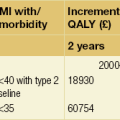
19
Oesophageal emergencies
Perforation of the oesophagus
Aetiology and pathophysiology
Iatrogenic perforation of the oesophagus
Iatrogenic damage to the oesophagus leading to full-thickness disruption occurs from within in 60–70% of cases, such as during endoscopic instrumentation, or from without, such as during para-oesophageal surgery. Although flexible video endoscopy is safe and has almost totally replaced rigid oesophagoscopy (0.03% perforation risk compared to 0.11% for rigid endoscopy), the dramatic increase in the number of examinations performed has led to an increase in the number of associated injuries. Intubation of the oesophagus can cause proximal perforation with risk increased by hyper-extension of the neck and the presence of arthritic cervical osteophytes or an oesophageal diverticulum. However, in 75–90% of diagnostic cases, trauma is sustained to the distal oesophagus, often in conjunction with an abnormality (Table 19.1). Therapeutic endoscopy carries a significantly higher perforation risk (200-fold), around 5%, that is further increased in patients who have received prior radiotherapy or chemotherapy (as the majority of therapeutic endoscopy is for palliation). Dilatation accounts for the majority of injuries and with a lower risk of perforation when placing self-expanding metal stents. Benign pneumatic dilatation for achalasia carries a higher risk than graded dilatation, due to higher pressures and large balloon size.1 Transoesophageal echocardiography carries risk not only for perforation during blind placement but also when placed for perioperative monitoring due to pressure necrosis. Similarly, any intubation such as placement of a nasogastric tube or inadvertent oesophageal placement of an endotracheal tube may all cause direct trauma. A case review of 75 patients with iatrogenic perforation of the oesophagus reported a not insubstantial overall mortality rate of 19%. Prevention is therefore the best solution, with increasing awareness and training likely to reduce the incidence.2
Table 19.1
Risk of iatrogenic oesophageal disruption through instrumentation
| Medical instrumentation | Percentage risk of iatrogenic oesophageal disruption |
| Dilatation | 0.5 |
| Dilatation for achalasia | 2 |
| Endoscopic thermal therapy | 1–2 |
| Treatment of variceal bleeding | 1–6 |
| Endoscopic laser therapy | 1–5 |
| Photodynamic therapy | 5 |
| Stent placement | 5–25 |
Spontaneous perforation of the oesophagus
Boerhaave’s syndrome is characterised by barogenic oesophageal injury leading to immediate and gross gastric content contamination of the pleural cavity. However, various degrees of damage and contamination are possible. As a result, a number of clinical terms have evolved to describe these events: this text will only use the term ‘spontaneous perforation of the oesophagus’, with the term ‘disruption’ used to describe the ‘process’ of perforation. Spontaneous perforation of the oesophagus is most accurately defined as complete disruption of the oesophageal wall occurring in the absence of pre-existing pathology. Since the oesophagus possesses no serosa, transgression of oesophagogastric contents leads rapidly to chemical and septic mediastinitis. In 80–90% of cases, this disruption is associated with a sudden rise in intra-abdominal pressure, most usually as a result of retching or vomiting; however, blunt trauma, weightlifting, parturition, defecation, the Heimlich manoeuvre or status epilepticus have all been cited as causal factors. Although vomiting is commonplace, spontaneous oesophageal perforation is not, which suggests that other as yet unidentified factors may be important, such as pre-existing anatomical or pathological abnormalities. However, an underlying pathology is identified in only 10–20% of cases, such as malignancy, peptic ulceration or infection (as such not truly spontaneous perforation). A common misconception is that Mallory–Weiss tears represent part of the spectrum of spontaneous perforation but it is likely that these mucosal injuries reflect ‘shearing’ rather than ‘barogenic’ trauma.3 Equally, eosinophilic oesophagitis has also been associated with an increased risk of both mucosal tears and full-thickness perforation either spontaneously induced by vomiting to dislodge impacted food or following endoscopic procedures.4
Clinical presentation
As a result, in spontaneous perforation, the diagnostic error is high, with only 5% of cases diagnosed at presentation. This leads to diagnostic delay of greater than 12 hours in the majority of cases.7 It may be that less than 35% of cases are correctly diagnosed pre-mortem8 (Box 19.1). As time passes, the critical condition of the patient further obscures relevant clinical features and the pursuit of incorrect investigations makes the diagnosis even more elusive.
Depending on the aetiology and amount of contamination, pain may be severe, constant, retrosternal or epigastric, distressing, exacerbated by movement and poorly relieved by narcotics or relatively mild. Dysphagia and odynophagia are common. Patients can be tachypnoeic and may sit up to splint their diaphragm. Abdominal pain or tenderness are not uncommon and can lead to a negative laparotomy.6 Similarly, subcutaneous emphysema takes time to develop; mediastinal emphysema precedes this and may be visible on a plain chest radiograph. With time the negative intrathoracic pressure draws air, food and fluids into the mediastinum and pleural cavities and a chemical pleuromediastinitis develops. A low-grade pyrexia ensues, and a sympathetic nervous system response develops with pallor, sweating, peripheral circulatory shutdown, tachycardia, tachypnoea and overt haemodynamic shock, which worsens as the systemic inflammatory response gives way to sepsis. Within 24–48 hours cardiopulmonary embarrassment and collapse develop as a consequence of overwhelming bacterial mediastinitis and septic shock. The combination of chest pain and shock may inappropriately, but all too commonly lead to a cardiological referral. Survival is dependent on the evacuation of the contamination, from the mediastinal and pleural cavities at the earliest possible opportunity.9 Systemic effects are less common when the cervical oesophagus is damaged, with neck pain, torticollis, dysphonia, cervical dysphagia, hoarseness and subcutaneous emphysema predominating.
