Chapter 40 Occupational Asthma
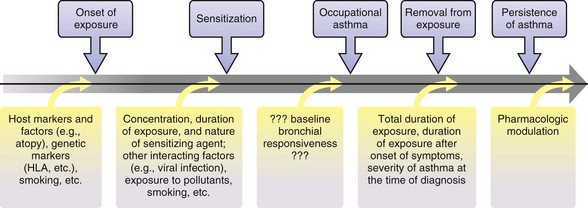
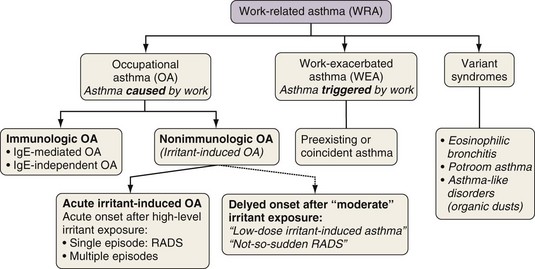
The workplace environment can lead to the development of different types of work-related asthma (Figure 40-1), including occupational asthma (OA) (i.e., asthma caused by work) and work-exacerbated asthma (i.e., preexisting or coincident asthma exacerbated by nonspecific stimuli at work). OA is defined as a disease characterized by variable airflow limitation and/or bronchial hyperresponsiveness and/or airway inflammation secondary to factors and conditions attributable to a particular working environment and not to stimuli encountered outside the workplace. OA may result either from immunologically mediated sensitization to occupational agents (i.e., “allergic” OA, or “OA with a latency period”) or from exposure(s) to high concentrations of irritant compounds (i.e., irritant-induced asthma [IrIA], best typified by the reactive airways dysfunction syndrome [RADS]).
In recent years, a growing interest in occupational asthma (OA) has emerged, for several reasons:
• The frequency of asthma has increased progressively during the past 2 decades, with a recent plateau, and occupational exposure may be a contributing factor.
• Epidemiologic data indicate that approximately 18% of cases of asthma in adults are attributable to workplace exposures.
• The number of agents that can cause OA is steadily increasing (www.asthme.csst.qc.ca).
• OA, together with diseases related to exposure to asbestos dust, has become the most prevalent occupational lung disease in many developed countries, resulting in an increased burden to society.
• OA is an excellent model to study the epidemiology, pathophysiology, genetics, and other aspects of asthma in humans.
Epidemiology
Causal Agents
A very large number of substances (more than 400) used at work can cause the development of immunologically mediated OA. The most common causal agents and occupations are listed in Table 40-1. A few agents—specifically, flour, diisocyanates, latex, persulfate salts, aldehydes, animals, wood dusts, metals, and enzymes—account for 50% to 90% of OA cases. Nevertheless, the distribution of causal agents may vary widely across geographic areas, depending on the pattern of industrial activities. The highest rates of OA occur in bakers and pastry makers, other food processors, spray painters, hairdressers, wood workers, health care workers, cleaners, farmers, laboratory technicians, and welders.
