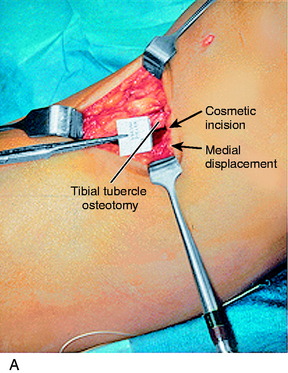
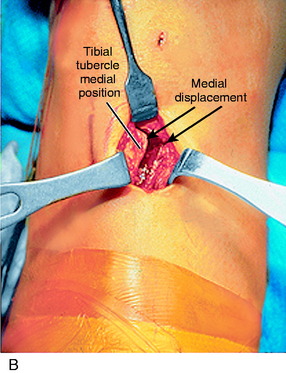
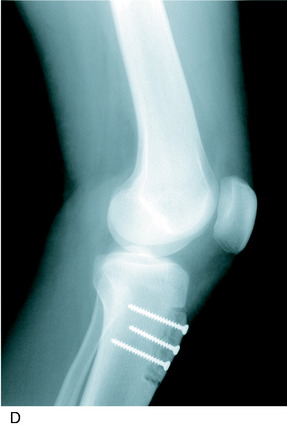
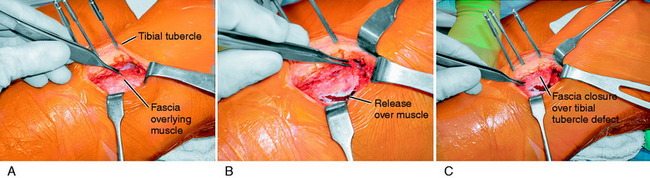
A lateral radiograph is used to confirm that a normal patella tendon length and patella height exist, as presented in Chapter 41, Prevention and Treatment of Knee Arthrofibrosis. A 3-cm vertical skin incision is placed lateral to the tibial tubercle (Fig. 39-11). The subcutaneous tissues are undermined sufficiently to create a skin flap that would allow the skin incision to be moved in all directions and maintain a small cosmetic incision. The patellar tendon insertion is identified and a retractor placed behind it to identify its insertion site on the tibial tubercle. A longitudinal incision is made over the periosteum, along the lateral border of patellar tendon. Subperiosteal dissection is carefully performed to expose the anterolateral aspect of the tibia, reflecting the periosteum and the muscular origin of the anterior compartment. Care is taken in the dissection not to enter into the muscle tissues to decrease postoperative pain and swelling. The planned tibial tubercle osteotomy measures 12 to 15 mm width, 8 mm thick, and 35 mm long. At the distal extent of the osteotomy, a hole is drilled through the tibial cortex, using a 3.2-mm drill bit and drill guide. This is to prevent the osteotomy from extending distally. The drill hole is placed beneath the anterior tibial periosteum, which maintains the normal proximal-to-distal position of the tibial tubercle. Similarly, four to five holes are drilled on the anterolateral tibial cortex, along the line of the planned osteotomy; the angle of the osteotomy is oblique by 15° to 20° from a posterolateral-to-anteromedial direction. An axial 90° cut is made just proximal to the patellar tendon insertion to mark the proximal extent of the osteotomy. This step-cut produces a bony buttress to prevent proximal migration of the tibial tubercle. The drill holes along the anterolateral surface of the tibia are then connected using a ½-inch osteotome. The osteotome is carried through the medial cortex directly adjacent to the tibial tubercle, while the distal periosteal hinge remains intact. The bone fragment is carefully mobilized and translated medially, according to a predetermined amount based on TT/TG distance correction. This distance is usually 8 to 10 mm. The tibial tubercle is secured with a 3.2-mm drill bit.
At this point, hemostasis is achieved, followed by the closure of the wound in layers. There is a lateral tibial tubercle defect after the medial displacement that requires a simple shifting of the anterolateral tissues (Fig. 39-12), which closes the defect and prevents a cosmetically unappealing indentation and concavity in this area after healing of the incision. The skin is closed using subcuticular stitches. A Hemovac drain is not routinely used. A double-cotton, double-Ace compression dressing is applied, and the lower extremity is placed in a brace. Cryotherapy is instituted immediately after surgery in the operating room.
Lateral Patellofemoral (Iliopatellar Tract) Reconstruction
The anatomy of the lateral iliopatellar tract is presented in detail in Chapter 1, Medial and Anterior Knee Anatomy. The iliopatellar tract is divided into the superficial oblique retinaculum and a second layer termed the deep transverse fibers. The attachment of the VLO to the superolateral aspect of the patellae is shown in Chapter 1, Medial and Anterior Knee Anatomy (see Fig. 1-13).
As previously discussed, a lateral release should be performed only when there is an abnormal contracture of these tissues, with an inability to perform a manual lateral translation (glide) at 30° of flexion. This condition may occur with joint arthrofibrosis or from a developmental standpoint referred to as the lateral patellar compression syndrome that may be associated with a bipartite patella. In the 1970s and 1980s, some authors recommended a lateral release that included resection of the VLO attachment for lateral patellar subluxation.57,59,60 It is now appreciated that the appropriate treatment of a lateral patellar subluxation is restoration of the MPFL, and not by weakening the VLO. There remain descriptions of the lateral release of soft tissues and VLO to the extent that the patella can be everted, which should be avoided.
The syndrome of a medial subluxation after a release of the lateral restraining structures and VLO has been identified by numerous authors.5,42,44,69,84 Nonweiler and DeLee69 reported on a series of five patients who presented with medial subluxation, pain, swelling, and giving-way after isolated lateral release. All underwent reconstruction of the lateral retinaculum. An average of 3.3 years postoperatively, four of the five had no symptoms of instability and the patellar stability was similar to that of the contralateral limb. Hughston and Deese42 described a series of 60 knees treated after failure of a lateral release to improve symptoms. Thirty knees had developed medial subluxation of the patella, 17 of whom underwent an isolated lateral release and 13 of whom underwent a concomitant proximal or distal realignment. Twenty-seven of these 30 knees had disabling symptoms postoperatively, and all demonstrated marked quadriceps atrophy and retraction of the VLO. Biedert and Friederich5 described 41 cases of patients who had pain after a lateral release; 32 had also had medial subluxation of the patella.