Investigations
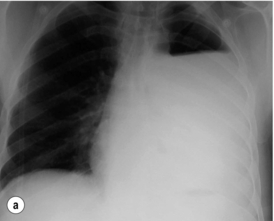
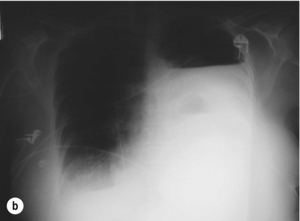
The typical findings on plain chest radiography are subtle – dependent on the site and the time interval following the insult. These are documented in Box 19.2 and Fig. 19.1. A plain abdominal radiograph may help to exclude a perforated intra-abdominal viscus.7
Contrast radiography
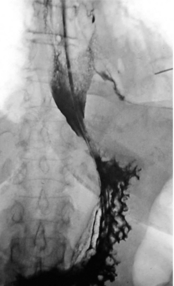
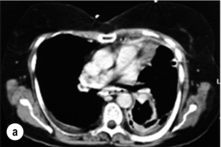
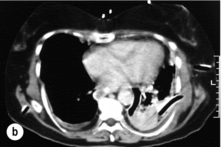
Oral water-soluble contrast radiography ascertains the site, the degree of containment and the degree of drainage of the perforation (Fig. 19.2). Aqueous agents are rapidly absorbed, do not exacerbate inflammation and have minimal tissue effects. However, false-negative results in 27–66% and the limited applicability to a collapsed, unwell patient have downgraded their usefulness.
Upper gastrointestinal endoscopy
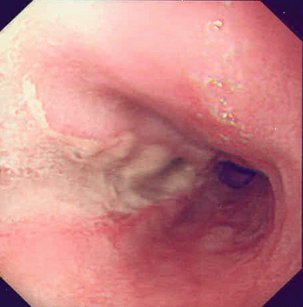
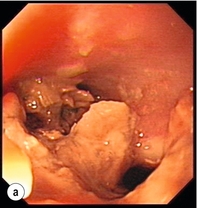
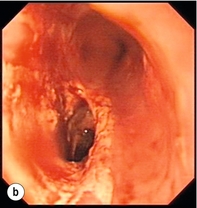
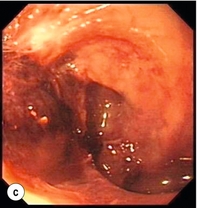
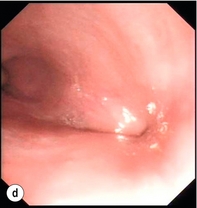
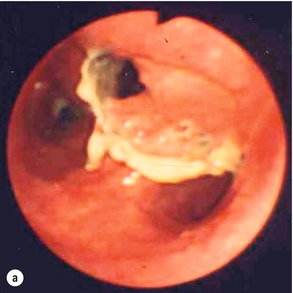
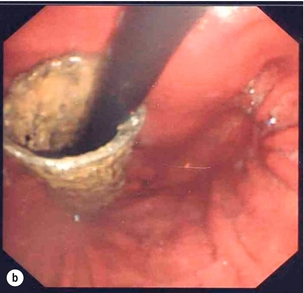
Endoscopic assessment excludes the diagnosis if normal, influences management if underlying pathology is discovered and facilitates the placement of a nasojejunal tube to allow enteral feeding. Risks are minimised using modern, flexible videoscopes together with fluoroscopic guidance, but should only be performed by a highly experienced endoscopist conversant with the consequences of their actions (Figs 19.3 and 19.4). Endoscopy can be performed in the sickest of patients, if necessary ‘on table’, when other injuries or instability of the patient preclude radiological assessment.
Management
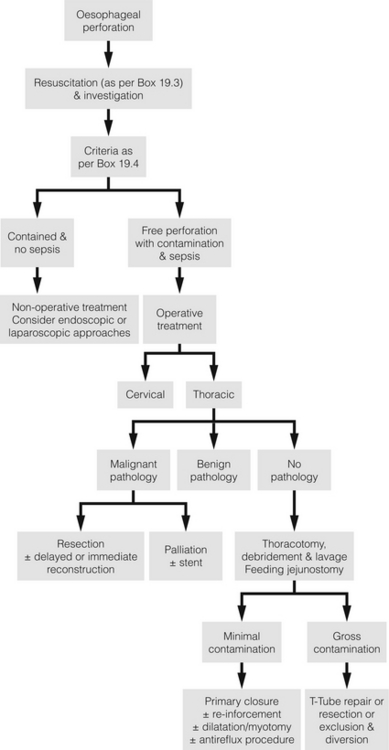
All patients with an oesophageal perforation are critically ill. The immediate priorities are the establishment of a secure and adequate airway, stabilisation of cardiovascular status and relief of pain, often using opiate-based analgesia. Regular reassessment is obligatory as an initially stable patient can rapidly decompensate. An early anaesthetic review is recommended. Box 19.3 documents the initial resuscitation.
Non-operative management
Non-operative management, endoscopic and minimally invasive operative management have all been shown to be safe and feasible in carefully selected patients who have either been diagnosed with minimal contamination and no mediastinitis or with a contained perforation. It may also be considered in those with a delayed diagnosis who have demonstrated tolerance.9
Criteria have been developed to aid the selection of suitable patients for non-operative management. These are detailed in Box 19.4. Case series applying these criteria demonstrate a mortality rate between zero and 16%, but numbers are small and results are skewed by both selection and publication bias.
Adjuncts to non-operative management
Closure: clips and sealants: Endoclips are well established in closing small, clean defects after endoscopic mucosal resection or submucosal dissection for early cancer.14,15 In the absence of significant contamination, small iatrogenic perforations may be closed immediately using endoclips in addition to supportive non-operative treatment. However, endoclipping ‘en face’ in the oesophagus is extremely challenging and should only be attempted by highly skilled endoscopists. It is also debatable whether this significantly alters the clinical course over a simple non-operative approach.2 There is at least one case report of clipping a spontaneous oesophageal perforation but this cannot be recommended in the face of gross contamination.16
Diversion: stents: Self-expanding stents have been used to seal oesophageal perforations, chronic fistulas and even postoperative anastomotic leaks.17–19 Stents were not designed for use in a normal oesophagus and migration rates approach 30%, and concerns have been raised in terms of extending the defect through pressure necrosis and through the trauma of their subsequent removal.14,20 Publication bias means that failure and the consequences of failure remain unknown. There is considerable variation in the timing of stent placement and number of stents used. It is evident that the majority of cases also involve aggressive non-operative management.21–24 It is therefore difficult to attribute successful outcomes to the stent placement alone. For example, one ‘successful’ report documents a patient who had five stents placed over an 8-month period before eventually proceeding to oesophagectomy at a tertiary referral centre.21 The one prospective stenting study lists 10 patients with a Boerhaave perforation.25 Stent migration was high (11 out of 33) and there was a 50% complication rate (bleeding/stent fracture/impacted stent) if stents were not removed before 6 weeks.