Table 40-1 Principal Agents Causing Immunologic Occupational Asthma
| Agent | Occupation/Industry | |
|---|---|---|
| High-Molecular-Weight Agents | ||
| Cereals, flour | Wheat, rye, barley, buckwheat | Flour milling, bakers, pastry makers |
| Latex | Health care workers, laboratory technicians | |
| Animals (food animals, other) | Mice, rats, cows, seafood | Laboratory workers, farmers, seafood processors |
| Enzymes | α-Amylase, maxatase, alcalase, papain, bromelain, pancreatin | Baking products manufacture, bakers, detergent production, pharmaceutical industry, food industry |
| Low-Molecular-Weight Agents | ||
| Diisocyanates | Toluene diisocyanate (TDI), methylene diphenyl-diisocyanate (MDI), hexamethylene diisocyanate (HDI) | Polyurethane production, plastic industry, molding, spray painters |
| Metals | Chromium, nickel, cobalt, platinum | Metal refinery, metal alloy production, electroplating, welding |
| Biocides | Aldehydes, quaternary ammonium compounds | Health care workers, cleaners |
| Persulfate salts | Hairdressers | |
| Acid anhydrides | Phthalic, trimellitic, maleic, tetrachlorophthalic acids | Epoxy resin workers |
| Reactive dyes | Reactive black 5, pyrazolone derivatives, vinyl sulfones, carmine | Textile workers, printers, food industry workers |
| Woods | Red cedar, iroko, obeche, oak, others | Sawmill workers, carpenters, cabinet and furniture makers |
Prevalence and Incidence
Cross-sectional surveys of workforces exposed to sensitizing agents found highly variable prevalence rates of OA. In general, the prevalence of OA caused by HMW agents is less than 5%, and that for LMW agents ranges from 5% to 10%. Cohort studies reported incidence rates of 2.7 to 3.5 cases of OA per 100 person-years among workers exposed to laboratory animals, 4.1 per 100 person-years among those exposed to wheat flour, and 1.8 per 100 person-years among dental health apprentices exposed to natural rubber latex. Estimates of the incidence of OA in the general population provided by voluntary notification schemes, medicolegal statistics, and population-based surveys are summarized in Table 40-2. Acute IrIA accounts for about 10% of all reported cases of OA.
Table 40-2 Estimates of Incidence of Occupational Asthma (OA)
| Country | Period | Incidence of OA (Cases per 106 Workers) |
|---|---|---|
| Physician-Based Notification Schemes | ||
| United Kingdom (SWORD) | 1989-1992 | 22 |
| 1992-1993 | 37 | |
| 1992-1097 | 38 (34-41)* | |
| 1992-2001 | 87 | |
| West Midlands (SHIELD) | 1991-2005 | 42 (37-45)* |
| United States (SENSOR) | ||
| Michigan | 1988-1994 | 29 |
| 1995 | 27 (58-204) | |
| California | 1993-1996 | 25 (23-27)* |
| Canada | ||
| British Columbia | 1991 | 92 |
| Quebec (PROPULSE) | 1992-1993 | 42-79 |
| France (ONAP) | 1996-1999 | 24 (22-25)* |
| Italy (PRIOR) | 1996-1997 | 24 (18-30)* |
| South Africa (SORDSA) | 1997-1999 | 18 |
| Australia (SABRE) | 1997-2001 | 31 (27-36)* |
| Spain | ||
| Catalonia | 2002 | 77 (66-90)* |
| Belgium (WAB) | 2000-2002 | 24 (19-29)* |
| Medicolegal Statistics | ||
| Finland | 1976 | 36 |
| 1989-1995 | 174 | |
| Canada | ||
| Quebec | 1986-1988 | 25 |
| 1989-1999 | 13-24 | |
| Sweden | 1990-1992 | 80 (70-90)* |
| Germany | 1995 | 51 |
| Belgium | 1993-2002 | 29 (28-31)* |
| Population-Based Surveys | ||
| Finland | 1986-1998 | Men: 478† Women: 419† |
| ECRHS | 1990-1995 1998-2003 |
250-300 |
ECRHS, European Community Respiratory Health Survey; ONAP, Observatoire National des Asthmes Professionnels; PROPULSE, Projet Pulmonaire Sentinelle; SABRE, Surveillance of Australian Workplace-Based Respiratory Events; SENSOR, Sentinel Event Notification System for Occupational Risks; SHIELD, Midland Thoracic Society Rare Respiratory Disease Registry Surveillance Scheme of Occupational Asthma; SORDSA, Surveillance of Work-Related and Occupational Respiratory Diseases in South Africa; SWORD, Surveillance of Work-Related and Occupational Respiratory Diseases; WAB, Work-Related Asthma in Belgium.
