CHAPTER 2 Oncologic Considerations for Breast Reconstruction
Timing of Reconstruction
Although many patients are able to be treated with breast conservation, a subset of patients will either require a mastectomy or choose mastectomy as their surgical option. For these patients, the plastic surgeon is critical in helping the patient determine whether they wish to have breast reconstruction. However, controversy exists regarding the timing of breast reconstruction – immediate versus delayed. Immediate reconstruction employs the skin-sparing mastectomy technique that was developed by Toth and Lappert in 19911 and offers several advantages. The reconstructed breast has an improved cosmetic outcome following immediate reconstruction due to preservation of the skin envelope. Psychologically, the patient wakes up with a breast mound rather than a flat chest wall. The patient has one hospital stay and one anesthetic for the majority of the surgery followed by additional outpatient procedures for revisions, nipple reconstruction, etc. The skin-sparing mastectomy technique has been evaluated from an oncologic perspective and there does not appear to be any increased risk for local or distant recurrence.2–7 Rozen et al performed a Medline literature review to evaluate the psychosocial need for immediate breast reconstruction and the issues surrounding oncologic safety. The authors’ review of previous studies concluded that immediate reconstruction does not increase local recurrence rates and does not delay the initiation of adjuvant chemotherapy or radiation. There was not a higher rate of complications in the setting of chemotherapy although there was a higher rate of complications in patients receiving adjuvant radiation therapy. Immediate breast reconstruction did have a positive effect on psychosocial outcomes including depression, anxiety, body image, self-esteem, self-image, emotional function, social function and sexual function.8
The oncologic safety of immediate reconstruction was also reviewed by Taylor et al.9 This was also a literature review and included analysis of 84 papers. The authors’ analysis concluded that there was not an increased risk of local or distant recurrence (although the studies reviewed were small and retrospective), and that detection of recurrence was not impaired by immediate reconstruction. In addition, no delay in the delivery of adjuvant chemotherapy was identified. With respect to radiation, there was a significantly higher rate of complications (capsular contracture, implant rupture, wound infections) in patients undergoing mastectomy and expander or implant placement who had radiation before or after reconstruction. With respect to autologous tissue reconstruction, complication rates were similar between patients undergoing radiation followed by TRAM reconstruction versus patients undergoing immediate reconstruction followed by radiation. However, fat necrosis and flap volume loss were more common in the immediate reconstruction group.9
Postmastectomy Radiation
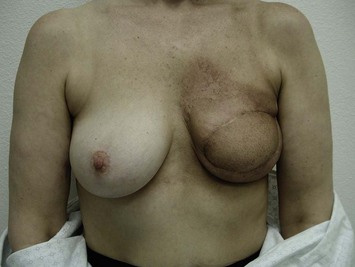
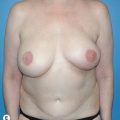
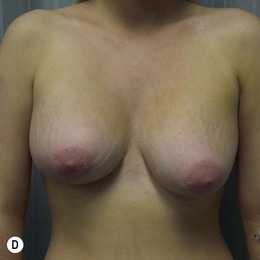
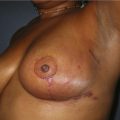
Current recommendations for postmastectomy radiation include four or more positive lymph nodes or advanced tumors (T3, T4, skin involvement). Close or positive margins are another indication for postmastectomy radiation. Based on recent Danish and Canadian trials, postmastectomy radiation for patients with one to three positive lymph nodes is becoming more common and therefore the overall use of postmastectomy radiation is increasing.10–12 The radiation may adversely affect the cosmetic outcome of the immediate reconstruction (Fig. 2.1) and there is some concern that the reconstructed breast may result in technical difficulties in the delivery of radiation therapy. This includes increased doses of radiation to the lungs or reduced radiation delivery to the internal mammary lymph nodes. If breast reconstruction is delayed until after radiation, however, the mastectomy skin is often compromised, and the shape of the native breast skin envelope lost. Delayed breast reconstruction with implants following postmastectomy radiation can result in wound healing problems, capsular contracture with subsequent implant displacement, and painful constriction against the chest wall.10 Many plastic surgeons will opt for autologous tissue when delayed reconstruction after radiation is performed.
