Chapter 16 Ocular Trauma
Introduction
Ocular trauma is a major cause of vision loss, particularly among younger patient populations. Traumatic injuries also represent one of the more difficult clinical examinations due to the severe pain associated with such injuries. Since examination of the traumatized globe may be limited to only a brief clinical examination secondary to pain, it is important that the echographer be aware of the various types of pathologic changes that are most likely to occur with certain types of traumatic injuries. Vitreoretinal involvement is present in nearly 50% of all severe eye injuries secondary to blunt or penetrating trauma.1
Clinical examination of the posterior segment can, however, be limited in such cases by factors related to the injury. Coexisting anterior segment injury can result in hyphema or corneal edema and opacification. Traumatic posterior segment pathology, such as vitreous hemorrhage can limit the diagnostic information obtained from clinical examination. In such cases, B-scan ocular ultrasonography has been shown to yield valuable diagnostic and prognostic information to define the nature of the pathology and guide management.2,3 In a review of 154 eyes with various posterior segment disorders for which ultrasound was ordered at one institution, it was reported that ultrasonography data made an impact on disease diagnosis or management in 83% of cases and were “pivotal” in 14% of cases.4 Although ultrasonography (B-scan and ultrasound biomicroscopy) is a valuable adjunct to management of trauma, one must exercise caution that it alone does not guide surgical intervention. Ultrasonography must be used in conjunction with other imaging modalities and the clinical examination, with particular attention to intraocular pressure.
Anterior segment
In the setting of trauma, ultrasound can be used to assess the anterior segment in patients with hyphemas or corneal opacification. In the setting of hyphema, an ultrasound can be instrumental in demonstrating the presence or absence of a clot and the depth of the anterior chamber.5
Angle trauma
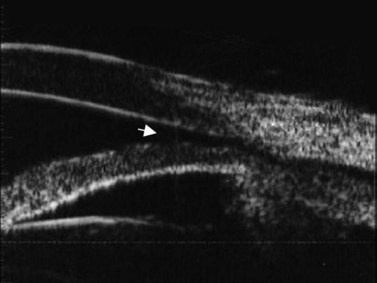
Ultrasound can also be utilized to evaluate delicate angle anatomy in order to examine for iridodialysis, angle recession, or cyclodialysis. The examination for a cyclodialysis cleft is especially important in a patient with persistent hypotony following trauma. The sequelae of cyclodialysis include shallow anterior chamber, cataract, retinal and choroidal folds, hypotonus maculopathy, and loss of vision in cases of prolonged hypotony.6 Clinically, a cyclodialysis cleft can be difficult to detect if the anterior chamber is shallow and there is corneal edema or anterior chamber hyphema (Figure 16.1). Ultrasound biomicroscopy (UBM) is a safe, accurate and noninvasive diagnostic tool in the diagnosis of cyclodialysis clefts (Chapter 4).7
Ultrasonography can also be instrumental in the evaluation of dislocated corneal grafts following Descemet stripping with endothelial keratoplasty (DSEK).8 In fact, ultrasonography can be useful in the evaluation of post-traumatic eyes that have undergone any type of endothelial keratoplasty, as donor dislocation can occur after any type of endothelial keratoplasty procedure, including posterior lamellar keratoplasty (PLK), deep lamellar endothelial keratoplasty (DLEK), or DSEK, because the donor tissue is not secured with any sutures.9
Lens dislocation
Blunt trauma can lead to the subluxation or dislocation of the crystalline lens or intraocular lens implants through the disruption of zonular fibers. Lens dislocation or subluxation has been reported from several sources of blunt trauma, including seizure-related injury,10 airbag deployment,11 paint-ball injuries,12,13 bottle corks,14 and plastic cord-related injuries.15–17 Subluxed lenses often can be identified with the slit-lamp, and subtle cases can be distinguished by looking for associated signs, such as iridodonesis and phakodonesis. Complete dislocation of an intraocular lens or crystalline lens can be identified in the posterior segment through indirect ophthalmoscopy, although dislocation into the anterior chamber or even the subconjunctival space also can occur.18–20