Christoforakis and colleagues14 measured the effects of lateral release on the lateral stability of the patella in cadaveric specimens (aged 65–82 yr). The patella was displaced 10 mm laterally while measuring the required force, with 175 N quadriceps force. Patellar force-displacement behavior was measured from 0° to 60° of knee flexion in intact knees and then after lateral release, which extended from the proximal limit of the lateral retinaculum to Gerdy’s tubercle. At 0°, 10°, and 20° of flexion, the mean force required to displace the patella (10 mm laterally) was significantly reduced after lateral release by 16% to 19% (P = .002–.001). The investigators concluded that the procedure decreased the lateral stability of the patella in normal elderly knees.
The reconstruction of the lateral soft tissue restraints is accomplished with a semitendinosus tendon autograft, which is preferred over an allograft to avoid delayed remodeling (Fig. 39-13). If the semitendinosus tendon is small in diameter, the gracilis tendon is also incorporated into the construct. The tendon is placed through a lateral patellar tunnel, which enters and exits at the one third and two thirds junction points along the vertical height of the patella. The lateral patella tunnel for the graft is approximately 10 mm in length, with a diameter matching that of the tendon. Each end of the tendon is sutured with a baseball-type stitch and the length adjusted so that 25 mm of tendon will pass into the femoral tunnel used for the posterior attachment of the graft. An incision is made at the junction of the iliopatellar tract and the ITB to expose the lateral aspect of the femoral condyle and lateral intramuscular septum. The isometric point for the femoral tunnel on the lateral femoral condyle is identified by using a suture fixed to the lateral patella tunnel site and a guidewire placed at the lateral femur with full extension-flexion produced. The usual site for the graft is posterior just above the lateral intermuscular septum and proximal to the lateral epicondyle. A drill hole is placed at this site and a Beath pin used to pass the two ends of the tendon into the tunnel. A soft tissue interference screw is used for fixation. The tension in the graft prior to fixation is adjusted by placing the knee at 30° of flexion and allowing a normal 10 to 12 mm manual medial glide. The graft should be under no tension in its resting state and under tension only to resist manual medial patellar displacement. It is important not to overtension the graft and produce a lateral soft tissue contracture, which would potentially lead to articular cartilage damage (iatrogenic lateral patellar compression syndrome). Following tensioning, the knee is taken through a full range of motion. The wound closure is routine and a drain is not necessary. The rehabilitation program is the same as that described for an MPFL reconstruction.
Patella Alta Correction
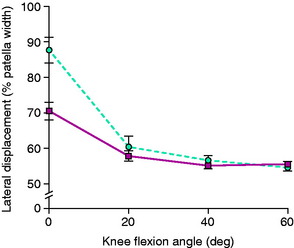
Patella alta represents a congenital abnormality of an increased vertical position of the patella due to an elongated patellar tendon resulting in the patella not engaging within the trochlea until a midflexion range of motion. Patients typically present with significant complaints related to lateral patellar subluxation or dislocation that can affect all activities of daily living. Ward and coworkers95 studied patients with patella alta with MRI under partial weight-bearing loads that induced quadriceps tension at various knee flexion angles and reported increased lateral displacement and lateral tilt at 0° degrees of knee flexion only (Fig. 39-14). There was a decrease in patellofemoral joint contact area in subjects with patella alta at all knee flexion angles. The data did not explain the basis for anterior knee pain in symptomatic patella alta patients, and the authors recommended the need for further studies. In most patients, the patella alta abnormality does not occur in isolation and other abnormalities of the extensor mechanism are usually present. Some patients with patella alta and anterior knee pain and joint swelling have signs of patellofemoral crepitus and arthritis, but do not experience subluxation symptoms.
The major factors for patellar instability include patella alta, MPFL deficiency, trochlear dysplasia, and patellar tendon lateral offset, as already discussed. Associated increased femoral anteversion and increased external tibial torsion add to the predisposition for lateral patellar instability. The decision to correct an abnormal patellar tendon length to allow the patella to engage within the trochlear would address only one aspect of the problem in cases of lateral patellar subluxation or dislocation. Additional correction of an MPFL deficiency and lateral patellar offset may be required. At surgery, the tibial tubercle is advanced distally to restore a normal patellotrochlear relationship. In this corrected position, the medial-lateral manual translation tests determine the new tension relationship of the MPFL and lateral restraints and the need for rebalancing the tension by surgical correction. The senior author has no experience with correcting trochlear dysplasia and this has not been a part of the operative procedure. Dejour and Walch22 analyzed the radiographs and CT scans of 143 knees with symptomatic patellar instability and 67 contralateral asymptomatic knees to determine the factors affecting patellar instability. These authors reported that trochlear dysplasia was present in 96% of unstable patella. Although it would be ideal to correct a trochlear dysplasia, the problem remains that the patella is still flattened and dysplastic and it is therefore not possible to restore a normal patellofemoral contact pattern by surgically deepening the trochlear groove. Abnormally high patellofemoral contact pressures would be expected after trochlearplasty procedures that may lead to short-term cartilage deterioration. Rather, the approach taken is to restore a normal patellotrochlear contact and MPFL function and correct an abnormal lateral patellar offset when present.
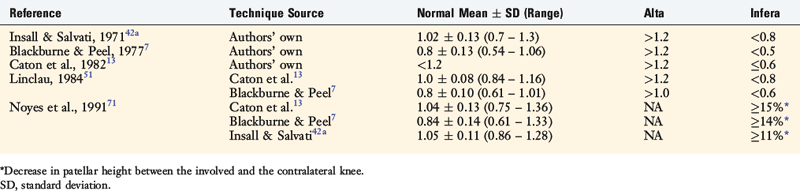
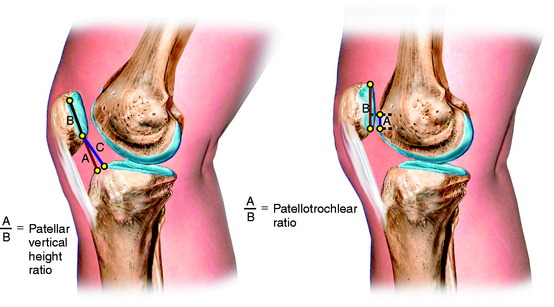
There are differences in the methods used for measurement of patellar height regarding the anatomic points selected and the values used for the classification of an alta, normal, or infera position of the patella. Seil and colleagues80 compared five different patellar height ratio techniques in 21 patients and reported that the classification of alta, normal, and infera depended on the normative data chosen for each technique. The normal values for patellar height, and those selected to determine the presence of patella alta and patella infera, are shown in Table 39-2. Noyes and colleagues71 conducted a study to determine normal right to left patellar vertical height ratios within the same individual in a group of 51 patients (102 knees). The difference in the vertical height ratio of the patella between these two methods ranged from 0% to 9%, with an average difference of 3%. Large variations existed in the ratios between individual patients (range, 0.75–1.46).