† Estimated from the work-attributable fraction of asthma derived through linkage of two national registries—the Medication Reimbursement of the Social Insurance Institution for Asthma and the Finnish Register of Occupational Diseases for Occupational Asthma.
Risk Factors
OA results from complex interactions between environmental factors and individual susceptibility. The environmental and individual risk factors are summarized in Table 40-3, together with the level of evidence supporting their role. The intensity of exposure to sensitizing agents currently is the best-identified and the most important environmental risk factor for the development of OA. Characterization of the relationship between the level of exposure to occupational agents and the development of IgE sensitization and OA has been greatly enhanced by the use of personal sampling techniques, direct analytic methods for chemicals, and immunoassay techniques for the quantification of airborne protein allergens. Exposure-response relationships may be affected by the nature of the sensitizing agent, individual susceptibility, and timing of exposure. Some agents seem to be more potent than others in inducing sensitization; the dose-response relationship for IgE sensitization is steeper for the bakery enzyme alpha-amylase than for wheat allergens. Some evidence indicates that the exposure-response relationships are not linear for certain occupational agents (e.g., laboratory animals, wheat flour), thereby suggesting an unexplained protective effect of high-level exposures. The role of genetic susceptibility markers, such as certain human leukocyte antigen (HLA) class II alleles, may become more apparent at low levels of exposure to occupational agents. The timing of exposure also may play a role, because the frequency of onset of work-related asthma symptoms is consistently higher within the first 1 to 4 years of exposure to HMW agents, and exposure-response gradients are more clearly documented in this early period of exposure.
Table 40-3 Summary of Potential Risk Factors for Development of Occupational Asthma (OA)
| Risk Factor | Strength of Evidence | Agents/Settings |
|---|---|---|
| Environmental Risk Factors | ||
| High level of exposure | +++ | HMW agents: Wheat flour, α-amylase, laboratory animals, detergent enzymes, snow crab allergens LMW agents: Platinum salts, acid anhydrides |
| ++ | Diisocyanates | |
| Skin exposure | + | Diisocyanates |
| Cigarette smoking | ++ | IgE sensitization: Laboratory animals, snow crab, shrimp, salmon, psyllium, green coffee, enzymes, acid anhydrides, platinum, reactive dyes |
| + | Clinical OA: Laboratory animals, enzymes | |
| Individual Risk Factors | ||
| Atopy | +++ | HMW agents: Flour, laboratory animals, snow crab, psyllium, detergent enzymes, α-amylase |
| + | LMW agents: Platinum, acid anhydrides | |
| Genetic Markers | ||
| HLA class II alleles | ++ | LMW agents: Diisocyanates, red cedar, acid anhydrides, platinum salts HMW agents: Laboratory animals, latex |
| Antioxidant enzyme* variants | ++ | Diisocyanates |
| TLR-4/8551 G variant | + | Laboratory animals |
| IL-4RA (I50V) II variant | + | Diisocyanates |
| Preexisting nonspecific bronchial hyperresponsiveness | + | Apprentices exposed to HMW agents (laboratory animals, flour, latex) |
| Preexisting rhinitis | + | IgE sensitization to HMW agents (laboratory animals, flour, latex) |
| Work-related rhinitis | +++ | Nonoccupational asthma in the general population and OA in cohorts of workers exposed to laboratory animals |
| Gender—female | + | Snow crab processors |
HLA, human leukocyte antigen; HMW, high-molecular-weight; IgE, immunoglobulin E; IL-4RA, interleukin-4 receptor alpha chain; LMW, low-molecular-weight; TLR-4, Toll-like receptor-4.
* Glutathione S-transferase (GSTM) and N-acetyltransferase (NAT).