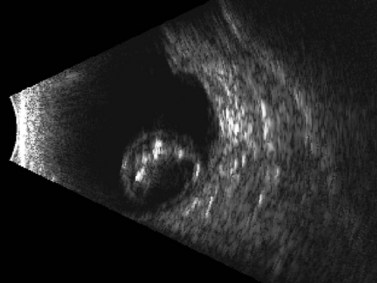
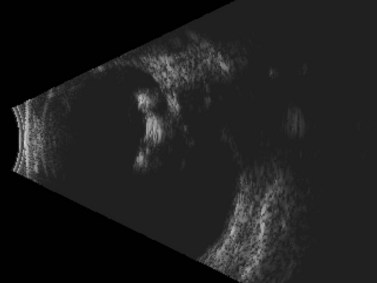
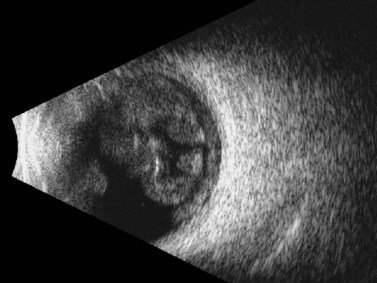
In cases in which other trauma-associated pathology, such as corneal opacification, hyphema, and vitreous hemorrhage, preclude posterior segment examination, dynamic ultrasonography can help to determine the position of the dislocated lens. On B-scan, a posteriorly dislocated crystalline lens appears as an oval-shaped, highly reflective mass that can be misdiagnosed as an intraocular tumor without careful attention to internal reflectivity and mobility (Figure 16.2).![]() See Clip 16.1 A traumatically displaced intraocular lens appears as a highly reflective linear body with marked reverberations along the echoic plane and two focal areas of reverberations corresponding to the intraocular lens haptics (Figure 16.3). Management options for crystalline lens injury range from observation in cases of mild subluxation without significant traumatic cataract to lens removal using vitrectomy techniques in cases of complete posterior dislocation.21 Finally, ultrasonography can be used to determine whether a lens (either crystalline or pseudophakic) has been extruded from the eye altogether.
See Clip 16.1 A traumatically displaced intraocular lens appears as a highly reflective linear body with marked reverberations along the echoic plane and two focal areas of reverberations corresponding to the intraocular lens haptics (Figure 16.3). Management options for crystalline lens injury range from observation in cases of mild subluxation without significant traumatic cataract to lens removal using vitrectomy techniques in cases of complete posterior dislocation.21 Finally, ultrasonography can be used to determine whether a lens (either crystalline or pseudophakic) has been extruded from the eye altogether.
Posterior segment
Rhegmatogenous retinal detachment
Trauma is the most common cause of retinal detachment in children and may play a role in approximately 10% of retinal detachments overall.1,22 Retinal detachment after blunt trauma may develop as a result of retinal dialysis, flap tears, operculated tears, macular hole, and giant retinal tears through rapid compression–decompression forces that result in transient anteroposterior shortening and equatorial elongation of the globe. It is estimated that 70% of hemorrhagic posterior vitreous detachments may have an associated retinal tear,23 and this association is likely even greater in the specific setting of trauma. Peripheral tears also can occur as a result of trauma-induced vitreous detachment.
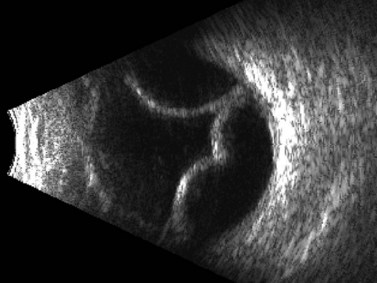
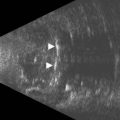
Although indirect ophthalmoscopy remains the technique of choice for diagnosing a retinal tear or detachment in this setting, ultrasonography can be of value when the view of the posterior segment is obscured by dense vitreous hemorrhage (Chapter 10). A small, peripheral retinal tear appears as a focal, highly reflective flap on B-scan (Figure 16.4). The posterior vitreous is usually thickened and partially detached but remains adherent to the retina at the location of the tear. In addition to the dense, vitreous hemorrhage, this adhesion sometimes can make the diagnosis of a peripheral retinal tear difficult and can lead to false-negative results. In a review of 106 eyes undergoing ultrasonography for dense vitreous hemorrhage, only 44% of retinal tears were diagnosed accurately.24 To maximize diagnostic sensitivity, examination of a suspicious area at a low gain and focally guided ocular movements to access flap mobility are essential.