At present there is insufficient evidence to support the use of oesophageal stents in oesophageal perforations. The authors suggest that their use is highly selective and should always be viewed as a temporary solution. However, in patients whose physical condition precludes more aggressive treatments and those in whom resection is not deemed suitable, stents do offer a serious alternative. If utilised then the stent should be removed within 3 months to avoid long-term complications since the biggest concern is septic erosion into surrounding structures. This horrendous situation does not appear to be represented in the literature (Fig. 19.6).25
Drainage: repeated endoscopy: Endoscopic lavage and drainage of contained mediastinal perforations or even endoscopic placement of a vacuum sponge drainage system has been used for liquid contamination. This is certainly a novel approach but labour intensive and not suitable for gross contamination. Success may again simply reflect patients who would have done well with more simple non-operative treatment.26,27
Operative management
Open surgery: Surgery is advocated if the patient has overt signs of sepsis, shock, gross contamination, an obstructing pathology, a retained foreign body, a major caustic injury or has failed non-operative management. Virtually all gunshot wounds require surgery. The primary objective of surgical intervention is to restore oesophageal integrity and prevent further soiling. Thorough debridement, drainage, lavage and irrigation are more important for survival than the type of repair.9 A feeding jejunostomy should also be fashioned as a routine to facilitate enteral feeding, usually following thoracotomy once the patient can be turned into a supine position. Management of the patients by a multidisciplinary team is again emphasised. Underlying pathology should be dealt with. Spontaneous perforation of the oesophagus carries a considerable mortality risk and a long in-hospital recovery period should the patient survive.
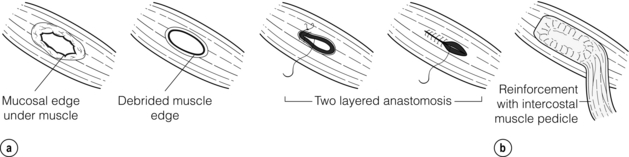
A posterolateral thoracotomy is used to approach the oesophagus, most commonly on the left in the seventh or eighth intercostal space. Solid debris is removed and the pleural cavity thoroughly cleaned. The mediastinal pleura is widely incised to expose the injury, and necrotic, devitalised tissue debrided. A longitudinal myotomy is made (as the mucosal injury is usually longer than the muscular one) and the oesophagus repaired.29
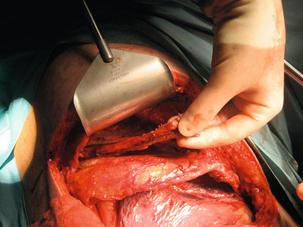
Primary repair with or without reinforcement: A simple, single- or two-layered, primary repair can be fashioned using 2/0 or 3/0 interrupted absorbable sutures with or without a small-diameter bougie (40–46 F) in situ (Fig. 19.7). However, primary repair is associated with a significant leak rate (20–50%) and should be reserved for those operated on rapidly with demonstrably healthy tissue and limited soiling.30 There is circumstantial evidence that reinforcing the suture line with an onlay patch of nearby tissues (such as omentum, pleura, lung, pedicled intercostal muscle grafts, gastric fundus, pericardium or diaphragm) may reduce the leak rate.31 Regardless, the authors would suggest that all primary repairs will leak and appropriate drains should be placed around the repair (Fig. 19.8).
T-tube repair: The concept of repair over a T-tube is to form a controlled oesophagocutaneous fistula.32 A large-diameter (6–10 mm) T-tube is placed through the tear with the limbs lying beyond the boundaries of the perforation and the oesophageal wall is closed loosely around the tube with fine interrupted, absorbable sutures (Fig. 19.9). The authors suggest anchoring the tube to the diaphragm, as originally described, as aortic erosion due to sepsis and pressure necrosis is possible.33 The tube is externalised and secured, a further drain is placed down to the repair, and apical and basal intercostal chest drains are sited. Healing is monitored by contrast radiology and CT scans. The T-tube is left until a defined tract is established, with the majority removed around 6 weeks.
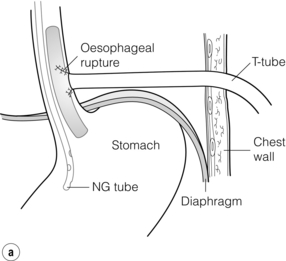
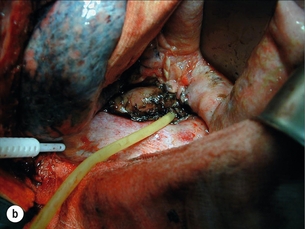
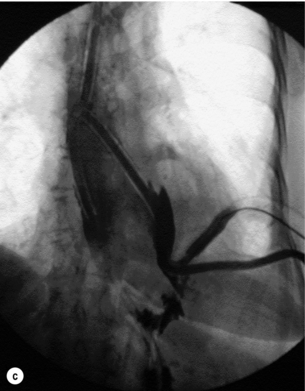
Figure 19.9 (a) Diagrammatic representation of T-tube repair of spontaneous oesophageal perforation with T-tube in situ. (b) Operative photograph. (c) Contrast radiological image of the same patient as in (b); note additional intercostal chest drain with small contrast leak directly into this drain.
Resection: Oesophageal resection in the presence of a perforation is a major undertaking with an extremely high mortality. It is to be reserved for damage to a diseased oesophagus or in cases of extensive oesophageal trauma. This has even been performed in this setting as a minimally invasive approach.38 If contamination is minimal then immediate reconstruction would be appropriate but a delayed approach may also be taken with limited differences in outcome.39 The use of non-definitive exclusion and diversion techniques is mostly historical but remains in the armamentarium of an oesophageal surgeon and may occasionally be useful, such as in extensive caustic injuries.
Minimally invasive surgery: laparoscopic/thoracoscopic: Distal, clean and immediately recognised iatrogenic perforations may be suitable to approach laparoscopically (transperitoneally) to attempt repair and drainage by surgeons used to working at the hiatus. This requires advanced laparoscopic skills in specialist centres with appropriate facilities.34 Equally, selected cases can be managed thoracoscopically.35,36
Surgical repair over a stent: In view of the high leak rate of primary repair, some authors advocate a surgical repair over a stent (sutured transluminal or externally to prevent migration). This theoretically expedites a return to enteral nutrition.37 However, the problems of stent placement remain in terms of the risks of a foreign body in the site of sepsis.
