Chapter 8 Office Management of Tympanic Membrane Perforation and the Draining Ear
OTORRHEA FROM CEREBROSPINAL FLUID FISTULA
Brodie and Thompson1 observed that CSF otorrhea resulting from temporal bone fractures resolved within 1 week of onset in 95 of 122 subjects (77.8%). They also showed that leaks persisting beyond 7 days had a much higher incidence of meningitis (23% versus 3%). Iatrogenic CSF otorrhea may occur immediately after surgery or may be delayed for days or years.
Spontaneous CSF otorrhea may result from congenital temporal bone abnormalities, or can arise in the setting of normal temporal bone morphology as a consequence of arachnoid granulations, or increased intracranial pressure.2,3 More recent studies have shown that subjects with spontaneous CSF otorrhea are often morbidly obese and often have empty or partially empty sella on radiographic examination.3,4
CHRONIC SUPPURATIVE OTITIS MEDIA
Definition
Etiology
Many, perhaps most, cases of CSOM arise from acute infections that have not resolved. Whether or not all cases of CSOM have an infectious etiology is unclear. Antonelli and colleagues5,6 have suggested that some cases may be due simply to “bacterial colonization and overgrowth in an ear that remains moist because of tubal pathology.” Many authors believe that allergy alone can result in chronic drainage through tympanic membrane perforation, at least occasionally.
As noted by Bluestone and others,7,8 a nonintact tympanic membrane (perforation or tympanostomy tube or both) eliminates the “middle ear cushion” and facilitates eustachian tube reflux. By eliminating the middle ear cushion, a tympanostomy tube or tympanic membrane perforation allows air to escape from the middle ear space, which eases the retrograde reflux of nasopharyngeal secretions into the middle ear. Eustachian tube reflux may be an especially important etiologic factor in populations who are otherwise prone to it (e.g., aboriginal North Americans).
Many cases of CSOM arise from inadequately or incompletely treated cases of acute otitis media (AOM). Gibney and coworkers9 showed that aggressive treatment of AOM in aboriginal children in Australia reduces the incidence of CSOM. It is usually unclear why an acute infection fails to resolve and becomes chronic. We do know that AOM causes mucosal sloughing, causes impairment of ciliary clearance, and exposes microbial binding sites.5.6 Even so, only a few episodes of AOM evolve into CSOM.
The onset of CSOM is characterized initially by increased vascularity of the mucosa and submucosa. As CSOM persists, the proportion of chronic inflammatory cells increases. This increase in cells leads to osteitis and mucosal edema with ulceration, and two important pathophysiologic events follow: (1) capillary proliferation, which results in formation of granulation tissue and polyps, and (2) a rarifying osteitis, which ultimately produces new bone formation and fibrosis. Osteitis is present in virtually 100% of CSOM patients, a finding that distinguishes CSOM from more transient pathologic alterations in the middle ear cleft.10
Bacteriology
CSOM is predominantly a gram-negative infection, although staphylococcal species occur with sufficient frequency that they must be taken into consideration when any microbial therapy is considered. Pseudomonas aeruginosa is generally the most common gram-negative organism, but other gram-negative organisms are commonly encountered, especially species of Enterobacteriaceae.11 The extent to which anaerobic organisms or fungi or both are pathogenically involved is controversial. Anaerobic organisms are often present, but whether or not they are cultured depends on how rigorously they are sought.12,13 The contributions of anaerobes to pathophysiology remain unclear. Fungi are commonly recovered, but the extent to which they are pathogens as opposed to saprophytes is unresolved, and probably variable.
