Chapter 60 Obstructive Sleep Apnea
Epidemiology, Risk Factors, and Pathophysiology
Epidemiology
Although obstructive sleep apnea clearly is a common disorder within the general population, its incidence is hard to establish, because methodologic differences among the various epidemiologic studies have made comparisons difficult. First, different tests have been used to diagnose OSA. Overnight polysomnography (PSG) is considered the “gold standard” diagnostic modality, but assessments have been made using other tests instead, such as unattended in-home PSG or respiratory polygraphy, pulse oximetry, and even clinical questionnaires (see Chapter 61). Second, variability in the definitions of different respiratory events (especially hypopnea) and the apnea-hypopnea index (AHI) cutoff value that defines OSA, or clinically significant OSA, is well recognized. In this sense, the chosen oxyhemoglobin desaturation threshold, typically 3% or 4%, used to define hypopnea can lead to different AHI scores; accordingly, estimates of disease severity will vary. Third, differences in sampling of populations (for example, the percentages of elderly and female subjects included) have been noted. Fourth, disparities in signal processing and a lack of standardization in the quantification of airflow (including thermistor, inductance plethysmography, and nasal cannula–pressure transduction) are common. Finally, the quality of validation of equipment and the conclusions of some studies have been questioned because of methodologic limitations such as small sample sizes or inadequate controls for potential confounding variables.
Middle-Aged Population
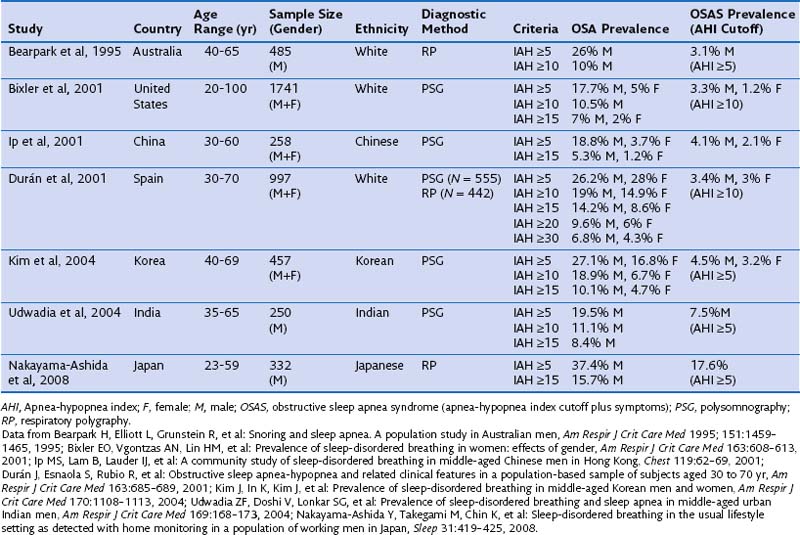
It is estimated that 24% of men and 9% of women have OSA, as defined by an AHI of 5 or greater, and that 15% of men and 5% of women between 30 and 60 years have an AHI of 10 or higher. With use of daytime sleepiness as a clinical syndrome, the prevalence ranges between 3% and 7% in men and 2% and 5% in women in the general population (Box 60-1). Until recently, population-based epidemiologic studies of OSA were available only for North America, Europe, and Australia. However, more recent studies undertaken in other countries, including China, India, and Korea, report similar prevalence rates. The overall incidence of moderate to severe OSA (defined by an AHI of 15 or higher) occurring over a 5-year period is 11% and 5% in men and in women, respectively, which persists even after adjustment for confounding variables. This means that, even in the absence of any weight change, approximately 20% of men and 10% of women will develop moderate to severe OSA in that period of time.
Box 60-1
Epidemiologic Features of Obstructive Sleep Apnea (OSA)
• 24% of men and 9% of women have OSA.
• 3% to 7% men and 2% to 5% women have OSA syndrome.
• 65% of older men and 56% of older women have OSA.
• 20% of older men and 15% of older women have OSA syndrome.
• In postmenopausal status, women reach similar incidence rates of OSA than men.
