Ocular Surface Disease
Surgical Management
Anterior Stromal Puncture
In 1986, McLean and MacRae observed that recurrent corneal erosion (RCE) often followed superficial corneal trauma but was not seen after deep stromal laceration.1 In light of this, they described the technique of anterior stromal puncture (ASP) for the treatment of RCE, which employed a 20G needle at the slit lamp to make multiple punctures through loose epithelium and Bowman’s layer into the anterior stroma. This created a more secure bonding of the epithelium to the underlying basement membrane (BM), Bowman’s layer, and stroma. This treatment has been shown to be effective, particularly in post-traumatic corneal erosions.
Indication
The therapeutic aim of ASP is to enhance epithelial adhesion to the BM by microscopic, superficial scar formation. In RCE, the cause is thought to be failure of the wounded epithelial cells to adhere to the underlying stroma. This could be secondary to weak hemidesmosomal attachment, reduplication of the BM, the action of metalloproteinases,2 and/or disruption of type VII collagen fibrils.3
ASP is a well-accepted treatment for RCE.1,4,5 ASP induces reactive subepithelial fibrosis or production of extracellular matrix proteins, both of which may be responsible for increased adhesion of the epithelium.6 Success rates of up to 80% have been reported in recalcitrant RCE.1,4,5
Recently, ASP was reported to be used for RCE after laser in situ keratomileusis (LASIK), which helped in resolving secondary diffuse lamellar keratitis.4 However, performing ASP immediately after LASIK may cause flap displacement and must be used judiciously.
Procedure
ASP can also be performed with a short-pulsed Nd:YAG laser.7 The Nd:YAG laser (1.8–2.2 mJ) is focused at the BM zone after epithelial debridement. Spots are placed in rows approximately 0.20–0.25 mm apart. The advantage of laser over needle puncture is that the laser puncture is more uniform, shallow and translucent. There may also be less corneal scarring, so the procedure can be repeated and can be used closer to the visual axis.7
Complications
One of the major concerns with regard to the safety of ASP is corneal perforation. To address this concern, Rubinfeld and Laibson designed a specially bent needle for use on a disposable handle.5 An insertion depth of 0.1 mm was found to be sufficient to cause a therapeutic, fibrocytic reaction.6
ASP can be a safe and effective therapy if performed under a magnified view at the slit lamp.
Punctal Occlusion
Occlusion of the nasolacrimal outflow tract was first described by Beetham in 1935.8 He reported resolution of dry eye in eight eyes with filamentary keratitis with electrocautery or diathermy to occlude the puncta. In 1975, Freeman9 proposed the use of punctal plugs to provide a reversible blockade of the nasolacrimal tract at the punctum. Semi-permanent and permanent punctal plugs are now widely used and are available in a range of sizes to ensure a good fit.
Indication
Patients with severe keratoconjunctivitis sicca with or without underlying systemic collagen vascular disease often require permanent punctal occlusion. Punctal occlusion also plays a beneficial role in aqueous tear deficiencies secondary to ocular surface conditions, such as ocular cicatricial pemphigoid, Stevens–Johnson syndrome, and Sjögren syndrome.10,11 Dry eye secondary to reduced reflex tearing found in neurotrophic keratitis can also be effectively managed with this procedure. Finally, punctal occlusion may be beneficial in patients with dry eyes, due to increased evaporation from an exposed ocular surface. This may occur with lagophthalmos, exophthalmos seen with thyroid conditions, and following blepharoplasties. Other than dry eye syndromes, punctal occlusion has been shown to improve contact lens comfort and also may be helpful in the management of superior limbic keratoconjunctivitis.12
Procedure
Permanent Procedures
Thermal Methods
Thermal methods involve occlusion of the punctum by shrinking the canalicular walls with argon laser, cautery, or diathermy.13,14 Even with thorough cauterization, the canaliculus may reopen in time. One of the most common techniques to accomplish permanent punctual closure is electrocautery. The eyelid adjacent to the punctum is infiltrated with local anesthetic (LA). A topical anesthetic is instilled in the cul-de-sac. The electrocautery instrument (Hyfrecator, Birtcher Corp., Los Angeles, CA) with a fine-needle tip is threaded into the punctum and along the canaliculus. The instrument is engaged while withdrawing the instrument slowly. The canaliculus is thermally de-epithelialized. Additional cautery may be performed at the punctal opening.
Thermal cauterization has been reported to be efficient in achieving punctal occlusion, with a reported recanalization rate of 26.1%.10 Recently, a recanalization rate of 1.4% was reported with a high-heat-energy-releasing cautery device.14 Thermal cauterization is performed similarly to electrocautery. Following LA, the loop tip of a disposable cautery is pinched together with sterile forceps to create a needle-tipped probe. This tip is resterilized at any time by turning the cautery on. If the probe does not insert into the canaliculus, the punctum is dilated with a dilator. The tip is threaded into the punctum and along the canaliculus. The instrument is then engaged while withdrawing the instrument slowly.
Key Surgical Points
 Although permanent punctal occlusion can significantly reduce the symptoms of dry eye, epiphora can result with improper patient selection.
Although permanent punctal occlusion can significantly reduce the symptoms of dry eye, epiphora can result with improper patient selection.
 Blepharitis and dry eyes frequently coexist and treatment of both is necessary.
Blepharitis and dry eyes frequently coexist and treatment of both is necessary.
 Patients with severe chronic dry eye or those who benefit from punctal plugs but experience repeated plug loss should be considered for permanent punctal occlusion.
Patients with severe chronic dry eye or those who benefit from punctal plugs but experience repeated plug loss should be considered for permanent punctal occlusion.
 As occlusion of upper and lower puncta at the same time may increase the risk of epiphora, the procedures should be performed one at a time.
As occlusion of upper and lower puncta at the same time may increase the risk of epiphora, the procedures should be performed one at a time.
 Even with cautery, permanent punctal occlusion may not be achieved and the procedure may need to be repeated.
Even with cautery, permanent punctal occlusion may not be achieved and the procedure may need to be repeated.
 If granulation around a silicone plug is noted, it should resolve with removal of the punctual plug.
If granulation around a silicone plug is noted, it should resolve with removal of the punctual plug.
Complications
Punctal plugs can cause ocular discomfort and complications,15,16 such as punctum enlargement, loss or migration or extrusion of the implant, epiphora, pyogenic granuloma, local inflammatory reaction to silicone, dacryocystitis, and canaliculitis.16,17
Granuloma formation is thought to be caused by local stimulation by the plug. Intracanalicular plugs are associated with higher rate of granulation tissue formation in the lacrimal tract when, compared with other forms of punctal plugs. Cases have been reported in which plug migration inside the lacrimal passage resulted in peripheral inflammations and infections, requiring surgical removal.18
Phototherapeutic Keratectomy
The laser–tissue phenomenon of photoablation was first demonstrated in 1983 by Trokel and Srinivasan,19 who were working on the ultraviolet 193-nm excimer laser. Its potential role in refractive and therapeutic corneal surgery was quickly recognized. The excimer laser underwent extensive preclinical trials before it was applied to human eyes, and it is now being used for photorefractive keratectomy (PRK), LASIK and PTK.