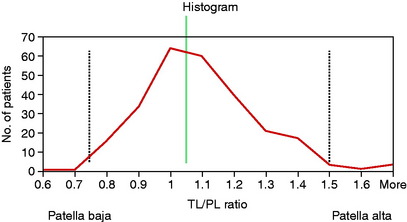
Shabshin and coworkers83 reported patellar tendon length to patellar length on MRI in 245 patients using the Insall-Salvati method. The results of the measurement are shown in Figure 39-15. The patients were not selected out as to diagnosis and were referred for orthopaedic evaluation for a number of disorders; thus, it is not known how many patients had a clinical diagnosis of a patellar subluxation or dislocation, which is an unaccounted variable in the study. In addition, the measurements were made with the knee in full extension with the possibility of an inaccurate length for the patellar tendon in a resting position.
Biedert and Albrecht6 described an MRI method to measure the true articular cartilage sagittal patellotrochlear relationship at 0° extension. The ratio of the patella to trochlea cartilage contact was described as a percentage and the mean index was 32% ± 12%. An index value greater than 50% documented a patella infera and less than 12.5% documented a patella alta (Fig. 39-16). This is a useful index in surgical cases of patella alta in terms of determining at surgery the distal displacement of the tibial tubercle required to establish a normal patellotrochlear relationship. The authors believed this index is more accurate than the numerous published indices (e.g., Blackburne and Peel,7 Linclau,51 and Caton and colleagues13), which rely on the length of the patella articular cartilage to a defined tibial reference point, but do not indicate the final patellofemoral joint position or define an alta or an infera relationship. The main problem with the patellotrochlear index method is the inability to obtain a quadriceps contraction during the MRI to define that the patella is at a maximum proximal relationship. In the absence of a quadriceps contraction, the patellar may appear to be lower and the maximum patellar height will not be measured.
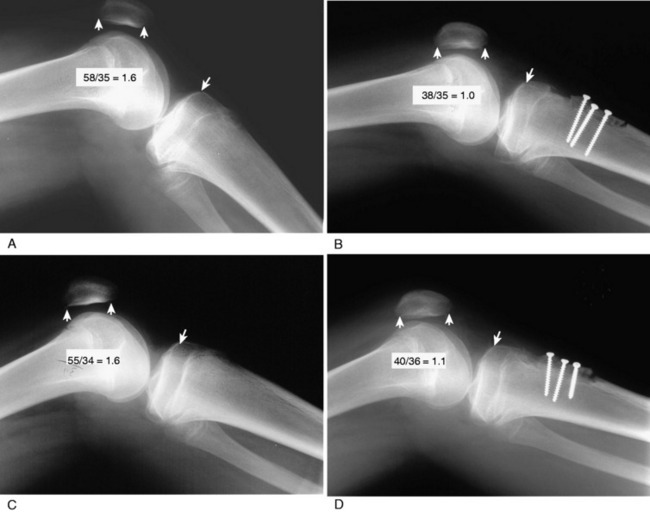
In Figure 39-17, radiographs of a 16-year-old male who had multiple recurrent lateral patellar dislocations are shown that demonstrate bilateral patella alta. The Linclau ratio was 1.6 and the patellotrochlear index demonstrated no patellar contact at full extension. The corrective bilateral operative procedures had to be delayed 2 years to allow for completion of growth. A distal transfer of the tibial tubercle was performed to restore these two indices to normal values. Lateral radiographs taken at surgery are required to measure that the normal patellar height ratio has been restored and to prevent too distal a transfer and a patella infera condition. A concurrent MPFL reconstruction was also performed.
Neyret and colleagues66 measured the patellar tendon length on lateral radiographs and MRI in 42 knees with patellar dislocation and 51 control knees and reported that the patellar tendon was 8 mm longer in the former group (mean length, 52 ± 6 mm, and range, 39–61 mm; and 44 ± 7 mm and range, 32–62 mm, respectively; P < .0001). The wide range in patellar length shows the marked variation in patellar length from patient to patient even in the dislocation group. The Caton-Deschamps index was abnormal (>1.20) in 48% of the dislocation group and 12% of controls, and the values for the MRI were 60% and 12%, respectively. There was no difference in the distance of the tendon insertion on the tibia compared with that on the tibial plateau. The authors suggested that when a distal transfer of the patellar tendon in patellar alta cases is performed, a tenodesis of the tendon at the tibial insertion site would restore normal tendon length and decrease side-to-side patellar mobility, given the high percentage of associated trochlear dysplasia. The senior author of this chapter has no experience with this operative technique.
Lancourt and Cristini49 used the Insall-Salvati method; however, these authors used the patellar tendon length as the denominator and the patellar length as the numerator, which is the reverse of the original description of this method. They reported that the Insall-Salvati index was 1.0 in normal patients, 0.80 in dislocating patella (alta), and 0.86 (infera) in chondromalacia (patella grating, crepitus), with the differences statistically significant (P < .05). The authors believed that patella alta results in patellotrochlear incongruity and risk for early patellofemoral arthritis. Marks and Bentley56 used a similar Insall-Salvati method in 51 patients with patellar chondromalacia graded at arthroscopy and reported no definite relationship to patella alta, suggesting that recurrent dislocation provided a stronger relationship.
