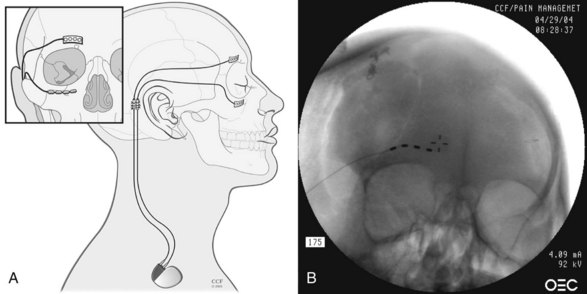
Chapter 19 Occipital Neurostimulation
 Stimulation of the occipital nerve, which originates from the C2 nerve root, is a valuable tool in treating cervicogenic headache, occipital neuritis, chronic migraine, and chronic cluster headache.
Stimulation of the occipital nerve, which originates from the C2 nerve root, is a valuable tool in treating cervicogenic headache, occipital neuritis, chronic migraine, and chronic cluster headache. Current prospective randomized studies are pending to access the evidence-based impact of this therapy on migraine.
Current prospective randomized studies are pending to access the evidence-based impact of this therapy on migraine.Indications
ONS has been used successfully in the treatment of occipital neuralgia1–4 and many primary headache disorders such as migraine,5 transformed migraine,4 cluster headache,5–9 and hemicrania continua.6,10 Few reports also demonstrated its efficacy in secondary headache disorders, including cervicogenic headache,11 C2-mediated headaches,12 posttraumatic,13 and postsurgical headaches.14
Mechanism of Action
The most accepted mechanism of action is that stimulation of the distal branches of C2 and C3, being the peripheral anatomical and functional extension of the trigeminocervical complex, may inhibit central nociceptive impulses.15 Positron emission tomography (PET) scan studies showed increased regional cerebral blood flow in areas involved in central neuromodulation in chronic migraine patients with occipital nerve electrical stimulation.16 Additional functional imaging may further define the exact mechanism of action as these studies become more widely available in multicenter studies.
Efficacy and Safety
Recently the preliminary results of a multicenter prospective randomized single-blind controlled feasibility study that was conducted to examine the safety and efficacy of ONS for treatment of intractable chronic migraine were reported.17 Patients who responded favorably to occipital nerve block (ONB) were randomized 2 : 1 : 1 to adjustable stimulation (AS), preset stimulation (PS), or medical management (MM). Those who did not respond to ONB formed an ancillary group (AG).
Reduction in overall pain intensity was 1.5 (AS), 0.5 (PS) (p = 0.076), 0.6 (MM) (p = 0.092), and 1.9 (AG) (p = 0.503). Responder rate was 39% (AS), 6% (PS) (p = 0.032), 0% (MM) (p = 0.003), and 40% (AG) (p = 1.000). The authors concluded that ONS may be a promising treatment for intractable chronic migraine and ONB may not be predictive of response to ONS.17
Anatomy
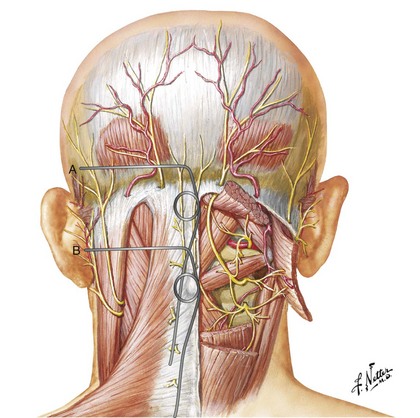
The GON arises from the C2 dorsal ramus and curves around the inferior border of the inferior oblique muscle (IOM) to ascend on its superficial surface between the IOM and the semispinalis capitis at the C1 level. Then it penetrates the semispinalis capitis and invariably the splenius muscle to end subcutaneously near the nuchal line by penetrating the trapezius muscle or its apponeurosis.18–20 There is considerable anatomical variation in the course of the GON. Bovim and colleagues18 found that the GON pierces the trapezius in nearly half the subjects; however, others have described a much lower likelihood of penetration of the trapezius muscle. The GON was invested in the aponeurosis of the trapezius at its insertion.19,20
The GON usually penetrates the semispinalis capitis muscle fibers at a distance between 2 and 5 cm caudad to the occipital protuberance.18 More cranially it may also penetrate the trapezius muscle fibers or aponeurosis, becoming superficial between 5 and 18 mm below the intermastoid line.19
Technique for Occipital Neurostimulation
The technique was originally reported by Weiner and Reed in 1999.1 Earlier reports involve placement of the leads subcutaneously at the C1 level. The stimulator lead can be directed medially from a lateral entry point medial and inferior to the mastoid process1,4,9,12,21,22 or laterally from a midline entry point.2,7,11,13,23,24
Level and Depth of Lead Placement
The level and depth of lead placement are crucial for a successful ONS trial. Placing the leads too superficially risks failure of nerve stimulation and lead erosion through the skin or patients experiencing unpleasant burning sensations. On the other hand, leads placed too deep risk stimulating posterior neck muscles and causing unpleasant muscle spasms.25
Positioning the stimulator lead subcutaneously at the C1 level places it at a significant distance from the nerve, with the posterior neck muscles (mainly trapezius and semispinalis capitis) intervening. Thus, to stimulate the GON itself, the intervening muscles are likely to be recruited as well (Fig. 19-1).