Management of penetrating injuries
Thoracic
Virtually all transthoracic gunshot wounds will require surgical exploration, and life-threatening cardiovascular, pulmonary and tracheobronchial injuries take precedence. Specialist advice and input should be sought but the majority of the oesophageal injuries will be able to be dealt with using the techniques described previously. The overall mortality of penetrating thoracic oesophageal injuries is hard to ascertain but lies between 15% and 27% (lower for cervical trauma at 1–16%).40 The morbidity arises mostly from associated spinal and airway trauma for cervical injuries and from cardiorespiratory damage in thoracic trauma.
Management of underlying pathology
Patients who sustain a perforation of a malignant stricture constitute a difficult group to manage. Those who have known inoperable disease due to metastatic spread or who are unfit for surgery should be managed non-operatively, and in this situation the use of a sealing palliative stent is appropriate. In patients with less clearly defined operability most authors recommend resection with a view to control of contamination and potential cure, but this strategy carries a considerable mortality rate (11–75%).39,41
Iatrogenic perforation of achalasia is uncommon (1–5%) and usually managed non-operatively or endoscopically as they are usually small, clean, immediately recognised and well contained. Other pathologies such as peptic stricture, infections or treatments can also predispose to perforation, e.g. radio/chemotherapy. Specific operative intervention may be required and, despite reduced contamination, the associated surgical mortality is increased. The indications for operative management are the corollary of those documented in Box 19.4.
Paraoesophageal surgery and procedural injuries
Direct oesophageal trauma is most commonly sustained during antireflux surgery, both open and laparoscopic, but the risk is low, of the order of 0–1.2%.43 The risk increases with an intrathoracic approach, a previous hiatal operation and suturing of the wrap to the oesophagus. The majority of injuries are recognised and repaired immediately with buttressing using the fundoplication wrap. Drainage is advised and it may also be appropriate to form a feeding jejunostomy or to place a nasojejunal tube until the repair is deemed safe by contrast radiology. The mortality of unrecognised and uncontained perforations approaches 20%.
Management algorithm
Diagnostic delay beyond 24 hours is classically associated with a poor outcome, but even when managed promptly and aggressively, perforation of the oesophagus, especially Boerhaave-type disruption, carries a significant mortality rate and reports to the contrary reflect selection bias. A management algorithm based on the therapeutic strategies outlined by the literature is demonstrated in Fig. 19.10. This is for guidance only and cases should be dealt with individually. Personal experience and expertise may well determine the best management.
Non-perforated spontaneous injuries of the oesophagus
Full-thickness oesophageal perforation contained by the mediastinal pleura is termed ‘intramural rupture’.44 This can occur spontaneously or secondary to instrumentation, food impaction or coagulopathies. Non-operative treatments with or without endoscopic adjuncts are usually successful as the perforation is contained, but a minority may require surgical intervention.44
‘Black oesophagus syndrome’ or acute oesophageal necrosis is extremely rare. This is circumferential mucosal and submucosal necrosis that ends sharply at the oesophagogastric junction in the absence of a caustic injury, most commonly presenting with upper gastrointestinal bleeding.45 The most likely cause is vascular insufficiency from venous thrombosis as part of a ‘two-hit’ traumatic phenomenon associated with systemic hypotension from another cause. It has also been associated with thrombotic disorders. Diagnosis is endoscopic and treatment expectant with a low threshold for surgical resection as the condition can rapidly progress to perforation. Mortality is high, often secondary to the underlying cause.
Caustic injuries
There are two important misconceptions about caustic injuries:
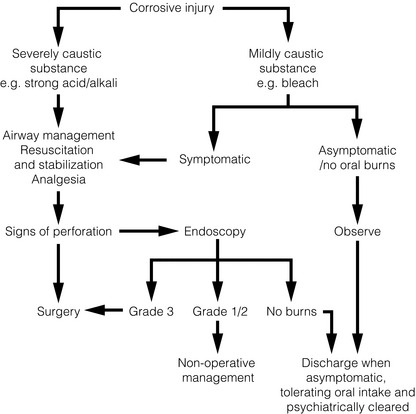
Misconception 1: tissue penetration by acids is minimised by coagulative necrosis whereas alkalis more rapidly penetrate transmurally through liquefactive necrosis. Although pathologically correct, this is clinically irrelevant as the ingestion of any strong caustic agent in sufficient quantity will inflict a potentially fatal oesophageal injury. Furthermore, there is evidence to suggest that strong acid ingestion is associated with greater systemic effects, a higher perforation rate and a higher mortality than alkali ingestion.46
Misconception 2: acid ingestion causes gastric damage whereas alkali ingestion causes oesophageal injury. Although commonly cited, there is no evidence to support this.47,48
Clinical presentation
Presentation can be varied and confusing. In accidental ingestion, symptoms and signs may not have developed due to rapid presentation since clinical features are dependent on the substance and the time since ingestion. Equally, the absence of oral burns or pharyngo-oesophageal symptoms does not exclude more distal injury as the caustic agent may have passed rapidly through the mouth and pharynx. Furthermore, in deliberate ingestion the clinical features may be ‘underplayed’ by the patients. The clinical features of a caustic injury of the oesophagus are documented in Box 19.5. Most patients survive to reach the hospital unless aspiration has occurred. Glossopharyngeal burns cause oedema that may threaten the airway and prevent clearance of secretions with drooling and hypersalivation, and injury to the epiglottis and larynx leads to stridor and a hoarse voice. Dyspnoea is uncommon unless aspiration has occurred. On inspection, oropharyngeal burns can range from mild oedema and superficial erosions to extensive mucosal sloughing and necrosis. Acid burns form a black eschar whereas alkali burns look grey and dull. Oesophageal injury is suggested by dysphagia and odynophagia, and gastric injury by epigastric pain, nausea, anorexia, retching, vomiting and haematemesis. Patients may present shocked or in respiratory distress.
Investigation and management
The immediate priorities are the establishment of a secure airway, the stabilisation of cardiovascular status and the relief of pain. Severe laryngopharyngeal burns or respiratory compromise may require early tracheal intubation and general anaesthesia. Concurrent facial or eye burns should be irrigated and ophthalmology and plastic surgery specialist involvement should be sought. Oral intake is prohibited. Gastric lavage, induced emesis, nasogastric aspiration and the use of neutralising chemicals are contraindicated. Where possible the ingested agent and amount swallowed should be identified and regional poison centres can provide information regarding the properties of specific agents. Endoscopic staging of the burn determines the optimum management, likelihood of subsequent stricture formation and is the only accurate predictor of systemic complications and death46 (Fig. 19.11).