Pathophysiology
Immunologic, Non–immunoglobulin E–mediated Occupational Asthma
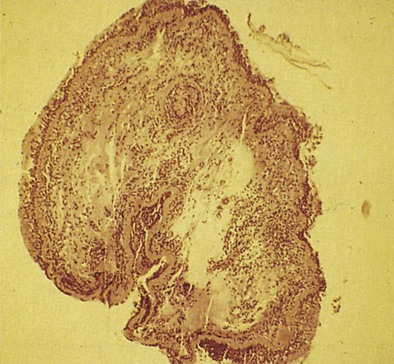
Early asthmatic reactions induced by occupational allergens probably are associated with smooth muscle contraction and edema induced by inflammatory mediators such as histamine and leukotrienes but not cellular infiltration. Late asthmatic reactions are associated with influx of inflammatory cells. Although both asthma and OA have been identified as diseases in which eosinophilic inflammation plays a key role, the role of neutrophils has recently been examined. Induced sputum examination is a noninvasive means to assess cell profiles and currently is more often used as an interesting investigative tool. Eosinophilic and neutrophilic variants of OA have been found in the case of OA due to LMW agents, especially diisocyanates. Some LMW agents have pharmacologic properties that cause bronchoconstriction. For example, diisocyanates may block the β2-adrenergic receptor. Diisocyanates and other occupational agents also may stimulate sensory nerves to release substance P and other peptides that have been shown to inhibit neutral endopeptidases necessary for the inactivation of neuropeptides. Neuropeptides affect many cells in the airways and may participate in airway inflammation by causing smooth muscle contraction, mucus production, and recruitment and activation of inflammatory cells. Thus, LMW occupational agents such as diisocyanates may have a variety of proinflammatory effects and induce asthma through more than one mechanism. An autopsy study of the lung of a person with isocyanate-induced asthma who died after reexposure showed denudation of airway epithelium, subepithelial fibrosis, infiltration of the lamina propria by leukocytes (mainly eosinophils), and diffuse mucous plugging of the bronchioles, similar to findings in persons who died from non-OA. Bronchial biopsy specimens of 18 patients with proven OA also have shown extensive epithelial desquamation, ciliary abnormalities of the epithelial cells, smooth muscle hyperplasia, and subepithelial fibrosis (Figure 40-2). The total cell count, eosinophils, and lymphocytes were increased compared with those in healthy control subjects.
Nonimmunologic Occupational Asthma
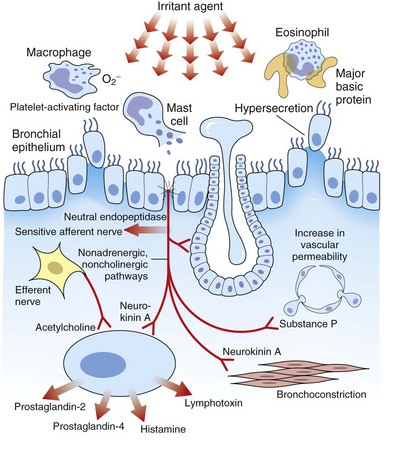
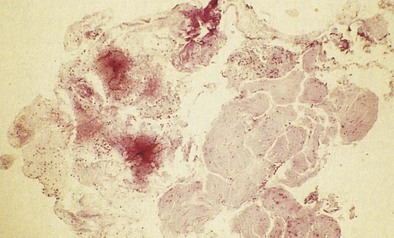
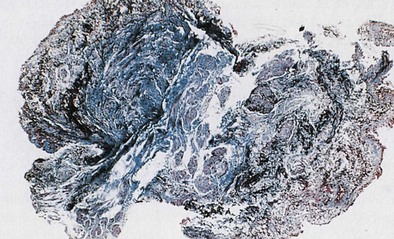
OA resulting from nonimmunologic mechanisms is characterized by the absence of latency. The underlying mechanism of IrIA is not known. It has been postulated that the extensive denudation of the epithelium in these conditions leads to airway inflammation and airway hyperresponsiveness by several mechanisms, including loss of the epithelial cell–derived relaxing factors, exposure of the nerve endings leading to neurogenic inflammation, and nonspecific activation of mast cells with release of inflammatory mediators and cytokines (Figure 40-3). Secretion of growth factors for epithelial cells, smooth muscle, and fibroblasts may lead to airway remodeling. Sequential changes in the airways of a patient with IrIA have been described. In the acute phase of IrIA, rapid denudation of the mucosa with fibrinohemorrhagic exudate in the submucosa is followed by regeneration of the epithelium with proliferation of basal and parabasal cells and subepithelial edema (Figure 40-4). In the chronic phase of IrIA, marked thickening of the airway wall is seen (Figure 40-5). In a study of IrIA caused by multiple exposures to an irritant, inflammatory infiltrate with eosinophils and lymphocytes and diffuse deposition with collagen fibers were found. Similar sequential changes have been reproduced in animal models of IrIA.
