Chapter 12 Ocular Inflammatory Diseases
Introduction
Uveitis comprises a large group of inflammatory eye diseases involving the iris, ciliary body and choroid. The incidence of uveitis in the United States has been reported to be as high as 52.4/100 000 person-years in large population-based studies.1 Several classification schemes currently exist, based on etiology (infectious and non-infectious), clinical course (acute, chronic, and recurrent), anatomy (primary site of inflammation), and histopathology (granulomatous or nongranulomatous). Unique ultrasonography features may aid in the diagnosis of certain inflammatory conditions, such as toxocariasis.
Anterior uveitis
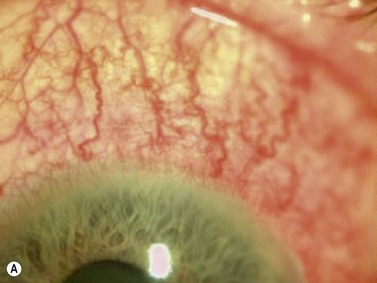
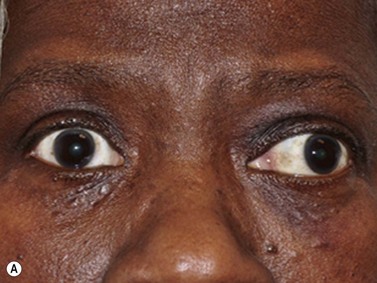
Anterior uveitis is the most common form of uveitis, accounting for approximately 90% of the cases. The inflammation is localized predominantly in the iris and pars plicata of the ciliary body, associated with a breakdown of the blood–aqueous barrier and increased aqueous protein and cells. The classic presentation of acute anterior uveitis is the sudden onset of pain, photophobia and redness, which may be accompanied by a decrease in visual acuity. There are numerous causes of anterior uveitis, including non-infectious disorders, such as sarcoidosis, Reiter’s syndrome, HLA B27 related iridocyclitis, inflammatory bowel disease, juvenile rheumatoid arthritis; as well as infectious conditions, related to agents such as herpesvirus and varicella-zoster virus.2
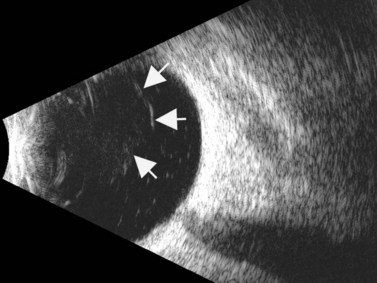
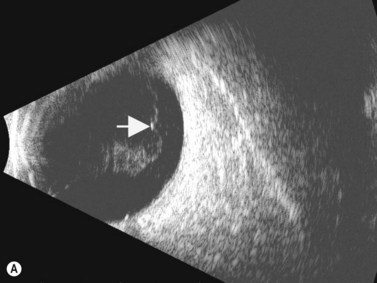
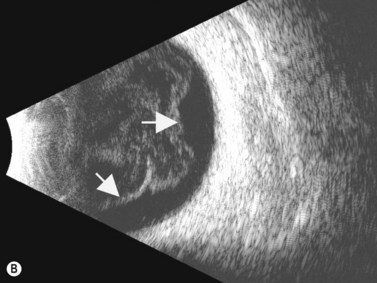
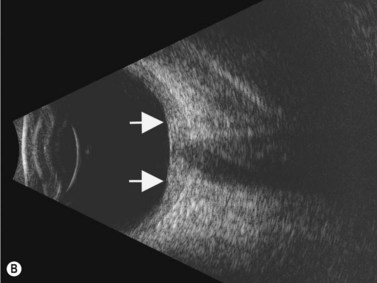