Lead placement adjacent to the nuchal line would be less prone to muscle stimulation because the GON is superficial at this level.25
Lead Type
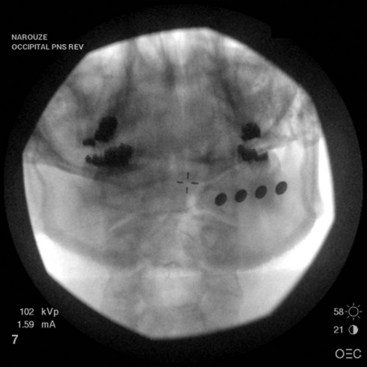
Original reports of the procedure described using quadripolar leads, although recent technical and practice trends favor the use of octipolar leads. There are no comparative studies of quadripolar vs. octipolar lead use in ONS. However, the added electrode contacts in the octipolar leads allow for exponentially more stimulation configuration arrays.25 Because the lesser occipital nerve runs laterally to the GON at the level of the nuchal line, longer octipolar leads also capture lesser occipital nerve branches, which leads to better coverage (Fig. 19-2). Paddle-type leads deliver electric current in one direction only, whereas cylindrical percutaneous leads deliver current circumferentially. Some clinicians prefer the paddle-type leads in redo cases secondary to percutaneous lead migrations because the paddle leads can be easily sutured into the surrounding fascia. The downside of the paddle is that the larger profile may lead to discomfort or erosion. The implanting doctor must weigh the benefits and the risks (Fig. 19-3).
Ultrasound-Guided Occipital Neurostimulation Placement
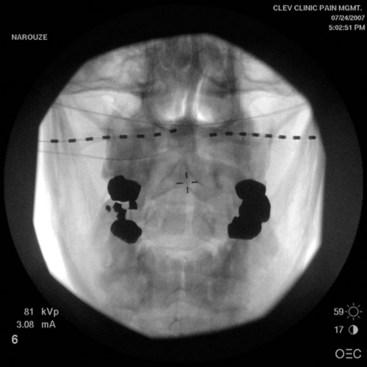
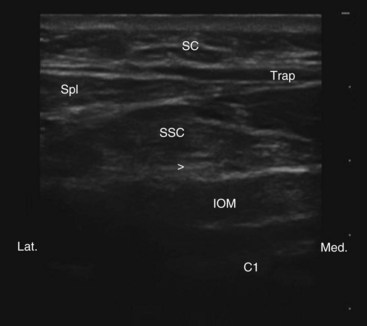
Traditionally the lead is placed with fluoroscopy; if it is too superficial, one may experience unpleasant dysthesias in the overlying skin area, and if placed deep it may invariably penetrate the occipital muscles, which usually leads to painful muscle spasms on stimulation. Since ultrasound is a great tool in visualizing soft tissue structures, with ultrasound-guided technique the lead can be placed subcutaneously near the nuchal line where the GON is superficial without intervening muscle; or the GON can be recognized, and the lead can be placed intentionally between the inferior oblique and semispinalis muscle (where the nerve runs) at the C1-C2 level (Fig. 19-4). In the latter case the GON can be stimulated with minimal settings; this can save the life of the battery. We refer to this latter approach as occipital PNS.26
Nerve Stimulation–Guided Occipital Neurostimulation Placement
Recently the possibility of using sensory nerve stimulation as a tool to determine depth of lead placement has been recommended and investigated. In 20 patients the mean subcutaneous depth of implant was 11 mm based on sensory nerve stimulation. Although this evaluation was performed prospectively, it is important that this finding be reproduced in a multicenter setting.27
Technical Problems and Complications
The major technical problem with ONS is lead migrations. The incidence of lead migration was 24% after 3 months.17 In another review it was found to be 60% 1 year after implant and 100% 3 years after implant.5 This led some practitioners to consider the use of self-anchoring leads in ONS with encouraging preliminary results (Fig. 19-5). None of 12 patients required a surgical revision for lead migration for a mean follow-up period of 13 months.28
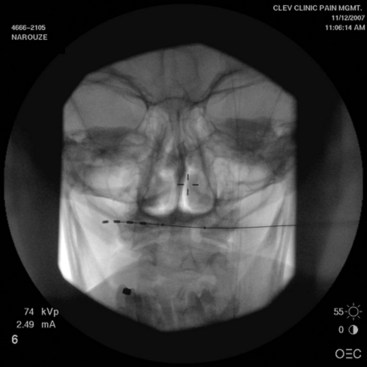
Fig. 19-5 Self-anchoring percutaneous lead.
