Chapter 18 Ocular Ultrasound Guided Anesthesia
Introduction
Retrobulbar block
Until recently, the RBB has been the most common regional ophthalmic anesthetic technique. Its roots date to the late 1800s and it was formally described by Atkinson in the early twentieth century.1 Advantages of RBBs include rapid onset of profound akinesia and analgesia while using a minimal volume of local anesthetic. However, complications have been associated with this technique including brainstem anesthesia, globe puncture, and retrobulbar hemorrhage. Therefore, this modality has been largely superseded by other methods.
Peribulbar block
Peribulbar blocks which are placed into the extraconal space may provide a superior safety profile since needles are intentionally guided outside of the orbits’ muscle cone with less depth and angulation.2 Disadvantages of the PBB technique include a longer latency of onset for total block, and the need for greater volumes of local anesthetic agents. Unfortunately, the potential for serious sequelae remain.3
Sub-Tenon’s block
While STBs, using cannulae instead of needles, have been touted by some as being less prone to major complications such as globe puncture and serious hemorrhage, minor complications including conjunctival bleeding and chemosis are more commonly observed. Notably, as more and more STBs are performed, the number of these aforementioned serious complications have been increasingly reported in the literature.4,5
Rationale for USG-guided anesthesia
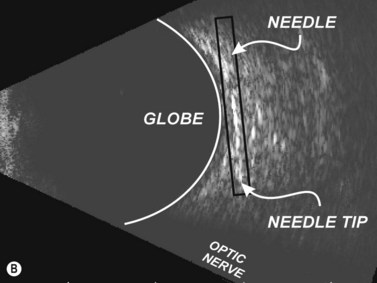
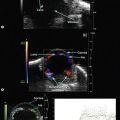
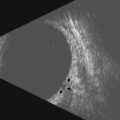
Needle misadventure and penetration or perforation of the globe, leading to potential long-term visual compromise, are arguably the most feared complications of ophthalmic anesthesia. Fortunately, in the hands of trained and experienced physicians, such complications are exceedingly rare.6 Needle-based eye blocks are “blind” techniques that are dependent upon surface anatomy landmarks in order to position the needle correctly. An office-based ultrasound A- or B-scan may provide information as to the globe’s axial length and morphology of the eye in order to detect an atypically long or asymmetric, possibly staphylomatous eye.7 Fundamentally, a RBB is conducted by intentionally angling the needle steeply and deeply within the orbit behind the globe. If the globe is longer than anticipated, or a staphyloma is prominent, greater risk of needle injury to the eye’s posterior portion exists.8 Using ultrasound to visualize important anatomy during the procedure may be of utility (Figure 18.1). Training and experience is of paramount importance in order to use this method with skill.
Purportedly, there is less likelihood to puncture or perforate the globe when one is using a PBB, which entails shallower needle placement with less angulation towards the orbital apex. However, longer globes have greater volume and increased superior/inferior girth, thus exposing the inferior aspect to needle trauma. Therefore, the risk of globe puncture remains.8
Published studies
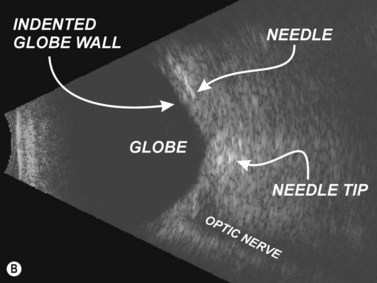
In 1995, Birch et al described the use of ultrasound block to localize 25 gauge 38 mm needles during RBB.9 Initially, each ophthalmologist performing a RBB estimated the distance between needle tip and the posterior globe as being greater than 5 mm. Sonographic images were obtained prior to needle placement, with the needle in situ, and upon injection of local anesthetic. In each block, the needle tip, according to ultrasonographic interpretation, was noted to be significantly closer to the globe than each ophthalmologist had estimated. True needle tip distances were found to be as little as 0.2 mm behind the globe’s posterior pole. The greatest distance was 3.3 mm! While no globe perforations or penetrations were encountered, the needle shaft was observed to indent the globe in over half of the RBBs in this series. (Others have not reproduced this finding.)10 Probe tension on the eye can deform the globe by causing it to be balloted against the needle shaft (Figure 18.2). Releasing pressure from the array allows the eye to revert to its usual position. Ultimately, Birch et al concluded that the use of external anatomic landmarks alone might be insufficient.9
Winder et al studied both the RBB and PBB ultrasound-guided techniques.10 Ultrasonography was performed prior to, during and 10 minutes after injection of local anesthetics. As expected, anechoic local anesthetic was initially seen within the muscle cone for RBBs, and outside of the cone for PBBs. Ten minutes after PBB, sonography demonstrated that the local anesthetics, placed extraconally, had travelled into the intraconal space thus confirming the mechanism of achieving orbital anesthesia with peribulbar injection. In contrast to Birch et al, there were no cases of needle contact with sclera.