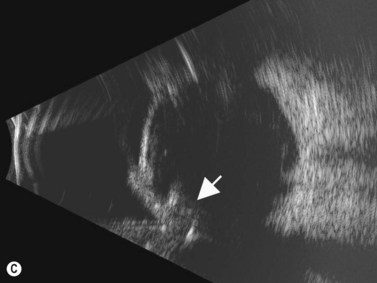
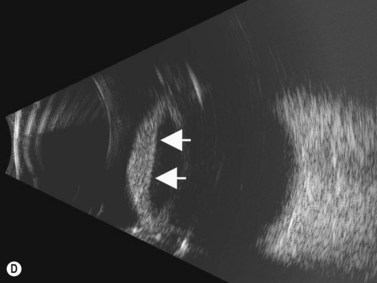
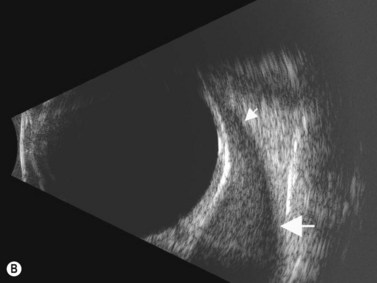
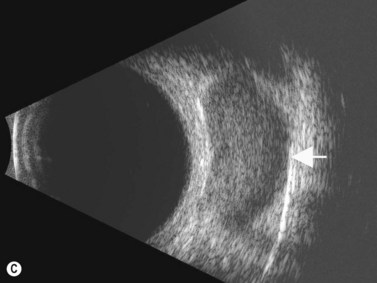
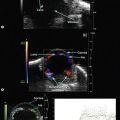
Although slit-lamp examination is sufficient to provide the diagnosis in the majority of cases, the use of ultrasound biomicroscopy (UBM) may disclose information on morphologic features of the anterior segment of the eye, not observed on clinical examination. Features that may be demonstrated by UBM include edema of the iris and ciliary body, demonstrating an irregular pattern of hyporeflectivity, and the presence of exudates adjacent to the ciliary body, as well as the vitreous base (Figure 12.1). Formation of cysts within the posterior iris and ciliary body epithelium at the iridociliary junction has also been described, more commonly related to nongranulomatous uveitis (Figure 12.2).3 A study by Peizeng et al reported that inflammatory changes could still be detected by UBM at 6 weeks after onset of uveitis, when the slit-lamp examination was unremarkable, indicating the need for further treatment, despite the disappearance of inflammatory cells from the anterior chamber.4
Intermediate uveitis
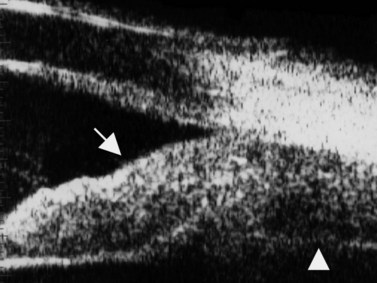
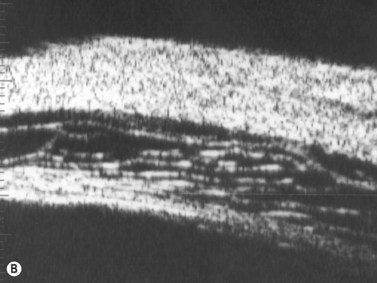
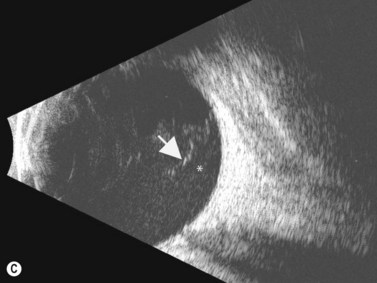
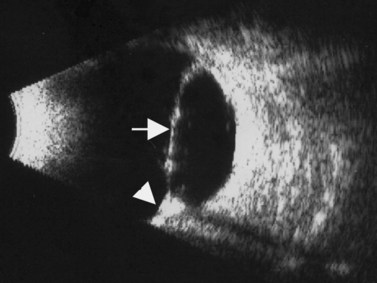
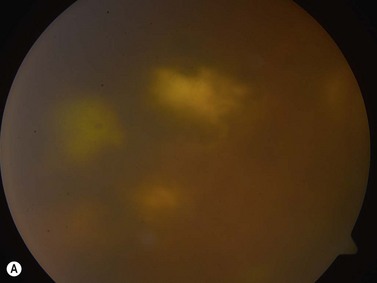
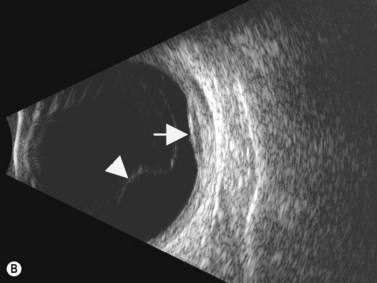
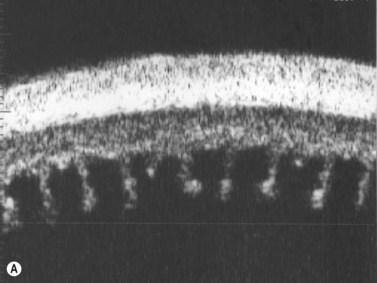
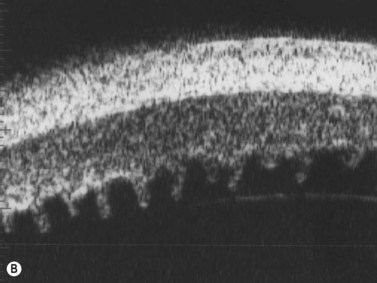
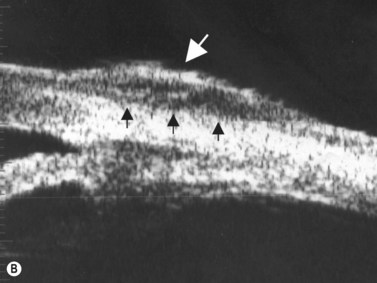
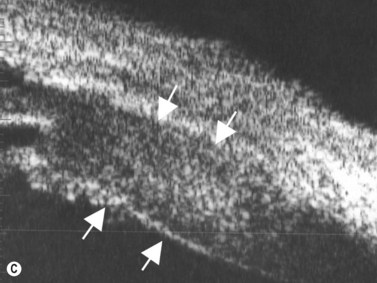
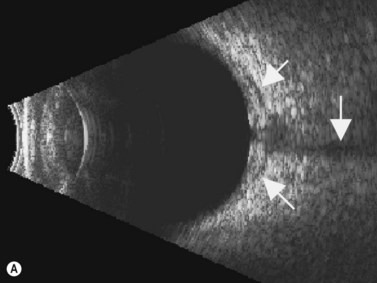
