Obstructive Sleep Apnea
Evaluation and Treatment
Researchers are increasingly finding that a lack of sleep is detrimental for our health. Sleepiness has been linked to increased rates of heart disease, obesity, stroke, and even certain cancers. The exact reasons for these effects are still largely unknown, but they give support to the theory that sleep is the time when our bodies naturally repair themselves on a cellular level.150 Sleep—or the lack of it—is now thought to be a complex process that underpins everything from the ability to learn a new skill to how likely we are to find a novel solution to a problem.243 It is also considered a vital part of happiness and one of the best forms of preventative medicine.288
Sleep disorders include conditions that are characterized by repeated pauses in breathing during sleep, which lead to the fragmentation of sleep and decreases in oxyhemoglobin saturation. The physiologic spectrum of sleep-disordered breathing ranges from partial airway collapse with increased upper airway resistance, which is manifested as loud snoring and episodes of hypopnea, to complete airway collapse and prolonged episodes of apnea. Obstructive sleep apnea (OSA) is clinically defined by frequent episodes of apnea and hypopnea as well as symptoms of functional impairment.13,14,129,130
According to the National Sleep Foundation, some form of snoring or OSA occurs in 90 million Americans.245,246 Approximately 40% of individuals who are more than 40 years old snore, and half of them snore every night. Among habitually loud snorers, the incidence of OSA is at least 17% in men and 15% in women. The Foundation estimates that 16 million of the 20 million Americans with OSA remain undiagnosed. Affected individuals are often aware of snoring, daytime sleepiness, poor sleep patterns, and complaints from bed partners.352 To the educated clinician, the diagnosis of OSA may seem clear, but the affected individuals and their families are often unaware.9,106
Young and colleagues carried out a study to determine the prevalence of sleep-disordered breathing.393–395 With the use of a random sample of 602 employed men and women who were between the ages of 30 to 60 years old and who were studied via overnight polysomnography (PSG), these authors assessed the frequency of episodes of apnea and hypopnea per hour of sleep. The authors determined that this condition affected up to 9% of middle-aged women and 24% of middle-aged men (i.e., an apnea–hypopnea index [AHI] of 5 or more and daytime hypersomnolence).185 Both male and female habitual snorers tended to have a higher AHI of 15 or more.
Rationale for Treatment
The two major rationales for the treatment of OSA are to address 1) pathophysiologic derangements and 2) behavioral derangements.263
The pathophysiologic derangements related to OSA are primarily cardiorespiratory in nature.* They include increased risks of myocardial infarction, stroke, and sudden death.153 Sudden death may be caused by tachycardia and tachyarrhythmias (i.e., atrial fibrillation) as well as by bradyarrhythmias. The documented high prevalence of atherosclerotic coronary artery disease among patients with OSA is possibly related to endothelial damage, with platelet activation and the presence of inflammatory mediators also being involved.153 When OSA is combined with systemic hypertension, an increased incidence of stroke has been reported.138,140,392 A higher-than-average occurrence of idiopathic dilated cardiomyopathy and more frequent episodes of ST-segment depression have also been recognized.118,125,153 The physiologic processes that are believed to be involved in OSA and that predispose patients to these risks include hypoxemia, negative intrathoracic pressure, and the disequilibrium of the autonomic nervous system.42
The behavioral derangements caused by OSA may be the result of excessive daytime sleepiness.161,367 OSA is associated with nocturnal arousals during sleep that occur in response to airway collapse and that result in poor sleep. Excessive daytime sleepiness manifests as sleepiness or fatigue of such a degree that the individual lacks vigilance and does not function as well as he or she should during the day. Other symptoms typically include heavy snoring, known apnea, headaches, difficulty waking in the morning, fatigue, sleepiness after eating, memory loss, having difficultly with work interactions, stressed relationships and sexual performance complaints. There is a documented increased incidence of motor vehicle accidents and job-related injuries among individuals with OSA (e.g., individuals with OSA are 10 times more likely to die in an automobile accident).26,85,103,292 OSA is also known to be a cause of difficulty with concentration at school or during work activities. Over time, the hypoxia that is caused by OSA is believed to result in changes in the neurons of the hippocampus and the right frontal cortex of the brain. Neuroimaging studies have revealed evidence of hippocampal atrophy in people who chronically suffer from OSA. Clinically, it is documented that a greater percentage of patients with OSA have problems with mentally manipulating nonverbal information and with executive processing.100 Interestingly, there is not a direct correlation between these behavioral derangements and the severity of OSA as measured with the AHI or with specific levels of oxygen desaturation. It is important to note that there are multiple causes of excessive daytime sleepiness other than OSA, including volitional sleep deprivation, alcoholism, insomnia, and narcolepsy.
Diagnosis, Characterization, and Assessment of Obstructive Sleep Apnea Severity
Pathophysiology
OSA is characterized by symptoms that result from the recurrent sleep-associated collapse of the pharyngeal airway and that lead to hypoxemia, hypercapnia, and fluctuations in intrathoracic pressure caused by increased respiratory effort, with arousal from sleep required to reestablish airway patency. When awake, protective mechanisms increase the activity of the pharyngeal dilator muscles; however, during sleep, these mechanisms may fail and result in the collapse of the pharyngeal airway.45,73,120,293,329,346,349,387 This is made worse by nighttime horizontal and supine sleep positioning.244,256 Frequent locations of pharyngeal collapse include the velopharynx (behind the soft palate), the oropharynx (behind the tongue), or both.240
Schwab and colleagues have documented an increase in adipose hypertrophy within the floor of the mouth, the soft palate, the pharyngeal fat pads, and the lateral pharyngeal walls in many individuals with OSA.324 In patients with obesity, there is a direct deposition of fat that causes submucosal adipose volume expansion within the pharyngeal walls (thus narrowing the lumen) and the floor of the mouth (thus causing elevation and retro positioning of the tongue), thereby diminishing the actual upper airway breathing space and, in many cases, explaining the greater extraluminal critical closing pressure documented in patients with OSA. Evidence supports the concept that many individuals with OSA have an anatomic predisposition to airway collapse as a result of retrusive jaws and obstructed nasal cavities. An individual’s unique baseline skeletal (i.e., jaw) anatomy may have a dramatic effect on the size (i.e., the luminal diameter) of the upper airway. It is now also appreciated that the variability in upper airway breathing space from one person to the next is influenced by the subject’s developmental or congenital maxillofacial skeletal morphology. Deficient maxillomandibular structural support adds to the environmental causes (i.e., weight gain) that thicken the pharyngeal soft tissues, thereby further narrowing the lumen and diminishing the patency afforded by pharyngeal dilator muscle contraction forces that work to maintain and open the airway. Genetics plays a lesser role in the observed volumes of the tongue, the soft palate, the adenoids, the turbinates, and the tonsillar tissues seen in individuals with OSA. These soft-tissue structures are more likely to be affected by acquired conditions such as weight gain, infections, or allergies. With obesity, there may also be alterations of pharyngeal muscle orientation and function. These factors combine to determine overall upper airway breathing space, pharyngeal muscle function, and, as a consequence, the occurrence of apnea events during sleep.
Schwartz and colleagues measured the critical closing pressures during sleep in individuals with OSA and compared these values to those of controls (i.e., individuals without OSA).327 Individuals with baseline anatomically “large-volume” airways are less dependent on pharyngeal dilator muscles to maintain airway patency than those with intrinsic “small-volume” anatomic airways. Schwartz and colleagues showed that control subjects (i.e., individuals without OSA) typically have critical closing airway values of less than 0 cm of water, whereas those with severe OSA have values of more than 8 cm of water. Multiple diagnostic modalities (e.g., cephalometric x-rays, computed tomography [CT] scans, acoustic reflection, magnetic resonance imaging) confirm that individuals with OSA generally have “smaller-volume” baseline awake airway lumens as compared with controls.264
According to Fogel and colleagues the pharyngeal muscles of primary importance fall into three groups87:
1. The muscles that influence the hyoid bone position (i.e., geniohyoid, sternohyoid)
2. The muscles of the tongue (i.e., genioglossus)
3. The muscles of the soft palate (i.e., tensor palatine and levator palatine)
The activity of these muscles is normally increased during inspiration to stiffen and expand (i.e., open) the upper airway. This counteracts the collapsing influences that would otherwise occur with inspiratory negative airway pressure. During expiration, the muscles normally substantially relax while positive pressure is flowing through the respiratory tree. The tensor palatine is an exception in that it must maintain a constant level of tone throughout the respiratory cycle to preserve a patent airway. Fogel and colleagues stated that the activity of the pharyngeal dilator muscles during wakefulness is tightly controlled to maintain pharyngeal patency.87 In individuals with an anatomically “small-volume” airway, the activation of this negative-pressure reflex (i.e., feedback mechanism) to increase the function of the pharyngeal muscles must occur to prevent or limit apnea. This reflex has been shown to be diminished in apnea-prone individuals for both the genioglossus and the tensor palatine muscles. An individual’s use of specific medications (e.g., alcohol, sedatives, narcotics) will also negatively influence the reflex arc, thereby causing the relaxation of the muscles and further diminishing airway patency.
At least until the age of 65 years, age is positively correlated with OSA severity as demonstrated by an increase in the AHI.152 This increase is substantially greater in the presence of baseline maxillomandibular sagittal deficiency. While a person is awake, neuromuscular mechanisms and body position (i.e., standing or sitting up) can positively influence and compensate for the “small-volume” airway. However, the pharyngeal dilator muscles are less able to effectively contract during sleep, which results in the more frequent collapse of the anatomically small breathing space. The ability of the pharyngeal muscles to compensate for and prevent apnea events becomes progressively more difficult with age and in the presence of weight gain.
Frieberg and colleagues hypothesized that a progressive lesion in the afferent and/or efferent nerve pathways caused by repetitive “snoring trauma” may also contribute to the collapsibility and obstruction of the upper airway that is seen in patients with OSA.89–91 Their study evaluated the hypothesis of the presence of a progressive neurogenic lesion in the pharyngeal muscles in patients with longstanding upper airway obstruction. Biopsies of palatopharyngeal muscle were obtained from patients (n = 21) with habitual snoring and degrees of upper airway obstruction and compared with those of non-snoring control subjects (n = 10). The degree of abnormality found was significantly increased in the study patients as compared with control subjects. The results of their research support the hypothesis of a progressive local neurogenic lesion that is caused by the “trauma of snoring” as a possible contributory factor in upper airway collapsibility.
Sleep electroencephalograms (EEGs) of patients with OSA will differ from those of individuals with upper airway resistance syndrome (UARS) and from those of control subjects.117,128 The arousal response of individuals with OSA is delayed, despite the fact that increased respiratory effort is seen during the obstructive phase. This delayed arousal response has been confirmed by differences in the patterns of EEG findings. Guilleminault and colleagues hypothesized that a difference in sensory input may be the responsible factor for the divergent responses to the abnormal breathing patterns that may exist between individuals with UARS and those with OSA.122 The authors set out to compare the results of a palatal mucosa two-point discrimination response in normal subjects (n = 15), those with OSA (n = 15), and those with UARS (n = 15). The subjects were matched for age, sex, and body mass index. The 45 subjects were submitted to a two-point discrimination study of the palatal mucosa during wakefulness. Individuals with OSA had a clear impairment of their palatal mucosa sensory input with a significant decrement in two-point discrimination. Interestingly, the subjects with UARS and the normal control subjects responded similarly to each other. The responses seen in individuals with UARS indicate that those individuals are more capable of transmitting sensory inputs than individuals with OSA. This may be at least one factor that explains the difference in arousal response that is been documented in patients with UARS as compared with those with OSA.122
The basic anatomic factors that reduce the size of the upper airway include the following:
1. Regional obesity (i.e., increased volume of the lateral pharyngeal fat pads, the soft palate, and the floor of the mouth
2. Baseline (inherent) abnormalities of the maxillomandibular skeletal structures (i.e., the maxilla, the mandible, and the nasal septum)
3. Volumetric enlargement of upper airway soft-tissue visceral structures (e.g., tongue, soft palate, tonsils, adenoids, inferior turbinates) as a result of congenital anomalies, allergies, infections, or tumors.
1. The intranasal region. This portion of the airway extends from and includes the external nasal valves, the internal nasal valves, the turbinates, the nasal septum, and the adenoids.
2. The retropalatal region. This portion of the airway is primarily affected by the position of the maxilla (i.e., the hard palate), the size and position of the soft palate, the tonsils, and the parapharyngeal fat pads.
3. The retroglossal region. This portion of the airway is primarily affected by the position of the mandible, the size and position of the tongue, the fullness of the soft tissues in the floor of the mouth, the parapharyngeal fat pads, and the tonsils.
An increased body mass index (BMI), which is also known as obesity, is a known risk factor for OSA in children, adolescents, and adults (Fig. 26-1).21,88,241,255,337 Peppard and colleagues outlined the cardiovascular risks associated with OSA for weight gain and weight loss.269,268 Excessive body weight was found to be positively associated with OSA,262 and the stability of the individual’s weight was also found to be an important factor. A 10% weight gain predicted a 32% increase in the AHI.319 The same 10% gain in weight also predicted a six-fold increase in the odds of moderate to severe OSA developing. Interestingly, a weight loss of 10% predicted a 26% decrease in the AHI.169 Unfortunately, studies consistently document that weight loss programs in patients with OSA frequently fail miserably. Any weight loss that the individual is able to initially achieve tends to be temporary.167
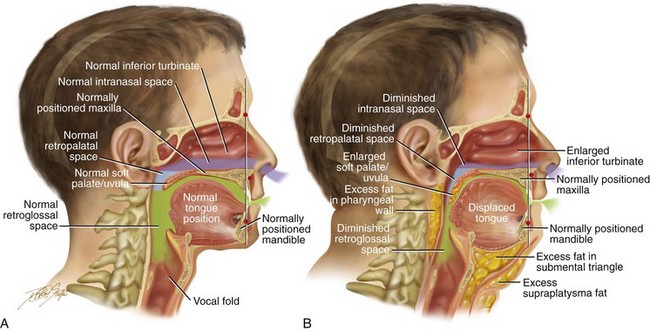
Figure 26-1 A, Midline sagittal cross-section head and neck illustration of a 20-year-old man with a normal body mass index to demonstrate upper airway space and associated maxillofacial anatomy. This individual has a normal upper facial skeleton (cranial vault, orbits, and zygomas), a normal lower facial skeleton (maxilla and mandible), and normal soft tissues (soft palate, tongue, tonsils, and adenoids). The intranasal cavity, which includes the turbinates and the septum, is also normal. B, The same individual when he is 50 years old with an elevated body mass index. The upper and lower facial skeleton remain unchanged with age. The adipose cells, which are scattered throughout and within the upper airway soft tissues, have now increased in volume. This includes the retropharyngeal and the lateral pharyngeal tissues, the soft palate, and the floor of the mouth. As a result, the soft palate is long, thick, and in close proximity to the posterior and lateral pharyngeal walls, thereby diminishing the retropalatal space. As a result of increased adipose volume in the floor of the mouth, the tongue has been displaced superiorly and posteriorly, thus decreasing the retroglossal airway space. As a result of chronic allergies, the patient’s turbinates have enlarged to cause increased nasal airway resistance (i.e., diminished intranasal airway space). There is also excess adipose volume in both the submental triangle and the supraplatysmal locations, thereby resulting in a full neck and a “double chin” appearance. In review, when the patient was 20 years old, he had normal upper airway space, including the intranasal site; the retropalatal site; and the retroglossal sites. When he was 50 years old, the upper airway space is considerably smaller, including the intranasal space, the retropalatal space, and the retroglossal space. The patient depicted here now has documented obstructive sleep apnea.
When an individual’s BMI is elevated, the volume of fat in the pharynx and the floor of the mouth also increases. It is estimated that more than 66% of individuals with OSA are obese. On the basis of the latest World Health Organization statistics, it is estimated that there are more than 1.6 billion overweight adults (from a total population of 6.5 billion) and at least 400 million obese individuals (BMI ≥ 25 kg/m2) on Earth.72 Obesity has both a genetic and an environmental basis.37,63,102,165,237 A review of population studies suggests that heredity accounts for at least 40% of the variance in BMI.219,286 Studies have demonstrated a strong relationship between the BMIs of biologic parents and their children. Interestingly, no relationship between the BMI of adoptive parents and their adopted children has been documented. The best indirect indicator of excessive fat accumulation in the pharyngeal region and the floor of the mouth is a person’s BMI.
Studies have also documented that relatives of individuals with OSA demonstrate a higher incidence of hypoplastic maxillae and mandibles, longer soft palates, and wider uvulas as compared with matched controls.219,326 This also speaks to the hereditary component of a small pharyngeal airway space and to an individual’s genetic predisposition to OSA.295,388
There are specific syndromes and conditions that result in severe maxillomandibular skeleton dysmorphology and that also negatively affect the upper airway (e.g., Crouzon syndrome, Apert syndrome, Treacher Collins syndrome, Pierre Robin sequence).17,38,270,271,287,341,354,390 It is documented that more than 50% of individuals with Down syndrome suffer from OSA.66 Interestingly, a similar percentage of individuals with Down syndrome are known to have a baseline intrinsic developmental jaw deformity that is characterized primarily by maxillary deficiency. The surgical correction of the maxillofacial deformities associated with these syndromes have been documented to enlarge the upper airway space and to improve and often resolve breathing difficulties, including OSA (see Chapters 27, 28, 30 and 31). The surgical management of velopharyngeal insufficiency (i.e., superiorly based pharyngeal flap or sphincteroplasty) in individuals with a repaired cleft palate is also known to result in OSA in some cases.4,39,157,176,187,205,257,258,339,340,343,373,389,396 Interestingly, the non-syndromal developmental dentofacial deformities (e.g., long face growth pattern, short face growth pattern, primary mandibular deficiency, maxillary deficiency with relative mandibular excess, maxillary hypoplasia associated with repaired cleft palate), which affect at least 5% of the general population, also negatively affect the upper airway.212 Unfortunately, these more common patterns of jaw disharmony frequently go unrecognized as significant etiologic factors for OSA (Figs. 26-2 and 26-3). It is incumbent on the otolaryngologist, the pulmonologist or sleep specialist, the pediatrician or internist, the orthognathic or maxillofacial surgeon, and the general dentist or orthodontist to understand the fundamental relationship between developmental jaw deformities and upper airway obstruction in each patient who is evaluated.
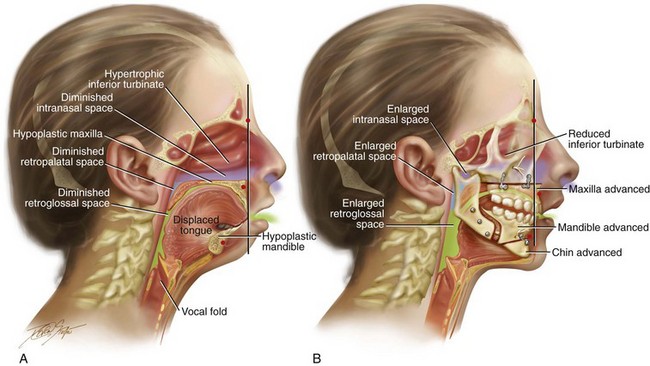
Figure 26-2 Midline sagittal cross-sectional head and neck illustrations of a 16-year-old girl with primary mandibular deficiency, maxillary arch constriction, and Class II excess overjet malocclusion (see Fig. 19-4). The illustrations demonstrate the upper airway space and the associated maxillofacial anatomy before and after jaw and intranasal reconstruction. A, Illustration when the patient was 16 years old, before intervention. The inferior turbinates are hypertrophic as a result of chronic allergies, and the nasal septum is deviated. These findings result in a diminished intranasal airway space. As part of the developmental jaw deformity, the maxilla is retropositioned, and there is a narrow arch width. Therefore, the soft palate is posterior, which results in diminished retropalatal space. The mandible is horizontally (sagittally) deficient, with a posterior displaced tongue. As a result, there is diminished retroglossal space. The tonsils, the adenoids, the tongue, and the soft palate are displaced but otherwise normal. Although the patient is young and has a normal body mass index, the intranasal and jaw deformities result in diminished upper airway space. The patient has difficulty breathing through the nose while she is awake, and is with heavy snoring at night. Obstructive sleep apnea has been documented. B, The patient agreed to a comprehensive orthodontic and surgical approach. Lower bicuspid extractions allowed for the relief of dental compensation. The patient’s surgery included maxillary Le Fort I osteotomy in segments (arch expansion and horizontal advancement); bilateral sagittal split ramus osteotomies (horizontal advancement); osseous genioplasty (horizontal advancement); and septoplasty and inferior turbinate reduction. The postoperative illustration indicates maxillofacial changes that resulted from the reconstruction. Now there is an expanded intranasal, retropalatal, and retroglossal airway space. There is improved nasal breathing during the day and reduced snoring and apnea events at night.
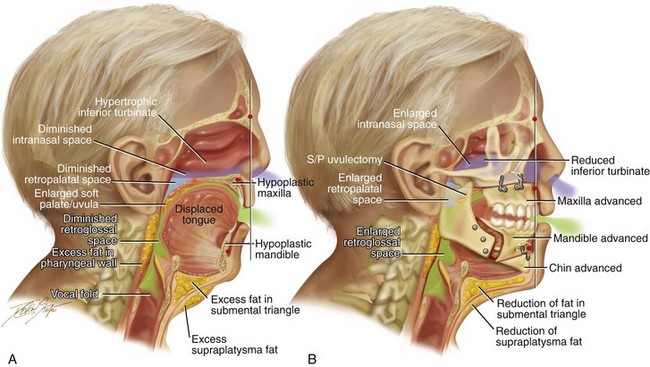
Figure 26-3 Midline sagittal cross-sectional head and neck illustrations of a 59-year-old man to demonstrate upper airway space and associated maxillofacial anatomy before and after jaw, intranasal, and facial surgery carried out primarily to open the airway (see Fig. 26-6). This patient has documented severe obstructive sleep apnea, including a respiratory disturbance index of 51 events/hour with desaturations reaching as low as 82%. The patient has an elevated body mass index and a short face growth pattern that is characterized by maxillomandibular vertical and horizontal deficiency. At the time of presentation to this surgeon, he had already undergone “Phase I” procedures including uvulopalatopharyngoplasty, septoplasty, and inferior turbinate reduction without relief of obstructive sleep apnea. A, Illustration of the patient’s condition before surgery demonstrates hypertrophic inferior turbinates and septal deviation that result in a diminished intranasal space. The maxilla is vertically short and horizontally deficient. The soft palate is long and thick as a result of excess fat accumulation. For all of these reasons, a diminished retropalatal airway space is present. The mandible is horizontally deficient, and the tongue is elevated as a result of excess fat in the floor of the mouth. The lateral and retropharyngeal walls also show excess fat accumulation. These findings account for the diminished retroglossal airway space. B, After the patient was referred to this surgeon, he underwent simultaneous procedures that included maxillary Le Fort I osteotomy (horizontal advancement of 10 mm and vertical lengthening of 5 mm) with interpositional graft; bilateral sagittal split osteotomies of the mandible (horizontal advancement of 12 mm); osseous genioplasty (horizontal advancement); redo septoplasty; and anterior neck soft-tissue procedures (cervical flap elevation, fat removal, and platysma muscle plication). The illustration after maxillary–mandibular–chin advancement demonstrates the enlarged upper airway space, including the intranasal space, the retropalatal space, and the retroglossal space. A 3-month postoperative attended polysomnogram confirms a respiratory disturbance index of less than 5 events/hour.
Not infrequently, the individual with “mandibular prognathism” has undergone a lower jaw set-back treatment to improve the occlusion. Historically, such procedures have been preferred by many clinicians for the treatment of skeletal Class III patterns. However, it is now known that this approach is associated with a risk to the airway size and shape.6,18,41,48,67,68,74,78,84,113,115,171,172,174,314,316,370,386 Demetriades and colleagues confirmed the clinical importance of considering the airway when formulating a treatment plan for the correction of so called “mandibular prognathism.”69 They described 26 patients who presented with developmental dentofacial deformities that were characterized by maxillary deficiencies in combination with relative mandibular excesses. Each patient underwent mandibular ramus osteotomies with at least some set-back. The patients were divided into two groups. The Group 1 patients (n = 13) underwent mandibular setback of at least 5 mm without simultaneous Le Fort I osteotomies. The Group 2 patients (n = 13) also underwent a degree of mandibular set-back, but in conjunction with Le Fort I advancement. All study patients (Groups 1 and 2) underwent preoperative and postoperative lateral cephalometric radiography and portable PSG. The preoperative and postoperative radiographs were compared, and any changes that occurred in the position of the hyoid bone, the distance from the posterior nasal spine to the palate, the retrolingual space, and the hypopharyngeal space were measured. The authors confirmed that mandibular retropositioning of at least 5 mm can significantly decrease the posterior airway space and cause mild to moderate OSA. The study also suggested that, when a limited mandibular set-back is carried out in combination with maxillary Le Fort I advancement, significant negative effects on the airway are likely to be prevented.
In summary, anatomic upper airway risk factors for OSA include the following: 1) regional obesity (i.e., increased upper airway submucosal adipose tissue deposition); 2) retrusive maxillomandibular skeleton (e.g., hypoplasia of the maxilla or mandible); 3) alterations in the intranasal cavity (e.g., septal deviations, hypertrophic inferior turbinates, tight nasal aperture, elevated nasal floor, adenoid hypertrophy); 4) enlargement of other critical upper-airway soft-tissue visceral structures (e.g., soft palate, tonsils, tongue); and 5) inadequate pharyngeal muscular tone (i.e., hereditary and environmental factors).320,332,348,378 Each of these head and neck structural aspects predispose individuals to OSA by causing upper-airway narrowing during wakefulness and airway collapse during sleep. When these aspects occur in combination, the risk is greatly exacerbated. There are also specific medical conditions (e.g., myasthenia gravis, Parkinson disease, amyotrophic lateral sclerosis) as well as certain medications and drugs (e.g. alcohol, narcotics, sedatives, muscle relaxants) that are known to negatively affect the neuromotor tone of the chest wall and the pharyngeal musculature and thus predispose an individual to apnea.