Al-Sayyad and Cameron1 reported short-term improvements in patellofemoral scores (1–4 yr postoperatively) in 25 patients with painful patella alta and no history of patellar dislocation who underwent distal transfer of the tibial tubercle. The authors did not provide a system for computing the measurement of the millimeters of distal transfer of the tibial tubercle, except for maintaining a minimum of 13 mm from the inferior patella pole and proximal tibial surface. A patient satisfaction rate of 88% was reported. Patients with a normal-appearing trochlea had higher scores compared with those with trochlea articular cartilage damage. The study reported cartilage lesions typically involved the inferolateral portion of the lateral patella facet, with possible involvement of the lateral region of the trochlea. The authors stressed that patella alta may be a source of anterior knee pain not responsive to nonoperative treatment as well as a leader to the development of patellofemoral arthritis.
The indications for correcting a patella alta are recurrent dislocations and symptomatic anterior knee pain (and an obvious patella alta) that has not responded to conservative treatment. Commonly, there are associated patellar crepitus and articular cartilage degeneration, and the patient is advised that symptoms of anterior knee pain related to the arthritis will continue. It is thus preferable to correct a symptomatic patellar alta condition early prior to the development of cartilage deterioration. At the time of a proximal or distal realignment, a patella alta results in the distal patella articular cartilage not engaging the trochlea, but lying in a more cephalad position. It is at this point that lateral subluxation or dislocation events occur, particularly with a dysplastic shallow trochlea. In addition to correcting the patella alta, a functional MPFL is required, because there is a loss of the normal geometric restraint provided by the trochlear groove. There are no established clinically proven rules regarding when correction of an abnormal patella height is required or the amount of correction to be obtained at surgery. The goal is to restore a normal patellar height index for the index chosen and to confirm that patellotrochlear contact (∼30% of the inferior patellar articular cartilage) has engaged the trochlear at full extension. These rules are empirical; however, they do provide the surgeon with guidelines to follow during the surgical technique. The operative steps for the correction of a patella alta are shown in Table 39-3.
TABLE 39-3 Operative Steps in the Technique to Correct Patella Alta
MPFL, medial patellofemoral ligament; TT/TG, tibial tubercle/trochlear groove.
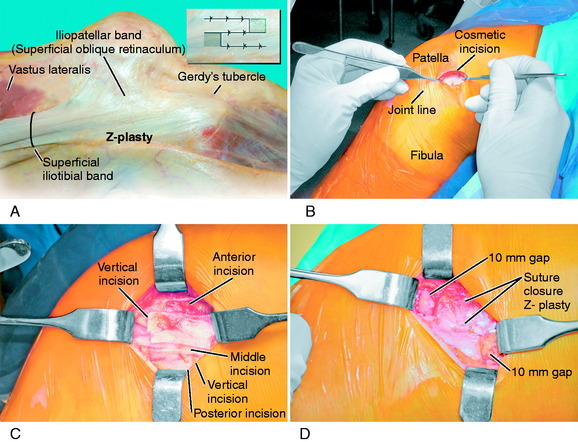
Iliotibial Band Z-Plasty Release for Contracture
The technique for a Z-plasty release of a tight ITB is demonstrated in Figure 39-18. After conservative modalities have failed, including one or two local cortical steroid injections, ITB stretching exercises over 4 to 6 months, and a return of symptoms, an ITB release may be indicated. The goal of the procedure is to restore the normal length of the ITB and the normal tension in both arms of the released ITB. The alternative technique that has been described is to remove a window of the ITB, which has the disadvantage of essentially removing the function of the ITB. In addition, there may be remaining anterior and posterior portions of the ITB that still require release. The Z-plasty release has the advantage of restoring normal anatomy. It is important to explore the soft tissues beneath the ITB for abnormal bursae tissue and a sensory nerve.
POSTOPERATIVE MANAGEMENT
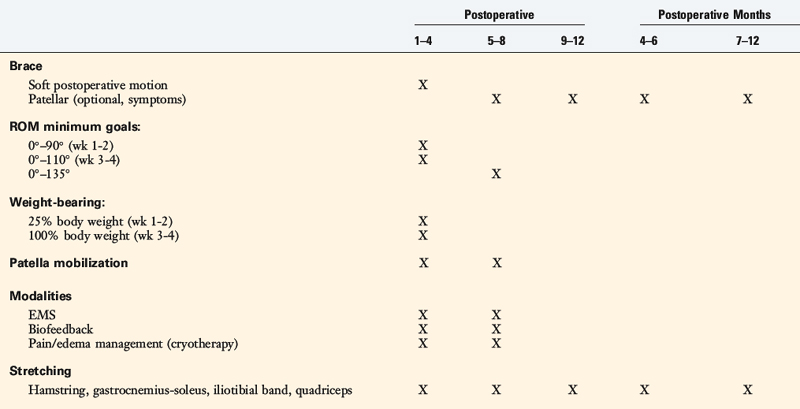
The postoperative rehabilitation protocol is summarized in Table 39-4. This protocol was developed for patients undergoing proximal and distal extensor mechanism realignment procedure, including MPFL reconstruction. Patients are placed into a postoperative long-leg brace for the first 4 weeks. Patellar mobilization in superior-inferior and medial-lateral directions is begun immediately after surgery to prevent parapatellar contractures. The goal for the 1st week is to obtain 0° to 90° of motion. Knee flexion is gradually increased to 110° by the 4th week and then full motion of at least 135° is allowed by the 8th week. This limitation of flexion in the first 4 weeks is designed to protect the suture lines and the repair when a proximal realignment procedure is performed. Patients are allowed to bear 25% of their body weight for 2 weeks; full weight-bearing is allowed between the 4th and the 6th week. When a distal realignment osteotomy has not been performed, full weight-bearing after 3 to 4 weeks is allowed based on patient control and muscle strength parameters.
COMPLICATIONS
CLINICAL STUDIES
MPFL Reconstruction
A summary of the published clinical studies on MPFL graft reconstructions is shown in Table 39-5. A variety of grafts were used, including the gracilis and semitendinosus tendons, the QT, the patellar tendon, the adductor magnus, and artificial ligaments, along with different fixation methods. In the majority of studies, the prevention of recurrent dislocation or subluxation episodes was used as a primary outcome factor, along with rating activities of daily living. Typically, fewer than 5% of patients suffered dislocation or subluxation episodes postoperatively. The most widely used outcome rating instrument was the Kujala score48 (0–100 points), which was designed to rate patellofemoral disorders by measuring the following factors: limp, support, walking, stairs, squatting, running, jumping, prolonged sitting with knees flexed, pain, swelling, knee subluxation, thigh atrophy, and flexion deficiency. All of the studies reviewed reported statistically significant improvement in this score postoperatively.