Diagnosis
An algorithm for the clinical investigation of OA is shown in Figure 40-6. The advantages and pitfalls of the various tools in confirming the diagnosis of OA are listed in Table 40-4. The presence of sensitization to occupational agents can be detected either by skin testing or by radioallergosorbent test (RAST) or enzyme immunosorbent assay (ELISA) techniques. In patients with a compatible clinical history of OA and bronchial hyperresponsiveness, a positive result on a skin test or RAST probably has a diagnostic accuracy close to 80% in the case of HMW agents. Unfortunately, very few standardized testing materials are commercially available for skin tests or for RASTs in OA, and for most LMW agents, an IgE-mediated mechanism has not been confirmed
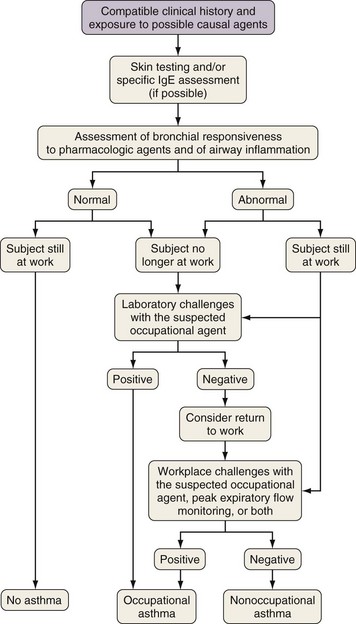
Figure 40-6 Clinical investigation of occupational asthma. IgE, immunoglobulin E.
(From Chan-Yeung M, Malo JL: Occupational asthma, N Engl J Med 333:107–112, 1995.)
Table 40-4 Advantages and Disadvantages of Diagnostic Methods in Occupational Asthma
| Method | Advantages | Disadvantages |
|---|---|---|
| Questionnaire | Simple, sensitive | Low specificity |
| Immunologic testing | Simple, sensitive | Only for agents of high molecular weight and for some of low molecular weight; identifies sensitization, not disease; no “standardized” or commercially available agents |
| Bronchial responsiveness to methacholine/histamine | Simple, sensitive | Not specific for asthma or occupational asthma; occupational asthma not ruled out by a negative test result if subject is no longer exposed |
| Measurement of forced expiratory volume in 1 second (FEV1) before and after a work shift | Simple, inexpensive | Low sensitivity and specificity |
| Assessment of airway inflammation (induced sputum, exhaled nitric oxide [NO]) | Addresses physiopathology of asthma; identifies eosinophilic bronchitis | Not specific for occupational asthma; limited to specialized centers |
| Peak expiratory flow monitoring | Relatively simple, inexpensive | Requires patient’s cooperation and honesty; not as sensitive as FEV1 or a computerized method to assess airway caliber to interpret changes |
| Specific inhalation challenges in a hospital laboratory | Positive result is confirmatory | Diagnosis not ruled out by a negative result on confirmatory testing (e.g., with use of wrong agent or with cessation of work exposure); expensive; few referral centers |
| Serial FEV1 measurement at work under supervision | Negative result rules out diagnosis when patient tested under usual work conditions | A positive result may be obtained in conditions of irritation; requires collaboration of employer |
From Chan-Yeung M, Malo JL: Occupational asthma, N Engl J Med 333:107–112, 1995.