Luyet et al performed a series of ultrasound-guided needle-based eye blocks on cadaveric specimens.11 They found that key structures within the orbit, notably the globe’s rim and optic nerve, as well as the needle and injected fluids were readily visualized. Notably, visualization was facilitated due to use of needles that were considerably larger than those typically used on live patients. Nonetheless, ultrasound-guided procedure feasibility was demonstrated. The need for additional research in this arena is warranted.
Technique
The pyramidal bony orbit, with a scant volume of approximately 30 mm3, is packed with globe, muscles, nerves, arteries, veins, connective tissue, and fat. There are no interior gas-filled or bony structures, making the orbit an ideal area for ultrasonic imaging. Injection at the superior aspect between the globe and orbital roof is not ideal since the space in that area is constricted, the ophthalmic vessels tend to reside in the upper orbit, and the superior oblique’s trochlea can be exposed to trauma.12 The conventional needle-entry point at the junction of the medial two-thirds and lateral one-third of the inferior orbital rim is shifted laterally to decrease the likelihood of inferior rectus or oblique trauma thought to be caused by injecting local anesthetics directly into those muscles.13
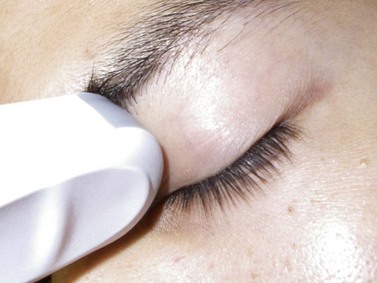
A regional ophthalmic block is performed only after confirming the correct patient, procedure, and side of surgery. In order to maintain the optic nerve in a slack state, such that it does not impinge upon the needle’s path, the patient is instructed to remain in neutral gaze. The proper orientation between the operator and the ultrasound monitor is obtained by standing nearest to the side to be blocked and positioning the monitor in line of sight on the opposite side of the patient’s head, such that one may visualize the surgical field and the monitor simultaneously. Water-soluble ultrasound transmission gel is liberally applied to the eyelid (Chapter 3). The transducer is positioned just below the supraorbital rim over the upper eyelid and a transocular image of the globe wall and surrounding orbital space are obtained. Incremental angulation of the transducer can bring the needle into clearer view. Altering the depth and sound wave frequency, using medium gain, is also useful.