Few authors assessed activity levels postoperatively with a validated rating system or determined the effect of articular cartilage damage on outcome. Various techniques were used to ascertain patellar stability, including physical examination and stress radiographs.19,20,62,63 Because most of the study cohorts were followed short- to mid-term postoperatively (<10 yr), the percentage of patients in whom progression of patellofemoral arthritis occurs after this operation is unknown.
Smith and coworkers86 conducted a systematic review of the existing literature of MPFL reconstruction and found eight studies that met their selection criteria. The authors concluded that the procedure may provide a favorable outcome; however, many methodological problems exist in the published investigations that should be appropriately resolved in future studies. Owing to the heterogeneity of the studies, a formal meta-analysis could not be conducted.
Autologous Chondrocyte Implantation
The initial report of autologous chondrocyte implantation (ACI) used for patellar articular cartilage lesions noted disappointing results,8 although improvements were noted in two small series of patients75,77 when the procedure was done with an extensor mechanism realignment. Minas and Bryant64 reported on 45 patients followed a mean of 46.4 months (range, 2–7 yr) after ACI for lesions in the patellofemoral joint. The lesions were either isolated to the patella or trochlea or combined with condylar defects. Either a concomitant tibial tubercle osteotomy or a high tibial osteotomy was done in 29 of the patients (64%). At follow-up, significant improvements were reported in several subjective scores (Medical Outcomes Study Short-Form 36-item questionnaire [SF-36], Western Ontario and MacMaster Universities [WOMAC], modified Cincinnati activity, all P < .001). There were 8 failures caused by a patella or trochlea graft failure. Seventy-one percent of the patients were satisfied with the outcome.
Mandelbaum and associates54 reported the outcomes of 40 patients (mean age, 37.1 ± 8.5 yr) from 34 centers treated with ACI for isolated trochlear lesions. Before the operation, 48% had undergone a failed microfracture, abrasion arthroplasty, or drilling procedure; 23% had a tibiofemoral osteotomy; and 13% had a lateral release or Fulkerson procedure. At a mean of 59 months (range, 24–84 mo) postoperatively, significant improvements were noted in scores for pain, swelling, and overall function of the knee. The pain score improved from 2.6 ± 1.7 to 6.2 ± 2.4 points (modified Cincinnati Knee Rating System, scale 0–10 points, P < .0001), the swelling score improved from 3.9 ± 2.7 to 6.3 ± 2.7 points (P < .0001), and the overall condition score improved from 3.1 ± 1.0 and 6.4 ± 1.7 points (P < .0001).
Gobbi and colleagues35 followed 32 patients who received a “second-generation ACI” Hyalogaft-C (Fidia Advanced Biopolymers, Abano Terme, Italy), a tissue-engineered graft composed of autologous chondrocytes grown on a hyaluronan-based three-dimensional scaffold, for isolated defects in the patellofemoral joint. Twenty-two knees had lesions on the patella and 10 on the trochlea. At follow-up, 24 months postoperatively, significant improvement was noted in the mean International Knee Documentation Committee (IKDC) subjective score from 43.2 to 73.6 points (P < .001), as well in the objective IKDC rating (P < .001). Before the operation, 6 patients were rated on the IKDC objective knee evaluation as nearly normal and the remaining 26, as abnormal or severely abnormal. At follow-up, 29 patients were rated as normal or nearly normal and only 3 as abnormal on this scale. MRI at 24 months postoperatively showed 23 of the grafted defects had greater than 50% fill to complete fill, 25 had a normal or nearly normal signal, and none demonstrated hypertrophy or delamination.
Farr26 presented the results of a series of 34 knees that received ACI for isolated patella or trochlear lesions. The majority (95%) underwent a staged or concomitant procedure to correct an anatomic disorder, such as anteromedialization (Fulkerson). At follow-up, 2 years postoperatively, a significant improvement was noted in the Cincinnati overall condition rating score (P < .01). Nine patients rated their knee condition as excellent, 11 as very good, 11 as good, and 8 as fair or poor. Seven patients required follow-up surgery for mechanical symptoms related to the graft and 5 patients required débridement for arthrofibrosis. There were 3 treatment failures. The author concluded that combining ACI and corrective procedures is worthy of further study.
Henderson and Lavigne41 compared the results of a group of 22 patients who underwent ACI and concomitant proximal and distal extensor realignment with a group of 22 patients who underwent ACI only, without patellar malalignment. All patients had isolated patellar lesions and were followed from 9 to 55 months postoperatively. There was an even distribution between groups regarding the severity of patellar articular cartilage lesions. At follow-up, significantly higher mean scores were reported in the combined procedure group in the modified Cincinnati overall rating of the knee condition (P = .001) score, the SF-36 physical component score (P < .001), and IKDC clinical outcome score (P < .001). Nine reoperations were required for hypertrophy or extrusion of the graft, but there were no graft failures. The authors postulated that the difference in outcome between the groups may have been from the unloading effect of the realignment (osteotomy), because patellar tracking was normal in all patients postoperatively. The suggestion was made to unload the patellofemoral joint via a realignment procedure when ACI is performed, even in knees with normal patellar tracking.
Peterson and coworkers74 reported on 17 patients who received ACI for isolated grade III to IV (Outerbridge) patellar lesions. All patients were followed a mean of 7.4 years (range, 5–11 yr) postoperatively and none underwent a concomitant procedure with the ACI. The overall Brittberg clinical score was good or excellent in 65%. Significant improvements were noted in the modified Cincinnati grading of the knee condition score (from 1.6 to 6.8 points), the Lysholm score, and the Brittberg score visual analog score (P < .001). There were 4 treatment failures of patients who did not improve in symptoms; however, none of the grafts failed. The authors did not comment on the potential necessity to perform a concomitant proximal-distal realignment with ACI.