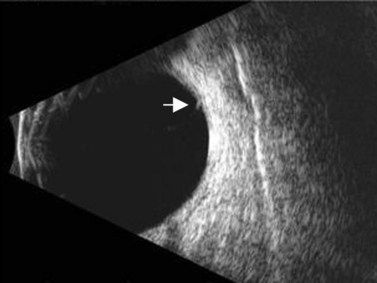
Figure 16.4 Peripheral retinal tear. Longitudinal B-scan at a low gain displays small, highly reflective flap (arrow).
Reproduced with permission from: Dadgostar H, Ventura ACM, Hayden BC. Posterior segment trauma. Ultrasound Clin 2008; 3:267–272.
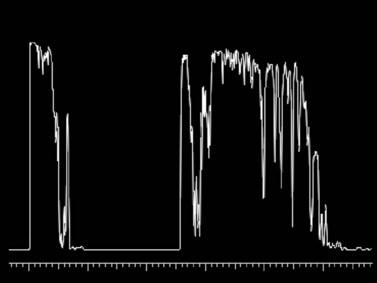
Because many cases involve younger patients with formed vitreous, the progression of a tear or dialysis to a detachment may take weeks to months;25 however, giant retinal tears have a much higher chance of progressing rapidly to retinal detachment.26 Giant retinal tears present on ultrasonography as highly reflective, discontinuous, rope-like membranes within the vitreous space (Figure 16.5). In the presence of dense hemorrhage, standardized diagnostic A-scan is an essential tool in the differentiation of a discontinuous, thickened posterior vitreous detachment from a giant retinal tear (Figure 16.6).
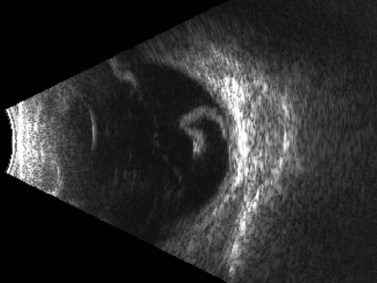
Figure 16.5 Giant retinal tear. Transverse view shows discontinuous, folded, hyperechoic retina.
Reproduced with permission from: Dadgostar H, Ventura ACM, Hayden BC. Posterior segment trauma. Ultrasound Clin 2008; 3:267–272.
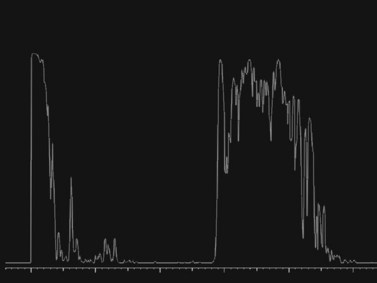
Figure 16.6 Diagnostic A-scan directed perpendicular to the retinal tear shows a 100% spike at tissue sensitivity.
Reproduced with permission from: Dadgostar H, Ventura ACM, Hayden BC. Posterior segment trauma. Ultrasound Clin 2008; 3:267–272.
Some injuries such as giant retinal tear and detachment may appear “flat” on B-scan when in fact the retina is folded over under dense hemorrhage. For this reason, the intraocular pressure becomes a valuable characteristic in determining which eyes should be explored. In cases of low intraocular pressure even after primary closure, exploration within the first 1–2 weeks should be considered. In addition, any suspicion of retinal tear in cases of dense vitreous hemorrhage warrant exploration by vitrectomy and scleral buckle because of the high risk of proliferative vitreoretinopathy.27
Penetrating ocular injury that tears the retina directly typically does not result in immediate rhegmatogenous detachment. In these cases, a delayed combined tractional and rhegmatogenous detachment often results from intraocular fibrovascular proliferation that originates from the site of injury (Figure 16.7).28 As demonstrated in animal models, this progressive proliferative vitreoretinopathy ultimately can lead to total retinal detachment, hypotony, and phthisis, if untreated.29,30
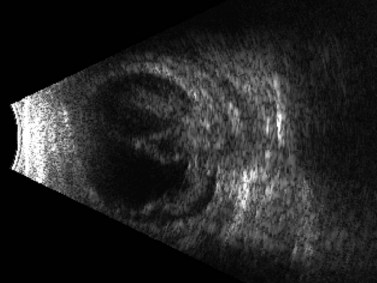
Figure 16.7 Combined tractional and rhegmatogenous retinal detachment at the site of globe penetration.