Basic Clinical Evaluation
1. The clinician should determine at what specific maxillofacial skeletal locations (e.g., nasal septum, turbinates, maxilla, mandible), if any, there are negative effects occurring in the upper airway during sleep or while awake.
2. If the patient also has a high BMI (i.e., >25 kg/m2), the clinician should determine at what regional soft-tissue locations (e.g., nasopharyngeal, palatopharyngeal, glossopharyngeal), if any, there is excessive submucosal adipose and other soft-tissue volume that may have a negative impact on the airway.
3. The clinician should determine if there are baseline physiologic dysfunctions of the lungs and chest walls (e.g., chronic obstructive pulmonary disease) that negatively affect the airway.
4. The clinician should consider the effects of baseline neuromotor disorders (e.g., Parkinson disease) and currently used medications (including non-prescription drugs and alcohol) that may negatively affect the airway.
It is also recommended that a subjective questionnaire be used to assess for OSA. The Epworth Sleepiness Scale (ESS) is considered the reference standard.86,163,164 The survey assesses the respondent’s propensity for sleep in eight different situations. Although the survey is not perfect, it does provide useful information about the patient’s waking sleepiness.
An airway analysis that is based on CT scan data may also be a useful tool to assess upper airway space. A recent study used three-dimensional computer analysis from cone-beam CT to present normative upper airway size reference data. The collected data confirm that, in humans, the airway size and length increases until the age of 20 years, at which time there is a variable period of stability. After this stable period, the airway at first decreases slowly in size; then, after the age of 50 years, it decreases more rapidly.322 Li and colleagues demonstrated a correlation between the airway area and the likelihood of OSA.197 There is a high probability of severe OSA with an airway area of less than 52 mm2; an intermediate probability if the airway is between 52 mm2 and 110 mm2; and a low probability if the airway area is more than 110 mm2.190 Cillo and colleagues attempted to correlate specific cephalometric measurements with the AHI in a consecutive series of patients (N = 101) who were documented to have OSA. Unfortunately, no one skeletal or soft-tissue parameter could be linked to OSA.51 Three-dimensional CT airway analysis has documented quantifiable changes in patients’ oropharynges after specific surgical interventions. This type of imaging may also be used to evaluate changes in the airway after specific medical interventions (e.g., weight loss).75
Susarla and colleagues used a case–control study that was designed to analyze the value of the cephalometric upper airway length (UAL) measurement as an indirect predictor of the presence and severity of OSA.360,361 The study group included adults (n = 96) with PSG-documented severe OSA and a control group of adults (n = 56) with skeletal Class II malocclusion without known OSA. Although significant attention has been devoted to describing and measuring upper airway width, little attention has been devoted to measuring UAL. The theory behind this study is that an important component of the airway resistance is the airway length (i.e., the vertical dimension), which is known to be directly correlated with airway resistance per Poiseuille’s law: resistance equals 8 ηL/πr4 (L = length). The results of the study suggest that the UAL (i.e., the distance between the posterior hard palate and the most superior aspect of the hyoid bone on a line parallel to the long access of the airway) is predictive of the presence of OSA in both men and women. The study results indicate that a cephalometric UAL of 72 mm or more in men and 62 mm in women was diagnostic for OSA with high sensitivity and specificity. It would appear that both a narrow posterior airway space and a long UAL predispose individuals to upper airway obstruction and that both conditions are associated with increased odds of OSA.
A reasonable predictor of OSA is a clinical triad of symptoms that includes loud snoring, apnea witnessed by a bed partner, and excessive daytime sleepiness. When the sleep and daytime history is combined with attended PSG, the patient’s clinical head and neck examination (including instrumentation), and a focused radiographic analysis, then an overall picture of the upper airway anatomy and function can be seen.*
Attended Polysomnography
Overnight PSG conducted in a sleep laboratory remains the reference standard for the diagnosis of central sleep apnea or OSA.92,111,155,180,365 PSG requires the use of several monitoring parameters, including EEG, electrooculography, electromyography, electrocardiography, the microphone measurement of snoring, pulse oximetry, respiratory inductance plethysmography, airflow measurement, and the recording of the body position. PSG primarily evaluates sleep-disordered breathing, sleep architecture, and oxygen desaturations. During PSG, episodes of apnea and hypopnea are determined from a reduction in airflow in combination with oxygen desaturation during a 4- to 8-hour time frame.
• Sleep stages (both rapid eye movement and non–rapid eye movement sleep), including the percentage of sleep in each.
• Awake oxygen saturation, including the lowest levels and the stratifications of the percentage of time below specific percentiles (i.e., 90%, 80%, and 70%)
• Heart rate fluctuations (e.g., brachyarrhythmia, tachyarrhythmia)
Characterization of Apnea
Interestingly, it is recognized that 10% to 20% of individuals with known OSA will fail to respond to successful anatomic surgical expansion of the maxillomandibular complex. Another difficult to explain subgroup of individuals with documented OSA is that group with apparent normal upper airway morphology (i.e., no significant narrowing). Complex sleep apnea is a newly recognized category in the spectrum of sleep apnea syndromes that helps to explain these outliers. According to Susarla and colleagues, complex sleep apnea often presents with morphologic features that are typical of obstructive disease but that are combined with PSG evidence of strongly chemoreflex-modulated sleep breathing.360 Complex sleep apnea is distinguished from other forms of OSA by the following:
• Non–rapid eye movement sleep dominance of obstructive disease;
• Oscillations of respiration with or without flow limitation; and
• Induction of central apneas or periodic breathing with the application of positive airway pressure therapy.
Susarla and colleagues believe that a lack of resolution of OSA after successful maxillomandibular advancement surgery may occur if a ventilation control disturbance either remains or is amplified.360 These individuals with chemoreflex-sensitive apnea can be identified, because they are extremely prone to changes in their carbon dioxide levels. It is important to make an accurate diagnosis, because these patients can be expected to require further management of the central component to their apnea. Helpful treatment for this unique subgroup may include the use of nasal oxygen, pharmacologic agents, or positive-pressure–based adaptive ventilation. Affected individuals require continued reassessment and management by a knowledgeable clinical sleep specialist.
Sites of Functional Obstruction
Studies that attempt to localize the site of upper airway obstruction in an individual with moderate to severe OSA have shown that there is rarely a single anatomic site of blockage (see Figs. 26-1 through 26-3). More commonly, multiple sites of upper airway obstruction occur during episodes of hypopnea and apnea. As stated, the potential upper airway sites of obstruction are somewhat artificially grouped as 1) intranasal; 2) retropalatal; and (3) retroglossal. In addition to taking a history and a physical examination, diagnostic studies to further clarify the anatomic levels of obstruction include the Müller maneuver, which is observed during nasoendoscopic instrumentation; lateral cephalometric radiographs taken during end-tidal volume measurement with the Müller maneuver; helical CT; and magnetic resonance imaging.
Intranasal Site
When a history of chronic obstructive nasal breathing is combined with an intranasal clinical examination, instrumentation (e.g., speculum, endoscope), and radiography (e.g., sinus CT scan), the causes of the intranasal obstruction are generally identified. A thickened, buckled, deviated septum (bone and cartilage), enlarged (hypertrophic) inferior turbinates, an elevated nasal floor, and narrow lateral walls (tight nasal aperture) are frequent causes (see Chapter 10). Occasionally, crooked nasal bones, stenotic external nasal valves (after cleft lip repair), or collapsing internal nasal valves (previous nasal surgery) may also be responsible. Enlarged adenoids or nasal polyps may also be causative factors.
Obstructive Sleep Apnea during Childhood: Diagnosis and Management
“The [American Academy of Pediatrics] guidelines contain the following recommendations for the diagnosis of OSA: (1) all children should be screened for snoring; (2) complex high-risk patients should be referred to a specialist; (3) patients with cardiorespiratory failure cannot await elective evaluation; (4) diagnostic evaluation is useful in discriminating between primary snoring and OSA, the reference standard being polysomnography; (5) adenotonsillectomy is the first line of treatment for most children, and continuous positive airway pressure is an option for those who are not candidates for surgery or do not respond to surgery; (6) high-risk patients should be monitored as inpatients postoperatively; and (7) patients should be reevaluated postoperatively to determine whether additional treatment is required.”12,57,328
“This clinical practice guideline is not intended as a sole source of guidance in the evaluation of children with OSA.15,16,35,46,50,108,110,127,170,216,223,235,296 Rather, it is designed to assist primary care clinicians by providing a framework for diagnostic decision making. It is not intended to replace clinical judgment or to establish a protocol for all children with this condition and may not provide the only appropriate approach to this problem.”223
Manifestations in Children
Snoring and difficulty breathing during sleep are the most common complaints reported by parents of children with OSA.54,249,400 However, in contrast with reports of OSA in adults, excessive daytime somnolence is believed to be less common in children, with only 7% of 10% of pediatric patients with OSA experiencing this symptom. Neurocognitive deficits and behavioral manifestations are more frequent in children and may be the result of the chronic exposure to intermittent hypoxemia and sleep disruption caused by sleep fragmentation. Children with OSA are more likely to present with symptoms of hyperactivity, inattentiveness, poor academic performance, and behavioral problems.30,49,60,112,152,166,229,231,253,254,312 Such symptoms are often attributed to attention-deficit/hyperactivity disorder; treatments prescribed for this disorder (e.g., pharmacologic therapy with stimulants) can actually worsen sleep symptoms.
Diagnostic Criteria in Children
Diagnostic criteria for OSA in adults are typically the product of consensus and often include an AHI of 5 or more as documented by nocturnal PSG as well as evidence of disturbed or unrefreshing sleep, daytime sleepiness, or other daytime symptoms.313,382 The rationale for specific AHI diagnostic criteria in children as compared with adults suffers from less available data and heterogeneity across studies. Part of the problem is that few studies have been performed to link specified levels of pediatric OSA with adverse outcomes. At present, an AHI or RDI of as low as 1 event/hour is used to identify children with OSA. Pediatric criteria for scoring AHI or RDI are used until the age of 13 years as a matter of routine. According to the most recent American Sleep Disorder Association guidelines, either the pediatric or adult criteria for OSA may be used for adolescent patients who are between 13 and 18 years old at the discretion of the scoring sleep specialist.12–16 It is also unclear how many events per hour should be considered significant in the adolescent patient as compared with pediatric and adult patients.5
Treatment Considerations in Children
Adenotonsillectomy (T&A) is generally considered to be the standard first-line treatment for a child with OSA who does not have complex medical conditions that would make surgery an unacceptable risk.34,121,124,183,188,220,224,230,232–234,298,317,344,351,355,366,384 A child with OSA should not undergo T&A without receiving a thorough evaluation of the entire upper airway to confirm the actual need for an operation. Nevertheless, the overall cure rate of T&A during childhood is believed to be as high as 82.9%. Studies indicate that, for children with documented OSA and a preoperative RDI of less than 19 events/hour, a T&A will frequently reduce the RDI to less than 5 events/hour. Alternatively, if the preoperative RDI is greater than 19 events/hour, then the T&A has a low probability of reducing the RDI to less than 5 events/hour.355 Other anatomic causes for the child’s OSA should be considered (e.g., maxillomandibular hypoplasia).
A tight nasal inlet is another common cause of upper airway obstruction that may be first recognized during childhood (see Chapter 10). Clinical studies have documented that maxillary arch expansion will improve a tight nasal inlet and thereby decrease nasal airway resistance. Maxillary arch expansion, which is accomplished with the use of a rapid palatal expansion appliance, is commonly carried out during childhood with little morbidity.8,27,36,79,399 Unfortunately, rapid palatal expansion will only address the one site of obstruction and in a limited way. No studies have confirmed that this approach alone will be effective for OSA. For children in whom T&A or rapid palatal expansion is required but does not lead to the resolution of OSA, evaluation to confirm the additional sites of obstruction should follow and appropriate interventions considered. The option of the administration of CPAP should be entertained, but the overall long-term adherence rate for CPAP is unknown and felt to be problematic in this population. Skeletal reconstruction is likely to be beneficial in a child with a craniofacial anomaly that is known to obstruct the upper airway and in whom OSA has been documented.17,38,53,270,271,287,341,354,390 For example, maxillary advancement in a child with either Apert or Crouzon syndrome and documented OSA has proven value (see Chapter 30). When a small lower jaw with a retro positioned tongue (i.e., some children with Treacher Collins Syndrome or Pierre Robin sequence) is the cause of OSA, then mandibular advancement can also be helpful (see Chapter 19). Developmental jaw deformities (i.e., long face growth pattern, short face growth pattern, and isolated mandibular deficiency) occur frequently in the general population, but they often go unrecognized as causes of OSA in children and teenagers (see Chapters 19, 21, and 23). After a developmental jaw deformity has been recognized in a child, OSA should be ruled out.126,383 Referral to an orthognathic surgeon familiar with OSA is recommended if the PSG is positive (Figs. 26-4 and 26-5).
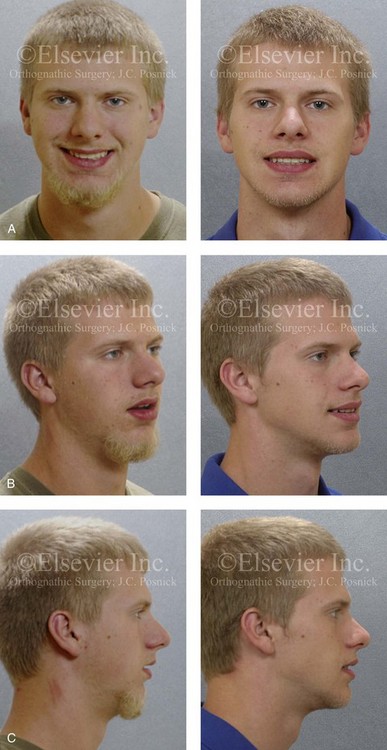
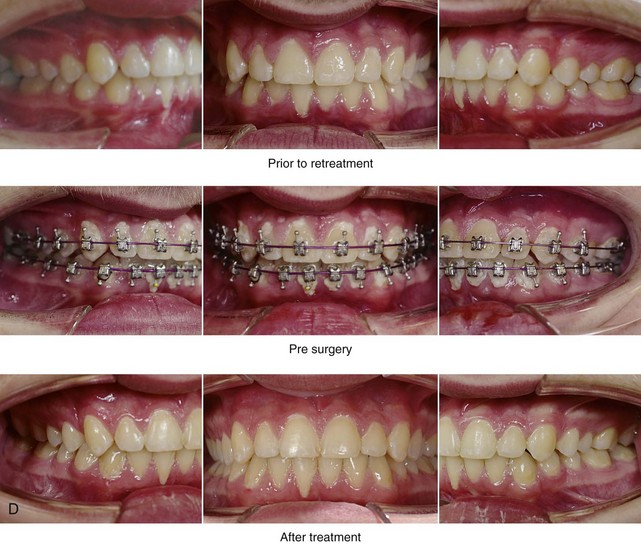
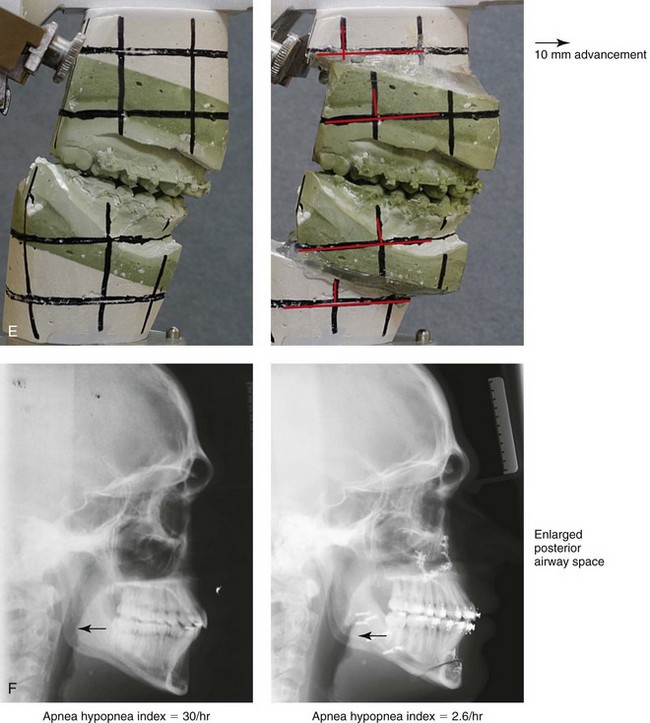
Figure 26-4 A 16-year-old boy was referred by his pediatrician for surgical evaluation. He had a history of heavy snoring, restless sleeping, daytime somnolence, attention-deficit/hyperactivity disorder and other behavioral problems, and poor school grades. An attended polysomnogram documented an apnea–hypopnea index of 30 events/hour. Head and neck examination confirmed a normal-sized and functioning soft palate and tongue. The tonsils and adenoids were not enlarged. The intranasal cavity was obstructed (septal deviation and inferior turbinate hypertrophy). The soft palate was resting close to the posterior pharyngeal wall as a result of maxillary retrusion. The tongue was retropositioned as a result of a retrognathic mandible. The patient had previously undergone 2 years of orthodontics and achieved a Class I canine and molar occlusion. The anterior and posterior facial heights were relatively normal. After consultation with his medical and dental team (general dentist, orthodontist, pediatrician, and sleep specialist), the patient agreed to a surgical approach. Orthodontic appliances were placed to protect the periodontium, and he underwent jaw and intranasal surgery. The patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement +10 mm); bilateral sagittal split ramus osteotomies (horizontal advancement +10 mm); osseous genioplasty (horizontal advancement +10 mm); and septoplasty and inferior turbinate reduction. Three months after surgery, he underwent an attended polysomnogram that confirmed the resolution of the obstructive sleep apnea with an apnea–hypopnea index of 2.6 events/hour. He no longer snored or experienced restless sleep. Daytime fatigue improved, as did his behavioral problems and his schoolwork. A, Frontal views with smile before and after surgery. B, Oblique facial views before and after surgery. C, Profile views before and after surgery. D, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. E, Articulated dental casts indicate analytic model planning. F, Lateral cephalometric radiographs before and after treatment.

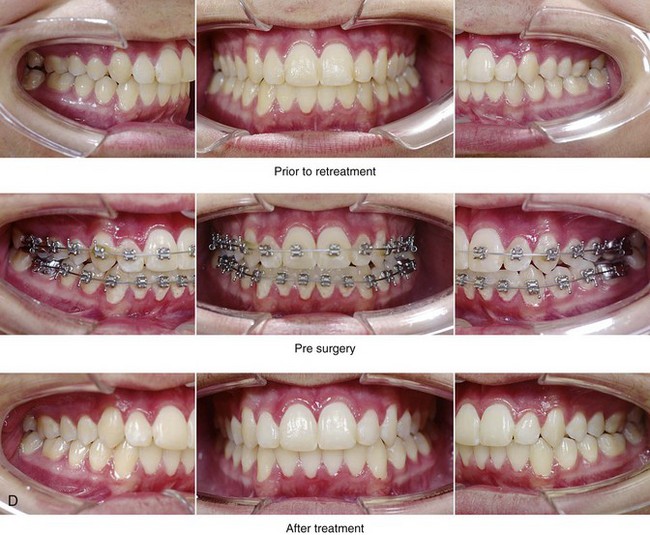
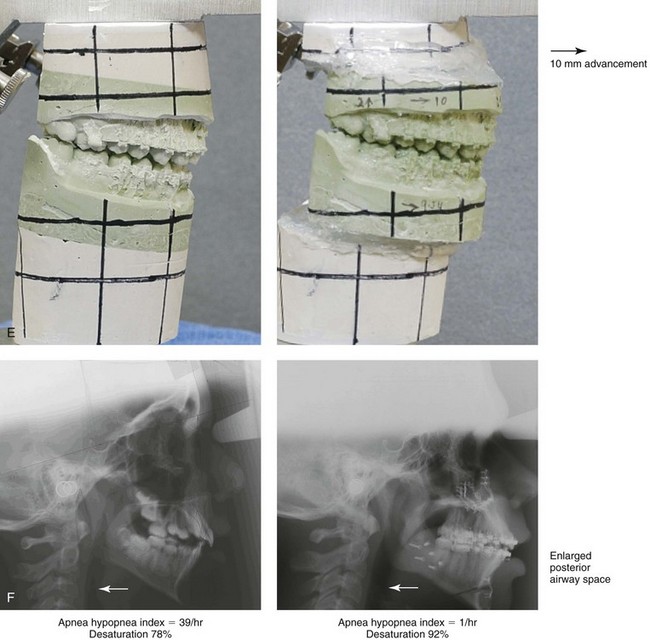
Figure 26-5 A 15-year-old boy was referred by his pediatrician and arrived with his parents for the surgical evaluation of documented obstructive sleep apnea. He had a history of heavy snoring, restless sleeping, and daytime somnolence. He was diagnosed with attention-deficit/hyperactivity disorder when he was 3 years old, and his school grades are poor. An attended polysomnogram confirmed an overall apnea–hypopnea index of 17 events/hour. In the supine position, his apnea–hypopnea index was 39 events/hour with desaturation to 78%. Head and neck examination confirmed a normal-sized and functioning soft palate and tongue. The tonsils and adenoids had previously been removed, and the intranasal cavity was obstructed (septal deviation and inferior turbinate hypertrophy). The soft palate was resting close to the posterior pharyngeal wall, and the tongue was retropositioned. Both the maxilla and the mandible were deficient in horizontal projection, which explained the reduced airway space. The anterior facial height was mildly deficient. The patient previously underwent orthodontics and had achieved a Class I canine and molar occlusion. In consultation with medical and dental specialists (general dentist, orthodontist, pediatrician, and pulmonologist), the patient agreed to a surgical approach. Orthodontic appliances were placed to protect the periodontium, and he underwent jaw and intranasal surgery. The patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement +10 mm); bilateral sagittal split ramus osteotomies (horizontal advancement +10 mm); osseous genioplasty (horizontal advancement); and septoplasty and inferior turbinate reduction. Three months after surgery, he underwent an attended polysomnogram that confirmed the resolution of the obstructive sleep apnea with an apnea–hypopnea index of 0.9 events/hour and a maximum desaturation of 92%. He was no longer snoring or experiencing restless sleep. His daytime fatigue, behavioral problems, and schoolwork all improved. A, Frontal views with smile before and after surgery. B, Oblique facial views before and after surgery. C, Profile views before and after surgery. D, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. E, Articulated dental casts indicate analytic model planning. F, Lateral cephalometric radiographs before and after treatment.
Managing the Airway during a Surgical Procedure in the Individual with Obstructive Sleep Apnea
A question has been raised regarding the risk of intubation in a morbidly obese individual with a baseline diagnosis of severe OSA. Neligan and colleagues completed a prospective study to assess whether severity of OSA, neck circumference, and BMI were risk factors for difficult intubation in morbidly obese patients.248 Each study patient (n = 180) underwent preoperative PSG, Mallampati scoring, thyromental distance measurement, mouth opening assessment, and neck circumference measurement. Each study patient was scheduled to undergo a surgical procedure that required orotracheal intubation. A systematic approach to the intubation was developed by the authors that included the placement of the individual into the “ramp” position to facilitate intubation by aligning the three axes: the pharyngeal axis, the laryngeal axis, and the tracheal axis. The ramp position was achieved by placing padding below the individual’s back to elevate the head, upper body, and shoulders above the level of the chest. This was judged by positioning the external auditory meatus at the same horizontal level as the sternal notch. Of the 180 patients enrolled in the study, 124 were documented by PSG to have severe OSA (mean AHI, 31.3 events/hour). The median Mallampati score was 2; the mean neck circumference was 43.9 cm; and the mean Cormack and Lehane grade was 1. The incidence of anticipated difficult laryngoscopy, which was defined as a Cormack and Lehane grade of 3 or 4, was 8.3%. Of the study patients with documented OSA (n = 124), only six required three or more intubation attempts, and only 3.3% were judged as being “difficult to intubate.” A Mallampati score of 3 or 4 predicted difficult intubation, and a mean neck circumference of 43.9 predicted difficulty with laryngoscopy. The study showed no direct relationship between the diagnosis or severity of OSA and the difficulty of laryngoscopy or intubation. There was no relationship between neck circumference or BMI and difficult intubation. The authors believe that their successful management of intubation requirements in this morbidly obese group related to their meticulous approach to airway management and their technique of using the “ramp” position, which aligns the airway axes and facilitates intubation. Some anesthesiologists prefer to use either a GlideScope (Verathon Medical, Bothell, Wash) or an awake fiber-optic nasotracheal laryngoscopy technique when faced with a morbidly obese individual who requires intubation (see Chapter 11).
Treatment Options for Obstructive Sleep Apnea
Recognizing that a significant subgroup of adults who have been diagnosed with OSA have a chronic illness is essential when considering treatment options. In general, obese adults with OSA should be thought of in a similar way to those with diabetes or hypertension. For these individuals, the total elimination of the disease is not generally feasible.63,147,169,344 The goal of any treatment modality in this situation is to improve or control the symptoms and the health risks by reducing the severity. A clear exception to this way of thinking is in the individual with a small-lumen upper airway that is primarily the result of retrognathic jaws. For these individuals, who make up approximately one third of patients with OSA, the correction of the jaw deficiency is expected to be successful for most.29,44,82,144,149,186,197,283,285,311,312
A review of the literature that addresses the management of OSA confirms that a plethora of publications discuss a spectrum of medical and surgical treatment options.181,207 More than a dozen “non-surgical” treatment options are mentioned, including palatal, tongue, and jaw training exercises; controlled sleep positioning; spray medications to reduce soft-tissue edema or to improve muscle tone; the use of a spectrum of oral appliances that have been designed to protrude the lower jaw; and the use of CPAP delivered through a variety of occlusive devices over the mouth and nose. The majority of these treatment recommendations (with the exception of CPAP) are empiric and frequently recommended as “simple” options in an attempt to avoid surgery or the need for CPAP. In addition, more than a dozen surgical options are offered with the intention of affecting either a specific region of the pharynx or a tissue type within the head and neck (e.g., external nasal valves, internal nasal valves, nasal septum, nasal turbinates, soft palate, adenoids, uvula, tonsillar pillars, tonsils, tongue, maxilla, mandible, chin, hyoid bone). Ideally, to ensure compliance with the recommended treatment, 1) the medical or surgical approach should have a biologic basis that is directed at the sites of pathology; 2) a reasonable probability of success should be anticipated; 3) an acceptable level of risk should be clarified; and 4) a realistic level of patient acceptance should be expected.