Reprinted with permission from Ohio Headache and Pain Institute.
The second most common problem is occipital muscle spasms caused by occipital muscle stimulation secondary to improper lead placement as described above.25
Other rare complications may include lead fracture or disconnect, lead tip erosion, infection, unpleasant stimulation, and localized pain at implant sites.29–31
Mixed Causes of Cephalgia
In some patients stimulation of the ONS only captures a single aspect of a complicated problem. Many patients suffer from pain in the occiput mixed with other neuropathic pain syndromes of the face. Commonly this involves the trigeminal nerve or its nerve innervation pathways. In these cases it may be necessary to combine ONS with cranial nerve stimulation of the face. These mixed devices may require repositioning and always require a careful analysis of how the 16 electrode contacts can be used to best cover the area of paresthesia. When a mixed nerve trial is required, it is important that each nerve be assessed in an independent fashion and also combined for an overall impact and decision on the appropriateness of a permanent implant. This combination of two or more peripheral nerve stimulation (PNS) targets may have fewer risks than motor cortex stimulation and may be more acceptable to the patient and insurer. In the event of failure of PNS, motor cortex stimulation should be considered.32
Stimulation of the Gasserian Ganglion and Peripheral Nerve Targets
In mixed neuropathic pain of the head and face, some implanters have focused on the gasserian ganglion. These results are often promising in the first few weeks of implant but have been technically challenging and have had mixed long-term results. Additional prospective studies are needed to evaluate the role of this target, as opposed to the branch of the trigeminal nerve, which may be easier to access with a simple percutaneous approach.33,34 Slavin and colleagues34 have shown positive outcomes combining ONS stimulation with infraorbital, supraorbital, or maxillary branches of the trigeminal nerve (Fig. 19-6). These PNS approaches are low risk and may be attractive when considering the option of significant intracranial procedures.35 Johnson and Burchiel36 reported a pilot study involving 10 patients who received subcutaneous infraorbital and/or supraorbital nerve stimulation for trigeminal neuropathic pain. The etiology of the pain was posttraumatic in six of the patients and postherpetic neuralgia in four. Greater than 50% pain relief and significant decrease in analgesic medication use occurred in 70% of patients, with more robust results occurring in the posttraumatic group than in the postherpetic group. Reoperation occurred in 30%, including wound breakdown over the extension in two of the 10 cases. There was a gradual decrease in efficacy of stimulation with time; 80% of patients still experienced ≥50% pain relief 2 years after implant.
1 Weiner RL, Reed KL. Peripheral Neurostimulation for Control of Intractable Occipital Neuralgia. Neuromodulation. 1999;2:217-221.
2 Kapural L, et al. Occipital nerve electrical stimulation via the midline approach and subcutaneous surgical leads for treatment of severe occipital neuralgia: a pilot study. Anesth Analg. 2005;101:171-174.
3 Johnstone CHS, Sundaraj R. Occipital nerve stimulation for the treatment of occipital neuralgia—eight case studies. Neuromodulation. 2006;9:41-47.
4 Oh MY, et al. Peripheral nerve stimulation for the treatment of occipital neuralgia and transformed migraine using a C1-2-3 subcutaneous paddle style electrode: a technical report. Neuromodulation. 2004;7:103-112.