Pathophysiology
Granulation tissue is almost an invariant accompaniment of CSOM. It can develop quickly in a draining ear, and is already present in many infections of less than 6 weeks’ duration. The presence of granulation tissue, especially when it is abundant, may be a factor that contributes to treatment failure of acute infections, and the evolution of AOM into CSOM. The formation of granulation tissue in the middle ear begins with a break in the basement membrane of the surface epithelial cells. Inflammatory cells in the underlying lamina propria traverse through the broken basement membrane and enter the lumen of the middle ear space. The rupture of the basement membrane and epithelial lining cell is caused by bacterial toxins, inflammatory mediators produced by ruptured liposomes, and the accumulation of subepithelial fluid and vacuoles, all of which exert pressure on the surface epithelium.14
The next step in the formulation of granulation tissue occurs when a small piece of the herniated lamina propria extrudes through the ruptured area between epithelial cells. Initially, the extruded lamina propria pushes into the middle ear without any epithelial covering. Angiogenic growth factors incite capillary budding, vascular hyperpermeability, and fibroblast recruitment—that is, granulation tissues form. If the growth of granulation tissue is vigorous and aggressive, polyps develop.15 Meyerhoff and colleagues10 evaluated temporal bone from subjects with CSOM and reported that granulation tissue develops in 90% of all CSOM, and in 100% of cases of CSOM that develop intracranial complications.
ACUTE OTITIS MEDIA WITH AN OPEN TYMPANIC MEMBRANE
Inadequately treated AOM with a nonintact tympanic membrane is a frequently cited cause of CSOM.16 Acute infections in an ear with a nonintact tympanic membrane are frequently inadequately or inappropriately treated (especially in children) because it is not universally recognized that in many ways an acute infection occurring in an open middle ear cavity is significantly different from classic AOM. Most importantly, the microbiology is significantly different. Although some cases of acute infection in an open middle ear arise from the typical AOM pathogens—Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis—most, especially in patients older than 2 years, are caused by species of Staphylococcus, Pseudomonas, or other gram-negative organisms.17 Yeasts (Candida spp.) are occasionally encountered when the eardrum is not intact, but rarely when the middle ear space is closed.17 The oral antibiotics usually prescribed for AOM are poorly active against Staphylococcus and completely inactive against gram-negative organisms. Individuals treated with these systemic antibiotics consequently are often treated inappropriately or inadequately. Aggressive, appropriate treatment of acute middle ear infections in individuals with a nonintact tympanic membrane is an important way of preventing the development of CSOM.
Another meaningful difference between AOM with an intact tympanic membrane and AOM in an open middle ear is that the natural history of these two infections seem to be different. It is well established that 80% or more of untreated children with AOM and intact eardrums recover spontaneously, and that suppurative complications are unlikely. Ruohola and associates18,19 found that AOM in children with tympanostomy tubes is significantly different in this respect: at the end of their study, 60% of nontreated children still had infection and drainage.
MANAGEMENT OF OTORRHEA
There are three principal components to effective management of the draining ear:
Antimicrobial Therapies
Elimination of infection is crucial to the successful management of a draining ear. Consequently, appropriate antimicrobial treatment is pivotal. Infection that is limited to the external auditory canal or an open mastoid cavity can sometimes be managed with the use of antiseptics or acidifying agents, or both, alone. When effective, these agents have several advantages: they are inexpensive, are easy to procure, do not promote bacterial resistance, and are generally effective against bacteria and fungi. The relative merits of one of these preparations versus another is generally unknown, although Tom20 showed that Cresylate is more effective in vitro than other antiseptics against fungal organisms.
When antibiotics are used, topical delivery in the form of otic drops is more effective and safer than the use of systemic antibiotics, either orally or parenterally.21–2527 By using a topical agent, the physician can deliver a concentration of medication that is several orders of magnitude higher in affected tissues than the concentration that can be achieved by using a systemic agent. A 3 to 5 drop dose of a 0.3% solution of antibiotic contains only 90 to 150 mg of medication, but its concentration is 3000 μg/mL, which exceeds the minimum inhibitory concentration of any known relevant pathogen. In contrast, consider the typical drug levels in middle ear fluid that can be achieved by three systemic antibiotics:
Skepticism has been voiced that topical drops would not provide sustained levels of antibiotic inside the middle ear space, especially if the perforation is small or delivery is through a tympanostomy tube. Ohyama and associates26 documented that topical antibiotic drops can be, and often are, effectively delivered into the middle ear space and have long dwell times. These investigators measured the persistence of a single dose of topically applied 0.3% ofloxacin in otorrhea fluid, serum, and middle ear mucosa at various time intervals after administration. They found a very high level of antibiotic in the otorrhea fluid several hours later. Perhaps the most surprising finding was the high concentration of drug in biopsy specimens of middle ear mucosa approximately 1 hour after the administration of ear drops. As expected, drug concentrations in serum were very low or nondetectable.