Reproduced with permission from: Dadgostar H, Ventura ACM, Hayden BC. Posterior segment trauma. Ultrasound Clin 2008; 3:267–272.
The treatment for traumatic rhegmatogenous retinal detachments typically involves scleral buckling and vitrectomy techniques used alone or in combination. Although the presence of vitreous hemorrhage, proliferative vitreoretinopathy, or other complicating factors (e.g., intraocular foreign body [IOFB]) often necessitates a combination of vitrectomy and scleral buckling, cases with a good view of the posterior segment and a well-defined tear or dialysis can be treated with scleral buckling alone.1 When a retinal tear is diagnosed in the presence of dense vitreous hemorrhage, a vitrectomy is usually required to prevent progression to retinal detachment. Ultrasound-guided external cryopexy in this setting has been reported as a less invasive alternative approach, however.31
Hemorrhagic choroidal detachment
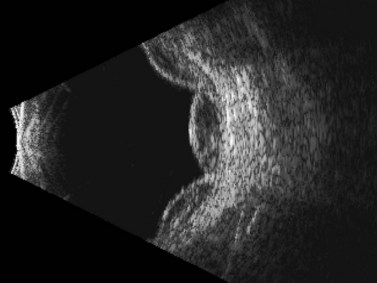
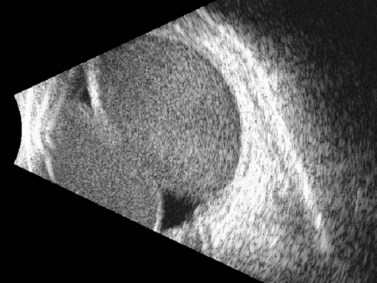
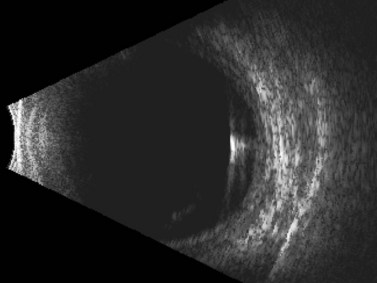
Although most commonly encountered as a postoperative complication, particularly after cataract surgery, glaucoma filtration surgery, or scleral buckling procedures, choroidal detachments are also known to occur in association with open-globe injury.32 Posterior segment ultrasonography can be useful for differentiating retinal and choroidal detachments, measuring the extent of choroidal detachments, and distinguishing hemorrhagic from exudative choroidal detachments (Figures 16.8–16.10). Typically, shallow to moderate choroidal detachments can be followed with serial examination and ultrasonography as needed, with prompt attention to the repair of the globe and any associated ocular injuries. Extensive, appositional (or “kissing”) choroidal detachments, on the other hand, often require surgical drainage, typically after a 1–2-week delay to allow liquefaction of the blood clot (Figure 16.11).33 Serial ultrasonography can be used to aid in the timing of surgery in these cases because clot liquefaction sometimes may become apparent on dynamic ultrasonography. Surgical management often involves transscleral drainage of the blood with or without concurrent vitrectomy.