5 Schwedt TJ, et al. Occipital nerve stimulation for chronic headache-long-term safety and efficacy. Cephalalgia. 2007;27:153-157.
6 Schwedt TJ, et al. Occipital nerve stimulation for chronic cluster headache and hemicrania continua: pain relief and persistence of autonomic features. Cephalalgia. 2006;26:1025-1027.
7 Burns B, Watkins L, Goadsby PJ. Treatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patients. Lancet. 2007;369:1099-1106.
8 Burns B, Watkins L, Goadsby PJ. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology. 2009;72:341-345.
9 Magis D, et al. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol. 2007;6:314-321.
10 Burns B, Watkins L, Goadsby PJ. Treatment of hemicrania continua by occipital nerve stimulation with a BION device: long-term follow-up of a crossover study. Lancet Neurol. 2008;7:1001-1012.
11 Rodrigo-Royo MD, et al. Peripheral neurostimulation in the management of cervicogenic headache: four case reports. Neuromodulation. 2005;8:241-248.
12 Melvin EAJr, et al. Using peripheral stimulation to reduce the pain of C2-mediated occipital headaches: a preliminary report. Pain Physician. 2007;10:453-460.
13 Schwedt TJ, et al. Occipital nerve stimulation for chronic headache—long-term safety and efficacy. Cephalalgia. 2007;27:153-157.
14 Ghaemi K, et al. Occipital nerve stimulation for refractory occipital pain after occipitocervical fusion: expanding indications. Stereotact Funct Neurosurg. 2008;86:391-393.
15 Goadsby PJ, Bartsch T, Dodick D. Occipital nerve stimulation for headache: mechanisms and efficacy. Headache. 2008;48:313-318.
16 Matharu MS, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulation: a PET study. Brain. 2004;127:120-130.
17 Saper J, et al. Occipital nerve stimulation (ONS) for treatment of intractable migraine headache: 3-month results from the ONSTIM feasibility study (abstract). Pain Med. 2009;10:225.
18 Bovim G, et al. Topographic variations in the peripheral course of the greater occipital nerve: autopsy study with clinical correlations. Spine. 1991;16:475-478.
19 Becser N, Bovim G, Sjaastad O. Extracranial nerves in the posterior part of the head: anatomic variations and their possible clinical significance. Spine. 1998;23:1435-1441.
20 Mosser SW, et al. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg. 2004;113:693-697.
21 Hammer M, Doleys DM. Perineuromal stimulation in the treatment of occipital neuralgia: a case study. Neuromodulation. 2001;4:47-51.
22 Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58:112-119.
23 Johnstone CSH, Sundaraj R. Occipital nerve stimulation for the treatment of occipital neuralgia-eight case studies. Neuromodulation. 2006;9:41-47.
24 Popeney CA, Alò KM. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache. 2003;43:369-375.
25 Hayek SM, et al. Occipital neurostimulation-induced muscle spasms: implications for lead placement. Pain Physician. 2009;12(5):867-876.
26 Narouze S. Ultrasonography in pain medicine: future directions. Tech Reg Anesth Pain Manage. 2009;13(3):198-202.
27 Abejon D, Deer T, Verrils P: Depth determination of subcutaneous stimulation by sensory nerve stimulation. Submitted to Neuromodulation.
28 Narouze S, et al. Occipital nerve stimulation with self-anchoring leads for the management of refractory chronic migraine headache (abstract). Pain Med. 2009;10:221.
29 Trentman TL, Zimmerman RS. Occipital nerve stimulation: technical and surgical aspects of implantation. Headache. 2008;48:319-327.
30 Jasper JF, Hayek SM. Implanted occipital nerve stimulators. Pain Physician. 2008;1:187-200.
31 Trentman TL, et al. Percutaneous occipital stimulator lead tip erosion: report of 2 cases. Pain Physician. 2008;11:253-256.
32 Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 2010;13(2):157-165.
33 Machado A, et al. A 12-month prospective study of gasserian ganglion stimulation for trigeminal neuropathic pain. Stereotact Funct Neurosurg. 2007;85(5):216-224. Epub May 25, 2007
34 Slavin KV, et al. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurg Focus. 2006;21(6):E5.
35 Thimineur M, De Ridder D. C2 area neurostimulation: a surgical treatment for fibromyalgia. Pain Med. 2007;8(8):639-646.
36 Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery. 2004;55(1):135-141.