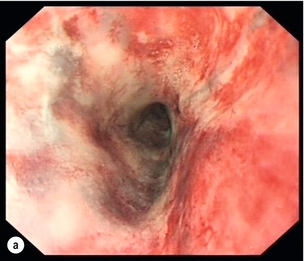
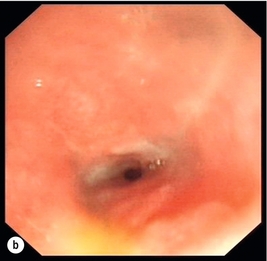
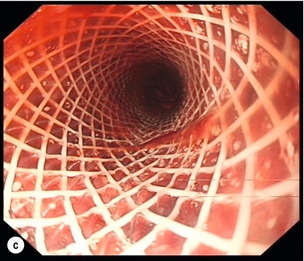
Figure 19.11 Endoscopic appearances of caustic injury to the oesophagus. (a) Acute grade 3a alkali injury. (b) Appearance after 8 weeks with pinhole stricture. (c) A year later and refractory stricture treated by placement of stent.
The severity of the injury is graded using a system similar to that for skin burns (Table 19.2) but differentiation between grades may be difficult, especially between second- and third-degree burns, with implications for management; consequently, some patients will benefit from repeated evaluation (Table 19.3).50 There has been interest in the use of oesophageal endosonography to assess depth of necrosis and damage to the muscle layers, but this currently offers no advantage over conventional endoscopic assessment.51 Equally, a modern CT grading system could be useful but is unlikely to replace an endoscopic assessment, which remains the gold standard.52
Table 19.2
| Depth of burn | Degree of burn | Endoscopic findings |
| Superficial | 1 | Mucosal oedema and hyperaemia |
| Transmucosal with or without involvement of the muscularis | 2a | Superficial ulcers, bleeding, exudates |
| 2b | Deep ulcers – focal or circumferential | |
| Full thickness with or without adjacent organ involvement | 3a | Full-thickness focal necrosis |
| 3b | Extensive necrosis |
There are a few interesting animal-based research projects looking at other stricture-preventing medical treatments such as ibuprofen after oesophageal caustic injuries, but these have not yet been tried in a human population.55
Asymptomatic patients with unintentional ingestion but no oropharyngeal burns and normal or minor oesophageal findings may be discharged once they are able to take oral fluids. Intravenous fluids, analgesia, nutritional support and antisecretory agents should be given to all other patients. Patients with grade 1 and 2a burns should be admitted and observed for 5–7 days, with diet reintroduced gradually over 24–48 hours, and endoscopy or contrast radiology studies should be arranged for 6–8 weeks after discharge to assess for strictures. Suicidal/intentional injury patients require psychiatric assessment prior to discharge. It is reasonable to observe patients with grade 2b and 3 burns, continuing nasojejunal feeding and if there is no evidence of progression to perforation then clear fluids can be introduced from 48 hours, but be aware that the perforation risk is present for at least 7 days. Those who present with a perforation or deteriorate will require an emergency oesophagogastrectomy as the stomach is almost always injured. The authors do not believe that laparoscopy has a role in assessing gastric viability. Immediate reconstruction with a substernal colonic interposition graft can be performed if there is minimal local contamination but more commonly oesophagostomy and delayed reconstruction 6–8 weeks later is preferred. It is reasonable to consider resection in patients with extensive circumferential mucosal injuries in view of the problem of refractory strictures and the long-term cancer risk. The mortality for these caustic injuries is 13–40%, with the majority of deaths occurring in the adult suicidal group.48 Mortality mainly stems from respiratory complications and delay in the aggressive surgical treatment of transmural necrosis. There is no place for ‘conservative’ treatment of a severe caustic oesophageal injury.
Long-term complications and outcomes
Strictures develop in 5–50% of patients, 95% of which are distal and can be graded according to the Marchand classification (Table 19.4).56
The procedure-related perforation incidence is less than 1%, but for safety the authors advise allowing approximately 6 weeks after injury before attempting dilatation. Antisecretory medication or even surgery may be required if reflux occurs after dilatation. Young patients with long, grade 3 or 4 strictures are likely to require a lifetime of repeated dilatations with a cumulative risk of iatrogenic perforation and ultimately of cancer, and in these patients other options should be considered. Surgical options are to bypass or resect the obstructive segment or to perform a stricturoplasty. Bypass avoids dissection through mediastinal fibrosis, and a retrosternal or subcutaneous route for the neo-oesophagus may avoid a thoracotomy. However, retaining the damaged oesophagus retains the long-term cancer risk and can lead to problems related to secretions and bacterial overgrowth. Thoracotomy, resection and colonic reconstruction (due to concurrent gastric damage) is therefore preferable. An alternative is an oesophageal stricturoplasty using a vascularised graft of colon, but again this retains the cancer risk. There is increasing interest in the use of removable or absorbable stents for refractory strictures but evidence is limited by numbers. However, these remain an alluring prospect and there is currently a National Institute for Health Research study investigating their use in benign oesophageal strictures.58 There is also some evidence for the use of endoscopic triamcinolone acetonide injection into the strictured segment to augment dilatation.59
Ingestion of foreign bodies
The oesophagus is the most common site for impaction of ingested foreign bodies within the gastrointestinal tract, accounting for 75% of cases.60 By far the majority occur in children under the age of 10 years, with coins, toys, crayons and batteries being the commonest objects swallowed.61 In adults, food boluses (predominantly meat) or impaction of food-related bone fragments are more common. This is especially the case in edentulous patients due to decreased palatal sensation. Cases also occur in people with mental or psychiatric difficulties, or related to drug and alcohol abuse, and in those seeking secondary gain such as prisoners, and most upper gastrointestinal units are aware of a number of recurrent offenders.