Excimer laser PTK utilizes 193-nm wavelength ultraviolet light to break the molecular bonds in corneal tissue, in turn ablating the anterior stroma in a highly predictable fashion. It was approved by the FDA in 1995 for the treatment of visually significant anterior corneal pathologies, namely superficial corneal dystrophies, epithelial basement membrane dystrophy (EBMD) with irregular corneal surfaces, corneal scars and opacities.20
Advantages
The main advantages of PTK over mechanical superficial keratectomy (SK) include precise tissue ablation with minimal damage to the surrounding tissues, a smooth residual bed for corneal re-epithelialization, and repeatability of the treatment. Compared to lamellar or penetrating keratoplasty (PKP), it is also less invasive, with more rapid visual recovery. PTK can be performed before or after PKP and does not impair the prognosis of PKP.21
Indications
Phototherapeutic keratectomy is best utilized for corneal pathologies affecting the epithelium or anterior 10–20% of the corneal stroma. For safety reasons, the residual corneal bed thickness must be greater than 300 µm at the end of the procedure. The indications for PTK can be separated into four broad categories,20 although they often overlap:
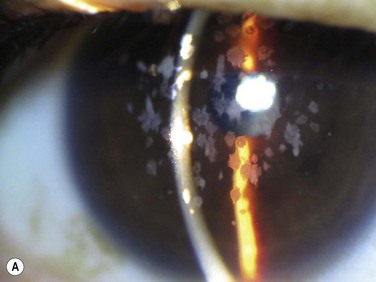
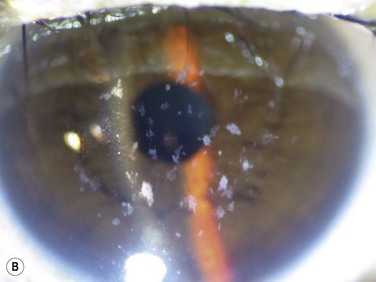
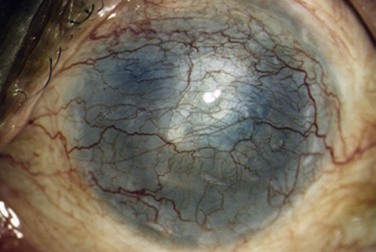
 superficial opacities, e.g. granular dystrophy (Fig. 36.1), scars from trauma or keratitis, post-PRK haze (Fig. 36.2)
superficial opacities, e.g. granular dystrophy (Fig. 36.1), scars from trauma or keratitis, post-PRK haze (Fig. 36.2)
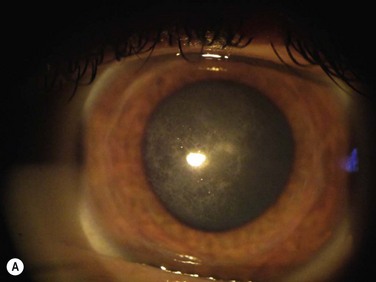
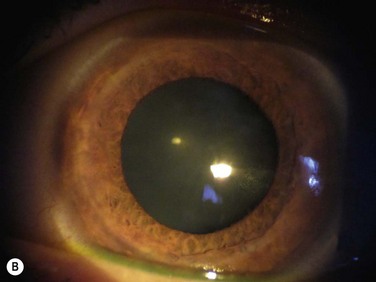
 elevated lesions or irregularities of the corneal surface, e.g. Salzmann’s nodular degeneration (SND), keratoconus nodules
elevated lesions or irregularities of the corneal surface, e.g. Salzmann’s nodular degeneration (SND), keratoconus nodules
Contraindications
It is important to identify patients who have conditions that predispose them to delayed epithelial healing and who would not be suitable candidates for PTK. They include immunocompromised individuals and patients with anesthetic corneas, severe dry eyes or uncontrolled uveitis.20 Others may require additional procedures prior to PTK, e.g. a patient with lagophthalmos secondary to paralytic ectropion may require ectropion repair first. Any deep corneal pathology requiring removal of more than 20–30% of corneal thickness may be more suitable for anterior lamellar keratoplasty rather than PTK. If there is significant thinning in the treatment zone, PTK may predispose them to ectasia and hence should be avoided.
Preoperative Assessment
Examination
Using slit lamp biomicroscopy, one should determine the size, depth, location, and density of the corneal abnormality, as well as any corneal thinning. In general, PTK is most suited to patients with superficial stromal opacities without significant irregularity and thinning, and those with small, central, elevated corneal lesions not amenable to SK.22 If the lesion is deep enough that the residual corneal thickness approaches 300 µm, one should exercise caution. In this scenario, either lamellar or penetrating keratoplasty may be the preferred treatment.
Imaging
Recent advancements in anterior segment imaging technology can be useful in the surgical planning of these cases. Subtle surface irregularities can be difficult to detect clinically. Corneal topography can highlight any irregularities of the cornea and document any irregular astigmatism. Pentacam® tomography (OCULUS Optikgerate GmbH, Watzlar, Germany) also provides Scheimflug imaging, which can illustrate the depth of the corneal lesions, although the resolution is limited. High-frequency ultrasound biomicroscopy may be of more use for large lesions. High-resolution anterior segment optical coherence tomography is a quick, non-contact imaging modality that can provide pachymetric mapping of corneal opacities.23 It could potentially result in more accurate resection of tissues during PTK. Sometimes, accurate depth is difficult to determine with these modalities as they all are subject to posterior shadowing which may overestimate the depth of lesions.
Procedure
 Maintain a smooth surface if possible, e.g. a patient with granular dystrophy with a smooth epithelium could be treated with transepithelial PTK, in which case the epithelium is used as the masking agent.
Maintain a smooth surface if possible, e.g. a patient with granular dystrophy with a smooth epithelium could be treated with transepithelial PTK, in which case the epithelium is used as the masking agent.
 In those with loose epithelium (e.g. RCE) or elevated lesions (e.g. SND), mechanical debridement (or with adjunctive 20–50% alcohol for 5–10 seconds) with a blade should be utilized prior to PTK.
In those with loose epithelium (e.g. RCE) or elevated lesions (e.g. SND), mechanical debridement (or with adjunctive 20–50% alcohol for 5–10 seconds) with a blade should be utilized prior to PTK.
 Remove the least amount of tissue to achieve the desired results by frequently stopping and checking the results under the microscope or the slit lamp before proceeding with further laser treatments.
Remove the least amount of tissue to achieve the desired results by frequently stopping and checking the results under the microscope or the slit lamp before proceeding with further laser treatments.
 Maintain centration of the treatment zone to avoid inducing irregular astigmatism.
Maintain centration of the treatment zone to avoid inducing irregular astigmatism.
 Use a controlled amount of modulating or masking agent to achieve a uniform corneal surface (e.g. artificial tears or balanced saline solution).
Use a controlled amount of modulating or masking agent to achieve a uniform corneal surface (e.g. artificial tears or balanced saline solution).
 For superficial opacities, the goal is to clear as much of the opacity centrally as possible while resisting the temptation to ablate deeper tissues (which can result in excessive induced hyperopia).
For superficial opacities, the goal is to clear as much of the opacity centrally as possible while resisting the temptation to ablate deeper tissues (which can result in excessive induced hyperopia).
 For RCE, only aim to remove 5–6 µm of the Bowman’s membrane.
For RCE, only aim to remove 5–6 µm of the Bowman’s membrane.
 For focal corneal scars, one may partially treat any induced refractive error, or use an opaque mask (e.g. use a specifically shaped weckcell sponge) if the treatment will remove tissue in an area where it is not desirable.
For focal corneal scars, one may partially treat any induced refractive error, or use an opaque mask (e.g. use a specifically shaped weckcell sponge) if the treatment will remove tissue in an area where it is not desirable.