Bariatric Surgery
Rasheid and colleagues completed a prospective study in a consecutive series of patients (n = 100) that underwent gastric bypass for the management of obesity to determine the effects of the surgery on OSA.290 Before surgery, all patients underwent PSG. The mean preoperative RDI was 40 ± 4. Thirteen patients had no OSA, 29 had mild OSA, and 58 had moderate to severe OSA. At 6 months after surgery, patients’ BMIs had improved (38 ± 1 kg/m2 versus 54 ± 1 kg/m2), and so had their ESS scores (6 ± 1 versus 12 ± 0.1). There were also a significant reductions in minimum desaturations during sleep as well as improvements in sleep efficiency. A shortcoming of the study was its conclusion at just 6 months after surgery.
A study by Pillar and colleagues describes the long-term follow up of patients with regard to both weight loss and upper airway findings after bariatric surgery.273 At 4 months after surgery, there was a significant improvement in the AI from 45 events/hour to 11 events/hour. Over a 7-year time frame, the AI regressed from a mean of 11 events/hour back up to 24 events/hour; however, the AI remained at a much lower level (24 events/hour) than it was before surgery (45 events/hour). In their report, the effectiveness of bariatric surgery was to reduce the mean BMI from 40 kg/m2 (i.e., morbid obesity) to a mean BMI of 35 kg/m2 (i.e., obesity) at 7 years after surgery. During the same time frame, the mean AI was reduced from 45 events/hour down to 24 events/hour. Other researchers have also studied the long-term effects of bariatric surgery on patient health.52,97,116,134
Continuous Positive Airway Pressure Option
The use of CPAP for OSA was first reported in 1981. Since its introduction, it has become the reference standard for non-surgical management.43,65,66,71,105,107,114,132,148,159,160,178,181,217,236,267,356,357 With this method, positive pressure can be continuously delivered through a sealed nasal or mouth mask that the patient wears while sleeping. The positive pressure has the potential to pneumatically splint open the whole pharyngeal airway by preventing the soft palate and the tongue from making a seal with the posterior pharyngeal mucosa (i.e., it acts as a counteracting force to the aforementioned collapsing force in the patient with an anatomic predisposition for airway obstruction). The pressure required to achieve this goal is titrated at a sleep laboratory during PSG. CPAP is a sound, evidence-based treatment option that has been shown to be effective when it is used correctly and consistently throughout the night as well as night after night throughout the patient’s life. If CPAP is used in this way, it will decrease daytime sleepiness and therefore likely improve the patient’s mood and quality of life. The discomfort and difficulty associated with the use of CPAP (e.g., drying of the nasal and oral mucous membranes, dislodgment during sleep, noise, inconvenience when transporting the unit, social displeasure, feelings of claustrophobia) makes long-term compliance a clear problem. Even when defining an acceptable CPAP compliance rate of just 4 hours per night for 70% of nights, only a limited percentage of patients (depending on the study reviewed) who consider themselves users of CPAP are adherent. This does not take into account the significant number of those with OSA who either will not consider trying CPAP or those who have tried it but then refuse to use it. Furthermore, this “acceptable compliance rate” represents only approximately 50% of functional sleep time.77,252,291,318,385 Sleep medicine clinicians continue to appreciate the complexity of the disorder (i.e., complex chemoreceptor apnea and apnea-related behavioral derangements), and they now acknowledge the inability of a majority of patients to stick with CPAP for the long term, even if they do use it for the short term. Nevertheless, all individuals should be advised of the availability of CPAP and encouraged to consider it.
The American Heart Association/American College of Cardiology Foundation released a position statement about the effects of sleep apnea on cardiovascular disease.133 The organizations summarized published studies that confirmed the moderate and variable effects of CPAP therapy for OSA on blood pressure. They concluded that individuals with more severe OSA, difficult-to-control hypertension, and better CPAP compliance are likely to have more substantial blood pressure reduction with CPAP. They also recommended that, for those hypertensive patients who were either not willing to use CPAP or not compliant, other effective methods of managing OSA should be considered (e.g., MMCA).
Oral Appliance Option
The objective of an oral appliance (OA) for the treatment of OSA is to mechanically reposition and hold the mandible in a protruded (forward) location during sleep.259 As the mandible moves forward, so does the tongue. An effective OA must prevent the sealing of the tongue to the posterior pharyngeal mucosa, thereby limiting obstruction at that site.374 The upper airway response to oral appliances varies among individuals. It has been stated that up to 50% of individuals with OSA who use an oral appliance will “respond to some extent.”218 Unfortunately, no matter how technically precise the OA is, it can only affect the retroglossal site.213,325 Most individuals with moderate to severe OSA have obstruction at multiple sites.377 According to published research, the median compliance rate with an OA after 1 year is 39% with a range of 4% to 82%, depending on the study reviewed.96 Frequently stated potential long-term negative effects of OAs include: tooth movement with changes in occlusion; injury to the periodontium; excessive salivation or dry mouth; and temporomandibular disorders including masticatory muscle discomfort.308,310 Other stated reasons for non-compliance include the insufficient reduction of snoring, continued OSA, or social concerns during use. Almeida and colleagues reviewed a series of patients (n = 70) who used OAs to treat snoring or OSA for more than 5 years.294 The authors found that 44% of those patients had suffered unfavorable occlusal, dental, or temporomandibular joint changes. In research that was carried out by Pancer and colleagues, 14% of study patients (n = 106) were found to have evidence of occlusal changes with the use of an OA for the treatment of OSA.261 The most common pathologic tooth movements were lingual tipping of the maxillary incisors, proclination of the mandibular incisors, and anterior shifting of the lower first molars. Although some patients were unaware of the occlusal changes, many were acutely bothered by the “shifts” (alterations) in their bites. Bondenmark and Bondenmark and Lindman observed patients (n = 32) after 2 years of continuous OA use.31,32 Occlusal changes were documented on lateral head radiographs in the study patients. The authors hypothesized that temporomandibular joint remodeling had occurred. To review, the use of OAs is not generally therapeutic for moderate to severe OSA, and OAs are often not well tolerated in the long term.
Indications for Upper Airway Surgery and Spectrum of Options
Surgery for OSA is indicated when applicable conservative therapies are unsuccessful, intolerable, or unacceptable to the patient with underlying specific anatomically correctible abnormalities that are causing the OSA.76,80,81,139,193–195,198,202,280,282,285,304,307,358,375 Unfortunately, the use of either CPAP or an OA requires continued personal application of a mechanical device. Likely for this reason, neither is consistently well tolerated or found to be acceptable to the patient in the long run. Indications for surgery to treat OSA are often stated to be a failure of medical management in the individual with one of the following symptoms:
• Excessive daytime sleepiness as a result of OSA
• RDI of more than 20 events/hour
• RDI of less than 20 events/hour but with marked excessive daytime sleepiness
• Oxygen desaturations of less than 90% with apnea events
• Documented hypertension, arrhythmia, or both believed to be triggered by OSA
• Negative esophageal pressures (i.e., >−10 cm of water)
• Anatomic abnormalities of the upper airway (i.e., jaw deformity, fixed intranasal obstructions, chronically enlarged tonsils, enlarged floppy soft palate) that result in apnea events
Although tracheostomy may be considered a way of achieving a “cure” for OSA because it completely bypasses all obstructions of the upper airway, secondary medical and social problems as a result of the tracheostomy cannot be overlooked.242,368,369 In general, this option is only considered for extreme cases (e.g., newborns or infants with congenital deformities such as the severe retrognathia seen with Treacher Collins syndrome) or as a temporary solution.
Uvulopalatopharyngoplasty (UPPP)—the surgical removal of the uvula, additional parts of the soft palate, the pharyngeal pillars, and the tonsils—to open the airway was originally described by Fujita in 1981.98,99 Although the effectiveness of this approach for the resolution of OSA is limited, it continues to be an often mentioned treatment option, because it is considered by some to be minimal surgery or a first treatment step (see Figs. 26-3 and 26-6).2,40,58,93,119,189,272,298,334,342 The reason for its limited success is because it addresses only the retropalatal site, and it does so in an incomplete way.209 In general, it will shorten the soft palate, but it may also result in a thicker and narrower retropalatal pharyngeal airway as a result of scarring. Interestingly, although improvements in snoring generally occur after UPPP, over time, the AHI actually increases in approximately 30% of patients. This is called silent apnea; the relief of the snoring may lead the individual to falsely believe that his or her apnea has also gone away. Realistically, UPPP is expected to be therapeutic only for those with massive soft-tissue redundancy of the pharynx and without concomitant intranasal and retroglossal obstruction.95,192,204,336,335,391 When either the adenoids, the tonsillar tissue, or the soft palate is significantly hypertrophic or redundant and causing physical blockage of the upper airway, surgery to remove this excessive tissue should be considered. In the absence of a specific syndrome or anomaly that causes increased tongue volume (e.g., Proteus syndrome, vascular anomalies, Beckwith-Wiedemann syndrome), this is rarely a cause of OSA.47,353 Frequent concerns after intrapharyngeal soft-tissue reduction procedures (e.g., UPPP, tonsillectomy, tongue reduction) include perioperative edema (airway compromise); moderate to severe pain for 2 to 3 weeks; limited success with a single-site approach for the resolution of OSA; velopharyngeal reflux; velopharyngeal insufficiency; pharyngeal scarring (stenosis); and silent apnea.173,226

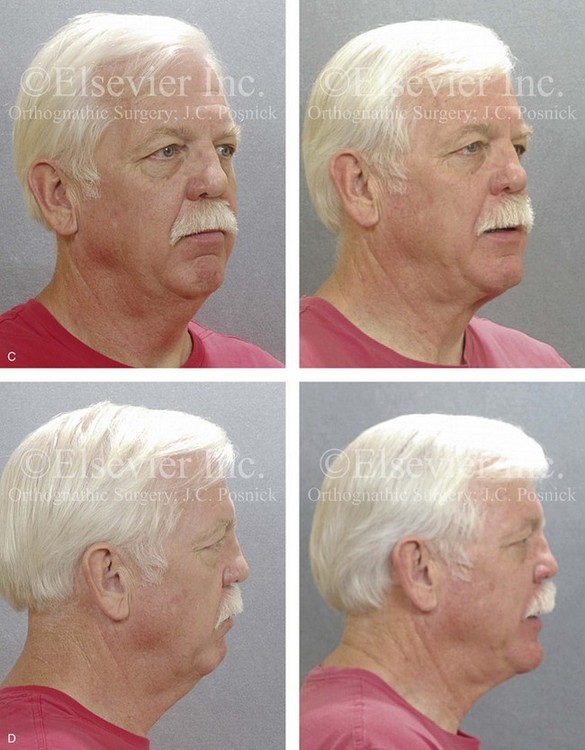
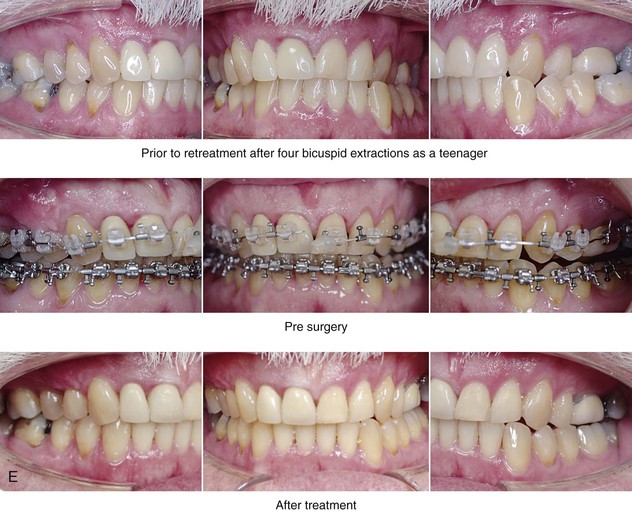
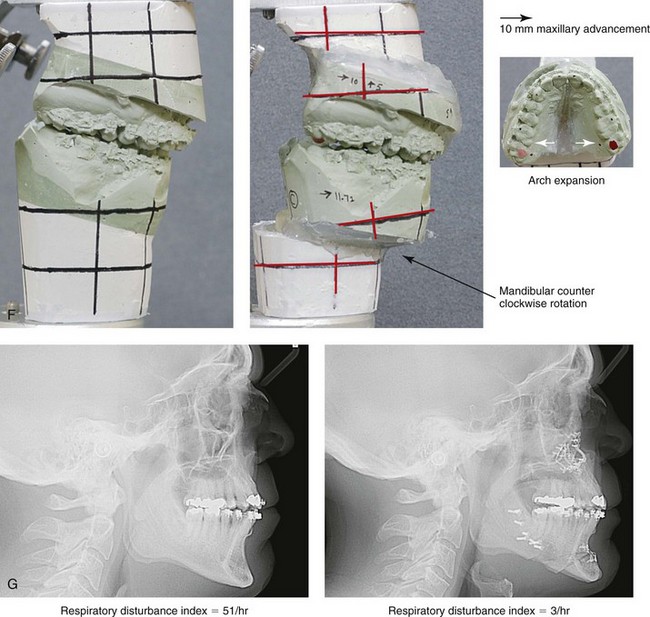
Figure 26-6 A 59-year-old man was referred by his otolaryngologist/head and neck surgeon to discuss further surgical options for the management of documented obstructive sleep apnea. He had already undergone uvulopalatopharyngoplasty, septoplasty, and inferior turbinate reduction. The procedures helped his snoring, but a postsurgery attended polysomnogram confirmed a respiratory disturbance index of 51 events/hour, with desaturations reaching as low as 82%. The patient was also seen by a pulmonary sleep specialist and a cardiologist; mild concentric left ventricular hypertrophy and mild pulmonary hypertension we found. The patient was unable to tolerate continuous positive airway pressure on a regular basis. He lost 20 pounds and was exercising regularly, but this did not resolve his obstructive sleep apnea. Examination confirmed a short face growth pattern with maxillomandibular deficiency (decreased vertical height and limited horizontal projection). During his teenage years, the patient had undergone orthodontic treatment that included four bicuspid extractions. He was now showing signs of periodontal deterioration, and he had a posterior crossbite on the left side. He underwent evaluation by a periodontist, a restorative dentist, and an orthodontist. He agreed to a comprehensive dental rehabilitation approach, with a primary interest in achieving an improved upper airway with the resolution of the obstructive sleep apnea. The sleep specialist and the otolaryngologist were also supportive of this approach. The patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement +10 mm and vertical lengthening +5 mm) with interpositional graft; bilateral sagittal split osteotomies of the mandible (horizontal advancement +12 mm); osseous genioplasty (horizontal advancement); redo septoplasty; and anterior arch soft-tissue procedures (cervical flap elevation, fat removal and platysma muscle plication). Three months after surgery, an attended polysomnogram was carried out, which confirmed a respiratory disturbance index of less than 5 events/hour with the lowest desaturations at 90%. Subjectively, the patient feels that his snoring has resolved, his nighttime sleeping is improved, and his daytime somnolence is greatly diminished. A, Frontal views in repose before and after surgery. B, Frontal views with smile before and after surgery. C, Oblique facial views before and after surgery. D, Profile views before and after surgery. E, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery.
If the maxilla and the mandible are retrusive then consideration should be given to the surgical advancement of these structures to correct the baseline anatomic deformity .193–195,201,215,277,345,398 MMCA has been shown to enlarge the pharyngeal and hypopharyngeal airway by physically expanding the whole facial skeletal framework (see Figs. 26-4 and 26-7 through 26-14). Because the lateral pharyngeal walls are dynamic, the skeletal expansion also tightens the soft tissues; this subsequently decreases lumen collapsibility. This skeletal approach address’s the “small airway” via an extrapharyngeal operation with minimal risk of early postoperative edema-induced upper airway compromise or long-term pharyngeal dysfunction. This operation is also thought to enhance the neuromuscular tone of the pharyngeal dilator musculature (e.g., tensor veli palatini, genioglossus). These anatomic facts explain the highly successful outcomes that have been documented after MMCA. Frequent short-term (perioperative) concerns in the adult patient after MMCA are most often related to: altered occlusion; dysesthesia (sensory loss); limited mouth opening; adjustment to the change in facial appearance; and the downtime required for recovery (see Chapter 25). If septal deviation or thickening or inferior turbinate enlargement or hypertrophy results in significant nasal airway resistance, then these abnormalities can be addressed simultaneously with MMCA (see Chapter 10).94,191,247,275,276,278 The medical risks associated with general anesthesia in conjunction with orthognathic and intranasal procedures are expected to be greater among adults with OSA than among teenagers. These risks may include the potential for stress to the cardiovascular, renal, and respiratory systems (see Chapters 11 and 16).

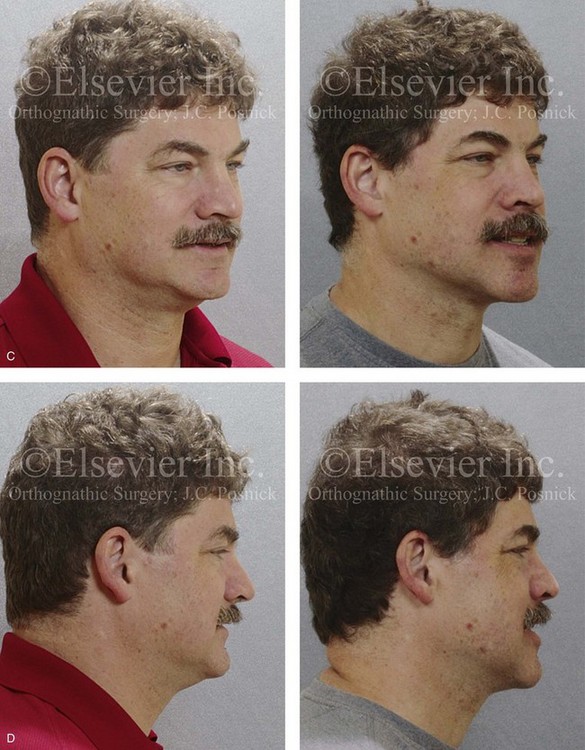
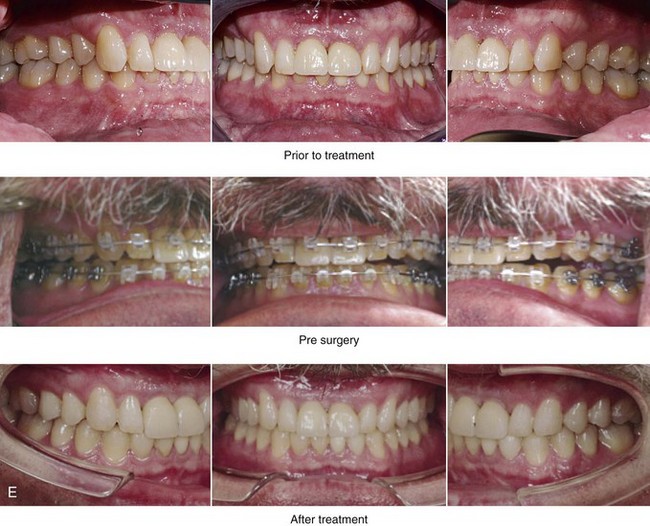
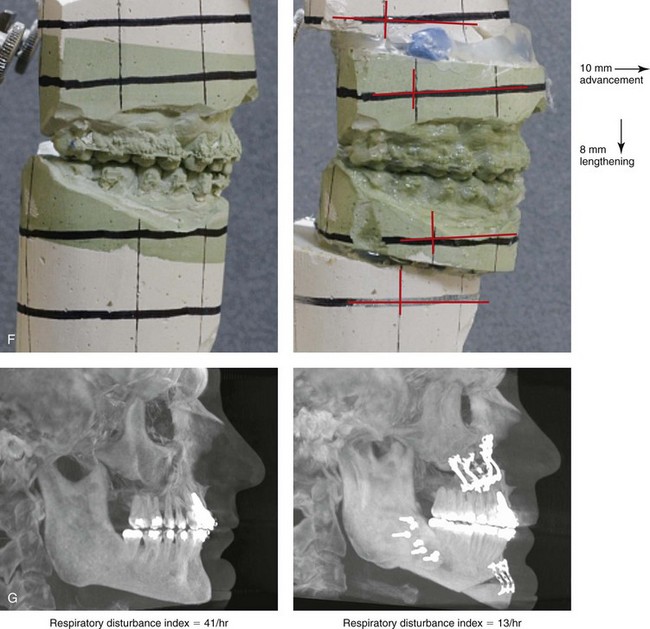
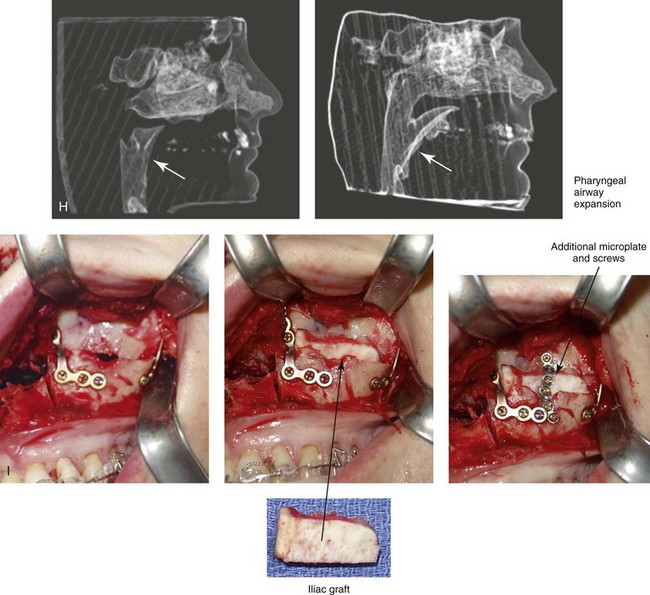
Figure 26-7 A 51-year-old man was referred by a neurologist to discuss surgical options for the management of documented obstructive sleep apnea. He had heavy snoring, restless sleeping, and significant daytime somnolence. A recent attended polysomnogram confirmed a respiratory disturbance index of 41 events/hour with oxygen desaturation of as low as 80%. A continuous positive airway pressure titration study was effective at 12 cm of pressure; however, the patient was not able to tolerate continuous positive airway pressure. Intranasal examination confirmed a deviated septum and hypertrophic inferior turbinates. The patient’s tonsils and adenoids were minimal in size. The soft palate functioned well, but the uvula was enlarged. The tongue was of normal size and function. During his teenage years, the patient had undergone orthodontic treatment, and he had a Class I deep bite. Examination confirmed a short face growth pattern that involved deficient vertical height and limited horizontal projection. Consultations with a sleep specialist, an internist, a general dentist, an otolaryngologist/head and neck surgeon, and an orthodontist were carried out. The patient agreed to a surgical approach that included orthodontics as needed to protect the periodontium. The procedures included maxillary Le Fort I osteotomy (horizontal advancement +10 mm and vertical lengthening +8 mm) with interpositional allograft; bilateral sagittal split osteotomies of the mandible (horizontal advancement); osseous genioplasty (horizontal advancement); uvulectomy; and septoplasty and inferior turbinate reduction. After surgery, the patient’s snoring was resolved, his nighttime sleeping improved, and his daytime somnolence was greatly diminished. A 1-year postoperative polysomnogram confirmed a respiratory disturbance index of 13 events/hour. A, Frontal views in repose before and after surgery. B, Frontal views with smile before and after surgery. C, Oblique facial views before and after surgery. D, Profile views before and after surgery. E, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery. H, Computed tomography scans reformatted to indicate pharyngeal airway space before and after surgery. I, Close-up intraoperative view of the left side Le Fort I osteotomy site with plate and screw fixation in place (zygomatic buttress and piriform aperture regions). Also shown is the corticocancellous iliac graft that was crafted and then inset in the defect; this was followed by additional plate and screw fixation of the graft.