• The overall 5-year incidence of moderate to severe OSA ranges between 5% and 11%.
• Referral to sleep centers for further investigation is four- to eight times more frequent in men than in women.
Most population-based studies have found a two- to three-fold higher prevalence of OSA in males than in females. The ratio is even higher for men treated in sleep centers, with reported ratios between 4 : 1 and 8 : 1 or higher. This higher ratio may be the result of multiple factors: Women do not show the “classic” OSA symptomatology—they typically have more comorbid illnesses, use more psychoactive drugs in the absence of a correct diagnosis, and often present with vague, nonspecific symptoms, which widens the differential diagnosis and leads to a higher level of underdiagnosis or misdiagnosis of OSA. An important finding from epidemiologic studies is that gender disparities in prevalence seem to decrease with age, and when women reach postmenopausal status (and are not receiving hormonal replacement treatment), incidence rates for men and women become similar. Table 60-1 summarizes the most important sleep apnea prevalence studies in middle-aged populations.
Elderly Population
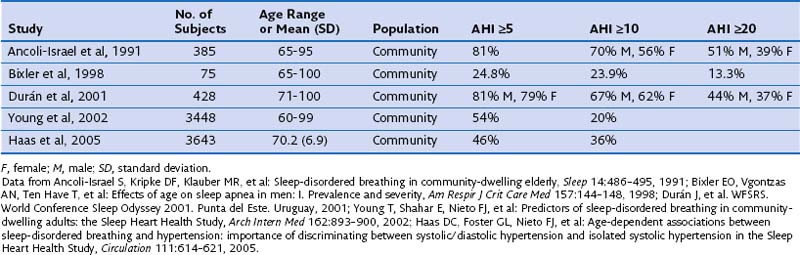
In any case, the two proposed types of OSA consist of (1) a pathologic form of OSA that appears in middle age in those patients who usually are diagnosed in sleep laboratories and (2) OSA that appears after the age of 60 years, with some overlap between the two, mainly caused by physiologic changes (increase in pharyngeal collapsibility) associated with aging, and of less clinical importance. Data also suggest that the interaction between body weight and OSA in elderly persons may be different from that in younger adults. Because of the population-wide increase in longevity, the proportion of elderly persons being treated at sleep units also is increasing; currently one in four sleep studies are performed in patients older than 65 years of age. This scenario will present a scientific challenge in the future, in view of the lack of scientific evidence available on OSA in the elderly. Table 60-2 summarizes the most important epidemiologic studies describing the prevalence of OSA in older people.
Risk Factors for Obstructive Sleep Apnea
Other Predisposing Factors
Box 60-2 shows other predisposing factors that have been associated with increased risk for OSA. Their mechanisms of action are multiple: anatomic variability (e.g., craniofacial malformations), increased airway inflammation (smoking or infections), physiologic factors (sleep position, REM sleep, pregnancy, or ethnicity-related predilection), and depression of dilator pharyngeal muscle function (alcohol consumption, benzodiazepine or sedative use, stroke, and other comorbid conditions).
Pathophysiology
OSA is characterized by repetitive collapse of the upper airway during sleep. The most frequent mechanism of collapse is that in which gravity pulls the tongue toward the back wall of the pharynx in a person who has assumed the supine position. Airway resistance increases during collapse, resulting in increased ventilatory effort, intrathoracic pressure swings, and disruption of sleep (arousals). The activation of the upper airway dilator muscles during arousals causes reopening of the airway and restores ventilation (Figure 60-1).
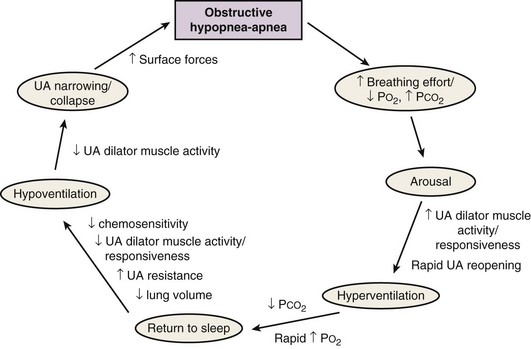
Figure 60-1 Typical pathophysiologic sequence in obstructive sleep apnea.