Osteochondral Autograft Transfer
Hangody and Fules38 summarized the results of 831 mosaicplasties, 118 of which were done for lesions located in the patellofemoral joint. The majority of patients (percentage unknown) underwent concomitant patellar realignment procedures. The results were assessed with a variety of scoring systems (modified Hospital for Special Surgery, modified Cincinnati, Lysholm). The clinical scores demonstrated “good-to-excellent” results in 79% of this subset of patients, which were the only data reported. This is the only series published to date to the authors’ knowledge on the results of this operation for patellofemoral articular cartilage lesions.
The 31 patellar osteochondral autograft transfer procedures that had survived at the time of writing were performed in 24 females and 7 males. The mean age at the time of the procedure was 32 years (range, 14–45 yr). Seventeen patients sustained an injury, and 14 patients had a gradual onset of symptoms without an injury. The mean time from the injury or onset of symptoms to the operation was 93 months (range, 6–283 mo). Significant improvements were found in the mean Cincinnati Knee Rating System scores (see Chapter 44, The Cincinnati Knee Rating System) from preoperative to follow-up for pain (2.4 ± 1.2 and 5.2 ± 1.9 points, respectively; P < .0001), swelling (3.8 ± 2.1 and 5.5 ± 1.9 points, respectively; P < .01), patient perception of the knee condition (2.6 ± 1.2 and 6.1 ± 2.1 points, respectively; P < .0001), walking (28 ± 9 and 36 ± 8 points, respectively; P < .001), and squatting (10 ± 2 and 16 ± 3 points, respectively; P = .01). Two patients rated their knee condition as poor, 3 as fair, 9 as good, 11 as very good, and 1 as normal. Complications included manipulation under anesthesia in 2 patients and reflex sympathetic dystrophy in two patients. The 5 patients who rated their knee condition as fair or poor did not improve in their subjective and functional scores.
ILLUSTRATIVE CASE
Acute Patellar Tendon Rupture
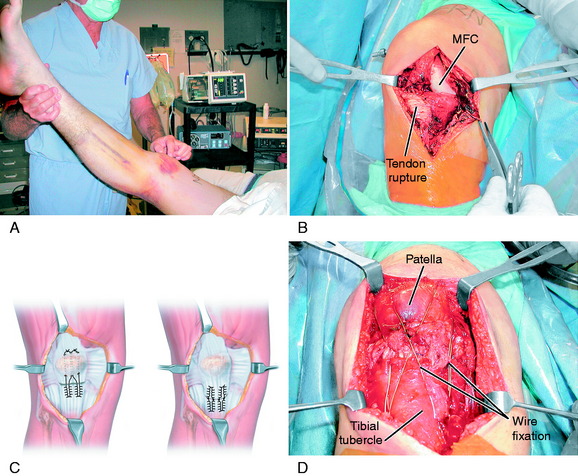
A demonstration of the surgical technique for an acute patellar tendon rupture is shown in Figure 39-19. In Figure 39-19A, the preoperative photograph shows subcutaneous hemorrhage along the anterior aspect of the knee in a patient who sustained a fall and acute rupture of the patellar tendon. The preoperative MRI showed extensive disruption throughout the entire patellar tendon. In Figure 39-19B, the initial exploration shows a “mop-end” appearance to the midsubstance patellar tendon rupture. Figure 39-19C and D shows the method of repair with interrupted baseball locking sutures from the distal tendon to the proximal tendon or through the patellar tunnel based on site of tendon rupture. In this case, a tendon-to-tendon repair was performed. Two wire sutures are placed through the tibial tubercle and distal one third of patella. One wire suture is placed through the tibial tubercle and distal quadriceps patellar attachment to function as a tension band. The use of wire fixation does involve the need for later removal; however, it provides a firm fixation of low cross-sectional area, which is an advantage over synthetic tape. The length of the repaired patellar tendon is adjusted at surgery and verified by fluoroscopy to be equal to the opposite lateral knee preoperative radiograph. It is important that immediate range of motion from 0° to 90° be initiated within the first 2 weeks after surgery, along with quadriceps muscle exercises to prevent a patella infera. The wire fixation allows initial weight-bearing with the knee in extension. A semitendinosus-gracilis graft augmentation was not required in this case, but may be indicated if the patellar tendon is severely disrupted, preventing primary tendon suture. This patient had a successful result and was weaned from crutches at 8 weeks postoperatively, regained a normal range of knee motion, and had a normal patellar height equal to that of the opposite knee. He was cautioned to avoid full weight-bearing on the operative limb in ascending and descending stairs until 20 weeks from surgery, and no sporting activities until 6 months after surgery.
1 Al-Sayyad M.J., Cameron J.C. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;396:152-162.
2 Amis A.A. Current concepts on anatomy and biomechanics of patellar stability. Sports Med Arthrosc. 2007;15:48-56.
3 Amis A.A., Firer P., Mountney J., et al. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10:215-220.
3a Avikainen V.J., Nikku R.K., Seppanen-Lehmonen T.K. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;297:12-16.
4 Bicos J., Fulkerson J.P., Amis A. Current concepts review: the medial patellofemoral ligament. Am J Sports Med. 2007;35:484-492.
5 Biedert R.M., Friederich N.F. Failed lateral retinacular release: clinical outcome. J Sports Traumotol. 1994;16:162-173.
6 Biedert R.M., Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14:707-712.
7 Blackburne J., Peel T. A new method for measuring patellar height. J Bone Joint Surg Br. 1977;58:241-242.
8 Brittberg M., Lindahl A., Nilsson A., et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895.
9 Bugbee W.D. Fresh osteochondral allografts. J Knee Surg. 2002;15:191-195.
10 Bugbee W.D., Convery F.R. Osteochondral allograft transplantation. Clin Sports Med. 1999;18:67-75.
11 Burks R.T., Luker M.G. Medial patellofemoral ligament reconstruction. Tech Orthop. 1997;12:185-191.
12 Cash J.D., Hughston J.C. Treatment of acute patellar dislocation. Am J Sports Med. 1988;16:244-249.
13 Caton J., Deschamps G., Chambat P., et al. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68:317-325.
13a Christiansen S.E., Jacobsen B.W., Lund B., Lind M. Reconstruction of the medial patellofemoral ligament with gracilis tendon autograft in transverse patellar drill holes. Arthroscopy. 2008;24:82-87.