The superior efficacy of topically applied antibiotics is well documented in the literature. Esposito and colleagues28 studied 60 subjects with CSOM who were randomly assigned to receive one of three treatment regimens: one group received oral ciprofloxacin twice a day, another received three drops of ciprofloxacin solution twice a day, and a third group received oral and topical ciprofloxacin doses twice a day. The topical group had a clinical response rate of 100% and a bacteriologic cure rate of 95%, and the topical/oral group had response rates of 95% and 85%. By contrast, the group that received oral antibiotics alone had a clinical response rate of 65% and a rate of bacterial eradication of only 40%. These differences were statistically significant.28 Two years later, Esposito and colleagues29 published the results of their comparison of topical ciprofloxacin versus intramuscular gentamicin in 60 adults. All patients had CSOM, and 40 of them had previously undergone systemic therapy. Half of the group received four drops of ciprofloxacin solutions twice a day, and the other half received twice-daily injections of 80 mg of gentamicin. Favorable clinical responses were seen in 87% of the topically treated patients and only 67% of patients who received systemic gentamicin. Only 43% of the patients receiving systemic gentamicin achieved microbiologic eradication compared with 83% of the group receiving topical drops.
Two studies have compared the relative efficacy of topical drop regimens with the use of systemic antibiotics with amoxicillin/clavulanic acid.30,31 Both studies showed enhanced efficacy with the topical treatment regimen. These studies were performed in children with acute otorrhea after insertion of a tympanostomy tube. Because Pseudomonas and Staphylococcus aureus are more prominent pathogens in CSOM, the superiority of a topical regimen over amoxicillin/clavulanic acid should be even greater because S. aureus is a more frequent pathogen in CSOM than an acute otitis media with a tympanostomy tube (AMOT).
In 1994, Yuen and coworkers32 published results of a prospective trial comparing 0.3% ofloxacin otic drops with oral amoxicillin/clavulanic acid in 56 patients. One week after completion of treatment, 76% of the ofloxacin group had dry ears compared with only 26% of the other group. de Miguel-Martinez and associates33 divided 125 patients into five treatment arms. The participants received one of the following treatments:
The best outcomes were achieved in the group that received 0.2% ciprofloxacin.
In 2000, the Cochrane database published a review by Acuin and coworkers.24 The review concluded that topical antibiotics are superior to systemic antibiotics for the treatment of CSOM, and that a combination of systemic plus topical antibiotic offers no therapeutic advantage. Fluoroquinolone topical antibiotics were seen to be more effective than other types of topical antibiotic drops. The systemic review emphasized the superior microbiologic eradication rates achieved with topical therapy, and emphasized the importance of bacterial eradication.
The addition of a steroid to a topical antibiotic was compared with the ototopical antibiotic alone in three studies by Roland and colleagues.15,34,35 Both studies showed that the steroids hastened the resolution of otorrhea, and in one study the final cure rate was higher in the group treated with a topical antibiotic that included a steroid. The efficacy of combination ototopical drops that contain an antibiotic and a steroid has not been specifically studied in the treatment of CSOM. The improved effectiveness of steroid-containing drops in the treatment of post-tympanostomy tube otorrhea and the management of granulation tissues has led many clinicians, however, to adopt them as the topical treatment of choice in individuals with CSOM.
Granulation Tissue
Otolaryngologists have long held as an article of faith that steroids are important in suppressing, eliminating, and preventing granulation tissue. Several animal studies have found that steroids are effective in controlling keloids and hypertrophic scarring, and reducing angiogenesis with subsequent formation of granulation tissue.39–43 More recently, evidence has shown that steroids are effective in humans as well. A randomized, controlled, double-blind drug comparison study of 599 children with patent tympanostomy tubes and AOM with otorrhea of 3 weeks or less duration provides such evidence. Children with granulation tissue associated with tympanostomy tubes were treated with either a quinolone plus a steroid or a quinolone alone. Not only did the combination product prove to be significantly more effective in establishing clinical and microbiologic cure in all patients, but also it was significantly more effective in eliminating granulation tissue.15,44 A more potent steroid seems to be more effective than a less potent steroid.