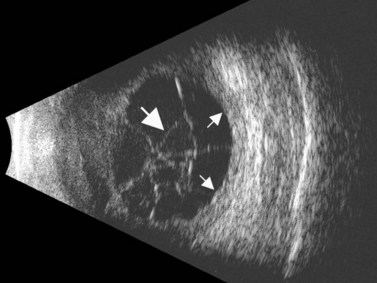
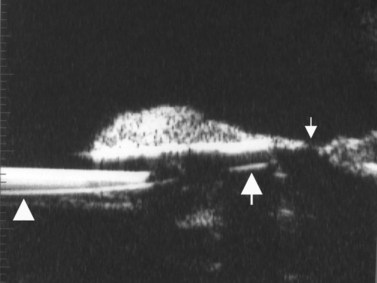
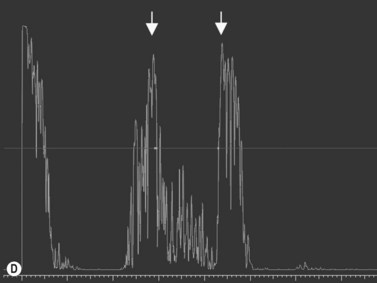
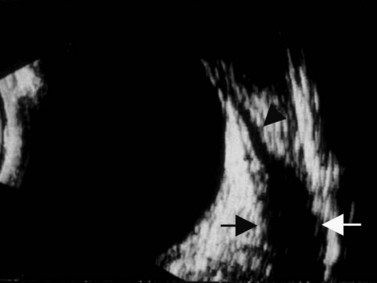
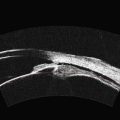
The Standardization of Uveitis Nomenclature (SUN) Working Group defines intermediate uveitis as the subset of uveitis where the primary site of inflammation is the vitreous (Figure 12.3). It may be associated with infections, such as Lyme disease, syphilis and tuberculosis, or other non-infectious conditions, such as sarcoidosis and multiple sclerosis. Inflammatory cells may aggregate in the vitreous (snowballs). In addition, exudates may be present in the pars plana (snowbanks), usually inferiorly and associated with more severe disease.5 These exudates are identified on UBM examination as highly reflective clumps of opacities adjacent or adherent to the pars plana (Figure 12.4).
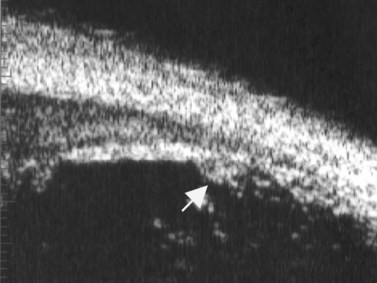
Figure 12.4 Pars planitis. UBM demonstrating white exudates, or “snowbanks” (arrow) over thickened pars plana.
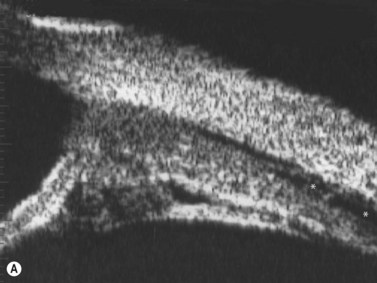
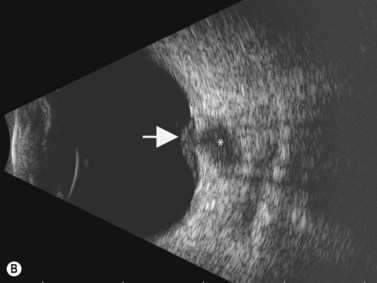
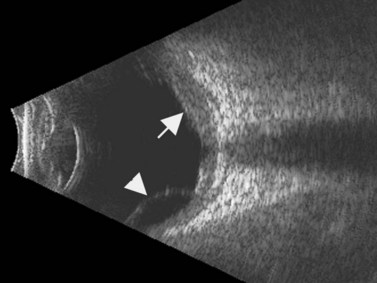
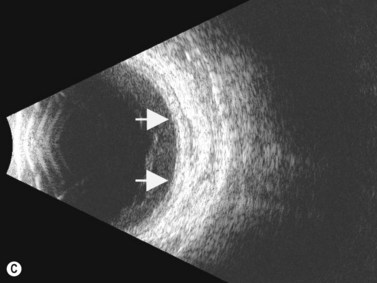
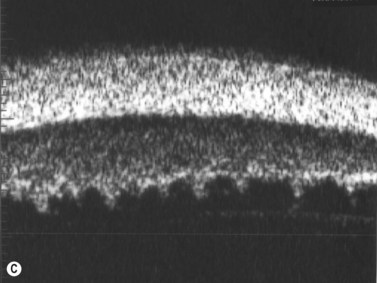
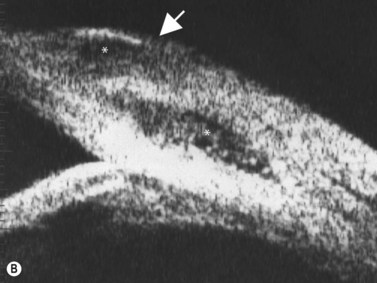
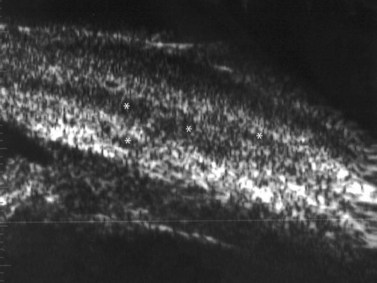
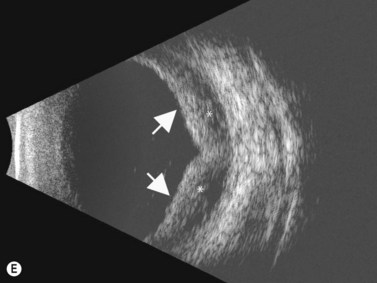
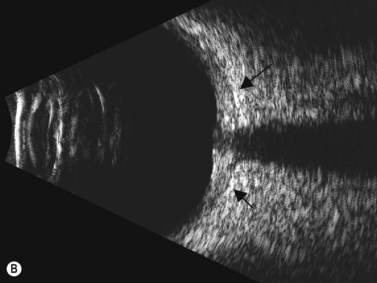
Direct visualization of the posterior pole may be limited in the presence of opaque media. Anatomical structures mostly affected by intermediate uveitis are often not readily visible on funduscopy. The presence of pathological structures such as spotted or membranous vitreous