To address this problem, surgeons at MD Anderson have described and published their experience with a two-stage approach that they have termed ‘delayed-immediate reconstruction.’ Stage 1 consists of a skin-sparing mastectomy with subpectoral insertion of a completely filled saline tissue expander to preserve the shape of the breast skin envelope. After review of the final pathology, patients who do not need radiation undergo immediate reconstruction within two weeks from the time of mastectomy (Stage 2). Patients who require radiation undergo therapy with the expander deflated to the chest wall. After radiation is complete, patients undergo re-expansion of the preserved breast skin.13
Nahabedian and Momen analyzed the local recurrence rate and survival of patients undergoing radiation therapy either before or after breast reconstruction. The patient cohort included 146 women who underwent breast reconstruction and radiation with a follow-up of at least 12 months. Tumor recurrence, survival, loss of implant and total flap necrosis were analyzed.14 The 59 women who underwent radiation after breast reconstruction had a higher local recurrence rate versus the 87 women who underwent radiation therapy prior to breast reconstruction (27% versus 15%). In addition there was a higher loss of life in the group undergoing breast reconstruction before radiation versus the group undergoing reconstruction after radiation (12% versus 7%). This raises concerns regarding the potential impact of immediate reconstruction on radiation delivery. This is a small study and it is difficult to draw definitive conclusions from this data. These questions may be better answered by a prospective trial.
BRCA Positive Patients
In these women who choose prophylactic surgery, consideration should be given to a coordinated approach for the mastectomies and bilateral salpingo-oophorectomies (BSO). Batista et al identified 12 patients who underwent combined mastectomies and BSO between 1996 at 2003. Ten of these patients underwent bilateral autologous tissue reconstruction. Six of these ten patients also underwent total abdominal hysterectomy during the same procedure. In this small retrospective study, there were no significant complications related to the gynecologic procedures.15 Therefore, it is important that these patients are cared for by a multidisciplinary team who can determine whether a combined approach is feasible.
Young Patients Considering Future Child-Bearing
Young women with breast cancer present additional challenges with respect to surgical options and reconstruction. Some of these patients will carry a BRCA mutation and may consider undergoing bilateral prophylactic mastectomy as discussed earlier. There also exists the issue of future pregnancy after autologous tissue reconstruction. Collin and Coady reported a case of a 33-year-old female who underwent a free transverse rectus abdominis myocutaneous (TRAM) reconstruction and subsequently became pregnant 1 year later. She was able to successfully carry her infant to term without any abdominal wall complications including width of donor site scar.16 Patients need to be counseled about the potential risks of pregnancy after TRAM including fascial tears, herniation, and scar widening. The exact safe time interval between TRAM and pregnancy is unknown. For breast cancer patients, we generally advise them to wait 2 years post treatment prior to considering pregnancy.
Locally Advanced Breast Cancer/Inflammatory Breast Cancer
Many patients have concerns regarding the oncologic safety of immediate reconstruction in the face of locally advanced breast cancer. This was addressed in a study by Newman et al, in which 540 patients undergoing immediate reconstruction following mastectomy were evaluated. Fifty of these patients had locally advanced breast cancer and all underwent postoperative chemotherapy. Forty percent of these patients underwent postoperative radiation therapy. At median follow-up of 58.5 months, there was no difference in local or distant recurrence between these 50 patients and 72 matched controls with locally advanced breast cancer who did not undergo reconstruction.17
In today’s paradigm, most patients with locally advanced breast cancer are treated with neoadjuvant chemotherapy. This allows not only for evaluation of how the chosen chemotherapy regimen is working on a patient’s particular tumor type, but it may shrink the tumor such that subsequent surgery is technically easier to perform. It also eliminates the concern about postoperative wound complications delaying the delivery of chemotherapy. Patients who develop wound infections or necrotic tissue must be completely healed prior to commencing chemotherapy.18 In patients with aggressive tumors, this can lead to concern regarding spread of disease.