(From Eckert DJ, Malhotra A: Pathophysiology of adult obstructive sleep apnea, Proc Am Thorac Soc 5:144–153, 2008.)
Upper Airway in Normal Subjects
It has long been recognized that, for what on casual consideration may appear to be a simple tube, the human upper airway is deceptively and enigmatically complex, performing functional tasks such as swallowing and the passage of air for breathing and speech, governed by anatomy and neural controls. Under normal physiologic conditions, the pharynx remains open at all times, except during short closures associated with swallowing, regurgitation, and speech. It is separated into three regions: the nasopharynx, extending from the nasal turbinates to the hard palate; the oropharynx, composed of the retropalatal region (from the hard palate to the caudal margin of the soft palate) and the retroglossal region (from the caudal margin of the soft palate to the base of the epiglottis); and the hypopharynx, extending from the base of the tongue to the larynx. It is composed of more than 20 muscles and soft tissues without rigid or bony support. Pharyngeal muscles can be divided into three groups: muscles controlling the position of the hyoid bone (geniohyoid and sternohyoid), muscles of the tongue (genioglossus), and palatal muscles (palatopharyngeus, tensor palatini, and levator palatini). The largest of the dilator pharyngeal muscles is the genioglossus, the muscle that forms the major portion of the body of the tongue (Figure 60-2).
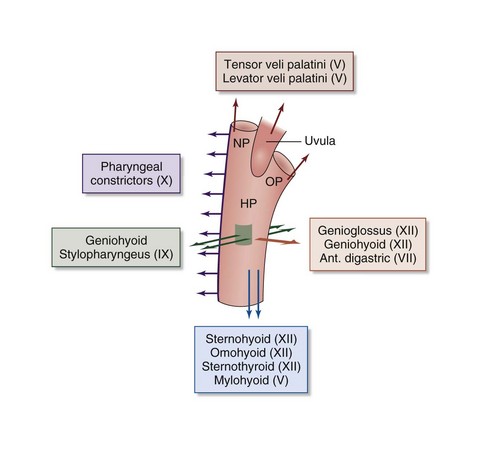
Figure 60-2 Action of upper airway muscles. Ant., anterior, HP, hypopharynx; NP, nasopharynx; OP, oropharynx.
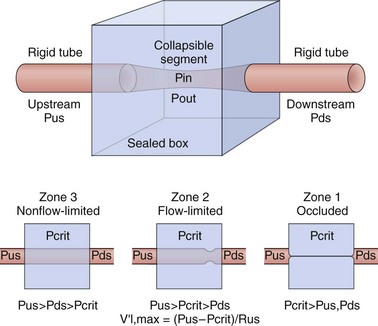
One mechanistic physiologic representation of this collapsible model is the Starling resistor (Figure 60-3). According to this model, when air pressure is applied to the upper airway, the collapsible portion of the upper airway is bound by an upstream segment (nasal segment) and a downstream segment (tracheal segment). Nasal and tracheal segments have their corresponding intraluminal pressures and resistances. The nasal segment has atmospheric pressure at the airway opening. In the surrounding collapsible segment, pressure is generated by soft tissues and structures surrounding the pharynx. Occlusion of the collapsible portions occurs when the surrounding pressure (Pout) becomes greater than the intraluminal pressure (Pin) (transmural pressure [Pout − Pin] above 0).