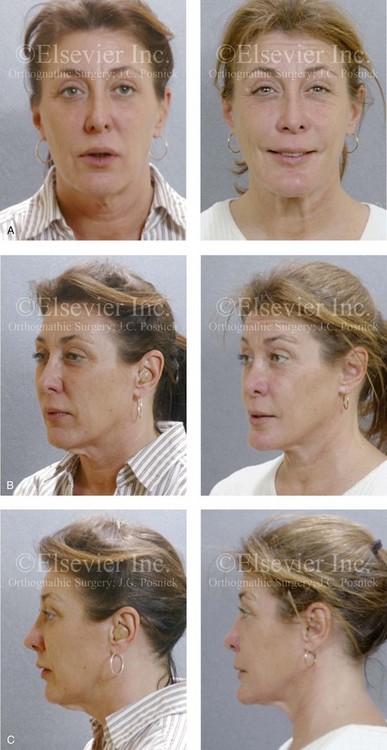
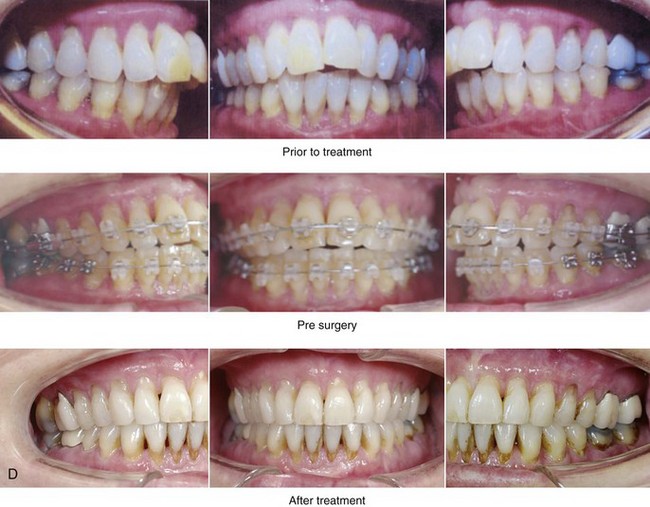
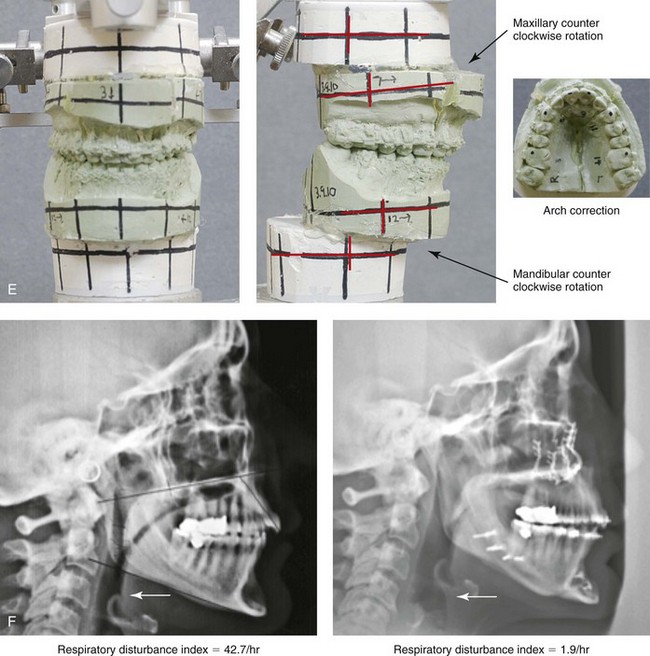
Figure 26-8 A 50-year-old woman was referred by her general dentist to an orthodontist and then for surgical evaluation. She had a mandibular deficiency and a constricted upper jaw width growth pattern that resulted in a Class II deep-bite excess overjet malocclusion. Over the years, there was destruction to the dentition and the periodontal apparatus with generalized bone loss and gingival recession. She was now under the care of a restorative dentist and a periodontist. To resolve the malocclusion, maxillary expansion and mandibular advancement in combination with orthodontics were recommended. As part of her referral to this surgeon, a history was taken that documented heavy snoring, restless sleeping, and daytime somnolence. An attended polysomnogram confirmed a respiratory disturbance index of 42.7 events/hour. There was intranasal site obstruction as a result of septal deviation and inferior turbinate enlargement. There was also retropalatal obstruction caused by maxillary retrusion and a retropositioned tongue that resulted from mandibular retrusion. The soft palate and the tongue were of normal size and function. There were minimum tonsil and adenoid structures. Continuous positive airway pressure was tried but not well tolerated. Surgery was agreed to in combination with orthodontics to improve the malocclusion and open the airway. The procedures that were carried out included maxillary Le Fort I osteotomy (horizontal advancement +7 mm, vertical lengthening +3 mm, and arch form correction); bilateral sagittal split osteotomies of the mandible (horizontal advancement +12 mm); osseous genioplasty (horizontal advancement); septoplasty and inferior turbinate reduction; and an anterior approach to the neck (cervical flap elevation, supraplatysmal defatting, and vertical platysma muscle plication). Subjectively, there was good relief of snoring, restless sleeping, and daytime fatigue. One year after surgery, an attended polysomnogram confirmed the resolution of the obstructive sleep apnea, with a respiratory disturbance index of 1.9 events/hour. A, Frontal views in repose before and after treatment. B, Oblique facial views before and after treatment. C, Profile views before and after treatment. D, Occlusal views before treatment, with orthodontics in progress, and after treatment. E, Articulated dental casts that indicate analytic model planning. F, Lateral cephalometric radiographs before and after surgery.

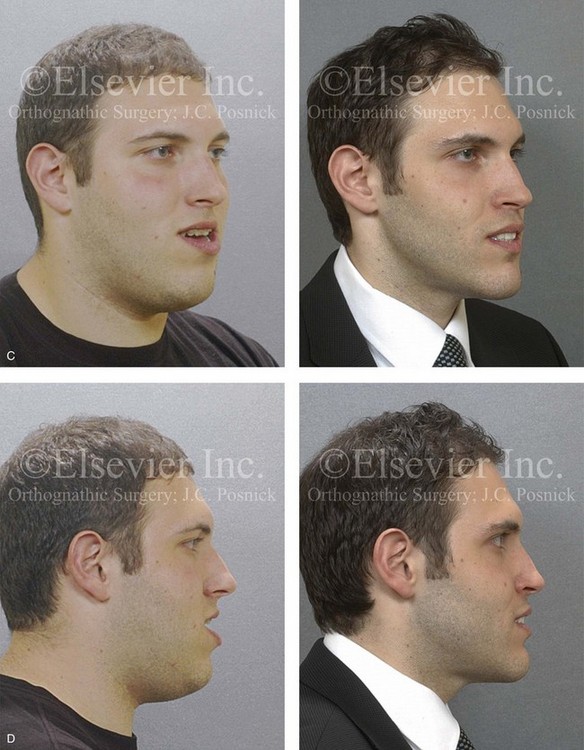
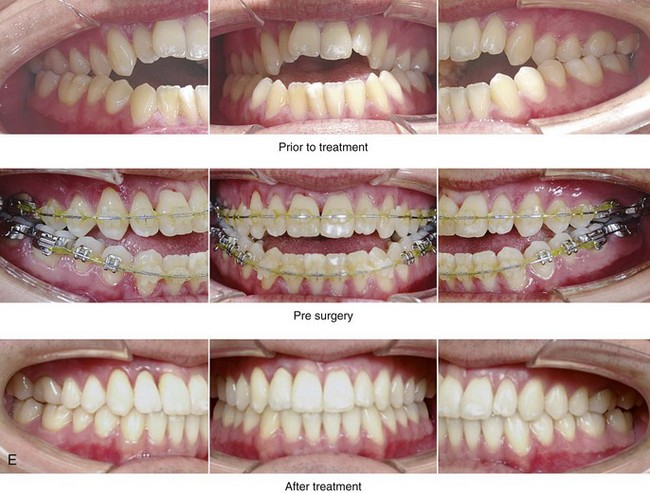
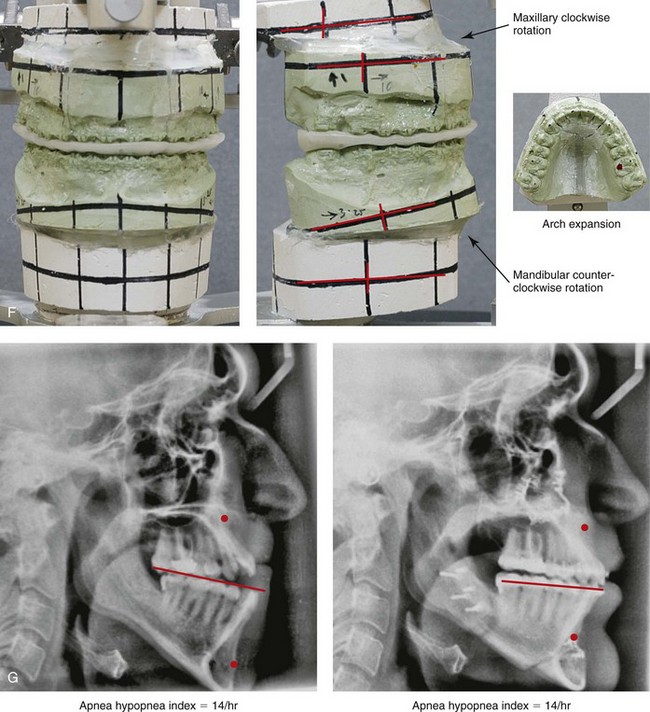
Figure 26-9 A 25-year-old man was referred by his general dentist to an orthodontist and then to this surgeon for the evaluation of a dentofacial deformity. Examination confirmed a maxillary deficiency and a relative mandibular excess growth pattern; this resulted in a severe Class III negative overjet anterior open-bite malocclusion. The maxillomandibular deformities had a negative impact on the patient’s speech articulation, swallowing, chewing, breathing, and lip closure/posture. The patient’s history confirmed lifelong obstructed nasal breathing, restless sleeping, heavy snoring, and daytime fatigue. An attended polysomnogram was carried out and documented a respiratory disturbance index of 14 events/hour, with desaturations of as low as 87%. The patient underwent additional evaluations by a sleep specialist, an otolaryngologist, a speech pathologist, a restorative dentist, and a periodontist. He agreed to a comprehensive surgical and dental rehabilitative approach, and his periodontal treatment was followed by orthodontic decompensation. The patient’s procedures included maxillary Le Fort I osteotomy in segments (horizontal advancement +10 mm, arch expansion and minimal clockwise rotation) with interpositional graft; bilateral sagittal split osteotomies of the mandible (horizontal advancement +4 mm and counterclockwise rotation); osseous genioplasty (horizontal advancement); and inferior turbinate reduction and septoplasty. The patient experienced relief of his snoring, improvements in his sleep quality, less daytime fatigue, and improved breathing during the day. Interestingly, the 4-month postoperative attended polysomnogram indicated only mild reduction of the sleep indexes. Reevaluation by the sleep specialist recommended the avoidance of supine sleep, with no need for further treatment. A, Frontal views in repose before and after surgery. B, Frontal views with smile before and after surgery. C, Oblique facial views before and after surgery. D, Profile views before and after surgery. E, Occlusal views before treatment, with orthodontic appliances in place, and after treatment. Note the pulpal necrosis of the right maxillary lateral incisor, which required root canal therapy and crown coverage. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery.
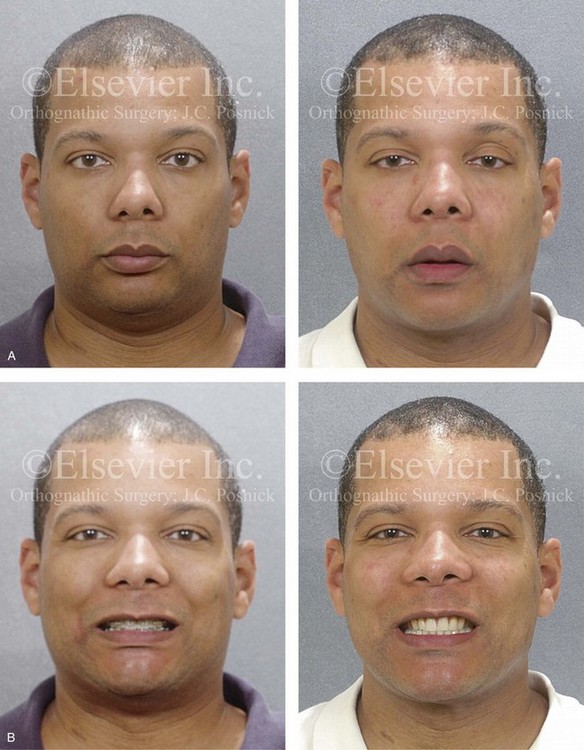
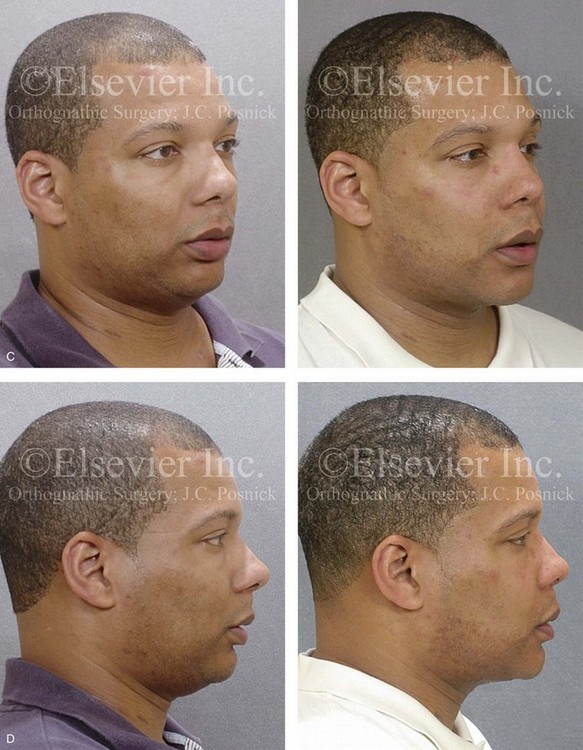
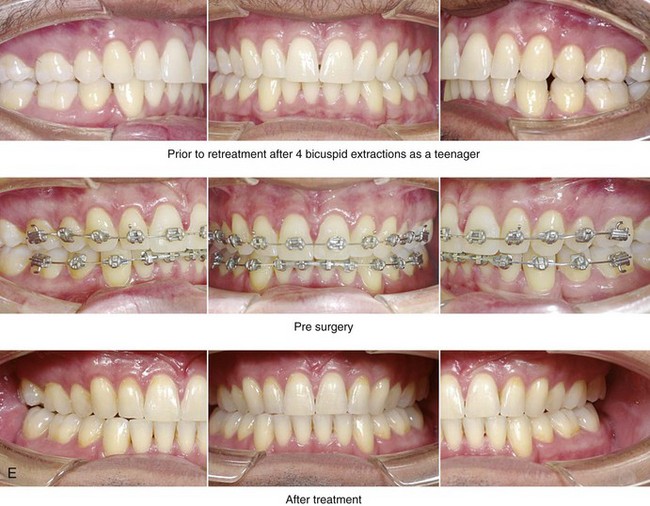
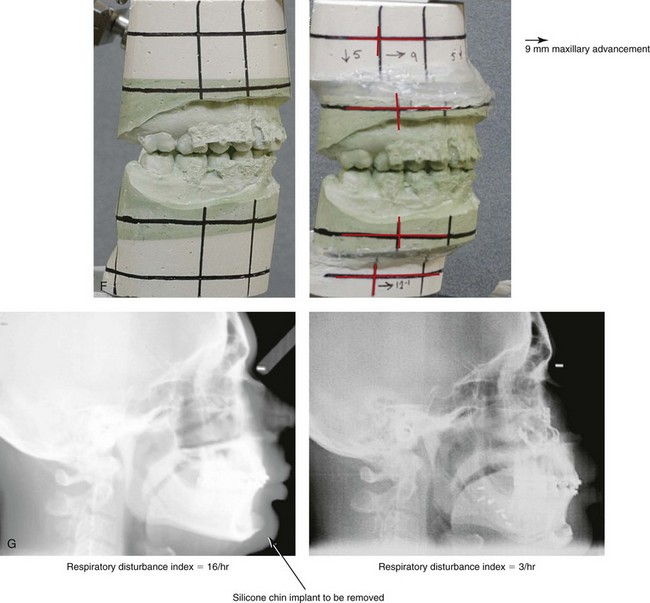
Figure 26-10 A 37-year-old man was referred to this surgeon with a request to have his chin implant removed because he was displeased with the aesthetic results. When he was 20 years old, he had undergone a chin augmentation (silicone implant) in an attempt to camouflage the small lower jaw profile. Ten years later, he underwent four bicuspid extractions and orthodontic alignment to improve the occlusion. He had undergone a septorhinoplasty procedure, but he was still experiencing continued difficulty breathing through the nose. During the patient’s referral to this surgeon, an examination confirmed maxillomandibular deficiency. He was recognized as having a short face growth pattern with decreased lower anterior facial height and limited facial projection. There was an accentuated labiomental “lip curl” and a “button appearance” of the chin. It was clear that the patient suffered with snoring, restless sleeping, and excessive daytime fatigue. An attended polysomnogram confirmed a respiratory disturbance index of 16 events/hour, with desaturations of 89%. A titration study confirmed the need for 12 cm of water pressure to reduce the respiratory disturbance index to 3 events/hour. A, Frontal views in repose before and after surgery. B, Frontal views with smile before and after surgery. The use of continuous positive airway pressure was attempted, but the patient found it to be uncomfortable and difficult to use. Consultations with a sleep specialist, an otolaryngologist, and an orthodontist confirmed the advantage of an intranasal and orthognathic surgical approach. The patient agreed to perioperative orthodontics and surgery to improve the airway and facial appearance. The patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement +9 mm, vertical lengthening +5 mm) and interpositional grafting; bilateral sagittal split osteotomies of the mandible (horizontal advancement +12 mm); removal of the chin implant; osseous genioplasty (horizontal advancement and vertical lengthening) with interpositional grafting; reduction of the inferior turbinates; redo septoplasty; and an anterior approach to the neck (cervical flap elevation, supraplatysmal defatting, and vertical platysma muscle plication). As a result of the procedures, the patient experienced relief of his snoring, improved sleep quality, diminished daytime fatigue, and improved breathing during the day. Six months after surgery, he underwent an attended polysomnogram that indicated a normal sleep study, with no snoring or apnea events. C, Oblique facial views before and after surgery. D, Profile views before and after surgery. E, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery (note the preoperative chin implant).

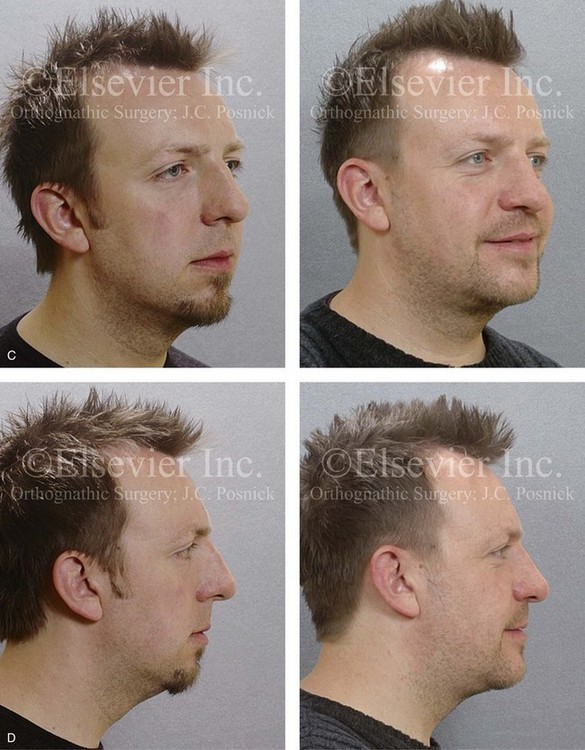
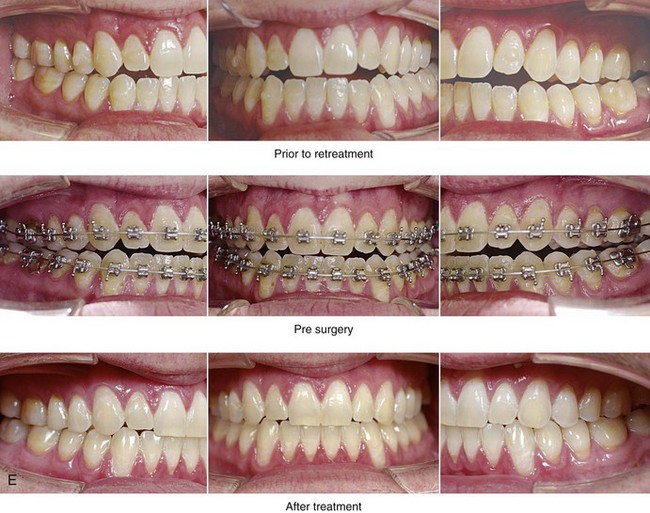
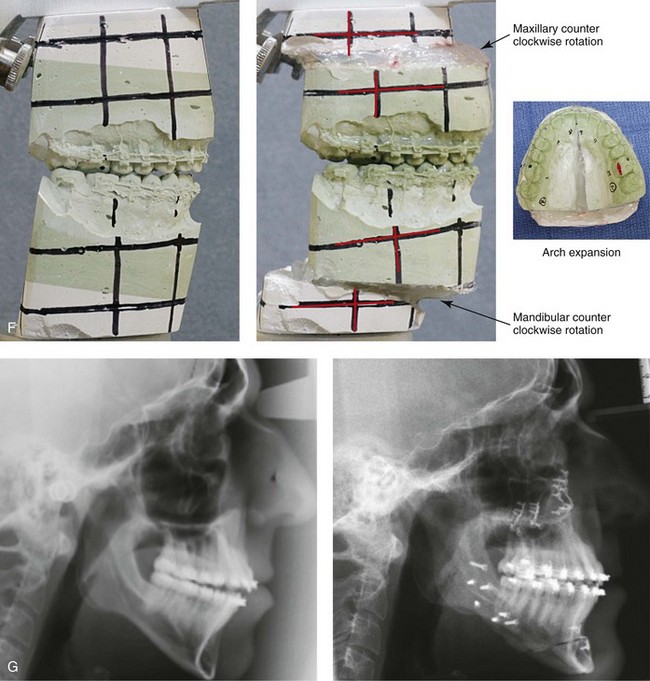
Figure 26-11 A 33-year-old man was referred by his orthodontist for the surgical evaluation of a dentofacial deformity. Examination confirmed a long face growth pattern with a Class III anterior open-bite malocclusion. During the mixed dentition, the patient underwent growth modification and orthodontic treatment that included the use of a Frankel appliance, a chin cup, and rapid palatal expansion. By the end of high school, he was left with a residual anterior open bite, a constricted maxillary arch, and a gummy smile. When he was 29 years old and had a lifelong history of heavy snoring, difficulty sleeping at night, and daytime fatigue, he underwent a sleep study. The polysomnogram revealed moderate obstructive sleep apnea with a respiratory disturbance index of 16 events/hour and oxygen desaturations down to 86%. A continuous positive airway pressure titration study confirmed the need for 10 cm of water pressure to resolve the respiratory events. The current head and neck examination documented a deviated septum; enlarged inferior turbinates; an elevated nasal floor; narrow pyriform rims; a soft palate close to the pharyngeal wall; and a retropositioned tongue. The tonsils were not enlarged, and the tongue and soft palate were of normal size. A, Frontal views in repose before and after surgery. B, Frontal views with smile before and after surgery. The patient hoped to achieve improved breathing during the day, better sleeping at night, and resolution of his obstructive sleep apnea. He also wanted correction of his malocclusion, improvements in his lip closure/posture, and an enhanced profile and smile. He agreed to a comprehensive orthodontic and surgical approach. With the orthodontic relief of his dental compensation, intranasal, orthognathic, and facial surgery was carried out. The patient’s procedures included maxillary Le Fort I osteotomy in three segments (horizontal advancement +8 mm, vertical shortening −4 mm, and arch expansion); bilateral sagittal split osteotomies of the mandible (horizontal advancement +12 mm and counterclockwise rotation); osseous genioplasty (horizontal advancement and vertical shortening); reduction of the inferior turbinates, septoplasty, and recontouring of the nasal floor; and an anterior approach to the neck (cervical flap elevation, supraplatysmal defatting, and vertical platysma muscle plication). He experienced the relief of his snoring, improvement in his quality of sleep, the ability to breathe through the nose during the day, and less daytime fatigue. He had not yet undergone a formal posttreatment polysomnogram. C, Oblique facial views before and after surgery. D, Profile views before and after surgery. E, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery.