14 Christoforakis J., Bull A.M., Strachan R.K., et al. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14:273-277.
15 Cofield R.H., Bryan R.S. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17:526-531.
16 Conlan T., Garth W.P.Jr., Lemons J.E. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75:682-693.
16a Cossey A.J., Paterson R. A new technique for reconstructing the medial patellofemoral ligament. Knee. 2005;12:93-98.
17 Cowan S.M., Bennell K.L., Crossley K.M., et al. Physical therapy alters recruitment of the vasti in patellofemoral pain syndrome. Med Sci Sports Exerc. 2002;34:1879-1885.
18 Crossley K., Bennell K., Green S., et al. Physical therapy for patellofemoral pain: a randomized, double-blinded, placebo-controlled trial. Am J Sports Med. 2002;30:857-865.
19 Deie M., Ochi M., Sumen Y., et al. A long-term follow-up study after medial patellofemoral ligament reconstruction using the transferred semitendinosus tendon for patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13:522-528.
20 Deie M., Ochi M., Sumen Y., et al. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85:887-890.
21 Dejour H., Neyret P., Walch G. Factors in patellar instability. In: Aichroth P.M., Cannon W.D., editors. Knee Surgery. Current Practice. New York: Raven; 1992:403-412.
22 Dejour H., Walch G., Nove-Josserand L., Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2:19-26.
23 Desio S.M., Burks R.T., Bachus K.N. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26:59-65.
23a Drez D.Jr., Edwards T.B., Williams C.S. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17:298-306.
24 Dye S.F. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;436:100-110.
25 Eckhoff D.G., Brown A.W., Kilcoyne R.F., Stamm E.R. Knee version associated with anterior knee pain. Clin Orthop Relat Res. 1997;339:152-155.
25a Ellera Gomes J.L., Stigler Marczyk L.R., Cesar de Cesar P., Jungblut C.F. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthroscopy. 2004;20:147-151.
26 Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment outcomes. Clin Orthop Relat Res. 2007;463:187-194.
27 Feller J.A., Amis A.A., Andrish J.T., et al. Surgical biomechanics of the patellofemoral joint. Arthroscopy. 2007;23:542-553.
27a Fernandez E., Sala D., Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71:303-308.
28 Ferris B., Aichroth P. The treatment of congenital knee dislocation. A review of nineteen knees. Clin Orthop Relat Res. 1987;216:135-140.
29 Fithian D.C., Mishra D.K., Balen P.F., et al. Instrumented measurement of patellar mobility. Am J Sports Med. 1995;23:607-615.
30 Fithian D.C., Paxton E.W., Stone M.L., et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32:1114-1121.
31 Froelke B.M., Elias J.J., Cosgarea A.J. Surgical options for treating injuries to the medial patellofemoral ligament. J Knee Surg. 2006;19:296-306.
32 Fulkerson J.P. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30:447-456.
33 Fulkerson J.P., Becker G.J., Meaney J.A., et al. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18:490-496. discussion 496–497
34 Garth W.P.Jr., DiChristina D.G., Holt G. Delayed proximal repair and distal realignment after patellar dislocation. Clin Orthop Relat Res. 2000;377:132-144.
35 Gobbi A., Kon E., Berruto M., et al. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006;34:1763-1773.
35a Gomes J.E. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24:430-435.
36 Grelsamer R.P., Stein D.A. Patellofemoral arthritis. J Bone Joint Surg Am. 2006;88:1849-1860.
37 Guenther K.P., Tomczak R., Kessler S., et al. Measurement of femoral anteversion by magnetic resonance imaging—evaluation of a new technique in children and adolescents. Eur J Radiol. 1995;21:47-52.
38 Hangody L., Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
39 Hautamaa P.V., Fithian D.C., Kaufman K.R., et al. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;349:174-182.
40 Hawkins R.J., Bell R.H., Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14:117-120.
41 Henderson I.J., Lavigne P. Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee. 2006;13:274-279.
42 Hughston J.C., Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16:383-388.
42a Insall J., Salvati E. Patella position in the normal knee joint. Radiology. 1971;101:101-104.
43 Jamali A.A., Emmerson B.C., Chung C., et al. Fresh osteochondral allografts. Clin Orthop Relat Res. 2005;437:176-185.
44 Johnson D.P., Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10:361-363.
45 Kannus P., Nittymaki S. Which factors predict outcome in the nonoperative treatment of patellofemoral pain syndrome? A prospective follow-up study. Med Sci Sports Exerc. 1994;26:289-296.
46 Koeter S., Diks M.J., Anderson P.G., Wymenga A.B. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89:180-185.
47 Kolowich P.A., Paulos L.E., Rosenberg T.D., et al. Lateral release of the patella: indications and contraindications. Am J Sports Med. 1990;18:359-365.
48 Kujala U.M., Jaakkola L.H., Koskinen S.K., et al. Scoring of patellofemoral disorders. Arthroscopy. 1993;9:159-163.
49 Lancourt J.E., Cristini J.A. Patella alta and patella infera. Their etiological role in patellar dislocation, chondromalacia, and apophysitis of the tibial tubercle. J Bone Joint Surg Am. 1975;57:1112-1115.
50 Larsen E., Lauridsen F. Conservative treatment of patellar dislocations. Influence of evident factors on the tendency to redislocation and the therapeutic result. Clin Orthop Relat Res. 1982;171:131-136.
51 Linclau L. Measuring patellar height. Acta Orthop Belg. 1984;50:70-74.
52 Maenpaa H., Huhtala H., Lehto M.U. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68:424-426.
53 Maenpaa H., Lehto M.U. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25:213-217.
54 Mandelbaum B., Browne J.E., Fu F., et al. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35:915-921.
55 Maquet P. Valgus osteotomy for osteoarthritis of the knee. Clin Orthop Relat Res. 1976;120:143-148.
56 Marks K.E., Bentley G. Patella alta and chondromalacia. J Bone Joint Surg Br. 1978;60:71-73.
57 McGinty J.B., McCarthy J.C. Endoscopic lateral retinacular release: a preliminary report. Clin Orthop Relat Res. 1981;158:120-125.