In general, the treatment zone should be tailored to the abnormality. For example, granular dystrophy could be treated with a larger treatment zone of around 6–7 mm diameter, while a 2-mm Salzmann’s nodule centrally should be treated with a 2–3-mm spot size. After laser ablation has been completed, topical antibiotics and steroids are applied along with a soft bandage contact lens (BCL). However, some advocate the use of a pressure patch rather than a BCL if there is a history of HSK or in a neurotrophic cornea. The patient should be reviewed frequently until re-epithelialization is complete. For RCE patients, after removal of the BCL, the use of hyperosmotic 5% sodium chloride ointment at bedtime may reduce the recurrence of erosions.
Adjunct Therapy
In recent years, mitomycin C (MMC) has been shown to be effective in reducing the occurrence of corneal haze following excimer laser ablation.24 Corneas requiring PTK are inherently at higher risk of haze, due to the presence of stimulated fibroblasts, surface irregularities (e.g. SND) and potential suboptimal healing ability (e.g. RCE). The ideal concentration and duration of MMC exposure remains controversial. Depending on the surgeon’s preference and the pathology, the dosage may range from 0.001% to 0.04%, with a duration of application between 12 seconds to 2 minutes.24 For high-risk patients, such as PTK post-corneal transplant or repeat treatments, the authors recommend 0.02% with a minimum application of 30 seconds.
Hyperopic shifts are common after PTK, due to central flattening, particularly in those with deeper ablations.25 This could be managed post-PTK with contact lenses or sequential hyperopic PRK treatment. If the patient has pre-existing hyperopia, one could combine PTK and hyperopic PRK treatment at the same time. With deeper ablations, one could perform a simple anti-hyperopic ablation concurrent with the PTK treatment. The authors recommend setting the laser with a standard +1.00 D treatment (ablation zone 9 mm, correction diameter 5 mm) to smooth out the mid periphery and increase the peripheral zone treated and the curvature. Others recommend using the joystick to maneuver a 2 mm diameter circular spot around the periphery of the central ablation (between 5 and 6 mm) to smooth out the edge of the ablation zone, thus counteracting the induced hyperopia.26
Outcomes
The outcome of PTK in RCE is related to the etiology, with traumatic etiology (success rate 74.4–80.0%) being better than EBMD (success rate 53.8%).27 In general, several studies have reported good results in anterior corneal dystrophies of the Bowman’s and stroma.28,29 However, recurrences are not uncommon, especially in Reis–Buckler and granular dystrophy.28 Fortunately, recurrences are often superficial and amenable to repeat PTK with good results. One must distinguish the difference between early haze from laser ablation (homogenous opacity over ablation zone) and recurrences (more heterogeneous with patterns resembling the underlying dystrophy).28 There are also reports of differential prognosis depending on the genotypes of the corneal dystrophy, with Gly623Arg mutation responding better to PTK.30 Others have shown success in treating lattice dystrophy,28 SND,31 keratoconus nodules,32 LASIK flap striae33 and symptomatic bullous keratopathy.
Key Surgical Points
 Careful selection of patients who are good candidates for PTK avoids any disappointments or unwanted complications.
Careful selection of patients who are good candidates for PTK avoids any disappointments or unwanted complications.
 PTK is best for corneal pathologies affecting the epithelium or anterior 10–20% of the stroma.
PTK is best for corneal pathologies affecting the epithelium or anterior 10–20% of the stroma.
 For patient safety, the residual corneal bed thickness must be greater than 300 µm.
For patient safety, the residual corneal bed thickness must be greater than 300 µm.
 Try to maintain a smooth surface during PTK; either perform a transepithelial PTK if able, or use masking agents.
Try to maintain a smooth surface during PTK; either perform a transepithelial PTK if able, or use masking agents.
 Remove the least amount of tissue required to achieve the intended goal.
Remove the least amount of tissue required to achieve the intended goal.
 Be aware of hyperopic shift and try and correct it within the same setting if possible.
Be aware of hyperopic shift and try and correct it within the same setting if possible.
Complications
In the immediate postoperative period, delayed epithelial healing can present a challenge. The time to re-epithelialization ranges between 2 and 6 days.34 Poor healing can be associated with more severe consequences, such as scarring, irregular astigmatism and infection. Induced hyperopia is the most common refractive change.35 However, induced myopia and irregular astigmatism have also been reported. Combined symptomatic and morphologic recurrence for RCE related to EBMD occurs in 14% of patients.36 Other side effects post-PTK include haze, graft rejection and reactivation of HSK.
Superficial Keratectomy
Treatment of RCE with superficial debridement of loose epithelium was reported as early as 1900.37 This, combined with chemical cautery, was advocated by Chandler in 1944, as he recognized that the pathology is one of epithelial adherence.37 However, this resulted in clouding of the cornea. Few decades later, Buxton and Fox reported their series of SK in patients with EBMD in 1983.38 They performed a total superficial epithelial keratectomy from limbus to limbus with a blade and then applied diamond drill to areas of persistent abnormalities. Eleven out of 13 patients were relieved of their symptoms. However, given the potential limbal stem cell damage from the large debridement, others advocate sparing of the peripheral corneal epithelium.39
Procedure
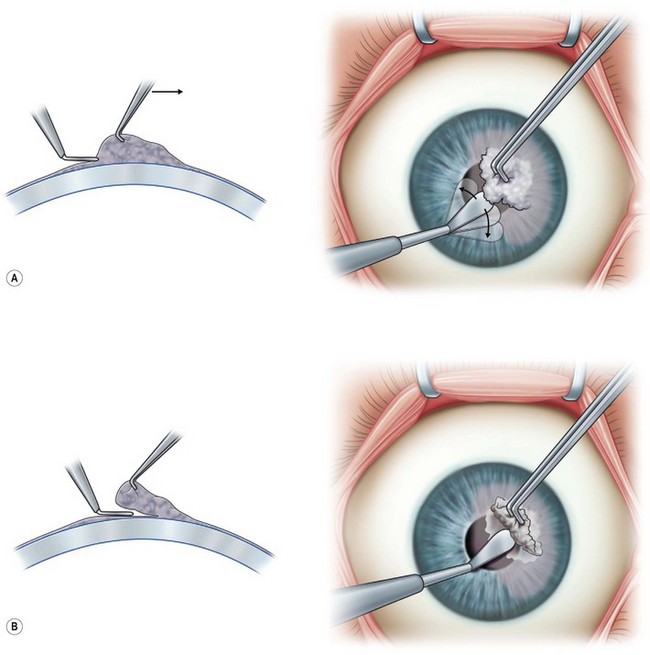
This is performed under topical or local anesthesia. It can be performed at the slit lamp or under an operating microscope in a minor operating room. For RCE, use a sharp instrument (e.g. a 64 Beaver blade) to debride the loose epithelium in a sheet, combined with gentle wiping with a cellulose sponge. Care should be taken to leave a 1 2-mm rim of peripheral corneal epithelium to avoid stem cell damage. This can be combined with the use of a fine diamond burr to polish in a gentle circular motion in the affected areas, which is shown to reduce recurrence rates with similar efficacy to PTK.40–42 For elevated lesions, such as a Salzmann’s nodule, grasp the nodule with a toothed forceps, e.g. Colibri, and dissect with a crescent or a 64 beaver blade. Scraping with the blade at 90 degrees to the surface of the cornea will often reveal a clear plane of dissection at the level of the Bowman’s membrane, leaving a smooth bed (Fig. 36.3. Some also promote the use of MMC intraoperatively to prevent recurrence and haze.42 A soft BCL is then applied and the patient is commenced on topical antibiotics for 1 week and topical steroids on a tapering regimen over 2–3 weeks. Frequent review is required until the epithelium is healed.