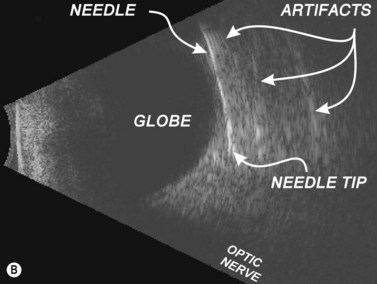
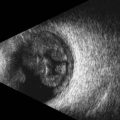
The edge between the globe and orbit are easily visualized as these tissue densities have markedly different acoustic impedances. The vitreous fluid that comprises the majority of the globe’s contents appears anechoic, while external orbital contents appear more echogenic. Reverberations and reflections from needle artifacts are not uncommon within the orbit (Figure 18.3). The optic nerve is less echogenic than orbital fat and tends to create an acoustic void appearing as a wedge-shaped echolucent defect. The bony infraorbital rim may become apparent vis-à-vis rotation of the transducer. Vascular landmarks are not used with ophthalmic regional anesthesia, hence Doppler color mode is not employed; however, incidental pulsations of the ophthalmic artery can occasionally be noted.
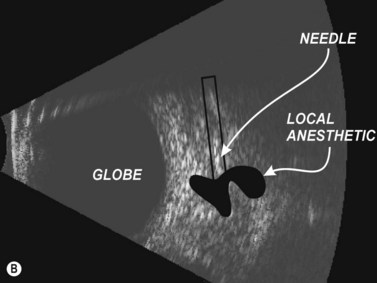
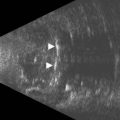
The needle is advanced to an appropriate depth and angulation depending on whether one is performing a RBB or PBB. The transducer probe is oriented in-plane with the needle. Slight side-to-side tether testing of the needle may affirm that the globe has not been engaged by the needle and it may also enhance needle visualization.14 Upon confir mation that the needle is not in key structures, local anesthetic, which appears anechoic compared to the more reflective periorbital fat, is injected and the needle is removed (Figure 18.4).
Ultrasound devices
Two key issues worth noting relate to transducer probe features and the bioeffects of ultrasound.
Probe shape, size, configuration
The geometric morphology of a probe may limit its utility for imaging of orbital structures (Chapters 2 and 3). Its size and configuration may restrict positioning options and may inhibit longitudinal alignment of the transducer to the needle, making visualization of the needle tip difficult to achieve. In contrast, smaller sized and circular profile probes may allow for more facile positioning and rotation of planes of view (Figure 18.5). Frequency ranges vary greatly across devices. While lower frequencies may be beneficial for visualizing deep structures, they will not provide optimal imaging of superficial orbital structures. The operator should select a device according to its physical structure, transducer frequency range (most B-scan probes function at a frequency in the 10 MHz range) and array configuration (curved versus linear).
Ultrasound bioeffects
Bioeffects on orbital tissue must be considered when using ultrasound for ophthalmic anesthesia.15 Since sonographic waves are no more than pulsed energy, they create thermal and mechanical responses in tissue and can induce heating and acoustic pressure. Thermal index (TI) and mechanical index (MI) are denotative of heat and mechanical alterations that may be generated by certain ultrasonic transducers.16
In the United States, ultrasound transducers are either FDA-rated for ophthalmic use, or not.17 Many of the ultrasound transducers currently found in operating room suites are not FDA-approved ophthalmically rated devices. In a recent translational study, using the Sonosite Micromaxx with a 8–4 MHz 10-mm broadband non-ophthalmic rated linear phased array transducer versus a FDA-rated transducer, data suggests that both the bedside (non-ophthalmic rated) and ophthalmic-rated ophthalmology-purposed ultrasonic devices had no significant thermal or mechanical effect in the rabbit eye.18 This outcome suggests that conventional devices may be safe if used for ophthalmic regional anesthesia applications.
Limitations
Visualizing the needle tip is key and is not always an easy task. Winder et al encountered difficulty attaining clear ultrasonic delineation of 25 gauge orbital block needles.10 As mentioned earlier, Greif et al readily visualized large bore 22 gauge needles during their cadaver experiment, but that needle gauge is not clinically applicable. Finer needles are less painful to insert and may produce less damage in case of inadvertent globe puncture, but appreciation of ocular injury may not be grossly apparent.19 Additionally, finer needles may create fewer sonographic artifacts.14,20 Blunt larger bore needles, similar to those used by Greif, are easily imaged with ultrasound, provide greater tactile feedback and require greater force to penetrate the globe, but may cause more damage in the rare case of traumatic injury to the eye.19,20
1 Atkinson WS. Retrobulbar injection of anaesthetic within the muscular cone. Arch Ophthalmol. 1936;16:494.
2 Davis DB2nd, Mandel MR. Efficacy and complication rate of 16,224 consecutive peribulbar blocks. A prospective multicenter study. J Cataract Refract Surg. 1994;20(3):327-337.