58 Meister K., James S.L. Proximal tibial derotation osteotomy for anterior knee pain in the miserably malaligned extremity. Am J Orthop. 1995;24:149-155.
59 Merchant A.C., Mercer R.L., Jacobsen R.H., Cool C. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg Am. 1974;56:1391-1396.
60 Metcalf R.W. An arthroscopic method for lateral release of subluxating or dislocating patella. Clin Orthop Relat Res. 1982;167:9-18.
61 Mihalko W.M., Boachie-Adjei Y., Spang J.T., et al. Controversies and techniques in the surgical management of patellofemoral arthritis. Instr Course Lect. 2008;57:365-380.
62 Mikashima Y., Kimura M., Kobayashi Y., et al. Medial patellofemoral ligament reconstruction for recurrent patellar instability. Acta Orthop Belg. 2004;70:545-550.
63 Mikashima Y., Kimura M., Kobayashi Y., et al. Clinical results of isolated reconstruction of the medial patellofemoral ligament for recurrent dislocation and subluxation of the patella. Acta Orthop Belg. 2006;72:65-71.
64 Minas T., Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005;436:30-39.
64a Muneta T., Sekiya I., Tsuchiya M., Shinomiya K. A technique for reconstruction of the medial patellofemoral ligament. Clin Orthop Relat Res. 1999;359:151-155.
65 Murphy S.B., Simon S.R., Kijewski P.K., et al. Femoral anteversion. J Bone Joint Surg Am. 1987;69:1169-1176.
66 Neyret P., Robinson A.H., Le Coultre B., et al. Patellar tendon length—the factor in patellar instability? Knee. 2002;9:3-6.
67 Nomura E. Classification of lesions of the medial patello-femoral ligament in patellar dislocation. Int Orthop. 1999;23:260-263.
67a Nomura E., Inoue M. Hybrid medial patellofemoral ligament reconstruction using the semitendinous tendon for recurrent patellar dislocation: minimum 3 years’ follow-up. Arthroscopy. 2006;22:787-793.
68 Nomura E., Horiuchi Y., Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7:121-127.
68a Nomura E., Inoue M., Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35:1851-1858.
69 Nonweiler D.E., DeLee J.C. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22:680-686.
70 Noyes F.R., Stabler C.L. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505-513.
71 Noyes F.R., Wojtys E.M., Marshall M.T. The early diagnosis and treatment of developmental patella infera syndrome. Clin Orthop. 1991;265:241-252.
72 Palmu S., Kallio P.E., Donell S.T., et al. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90:463-470.
73 Panagiotopoulos E., Strzelczyk P., Herrmann M., Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14:7-12.
74 Peterson L., Brittberg M., Kiviranta I., et al. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12.
75 Peterson L., Karlsson J., Brittberg M. Patellar instability with recurrent dislocation due to patellofemoral dysplasia. Results after surgical treatment. Bull Hosp Jt Dis Orthop Inst. 1988;48:130-139.
76 Peterson L., Minas T., Brittberg M., Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation. Results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
77 Peterson L., Minas T., Brittberg M., Nilsson A., et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212-234.
78 Sallay P.I., Poggi J., Speer K.P., Garrett W.E. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24:52-60.
79 Sayli U., Bolukbasi S., Atik O.S., Gundogdu S. Determination of tibial torsion by computed tomography. J Foot Ankle Surg. 1994;33:144-147.
79a Schottle P.B., Fucentese S.F., Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13:516-521.
80 Seil R., Muller B., Georg T., et al. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8:231-236.
81 Senavongse W., Amis A.A. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability. J Bone Joint Surg Br. 2005;87:577-582.
82 Servien E., Verdonk P.C., Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15:61-67.
83 Shabshin N., Schweitzer M.E., Morrison W.B., Parker L. MRI criteria for patella alta and baja. Skeletal Radiol. 2004;33:445-450.
84 Shea K.P., Fulkerson J.P. Preoperative computed tomography scanning and arthroscopy in predicting outcome after lateral retinacular release. Arthroscopy. 1992;8:327-334.
85 Sillanpaa P., Mattila V.M., Iivonen T., et al. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40:606-611.
86 Smith T.O., Walker J., Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2007;15:1301-1314.
87 Stefancin J.J., Parker R.D. First-time traumatic patellar dislocation: a systematic review. Clin Orthop Relat Res. 2007;455:93-101.
87a Steiner T.M., Torga-Spak R., Teitge R.A. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34:1254-1261.
88 Tamari K., Tinley P., Briffa K., Breidahl W. Validity and reliability of existing and modified clinical methods of measuring femoral and tibiofibular torsion in healthy subjects: use of different reference axes may improve reliability. Clin Anat. 2005;18:46-55.
89 Teitge R.A. Osteotomy in the treatment of patellofemoral instability. Tech Knee Surg. 2006;5:2-18.
90 Teitge R.A., Faerber W.W., Des Madryl P., Matelic T.M. Stress radiographs of the patellofemoral joint. J Bone Joint Surg Am. 1996;78:193-203.
91 Thomee R., Renstrom P., Karlsson J., Grimby G. Patellofemoral pain syndrome in young women. I. A clinical analysis of alignment, pain parameters, common symptoms and functional activity level. Scand J Med Sci Sports. 1995;5:237-244.
92 Trikha S.P., Acton D., O’Reilly M., et al. Acute lateral dislocation of the patella: correlation of ultrasound scanning with operative findings. Injury. 2003;34:568-571.
93 Trillat A., Dejour H., Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:813-824.
94 Turner M.S. The association between tibial torsion and knee joint pathology. Clin Orthop Relat Res. 1994;302:47-51.
95 Ward S.R., Terk M.R., Powers C.M. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89:1749-1755.
96 Witvrouw E., Danneels L., Van Tiggelen D., et al. Open versus closed kinetic chain exercises in patellofemoral pain: a 5-year prospective randomized study. Am J Sports Med. 2004;32:1122-1130.
97 Yoshioka Y., Cooke T.D. Femoral anteversion: assessment based on function axes. J Orthop Res. 1987;5:86-91.
98 Yoshioka Y., Siu D.W., Scudamore R.A., Cooke T.D. Tibial anatomy and functional axes. J Orthop Res. 1989;7:132-137.