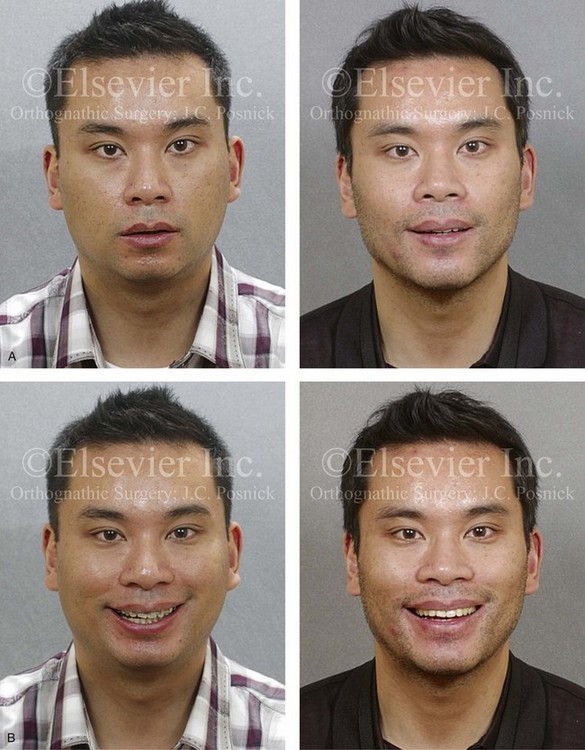
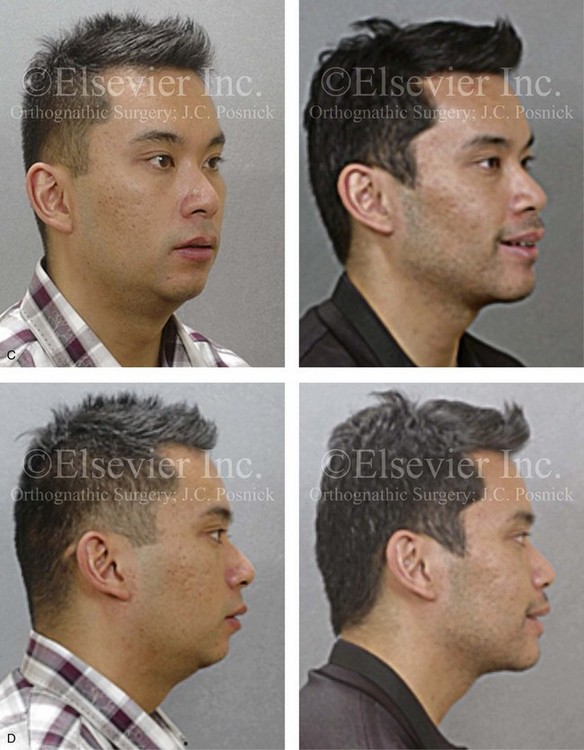
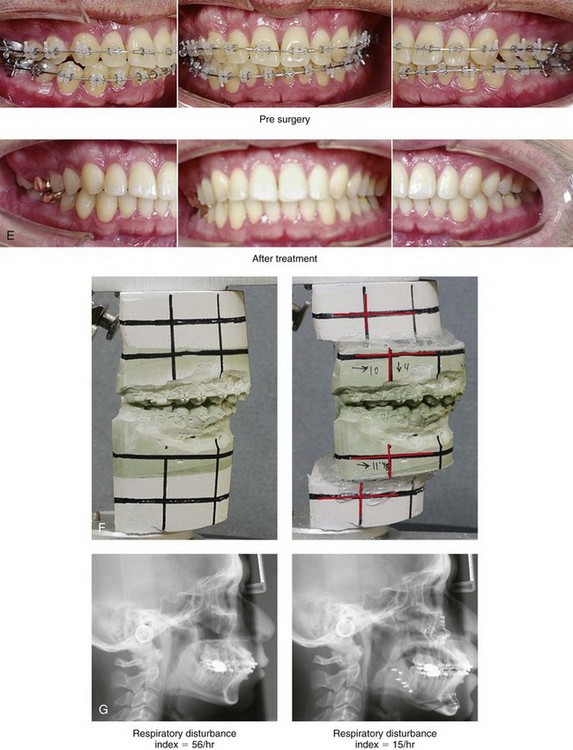
Figure 26-12 A 32-year-old man was referred by his internist and otolaryngologist/head and neck surgeon to discuss further surgical options for the management of document obstructive sleep apnea. An attended polysomnogram confirmed a respiratory disturbance index of 56 events/hour, with oxygen desaturations reaching as low as 84%. The patient’s sleep efficiency was reduced to 46%. He underwent a split study that confirmed the need for 8 cm of continuous positive airway pressure to significantly reduce the respiratory disturbance index. He was unable to effectively use the continuous positive airway pressure device as a result of claustrophobia, excessive noise, and poor mask fit. Since his early childhood years, the patient was known to have a jaw deformity with malocclusion. He underwent 2 years of orthodontic treatment when he was 12 to 14 years old; this resulted in improvement of the occlusion but residual jaw deformity. Examination confirmed a short face growth pattern with maxillomandibular deficiency (decreased vertical height and limited horizontal projection). The tonsils were not inflamed, but they were moderately enlarged, and the uvula was mildly long and floppy. The tongue was of normal size, the septum was straight, and the inferior turbinates were enlarged. A, Frontal views in repose before and after surgery. B, Frontal views with smile before and after surgery. The patient underwent evaluation by a restorative dentist, an orthodontist, a sleep specialist, an otolaryngologist/head and neck surgeon, and an internist. His primary motivations were to achieve an improved airway and to resolve his obstructive sleep apnea. He was also interested in improved profile aesthetics and long-term dental health. We discussed potential benefits of tonsillectomy and of the reduction of the size of the soft palate, but he preferred a skeletal approach, thus reserving the option of soft-tissue reduction if needed as a secondary procedure. With orthodontic appliances in place, the patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement +10 mm and vertical lengthening +4 mm) with interpositional allogenic graft; bilateral sagittal split osteotomies of the mandible (horizontal advancement +12); osseous genioplasty (horizontal advancement); and inferior turbinate reduction. He experienced relief of his snoring, improvement in his quality of sleep, better breathing during the day, and less daytime fatigue. Three months postoperatively, he underwent an attended polysomnogram. Interestingly, after surgery, he chose to sleep with his lips taped together out of the belief that this improves sleep hygiene, including air humidification. Thus, the sleep study was carried out with the patient’s lips taped together. An overall respiratory disturbance index of 15 events/hour was documented, with average desaturations to 95%. C, Oblique facial views before and after surgery. D, Profile views before and after surgery. E, Occlusal views before and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery.
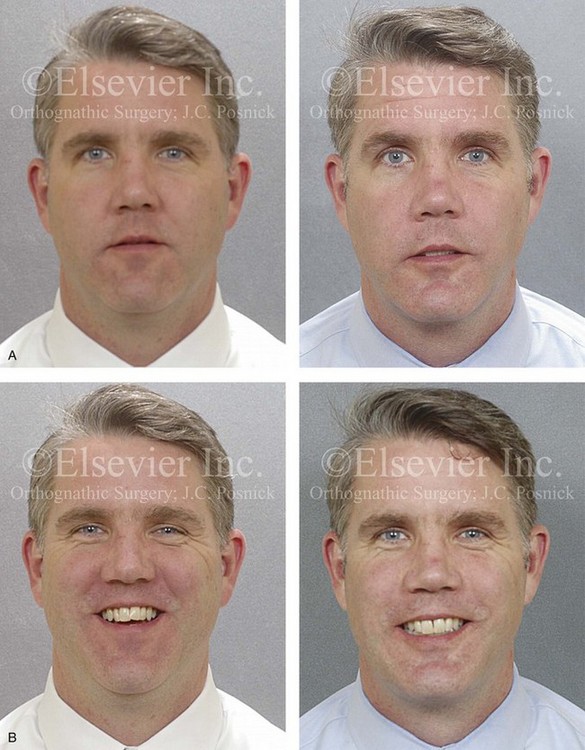
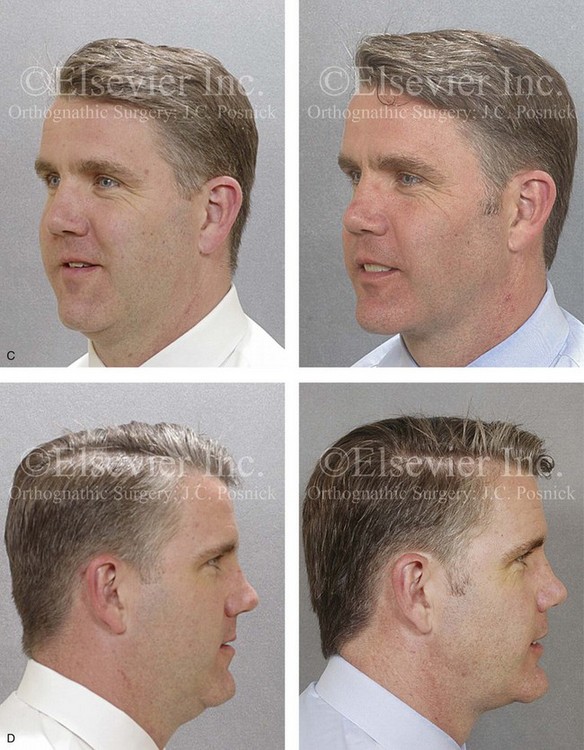
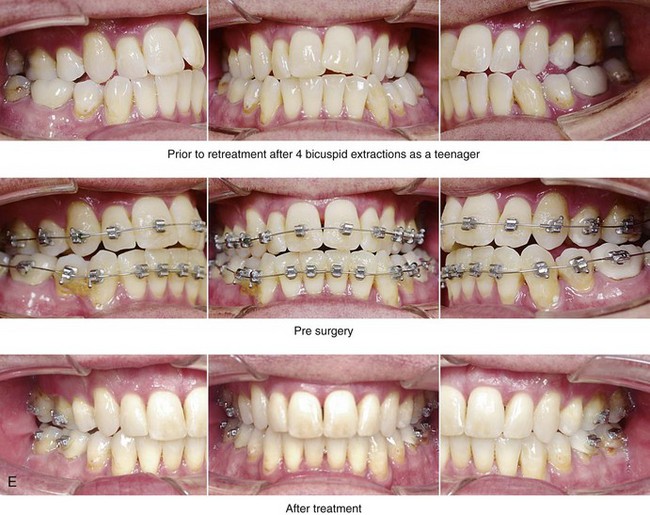
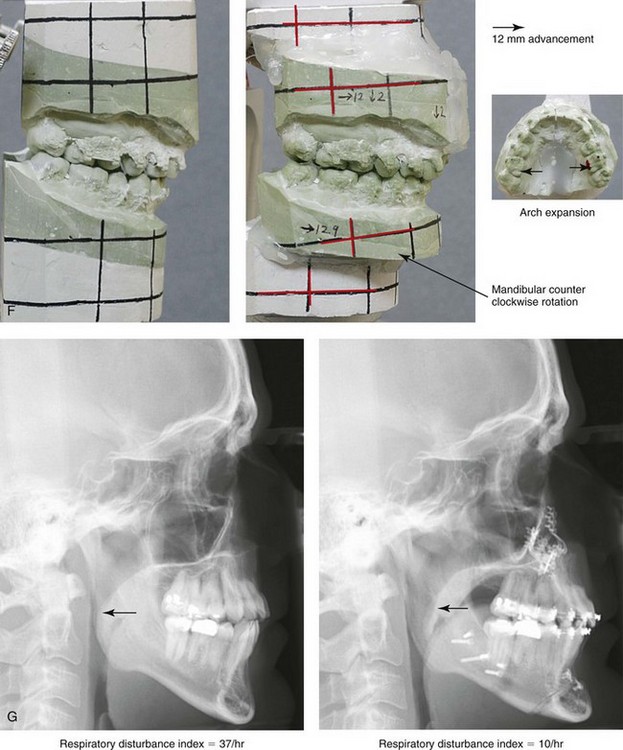
Figure 26-13 A 43-year-old man was referred by a restorative dentist to an orthodontist and then for surgical evaluation of a dentofacial deformity characterized by maxillomandibular deficiency. Despite orthodontic treatment (including four bicuspid extractions when the patient was a teenager), there remained a Class III negative overjet bilateral posterior cross-bite malocclusion. A degree of labial bone loss and gingival recession was appreciated. Earlier during his life, the patient’s nose was broken during a sports injury, and he had since experience chronic obstructive breathing. Examination confirmed a deviated septum and inferior turbinate enlargement. The tonsils, the tongue, and the soft palate were essentially normal; the uvula was somewhat enlarged. He admitted to heavy snoring, restless sleeping, and excessive daytime fatigue. A sleep study confirming a respiratory disturbance index of 37 events/hour with desaturations reaching a low of 86%. The patient was fitted with a continuous positive airway pressure device (8 cm of water pressure per hour) as temporary treatment while his orthodontics were initiated. After the orthodontic relief of dental compensation, surgery was carried out. The patient’s procedures included maxillary Le Fort I osteotomy in two segments (horizontal advancement +10 mm, vertical lengthening −2 mm, and arch expansion) with allogenic interpositional grafting; bilateral sagittal split osteotomies of the mandible (horizontal advancement +13 mm and counterclockwise rotation); osseous genioplasty (horizontal advancement); inferior turbinate reduction and septoplasty; and an anterior approach to the neck (cervical flap elevation, supraplatysmal defatting, and vertical platysma muscle plication). After surgery, there was an immediate relief of the patient’s snoring as well as improvements in his quality of sleep, his ability to breathe through his nose, his concentration, and his daytime fatigue. The 6-month postoperative attended polysomnogram confirmed a reduction in the respiratory disturbance index to 10 events/hour. A, Frontal views in repose before and after surgery. B, Frontal views with smile before and after surgery. C, Oblique facial views before and after surgery. D, Profile views before and after surgery. E, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery.

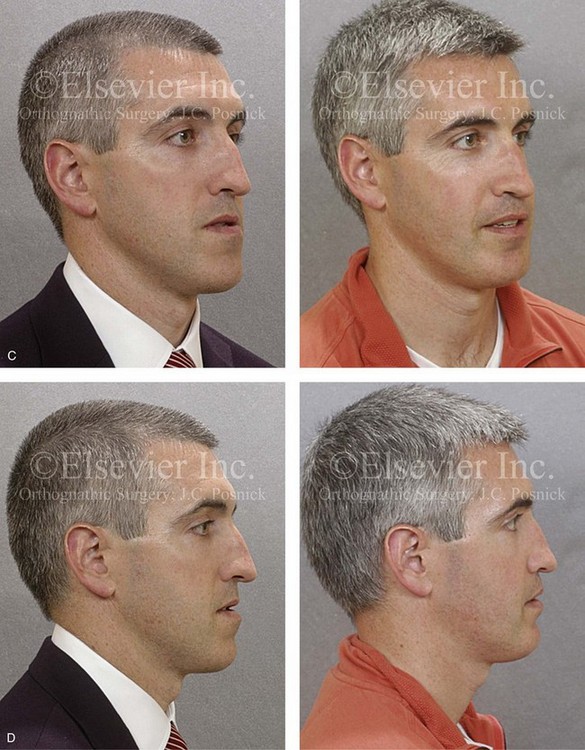
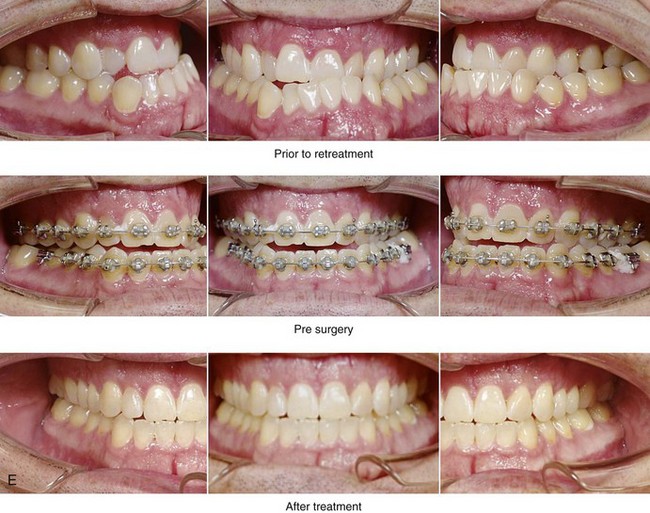
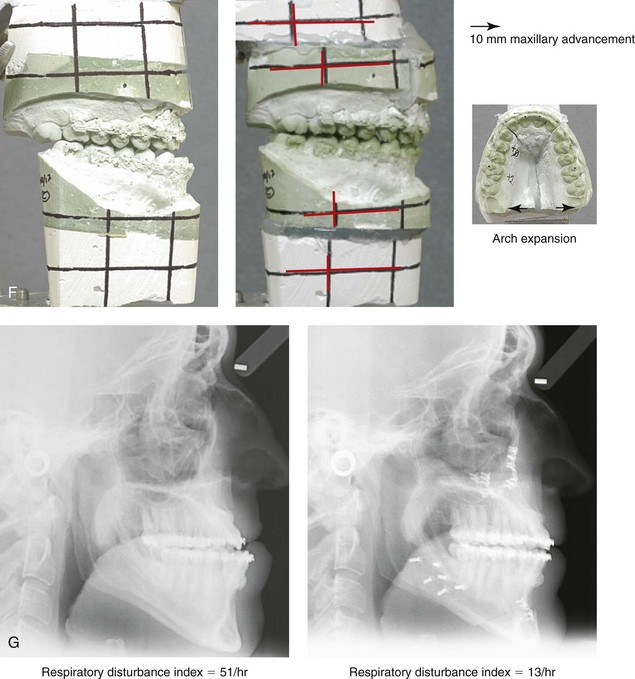
Figure 26-14 A 34-year-old man was referred by his orthodontist for a surgical evaluation of a longstanding dentofacial deformity with malocclusion. Examination confirmed a long face asymmetric Class III growth pattern with an anterior open-bite, negative overjet, posterior crossbite malocclusion. During the mixed dentition, growth modification maneuvers were attempted. Compensatory orthodontic mechanics were then used but did not result in correction. A residual gummy smile with lip incompetence and asymmetric Class III negative overjet malocclusion remained. The patient had undergone septoplasty and tonsillectomy when he was 20 years old in an attempt to improve the airway but without success. At referral to this surgeon, the patient’s history confirmed ongoing heavy snoring, difficulty sleeping at night, an inability to breathe through the nose, and excessive daytime fatigue. The patient underwent an attended polysomnogram that confirmed a respiratory disturbance index of 19 events/hour, with desaturations reaching as low as 84%. A continuous positive airway pressure titration study was carried out, and the use of continuous positive airway pressure was attempted; however, as a result of side effects, it was not practical for this patient. A head and neck examination documented a severely deviated septum, enlarged inferior turbinates, an elevated nasal floor, narrow pyriform rims, a soft palate that was close to the pharyngeal wall, and a retropositioned tongue. The tonsils had previously been removed, and the tongue and soft palate were of normal size. A, Frontal views in repose before and after surgery. B, Frontal views with smile before and after surgery. The patient hoped to achieve improved breathing during the day, better sleeping at night, and the relief of excessive daytime fatigue. He also desired the correction of his malocclusion to improve swallowing, speech, chewing, and lip closure as well as to enhance his profile and smile aesthetics. He agreed to a comprehensive orthodontic and surgical approach. No extractions were required, and the orthodontic relief of dental compensation was accomplished. The patient’s jaw and intranasal procedures included maxillary Le Fort I osteotomy in three segments (horizontal advancement +8 mm, vertical shortening −1.0 mm, dental midline correction, clockwise rotation of 2 mm, and arch expansion) with interpositional graft; bilateral sagittal split osteotomies of the mandible (horizontal advancement +2 mm); osseous genioplasty (vertical shortening and minimal horizontal advancement); and inferior turbinate reduction, septoplasty, and recontouring of the nasal floor. The patient experienced immediate relief of his snoring as well as improvements in quality of sleep, ability to breathe through the nose during the day, and daytime fatigue. An attended polysomnogram was carried out 4 months after surgery and demonstrated the relief of apnea in all sleep positions other than supine. When sleeping supine, mild obstructive sleep apnea remained, but there were no longer any significant desaturations. C, Oblique facial views before and after surgery. D, Profile views before and after surgery. E, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery.
The advantage of a combined intranasal and jaw operation for the individual with OSA is that, like CPAP, such a procedure effectively addresses all of the obstructing upper airway sites simultaneously (i.e., intranasal, retropalatal, and retroglossal). Straightening the septum; reducing the inferior turbinates; widening the maxilla and the piriform rim (i.e., segmental Le Fort I osteotomy), if indicated; and recontouring (deepening) the nasal floor, if indicated—in combination with MMCA—dramatically opens the whole upper airway. Posnick and colleagues have confirmed that jaw and intranasal procedures can be carried out simultaneously without increasing complications.278
The Maxillary–Mandibular–Chin Advancement Option
Background
Clinicians now recognize that maxillomandibular deficiency results in reduced pharyngeal airway dimensions and that it can, on its own, lead to or be an additive factor for OSA in both children and adults (see Figs. 26-4 through 26-14).11,17,154,158,162,184,239,271,285,341,354,363,380,383,390 Mandibular advancement was first suggested in 1979 by West and colleagues as an alternative to tracheostomy for the treatment of OSA.181 By the early 1980s, Riley and colleagues further documented the value of mandibular advancement surgery that is specifically carried out for the treatment of OSA.297,306 They advocated simultaneous maxillary and mandibular advancement (MMA) to open the upper airway and to diminish apnea episodes among appropriate individuals with OSA. This surgical approach has consistently been shown to be a highly effective modality for opening the whole upper airway passage for individuals with OSA* (see Figs. 26-4 through 26-14).
Prinsell and colleagues stated the following: “MMA surgery for obstructive sleep apnea syndrome is a highly successful and potentially definitive primary single-stage surgery that may result in a significant reduction in OSA-related health risks, as well as financial savings for the health care system.”284,285 Li and colleagues reached the same conclusion and stated the following: “Maxillomandibular advancement surgery is the most effective procedure for OSA.”197 In a surgical update published by the American Association of Oral and Maxillofacial Surgeons, Moore affirmed these conclusions: “Jaw advancement surgery is better tolerated and has a far more significant and beneficial effect than most airway soft tissue surgical procedures [for OSA]. Excellent long-term [5-year] results have been documented with favorable effects on quality of life.”238
The abundance of published data now confirms that those individuals with documented OSA; anatomic findings of minimal tonsillar tissue and minimal redundant pharyngeal soft tissues; and physical findings of recessive jaws will not sufficiently respond to surgical procedures that are less thorough than MMCA. With today’s state of knowledge, a treatment philosophy that suggests that an individual with documented OSA must “fail” Phase I soft-tissue reduction surgery before proceeding to Phase II skeletal expansion surgery” is like saying that a patient with a complex tumor defect must first fail a skin graft before undergoing an effective vascularized flap reconstruction.209 Although an automatic “soft-tissue first” and “step-wise” surgical approach to OSA made sense during the 1980s, it does not make sense for the 21st century. The soft palate is a dynamic tissue organ with the primary function of preventing the reflux of air and liquids into the nasopharynx during speech and swallowing. Unfortunately, partial surgical ablation or resection of the soft palate (i.e., UPPP) may produce dysfunction, such as velopharyngeal insufficiency, stenosis, voice changes, dysphagia, or silent apnea. In addition, immediate postoperative concerns of pain, hemorrhage, and upper airway obstruction must also be considered. For these reasons, a case can be made for treating the most severe and critical site or area of obstruction first. This will frequently mean performing MMCA initially and then following up later with soft-tissue procedures, when indicated. This skeletal approach will enlarge and stabilize the entire velo-orohypopharyngeal upper airway to definitively treat the OSA and frequently to obviate the need for other soft-tissue procedures (e.g., UPPP, tongue reduction, tonsillectomy).
To review, treatment recommendations for OSA should be made in accordance with the patient’s specific anatomic, physiologic, and psychosocial factors. OSA is commonly the result of multiple anatomic deformities that are correctable. The concept of a Phase I soft-tissue approach that is followed by Phase II skeletal procedures as a last resort should be discarded in favor of treating the specific anatomic abnormalities that are contributing to the individual’s OSA, preferably during a single operation.104
Review of Published Studies concerning Maxillomandibular Advancement Surgery
Critical commentary in the earlier medical literature regarding the spectrum of surgical options may leave sleep physicians and others skeptical of the efficacy of any operative procedure for their patients with OSA. With the use of nasal CPAP as the reference for OSA therapy, a standard for a successful outcome has been established; this standard defines an acceptable compliance rate as 4 hours of CPAP per night for 70% of nights. However, this represents only approximately 50% of sleep time involving a normal airway. Li suggested that an equivalent standard for rating a successful surgical outcome is a 50% reduction in the RDI and fewer than 20 events/hour.200 If we keep this proposed standard for treatment success in mind, then the literature clearly supports an MMA approach to OSA. Clinical studies that were published between 1989 and 2012 and that were carried out to evaluate the effectiveness of MMA as a treatment approach for OSA are reviewed in the remainder of this section.
Waite and colleagues described 23 consecutive patients with OSA documented by PSG (RDI range, 11 events/hour to 104 events/hour) who underwent MMA in addition to septoplasty and inferior turbinate reduction.381 Twenty-two of 23 (96%) of the study patients were successfully treated. Successful treatment was based on achieving an RDI of less than 20 events/hour or that represented a more than 50% reduction in RDI. Sixty-five percent of the patients (15/23) achieved an RDI of less than 10 events/hour.
Riley and colleagues reported on 40 patients with OSA documented by PSG who underwent maxillomandibular advancement.302 Thirty-nine of the 40 study patients (97%) were successfully treated. Successful treatment was based on achieving a postoperative RDI of less than 20 events/hour or a more than 50% reduction in RDI and normal or near normal oxygen saturation. A mean presurgical RDI of 67 events/hour was reduced to 9 events/hour.
Riley and colleagues described 306 consecutively treated surgical patients with PSG-documented OSA.305 A two-phase surgical protocol was used to reconstruct the upper airway. Phase I surgery consisted of UPPP and genioglossus advancement with hyoid myotomy suspension. Patients for whom Phase I surgery was unsuccessful were offered Phase II reconstruction in the form of maxillomandibular advancement. Surgery was considered successful if the postoperative RDI was less than 20 events/hour with normal oxygenation. Ninety-one patients who failed Phase I treatment entered the Phase II protocol. All patients who underwent Phase II treatment (i.e., maxillomandibular advancement) also had perioperative orthodontics. The success of Phase II therapy was 97% (i.e., RDI <20 events/hour with normal oxygenation).
Hochban and colleagues discussed 38 consecutive patients with OSA documented by PSG (RDI >20 events/hour) who underwent MMA (10-mm maxillary advancement); 37 of 38 of the study patients (95%) were successfully treated.144 Successful treatment was based on achieving a postoperative AHI of less than 10 events/hour. This clinical series included only healthy, non-obese patients with a documented dentofacial skeletal deformity.
Prinsell reported on 50 consecutive patients with PSG-documented OSA (mean AHI, 59 events/hour) who underwent MMA, with or without adjunctive procedures.283 All 50 patients were successfully treated. Successful treatment was based on the achievement of a postoperative AHI of 5 events/hour or less or a more than 60% reduction in RDI.
Li and colleagues described 175 patients with PSG-documented OSA (mean RDI, 72 events/hour) who underwent MMA.200 One hundred sixty-six of the 175 patients (95%) were successfully treated. Successful treatment was based on achieving a postoperative RDI of less than 20 events/hour or a more than 50% reduction in RDI. The mean preoperative RDI of 72 events/hour was reduced to a mean postoperative RDI of 7.2 events/hour. Eighty-six of the 175 patients (49%) had undergone ineffective Phase I surgery before MMA. The cure rate for these patients in this study was 97% (83 out of 86). The mean age of the patients in this study who underwent Phase II treatment (i.e., MMA) was 44 years.
Bettega and colleagues completed a prospective study of a consecutive series of individuals (n = 51) with severe OSA (mean AHI, 59 events/hour).29 All of these patients underwent surgical therapy. The surgery was considered successful if the postoperative AHI was less than 15 events/hour with at least a 50% reduction from presurgical rates. Forty-four of the patients initially underwent Phase I surgery, and the success rate of the Phase I procedures was only 22.7% (10 of 44 patients). Twenty of the 51 patients underwent MMA, including 13 patients for whom Phase I surgery had failed and 7 who underwent MMA as the primary treatment. On average, the AHI decreased from 59 events/hour to 11 events/hour after the MMA procedure. Overall, 15 of the 20 individuals undergoing MMA (75%) were considered to have successful outcomes.
Li and colleagues studied a consecutive series of 12 individuals with OSA that had been documented by PSG (mean RDI, 75 events/hour) and who had undergone MMA.197 The authors were able to demonstrate a decrease in airway obstruction and collapse after MMA as documented by lateral cephalometric radiographs and by fiber-optic nasopharyngoscopy during passive respiration. Postoperative fiber-optic nasopharyngoscopy with the Müller maneuver specifically documented a decrease in airway collapsibility; an improvement in the retro displacement of the tongue base; and an improvement in lateral wall stability. The mean preoperative RDI was 75 events/hour, which improved by 6 months after surgery to 10.8 events/hour. Ten of the 12 patients who had been treated with MMA (83%) were felt to be cured (RDI <20 events/hour).