Outcomes
In general, SK with or without diamond burr polishing is thought to be a safe and cost-effective treatment option. In RCE associated with EBMD, diamond burr polishing has been shown to be as efficacious as PTK, and it has a lower recurrence rate of 11.1% compare to 26.7% in the PTK group.42 Diamond burr SK is also thought to be more effective, compared to epithelial debridement alone, with less recurrences and the need for repeated surgical interventions.41 In a retrospective study of patients with SND treated with SK combined with MMC, there were no recurrences and most had improvement in their symptoms.43
Complications
Similar to PTK, early postoperative complications include delayed epithelialization and infection. Care needs to be taken to avoid induced astigmatism after diamond burr SK by using only gentle small circular motions.44 Postoperative subepithelial haze was reported in around 23–26% of patients, which can occur at any time after surgery for RCE.45,46
Tarsorrhaphy
Temporary Suture Tarsorrhaphy
Temporary suture tarsorrhaphy was first described by Koenig and Harris47 in 1991, with various modifications published since then, including without the bolsters48 and a drawstring technique.49 The most common type is a partial lateral tarsorrhaphy, which permits examination of the ocular surface and preserves the patient’s visual axis. If additional protection is required, one could also add a medial tarsorrhaphy. Often, complete temporary closure may be needed to achieve epithelial healing and should not be withheld.
Procedure
1. Assess the amount of tarsorrhaphy that is required (usually one-third to one-quarter length of the eyelid).
2. Infiltrate the eyelids with 2–3 mL of LA with 1 : 100 000 epinephrine (for hemostasis). Flip over the lid and inject above the superior tarsus and below the inferior tarsus to achieve complete anesthesia.
3. Prepare two 1–1.5-cm silicone or rubber bolsters. These prevent erosion of the sutures through the eyelids and aid eversion of the lids to prevent trichiasis.
4. Use a double-armed 5-0 nylon or 6-0 silk suture (the authors prefer nylon as it is easier to remove later on), pass them through one of the bolsters completely at one end, then through the skin 3–4 mm from the upper eyelid margin, into the tarsus, and exits at the gray line of the upper lid. The needle is then passed through the lower lid gray line and exits 2–3 mm inferior to the lid margin. Repeat the same maneuver with the other end of the needle, but displace it laterally. Tie the sutures externally over the bolsters. Ensure that there are no sutures internally that could cause further irritation of the ocular surface.
5. One modification is the drawstring method49 which allows complete closure but also easy access to the ocular surface. This technique uses three bolsters, two 2 cm and one 1 cm sections. The first step is as described above, but using the two 2-cm bolsters. Then the two suture arms below the lower lid margin are passed through the 1-cm bolster. This is tied, but 2–3 cm of slack is left in the suture to allow the surgeon to loosen the bolsters (move the smaller bolster away from the larger bolster) and examine the ocular surface as required. In the interim, the loop can be taped to the skin with steri-strips.
Permanent Tarsorrhaphy
Procedure
1. As with temporary tarsorrhaphy, infiltrate the eyelids with local anesthetic with epinephrine.
2. Use a sharp blade (e.g. No.11 stab blade) and split the eyelids down the gray line, approximately 2–3 mm deep. Then make two relieving incisions perpendicular to the gray line incision, directed posteriorly.
3. Use Westcott scissors to excise a strip of the posterior eyelid margin in both the upper and the lower lids.
4. Appose the denuded posterior lid margin using multiple interrupted 5-0 or 6-0 Vicryl mattress sutures through the tarsus and tie the knots anteriorly away from the cornea.
5. Close the anterior lamellar with multiple interrupted 6-0 vicryl or 6-0 double-armed nylon through a bolster (as for temporary tarsorrhaphy) as it everts the lid during healing. The nylon and bolster can be removed after 2 weeks.
Postoperatively, topical antibiotic ointment is applied to the eyelids for 2 weeks. The lid margin will be fused laterally, but this can be reversed at any stage by dividing across the intermarginal adhesion.
Botox Tarsorrhaphy
Botulinum toxin was first used for ocular disorders for the treatment of strabismus. In 1987, Adams and Kirkness50 identified that temporary induced ptosis from injection of botulinum toxin into the levator palpebrae superioris (LPS) could act as a protector of a compromised ocular surface. For patients unwilling or medically unable to undergo a surgical procedure, such as suture tarsorrhaphy, this may be an alternative. It provides a complete ptosis and the ocular surface is easily accessible to the examiner and for instilling drops. However, it has the expense of the neurotoxin, it is mildly invasive, with more obvious appearance asymmetry. It also commits the patient to monovision for 6–9 weeks until the ptosis recovers.
There are several types of botulinum toxin, but the majority of studies were based on Dysport (Ipsen, Slough, United Kingdom) and Type A Botox (Allergan, Irvine, California). These are not bioequivalent; hence, one must be careful when reconstituting the drug and should also be aware of the difference in concentrations. The suggested relative bioequivalence or per-unit strength ratio of Dysport compared to Botox is 3 : 1.51 The earlier reports described administration of the botulinum toxin with a 25-mm needle, through the eyelid, aiming at the levator palpebrae superioris muscle belly.50,52,53 The toxin doses were 0.0625 ng for Dysport50,52 and 2.5–5 units for Botox.53 The mean duration of ptosis ranged between 6.5 and 8.5 weeks. However, this was associated with 24–80% superior rectus underaction,50,52,53 which could exacerbate exposure keratopathy. A recent report suggested a more anterior chemo-denervation of the LPS, which was associated with no superior rectus dysfunction.54 In this approach, the clinician used a tuberculin syringe and a half-inch 26G or 30G needle and placed the needle tip near the anterior orbital roof just behind the superior orbital rim in the mid-pupillary axis before injection. The mean effective dose was 12.5 units and the mean ptosis duration was 9.2 weeks. In general, the time from injection to complete ptosis is 3.6–4 days.53 The patient should be continued on appropriate topical medications as prescribed by the surgeon.
Other Modalities
Other types of tarsorrhaphies that have been described which may be useful in selected patients. Cyanoacrylate adhesion of the eyelids is an easy and effective method of closing the palpebral aperture.55 However, it does not permit easy examination of the eye and its duration is unpredictable (range 1–15 days). This may be useful as a short-term solution in those who have contraindications or are unwilling to have other surgical procedures (e.g. on anticoagulants). Mulhern and Rootman described the use of the Stamler temporary lid splints,56 which are inexpensive, non-invasive and easy to apply. However, these only last for a short period of time (average 3–7 days) and require frequent re-application. Their role is limited to application in those patients requiring a total therapeutic ptosis for a short period of time (less than 2 weeks) and to those who are able to return, or to be taught how to reapply the splint.
Key Surgical Points
 The decision on the type of tarsorrhaphy depends on the diagnosis and the expected recovery time.
The decision on the type of tarsorrhaphy depends on the diagnosis and the expected recovery time.
 For temporary and permanent suture tarsorrhaphy, avoid leaving any internal sutures that may inadvertently rub against the ocular surface.
For temporary and permanent suture tarsorrhaphy, avoid leaving any internal sutures that may inadvertently rub against the ocular surface.
 Using the drawstring technique can make it easier to access and examine the ocular surface.
Using the drawstring technique can make it easier to access and examine the ocular surface.
 For botulinum toxin ptosis, one must be mindful of which serotype and product, as they are not all bioequivalent.
For botulinum toxin ptosis, one must be mindful of which serotype and product, as they are not all bioequivalent.