Intraocular foreign body
IOFBs are encountered in 18–41% of cases that involve open globe trauma.34 Young men are the most frequently affected, and hammering is the most frequent predisposing activity, accounting for 60–80% of cases.35–37 Although pain and vision loss are common presenting symptoms, both of these symptoms may be absent.38,39 In any open globe injury, the clinician must maintain a high index of suspicion for the presence of IOFB. In one study, up to 56% of all trauma-related legal claims were related to missed IOFBs.40
In addition to clinical examination at the slit-lamp and indirect ophthalmoscopy, various imaging modalities are valuable for the identification and localization of IOFB. Although plain radiographs have been used in the past, modern computed tomography scans have a much higher sensitivity.41,42 Magnetic angiographic imaging is contraindicated when metallic IOFB is suspected. Adjunctive use of ultrasonography by a skilled technician can increase the likelihood of detecting IOFB. Using a porcine eye model, one study demonstrated a 93% IOFB detection rate with ultrasonography.43 In another experimental model using metallic IOFBs of different sizes, sensitivity, specificity, positive predictive value, and negative predictive value of detection by ultrasonography were 87.5%, 95.8%, 96.5%, and 85.2%, respectively.44
Diagnostic ultrasonography is valuable in determining the precise location and orientation of small IOFBs and distinguishing between objects composed of different materials. Extremely thin IOFBs (<100 µm), such as a metallic wire or a splinter of wood, can be differentiated, localized, and measured with B-scan (Figure 16.12). Metallic IOFBs are echo dense even at low gain settings and often produce shadowing of intraocular structures and the orbit. Organic IOFBs produce various findings on ultrasonography depending on the shape and structure of the material. These foreign bodies are usually echo dense at low gains. Intraocular sutures, air bubbles, and retained lens fragments can closely resemble true IOFBs, frequently presenting as small points of highly reflective echoes with a combination of reverberation echoes and shadowing present.2,45
Management of trauma with retained IOFBs involves surgical removal of the foreign body, most commonly performed using vitrectomy techniques. Early removal of the foreign body (i.e., within 24 hours of injury) seems to be one of the variables associated with better visual outcome in such cases. IOFB size and composition, presenting visual acuity, and the extent of associated ocular injuries also seem to be important indicators of subsequent course and visual prognosis.46–49 In general, it is preferable to remove IOFBs early so as to minimize the risk of endophthalmitis, although delayed intervention may achieve a good outcome in some settings.50 The risk of endophthalmitis is related to the nature of the foreign body, as high-velocity metallic particles are often sterilized prior to penetration. In addition, entry posterior to the limbus outside of the anterior chamber increases the risk of endophthalmitis.
Posterior scleral rupture
A posterior rupture of the globe can result from severe blunt trauma. Often, these patients present clinically with hemorrhagic chemosis with concomitant vitreous hemorrhage which obscures the posterior intraocular view. Thus, these ruptures are often occult and must be evaluated by imaging such as ultrasonography. On ultrasonography, the sclera in the area of rupture may show an irregular contour, thickening, and decreased reflectivity.51 An actual splitting of the scleral fibers may not be visualized with ultrasonography in some posterior ruptures. Prolapsed vitreous and/or retina may also become incarcerated in the scleral rupture. The incarcerated vitreous and/or retina may produce traction folds or bands that extend across the posterior segment toward the site of incarceration.
Optic nerve avulsion
Avulsion of the optic nerve is an uncommon, but devastating, sequela of blunt trauma. A meta-analysis of 63 patients with optic nerve avulsions found that in 31 patients (49%) the etiology of the optic nerve avulsion was a small blunt object or finger that struck the eye or entered the orbit. The authors of the meta-analysis concluded that the most common mechanism of avulsion injury is a severe rotation of the eye leading to rupture of the optic nerve fibers with an anterior displacement of the globe.52 Since vitreous hemorrhage can clinically obscure optic nerve details in patients with suspected optic nerve avulsion, ultrasonographic evaluation for avulsion has been described.53 On ultrasonography, one finds full-thickness lamina cribrosa defects and retraction of the edematous optic nerve into its sheath posterior to the lamina cribrosa.54 A small orbital hemorrhage may also be evident. Fibroglial scarring from the posterior scleral outlet into the vitreous cavity is a reported late finding.54
Endophthalmitis
Endophthalmitis can occur in the setting of open-globe injury. Reported rates vary from 0% to 16.5%, but the average incidence of endophthalmitis in open-globe trauma is likely around 7%.55–59 According to the United States Eye Injury Registry database, out of 10 309 serious ocular trauma cases, 39% presented with an open globe, and of these, 3.4% developed endophthalmitis.60 Retained foreign body composed of vegetative material, delayed repair (>24 hours), lens capsule disruption, and the presence of a contaminated wound are important risk factors for development of endophthalmitis.60–62 Although endophthalmitis is diagnosed clinically based on such signs as conjunctival injection and chemosis, anterior chamber fibrin, hypopyon, and vitreitis, ultrasonography can aid in the examination of an often poorly visible posterior segment. B-scan ultrasonography of an affected eye typically reveals dense vitreous opacities and moderate to marked, irregular, web-like vitreous membrane formation (Figure 16.13).