Clinical presentation
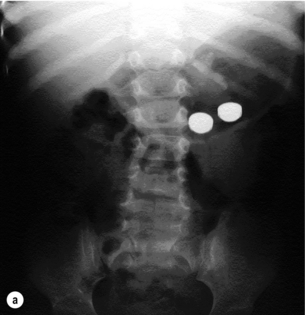
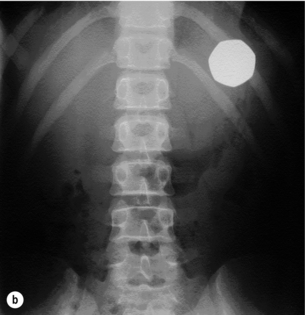
In over 90% there is a clear history of ingestion associated with acute dysphagia at the level of the impaction and thus a rapid diagnosis.62 However, in young children and uncooperative adults the diagnosis may not be so clear-cut. Suspicious symptoms in children are refusal of feeds, gagging and choking, but some cases may remain concealed for months or even years and chronic aspiration or reflux may represent long-standing impaction. A high index of suspicion is also required for psychiatric patients with features suggestive of foreign body ingestion. Respiratory symptoms occur in 5–15%, especially in children and in cervical impaction, leading to coughing, wheezing, stridor and dyspnoea. In adults, acute impaction in the cervical oesophagus can cause tracheal obstruction leading to the so-called ‘café coronary’ or ‘steakhouse syndrome’. Typically, sharp object ingestion (e.g. fish bones) can cause a persistent foreign body sensation despite easy passage through the oesophagus without impaction. Physical signs are usually limited unless impaction causes obstruction leading to drooling or perforation leading to neck swelling, erythema, tenderness, subcutaneous emphysema or systemic effects. Long-standing impaction may lead to recurrent aspiration, empyema of the lung, perioesophagitis, oesophageal stenosis or fistulation into the airways or major vessels.
Diagnosis
Plain radiographs may localise both radio-opaque and non-radio-opaque objects and are useful if perforation is suspected (Fig. 19.13). Both anteroposterior and lateral projections should be obtained as objects may not be visible if overlying the vertebrae, and this also helps to distinguish whether objects are in the digestive or tracheobronchial tract. In young children and infants, extensive plain radiography may be required to confirm or refute the diagnosis of a swallowed radio-opaque foreign body. Even in the absence of symptoms or physical signs a potential history of ingestion should prompt the use of radiography, as in one study 17% of asymptomatic children with a history of coin ingestion had an impacted oesophageal coin.63 Water-soluble contrast studies or CT scans may occasionally be required in cases of non-radio-opaque objects such as wood, aluminium, glass and plastics, but the use of hypertonic contrast media and barium should be avoided.
Management
The indications for urgent intervention are:
2. Absolute dysphagia with aspiration risk.
A number of techniques to dislodge food boluses without recourse to endoscopy have been reported. Proteolytic agents (e.g. papain) that dissolve the food bolus may cause oesophageal trauma and are dangerous if aspirated and they are not recommended. Effervescent agents, such as carbonated drinks and intravenous glucagon, which causes smooth muscle relaxation, were thought to help disimpact the food bolus but there is no good evidence to support their use.67 Failure to progress through the gastrointestinal tract or symptomatic deterioration should prompt surgical review. Therapeutic video endoscopy is as successful in object removal as rigid endoscopy but with a significantly lower complication rate (5% vs. 10%) and avoids general anaesthesia in the majority of cases. However, in a minority, the type of object, number of objects or the inability of the patient to cooperate (i.e. young children) may dictate that general anaesthesia is required and rigid endoscopy is still useful for impaction in the pharynx as the view and access are superior, but the authors feel that it should be abandoned for distal obstructions. Patience is important in endoscopic removal but is rewarded with a high success rate (around 95%).64 Failure is most likely to occur with long (>10 cm) or complex objects such as dental prostheses.
Surgery may be necessary when endoscopy fails for large objects, for objects embedded in the oesophageal wall or when there has been an associated or iatrogenic perforation. The surgical approach depends not only on the site and severity of the injury, but also associated inflammation and any underlying oesophageal pathology. Deliberate narcotic packet ingestion is a particularly taxing scenario. The authors suggest that endoscopic removal should not be attempted as successful retrieval is outweighed by the risk of rupture.68 Most packets will pass safely through the bowel but urgent surgery is indicated in cases where there is failure to progress, obstruction or rupture, in conjunction with medical support for absorption of relevant contents.
References
1. Borotto, E., Gaudric, M., Danel, B., et al, Risk factors of oesophageal perforation during pneumatic dilatation for achalasia. Gut. 1996;39(1):9–12. 8881799
2. Fernandez, F.F., Richter, A., Freudenberg, S., et al, Treatment of endoscopic esophageal perforation. Surg Endosc. 1999;13(10):962–966. 10526027
3. Hayes, N., Waterworth, P.D., Griffin, S.M., Avulsion of short gastric arteries caused by vomiting. Gut. 1994;35(8):1137–1138. 7926919
4. Lucendo, A.J., Friginal-Ruiz, A.B., Rodríguez, B., Boerhaave’s syndrome as the primary manifestation of adult eosinophilic esophagitis. Two case reports and a review of the literature. Dis Esophagus. 2011;24(2):E11–E15. 21309916
5. Mackler, S., Spontaneous rupture of the oesophagus; an experimental and clinical study. Surg Gynecol Obst 1952; 95:345–356. 14950670
6. Griffin, S.M., Lamb, P.J., Shenfine, J., et al, Spontaneous rupture of the oesophagus. Br J Surg. 2008;95(9):1115–1120. 18655213
7. Shenfine, J., Dresner, S.M., Vishwanath, Y., et al, Management of spontaneous rupture of the oesophagus. Br J Surg. 2000;87(3):362–373. 10718969
8. Levine, P.H., Kelley, M.L., Jr., Spontaneous perforation of esophagus simulating acute pancreatitis. JAMA. 1965;191(4):342–345. 5899759