condensations, ciliary body detachment and vitreoretinal adhesions have been documented by ultrasonography (Figure 12.5).6 In a study by Häring et al UBM demonstrated pathological lesions in almost 70% of cases of intermediate uveitis. Lesions appeared as vitreous condensations or membranes of various configurations and extent, located over the peripheral retina and pars plana, and sometimes also extending towards the pars plicata. In 34.6% of eyes enrolled in the study, UBM disclosed pathological lesions not identified on ophthalmoscopy with scleral indentation.7 Additionally, a good correlation of the activity of pars planitis evaluated by UBM as compared to that by indirect ophthalmoscopy has been demonstrated.8 Therefore, UBM can be helpful in the management of patients, grading severity and guiding treatment decisions (Figure 12.6).
Posterior uveitis
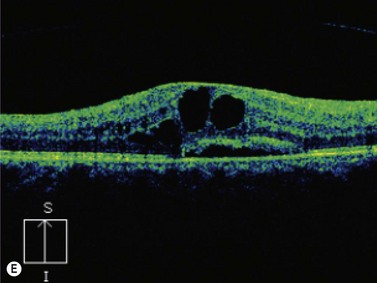
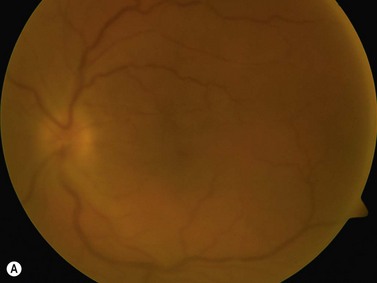
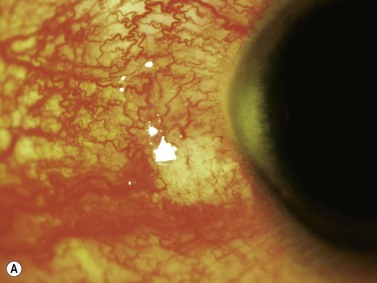
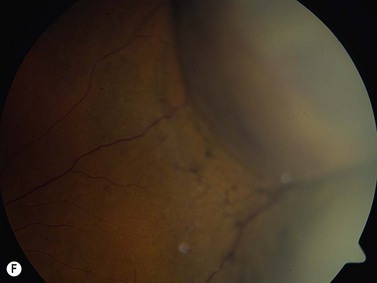
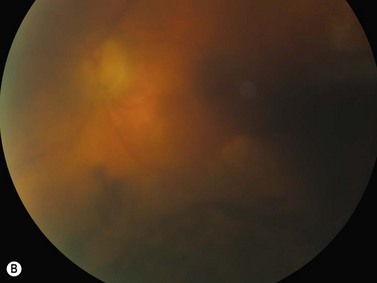
The primary site of inflammation in posterior uveitis is the choroid, the retina or both. Inflammatory cells may be seen diffusely throughout the vitreous cavity. Many posterior uveitis syndromes are the result of an underlying infection, including toxoplasmosis, syphilis, toxocariasis, tuberculosis, cytomegalovirus retinitis and ocular histoplasmosis syndrome. Non-infectious etiologies include disorders such as sarcoidosis, white dot syndromes and collagen vascular diseases (Figure 12.7).9 The signs and symptoms of posterior uveitis are variable, depending on the location and extent of inflammation.