Office débridement of granulation tissue can be performed using the microscope and microinstrumentation, but it is most commonly performed using chemical cautery. Silver nitrate is the agent most commonly used, although trichloroacetic acid is used by some clinicians. Chemical cautery is, in effect, a chemical burn, and control over the depth and severity of that burn is limited.45 If care is not exercised, vital structures (i.e., facial nerve) can be permanently injured. Mechanical removal should be done sharply, with clear visualization of exactly what tissues are being incised. Avulsion of aural polyps is not usually recommended because when the polyp is removed, the otolaryngologist might find that the stapes is attached to it. Using sharp techniques under direct visualization avoids inadvertent injury to important structures. Tympanomastoid surgery for chronic otitis media is an aggressive form of débridement and is often very effective.
Preventing Bacterial Resistance
Applying the term resistant to a bacterial species is based on two determinations:
As a practical matter, increases in bacterial resistance to a specific antibiotic are manifest by increasing MICs for that particular antibiotic. An analysis of clinical failures from several large clinical trials verified that changes in MIC have not occurred as a result of treatment using topical antibiotic drops.35
Causes of Failed Therapy
Proper technique requires that the drops are delivered into the internal auditory canal, which is often difficult with a squirming child. “Tragal” pumping (rhythmic pressure just anterior to the tragal cartilage) is important in ensuring that drops penetrate through a tympanostomy tube or tympanic membrane perforation into the middle ear space.46 Issues related to management of granulation tissue have been discussed previously. Occasionally, mucosal edema is sufficient to prevent adequate delivery of antibiotics into and through the middle ear space. When this is the case, patience is required. The use of a steroid-containing antibiotic drop generally results in progressively increasing middle ear mucosal edema over several days. Voluminous drainage is managed as mentioned earlier by effective aural toilet.
Biofilms have been shown not only on tympanostomy tubes, but also in the middle ear and mastoid with suppurative otitis media. The biofilms seen in patients with CSOM resemble those found on the surface of the middle ear mucosa of chinchillas after inoculation with nontypable Haemophilus influenzae. Although the role of biofilms in the pathogenesis of culture-negative otitis media with effusion is outside the scope of this chapter, the presence of biofilms in individuals with persistent/recurrent otologic infections does suggest a possible pathogenic role for biofilms.47,48 It is widely recognized that patients who fail combined topical/systemic therapy often resolve the infection after the removal of a tympanostomy tube.
OFFICE TECHNIQUES FOR CLOSURE OF TYMPANIC MEMBRANE PERFORATIONS
Cauterization and Paper Patching
Rose and Politzer were among the first to use silver nitrate cauterization of the margins of a perforation to promote healing.49 Dunlap and Schuknecht reported that closure generally requires repeated application of cautery with removal of dried exudates every 2 weeks; closure was complete, on average, only after 6 to 12 months.49 Jeurs and Wright reported success in closing perforations by marginal stimulation in 80% to 90% of cases; multiple treatment episodes were required.50 Derlacki51,52 was able to close 75% of 131 perforations he treated, with an average of 14 treatments required. Although Derlacki51,52 had some success in larger perforations, most successes are reported for small to medium-sized perforations. Repeated cauterizations are always necessary.