9. Altorjay, A., Kiss, J., Voros, A., et al, The role of esophagectomy in the management of esophageal perforations. Ann Thorac Surg. 1998;65(5):1433–1436. 9594880
10. Horwitz, B., Krevsky, B., Buckman, R.F., Jr., et al, Endoscopic evaluation of penetrating esophageal injuries. Am J Gastroenterol. 1993;88(8):1249–1253. 8338093
11. Srinivasan, R., Haywood, T., Horwitz, B., et al, Role of flexible endoscopy in the evaluation of possible esophageal trauma after penetrating injuries. Am J Gastroenterol. 2000;95(7):1725–1729. 10925975
12. Griffin, S.M., Lamb, P.J., Dresner, S.M., et al, Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg. 2001;88(10):1346–1351. 11578290
13. White, C.S., Templeton, P.A., Attar, S., Esophageal perforation: CT findings. Am J Roentgenol. 1993;160(4):767–770. 8456662
14. Qadeer, M.A., Dumot, J.A., Vargo, J.J., et al, Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007;66(3):605–611. 17725956
15. Fritscher-Ravens, A., Hampe, J., Grange, P., et al, Clip closure versus endoscopic suturing versus thoracoscopic repair of an iatrogenic esophageal perforation: a randomized, comparative, long-term survival study in a porcine model (with videos). Gastrointest Endosc. 2010;72(5):1020–1026. 21034902
16. Rokszin, R., Simonka, Z., Paszt, A., et al, Successful endoscopic clipping in the early treatment of spontaneous esophageal perforation. Surg Laparosc Endosc Percutan Tech. 2011;21(6):e311–e312. 22146179
17. Kiev, J., Amendola, M., Bouhaidar, D., et al, A management algorithm for esophageal perforation. Am J Surg. 2007;194(1):103–106. 17560919
18. Adam, A., Watkinson, A.F., Dussek, J., Boerhaave syndrome: to treat or not to treat by means of insertion of a metallic stent. J Vasc Interv Radiol. 1995;6(5):741–746. 8541678
19. Doniec, J.M., Schniewind, B., Kahlke, V., et al, Therapy of anastomotic leaks by means of covered self-expanding metallic stents after esophagogastrectomy. Endoscopy. 2003;35(8):652–658. 12929059
20. Radecke, K., Gerken, G., Treichel, U., Impact of a self-expanding, plastic esophageal stent on various esophageal stenoses, fistulas, and leakages: a single-center experience in 39 patients. Gastrointest Endosc. 2005;61(7):812–818. 15933681
21. Odell, J.A., DeVault, K.R., Extended stent usage for persistent esophageal leak. Ann Thorac Surg 2010; 90:1707–1708. 20971302
22. Eubanks, P.J., Hu, E., Nguyen, D., et al, Case of Boerhaave’s syndrome treated with a self-expanding metallic stent. Gastrointest Endosc 1999; 49:780–783. 10343228
23. Davies, A.P., Vaughan, R., Expanding mesh stent in the emergency treatment of Boerhaave’s syndrome. Ann Thorac Surg 1999; 67:1482–1483. 10355440
24. Yuasa, N., Hattori, T., Kobayhashi, Y., et al, Treatment of spontaneous esophageal rupture with a covered self-expanding metal stent. Gastrointest Endosc 1999; 49:777–780. 10343227
25. Van Heel, N.C., Haringsma, J., Spaander, M.C., et al, Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol 2010; 105:1515–1520. 20234349
26. Loske, G., Schorsch, T., Müller, C., Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy. 2011;43(6):540–544. 21448855
27. Ahrens, M., Schulte, T., Egberts, J., et al, Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy. 2010;42(9):693–698. 20806153
28. Petruzziello, L., Tringali, A., Riccioni, M.E., et al, Successful early treatment of Boerhaave’s syndrome by endoscopic placement of a temporary self-expandable plastic stent without fluoroscopy. Gastrointest Endosc. 2003;58(4):608–612. 14520304
29. Walker, W.S., Cameron, E.W., Walbaum, P.R., Diagnosis and management of spontaneous transmural rupture of the oesophagus (Boerhaave’s syndrome). Br J Surg. 1985;72(3):204–207. 3978378
30. Lawrence, D.R., Ohri, S.K., Moxon, R.E., et al, Primary esophageal repair for Boerhaave’s syndrome. Ann Thorac Surg. 1999;67(3):818–820. 10215235
31. Wright, C.D., Mathisen, D.J., Wain, J.C., et al, Reinforced primary repair of thoracic esophageal perforation. Ann Thorac Surg. 1995;60(2):245–249. 7646082
32. Mansour, K.A., Wenger, R.K., T-tube management of late esophageal perforations. Surg Gynecol Obstet. 1992;175(6):571–572. 1448740
33. Naylor, A.R., Walker, W.S., Dark, J., et al, T tube intubation in the management of seriously ill patients with oesophagopleural fistulae. Br J Surg. 1990;77(1):40–42. 2302511
34. Bell, R.C., Laparoscopic closure of esophageal perforation following pneumatic dilatation for achalasia. Report of two cases. Surg Endosc. 1997;11(5):476–478. 9153180
35. Cho, J.S., Kim, Y.D., Kim, J.W., et al, Thoracoscopic primary esophageal repair in patients with Boerhaave’s syndrome. Ann Thorac Surg. 2011;91(5):1552–1555. 21435633
36. Haveman, J.W., Nieuwenhuijs, V.B., Kobold, J.P., et al, Adequate debridement and drainage of the mediastinum using open thoracotomy or video-assisted thoracoscopic surgery for Boerhaave’s syndrome. Surg Endosc. 2011;25(8):2492–2497. 21359901
37. Babor, R., Talbot, M., Tyndal, A., Treatment of upper gastrointestinal leaks with a removable, covered, self-expanding metallic stent. Surg Laparosc Endosc Percutan Tech. 2009;19(1):e1–e4. 19238047
38. Grewal, N., El-Badawi, K., Nguyen, N.T., Minimally invasive Ivor Lewis esophagectomy for the management of iatrogenic esophageal perforation in a patient with esophageal cancer. Surg Technol Int 2009; 18:82–85. 19585419
39. Orringer, M.B., Stirling, M.C., Esophagectomy for esophageal disruption. Ann Thorac Surg. 1990;49(1):35–43. 2297275
40. Pass, L.J., LeNarz, L.A., Schreiber, J.T., et al, Management of esophageal gunshot wounds. Ann Thorac Surg. 1987;44(3):253–256. 3632110
41. Adam, D.J., Thompson, A.M., Walker, W.S., et al, Oesophagogastrectomy for iatrogenic perforation of oesophageal and cardia carcinoma. Br J Surg. 1996;83(10):1429–1432. 8944464
42. Dresner, S.M., Lamb, P.J., Viswanath, Y.K.S., et al. Oesophagectomy following iatrogenic perforation of operable oesophageal carcinoma. Br J Surg. 2000; 87(S1):29.