Fairburn and colleagues described 20 consecutive patients with PSG-documented OSA (AHI range, 26 events/hour to 134 events/hour) who had undergone MMA with at least 10 mm of mandibular advancement.82 Eighteen of the 20 study patients (90%) were successfully treated. Successful treatment was based on the achievement of a significant reduction in the AHI. Ten of the 20 study patients (50%) achieved an AHI of less than 10 events/hour. There was a mean improvement of 60 events/hour. In addition, there was a measured enlargement of the upper airway space (in both the lateral and the anteroposterior dimensions) for all patients at all levels.
Vicini and colleagues completed a prospective, randomized, controlled trial to assess the effectiveness of surgery versus ventilation in adults with documented severe OSA (AHI average of >30 events/hour; range, 31 events/hour to 87 events/hour).376 Fifty consecutive subjects who had severe OSA were enrolled and randomized into a conservative treatment group (i.e., auto-titrating positive air pressure) or a surgical treatment group (i.e., MMA). One year after MMA surgery or continuous auto-titrating positive air pressure treatment, both groups showed a remarkable improvement of mean AHI and ESS levels. The degree of improvement was not statistically different between the two groups. The MMA group (n = 25) had a mean age of 49 years, with a mean BMI of 32.7 kg/m2 and a mean preoperative AHI of 56.8 events/hour. After MMA surgery, the mean postoperative AHI dropped to 8.1 events/hour ± 7.0. There were no fatal events, no significant bleeding events, and no complications that required a surgical revision procedure. According to a survey questionnaire, the subject’s personal perception of the overall surgical experience was highly satisfactory. Twenty-four of the 25 patients stated they could “go through the operation all over again.” MMA proved to be a valuable alternative therapeutic tool in adults with severe OSA, with a success rate equivalent to that of therapeutic ventilation treatment.
Ronchi and colleagues completed a study to evaluate the effectiveness of MMA in patients with PSG-documented OSA.311 Two groups with different skeletal patterns were studied. Group I (n = 11) had moderate or severe OSA and cephalometrically documented mandibular deficiency (i.e., a sella–nasion–A-point angle of ≤78 degrees or a sella–nasion–B-point angle of <65 degrees and a Class II malocclusion). Group II (n = 11) had moderate or severe OSA without cephalometrically documented maxillomandibular hypoplasia (i.e., a sella–nasion–A-point angle of >80 degrees and a Class I malocclusion). Preoperative and postoperative data collected included AHI; posterior airway space measurement; sella–nasion–A-point and sella–nasion–B-point angles; ESS scores; body mass index; and a subjective standardized questionnaire about facial aesthetics. Results documented that all patients (Groups I and II) achieved increased posterior airway space and the remission of OSA and that both groups were comparable. All patients were satisfied with the functional and aesthetic results achieved. In Group I, AHI decreased from 57.9 events/hour to 7.0 events/hour after surgery. Five patients reported slight improvement and six reported great improvement in their aesthetic results. In Group II, AHI decreased from 50.7 events/hour before surgery to 7.6 events/hour after surgery. Five patients reported slight improvement, five reported no changed, and one reported great improvement in facial appearance. No patients (Group I or II) reported a worsening of the aesthetic appearance. The study confirms that MMA is effective for patients with moderate to severe OSA and even in those who are considered to have cephalometrically normal skeletal and Angle-classified occlusions.
Holty and Guilleminault completed an extensive meta-analysis of the outcomes of MMA surgery. A total of 627 patients from 42 study populations were identified from a review of 914 reports.149 The mean age was 44.4 years, and 88% of the study subjects were men. The mean postsurgical follow-up period was 5.3 months, and 67% of patients had previously undergone less invasive OSA treatment procedures without success. The MMA surgical success rate was 86%. As a result of the MMA procedures, the following occurred:
1. BMI decreased from 30.4 kg/m2 to 29.4 kg/m2.
2. AHI improved from 63.9 events/hour to 9.5 events/hour.
3. Oxygen nadir improved from 71.9% to 87.7%.
4. AI improved from 34.7 events/hour to an average of 1.6 events/hour.
5. Quality of life assessments documented a 72% absolute reduction in depression and irritability.
6. Systolic blood pressure improved from 139 mm Hg to 124 mm Hg.
7. Improvement in rapid eye movement sleep and Stage III or IV sleep was significant.
Prinsell completed a literature survey and made treatment recommendations regarding both primary and secondary MMA with or without adjunctive procedures for adults with OSA.44,285 A critical review of all published studies that included six or more cases per series of MMA and other multilevel surgery in adults that also documented preoperative and postoperative AHI was conducted. The AHI data from the case series were tabulated into categories of surgery from which the mean percent reduction of AHI was calculated and used as the primary measure of PSG therapeutic efficacy. The review of published studies indicates a mean percent reduction in AHI as follows:
• An 88.4% reduction of AHI for primary MMA
• An 86.6% reduction of AHI for secondary MMA
• A 79.4% reduction of AHI for primary MMA with combined intrapharyngeal procedures
• A 92.1% reduction of AHI for primary MMA with combined extrapharyngeal procedures
• A 53% reduction of AHI for non-MMA multilevel surgery
References
1. Abbott, MB, Donnelly, LF, Dardzinski, BJ, et al. Obstructive sleep apnea: MR imaging volume segmentation analysis. Radiology. 2004; 232(3):889–895.
2. Aboussouan, LS, Golish, JA, Wood, BG, et al. Dynamic pharyngoscopy in predicting outcome of uvulopalatopharyngoplasty for moderate and severe obstructive sleep apnea. Chest. 1995; 107:946.
3. Abramson, Z, Susarla, AM, Lawler, M, et al. Three-dimensional computed tomographic airway analysis of patients with obstructive sleep apnea treated by maxillomandibular advancement. J Oral Maxillofac Surg. 2011; 69:677–686.
4. Abyholm, F, D’Antonio, L, Davidson Ward, SL, et al. Pharyngeal flap and sphincteroplasty for velopharyngeal insufficiency have equal outcome at 1 year postoperatively: Results of a randomized trial. Cleft Palate Craniofac J. 2005; 42(5):501–511.
5. Accardo, JA, Shults, J, Leonard, MB, et al. Differences in overnight polysomnography scores using the adult and pediatric criteria for respiratory events in adolescents. Sleep. 2010; 33(10):1333–1339.
6. Achilleos, S, Krogstad, O, Lyberg, T. Surgical mandibular setback and changes in uvuloglossopharyngeal morphology and head posture: A short- and long-term cephalometric study in males. Eur J Orthod. 2000; 22:383–394.
7. Adams, GL, Gansky, SA, Miller, AJ, et al. Comparison between traditional 2-dimensonal cephalometry and a 3-dimensional approach on human dry skulls. Am J Orthod Dentofacial Orthop. 2004; 126(4):397–409.
8. Agren, K, Nordlander, B, Linder-Aronsson, S, et al. Children with nocturnal upper airway obstruction: Postoperative orthodontic and respiratory improvement. Acta Otolaryngol. 1998; 118:581–587.
9. Akashiba, T, Kawahara, S, Kosaka, N, et al. Determinants of chronic hypercapnia in Japanese men with obstructive sleep apnea syndrome. Chest. 2002; 121:415–421.
10. Alajmi, M, Mulgrew, AT, Fox, J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea. Hypopnea: A meta-analysis of randomized controlled trials. Lung. 2007; 185:67–72.
11. Al-Saleh, A, Riekstins, A, Forrest, CR, et al. Sleep-related disordered breathing in children with syndromic craniosynostosis. J Craniomaxillofac Surg. 2011; 39:153–157.
12. American Academy of Pediatrics. Clinical practice guideline: Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002; 109:704–712.
13. American Academy of Sleep Medicine (AASM) Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999; 22:667–689.
14. American Academy of Sleep Medicine. International classification of sleep disorders, ed 2, Diagnostic and coding manual. Westchester, Ill: American Academy of Sleep Medicine; 2005.
15. American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996; 153:866–878.
16. American Thoracic Society Workshop Summary. Cardiorespiratory sleep studies in children. Establishment of normative data and polysomnographic predictors of morbidity. Am J Respir Crit Care Med. 1999; 160:1381–1387.
17. Anderson, P, Netherway, D, Abbott, A, et al. Mandibular lengthening by distraction for airway obstruction in Treacher-Collins syndrome: The long-term results. J Craniofac Surg. 2004; 15:47.
18. Athanasiou, AE, Toutountzakis, N, Mavreas, D, et al. Alterations of hyoid bone position and pharyngeal depth and their relationship after surgical correction of mandibular prognathism. Am J Orthod Dentofacial Orthop. 1991; 100:259–265.
19. Avrahami, E, Englender, M. Relation between CT axial cross-sectional area of the oropharynx and obstructive sleep apnea syndrome in adults. AJNR Am J Neuroradiol. 1995; 16:135.
20. Bacon, WH, Turiot, JC, Krieger, J, Stierie, JL. Cephalometric evaluation of pharyngeal obstructive factors in patients with sleep apnea syndrome. Angle Orthod. 1989; 60:115–122.
21. Banerjee, D, Yee, BJ, Piper, AJ, et al. Obesity hypoventilation syndrome: Hypoxemia during continuous positive airway pressure. Chest. 2007; 131:1678–1684.
22. Battagel, JM, Lestange, PR. The cephalometric morphology of patients with obstructive sleep apnea (OSA). Eur J Orthod. 1996; 18:557–569.
23. Bazzano, LA, Khan, Z, Reynolds, K, He, J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007; 50:417–423.
24. Bear, SE, Priest, JH. Sleep apnea syndrome: Correction with surgical advancement of the mandible. J Oral Surg. 1980; 38:543–549.
25. Becker, HF, Jerrentrup, A, Ploch, T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003; 107:68–73.
26. Beebe, DW, Groesz, L, Wells, C, et al. The neuropsychological effects of obstructive sleep apnea: A meta-analysis of norm-referenced and case-controlled data. Sleep. 2003; 26(3):298–307.
27. Berretin-Felix, G, Yamashita, RP, Filho, HN, et al. Short- and long-term effect of surgically assisted maxillary expansion on nasal airway size. J Craniofac Surg. 2006; 17:1045–1049.
28. Borowiecki, B, Kukwa, A, Blanks, RHI, Irvine, CA. Cephalometric analysis for diagnosis and treatment of obstructive sleep apnea. Laryngoscope. 1988; 98:226–234.
29. Bettega, G, Pepin, JL, Veale, D, et al. Obstructive sleep apnea syndrome: Fifty-one consecutive patients treated by maxillofacial surgery. Am J Respir Crit Care Med. 2000; 162:641–649.
30. Blunden, S, Lushington, K, Kennedy, D, et al. Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol. 2000; 22:554–568.
31. Bondenmark, L. Does 2 years’ nocturnal treatment with mandibular advancement splint in adult patients with snoring and OSAS cause a change in the posture of the mandible? Am J Orthod Dentofacial Orthop. 1999; 116(6):621–628.
32. Bondenmark, L, Lindman, R. Craniomandibular status and function in patients with habitual snoring and obstructive sleep apnea after nocturnal treatment with a mandibular advancement splint: A 2-year follow-up. Eur J Orthod. 2000; 22:53–60.
33. Borowiecki, B, Pollak, CP, Weitzman, ED, et al. Fiberoptic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep apnea syndrome. Laryngoscope. 1978; 88:1310–1313.
34. Brietzke, SE, Gallagher, D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: A meta-analysis. Otolaryngol Head Neck Surg. 2006; 134:979–984.
35. Brietzke, SE, Katz, ES, Roberson, DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg. 2004; 131:827–832.
36. Buccheri, A, Dilella, G, Stella, R. Rapid palatal expansion and pharyngeal space: Cephalometric evaluation. Prog Orthod. 2004; 5(2):160–171.
37. Buchwald, J, Avidor, Y, Braunwald, E, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004; 292:1724.
38. Burstein, FD, Cohen, SR, Scott, PH, et al. Surgical therapy for severe refractory sleep apnea in infants and children: Application of the airway zone concept. Plast Reconstr Surg. 1995; 96:34.
39. Cadieux, RJ, Kales, A, McGlynn, T, et al. Sleep apnea precipitated by pharyngeal surgery in a patient with myotonic dystrophy. Arch Otolaryngol. 1984; 110(9):611–613.
40. Cahali, MB, Formigoni, GG, Gebrim, EM, et al. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: A clinical, polysomnographic and computed tomography measurement comparison. Sleep. 2004; 27(5):942–950.
41. Cakarne, D, Urtane, I, Skagers, A. Pharyngeal airway sagittal dimension in patients with Class III skeletal dentofacial deformity before and after bimaxillary surgery. Stomatologija. 2003; 5:13.
42. Campo, A, Fuhbeck, G, Zulueta, JJ, et al. Hyperleptinaemia, respiratory drive and hypercapnic response in obese patients. Eur Respir J. 2007; 30:223–231.
43. Campos-Rodriguez, F, Pena-Grinon, N, Reyes-Nunez, N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005; 128:624–633.
44. Caples, SM, Rowley, JA, Prinsell, JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: A systemic review and meta-analysis. Sleep. 2010; 33:1396.
45. Carlson, D, Onal, ME, Carley, DW, et al. Palatal muscle electromyogram activity in obstructive sleep apnea. Am J Respir Crit Care Med. 1995; 152:1022–1027.
46. Carroll, J, Loughlin, G. Diagnostic criteria for obstructive sleep apnea syndrome in children. Pediatr Pulmonol. 1992; 14:71–74.
47. Chabolle, F, Wagner, I, Blumen, MB, et al. Tongue base reduction with hyoepiglottoplasty: A treatment for severe obstructive sleep apnea. Laryngoscope. 1999; 109:1273–1280.
48. Chen, F, Terada, K, Hua, Y, Saito, I. Effects of bimaxillary surgery and mandibular setback surgery on pharyngeal airway measurements in patients with Class III skeletal deformities. Am J Orthod Dentofacial Orthop. 2007; 131:372–377.
49. Chervin, RD, Ruzicka, DL, Giodani, BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006; 117:769–778.
50. Chervin, RD, Weatherly, RA, Garetz, SL, et al. Pediatric sleep questionnaire: Prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007; 133:216–222.
51. Cillo, JE, Jr., Thayer, S, Dasheiff, RM, Finn, R. Relations between obstructive sleep apnea syndrome and specific cephalometric measurements, body mass index, and apnea-hypopnea index. J Oral Maxillofac Surg. 2012; 70:e278–e283.
52. Cleator, IGM, Birmingham, CL, Kovacevic, S, et al. Long-term effect of ileogastrostomy surgery for morbid obesity on diabetes mellitus and sleep apnea. Obes Surg. 2006; 16:1337–1341.
53. Coccagna, G, diDonato, G, Verucchi, R, et al. Hypersomnia with periodic apneas in acquired micrognathia: A bird-like face syndrome. Arch Neurol. 1976; 33:769–776.
54. Compadretti, GC, Tasca, I, Bonetti, GA. Nasal airway measurements in children treated by rapid maxillary expansion. Am J Rhinol. 2006; 20(4):385–393.
55. Conley, RS, Legan, HL. Correction of severe obstructive sleep apnea with bimaxillary transverse distraction osteogenesis and maxillomandibular advancement. Am J Orthod Dentofacial Orthop. 2006; 129(2):283–292.
56. Conradt, R, Hochban, W, Brandenburg, U, et al. Long-term follow-up after surgical treatment of obstructive sleep apnea by maxillomandibular advancement. Eur Respir J. 1997; 10:123–128.
57. Contencin, P, Guilleminault, C, Manach, Y. Long-term follow-up and mechanisms of obstructive sleep apnea (OSA) and related syndromes through infancy and childhood. Int J Pediatr Otorhinolaryngol. 2003; 67(Suppl 1):S119–S123.
58. Conway, W, Fujita, S, Zohck, F, et al. Uvulopalatopharyngoplasty: One year follow-up. Chest. 1985; 88:385.
59. Costello, BJ, Posnick, JC. The role of maxillofacial osteotomies in the treatment of obstructive sleep apnea. Curr Opin Otolaryngol Head Neck Surg. 2003; 11(4):267–274.
60. Crabtree, VM, Varni, JW, Gozal, D. Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing. Sleep. 2004; 15:1131–1138.
61. Crumley, RL, Stein, S, Ganser, G, et al. Determination of obstructive sites in obstructive sleep apnea. Laryngoscope. 1987; 97:301.
62. Dattilo, DJ, Drooger, SA. Outcome assessment of patients undergoing maxillofacial procedures for the treatment of sleep apnea: Comparison of subjective and objective results. J Oral Maxillofac Surg. 2004; 62(2):164–168.
63. Davies, RJ, Stradling, JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnea syndrome. Eur Respir J. 1990; 3:509–514.
64. De Berry-Borowieki, B, Kukwa, A, Banks, R. Cephalometric analysis for diagnosis and treatment of obstructive sleep apnea. Laryngoscope. 1988; 98:226.
65. De Lucas-Ramos, P, de Miguel-Diez, J, Santacruz-Siminiani, A, et al. Benefits at 1 year of nocturnal intermittent positive pressure ventilation in patients with obesity hypoventilation syndrome. Respir Med. 2004; 98:961–967.
66. De Miguel Diez, J, de Lucas Ramos, P, Perez Parra, JJ, et al. Analysis of withdrawal from noninvasive mechanical ventilation in patients with obesity-hypoventilation syndrome. Medium term results [Spanish]. Arch Bronconeumol. 2003; 39:292–297.
67. Degerliyurt, K, Ueki, K, Hashiba, Y, et al. A comparative CT evaluation of pharyngeal airway changes in class III patients receiving bimaxillary surgery or mandibular setback surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105:495–502.
68. Degerliyurt, K, Ueki, K, Hashiba, Y, et al. The effect of mandibular setback or two-jaws surgery on pharyngeal airway among different genders. Int J Oral Maxillofac Surg. 2009; 38:647–652.
69. Demetriades, N, Chang, DJ, Laskarides, C, Papageorge, M. Effects of mandibular retropositioning, with or without maxillary advancement, on the oro-naso-pharyngeal airway and development of sleep-related breathing disorders. J Oral Maxillofac Surg. 2010; 68:2431–2436.
70. Dempsey, JA, Skatrud, JB, Jacques, AJ, et al. Anatomic determinants of sleep disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002; 122:840–851.
71. Douglas, NJ, Engleman, HM. Effects of CPAP on vigilance and related functions in patients with the sleep apnea/hypopnea syndrome. Sleep. 2000; 23(Suppl 4):S147–S149.
72. Eckert, DJ, Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008; 5:144–153.
73. Edström, L, Larsson, H, Larsson, L. Neurogenic effects on the palatopharyngeal muscle in patients with obstructive sleep apnea: A muscle biopsy study. J Neurol Neurosurg Psychiatry. 1992; 55:916–920.
74. Eggensperger, N, Smolka, W, Iizuka, T. Long-term changes of hyoid bone position and pharyngeal airway size following mandibular setback by sagittal split ramus osteotomy. J Craniomaxillofac Surg. 2005; 33:111–117.
75. El, AS, El, H, Palomo, JM, Baur, DA. A 3-dimensional airway analysis of an obstructive sleep apnea surgical correction with cone beam computed tomography. J Oral Maxillofac Surg. 2011; 69:2424–2436.
76. Elshaug, AG, Moss, JR, Southcott, AM, et al. An analysis of the evidence-practice continuum: Is surgery for obstructive sleep apnea contraindicated? J Eval Clin Pract. 2007; 13:3.
77. Elshaug, AG, Moss, JR, Southcott, AM, et al. Redefining success in airway surgery for obstructive sleep apnea: A meta analysis and synthesis of the evidence. Sleep. 2007; 30:461.
78. Enacar, A, Aksoy, AU, Sençift, Y, et al. Changes in hypopharyngeal airway space and in tongue and hyoid bone positions following the surgical correction of mandibular prognathism. Int J Adult Orthodon Orthognath Surg. 1994; 9:285–290.
79. Enoki, C, Valera, FC, Lessa, FC, et al. Effect of rapid maxillary expansion on the dimension of the nasal cavity and on nasal air resistance. Int J Pediatr Otorhinolaryngol. 2006; 70:1225–1230.
80. Epstein, LJ, Kristo, D, Strollo, PJ, et al. Clinical guidelines for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009; 5:263.
81. Esclamado, RM, Glenn, MG, McCulloch, TM, Cummings, CW. Perioperative complications and risk factors in the surgical treatment of obstructive sleep apnea syndrome. Laryngoscope. 1989; 99:1125–1129.
82. Fairburn, SC, Waite, PD, Vilos, G, et al. Three-dimensional changes in upper airways of patients with obstructive sleep apnea following maxillomandibular advancement. J Oral Maxillofac Surg. 2007; 65:6–12.
83. Farole, A, Mundenar, MJ, Braitman, LE. Posterior airway changes associated with mandibular advancement surgery: Implications for patients with obstructive sleep apnea. Int J Adult Orthodon Orthognath Surg. 1990; 5:255–258.
84. Fengshan, C, Terada, K, Hua, Y, et al. Effects of bimaxillary surgery and mandibular setback surgery on pharyngeal airway measurements in patients with Class III skeletal deformities. Am J Orthod Dentofacial Orthop. 2007; 131:372.
85. Findley, LJ, Unverzagt, ME, Suratt, PM. Automobile accidents involving patients with obstructive sleep apnea. Am Rev Respir Dis. 1988; 138:337–340.
86. Flemons, WW, Reimer, MA. Development of a disease-specific health-related quality-of-life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998; 158:494–503.
87. Fogel, R, Mallhotra, A, Pillar, G, et al. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001; 164:2025–2030.
88. Foster, GD, Wadden, TA, Mullen, JL, et al. Resting energy expenditure, body composition, and excess weight in the obese. Metabolism. 1988; 37:467–472.
89. Friberg, D, Ansved, T, Borg, K, et al. Histological indications of a progressive snorers disease in an upper-airway muscle. Am J Respir Crit Care Med. 1998; 157:586–593.
90. Friberg, D, Gazelius, B, Holfelt, T, et al. Abnormal afferent nerve endings in the soft palatal mucosa of sleep apneics and habitual snorers. Regul Pept. 1997; 71:29–36.
91. Friberg, D, Gazelius, B, Linblad, LE, et al. Habitual snorers and sleep apneics have abnormal vascular reaction of the soft palatal mucosa or afferent nerve stimulation. Laryngoscope. 1998; 108:431–436.
92. Friedman, M, Ibrahim, H, Bass, I. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002; 127(1):13–21.
93. Friedman, M, Ibrahim, H, Lee, G, Joseph, NJ. Combined uvulopalatopharyngoplasty and radiofrequency tongue base reduction for treatment of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2003; 129:611–621.
94. Friedman, M, Tanyeri, H, Lim, JW, et al. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000; 122(1):71–74.
95. Friedman, M, Vidyasagar, R, Bliznikas, D, et al. Does severity of obstructive sleep apnea/hypopnea syndrome predict uvulopalatopharyngoplasty outcome? Laryngoscope. 2005; 115(12):2109–2113.
96. Fritsch, KM, Iseli, A, Russi, EW, Bloch, KE. Side effects of mandibular advancement devices for sleep apnea treatment. Am J Respir Crit Care Med. 2001; 164:813–818.
97. Fritscher, LG, Mottin, CC, Canani, S, Chatkin, JM. Obesity and obstructive sleep apnea-hypopnea syndrome: the impact of bariatric surgery. Obes Surg. 2007; 17:95–99.
98. Fujita, S. Pharyngeal surgery for obstructive sleep apnea. In: Fairbanks DNF, Fujita S, Ikermatsu T, et al, eds. Snoring and obstructive sleep apnea. New York: Raven Press; 1987:101–128.
99. Fujita, S, Conway, W, Zorich, F, Roth, T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: Uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981; 89:923–934.
100. Gale, SD, Hopkins, RO. Effects of hypoxia on the brain: Neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. J Int Neuropsychol Soc. 2004; 10:60–71.
101. Galvin, JR, Rooholamini, SA, Stanford, W. Obstructive sleep apnea: Diagnosis with ultrafast CT. Radiology. 1989; 171:775–778.
102. Gastaut, H, Tassinari, CA, Duron, B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1967; 1:167–186.
103. George, CFP, Smiley, A. Sleep apnea and automobile crashes. Sleep. 1999; 22:790–795.
104. Giarda, M, Brucoli, M, Arcuri, F, et al. Proposal of presurgical algorithm for patients affected by obstructive sleep apnea syndrome. J Oral Maxillofac Surg. 2012; 70:2433–2439.
105. Giles, TL, Lasserson, TJ, Smith, BH, et al. Continuous positive airways pressure for obstructive sleep apnea in adults. Cochrane Database Syst Rev. (3):2006.
106. Gislason, T, Almqvist, M, Eriksson, G, et al. Prevalence of sleep apnea syndrome among Swedish men—an epidemiological study. J Clin Epidemiol. 1988; 41:571–576.
107. Gold, AR, Schwartz, AR. The pharyngeal critical pressure: The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996; 110:1077.
108. Goldstein, NA, Pugazhendhi, V, Rao, SM, et al. Clinical assessment of pediatric obstructive sleep apnea. Pediatrics. 2004; 114:33–43.
109. Goncalves, JR, Buschang, PH, Goncalves, DG, et al. Postsurgical stability of oropharyngeal airway changes following counterclockwise maxillomandibular advancement surgery. J Oral Maxillofac Surg. 2006; 64:755–762.
110. Goodwin, JL, Kaemingk, KL, Fregosi, RF, et al. Clinical outcomes associated with sleep-disordered breathing in Caucasian and Hispanic children—the Tucson Children’s Assessment of Sleep Apnea study (TuCASA). Sleep. 2003; 26:587–591.
111. Gottlieb, DJ, Whitney, CW, Bonekat, WH, et al. Relation of sleepiness to respiratory disturbance index: The Sleep Heart Health study. Am J Respir Crit Care Med. 1999; 159:502.