For HMW agents, skin tests to detect immediate reactivity and measurements of specific IgE antibodies are important tools. The absence of nonspecific bronchial hyperresponsiveness in a subject at the end of 2 weeks of working under the usual conditions virtually excludes the diagnosis of asthma and OA. If nonspecific bronchial hyperresponsiveness is present, further testing is required. Spirometric measurements obtained before and after a work shift have not been found to be sensitive or specific. Two options (controlled exposure and PEF) can be considered for objective confirmation, depending on availability (see Figure 40-6). Exposure to the suspected agent under control conditions in a hospital laboratory can be done as originally described by Pepys and Hutchcroft in 1975. Attempts have been made to improve specific challenge tests by exposing subjects in the laboratory to low and stable levels of dry or wet aerosols and vapors to avoid nonspecific reactions. However, these tests can give false-negative results if an incorrect agent is used for testing or if the subject has been away from work for too long, although such occurrences are rare. In the latter instance, the subject should be instructed to return to the workplace, if feasible, and specific laboratory or worksite challenges should be repeated at a later time.
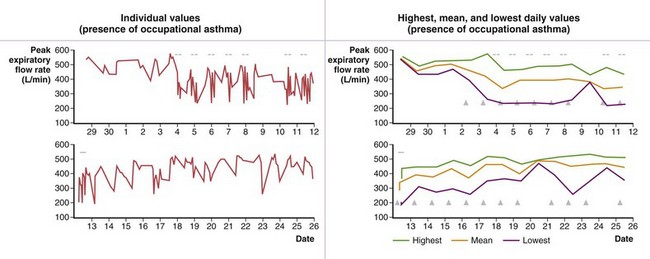
Burge and co-workers were the first to propose the use of serial measurement of peak expiratory flow (PEF) by use of portable devices in the diagnosis of OA. An example of serial PEF recording is shown in Figure 40-7. Although relatively good correlation has been found between the results of serial PEF monitoring and OA as confirmed by specific inhalation challenges in the laboratory, several limitations and pitfalls in PEF monitoring are recognized (see Table 40-4). When PEF monitoring is suggestive of OA and specific inhalation challenges in the laboratory are not possible or yield negative results, it is advisable to confirm OA by sending a technician to the workplace to record serial spirometric variables throughout a work shift. The use of computerized peak flowmeters is very helpful in overcoming some of the problems of PEF monitoring. Computerized programs to assess changes in PEF are currently available (e.g., OASYS [Occupational Asthma Expert System]). Combining PEF monitoring with serial assessments of nonallergic bronchial responsiveness can provide further objective evidence, although this approach does not add to the sensitivity and specificity of PEF monitoring alone. Finally, assessment of airway inflammation (percent eosinophils) in induced sputum has recently been found to be sensitive and specific in the diagnosis of OA. Such measurements improve the sensitivity and specificity of PEF monitoring. Assessing airway inflammation at work and away from work also can be done by assessing exhaled NO, but the reliability of this technique has not been demonstrated in the investigation of OA.
Clinical Course
Subjects with OA will deteriorate if they continue in the same job without protection. Fatalities in workers who continue to be exposed have been reported. A scheme of the progressive natural history of OA is shown in Figure 40-8. Most patients with OA improve but do not recover completely even several years after removal from exposure. Even those subjects apparently cured as indicated by clinical and functional assessments continue to show airway inflammation and remodeling on subsequently obtained bronchial biopsy specimens. Follow-up studies of various types of OA have shown that persons who became asymptomatic after leaving exposure had better lung function and a lower degree of nonallergic bronchial hyperresponsiveness at the time of diagnosis and a shorter duration of exposure after the onset of symptoms. These findings suggest that they were diagnosed at an earlier stage of the disease. Early diagnosis and removal from exposure are essential in ensuring recovery. Although symptoms subside and lung function improves within 1 year of leaving the job involving exposure, decrease in nonallergic bronchial hyperresponsiveness depends on the length of the interval from cessation of exposure. Specific IgE antibodies decrease even more slowly, with no plateau after 5 years as shown in persons with snow crab–induced asthma. It has been recommended that assessment of permanent respiratory impairment or disability take place after at least 2 years of cessation of exposure. The rate of decline in lung function of patients with OA with continuous exposure is higher than in patients without asthma. Moreover, specific bronchial reactivity to the offending occupational agents often persists after the person has left exposure for 2 or more years. Thus, it is not advisable for these patients to return to the same job after they became asymptomatic. Finally, although fewer follow-up studies are available, the outcome with IrIA seems similar to that with immunologic OA.