3 Ripart J, Nouvellon E, Chaumeron A. Regional anesthesia for eye surgery. Reg Anesth Pain Med. 2005;30(1):72-82.
4 Ruschen H, Bremner FD, Carr C. Complications after sub-Tenon’s eye block. Anesth Analg. 2003;96(1):273-277. table of contents
5 Frieman BJ, Friedberg MA. Globe perforation associated with subtenon’s anesthesia. Am J Ophthalmol. 2001;131(4):520-521.
6 Edge R, Navon S. Scleral perforation during retrobulbar and peribulbar anesthesia: risk factors and outcome in 50 000 consecutive injections. J Cataract Refract Surg. 1999;25(9):1237-1244.
7 Duker JS, Belmont JB, Benson WE, et al. Inadvertent globe perforation during retrobulbar and peribulbar anesthesia. Patient characteristics, surgical management, and visual outcome. Ophthalmology. 1991;98(4):519-526.
8 Vohra SB, Good PA. Altered globe dimensions of axial myopia as risk factors for penetrating ocular injury during peribulbar anaesthesia. Br J Anaesth. 2000;85(2):242-245.
9 Birch AA, Evans M, Redembo E. The ultrasonic localization of retrobulbar needles during retrobulbar block. Ophthalmology. 1995;102(5):824-826.
10 Winder S, Walker SB, Atta HR. Ultrasonic localization of anesthetic fluid in sub-Tenon’s, peribulbar, and retrobulbar techniques. J Cataract Refract Surg. 1999;25(1):56-59.
11 Luyet C, Eichenberger U, Moriggl B, et al. Real-time visualization of ultrasound-guided retrobulbar blockade: an imaging study. Br J Anaesth. 2008;101(6):855-859.
12 Dutton JJ. Clinical and Surgical Orbital Anatomy. Philadelphia: WB Saunders; 1994.
13 Brown SM, Coats DK, Collins ML, et al. Second cluster of strabismus cases after periocular anesthesia without hyaluronidase. J Cataract Refract Surg. 2001;27(11):1872-1875.
14 Chapman GA, Johnson D, Bodenham AR. Visualisation of needle position using ultrasonography. Anaesthesia. 2006;61(2):148-158.
15 Gayer S, Palte H, Kumar C. Real-time visualization of ultrasound-guided retrobulbar blockade: an imaging study. Br J Anaesth. 2009;102(4):561-562. author reply 62
16 Abbott JG. Rationale and derivation of MI and TI – a review. Ultrasound Med Biol. 1999;25(3):431-441.
17 Phillips R, Harris G. Information for manufacturers seeking marketing clearance of diagnostic ultrasound systems and transducers. www.fda.gov/cdrh/ode/guidance/560.pdf, 2008. Available from (accessed 18 March 2011)
18 Palte H, Gayer S, Arrieta-Quintero E, et al. A rabbit model comparative evaluation of two ultrasound devices for thermal and structural injury. [abstract]. Anesthesiology. 2010;113:A1169.
19 Waller SG, Taboada J, O’Connor P. Retrobulbar anesthesia risk. Do sharp needles really perforate the eye more easily than blunt needles? Ophthalmology. 1993;100(4):506-510.
20 Schafhalter-Zoppoth I, McCulloch CE, Gray AT. Ultrasound visibility of needles used for regional nerve block: an in vitro study. Reg Anesth Pain Med. 2004;29(5):480-488.