112. Gozal, D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998; 102(3 Pt 1):616–620.
113. Greco, JM, Frohberg, U, Van Sickels, JE. Long-term airway space changes after mandibular setback using bilateral sagittal split osteotomy. Int J Oral Maxillofac Surg. 1990; 19:103–105.
114. Grote, L, Hedner, J, Grunstein, R, et al. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000; 16:921.
115. Gu, G, Gu, G, Nagata, J, et al. Hyoid position, pharyngeal airway and head posture in relation to relapse after the mandibular setback in skeletal Class III. Clin Orthod Res. 2000; 3:67–77.
116. Guardiano, SA, Scott, JA, Ware, JC, Schechner, SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest. 2003; 124:1615–1619.
117. Guilleminault, C, Chowdhury, S. Upper airway resistance syndrome is a distinct syndrome. Am J Respir Crit Care Med. 2000; 161:1412–1413.
118. Guilleminault, C, Connolly, S, Winkle, R, et al. Cyclical variation of the heart rate in sleep apnea syndrome: Mechanisms and usefulness of 24 h electrocardiography as a screening technique. Lancet. 1984; 1:126–131.
119. Guilleminault, C, Hayes, B, Smith, FB. Palatopharyngoplasty and obstructive sleep apnea: A review of 35 cases. Bull Eur Physiopathol Respir. 1983; 19:595.
120. Guilleminault, C, Hill, MW, Simmons, FB. Obstructive sleep apnea: Electromyelographic and fiberoptic studies. Exp Neurol. 1978; 62:48.
121. Guilleminault, C, Huang, YS, Glamann, C, Chan, A. Adenotonsillectomy and obstructive sleep apnea in children: A prospective survey. Otolaryngol Head Neck Surg. 2007; 136:169–175.
122. Guilleminault, C, Li, K, Chen, NH, Poyares, D. Two-point palatal discrimination in patients with upper airway resistance syndrome, obstructive sleep apnea syndrome, and normal control subjects. Chest. 2002; 122:866–870.
123. Guilleminault, C, Li, KK. Maxillomandibular expansion for the treatment of sleep-disordered breathing: Preliminary result. Laryngoscope. 2004; 114(5):893–896.
124. Guilleminault, C, Li, KK, Quo, S, et al. A prospective study on the surgical outcome of children with sleep-disordered breathing. Sleep. 2004; 27(1):95–100.
125. Guilleminault, C, Motta, J, Mihm, F, Melvin, K. Obstructive sleep apnea and cardiac index. Chest. 1986; 89:331–334.
126. Guilleminault, C, Partinen, M, Praud, JP, et al. Morphometric facial changes and obstructive sleep apnea in adolescents. J Pediatr. 1989; 114:997–999.
127. Guilleminault, C, Partinen, M, Praud, JP, et al. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg. 2004; 131:827–832.
128. Guilleminault, C, Poyares, D, Palombini, L, et al. Variability of respiratory effort in relation to sleep stages in normal controls and upper airway resistance syndrome patients. Sleep Med. 2001; 2:397–406.
129. Guilleminault, C, Tilkian, AG, Dement, WC. Sleep apnea syndromes. Annu Rev Med. 1976; 27:465–484.
130. Guilleminault, C, Tilkian, AC, Eldridge, FL. Sleep apnea syndrome upper airway obstruction: A review of 25 cases. Arch Intern Med. 1977; 137:296.
131. Guven, O, Saracoglu, U. Changes in pharyngeal airway space and hyoid bone positions after body ostectomies and sagittal split ramus osteotomies. J Craniofac Surg. 2005; 16:23–30.
132. Hack, M, Davies, RJ, Mullins, R, et al. Randomized prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax. 2000; 55:224.
133. Haentjens, P, Meerhaeghe, AV, Moscariello, A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome. Evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007; 167(8):757–765.
134. Haines, KL, Nelson, LG, Gonzalez, R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007; 141:354–358.
135. Hans, MG, Goldberg, J. Cephalometric exam in obstructive sleep apnea. In Waite PD, editor: Oral and maxillofacial treatment of obstructive sleep apnea. Oral Maxillofac Surg Clin North Am. 1995; 7:209–281.
136. Haponik, EF, Smith, PL, Bohlman, ME, et al. Computerized tomography in obstructive sleep apnea. Am Rev Respir Dis. 1983; 127:221.
137. Hatcher, DC. Cone beam computed tomography: Craniofacial and airway analysis. Sleep Med Clin. 2010; 5:59–70.
138. He, J, Kryger, MH, Zorick, FJ, et al. Mortality and apnea index in obstructive sleep apnea: Experience in 385 male patients. Chest. 1988; 94:9–14.
139. Hendler, BH, Costello, BJ, Silverstein, K, et al. A protocol for uvulopalatopharyngoplasty, mortised genioplasty, and maxillomandibular advancement in patients with obstructive sleep apnea: An analysis of 40 cases. J Oral Maxillofac Surg. 2001; 59:892.
140. Hla, KM, Young, TB, Bidwell, T, et al. Sleep apnea and hypertension: A population-based study. Ann Intern Med. 1994; 120:382–388.
141. Hochban, W. Surgical treatment of obstructive sleep apnea. Otorhinolaryngol Nova. 2000; 10:149–156.
142. Hochban, W, Brandenburg, U. Morphology of the viscerocranium in obstructive sleep apnea syndrome—cephalometric evaluation of 400 patients. J Craniomaxillofac Surg. 1994; 22:205.
143. Hochban, W, Brandenburg, U, Peter, JH. Surgical treatment of obstructive sleep apnea by maxillomandibular advancement. Sleep. 1994; 17(7):624–629.
144. Hochban, W, Conradt, R, Brandenburg, U, et al. Surgical maxillofacial treatment of obstructive sleep apnea. Plast Reconstr Surg. 1997; 99:619–626.
145. Hochban, W, Schurmann, R, Brandenburg, U, Conradt, R. Mandibular setback for surgical correction of mandibular hyperplasia: Does it provoke sleep-related breathing disorders? Int J Oral Maxillofac Surg. 1996; 25:333–338.
146. Hoffman, EA, Gefter, WB. Multimodality imaging of the upper airway: MRI, MR spectroscopy, and ultrafast X-ray CT. Prog Clin Biol Res. 1990; 345:291–301.
147. Hoffstein, V, Mateika, S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnea. Eur Respir J. 1992; 5:377–381.
148. Hoffstein, V, Viner, S, Mateika, S, Conway, J. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure: Patient compliance, perception of benefits and side effects. Am Rev Respir Dis. 1992; 145:841–845.
149. Holty, JEC, Guilleminault, C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev. 2010; 14:287.
150. Horne, J. Why we sleep: The functions of sleep in humans and other mammals. Oxford, UK: Oxford University Press; 1988.
151. Hsu, PP, Tan, BY, Chan, YH, et al. Clinical predictors in obstructive sleep apnea patients with computer-assisted quantitative videoendoscopic upper airway analysis. Laryngoscope. 2004; 114(5):791–799.
152. Huang, YS, Guilleminault, C, Li, HY, et al. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: A treatment outcome study. Sleep Med. 2007; 8:18–30.
153. Hung, J, Whitford, EG, Parsons, RW, Hillman, DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990; 336:261–264.
154. Iatrou, I, Theologie-Lygidakis, N, Schoinohoriti, O. Mandibular distraction osteogenesis for severe airway obstruction in Robin sequence: Case report. J Craniomaxillofac Surg. 2010; 38:431–435.
155. Iber, C, Ancoli-Israel, S, Chesson, A, et al. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, Ill: American Academy of Sleep Medicine; 2007.
156. Ikeda, K, Ogura, M, Oshima, T, et al. Quantitative assessment of the pharyngeal airway by dynamic magnetic resonance imaging in obstructive sleep apnea syndrome. Ann Otol Rhinol Laryngol. 2001; 110:183–189.
157. Jackson, IT, Silverton, J. The sphincter pharyngoplasty as a secondary procedure in cleft palates. Plast Reconstr Surg. 1977; 59(4):518–524.
158. Jamieson, A, Guilleminault, C, Partinen, M, et al. Obstructive sleep apneic patients have craniomandibular abnormalities. Sleep. 1986; 9:469.
159. Jenkinson, C, Davies, RJ, Mullins, R, et al. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomized prospective parallel trial. Lancet. 1999; 353:2100.
160. Jenkinson, C, Stradling, J, Petersen, S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airway pressure therapy for sleep apnea. J Sleep Res. 1997; 6:199–204.
161. Jennum, P, Hein, HO, Suadicani, P, Gyntelberg, F. Cognitive functioning and snoring. Sleep. 1993; 16:562–564.
162. Johns, FR, Strollo, PJ, Jr., Buckley, M, et al. The influence of craniofacial structure on obstructive sleep apnea in young adults. J Oral Maxillofac Surg. 1998; 56:596.
163. Johns, MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991; 14:50–55.
164. Johns, MW. Reliability and factor analysis of the Epworth sleepiness scale. Sleep. 1992; 15:376–381.
165. Jung, R, Kuhlo, W. Neurophysiological studies of abnormal night sleep and the Pickwickian syndrome. Prog Brain Res. 1965; 18:140–159.
166. Kaemingk, KL, Pasvogel, AE, Goodwin, JL, et al. Learning in children and sleep disordered breathing: Findings of the Tucson Children’s Assessment of Sleep Apnea (TuCASA) prospective cohort study. J Int Neuropsychol Soc. 2003; 1016–1026.
167. Kajaste, S, Telakivi, T, Mustajoki, P, et al. Effects of a cognitive-behavioural weight loss programme on overweight obstructive sleep apnoea patients. J Sleep Res. 1994; 3:245–249.
168. Katsantonis GR Maas, CS, Walsh, JK. The predictive efficacy of the Mueller maneuver in uvulopalatopharyngoplasty. Laryngoscope. 1989; 99:677–680.
169. Katz, I, Stradling, J, Slutsky, AS, et al. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990; 141:1228–1231.
170. Katz, ES, D’Ambrosio, CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008; 5:253–262.
171. Kawakami, M, Yamamoto, K, Fujimoto, M, et al. Changes in tongue and hyoid positions, and posterior airway space following mandibular setback surgery. J Craniomaxillofac Surg. 2005; 33:107–110.
172. Kawamata, A, Fujishita, M, Ariji, Y, Ariji, E. Three-dimensional computed tomographic evaluation of morphologic airway changes after mandibular setback osteotomy for prognathism. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 89:278–287.
173. Kezirian, EJ, Weaver, EM, Yueh, B, et al. Incidence of serious complications after uvulopalatopharyngoplasty. Laryngoscope. 2004; 114(3):450–453.
174. Kitagawara, K, Kobayashi, T, Goto, H, et al. Effects of mandibular setback surgery on oropharyngeal airway and arterial oxygen saturation. Int J Oral Maxillofac Surg. 2008; 37:328–333.
175. Koskenvuo, M, Kaprio, J, Telakivi, T, et al. Snoring as a risk factor for ischaemic heart disease and stroke in men. BMJ. 1987; 294:16–19.
176. Kravath, RE, Pollak, C, Borowiecki, B, Weitzman, E. Obstructive sleep apnea and death associated with surgical correction of velopharyngeal incompetence. J Pediatr. 1980; 96(4):645–648.
177. Krekmanov, L, Andersson, L, Ringqvist, M, et al. Anterior-inferior mandibular osteotomy in treatment of obstructive sleep apnea syndrome. Int J Adult Orthodon Orthognath Surg. 1998; 13:289–297.
178. Kribbs, NB, Redline, S, Smith, PL, et al. Objective monitoring of nasal CPAP usage in OSAS patients. J Sleep Res. 1991; 20:270.
179. Kuo, PC, West, RA, Bloomquist, DS, McNeil, RW. The effect of mandibular osteotomy in three patients with hypersomnia sleep apnea. Oral Surg Oral Med Oral Pathol. 1979; 48:385–392.
180. Kushida, CA, Littner, MR, Morgenthaler, T, et al. Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep. 2005; 28(4):499–521.
181. Kushida, CA, Morgenthaler, T, Littner, MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: An update for 2005. Sleep. 2006; 29:240.
182. Laks, L, Krieger, J, Podszus, T. Pulmonary hypertension in obstructive sleep apnea: Retrospective multicentre study. Am Rev Respir Dis. 1992; 145:A865.
183. Lalakea, ML, Marquez-Biggs, I, Messner, AH. Safety of pediatric short-stay tonsillectomy. Arch Otolaryngol Head Neck Surg. 1999; 125:749–752.
184. Lam, B, Ip, MSM, Tench, E, et al. Craniofacial profile in Asian and white subjects with obstructive sleep apnea. Thorax. 2005; 60:504–510.
185. Lavie, P. Incidence of sleep apnea in a presumably healthy working population: A significant relationship with excessive daytime sleepiness. Sleep. 1983; 6:312–318.
186. Lee, NR, Givens, CD, Jr., Wilson, J, Robins, RB. Staged surgical treatment of obstructive sleep apnea syndrome. J Oral Maxillofac Surg. 1999; 57:382–385.
187. Lesavoy, MA, Borud, L, Thorson, T, et al. Upper airway obstruction after pharyngeal flap surgery. Ann Plast Surg. 1996; 36(1):26–30.
188. Li, AM, Hui, S, Wong, E, et al. Obstructive sleep apnea in children with adenotonsillar hypertrophy: Prospective study. Hong Kong Med J. 2001; 7:236–240.
189. Li, HY, Chen, NH, Shu, YH, et al. Changes in quality of life and respiratory disturbance after extended uvulopalatal flap surgery in patients with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2004; 130(2):195–200.
190. Li, HY, Chen, NH, Wang, CR, et al. Use of 3-dimensional computed tomography scan to evaluate upper airway patency for patients undergoing sleep-disordered breathing surgery. Otolaryngol Head Neck Surg. 2003; 129(4):336–342.
191. Li, HY, Lin, Y, Chen, NH, et al. Improvement in quality of life after nasal surgery alone for patients with obstructive sleep apnea and nasal obstruction. Arch Otolaryngol Head Neck Surg. 2008; 134(4):429–433.
192. Li, HY, Wang, PC, Lee, LA, et al. Prediction of uvulopalatopharyngoplasty outcome: Anatomy-based staging system versus severity-based staging system. Sleep. 2006; 29(12):1534–1541.
193. Li, KK. Surgical management of obstructive sleep apnea. Clin Chest Med. 2003; 24:365.
194. Li, KK. Surgical therapy for adult obstructive sleep apnea. Sleep Med Rev. 2005; 9:201–209.
195. Li, KK. Surgical therapy for obstructive sleep apnea syndrome. Semin Respir Crit Care Med. 2005; 26:80.
196. Li, KK. Maxillomandibular advancement for obstructive sleep apnea. J Oral Maxillofac Surg. 2011; 69:687–694.
197. Li, KK, Guilleminault, C, Riley, RW, Powell, NB. Obstructive sleep apnea and maxillomandibular advancement: An assessment of airway changes using radiographic and nasopharyngoscopic examinations. J Oral Maxillofac Surg. 2002; 60:526–530.
198. Li, KK, Powell, NB, Riley, RW, et al. Morbidly obese patients with severe obstructive sleep apnea: Is airway reconstructive surgery a viable treatment option? Laryngoscope. 2000; 110:982.
199. Li, KK, Powell, NB, Riley, RW, et al. Long-term results of maxillomandibular advancement surgery. Sleep Breath. 2000; 4:137–140.
200. Li, KK, Riley, RW, Powell, NB, et al. Overview of phase II surgery for obstructive sleep apnea syndrome. Ear Nose Throat J. 1999; 78:854.
201. Li, KK, Riley, RW, Powell, NB, et al. Maxillomandibular advancement for persistent OSA after phase I surgery in patients without maxillomandibular deficiency. Laryngoscope. 2000; 110:1684.
202. Li, KK, Riley, RW, Powell, NB, et al. Obstructive sleep apnea surgery: Patients’ perspective and polysomnographic results. Otolaryngol Head Neck Surg. 2000; 123:572.
203. Li, KK, Riley, RW, Powell, NB, Guilleminault, C. Patient’s perception of the facial appearance after maxillomandibular advancement for obstructive sleep apnea syndrome. J Oral Maxillofac Surg. 2001; 59:377–380.
204. Li, KK, Troell, RJ, Riley, RW, et al. Uvulopalatopharyngoplasty, maxillomandibular advancement, and the velopharynx. Laryngoscope. 2001; 111:1075.
205. Liao, YF, Chuang, M, Chen, P, et al. Incidence and severity of obstructive sleep apnea following pharyngeal flap surgery in patients with cleft palate. Cleft Palate Craniofac J. 2002; 39(3):312–316.
206. Liu, Y, Lowe, AA, Zeng, X, et al. Cephalometric comparisons between Chinese and Caucasian patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2000; 117:479–485.
207. Lojander, J, Maasilta, P, Partinen, M, et al. Nasal CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome: A randomized study. Chest. 1996; 110:114–119.
208. Louis, PJ, Austin, RB, Waite, PD, et al. Soft tissue changes of the upper lip associated with maxillary advancement in obstructive sleep apnea patients. J Oral Maxillofac Surg. 2001; 59:151–156.
209. Lowe, AA, Gionhaku, N, Takeuchi, K, Fleetham, JA. Three-dimensional CT reconstructions of tongue and airway in adult subjects with obstructive sleep apnea. Am J Orthod. 1986; 90:364–374.
210. Lowe, AA, Fleetham, JA, Adachi, S, Ryan, F. Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 1995; 107:589–595.
211. Lowe, AA, Ozbek, MM, Miyamoto, K, et al. Cephalometric and demographic characteristics of obstructive sleep apnea: An evaluation with partial least square analysis. Angle Orthod. 1997; 67:143–154.
212. Lowe, AA, Santamaria, JD, Fleetham, JA, Price, C. Facial morphology and obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1986; 90:484–491.
213. Lowe, AA, Sjoholm, T, Ryan, C, et al. Treatment, airway and compliance effects of a titratbale oral appliance. Sleep. 2000; 23:S172.
214. Lye, KW, Waite, PD, Meara, D, Wang, D. Quality of life evaluation of maxillomandibular advancement surgery for treatment of obstructive sleep apnea. J Oral Maxillofac Surg. 2008; 66:968–972.
215. Malkoç, S, Usümez, S, Iseri, H. Long-term effects of symphyseal distraction and rapid maxillary expansion on pharyngeal airway dimensions, tongue, and hyoid position. Am J Orthod Dentofacial Orthop. 2007; 132:769–775.
216. Marcus, CL, Omlin, KJ, Basinski, DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992; 146:1235–1239.
217. Marin, JM, Carrizo, SJ, Vicente, E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnea-hypopnea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005; 365:1046–1053.
218. Marklund, M, Stenlund, H, Franklin, KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring-tolerability and predictors of treatment success. Chest. 2004; 125:1270.
219. Mather, R, Douglas, NJ. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med. 1995; 122:174–178.
220. McColley, SA, April, MM, Carroll, JL, et al. Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992; 118:940–943.
221. McNicholas, WT, Bonsignore, MR. Sleep apnoea as an independent risk factor for cardiovascular disease: Current evidence, basic mechanisms and research priorities. Eur Respir J. 2007; 29:156–178.
222. Mehra, P, Downie, M, Pita, MC, et al. Pharyngeal airway space changes after counterclockwise rotation of the maxillomandibular complex. Am J Orthod Dentofacial Orthop. 2001; 120:154–159.
223. Meltzer, LJ, Johnson, C, Crosette, J, et al. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 2010; 125:1410–1418.
224. Messner, AH. Evaluation of obstructive sleep apnea by polysomnography prior to pediatric adenotonsillectomy. Arch Otolaryngol Head Neck Surg. 1999; 125:353–355.
225. Metes, A, Hoffstein, V, Direnfeld, V, et al. Three-dimensional CT reconstruction and volume measurements of the pharyngeal airway before and after maxillofacial surgery in obstructive sleep apnea. J Otol. 1993; 22:261.
226. Mickelson, SA, Hakim, I. Is postoperative intensive care monitoring necessary after uvulopalatopharyngoplasty? Otolaryngol Head Neck Surg. 1998; 119(4):352–356.
227. Miles, PJ, Nimkarn, Y. Maxillomandibular advancement surgery in patients with obstructive sleep apnea: Mandibular morphology and stability. Int J Adult Orthodon Orthognath Surg. 1995; 10:193–200.
228. Miller, WE. Cardiac arrhythmias and conduction disturbances in the sleep apnea syndrome: Prevalence and significance. Am J Med. 1982; 73:317–321.
229. Mitchell, RB. Adenotonsillectomy for obstructive sleep apnea in children: Outcome evaluated by pre- and postoperative polysomnography. Laryngoscope. 2007; 117:1844–1854.
230. Mitchell, RB, Kelly, J. Quality of life after adenotonsillectomy for SDB in children. Otolaryngol Head Neck Surg. 2005; 133:569–572.
231. Mitchell, RB, Kelly, J. Behavior, neurocognition and quality-of-life in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2006; 70(3):395–406.
232. Mitchell, RB, Kelly, J. Long-term changes in behavior after adenotonsillectomy for obstructive sleep apnea syndrome in children. Otolaryngol Head Neck Surg. 2006; 134(3):374–378.
233. Mitchell, RB, Kelly, J. Changes in behavior for children with mild sleep-disordered breathing (SDB) or obstructive sleep apnea (OSA) after adenotonsillectomy. Laryngoscope. 2007; 117:1685–1688.
234. Mitchell, RB, Kelly, J. Outcomes and quality of life following adenotonsillectomy for sleep-disordered breathing in children. ORL J Otorhinolaryngol Relat Spec. 2007; 69(6):345–348.
235. Mitchell, RB, Pereira, KD, Friedman, NR. Sleep-disordered breathing in children: Survey of current practice. Laryngoscope. 2006; 116:956–958.
236. Mokhlesi, B, Tulaimat, A, Evans, AT, et al. Impact of adherence with positive airway pressure therapy on hypercapnia in obstructive sleep apnea. J Clin Sleep Med. 2006; 2:57–62.
237. Mokhlesi, B, Tulaimat, A, Faibussowitsch, I, et al. Obesity hypoventilation syndrome: Prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007; 11:117–124.
238. Moore K: Obstructive Sleep Apnea. Surgical Update Vol 22, American Association of Oral and Maxillofacial Surgeons.
239. Moore, MH, Guzman-Stein, G, Prodman, TW, et al. Mandibular lengthening by distraction for airway obstruction in Treacher Collins syndrome. J Craniofac Surg. 1994; 5:22.
240. Morrison, DL, Launois, SH, Isono, S, et al. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993; 148:606–611.
241. Mortimore, IL, Marshall, I, Wraith, PK, et al. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998; 157:280–283.
242. Motta, J, Guilleminault, C, Schroeder, JS, et al. Tracheostomy and hemodynamic changes in sleep-inducing apnea. Ann Intern Med. 1978; 89(4):454–458.
243. Moyer, CA, Sonnad, SS, Garetz, SL, et al. Quality of life in obstructive sleep apnea: A systematic review of the literature. Sleep Med. 2001; 2:477–491.
244. Muto, T, Takeda, S, Kanazawa, M, et al. The effect of head posture on the pharyngeal airway space (PAS). Int J Oral Maxillofac Surg. 2002; 31:579–583.
245. National Commission on Sleep Disorders Research. Report of the National Commission on Sleep Disorders Research, Publication 92. Washington, DC: Superintendent of Documents, US Government Printing Office, Department of Health and Human Services; 1992.
246. National Institutes of Health Consensus Development Conference Statement. The treatment of sleep disorders of older people, March 26-28, 1990. Sleep. 1991; 14:169–177.
247. Neeley, WW, 2nd., Edgin, WA, Gonzales, DA. A review of the effects of expansion of the nasal base on nasal airflow and resistance. J Oral Maxillofac Surg. 2007; 65:1174–1179.
248. Neligan, PJ, Porter, S, Max, B, et al. Obstructive sleep apnea is not a risk factor for difficult intubation in morbidly obese patients. Anesth Analg. 2009; 109:1182.
249. Nieminen, P, Tolonen, U, Lopponen, H. Snoring and obstructive sleep apnea in children: A 6-month follow-up study. Arch Otolaryngol Head Neck Surg. 2000; 126:481–486.
250. Nieto, FJ, Young, TB, Lind, BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based. study. JAMA. 2000; 283:1829–1836.
251. Nimkarn, Y, Miles, PG, Waite, PD. Maxillomandibular advancement surgery in obstructive sleep apnea syndrome patients: Long-term surgical stability. J Oral Maxillofac Surg. 1995; 53:1414–1418.
252. Nino-Murcia, G, McCann, I, Bliwise, DL, et al. Compliance and side effects in sleep apnea patients treated with nasal continuous positive airway pressure. West J Med. 1989; 150:165–169.
253. O’Brien, LM, Holbrook, CR, Mervis, CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003; 111:554–563.
254. O’Brien, LM, Mervis, CB, Holbrook, CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004; 114:44–49.
255. Ogden, CL, Carroll, MD, Curtin, LR, et al. Prevalence of overweight and obesity in the United States 1999-2004. JAMA. 2006; 295:1549–1555.
256. Oksenberg, A, Khaymaysi, I, Silverberg, DS, et al. Association of body position with severity of apneic events in patients with severe nonpositional obstructive sleep apnea. Chest. 2000; 118:1018–1024.
257. Orr, WC, Levine, N, Buchanan, R. Effect of cleft palate repair and pharyngeal flap surgery on upper airway obstruction during sleep. Plast Reconstr Surg. 1987; 80(2):226–230.
258. Osman, EZ, Abo-Khatwa, MM, Hill, PD, et al. Palatal surgery for snoring: Objective long-term evaluation. Clin Otolaryngol Allied Sci. 2003; 28(3):257–261.
259. Otsuka, R, Ribeiro de Almeida, F, Lowe, AA. The effects of oral appliance therapy on occlusal function in patients with obstructive sleep apnea: A short-term prospective study. Am J Orthod Dentofacial Orthop. 2007; 131:176–183.