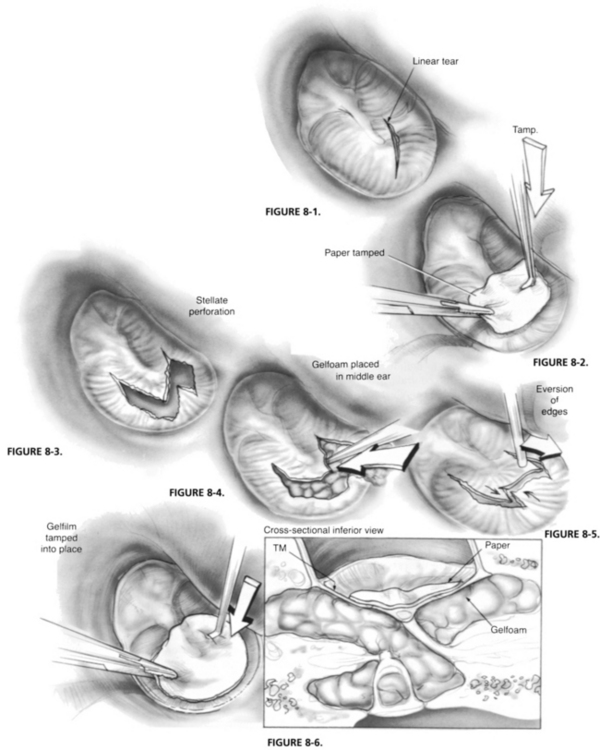
Some otologists believe that traumatic tympanic membrane perforations must be managed acutely. They believe the best results are achieved when torn areas of drum are immediately reapproximated, often over absorbable gelatin sponge (Gelfoam) placed through the perforation into the middle ear to support the reapproximated portions of drum (Figs. 8-1 through 8-6). Special emphasis is often placed on elevating segments of drum that are folded back on themselves after an implosion-type injury. Accurate reapproximation of drum fragments requires microinstrumentation and a microscope. Some authors recommend combining fragment reapproximation with paper patching.
Fat Graft Tympanoplasty
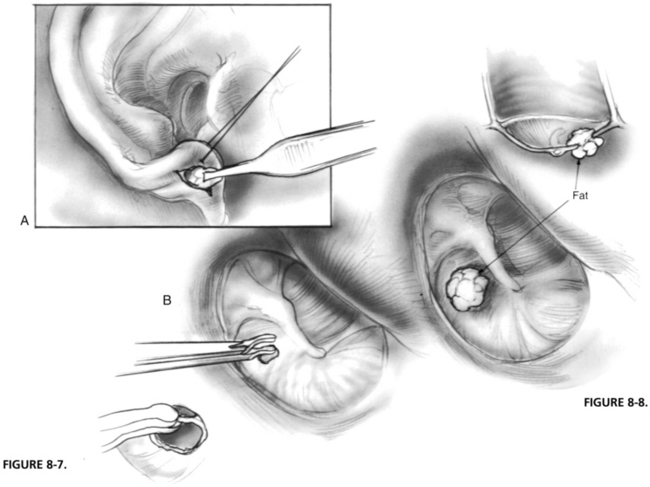
The technique of fat graft tympanoplasty was first described by Ringenberg in 1962,53 but differed substantially from the technique currently recommended in two respects: (1) Ringenberg turned a tympanomeatal flap and explored the middle ear as part of the procedure, and (2) the fat graft was placed lateral to (and not through) the perforation. Donor fat is generally harvested from the earlobe. Ringenberg53 evaluated fat from several anatomic sites, and determined that earlobe fat was more compact and contained more connective tissue than fat harvested from the abdomen or the buttocks. Other advantages include the ease with which lobule fat from the lobe can be obtained, and that enough donor fat can be taken from a single lobule to graft more than one tympanic membrane perforation.54
The technique as currently described is quite simple (Figs. 8-7 and 8-8). The epithelial margin of the perforation is removed so that the edges of the perforation are fresh and open. This is sometimes referred to as “rimming” the perforation. A globule of fat slightly larger than the perforation is pushed through the perforation so that a portion of the inserted fat is medial to the tympanic membrane, and a portion is lateral.
Histologically, the graft seems to epithelialize over 2 to 3 weeks. The graft atrophies in the first couple of weeks, but at 3 to 4 weeks a significant volume of fat can still be seen within the epithelialized medial and lateral surface. This fat atrophies almost completely in the following 3 to 6 weeks. Very little of the fat remains within the middle layer of the now healed tympanic membrane 6 to 8 weeks after grafting.53,55 It is widely agreed that the technique is most suitable for small perforations involving no more than 25% to 30% of the tympanic membrane.54,56–59 The technique should be limited to individuals whose hearing is normal or close to normal, and in whom there is little or no possibility of associated ossicular abnormality.54,56,57,60,61
Because this technique is most frequently used in pediatric patients for small perforations that persisted after extrusion of tympanostomy tubes, most cases have been done in a day surgery setting. The technique is suitable for office outpatient use, however, and can be performed with local anesthesia. Because the middle ear space is not entered, it is reasonable to perform the procedure bilaterally.54,57 Closure rates have been reported as 76% to 92% of cases.54,56–62 In a small series of 15 tympanostomy tubes removed for persistent unremitting otorrhea, Liew and associates63 reported a success rate of 100%.