Ultrasonography can be a very useful tool, particularly in cases in which the media is not clear, secondary to severe vitritis or vitreous hemorrhage.![]() See Clip 12.1A,B 9 Specific ultrasonographic findings may aid in the diagnosis of certain conditions. Characteristic pseudocystic degeneration of the peripheral vitreous has been described in patients with ocular toxocariasis.10 Other ultrasonographic features that can be observed in toxocariasis include mildly to moderately elevated granulomatous lesions that can be calcified, vitreous membranes extending from the granulomatous lesion to the posterior pole and posterior tractional retinal detachment or retinal fold (Figure 12.8). A retrospective study by Hercos et al reported ultrasonographic findings in patients with ocular toxoplasmosis. The most frequent features were intravitreal punctiform echoes, thickening of the posterior hyaloid, partial or total posterior vitreous detachment and focal retinochoroidal thickening (Figure 12.9).11
See Clip 12.1A,B 9 Specific ultrasonographic findings may aid in the diagnosis of certain conditions. Characteristic pseudocystic degeneration of the peripheral vitreous has been described in patients with ocular toxocariasis.10 Other ultrasonographic features that can be observed in toxocariasis include mildly to moderately elevated granulomatous lesions that can be calcified, vitreous membranes extending from the granulomatous lesion to the posterior pole and posterior tractional retinal detachment or retinal fold (Figure 12.8). A retrospective study by Hercos et al reported ultrasonographic findings in patients with ocular toxoplasmosis. The most frequent features were intravitreal punctiform echoes, thickening of the posterior hyaloid, partial or total posterior vitreous detachment and focal retinochoroidal thickening (Figure 12.9).11
Panuveitis
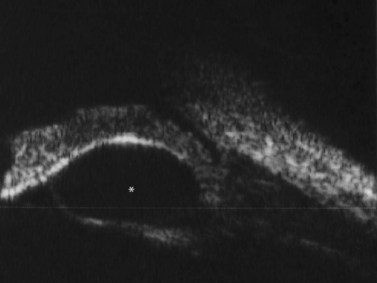
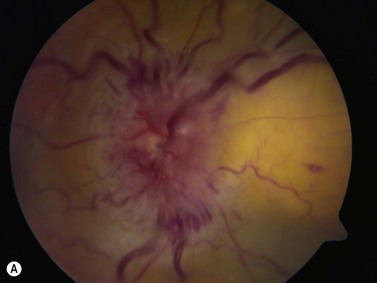
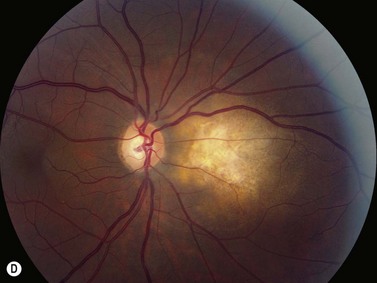
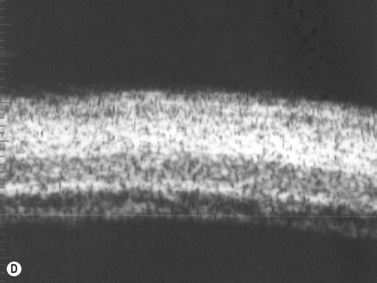
Panuveitis refers to a generalized inflammation of the whole uveal tract, with involvement of the retina and vitreous, with no single predominant site of inflammation. Among the causes of panuveitis are tuberculosis, syphilis, sarcoidosis, Vogt–Koyanagi–Harada (VKH) syndrome, sympathetic ophthalmia and Behçet’s disease.12 VKH is characterized by a spectrum of bilateral panuveitis with associated exudative retinal detachment and neurologic and dermatologic manifestations. UBM may demonstrate shallow anterior chambers during the active phase, angle closure, ciliary body thickening, supraciliary effusion, decreased internal echo reflection of the ciliary body stroma, obscured appearance of the ciliary processes and ciliochoroidal detachment in certain cases. These pathologic features have been shown to resolve in response to treatment and UBM may be used to monitor disease activity and treatment efficacy.13 Consistent ultrasonography findings in a study by Forster et al were diffuse, low to medium reflective thickening of the choroid posteriorly; serous retinal detachment, located inferiorly or in the posterior pole; mild vitreous opacities with no posterior vitreous detachment and thickening of the sclera and/or episclera posteriorly. The choroidal thickening observed in VKH is generally most pronounced in the peripapillary region (Figure 12.10). Resolution of these findings has been demonstrated after systemic corticosteroid therapy.14
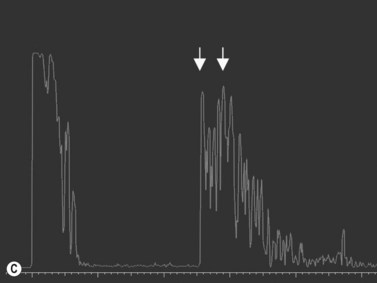
Behçet’s disease is a chronic, multisystemic inflammatory disorder of unknown etiology, characterized by relapsing occlusive vasculitis, and by a uveitis that can affect both the anterior and posterior segments of the eye, often simultaneously. Ultrasonography is used to document diffuse inflammation of the posterior fundus, sclera and adjacent orbital space. Decreased flow velocities in the ophthalmic artery and superior ophthalmic vein and increased resistance indices in the posterior ciliary artery and ophthalmic artery, measured by Doppler ultrasonography, have been associated with active ocular involvement.15
Hypotony
Chronic hypotony, defined as intraocular pressure of less than 6 mmHg, may occur as a result of chronic uveitis, and may or may not be associated with active inflammation (Figure 12.11). Other causes of hypotony include long-standing retinal detachment, ocular trauma and previous vitreous surgery. Hypotony in uveitis is multifactorial. Chronic inflammation may lead to the formation of ciliary membranes, damage to the secretory ciliary epithelium and tractional ciliary body detachment, resulting in decreased aqueous production and hypotony. Atrophy of the ciliary processes may cause permanent damage to the aqueous secretory mechanism.16
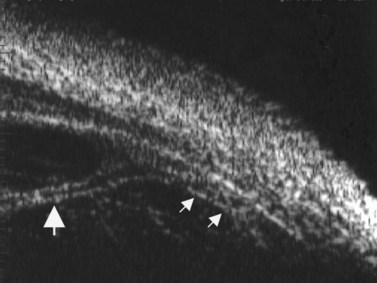