Outcomes
In general, temporary and permanent suture tarsorrhaphies are successful in achieving re-epithelialization. A series evaluating the outcome of suture tarsorrhaphies in a cohort of cornea and external eye disease patients showed that 90.9% of the epithelial defects completely resolved. The mean time to healing after tarsorrhaphy was 18 days.57 When comparing lateral tarsorrhaphy to patching in persistent post-keratoplasty epithelial defects, the epithelial healing was significantly faster (7.61 veersus 12.6 days) and the patients were more comfortable in the lateral tarsorrhaphy group.
Botox ptosis is effective in inducing complete ptosis in 75% of cases,52 although in some cases the patient may require a second injection to achieve the desired result.
Complications
Complications of suture temporary tarsorrhaphy include premature separation of tarsorrhaphy, trichiasis and cellulitis. When reversing permanent tarsorrhaphy, the lid margins can scar and deform with possible secondary cicatricial entropion. Beware of doing a lateral tarsorrhaphy in patients with a sixth nerve palsy as it may exacerbate the exposure. In patients with central melting, it may be better to completely close the lid fissure, as a lateral tarsorrhaphy may exacerbate the exposure and melting. Superior rectus underaction is the most commonly reported side effect (68–80%) following Botox ptosis, leading to hypotropia and reduced Bell’s phenomenon.50,52 This could exacerbate exposure keratopathy. The mean recovery time of superior rectus underaction was reported to be 6 weeks, although 16% persisted beyond resolution of ptosis.52 Diplopia occurs in 16–24%, with some persisting beyond the resolution of the ptosis.
Conjunctival Flaps
Conjunctival flaps have been used in the treatment of corneal diseases since the 1800s. Described in the German literature in the 1877, Gundersen58,59 reported the largest series and described his technique for thin conjunctival flaps in 1958. He advocated their use for corneal ulcerations and thinning disorders, such as neuroparalytic keratitis, marginal ulcerations, relapsing erosions, and herpetic ulcers. In the early 1900s this procedure was used to reinforce cataract wounds.58
Indication
The treatment of recalcitrant corneal surface disease can be a challenge. Although local and systemic treatment is often successful, there are situations in which medical or surgical therapy may fail, with resultant recurrent epithelial breakdown and stromal ulceration. In specific situations, the conjunctival flap is effective and definitive treatment for persistent ocular surface disease.59–62 Patients are relieved of pain, frequent regimen of topical medication, as well as more invasive surgery or enucleation.
The purpose of a conjunctival flap is to restore the integrity of a chronically compromised corneal surface,63,64 and provide metabolic and mechanical support for corneal healing.63,65 They do not add tectonic support and should not be used in very thin or perforated corneas. Conjunctival flaps act as biologic patches, conferring a trophic effect because of the nutritional and immunologic supply by its vascular connective tissue. A conjunctival flap will often provide comfort, reduce the ocular inflammation, and promote healing in these patients. This is particularly helpful in the setting of a blind painful eye where the flap can provide a new ocular surface for the placement of a cosmetic scleral shell (Fig. 36.4).
A conjunctival flap is rarely used in the management of microbial keratitis. Marginal fungal ulcers unresponsive to antimycotic therapy have been successfully treated with conjunctival flaps.66,67 This treatment is not appropriate in the setting of an active suppurative infection that is in danger of perforation.67 A repeat graft is generally preferable once the inflammation associated with graft infections has resolved. In cases where medical treatment has been exhausted, a conjunctival flap may offer a more effective means to resolve the infection.68 A repeat graft can then be safely performed at a later date.
In the technique described by Gunderson,58,59 coverage of the entire cornea with the conjunctiva obstructed any view of the anterior chamber58,69 and precluded monitoring of corneal disease progression. In addition, this procedure was challenging in patients with short fornices and had the potential to cause ptosis.58 It also required a more careful and extensive surgical procedure in the operating room. Any buttonholes or traction could ultimately lead to flap failure. In cases where only partial coverage of the cornea is required, a selective partial conjunctival flap tailored to cover the desired part of the diseased cornea provides an alternative to a total conjunctival flap.58 This is particularly helpful if the patient has short fonices. Visualization of the anterior segment is not limited with this technique, and therefore, progression of corneal disease can be monitored. Intraocular pressure can also be accurately measured. Following stabilization of the ocular surface with either a total or partial conjunctival flap, PKP may be considered.
Procedure
Total Conjunctival Flap
The most commonly employed technique for a total conjunctival flap is described by Gundersen58 or modifications thereof.60,70 Anesthesia may be local or general, but retrobulbar anesthesia is adequate in mos casest. Before the procedure, it is important to evaluate the availability and mobility of the conjunctiva. Conjunctival scarring in the area to be mobilized may preclude the success of this procedure.
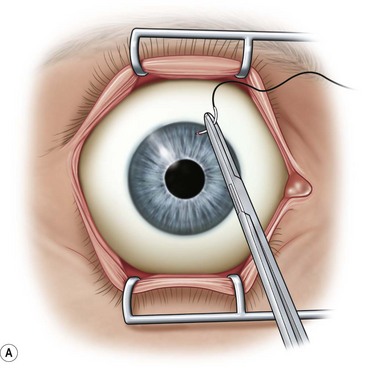
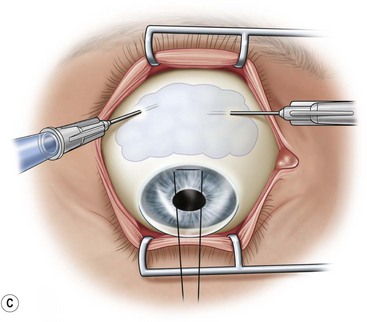
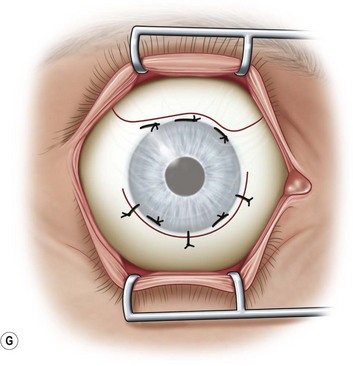
After placement of the lid speculum, a 7-0 silk traction suture is placed through the peripheral cornea at the superior limbus (Fig. 36.5A). This allows the surgeon to control the globe and rotate it downward to expose the entire upper bulbar conjunctiva. Removal of the corneal epithelium can be performed after mobilization of the conjunctival flap. However, if done first, it is completed in a bloodless field and ensures it is not forgotten.