1. Brodie H.A., Thompson T.C. Management of complications from 820 temporal bone fractures. Am J Otol. 1997;18:188-197.
2. Gacek R.R. Evaluation and management of temporal bone arachnoid granulations. Arch Otolaryngol Head Neck Surg. 1992;118:327-332.
3. Prichard C.N., Isaacson B., Oghalai J.S., et al. Adult spontaneous CSF otorrhea: Correlation with radiographic empty sella. Otolaryngol Head Neck Surg. 2006;134:767-771.
4. Scurry W.C.Jr., Ort S.A., Peterson W.M., et al. Idiopathic temporal bone encephaloceles in the obese patient. Otolaryngol Head Neck Surg. 2007;136:961-965.
5. Antonelli P.J., Juhn S.K., Goycoolea M.V., Giebink G.S. Middle ear susceptibility to Pseudomonas infection during acute otitis media. Ann Otol Rhinol Laryngol. 1993;102:531-536.
6. Antonelli P.J., Juhn S.K., Le C.T., Giebink G.S. Acute otitis media increases middle ear susceptibility to nasal injection of Pseudomonas aeruginosa. Otolaryngol Head Neck Surg. 1994;110:115-121.
7. Bluestone C.D. Pathogenesis of otitis media: Role of eustachian tube. Pediatr Infect Dis J. 1996;15:281-291.
8. Buchman C.A., Doyle W.J., Swarts J.D., Bluestone C.D. Effects of nasal obstruction on Eustachian tube function and middle ear pressure. Acta Otolaryngol. 1999;119:351-355.
9. Gibney K.B., Morris P.S., Carapetis J.R., et al. The clinical course of acute otitis media in high risk Australian aboriginal children: A longitudinal study. BMC Pediatr. 2005;5:16.
10. Meyerhoff W.L., Kim C.S., Paparella M.M. Pathology of chronic otitis media. Ann Otol Rhinol Laryngol. 1978;87(6 Pt 1):749-760.
11. Roland P.S. Chronic suppurative otitis media: A clinical overview. Ear Nose Throat J. 2002;81(8 Suppl 1):8-10.
12. Brook I. Microbiology and management of chronic suppurative otitis media in children. J Trop Pediatr. 2003;49:196-199.
13. Brook I. The role of anaerobic bacteria in acute and chronic mastoiditis. Anaerobe. 2005;11:252-257.
14. Hashimoto I., Nakanishi H., Shono Y., et al. Angiostatic effects of corticosteroid on wound healing of the rabbit ear. J Med Invest. 2002;49:61-66.
15. Roland P.S. The formation and management of middle ear granulation tissue in chronic ear disease. Ear Nose Throat J. 2004;83(Suppl 1):5-8.
16. Bluestone C.D. Epidemiology and pathogenesis of chronic suppurative otitis media: Implications for prevention and treatment. Int J Pediatr Otorhinolaryngol. 1998;42:207-223.
17. Roland P.S., Parry D.A., Stroman D.W. Microbiology of acute otitis media with tympanostomy tubes. Otolaryngol Head Neck Surg. 2005;133:585-595.
18. Ruohola A., Heikkinen T., Meurman O., et al. Antibiotic treatment of acute otorrhea through tympanostomy tube: Randomized double-blind placebo-controlled study with daily follow-up. Pediatrics. 2003;111(5 Pt 1):1061-1067.
19. Ruohola A., Meurman O., Nikkari S., et al. Microbiology of acute otitis media in children with tympanostomy tubes: Prevalences of bacteria and viruses. Clin Infect Dis. 2006;43:1417-1422.
20. Tom L.W. Ototoxicity of common topical antimycotic preparations. Laryngoscope. 2000;110:509-516.
21. Acuin J. Chronic suppurative otitis media. Clin Evid. 2006;15:772-787.
22. Roland P.S. Ototopical agents are superior to systemic therapy for the treatment of acute and chronic otitis media. Ear Nose Throat J. 2004;83(9 Suppl 4):9-12.