Uveitic patients with chronic hypotony and patients with opaque media, are among those that most benefited from UBM examination in a study by Tran et al, demonstrating great clinical value and improving the management in a significant manner.17 The length of ciliary processes may be measured by UBM. Patients with diffuse and recurrent uveitis have been shown to have a significant loss of the ciliary processes, particularly in the inferior quadrant. Patients who are found to have atrophic changes may need a more aggressive treatment approach for any signs of inflammation, to prevent further damage and eventual hypotony (Figure 12.12).18 Findings that have been described by UBM examination in chronic hypotony include epiciliary membranes, supraciliary effusion, ciliary traction and detachment, massive thickening of the anterior uvea and ciliary atrophy (Figure 12.13). The proliferating tissue covering or causing traction on the ciliary processes is a potentially reversible cause of chronic hypotony. The location and thickness of epiciliary membranes may guide the surgical approach. The absence of ciliary process atrophy may suggest a better surgical prognosis. In the setting of atrophy, surgery alone may not lead to a significant rise in intraocular pressure or improvement in vision.16,19–20
Scleral inflammatory disease
Episcleritis
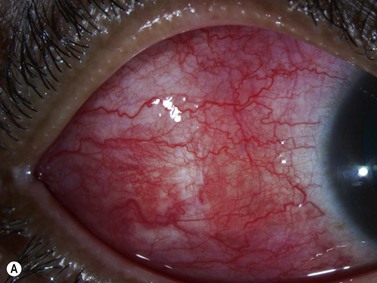
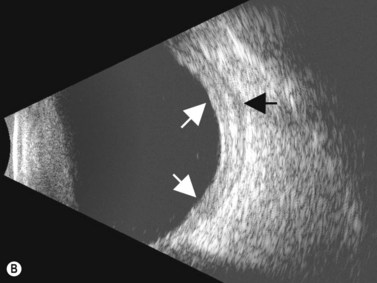
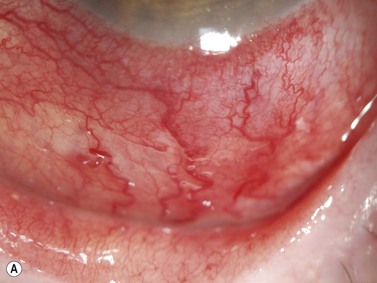
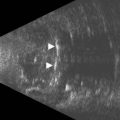
Episcleritis is an inflammatory condition that affects the episcleral tissue, which lies between the sclera and conjunctiva. Most cases are idiopathic, although up to a third may be related to an underlying systemic disorder, such as collagen vascular diseases or infectious entities. Most patients with episcleritis complain of an acute onset mild to moderate ocular discomfort, associated with a sectorial or diffuse injection. A freely mobile nodule may be noted in cases of nodular episcleritis. The diagnosis is primarily clinical, based on slit-lamp examination. When episcleritis is severe, UBM may be performed to demonstrate episcleral thickening and distinction of the low-to-medium reflective episcleral tissue from the underlying highly reflective scleral tissue (Figure 12.14).21
Scleritis
Scleritis is an inflammatory condition affecting the sclera and can occur in any age group, but typically presents between ages 30 and 50, affecting females more commonly than males. The prevalence in the general population is estimated to be 6 per 100 000 people.22
Anterior scleritis
Anterior scleritis is further subdivided into diffuse, nodular, necrotizing with inflammation and necrotizing without inflammation. Presenting symptoms of anterior scleritis typically include ocular pain poorly responsive to analgesics, associated with redness, which persists after application of topical phenylephrine. Ultrasonography in anterior scleritis can demonstrate thickening of the anterior sclera, as well as the presence of shallow ciliochoroidal detachments. UBM can be important in the evaluation and differentiation of anterior scleritis by defining the involved area and recognizing areas of scleral thinning, nodular lesions and regions of necrosis (Figure 12.15). Focal areas with decreased reflectivity and thickening can typically be detected, probably representing the perivascular or scleral infiltration and edema.23 Onset of necrosis has been characterized by the presence of low-reflectivity pockets by UBM (Figure 12.16). Necrotizing scleritis can be followed by marked tissue loss, but slit-lamp examination may be limited to determine the extent of scleral thinning. UBM examination can quantify the scleral thickness with a high degree of accuracy.23
Posterior scleritis
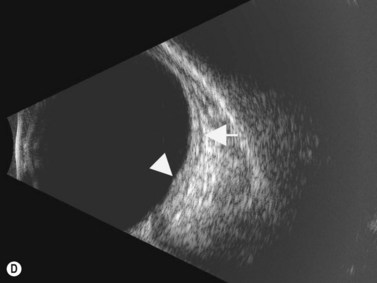
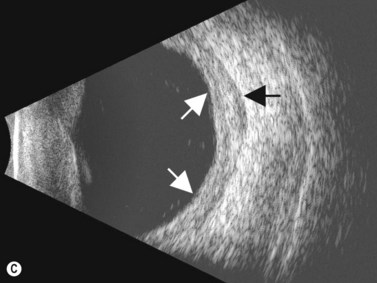
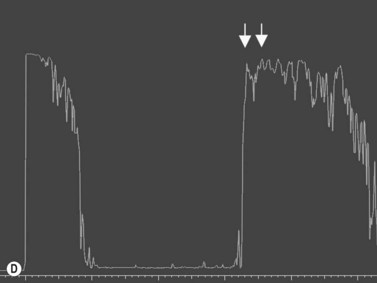
The diagnosis of posterior scleritis may be more challenging due to its non-specific clinical features. Certain patients can present with minimal clinical findings. Ocular examination may be entirely normal or may disclose the presence of exudative retinal detachment, chorioretinal lesions, optic nerve edema, subretinal mass, choroidal effusion and vasculitis. B-scan ultrasonography is an essential tool for an accurate diagnosis of posterior scleritis. The most important finding is thickening of the sclera, which can vary in degree and can be either diffuse or localized. Usually, the thickened sclera is highly reflective, with regular internal structure. Thickening of the retinochoroid layer can also be observed. There may be an associated inflammatory reaction in the episcleral region (Figure 12.17). In the peripapillary region, episcleral inflammation results in distention of sub-Tenon’s space, and may produce the echographic “T-sign” (Figure 12.18).