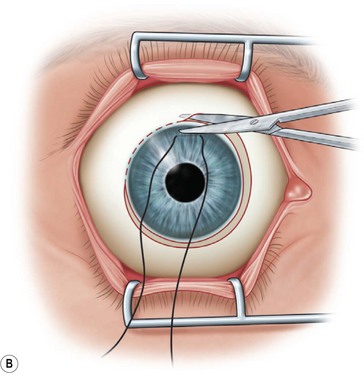
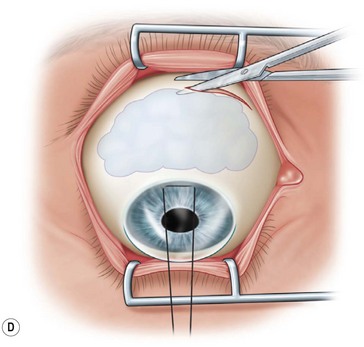
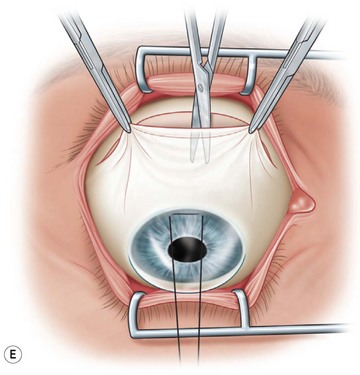
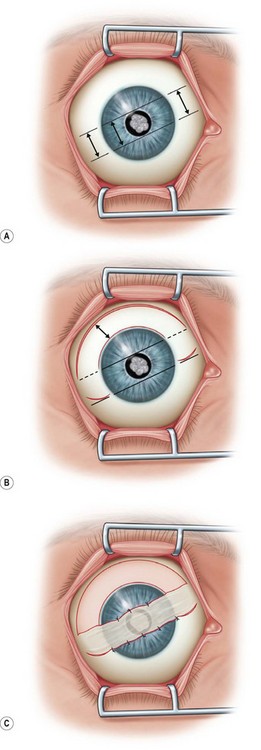
Next, the conjunctival flap is mobilized. A caliper is used to measure at least 14 mm from and concentric to the superior limbus into the fornix, the area necessary to cover the cornea. The incision is carried from medial to lateral canthal area superiorly in an arc (Fig. 36.5B). The conjunctiva is then ballooned up with a subconjunctival injection of 1% lidocaine with epinephrine (Fig. 36.5C). Some surgeons prefer not to inject subconjunctivally as it swells the Tenon’s capsule and makes dissection more difficult. The injection site should not reside within the area to be used to cover the cornea. With the globe rotated downward, an incision in the previously marked superior fornix is made, avoiding the underlying Tenon’s capsule (Fig. 36.5D). A very thin flap of conjunctiva is dissected downward towards the limbus without creating buttonholes (Fig. 36.5E). When the limbus is reached, the resultant flap is freed with a 360-degree peritomy. Bleeding in the dissection bed is controlled with cautery.
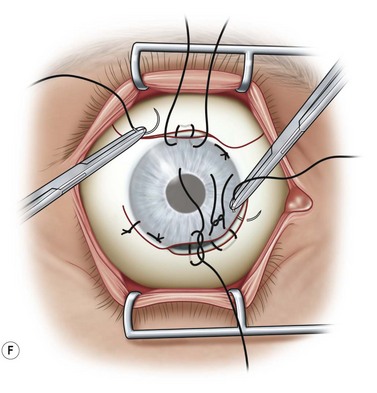
The traction suture is released and the conjunctival flap is pulled down over the cornea (Fig. 36.5F). Relaxing incisions may help to release tension. Finally, the flap is sutured into place with Vicryl or nylon sutures placed in the superficial sclera just outside the limbus (Fig. 36.5G).
Although most authors agree that the procedure of choice is performing thin conjunctival flaps, without Tenon’s capsule,60 there are special situations in which a keratectomy is necessary and where Tenon’s capsule may be useful to occupy the space.71 Sanitato et al.66 reported on a thick conjunctival flap with Tenon’s capsule for the treatment of peripheral corneal mycotic abscesses. The thicker flaps do thin out over time, thereby improving the overall appearance.
Bridge Flap
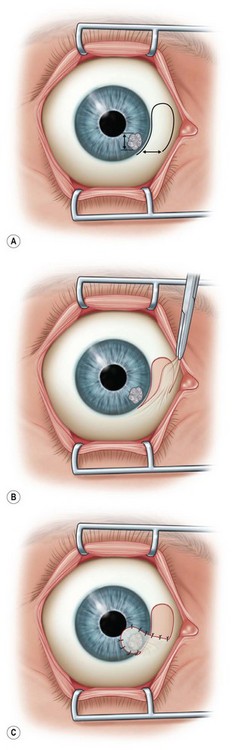
A bridge flap is used to cover focal central or paracentral corneal defects (Fig. 36.6A). This technique is similar to a Gundersen flap except that the width of the flap to be dissected is measured to be large enough to cover the corneal lesion (typically 20–30% larger). The corneal epithelium is removed in the area to be covered. Following appropriate measurements and marking in the superior bulbar conjunctiva, a thin flap is dissected from periphery to limbus, and released with a limited peritomy (Fig. 36.6B). The flap is then mobilized to cover the corneal lesion and fixed into place with Vicryl or nylon sutures (Fig. 36.6C).
Pedicle Flap
This flap is used to cover lesions near the limbus. After an LA, the corneal epithelium is removed from the area to be covered. As with a bridge flap, the size of the pedicle should be 20–30% larger than the lesion to be covered (Fig. 36.7A). Following appropriate measurements and marking in the conjunctiva to be used, a thin flap is dissected, mobilized to cover the corneal lesion, and fixed with nylon or Vicryl sutures (Fig. 36.7B,C).
Simple Advancement Flaps
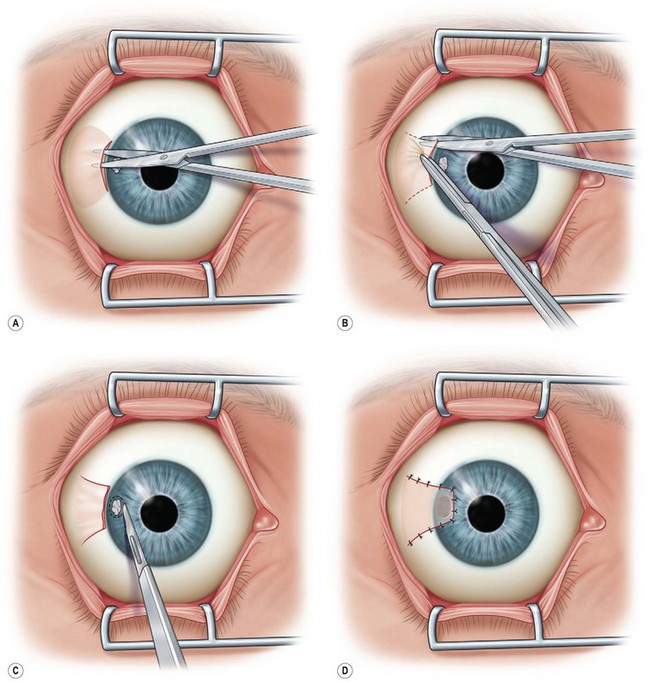
This flap may also be used to cover lesions near the limbus and can be used with a lamellar patch graft. After an LA, a limited peritomy is performed in the area of interest (Fig. 36.8A,B). The conjunctiva is dissected to create a flap without tension, which is then pulled forward into place to cover the corneal lesion. Nylon or Vicryl sutures may be used to fix the flap into place (Fig. 36.8C,D).
Key Surgical Points
 The key to creating a proper conjunctival flap involves selection of the appropriate site, mobilization of the flap without traction, and preservation of the flap’s blood supply. The surgeon should determine preoperatively how much conjunctiva is available. If necessary, an inferior flap may be combined to provide adequate coverage.
The key to creating a proper conjunctival flap involves selection of the appropriate site, mobilization of the flap without traction, and preservation of the flap’s blood supply. The surgeon should determine preoperatively how much conjunctiva is available. If necessary, an inferior flap may be combined to provide adequate coverage.
 If possible, bridge flaps should be created vertically rather than horizontally to prevent the flap displacement by the action of the lid. The flap must be maintained in position by mattress sutures into the stroma.
If possible, bridge flaps should be created vertically rather than horizontally to prevent the flap displacement by the action of the lid. The flap must be maintained in position by mattress sutures into the stroma.
 A small hole in a conjunctival flap will enlarge if the flap is under tension. If necessary, the hole may be closed using 11-0 nylon on a vascular needle suturing the conjunctiva to the underlying cornea. If necessary, a buttonhole may be moved off the cornea by shifting the whole flap medially or laterally.