Sympathetic ophthalmia
Sympathetic ophthalmia (SO) is a rare, bilateral, non-necrotizing, granulomatous uveitis that occurs after ocular trauma or surgical procedures to one eye threatening sight in the fellow (sympathizing) eye. The pathophysiology is not clearly understood, but it appears that the disrupted integrity of the inciting eye leads to an autoimmune hypersensitivity reaction against the exposed ocular antigens in the injured eye as well as in the sympathizing eye.63 The sympathizing eye usually presents with inflammation within 3 months after the injury but the range has been noted to be from 2 weeks to 50 years.64 About 80% of cases occur within a 3-month time frame and 90% occur within 1 year.65,66 B-scan ultrasonography is used to evaluate for choroidal thickening (Chapter 12).63 Prompt treatment with corticosteroids has improved outcomes in this traditionally devastating disease.67–69
1 Pieramici DJ. Vitreoretinal trauma. Ophthalmol Clin N Am. 2002;15(2):225-234.
2 McNicholas MM, Brophy DP, Power WJ, et al. Ocular trauma: evaluation with US. Radiology. 1995;195(2):423-427.
3 Rubsamen PE, Cousins SW, Winward KE, et al. Diagnostic ultrasound and pars plana vitrectomy in penetrating ocular trauma. Ophthalmology. 1994;101(5):809-814.
4 Scott IU, Smiddy WE, Feuer WJ, et al. The impact of echography on evaluation and management of posterior segment disorders. Am J Ophthalmol. 2004;137(1):24-29.
5 Allemann N, Silverman RH, Reinstein DZ, et al. High-frequency ultrasound imaging and spectral analysis in traumatic hyphema. Ophthalmology. 1993;100(9):1351-1357.
6 Malandrini A, Balestrazzi A, Martone G, et al. Diagnosis and management of traumatic cyclodialysis cleft. J Cataract Refract Surg. 2008;34(7):1213-1216.
7 Bhende M, Lekha T, Vijaya L, et al. Ultrasound biomiscroscopy in the diagnosis and management of cyclodialysis clefts. Ind J Ophthalmol. 1999;47(1):19-23.
8 Suh LH, Kymionis GD, Culbertson WW, et al. Descemet stripping with endothelial keratoplasty in aphakic eyes. Arch Ophthalmol. 2008;126(2):268-270.
9 Price FWJr, Price MO. A nonsurgical treatment for donor dislocation after descemet stripping endothelial keratoplasty (DSEK). Cornea. 2006;25(8):991.
10 Izadi S, Stewart RM, Jain S. Bilateral posterior dislocation of the crystalline lens after a head injury sustained during a seizure. Emerg Med J. 2007;24(1):e6.
11 Blackmon SM, Fekrat S, Setlik DE, et al. Posterior dislocation of a crystalline lens associated with airbag deployment. J Cataract Refract Surg. 2005;31(12):2431-2432.
12 Farr AK, Fekrat S. Eye injuries associated with paintball guns. Int Ophthalmol. 1998;22(3):169-173.
13 Thach AB, Ward TP, Hollifield RD, et al. Ocular injuries from paintball pellets. Ophthalmology. 1999;106(3):533-537.
14 Cavallini GM, Lugli N, Campi L, et al. Bottle-cork injury to the eye: a review of 13 cases. Eur J Ophthalmol. 2003;13(3):287-291.
15 Chorich LJ3rd, Davidorf FH, Chambers RB, et al. Bungee cord-associated ocular injuries. Am J Ophthalmol. 1998;125(2):270-272.
16 Cooney MJ, Pieramici DJ. Eye injuries caused by bungee cords. Ophthalmology. 1997;104(10):1644-1647.
17 Nichols CJ, Boldt HC, Mieler WF, et al. Ocular injuries caused by elastic cords. Arch Ophthalmol. 1991;109(3):371-372.
18 Gaur A, Sharma YR, Sudan R. Subconjunctival lens dislocation. J Cataract Refract Surg. 2005;31(1):13-14.
19 Sathish S, Chakrabarti A, Prajna V. Traumatic subconjunctival dislocation of the crystalline lens and its surgical management. Ophthalmic Surg Lasers. 1999;30(8):684-686.
20 Yurdakul NS, Ugurlu S, Yilmaz A, et al. Traumatic subconjunctival crystalline lens dislocation. J Cataract Refract Surg. 2003;29(12):2407-2410.