Inflammatory breast cancer is a very aggressive subtype of cancer with involvement of the dermal lymphatics. Despite multi-modality treatment of chemotherapy followed by mastectomy and then radiation, the overall 5-year survival is 46%.19 These patients tend to have a high-rate of local recurrence, which is why they have traditionally not been considered candidates for immediate breast reconstruction. Two studies have recently challenged this recommendation. Slavin et al evaluated 10 patients with inflammatory breast cancer who underwent immediate reconstruction with a myocutaneous flap. Six of these patients developed local recurrence, however there was no effect on patient survival.20 In another study by Chin et al, 23 women underwent breast reconstruction after surgery for inflammatory breast cancer. Fourteen patients had immediate reconstruction while 9 patients had delayed reconstruction. At median follow-up of 44 months, there were no differences in outcome between the two treatment groups.21
Nipple-sparing Mastectomy
A recent phenomenon is the increased use of nipple-sparing mastectomy or total skin-sparing mastectomy in patients undergoing either prophylactic surgery or treatment for breast cancer. This procedure involved ‘coring-out’ the ductal tissue from within the nipple–areola complex to reduce the likelihood of a recurrence in the area of the nipple. Recent studies have addressed the concerns regarding nipple–areola recurrences as well as technical issues related to nipple necrosis. Sacchini et al analyzed data on 123 patients who underwent nipple-sparing mastectomy with breast reconstruction. Forty-four patients had invasive cancer, 20 had ductal carcinoma in situ (DCIS), and four had phylloides tumors. There were two local recurrences with one being DCIS and the other invasive cancer. Two patients developed breast cancer after prophylactic mastectomy. None of these recurrences or new cancers was in the nipple–areola complex. Eleven percent of patients developed nipple necrosis but this was minimal in 59% of patients.22 The authors concluded that local relapse after nipple-sparing mastectomy was very low and that this procedure might be feasible in select patients.
Garwood et al recently compared the first 64 patients undergoing total skin-sparing mastectomy at University of California, San Francisco between 2001 and 2005 to 106 patients undergoing total skin-sparing mastectomy between 2005 and 2007. The first cohort was analyzed in 2005 and techniques were altered to minimize risk factors for complications. An incision involving 30% or less of the nipple areolar complex resulted in improved nipple viability. Between cohort 1 and cohort 2, nipple survival rates rose from 80% to 95% and complication rates declined. These included necrotic complications, implant loss and wound infections. Local recurrence at median follow-up of 13 months was 0.6% with no recurrences in the nipple–areolar complex.23 The authors concluded that total skin-sparing mastectomy is oncologically safe with high nipple viability.
Breast Imaging Following Breast Reconstruction
Confusion exists regarding the role for mammography following bilateral mastectomies with reconstruction. Many primary care physicians are unclear about the necessity of routine screening mammography in a patient who clinically appears to have breasts but has undergone mastectomy and reconstruction particularly in the case of autologous tissue transfer. A study by Lee et al aimed to determine the role of mammography after TRAM reconstruction in women treated for breast cancer. In this retrospective review, 264 patients who had undergone mastectomy with TRAM reconstruction and subsequent bilateral screening mammograms were evaluated. Over a median follow-up period of 4.9 years, the rate of detection of recurrent non-palpable cancer in TRAM reconstructions was 0%.24 In our practice, we do not perform routine mammograms of a breast that has undergone mastectomy followed by reconstruction. These patients should have yearly mammograms on the contralateral breast if prophylactic mastectomy has not been performed. Women who have undergone mastectomy with reconstruction have clinical breast exams and diagnostic imaging of any areas of concern. This imaging may include mammogram, ultrasound or MRI. The management of breast cancer local recurrence after mastectomy with reconstruction can be challenging and usually involves a combination of wide local excision, chemotherapy and radiation.