A small hole in a conjunctival flap will enlarge if the flap is under tension. If necessary, the hole may be closed using 11-0 nylon on a vascular needle suturing the conjunctiva to the underlying cornea. If necessary, a buttonhole may be moved off the cornea by shifting the whole flap medially or laterally.
 Epithelial cysts may form underneath the flap secondary to inadequate removal of corneal epithelium during the procedure. These may be excised as necessary.
Epithelial cysts may form underneath the flap secondary to inadequate removal of corneal epithelium during the procedure. These may be excised as necessary.
 Over time, there may be corneal vascularization and scarring underneath a conjunctival flap. This can influence the success of subsequent transplantation.
Over time, there may be corneal vascularization and scarring underneath a conjunctival flap. This can influence the success of subsequent transplantation.
Complications
Postoperative complications of conjunctival flaps include buttonholing of the conjunctiva, retraction of the flap, and formation of granulomas and inclusion cysts. Ptosis has been reported as a complication of conjunctival flap surgery.58,60 Gundersen thought the ptosis was caused by the incorporation of Tenon’s capsule within the flap or insufficient dissection of the flap from its lateral and medial attachments. Paton and Milsaukas60 similarly had attributed post-conjunctival flap ptosis caused by undue traction.
Vision may be affected unless a conjunctival flap procedure is performed in the peripheral cornea. However, in cases where a total flap is being considered, vision is not usually the primary concern. Similarly, total flaps may hinder examination of the anterior and posterior segments. Corneal perforation under a conjunctival flap has been reported.72 Intraocular pressure measurements may be a challenge, depending on the instrument and flap type. Penetration of topical medications (e.g., glaucoma medications) may also be compromised by a total conjunctival flap.
References
1. McLean, EN, MacRae, SM, Rich, LF, et al. Recurrent erosion. Treatment by anterior stromal puncture. Ophthalmology. 1986;93:784–788.
2. Garrana, RM, Zieske, JD, Assouline, M, et al. Matrix metalloproteinases in epithelia from human recurrent corneal erosion. Invest Ophthalmol Vis Sci. 1999;40:1266–1270.
3. Chen, YT, Huang, CW, Huang, FC, et al. The cleavage plane of corneal epithelial adhesion complex in traumatic recurrent corneal erosion. Mol Vis. 2006;12:196–204.
4. Malecha, MA. Anterior stromal puncture for recurrent corneal erosion after laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:496–498.
5. Rubinfeld, RS, Laibson, PR, Cohen, EJ, et al. Anterior stromal puncture for recurrent erosion: further experience and new instrumentation. Ophthalmic Surg. 1990;21:318–326.
6. Katsev, DA, Kincaid, MC, Fouraker, BD, et al. Recurrent corneal erosion: pathology of corneal puncture. Cornea. 1991;10:418–423.
7. Geggel, HS. Successful treatment of recurrent corneal erosion with Nd:YAG anterior stromal puncture. Am J Ophthalmol. 1990;110:404–407.
8. Beetham, WP. Filamentary keratitis. Trans Am Ophthalmol Soc. 1935;33:413–435.
9. Freeman, JM. The punctum plug: evaluation of a new treatment for the dry eye. Am Acad Ophthalmol Otolaryngol. 1975;79:874–879.
10. Kaido, M, Goto, E, Dogru, M, et al. Punctal occlusion in the management of chronic Stevens–Johnson syndrome. Ophthalmology. 2004;111:895–900.
11. Tsubota, K, Satake, Y, Kaido, M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. Br J Ophthalmol. 1999;340:1697–1703.
12. Yang, HY, Fujishima, H, Toda, I, et al. Lacrimal punctal occlusion for the treatment of superior limbic keratoconjunctivitis. Am J Ophthalmol. 1997;124:80–87.
13. Cartwright, MJ. A prospective, randomized comparison of thermal cautery and argon laser for permanent punctal occlusion. Am J Ophthalmol. 1994;117:414.
14. Ohba, E, Dogru, M, Hosaka, E, et al. Surgical punctal occlusion with a high heat-energy releasing cautery device for severe dry eye with recurrent punctal plug extrusion. Am J Ophthalmol. 2011;151:483–487. [e1].
15. Akova, YA, Demirhan, B, Cakmakci, S, et al. Pyogenic granuloma: a rare complication of silicone punctal plugs. Ophthalmic Surg Lasers. 1999;30:584–585.
16. Mazow, ML, McCall, T, Prager, TC. Lodged intracanalicular plugs as a cause of lacrimal obstruction. Ophthal Plast Reconstr Surg. 2007;23:138–142.
17. Horwath-Winter, J, Thaci, A, Gruber, A, et al. Long-term retention rates and complications of silicone punctal plugs in dry eye. Am J Ophthalmol. 2007;144:441–444.
18. Soparkar, CN, Patrinely, JR, Hunts, J, et al. The perils of permanent punctal plugs. Am J Ophthalmol. 1997;123:120–121.
19. Trokel, SL, Srinivasan, R, Braren, B. Eximer laser surgery of the cornea. Am J Ophthalmol. 1983;96:710–715.
20. Rapuano, CJ. Phototherapeutic keratectomy: who are the best candidates and how do you treat them? Curr Opin Ophthalmol. 2010;21:280–282.
21. Szentmary, N, Langenbucher, A, Hafner, A, et al. Impact of phototherapeutic keratectomy on the outcome of subsequent penetrating keratoplasty in patients with stromal corneal dystrophies. Am J Ophthalmol. 2004;137:301–307.
22. Rapuano, CJ. Excimer laser phototherapeutic keratectomy. Int Ophthalmol Clin. 1996;36:127–136.
23. Ma, JJK, Tseng, SS, Yarascavitch, BA. Anterior segment optical coherence tomography for transepithelial phototherapeutic keratectomy in central corneal stromal scarring. Cornea. 2009;28:927–929.
24. Shah, RA, Wilson, SE. Use of mitomycin-C for phototherapeutic keratectomy and photorefractive keratectomy surgery. Curr Opin Ophthalmol. 2010;21:269–273.
25. Dogru, M, Katakami, C, Yamanaka, A. Refractive changes after excimer laser phototherapeutic keratectomy. J Cataract Refract Surg. 2001;27:686–692.
26. Rapuano, CJ. Excimer laser phototherapeutic keratectomy in eyes with anterior corneal dystrophies: short-term clinical outcomes with and without an antihyperopia treatment and poor effectiveness of ultrasound biomicroscopic evaluation. Cornea. 2005;24:20–31.
27. Jain, S, Austin, DJ. Phototherapeutic keratectomy for treatment of recurrent corneal erosion. J Cataract Refract Surg. 1999;25:1610–1614.
28. Dinh, R, Rapuano, CJ, Cohen, EJ, et al. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology. 1999;106:1490–1497.
29. Orndahl, M, Fagerholm, P, Fitzsimmons, T, et al. Treatment of corneal dystrophies with excimer laser. Acta Ophthalmol (Copenh). 1994;72:235–240.
30. Gruenauer-Kloevekorn, C, Braeutigam, S, Froster, UG, et al. Surgical outcome after phototherapeutic keratectomy in patients with TGFBI-linked corneal dystrophies in relation to molecular genetic findings. Graefes Arch Clin Exp Ophthalmol. 2009;247:93–99.
31. Marcon, AS, Rapuano, CJ. Excimer laser phototherapeutic keratectomy retreatment of anterior basement membrane dystrophy and Salzmann’s nodular degeneration with topical mitomycin C. Cornea. 2002;21:828–830.