23. Macfadyen C.A., Acuin J.M., Gamble C. Systemic antibiotics versus topical treatments for chronically discharging ears with underlying eardrum perforations. Cochrane Database Syst Rev. (1):2006. CD005608
24. Acuin J., Smith A., Mackenzie I. Interventions for chronic suppurative otitis media. Cochrane Database Syst Rev. (2):2000. CD000473
25. Macfadyen C.A., Acuin J.M., Gamble C. Topical antibiotics without steroids for chronically discharging ears with underlying eardrum perforations. Cochrane Database Syst Rev. (4):2005. CD004618
26. Ohyama M., Furuta S., Ueno K., et al. Ofloxacin otic solution in patients with otitis media: An analysis of drug concentrations. Arch Otolaryngol Head Neck Surg. 1999;125:337-340.
27. Hannley M.T., Denneny J.C.III, Holzer S.S. Use of ototopical antibiotics in treating 3 common ear diseases. Otolaryngol Head Neck Surg. 2000;122:934-940.
28. Esposito S., D’Errico G., Montanaro C. Topical and oral treatment of chronic otitis media with ciprofloxacin: A preliminary study. Arch Otolaryngol Head Neck Surg. 1990;116:557-559.
29. Espisito S., Noviello S., D’Errico G., et al. Topical ciprofloxacin vs intramuscular gentamicin for chronic otitis media. Arch Otolaryngol Head Neck Surg. 1992;118:842-844.
30. Dohar J., Giles W., Roland P., et al. Topical ciprofloxacin/dexamethasone superior to oral amoxicillin/clavulanic acid in acute otitis media with otorrhea through tympanostomy tubes. Pediatrics. 2006;118:e561-e569.
31. Goldblatt E.L., Dohar J.E., Noza R.J., et al. Topical ofloxacin versus systemic amoxicillin/clavulanate in purulent otorrhea in children with tympanostomy tubes. Int J Pediatr Otorhinolaryngol. 1998;146:91-101.
32. Yuen P.W., Lou S.K., Chau P.Y., et al. Ofloxacin eardrop treatment for active chronic suppurative otitis media: Prospective randomized study. Am J Otol. 1994;15:670-673.
33. de Miguel-Martinez I., Ramos-Macias A., Martin-Sanchez A.M. Otitis media due to Corynebacterium jeikeium. Eur J Clin Microbiol Infect Dis. 1999;18:231-232.
34. Roland P.S., Anon J.B., Moe R.D., et al. Topical ciprofloxacin/dexamethasone is superior to ciprofloxacin alone in pediatric patients with acute otitis media and otorrhea through tympanostomy tubes. Laryngoscope. 2003;113:2116-2122.
35. Roland P.S., Kreisler L.S., Reese B., et al. Topical ciprofloxacin/dexamethasone otic suspension is superior to ofloxacin otic solution in the treatment of children with acute otitis media with otorrhea through tympanostomy tubes. Pediatrics. 2004;113:e40-e46.
36. Roland P.S., Stewart M.G., Hannley M., et al. Consensus panel on role of potentially ototoxic antibiotics for topical middle-ear use: Introduction, methodology, and recommendations. Otolaryngol Head Neck Surg. 2004;130(3 No. 3S):S51-S56.
37. Schapowal A. Contact dermatitis to antibiotic ear drops is due to neomycin but not to ciprofloxacin [Abstract]. Allergy. 2001;56(Suppl 68):148.
38. VanGinkel C.J., Bruintjes T.D., Huizing E.H. Allergy due to topical medications in chronic otitis externa and chronic otitis media. Clin Otolaryngol. 1995;20:326-328.
39. Sobol S.E., Keswani S., Parvadia J.K., et al. Effect of corticosteroids-antibiotic agents on granulation tissue in a murine model. Arch Otolaryngol Head Neck Surg. 2005;131:330-335.
40. Park S.N., Yeo S.W. Effects of antibiotics and steroids on middle ear mucosa in rats with experimental acute otitis media. Acta Otolaryngol. 2001;121:808-812.