260. Pae, EK, Ferguson, KA. Cephalometric characteristics of non-obese patients with severe OSA. Angle Orthod. 1999; 69:408–412.
261. Pancer, J, Al-Faifi, S, Al-Faifi, M, et al. Evaluation of variable mandibular advancement appliance for treatment of snoring and sleep apnea. Chest. 1999; 116:1511.
262. Pankow, W, Hijjeh, N, Schuttler, F, et al. Influence of noninvasive positive pressure ventilation on inspiratory muscle activity in obese subjects. Eur Respir J. 1997; 10:2847–2852.
263. Partinen, M, Guilleminault, C. Daytime sleepiness and vascular morbidity at seven-year follow-up in obstructive sleep apnea patients. Chest. 1990; 97:27–32.
264. Partinen, M, Guilleminanlt, C, Quera-Salva, MA, Jamieson, A. Obstructive sleep apnea and cephalometric roentgenograms: The role of anatomic upper airway abnormalities in the definition of abnormal breathing during sleep. Chest. 1988; 93:1199–1205.
265. Partinen, M, Jamieson, A, Guilleminault, C. Long-term outcome for obstructive sleep apnea syndrome patients: Mortality. Chest. 1988; 94:1200–1204.
266. Peker, Y, Kraiczi, H, Hedner, J, et al. An independent association between obstructive sleep apnea and coronary artery disease. Eur Respir J. 1999; 14:179.
267. Pepin, JL, Krieger, J, Rodenstein, D, et al. Effective compliance during the first three months of continuous positive airways pressure: A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999; 160:1124–1129.
268. Peppard, PE, Young, T, Palta, M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000; 284:3015.
269. Peppard, PE, Young, T, Palta, M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342:1378–1384.
270. Perkins, JA, Sie, KC, Milczuk, H, et al. Airway management in children with craniofacial anomalies. Cleft Palate Craniofac J. 1997; 34:135.
271. Perlyn, CA, Schmelzer, RE, Sutera, SP, et al. Effect of distraction osteogenesis of the mandible on upper airway volume and resistance in children with micrognathia. Plast Reconstr Surg. 2002; 109:1809–1818.
272. Petri, N, Suadicani, P, Wildschiodtz, G, et al. Predictive value of Muller maneuver, cephalometry and clinical features for the outcome of uvulopalatopharyngoplasty. Acta Otolaryngol. 1994; 114:565.
273. Pillar, G, Peled, R, Lavie, P. Recurrence of sleep apnea without concomitant weight increase 7. 5 years after weight reduction surgery. Chest. 1994; 106:1702–1704.
274. Pirklbauer, K, Russmueller, G, Stiebellehner, L, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea syndrome: A systematic review. J Oral Maxillofac Surg. 2011; 69:e165–e176.
275. Posnick, JC, Agnihotri, N. Consequences and management of nasal airway obstruction in the dentofacial deformity patient. Curr Opin Otolaryngol Head Neck Surg. 2010; 18:323–331.
276. Posnick, JC, Agnihotri, N. Managing chronic nasal airway obstruction at the time of orthognathic surgery: A twofer. J Oral Maxillofac Surg. 2011; 69:695–701.
277. Posnick, JC, Fantuzzo, J, Orchin, J. Deliberate operative rotation of the maxillomandibular complex to alter the A-point to B-point relationship for enhanced facial esthetics. J Oral Maxillofac Surg. 2006; 64:1687–1695.
278. Posnick, JC, Fantuzzo, JJ, Troost, T. Simultaneous intranasal procedures to improve chronic obstructive nasal breathing in patients undergoing maxillary (Le Fort I) osteotomy. J Oral Maxillofac Surg. 2007; 65:2273–2281.
279. Powell, NB, Guilleminault, C, Riley, RW, Smith, L. Mandibular advancement and obstructive sleep apnea syndrome. Bull Eur Physiopathol Respir. 1983; 19:607–610.
280. Powell, NB, Riley, RW. A surgical protocol for sleep disordered breathing. In Waite PD, editor. Oral Maxillofac Surg Clin North Am. 1995; 7:345–354.
281. Pracharktam, N, Nelson, S, Hans, MG, et al. Cephalometric assessment in obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1996; 109:410–419.
282. Practice parameters for the treatment of obstructive sleep apnea in adults: The efficacy of surgical modifications of the upper airway. Report of the American Sleep Disorders Association. Sleep. 1996; 19:152.
283. Prinsell, JR. Maxillomandibular advancement surgery in a site-specific treatment approach for obstructive sleep apnea in 50 consecutive patients. Chest. 1999; 116:1519–1529.
284. Prinsell, JR. Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc. 2002; 133:1489–1497.
285. Prinsell, JR. Primary and secondary telegnathic maxillomandibular advancement, with or without adjunctive procedures, for obstructive sleep apnea in adults: A literature review and treatment recommendations. J Oral Maxillofac Surg. 2012; 70:1659–1677.
286. Punjabi, NR. The epidemiology of adult sleep apnea. Proc Am Thorac Soc. 2008; 5:136–143.
287. Rachmiel, A, Aizenbud, D, Pillar, G, et al. Bilateral mandibular distraction for patients with compromised airway analyzed by three-dimensional CT. Int J Oral Maxillofac Surg. 2005; 34:9–18.
288. Randall, DK. Dreamland: Adventures in the strange science of sleep. W. W. Norton & Co. : New York; 2012.
289. Rapoport, DM, Garay, SM, Epstein, H, et al. Hypercapnia in the obstructive sleep apnea syndrome. A reevaluation of the “Pickwickian syndrome”. Chest. 1986; 89:627–635.
290. Rasheid, S, Banasiak, M, Gallagher, SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg. 2003; 13:58–61.
291. Reeves-Hoche, MK, Meck, R, Zwillich, CW, et al. An objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994; 149:149.
292. Reimer, MA, Flemons, WW. Quality of life in sleep disorders. Sleep Med Rev. 2003; 7:335–349.
293. Remmers, JE, deGroot, WJ, Sauerland, EK, Anch, AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978; 44:931–938.
294. Ribeiro de Almeida, F, Bittencourt, LR, de Almeida, CI, et al. Effects of mandibular posture on obstructive sleep apnea severity and the temporomandibular joint in patients fitted with an oral appliance. Sleep. 2002; 25(5):505–511.
295. Ricketts, RM. Respiratory obstruction syndrome. Am J Orthod. 1968; 54:495–507.
296. Rieder, AA, Flanary, V. The effect of polysomnography on pediatric adenotonsillectomy postoperative management. Otolaryngol Head Neck Surg. 2005; 132:263–267.
297. Riley, R, Guilleminault, C, Powell, N, Derman, S. Mandibular osteotomy and hyoid bone advancement for obstructive sleep apnea: A case report. Sleep. 1984; 7:79.
298. Riley, RW, Guilleminault, C, Powell, N. Palatopharyngoplasty failure, cephalometric roentgenograms and obstructive sleep apnea. Otolaryngol Head Neck Surg. 1985; 93:240.
299. Riley, RW, Powell, NB, Guilleminault, C. Inferior sagittal osteotomy of the mandible with hyoid myotomy suspension: A new procedure for obstructive sleep apnea. Otolaryngol Head Neck Surg. 1986; 94:589.
300. Riley, RW, Powell, NB, Guilleminault, C. Inferior mandibular osteotomy and hyoid myotomy suspension for obstructive sleep apnea: A review of 55 patients. J Oral Maxillofac Surg. 1989; 47:159.
301. Riley, RW, Powell, NB, Guilleminault, C. Maxillofacial surgery and obstructive sleep apnea: A review of 80 patients. Otolaryngol Head Neck Surg. 1989; 101:353.
302. Riley, RW, Powell, NB, Guilleminault, C. Maxillary, mandibular, and hyoid advancement for treatment of obstructive sleep apnea: A review of 40 patients. J Oral Maxillofac Surg. 1990; 48:20–26.
303. Riley, RW, Powell, NB, Guilleminault, C. Maxillofacial surgery and nasal CPAP: A comparison of treatment for obstructive sleep apnea syndrome. Chest. 1990; 98:1421–1425.
304. Riley, RW, Powell, NB, Guilleminault, C. Obstructive sleep apnea syndrome: A surgical protocol for dynamic upper airway reconstruction. J Oral Maxillofac Surg. 1993; 51:742–747.
305. Riley, RW, Powell, NB, Guilleminault, C. Obstructive sleep apnea syndrome: A review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg. 1993; 108:117–125.
306. Riley, RW, Powell, NB, Guilleminault, C, et al. Maxillary, mandibular and hyoid advancement: An alternative to tracheostomy in obstructive sleep apnea. Otolaryngol Head Neck Surg. 1986; 94:584.
307. Riley, RW, Powell, NB, Li, KK, et al. Surgery and obstructive sleep apnea: Long-term clinical outcomes. Otolaryngol Head Neck Surg. 2000; 122:415–421.
308. Ringqvist, M, Walker-Engstrom, ML, Tegelberg, A, Ringqvist, I. Dental and skeletal changes after 4 years of obstructive sleep apnea treatment with a mandibular advancement device: A prospective, randomized study. Am J Orthod Dentofacial Orthop. 2003; 124:54–60.
309. Ritter, CT, Trudo, FJ, Goldbert, AN, et al. Quantitative evaluation of the upper airway during nasopharyngoscopy with the Muller maneuver. Laryngoscope. 1999; 109:954.
310. Robertson, C, Herbison, P, Harkness, M. Dental and occlusal changes during mandibular advancement splint therapy in sleep disordered patients. Eur J Orthod. 2003; 25:371–376.
311. Ronchi, G, Novelli, G, Colombo, L, et al. Effectiveness of maxillomandibular advancement in obstructive sleep apnea patients with and without skeletal anomalies. Int J Oral Maxillofac Surg. 2010; 39:541–547.
312. Rosen, CL, Storfer-Isser, A, Taylor, HG, et al. Increased behavioral morbidity in school-aged children with sleep-disordered breathing. Pediatrics. 2004; 114:1640–1648.
313. Rosen, GM, Muckle, RP, Mahowald, MW, et al. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: Can it be anticipated? Pediatrics. 1994; 93:784–788.
314. Saitoh, K. Long-term changes in pharyngeal airway morphology after mandibular setback surgery. Am J Orthod Dentofacial Orthop. 2004; 125:556–561.
315. Sakakibara, H, Tong, M, Matsushita, K, et al. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnea. Eur Respir J. 1999; 13:403–410.
316. Samman, N, Tang, SS, Xia, J. Cephalometric study of the upper airway in surgically corrected class III skeletal deformity. Int J Adult Orthodon Orthognath Surg. 2002; 17:180–190.
317. Sanders, JC, King, MA, Mitchell, RB, Kelly, JP. Perioperative complications of adenotonsillectomy in children with obstructive sleep apnea syndrome. Anesth Analg. 2006; 103:1115–1121.
318. Sanders, MH, Gruendl, CA, Rogers, RM. Patient compliance with nasal CPAP therapy for sleep apnea. Chest. 1986; 90:330–333.
319. Santos, M, Laureano Filho, J, Campello, RI, et al. Improvement in respiration and craniofacial changes associated with weight loss after bariatric surgery. J Oral Maxillofac Surg. 2011; 69:e177–e185.
320. Shepard, JW, Jr., Thawley, SE. Localization of upper airway collapse during sleep in patients with obstructive sleep apnea. Am Rev Respir Dis. 1990; 141:1350–1355.
321. Schendel, S, Powell, N, Jacobson, R. Maxillary, mandibular, and chin advancement: Treatment planning based on airway anatomy in obstructive sleep apnea. J Oral Maxillofac Surg. 2011; 69:663–676.
322. Schendel, SA, Jacobson, R, Khalessi, S. Airway growth and development: A computerized three dimensional analysis. J Oral Maxillofac Surg. 2012; 70:2174–2183.
323. Schwab, RJ, Gefter, WB, Hoffman, EA, et al. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep-disordered breathing. Am Rev Respir Dis. 1993; 148(5):1385–1400.
324. Schwab, RJ, Gupta, KB, Gefter, WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing: Significance of lateral pharyngeal walls. Am J Respir Crit Care Med. 1995; 152:1673.
325. Schwab, RJ, Gupta, KB, Metzger, LJ, et al. Upper airway soft tissue structural changes with a dental appliance in apneics. Am J Respir Crit Care Med. 1996; 153:A719.
326. Schwab, RJ, Pierson, R, Pasirstein, M, et al. Family aggregation of upper airway soft tissue structures in normals and patients with sleep apnea. Am J Respir Crit Care Med. 2006; 173:453–463.
327. Schwartz, AR, Smith, PL, Wise, RA, et al. Induction of upper airway occlusion in sleeping individuals with subatmospheric negative pressure. J Appl Physiol. 1988; 64:535.
328. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002; 109:704–712.
329. Series, F, Simoneau, JA, St Pierre, S, et al. Characteristics of the genioglossus and musculus uvulae in sleep apnea/hypopnea syndrome and in snorers. Am J Respir Crit Care Med. 1996; 153:1870–1874.
330. Shamsuzzaman, AS, Somers, VK. Fibrinogen, stroke, and obstructive sleep apnea: An evolving paradigm of cardiovascular risk [editorial]. Am J Respir Crit Care Med. 2000; 162:2018.
331. Shamsuzzaman, ASM, Gersch, BJ, Somers, VK. Obstructive sleep apnea: Implications for cardiac and vascular disease. JAMA. 2003; 290:1906–1914.
332. Shelton, KE, Gay, SB, Woodson, H, et al. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993; 148:462.
333. Shepard, JW, Jr. Hypertension, cardiac arrhythmias, myocardial infarction and stroke in relation to obstructive sleep apnea. Clin Chest Med. 1992; 13:437–458.
334. Sher, AE. Upper airway surgery for obstructive sleep apnea. Sleep Med Rev. 2002; 6:195.
335. Sher, AE, Schechtman, KB, Piccirillo, JE. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996; 19:156–177.
336. Sher, AE, Thorpy, MJ, Shprintzen, RJ, et al. Predictive value of Muller’s maneuver in selection of patients for uvulopalatopharyngoplasty. Laryngoscope. 1988; 95:1483–1487.
337. Shimura, R, Tatsumi, K, Nakamura, A, et al. Fat accumulation, leptin, and hypercapnia in obstructive sleep apnea-hypopnea syndrome. Chest. 2005; 127:543–549.
338. Shintani, T, Kozawa, T, Himi, T. Obstructive sleep apnea by analysis of MRI findings. Int Congr Ser. 2003; 1257:99–102.
339. Shprintzen, RJ. Pharyngeal flap surgery and the pediatric upper airway. Int Anesthesiol Clin. 1988; 26(1):79–88.
340. Shprintzen, RJ, Singer, L, Sidoti, EJ, Argamaso, RV. Pharyngeal flap surgery: Postoperative complications. Int Anesthesiol Clin. 1992; 30(4):115–124.
341. Sidman, J, Sampson, D, Templeton, B. Distraction osteogenesis of the mandible for airway obstruction in children. Laryngoscope. 2001; 111:1137.
342. Simmons, FB, Guilleminanlt, C, Miles, LE. The palatopharyngoplasty operation for snoring and sleep apnea: An interim report. Otolaryngol Head Neck Surg. 1984; 92:375–380.
343. Sirois, M, Caouette-Laberge, L, Spier, S, et al. Sleep apnea following a pharyngeal flap: A feared complication. Plast Reconstr Surg. 1994; 93(5):943–947.
344. Slovik, Y, Tal, A, Shapira, Y, et al. Complications of adenotonsillectomy in children with OSAS younger than 2 years of age. Int J Pediatr Otorhinolaryngol. 2003; 67:847–851.
345. Smatt, Y, Ferri, J. Retrospective study of 18 patients treated by maxillomandibular advancement with adjunctive procedures for obstructive sleep apnea syndrome. J Craniofac Surg. 2005; 16:770–777.
346. Smirne, S, Iannaccone, S, Ferini-Strambi, L, et al. Muscle fibre type and habitual snoring. Lancet. 1991; 337:597–599.
347. Smith, CB, Waite, PD. Surgical management of obstructive sleep apnea in acromegaly with mandibular prognathism and macroglossia: A treatment dilemma. J Oral Maxillofac Surg. 2012; 70:207–210.
348. Solow, B, Siersbaek-Nelsen, S, Greve, EE. Airway adequacy, head posture, and craniofacial morphology. Am J Orthod. 1984; 86:214–223.
349. Somers, VK, Dyken, ME, Clary, MP, Abboud, FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995; 96:897–904.
350. Somers, VK, White, DP, Amin, R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008; 118:1080–1111.
351. Statham, MM, Elluru, RG, Buncher, R, Kalra, M. Adenotonsillectomy for obstructive sleep apnea syndrome in young children: Prevalence of pulmonary complications. Arch Otolaryngol Head Neck Surg. 2006; 132:476–480.
352. Stradling, JR, Crosby, JH. Predictors and prevalence of obstructive sleep apnea and snoring in 1001 middle aged men. Thorax. 1991; 46:85–90.
353. Straith, RE, Ritter, G. Partial resection of the tongue for the amelioration of obstructive sleep apnea: A report on 34 cases with long-term follow-up. J Craniomaxillofac Surg. 1997; 25:305–309.
354. Strelzow, VV, Blanks, RH, Basile, A, Strelzow, AE. Cephalometric airway analysis in obstructive sleep apnea syndrome. Laryngoscope. 1988; 98:1149–1158.
355. Suen, JS, Arnold, JE, Brooks, LJ. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg. 1995; 121:525–530.
356. Sugiura, T, Noda, A, Nakata, S, et al. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration. 2007; 74(1):56–60.
357. Sullivan, CE, Berthon-Jones, M, Issa, FG, et al. Reversal of obstructive sleep apnea by continuous positive airway pressure applied through the nares. Lancet. 1981; 1:862–865.
358. Sundaram, S, Bridgman, S, Lim, J, Lasserson, TJ. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 19(4), 2005.
359. Suratt, PM, Dee, P, Atkinson, RL, et al. Fluoroscopic and computer tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis. 1993; 127:487.
360. Susarla, SM, Abramson, ZR, Dodson, TB, Kaban, LB. Cephalometric measurement of upper airway length correlates with the presence and severity of obstructive sleep apnea. J Oral Maxillofac Surg. 2010; 68:2846–2855.
361. Susarla, SM, Thomas, RJ, Abramson, ZR, Kaban, LB. Biomechanics of the upper airway: Changing concepts in the pathogenesis of obstructive sleep apnea. Int J Oral Maxillofac Surg. 2010; 39:1149–1159.
362. Suto, Y, Matsuo, T, Kato, T, et al. Evaluation of pharyngeal airway in patients in sleep apnea: Value of ultrafast MR imaging. AJR Am J Roentgenol. 1993; 160:311.
363. Tallgren, A, Solow, B. Hyoid bone position, facial morphology and head posture in adults. Eur J Orthod. 1987; 9:1–8.
364. Tangugsorn, V, Skatvedt, O, Krogstad, O, Lyberg, T. Obstructive sleep apnea: A cephalometric study. Part I. Cervico-craniofacial skeletal morphology. Eur J Orthod. 1995; 17:45–56.
365. Tassinari, CA, Dalla Bernardina, B, Cirignotta, F, Ambrosetto, G. Apneaic periods and the respiratory related arousal patterns during sleep in the Pickwickian syndrome. A polygraphic study. Bull Physiopathol Respir (Nancy). 1972; 8:1087–1102.
366. Tauman, R, Gulliver, TE, Krishna, J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006; 149:803–808.
367. Telakivi, T, Kajaste, S, Partinert, M, et al. Cognitive function in obstructive sleep apnea. Sleep. 1993; 74–75.
368. Thatcher, GW, Maisel, RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope. 2003; 113:201.
369. Tilkian, AG, Guilleminault, C, Schroeder, JS. Sleep-induced apnea syndrome: Prevalence of cardiac arrhythmias and their reversal after tracheostomy. Am J Med. 1977; 63:348.
370. Tselnik, M, Pogrel, MA. Assessment of the pharyngeal airway space after mandibular setback surgery. J Oral Maxillofac Surg. 2000; 58:282–285.
371. Tsuiki, S, Ono, T, Kuroda, T. Mandibular advancement modulates respiratory-related genioglossus electromyographic activity. Sleep Breath. 2000; 4:53.
372. Turnbull, NR, Battagel, JM. The effects of orthognathic surgery on pharyngeal airway dimensions and quality of sleep. J Orthod. 2000; 27:235–247.
373. Valnicek, SM, Zuker, R, Halpern, L, Roy, W. Perioperative complications of superior pharyngeal flap surgery in children. Plast Reconstr Surg. 1994; 93(5):954–958.
374. Vanderveken, OM, Devolder, A, Marklund, M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008; 178:197.
375. Verse, T, Baisch, A, Maurer, JT, et al. Multilevel surgery for obstructive sleep apnea: Short-term results. Otolaryngol Head Neck Surg. 2006; 134:571–577.
376. Vicini, C, Dallan, I, Campanini, A, et al. Surgery vs ventilation in adult severe obstructive sleep apnea syndrome. Am J Otolaryngol. 2010; 31:14–20.
377. Villa, MP, Bernkopf, E, Pagani, J, et al. Randomized controlled study of an oral jaw-positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. Am J Respir Crit Care Med. 2002; 165:123.
378. Vos, W, De Backer, K, Devolder, A, et al. Correlation between severity of sleep apnea and upper airways morphology based on advanced anatomical and functional imaging. J Biomech. 2007; 40:2207–2213.
379. Waite, PD, Shettar, SM. Maxillomandibular advancement surgery: A cure for obstructive sleep apnea syndrome. In Waite PD, editor: Oral and maxillofacial treatment of obstructive sleep apnea. Oral Maxillofac Surg Clin North Am. 1995; 7:327–336.
380. Waite, PD, Tejera, TJ, Anucul, B. The stability of maxillary advancement using Le Fort I osteotomy with and without genial bone grafting. Int J Oral Maxillofac Surg. 1996; 25:264–267.
381. Waite, PD, Wooten, V, Lachner, J, Guyette, RF. Maxillomandibular advancement surgery in 23 patients with obstructive sleep apnea. J Oral Maxillofac Surg. 1989; 47:1256–1261.
382. Warwick, JP, Mason, DG. Obstructive sleep apnea in children. Anaesthesia. 1998; 53:571–579.
383. Watanabe, T, Isono, S, Tanaka, A, et al. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002; 165(2):260–265.
384. Weatherly, RA, Mai, EF, Ruzicka, DL, Chervin, RD. Identification and evaluation of obstructive sleep apnea prior to adenotonsillectomy in children: A survey of practice patterns. Sleep Med. 2003; 4:297–307.
385. Weaver, TE, Grunstein, RR. Adherence to continuous positive airway pressure therapy. Proc Am Thorac Soc. 2008; 5:173–178.
386. Wenzel, A, Williams, S, Ritzau, M. Changes in head posture and nasopharyngeal airway following surgical correction of mandibular prognathism. Eur J Orthod. 1989; 11:37–42.
387. Wheatley, JR, Tangel, DJ, Mezzanotte, WS, White, DP. Influence of sleep on response to negative airway pressure of tensor palatini muscle and retropalatal airway. J Appl Physiol. 1993; 75:2117–2124.
388. Winnberg, A, Pancherz, H, Westesson, PL. Head posture and hyomandibular function in man. A synchronized electromyographic and videofluorographic study of the open-close-clench cycle. Am J Orthod Dentofacial Orthop. 1988; 94:393–404.
389. Witt, PD, Marsh, J, Muntz, H, et al. Acute obstructive sleep apnea as a complication of sphincter pharyngoplasty. Cleft Palate Craniofac J. 1996; 33(3):183–189.
390. Wittenborn, W, Panchal, J, Marsh, J, et al. Neonatal distraction surgery for micrognathia reduces obstructive sleep apnea and the need for tracheotomy. J Craniofac Surg. 2004; 15:623.
391. Woodson, BT. Predicting which patients will benefit from surgery for obstructive sleep apnea. The ENT exam. ENT J. 1999; 78:792.
392. Yaggi, HK, Concato, J, Kernan, WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005; 353:2034–2041.
393. Young, T, Palta, M, Dempsey, J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993; 328:1230–1235.
394. Young, T, Peppard, P, Palta, M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997; 157:1745–1752.
395. Young, T, Peppard, PE, Taheri, S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005; 99:1592–1599.
396. Ysunza, A, Garcia-Velasco, M, Garcia-Garcia, M, et al. Obstructive sleep apnea secondary to surgery for velopharyngeal insufficiency. Cleft Palate Craniofac J. 1993; 30(4):387–390.
397. Yu, CC, Hsiao, HD, Lee, LC, et al. Computational fluid dynamic study on obstructive sleep apnea syndrome treated with maxillomandibular advancement. J Craniofac Surg. 2009; 20:426–430.
398. Yu, LF, Pogrel, MA, Ajayi, M. Pharyngeal airway changes associated with mandibular advancement. J Oral Maxillofac Surg. 1994; 52:40–43.
399. Zhao, Y, Nguyen, M, Gohl, E, et al. Oropharyngeal airway changes after rapid palatal expansion evaluated with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2010; 137(4 Suppl):S71–S78.
400. Zucconi, M, Strambli, LF, Pestalozza, G, et al. Habitual snoring and obstructive sleep apnea syndrome in children: Effects of early tonsil surgery. Int J Pediatr Otorhinolaryngol. 1993; 26:235–243.
*References 10, 23, 25, 42, 118, 125, 138, 140, 153, 175, 182, 221, 228, 250, 263, 265, 266, 289, 330, 331, 333, 350
*References 1, 7, 19, 20, 22, 28, 33, 61, 64, 70, 101, 135–137, 146, 151, 156, 168, 190, 206, 210–212, 260, 281, 309, 315, 323, 338, 359, 362, 364
*References 3, 17, 24, 29, 55, 56, 59, 62, 82, 83, 109, 123, 131, 141–145, 149, 177, 179, 196, 197, 199, 200, 203, 208, 214, 222, 225, 227, 251, 274, 279, 283, 284, 287, 299, 300–303, 306, 311, 321, 347, 371, 372, 379, 381, 382, 397