32. Elsahn, AF, Rapuano, CJ, Antunes, VA, et al. Excimer laser phototherapeutic keratectomy for keratoconus nodules. Cornea. 2009;28:144–147.
33. Ashrafzadeh, A, Steinert, RF. Results of phototherapeutic keratectomy in the management of flap striae after LASIK before and after developing a standardized protocol: long-term follow-up of an expanded patient population. Ophthalmology. 2007;114:1118–1123.
34. Rapuano, CJ. Excimer laser phototherapeutic keratectomy: long-term results and practical considerations. Cornea. 1997;16:151–157.
35. Rapuano, CJ. Excimer laser phototherapeutic keratectomy. Curr Opin Ophthalmol. 2001;12:288–293.
36. Germundsson, J, Fagerholm, P, Lagali, N. Clinical outcome and recurrence of epithelial basement membrane dystrophy after phototherapeutic keratectomy a cross-sectional study. Ophthalmology. 2011;118:515–522.
37. Chandler, PA. Recurrent erosion of the cornea. Trans Am Ophthalmol Soc. 1944;42:355–371.
38. Buxton, JN, Fox, ML. Superficial epithelial keratectomy in the treatment of epithelial basement membrane dystrophy. A preliminary report. Arch Ophthalmol. 1983;101:392–395.
39. Das, S, Seitz, B. Recurrent corneal erosion syndrome. Surv Ophthalmol. 2008;53:3–15.
40. Soong, HK, Farjo, Q, Meyer, RF, et al. Diamond burr superficial keratectomy for recurrent corneal erosions. Br J Ophthalmol. 2002;86:296–298.
41. Wong, VWY, Chi, SCC, Lam, DSC, et al. Diamond burr polishing for recurrent corneal erosions: results from a prospective randomized controlled trial. Cornea. 2009;28:152–156.
42. Sridhar, MS, Rapuano, CJ, Cosar, CB, et al. Phototherapeutic keratectomy versus diamond burr polishing of Bowman’s membrane in the treatment of recurrent corneal erosions associated with anterior basement membrane dystrophy. Ophthalmology. 2002;109:674–679.
43. Bowers, PJ, Jr., Price, MO, Zeldes, SS, et al. Superficial keratectomy with mitomycin-C for the treatment of Salzmann’s nodules. J Cataract Refract Surg. 2003;29:1302–1306.
44. Yoo, JH, Choi, DM. Induced astigmatism after diamond burr superficial keratectomy for recurrent corneal erosion. Eye Contact Lens. 2009;35:341–344.
45. Itty, S, Hamilton, SS, Baratz, KH, et al. Outcomes of epithelial debridement for anterior basement membrane dystrophy. Am J Ophthalmol. 2007;144:217–221.
46. Tzelikis, PF, Rapuano, CJ, Hammersmith, KM, et al. Diamond burr treatment of poor vision from anterior basement membrane dystrophy. Am J Ophthalmol. 2005;140:308–310.
47. Koenig, SB, Harris, GJ. Temporary suture tarsorrhaphy after penetrating keratoplasty. Cornea. 1991;10:121–122.
48. Quist, LH. A simple and effective tarsorrhaphy technique without the use of external bolsters. Ophthal Plast Reconstr Surg. 1993;9:148–149.
49. Kitchens, J, Kinder, J, Oetting, T. The drawstring temporary tarsorrhaphy technique. Arch Ophthalmol. 2002;120:187–190.
50. Adams, GG, Kirkness, CM, Lee, JP, et al. Botulinum toxin A induced protective ptosis. Eye. 1987;1(Pt5):603–608.
51. Khan, JA, Steinsapir, KD, McCracken, M, et al. Facial fillers, botulinum toxin, and facial rejuvenation. Am Acad Ophthalmol Focal Points. 2011;29:1–18.
52. Kirkness, CM, Adams, GG, Dilly, PN, et al. Botulinum toxin A-induced protective ptosis in corneal disease. Ophthalmology. 1988;95:473–480.
53. Ellis, MF, Daniell, M. An evaluation of the safety and efficacy of botulinum toxin type A (BOTOX) when used to produce a protective ptosis. Clin Experiment Ophthalmol. 2001;29:394–399.
54. Naik, MN, Gangopadhyay, N, Fernandes, M, et al. Anterior chemodenervation of levator palpebrae superioris with botulinum toxin type-A (Botox) to induce temporary ptosis for corneal protection. Eye. 2008;22:1132–1136.
55. Ehrenhaus, M, D’Arienzo, P. Improved technique for temporary tarsorrhaphy with a new cyanoacrylate gel. Arch Ophthalmol. 2003;121:1336–1337.
56. Mulhern, MG, Rootman, DS. The stamler lid splint: a new short-term technique for achieving therapeutic ptosis. Cornea. 2002;21:260–264.
57. Cosar, CB, Cohen, EJ, Rapuano, CJ, et al. Tarsorrhaphy: clinical experience from a cornea practice. Cornea. 2001;20:787–791.
58. Gundersen, T. Conjunctival flaps in the treatment of corneal disease with reference to a new technique of application. Am Arch Opthalmol. 1958;60:880–888.
59. Gundersen, T, Pearlson, HR. Conjunctival flaps for corneal disease: their usefulness and complications. Trans Am Ophthalmol Soc. 1969;67:78–95.
60. Paton, D, Milauskas, AT. Indications, surgical technique, and results of thin conjunctival flaps on the cornea: a review of 122 consecutive cases. Int Ophthalmol Clin. 1970;10:329–345.
61. Insler, MS, Pechous, B. Conjunctival flaps revisited. Ophthalmic Surg. 1987;18:455–458.
62. Lugo, M, Arentsen, JJ. Treatment of neurotrophic ulcers with conjunctival flaps. Am J Ophthalmol. 1987;103:711–712.
63. Thoft, RA. Conjunctival transplantation. Arch Ophthalmol. 1977;95:1425–1427.
64. Coster, DJ, Aggarwal, RK, Williams, KA. Surgical management of ocular surface disorders using conjunctival and stem cell allografts. Br J Ophthalmol. 1995;79:977–982.
65. Reim, M, Teping, C. Surgical procedures in the treatment of most severe eye burns. Revival of the artificial epithelium. Acta Ophthalmol Suppl. 1989;192:47–54.
66. Sanitato, JJ, Kelley, CG, Kaufman, HE. Surgical management of peripheral fungal keratitis (keratomycosis). Arch Ophthalmol. 1984;102:1506–1509.
67. Arentsen, JJ, Laibson, PR, Cohen, EJ. Management of corneal descemetoceles and perforations. Ophthalmic Surg. 1985;16:29–33.
68. Geria, RC, Wainsztein, RD, Brunzini, M. Infectious keratitis in the corneal graft: treatment with partial conjunctival flaps. Ophthalmic Surg Lasers Imaging. 2005;36:298–302.
69. Lin, DT, Webster, RG, Jr., Abbott, RL. Repair of corneal lacerations and perforations. Int Ophthalmol Clin. 1988;28:69–75.
70. Thoft, RA. Conjunctival surgery for corneal disease. In: Molin G, Thoft RA, eds. The cornea: scientific foundations and clinical practice. Boston: Little Brown; 1983:465–476.
71. Hvidberg-Hansen, J, Moller, PM. Lamellar keratectomy by the method of Gundersen. Acta Opthalmol (Copenh). 1973;51:142–151.
72. Lesher, MP, Lohman, LE, Yeakley, W, et al. Recurrence of herpetic stromal keratitis after a conjunctival flap surgical procedure. Am J Ophthalmol. 1992;114:231–233.