43. Pessaux, P., Arnaud, J.P., Ghavami, B., et al, Morbidity of laparoscopic fundoplication for gastroesophageal reflux: a retrospective study about 1470 patients. Hepatogastroenterology. 2002;49(44):447–450. 11995471
44. Steadman, C., Kerlin, P., Crimmins, F., et al, Spontaneous intramural rupture of the oesophagus. Gut. 1990;31(8):845–849. 2387502
45. Moreto, M., Ojembarrena, E., Zaballa, M., et al, Idiopathic acute esophageal necrosis: not necessarily a terminal event. Endoscopy. 1993;25(8):534–538. 8287816
46. Poley, J.W., Steyerberg, E.W., Kuipers, E.J., et al, Ingestion of acid and alkaline agents: outcome and prognostic value of early upper endoscopy. Gastrointest Endosc. 2004;60(3):372–377. 15332026
47. Zargar, S.A., Kochhar, R., Nagi, B., et al, Ingestion of corrosive acids. Spectrum of injury to upper gastrointestinal tract and natural history. Gastroenterology. 1989;97(3):702–707. 2753330
48. Zargar, S.A., Kochhar, R., Nagi, B., et al, Ingestion of strong corrosive alkalis: spectrum of injury to upper gastrointestinal tract and natural history. Am J Gastroenterol. 1992;87(3):337–341. 1539568
49. Wijburg, F.A., Heymans, H.S., Urbanus, N.A., Caustic esophageal lesions in childhood: prevention of stricture formation. J Pediatr Surg. 1989;24(2):171–173. 2724008
50. Zargar, S.A., Kochhar, R., Mehta, S., et al, The role of fiberoptic endoscopy in the management of corrosive ingestion and modified endoscopic classification of burns. Gastrointest Endosc. 1991;37(2):165–169. 2032601
51. Kamijo, Y., Kondo, I., Kokuto, M., et al, Miniprobe ultrasonography for determining prognosis in corrosive esophagitis. Am J Gastroenterol. 2004;99(5):851–854. 15128349
52. Ryu, H.H., Jeung, K.W., Lee, B.K., et al, Caustic injury: can CT grading system enable prediction of esophageal stricture? Clin Toxicol (Phila). 2010;48(2):137–142. 20199130
53. Anderson, K.D., Rouse, T.M., Randolph, J.G., A controlled trial of corticosteroids in children with corrosive injury of the esophagus. N Engl J Med. 1990;323(10):637–640. 2200966 A prospective randomised, controlled trial in 60 children with caustic injuries with a follow-up of 18 years comparing a steroid and antibiotic regimen with best supportive care. No benefit was demonstrated in the steroid and antibiotic group; the development of oesophageal strictures related only to the severity of the corrosive injury.
54. Bauer, T.M., Dupont, V., Zimmerli, W., Invasive candidiasis complicating spontaneous esophageal perforation (Boerhaave syndrome). Am J Gastroenterol. 1996;91(6):1248–1250. 8651181
55. Herek, O., Karabul, M., Yenisey, C., et al, Protective effects of ibuprofen against caustic esophageal burn injury in rats. Pediatr Surg Int. 2010;26(7):721–727. 20480167
56. Marchand, P., Caustic strictures of the oesophagus. Thorax. 1955;10(2):171–181. 14396853
57. Cox, J.G., Winter, R.K., Maslin, S.C., et al, Balloon or bougie for dilatation of benign esophageal stricture? Dig Dis Sci. 1994;39(4):776–781. 7818628 A randomised study in 93 adult patients demonstrating a better and longer-lasting symptomatic result for lower cost with Savary–Gilliard bougie dilatation than balloon dilatation.
58. Thomas, T., Abrams, K.R., Subramanian, V., et al, Esophageal stents for benign refractory strictures: a meta-analysis. Endoscopy. 2011;43(5):386–393. 21437850
59. Kochhar, R., Ray, J.D., Sriram, P.V., et al, Intralesional steroids augment the effects of endoscopic dilation in corrosive esophageal strictures. Gastrointest Endosc. 1999;49(4, Pt 1):509–513. 10202068
60. Webb, W.A., Management of foreign bodies of the upper gastrointestinal tract. Gastroenterology. 1988;94(1):204–216. 3275566
61. Nadir, A., Sahin, E., Nadir, I., et al, Esophageal foreign bodies: 177 cases. Dis Esophagus. 2011;24(1):6–9. 20626451
62. Ciriza, C., Garcia, L., Suarez, P., et al, What predictive parameters best indicate the need for emergent gastrointestinal endoscopy after foreign body ingestion? J Clin Gastroenterol. 2000;31(1):23–28. 10914771
63. Hodge, D., 3rd., Tecklenburg, F., Fleisher, G., Coin ingestion: does every child need a radiograph? Ann Emerg Med. 1985;14(5):443–446. 3985465
64. Li, Z.S., Sun, Z.X., Zou, D.W., et al, Endoscopic management of foreign bodies in the upper-GI tract: experience with 1088 cases in China. Gastrointest Endosc. 2006;64(4):485–492. 16996336
65. Eisen, G.M., Baron, T.H., Dominitz, J.A., et al, Guideline for the management of ingested foreign bodies. Gastrointest Endosc. 2002;55(7):802–806. 12024131
66. Waltzman, M.L., Baskin, M., Wypij, D., et al, A randomized clinical trial of the management of esophageal coins in children. Pediatrics. 2005;116(3):614–619. 16140701 A prospective randomised controlled trial of 60 children with an asymptomatic oesophageal coin, comparing immediate endoscopic removal with observation, including radiography and endoscopic removal where deemed necessary. This demonstrated no benefit from immediate endoscopic removal and 25–30% of coins passed spontaneously without complications. They conclude that treatment could reasonably include a short period of observation, particularly in older children with distally sited coins.
67. Tibbling, L., Bjorkhoel, A., Jansson, E., et al, Effect of spasmolytic drugs on esophageal foreign bodies. Dysphagia. 1995;10(2):126–127. 7600855 A multicentre placebo-controlled trial, showing no benefit of glucagon and diazepam over a placebo treatment in the treatment of food bolus impaction.
68. Lancashire, M.J., Legg, P.K., Lowe, M., et al, Surgical aspects of international drug smuggling. Br Med J (Clin Res Ed). 1988;296(6628):1035–1037. 3130126