41. Cutler J.L., Wall G.M., Labadie R.D. Effects of ototopic steroid and NSAIDs in clearing middle ear effusion in an animal model. Otolaryngol Head Neck Surg. 2006;135:585-589.
42. Florea A., Zwart J.E., Lee C.W., et al. Effect of topical dexamethasone versus rimexolone on middle ear inflammation in experimental otitis media with effusion. Acta Otolaryngol. 2006;126:910-915.
43. Alper C.M., Dohar J.e., Gulhan M., et al. Treatment of chronic suppurative otitis media with topical tobramycin and dexamethasone. Arch Otolaryngol Head Neck Surg. 2000;126:165-173.
44. Bertone A.L. Management of exuberant granulation tissue. Vet Clin North Am Equine Pract. 1989;5:551-562.
45. Wachter B.G., Leonetti J.P., Lee J.M., et al. Silver nitrate injury in the rat sciatic nerve: A model of facial nerve injury. Otolaryngol Head Neck Surg. 2002;127:48-54.
46. Hebert R.L.2nd, Vick M.L., King G.E., Bent J.P.3rd. Tympanostomy tubes and otic suspensions: Do they reach the middle ear space? Otolaryngol Head Neck Surg. 2000;122:330-333.
47. Post J.C., Hiller N.L., Nistico L., et al. The role of biofilms in otolaryngologic infections: Update 2007. Curr Opin Otolaryngol Head Neck Surg. 2007;15:347-351.
48. Dohar J.E., Hebda P.A., Veeh R., et al. Mucosal biofilm formation on middle-ear mucosa in a nonhuman primate model of chronic suppurative otitis media. Laryngoscope. 2005;115:1469-1472.
49. Stenfors L.E., Carlsöö B., Salen B., et al. Repair of experimental tympanic membrane perforations. Acta Otorhinolaryngol. 1980;90:332-341.
50. Glasscock M., Levine S., McKennan K. Materials used in tympanoplasty. In: Paparella M., et al, editors. Otolaryngology. Philadelphia: Saunders; 1991:1441-1447.
51. Derlacki E.L. Residual perforations after tympanoplasty: Office technique for closure. Otolaryngol Clin North Am. 1982;15:861-867.
52. Derlacki E.L. Office closure of central tympanic membrane perforations: A quarter century of experience. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:53-66.
53. Ringenberg J.C. Fat graft tympanoplasty. Laryngoscope. 1962;72:188-192.
54. Mitchell R.B., Pereira K.D., Younis R.T., et al. Bilateral fat graft myringoplasty in children. Ear Nose Throat J. 1996;75(652):655-656.
55. Imamoglu M., Isik A.U., Acuner O., et al. Fat-plug and paper-patch myringoplasty in rats. J Otolaryngol. 1998;27:318-321.
56. Mitchell R.B., Pereira K.D., Lazar R.H. Fat graft myringoplasty in children—a safe and successful day-stay procedure. J Laryngol Otol. 1997;111:106-108.
57. Ozgursoy O.B., Yorulmaz I. Fat graft myringoplasty: A cost-effective but underused procedure. J Laryngol Otol. 2005;119:277-279.
58. Terry R.M., Bellini M.J., Clayton M.I., Gandhi A.G. Fat graft myringoplasty—a prospective trial. Clin Otolaryngol Allied Sci. 1998;13:227-229.
59. Fiorino F., Barbieri F. Fat graft myringoplasty after unsuccessful tympanic membrane repair. Eur Arch Otorhinolaryngol. 2007;264(10):1125-1128.
60. Landsberg R., Rishman G., DeRowe A., et al. Fat graft myringoplasty: Results of a long-term follow-up. J Otolaryngol. 2006;35:44-47.
61. Hagemann M., Hausler R. Tympanoplasty with adipose tissue. Laryngorhinootologie. 2003;82:393-396.
62. Ayache S., Braccini F., Facon F., Thomassin J.M. Adipose graft: An original option in myringoplasty. Otol Neurotol. 2005;26:554-555.
63. Liew L., Daudia A., Narula A.A. Synchronous fat plug myringoplasty and tympanostomy tube removal in the management of refractory otorrhoea in younger patients. Int J Pediatr Otorhinolaryngol. 2002;66:291-296.