21 Greven CM, Collins AS, Slusher MM, et al. Visual results, prognostic indicators, and posterior segment findings following surgery for cataract/lens subluxation-dislocation secondary to ocular contusion injuries. Retina. 2002;22(5):575-580.
22 Haimann MH, Burton TC, Brown CK. Epidemiology of retinal detachment. Arch Ophthalmol. 1982;100(2):289-292.
23 Sarrafizadeh R, Hassan TS, Ruby AJ, et al. Incidence of retinal detachment and visual outcome in eyes presenting with posterior vitreous separation and dense fundus-obscuring vitreous hemorrhage. Ophthalmology. 2001;108(12):2273-2278.
24 Rabinowitz R, Yagev R, Shoham A, et al. Comparison between clinical and ultrasound findings in patients with vitreous hemorrhage. Eye (Lond). 2004;18(3):253-256.
25 Kennedy CJ, Parker CE, McAllister IL. Retinal detachment caused by retinal dialysis. Aust N Z J Ophthalmol. 1997;25(1):25-30.
26 Nacef L, Daghfous F, Chaabini M, et al. [Ocular contusions and giant retinal tears]. J Fr Ophtalmol. 1997;20(3):170-174.
27 Yoshino Y, Ideta H, Nagasaki H, et al. Comparative study of clinical factors predisposing patients to proliferative vitreoretinopathy. Retina. 1989;9(2):97-100.
28 Johnston S. Perforating eye injuries: a five year survey. Trans Ophthalmol Soc UK. 1971;91:895-921.
29 Cleary PE, Ryan SJ. Method of production and natural history of experimental posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol. 1979;88(2):212-220.
30 Cleary PE, Ryan SJ. Histology of wound, vitreous, and retina in experimental posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol. 1979;88(2):221-231.
31 Kelley LM, Walker JP, Wing GL, et al. Ultrasound-guided cryotherapy for retinal tears in patients with vitreous hemorrhage. Ophthalmic Surg Lasers. 1997;28(7):565-569.
32 Liggett PE, Mani N, Green RE, et al. Management of traumatic rupture of the globe in aphakic patients. Retina. 1990;10(Suppl 1):S59-S64.
33 Chu TG, Cano MR, Green RL, et al. Massive suprachoroidal hemorrhage with central retinal apposition. A clinical and echographic study. Arch Ophthalmol. 1991;109(11):1575-1581.
34 Mester V, Kuhn F. Intraocular foreign bodies. Ophthalmol Clin North Am. 2002;15(2):235-242.
35 Percival SP. A decade of intraocular foreign bodies. Br J Ophthalmol. 1972;56(6):454-461.
36 Roper-Hall MJ. Review of 555 cases of intra-ocular foreign body with special reference to prognosis. Br J Ophthalmol. 1954;38(2):65-99.
37 Williams DF, Mieler WF, Abrams GW, et al. Results and prognostic factors in penetrating ocular injuries with retained intraocular foreign bodies. Ophthalmology. 1988;95(7):911-916.
38 Kuhn F, Halda T, Witherspoon CD, et al. Intraocular foreign bodies: myths and truths. Eur J Ophthalmol. 1996;6(4):464-471.
39 Weiss MJ, Hofeldt AJ, Behrens M, et al. Ocular siderosis. Diagnosis and management. Retina. 1997;17(2):105-108.
40 Bettman JW. Seven hundred medicolegal cases in ophthalmology. Ophthalmology. 1990;97(10):1379-1384.
41 Chacko JG, Figueroa RE, Johnson MH, et al. Detection and localization of steel intraocular foreign bodies using computed tomography. A comparison of helical and conventional axial scanning. Ophthalmology. 1997;104(2):319-323.
42 Wu JT, Lam DS, Fan DS, et al. Intravitreal phaco chopper fragment missed by computed tomography. Br J Ophthalmol. 1998;82(4):460-461.
43 Bryden FM, Pyott AA, Bailey M, et al. Real time ultrasound in the assessment of intraocular foreign bodies. Eye (Lond). 1990;4(Pt 5):727-731.
44 Shiver SA, Lyon M, Blaivas M. Detection of metallic ocular foreign bodies with handheld sonography in a porcine model. J Ultrasound Med. 2005;24(10):1341-1346.