Prevention
Another approach is to identify susceptible persons at the time of preemployment examination and exclude them from employment or from high-risk jobs. Unfortunately, the currently identified markers of individual susceptibility (see Table 40-3) have a low positive predictive value for the development of OA. The high prevalence of these markers among the general population, compared with the relatively low risk of occupational sensitization, precludes such screening from being an effective strategy and would unduly exclude many workers in whom OA would never develop. Accordingly, primary prevention should be directed toward reducing exposure to low-enough levels to prevent the onset of asthma in all workers, irrespective of their individual susceptibility. Nevertheless, physicians caring for adolescents with asthma and allergic diseases may offer useful advice regarding careers in which their underlying atopic status increases the risks for work-related sensitization to HMW agents.
Becklake MR, Chan-Yeung M, Malo JL. Epidemiological approaches in occupational asthma. In: Bernstein IL, Chan-Yeung M, Malo JL, Bernstein DI. Asthma in the workplace. ed 3. New York: Taylor & Francis; 2006:37–85.
Dykewicz MS. Occupational asthma: current concepts in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2009;123:519–528.
Gautrin D, Bernstein IL, Brooks SM, Henneberger PK. Reactive airways dysfunction syndrome and irritant-induced asthma. In: Bernstein IL, Chan-Yeung M, Malo JL, Bernstein DI. Asthma in the workplace. ed 3. New York: Taylor & Francis; 2006:579–627.
Malo JL, Chan-Yeung M. Agents causing occupational asthma. J Allergy Clin Immunol. 2009;123:545–550.
Malo JL, Chan-Yeung M. Occupational asthma. J Allergy Clin Immunol. 2001;108:317–328.
Mapp CE, Boschetto P, Maestrelli P, Fabbri LM. Occupational asthma. Am J Respir Crit Care Med. 2005;172:280–305.
Newman-Taylor AJ, Yucesov B. Genetics and occupational asthma. In: Bernstein IL, Chan-Yeung M, Malo JL, Bernstein DI. Asthma in the workplace. ed 3. New York: Taylor & Francis; 2006:87–108.
Nicholson PJ, Cullinan P, Taylor AJ, et al. Evidence based guidelines for the prevention, identification, and management of occupational asthma. Occup Environ Med. 2005;62:290–299.
Tarlo SM, Balmes J, Balkissoon R, et al. Diagnosis and management of work-related asthma: American College Of Chest Physicians Consensus Statement. Chest. 2008;134:1S–41S.
Vandenplas O, Cartier A, Malo JL. Occupational challenge tests. In: Bernstein IL, Chan-Yeung M, Malo JL, Bernstein D. Asthma in the workplace. ed 3. New York: Taylor & Francis; 2006:227–252.
Vandenplas O, Malo JL. Definitions and types of work-related asthma: a nosological approach. Eur Respir J. 2003;21:706–712.
Vandenplas O, Toren K, Blanc PD. Health and socioeconomic impact of work-related asthma. Eur Respir J. 2003;22:689–697.

 C
C O), carbonyl (C
O), carbonyl (C O), and amine (NH2), particularly when two or more groups are present within the same molecule.
O), and amine (NH2), particularly when two or more groups are present within the same molecule.