Nodular posterior scleritis can present as an elevated choroidal mass and mimic an intraocular tumor (Chapter 11). The thickened sclera in these nodular lesions demonstrates high reflectivity with regular internal structure on ultrasonography. Choroidal and ciliary body detachments, as well as ciliochoroidal effusion syndrome may also occur in the setting of posterior scleritis and can be confirmed by ultrasonography (Figure 12.5).24
Specific entities
Intraocular tumor masquerading as scleritis
In rare circumstances, choroidal melanoma and metastatic carcinoma can masquerade as scleritis, confounding the diagnosis. Inflammation is usually secondary to tumor necrosis. Careful evaluation by ophthalmoscopy and ultrasonography is necessary to establish the correct diagnosis (Figure 12.19).25
Endophthalmitis
Infectious endophthalmitis is a potentially devastating condition that may result as a major complication of surgery or trauma or may develop from an endogenous source elsewhere in the body. Ultrasonography can be used to determine the extent and severity of the condition. Reported findings include dense vitreous opacities and membranes, posterior vitreous detachment, hyaloid thickening, large endovitreal vacuoles, choroidal thickening, macular edema, choroidal abscess or granuloma, optic nerve head swelling, choroidal detachment, retinal traction and detachment (Figure 12.20).26–27
Postoperative non-infectious inflammation after cataract surgery
The breakdown of the blood–aqueous barrier and release of inflammatory agents, due to excessive surgical manipulation or irritation of ocular tissue by an intraocular lens (IOL) is the major cause of postoperative noninfectious inflammation in pseudophakic eyes. An IOL that erodes into tissues may lead to chronic inflammation. A case series by Ozdal et al showed misplacement of one or both haptics by UBM in 68.5% of cases of chronic postoperative inflammation. Clinically relevant lens remnants were present in 11.1% of patients. Edematous ciliary body processes and thickened ciliary bodies were observed in 20.4% of cases. Treatment with anti-inflammatory agents or surgical repositioning or removal of IOL can be considered according to UBM findings (Figure 12.21).28
Inflammatory orbital diseases
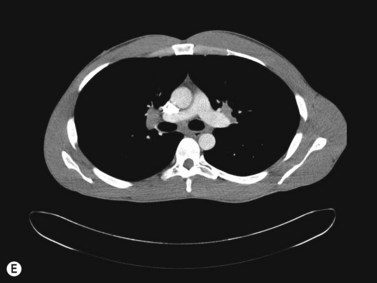
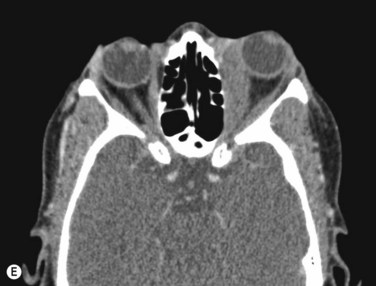
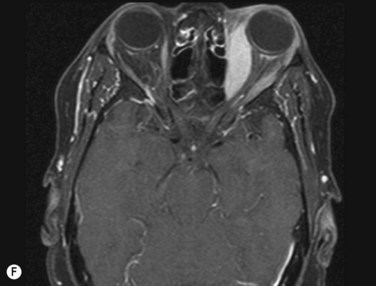
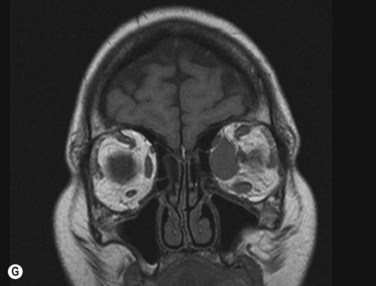
Inflammatory orbital diseases can be either infectious or non-infectious. Frequently the inflammation is idiopathic and referred to as “orbital pseudotumor” or “non-specific orbital inflammation”. Both children and adults may be affected. Symptoms depend on the involved tissue and can include proptosis, extraocular muscle restriction, conjunctival inflammation and chemosis, and soft tissue edema. Imaging studies with orbital computerized tomography (CT) scan, magnetic resonance imaging (MRI) and ultrasonography are important in the diagnosis. Pain associated with ocular rotations suggests myositis. Imaging shows enlargement of one or more extraocular muscles, involving the tendinous insertions (Figure 12.22). In contrast, Graves’ orbitopathy is characterized by enlargement of muscles but sparing of the muscle tendons. Orbital CT scan or MRI are the preferred imaging modalities for evaluation of the orbital muscles. Ultrasonographic diagnosis of Graves’ orbitopathy is based on the following features: absence of mass lesion, enlargement of orbital tissues with a heterogeneous reflectivity, thickening of the bellies of at least two extraocular muscles, and thickened periorbital tissue (Figure 12.23).29
1 Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491-500. discussion
2 Agrawal RV, Murthy S, Sangwan V, et al. Current approach in diagnosis and management of anterior uveitis. Ind J Ophthalmol. 2010;58:11-19.