45 Bhavsar AR, Fong DS, Kerman B, et al. Intraorbital air simulating an intraocular foreign body. Am J Ophthalmol. 1997;123(6):835-837.
46 Jonas JB, Knorr HL, Budde WM. Prognostic factors in ocular injuries caused by intraocular or retrobulbar foreign bodies. Ophthalmology. 2000;107(5):823-828.
47 Chaudhry IA, Shamsi FA, Al-Harthi E, et al. Incidence and visual outcome of endophthalmitis associated with intraocular foreign bodies. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):181-186.
48 Szijarto Z, Gaal V, Kovacs B, et al. Prognosis of penetrating eye injuries with posterior segment intraocular foreign body. Graefes Arch Clin Exp Ophthalmol. 2008;246(1):161-165.
49 Chiquet C, Zech JC, Gain P, et al. Visual outcome and prognostic factors after magnetic extraction of posterior segment foreign bodies in 40 cases. Br J Ophthalmol. 1998;82(7):801-806.
50 Colyer MH, Weber ED, Weichel ED, et al. Delayed intraocular foreign body removal without endophthalmitis during Operations Iraqi Freedom and Enduring Freedom. Ophthalmology. 2007;114(8):1439-1447.
51 Hughes JR, Byrne SF. Detection of Posterior Ruptures in Opaque Media. Dordrecht: Dr W Junk; 1987.
52 Buchwald HJ, Spraul CW, Wagner P, et al. [Optic nerve evulsion: Metaanalysis]. Klin Monbl Augenheilkd. 2001;218(10):635-644.
53 Talwar D, Kumar A, Verma L, et al. Ultrasonography in optic nerve head avulsion. Acta Ophthalmol (Copenh). 1991;69(1):121-123.
54 Oliver SC, Mandava N. Ultrasonographic signs in complete optic nerve avulsion. Arch Ophthalmol. 2007;125(5):716.
55 Affeldt JC, Flynn HWJr, Forster RK, et al. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987;94(4):407-413.
56 Brinton GS, Topping TM, Hyndiuk RA, et al. Posttraumatic endophthalmitis. Arch Ophthalmol. 1984;102(4):547-550.
57 Essex RW, Yi Q, Charles PG, et al. Post-traumatic endophthalmitis. Ophthalmology. 2004;111(11):2015-2022.
58 Thompson WS, Rubsamen PE, Flynn HWJr, et al. Endophthalmitis after penetrating trauma. Risk factors and visual acuity outcomes. Ophthalmology. 1995;102(11):1696-1701.
59 Verbraeken H, Rysselaere M. Post-traumatic endophthalmitis. Eur J Ophthalmol. 1994;4(1):1-5.
60 Danis RP. Endophthalmitis. Ophthalmol Clin North Am. 2002;15(2):243-248.
61 Boldt HC, Pulido JS, Blodi CF, et al. Rural endophthalmitis. Ophthalmology. 1989;96(12):1722-1726.
62 Lemley CA, Han DP. Endophthalmitis: a review of current evaluation and management. Retina. 2007;27(6):662-680.
63 Castiblanco CP, Adelman RA. Sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2009;247(3):289-302.
64 Bakri SJ, Peters GB3rd. Sympathetic ophthalmia after a hyphema due to nonpenetrating trauma. Ocul Immunol Inflamm. 2005;13(1):85-86.
65 Lubin JR, Albert DM, Weinstein M. Sixty-five years of sympathetic ophthalmia. A clinicopathologic review of 105 cases (1913–1978). Ophthalmology. 1980;87(2):109-121.
66 Goto H, Rao NA. Sympathetic ophthalmia and Vogt-Koyanagi-Harada syndrome. Int Ophthalmol Clin. 1990;30(4):279-285.
67 Makley TAJr, Azar A. Sympathetic ophthalmia. A long-term follow-up. Arch Ophthalmol. 1978;96(2):257-262.
68 Chan CC, Roberge RG, Whitcup SM, et al. 32 cases of sympathetic ophthalmia. A retrospective study at the National Eye Institute, Bethesda, Md., from 1982 to 1992. Arch Ophthalmol. 1995;113(5):597-600.
69 Kilmartin DJ, Dick AD, Forrester JV. Prospective surveillance of sympathetic ophthalmia in the UK and Republic of Ireland. Br J Ophthalmol. 2000;84(3):259-263.