1 Warren AG, Morris DJ, Houlihan MY, Slavin SA. Breast reconstruction in a changing breast cancer treatment paradigm. Plast Reconstr Surg. 2008;121(4):1116-1126.
2 Toth BA, Forley BG, Calabria R. Retrospective study of the skin-sparing mastectomy in breast reconstruction. Plast Reconstr Surg. 1999;104(1):77-84.
3 Simmons RM, Fish SK, Gayle L, et al. Local and distant recurrence rates in skin-sparing mastectomies compared with non-skin-sparing mastectomies. Ann Surg Oncol. 1999;6(7):676-681.
4 Carlson GW, Bostwick J, Styblo TM, et al. Skin-sparing mastectomy: oncologic and reconstructive considerations. Ann Surg. 1997;225(5):570-575.
5 Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence after skin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol. 1998;5(7):620-626.
6 Kroll SS, Khoo A, Singletary SE, et al. Local recurrence risk after skin-sparing and conventional mastectomy: a 6 year follow-up. Plast Reconstr Surg. 1999;104(2):421-425.
7 Slavin SA, Schnitt SJ, Duda RB, et al. Skin-sparing mastectomy and immediate reconstruction: Oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast Reconstr Surg. 1998;102(1):49-62.
8 Rozen WM, Ashton MW, Taylor GI. Defining the role for autologous breast reconstruction after mastectomy: social and oncologic implications. Clin Breast Cancer. 2008;8(2):134-142.
9 Taylor CW, Horgan K, Dodwell D. Oncological aspects of breast reconstruction. Breast. 2005;14(2):118-130.
10 Kronowitz SJ, Kuerer HM. Advances and surgical decision-making for breast reconstruction. Cancer. 2006;107(5):893-907.
11 Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337(14):956-962.
12 Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337(14):956-962.
13 Kronowitz SJ, Robb GL. Breast reconstruction with postmastectomy radiation therapy: current issues. Plast Reconstr Surg. 2004;114(4):950-960.
14 Nahabedian MY, Momen B. The impact of breast reconstruction on the oncologic efficacy of radiation therapy. Ann Plast Surg. 2008;60(3):244-250.
15 Batista LI, Lu KH, Beahm EK, Arun BK, Bodurka DC, Meric-Bernstam F. Coordinated prophylactic surgical management for women with hereditary breast-ovarian cancer syndrome. BMC Cancer. 2008;8:101-106.
16 Collin TW, Coady MSE. Is pregnancy contraindicated following free TRAM breast reconstruction? J. Plast Reconstruct Aesthet Surg. 2006;59(5):556-559.
17 Newman LA, Kuerer HM, Hunt KK, et al. Feasibility of immediate breast reconstruction for locally advanced breast cancer. Ann Surg Oncol. 1999;6(7):671-675.
18 Ananthakrishnan P, Lucas A. Options and considerations in the timing of breast reconstruction after mastectomy. Cleve Clin J Med. 2008;75(S1):S30-3.
19 Singletary E. Surgical management of inflammatory breast cancer. Semin Oncol. 2008;35(1):72-77.
20 Slavin SA, Love SM, Goldwyn RM. Recurrent breast cancer following immediate reconstruction with myocutaneous flaps. Plast Reconstr Surg. 1994;93(6):1191-1204.
21 Chin PL, Andersen JS, Somlo G, Chu DZ, Schwarz RE, Ellenhorn JD. Esthetic reconstruction after mastectomy for inflammatory breast cancer: is it worthwhile? J Am Coll Surg. 2000;190(3):304-309.
22 Sacchini V, Pinotti JA, Barros A, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg. 2006;203(5):704-714.
23 Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26-32.
24 Lee JM, Georgian-Smith D, Gazelle GS, et al. Detecting nonpalpable recurrent breast cancer: The role of routine mammographic screening of transverse rectus abdominis myocutaneous flap reconstructions. Radiology. 2008;248(2):398-405.