3 Marigo FA, Esaki K, Finger PT, et al. Differential diagnosis of anterior segment cysts by ultrasound biomicroscopy. Ophthalmology. 1999;106:2131-2135.
4 Peizeng Y, Qianli M, Xiangkun H, et al. Longitudinal study of anterior segment inflammation by ultrasound biomicroscopy in patients with acute anterior uveitis. Acta Ophthalmol. 2009;87:211-215.
5 Babu BM, Rathinam SR. Intermediate uveitis. Ind J Ophthalmol. 2010;58:21-27.
6 Wei W, Yang W, Zhang H, et al. [Ultrasound biomicroscopy in intermediate uveitis]. Zhonghua Yan Ke Za Zhi. 2002;38:207-209.
7 Haring G, Nolle B, Wiechens B. Ultrasound biomicroscopic imaging in intermediate uveitis. Br J Ophthalmol. 1998;82:625-629.
8 Greiner KH, Kilmartin DJ, Forrester JV, et al. Grading of pars planitis by ultrasound biomicroscopy–echographic and clinical study. Eur J Ultrasound. 2002;15:139-144.
9 Sudharshan S, Ganesh SK, Biswas J. Current approach in the diagnosis and management of posterior uveitis. Indian J Ophthalmol. 2010;58:29-43.
10 Tran VT, Lumbroso L, LeHoang P, et al. Ultrasound biomicroscopy in peripheral retinovitreal toxocariasis. Am J Ophthalmol. 1999;127:607-609.
11 Hercos BV, Muinos SJ, Casaroli-Marano RP. [Utility of ultrasonography in toxoplasmic uveitis]. Arch Soc Esp Oftalmol. 2004;79:59-65.
12 Bansal R, Gupta V, Gupta A. Current approach in the diagnosis and management of panuveitis. Ind J Ophthalmol. 2010;58:45-54.
13 Wada S, Kohno T, Yanagihara N, et al. Ultrasound biomicroscopic study of ciliary body changes in the post-treatment phase of Vogt–Koyanagi–Harada disease. Br J Ophthalmol. 2002;86:1374-1379.
14 Forster DJ, Cano MR, Green RL, et al. Echographic features of the Vogt-Koyanagi-Harada syndrome. Arch Ophthalmol. 1990;108:1421-1426.
15 Yanik B, Conkbayir I, Berker N, et al. Doppler ultrasonography findings in ocular Behcet’s disease. Clin Imaging. 2006;30:303-308.
16 Gupta P, Gupta A, Gupta V, et al. Successful outcome of pars plana vitreous surgery in chronic hypotony due to uveitis. Retina. 2009;29:638-643.
17 Tran VT, LeHoang P, Herbort CP. Value of high-frequency ultrasound biomicroscopy in uveitis. Eye (Lond). 2001;15:23-30.
18 da Costa DS, Lowder C, de Moraes HVJr, et al. [The relationship between the length of ciliary processes as measured by ultrasound biomicroscopy and the duration, localization and severity of uveitis]. Arq Bras Oftalmol. 2006;69:383-388.
19 de Smet MD, Gunning F, Feenstra R. The surgical management of chronic hypotony due to uveitis. Eye (Lond). 2005;19:60-64.
20 Roters S, Szurman P, Engels BF, et al. Ultrasound biomicroscopy in chronic ocular hypotony: its impact on diagnosis and management. Retina. 2002;22:581-588.
21 Pavlin CJ, Easterbrook M, Hurwitz JJ, et al. Ultrasound biomicroscopy in the assessment of anterior scleral disease. Am J Ophthalmol. 1993;116:628-635.
22 Galor A, Thorne JE. Scleritis and peripheral ulcerative keratitis. Rheum Dis Clin North Am. 2007;33:835-854. vii
23 Heiligenhaus A, Schilling M, Lung E, et al. Ultrasound biomicroscopy in scleritis. Ophthalmology. 1998;105:527-534.
24 Ikeda N, Ikeda T, Nomura C, et al. Ciliochoroidal effusion syndrome associated with posterior scleritis. Jpn J Ophthalmol. 2007;51:49-52.
25 Yap EY, Robertson DM, Buettner H. Scleritis as an initial manifestation of choroidal malignant melanoma. Ophthalmology. 1992;99:1693-1697.
26 Maneschg O, Csakany B, Nemeth J. [Ultrasonographic findings in endophthalmitis following cataract surgery : a review of 81 cases]. Ophthalmologe. 2009;106:1012-1015.
27 Marchini G, Pagliarusco A, Tosi R, et al. Ultrasonographic findings in endophthalmitis. Acta Ophthalmol Scand. 1995;73:446-449.
28 Ozdal PC, Mansour M, Deschenes J. Ultrasound biomicroscopy of pseudophakic eyes with chronic postoperative inflammation. J Cataract Refract Surg. 2003;29:1185-1191.
29 Kirsch E, Hammer B, von Arx G. Graves’ orbitopathy: current imaging procedures. Swiss Med Wkly. 2009;139:618-623.