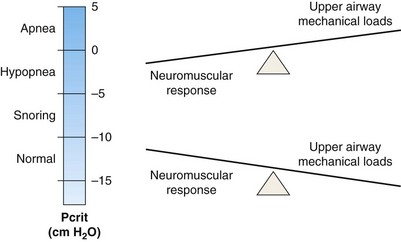
The oropharynx represents the collapsible segment, the critical closing pressure (Pcrit) of which is defined as the pressure inside the airway at which airway collapse occurs. Flow is partially limited when nasal pressure is higher than Pcrit, Pcrit being higher than tracheal pressure, and total occlusion occurs when Pcrit is greater even than nasal pressure. Pcrit in the human upper airway is determined by lowering the nasal pressure until inspiratory airflow ceases. Its measurements have been shown to define a spectrum of upper airway obstruction ranging from normal breathing to total collapse (apnea), depending on the equilibrium between mechanical upper airway loads that provoke collapse and neuromuscular dilator functions that maintain upper airway patency. In general, these measures when applied in sleeping or paralyzed humans have shown that passive Pcrit is below −10 cm H2O in normal subjects with lower airway resistance and minimal CO2 retention during non-REM sleep. In sleep-disordered breathing, however, it varies, with reported values of −10 to −5 cm H2O in snorers, −5 to 0 cm H2O in those with sufficiently high airway resistance to induce airflow limitation and transient hypopneas, and above 0 cm H2O in patients with apneas associated with complete airway obstruction (Figure 60-4). Increases in airway resistance or hypercapnia provoke the activation of the muscle through neural control pathways composed of pressure receptors, sleep-wake brain centers, chemoreceptors, and respiratory centers.
Upper Airway in Patients With Obstructive Sleep Apnea
The primary site of collapse is in the retropalatal and oropharyngeal area in a majority of patients (up to 75%), with frequent caudal extension to the base of the tongue (25% to 44%) and, less often, to the hypopharynx (0 to 33%). The site of collapse depends on the equilibrium of functions exercised by the skeletal structure, soft tissue, and pharyngeal muscles. In obese patients, the most frequent area of collapse evidently is the velopharynx, whereas in nonobese patients, both the velopharynx and the oropharynx constitute the primary site of collapse. The extent of collapse varies by sleep stage, with caudal collapse being more extensive in REM sleep owing to changes in neuromuscular activation (Box 60-3).
Box 60-3
Factors Contributing to Upper Airway Collapse in Obstructive Sleep Apnea
• Reduction in upper airway caliber by anatomic factors
• Reduction in upper airway dilator muscle activity
• Ventilatory control instability
• Decrease in lung volume during sleep
• Premature arousals during a respiratory event
• Edema and rostral fluid shifts
• Increase in respiratory events during REM sleep
• Reduction in intraluminal pressure due to Bernoulli effect
PathogenEsis
Anatomic Factors
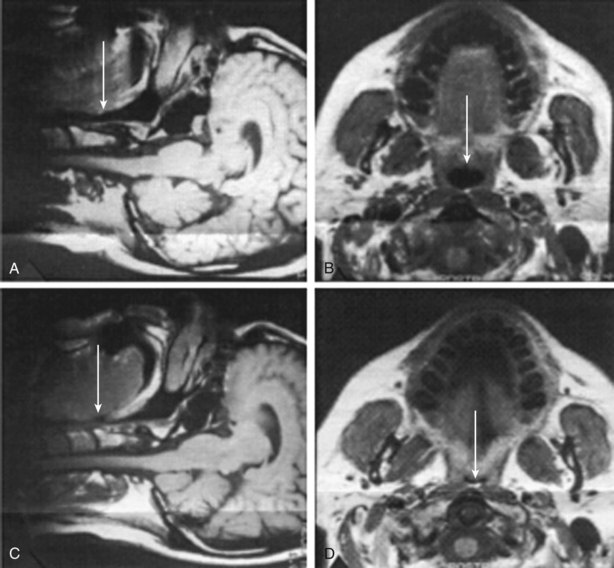
Anatomic factors include increased airway length, lateral wall thickness, tongue volume (macroglossia), skeletal structure (retrognathia or micrognathia), and nasal or pharyngeal abnormalities (nasal obstruction, polyps, or enlarged tonsils, adenoids, or uvula) and probably constitute the most important heritable determinants of OSA. Studies of cephalometric and computed tomography scans have demonstrated differences in the craniofacial structure of patients with OSA in comparison with that of normal subjects, including lower facial weight, retroposition of both the maxilla and the mandible, and shorter and medially displaced mandibular rami, all of them correlated with decreased pharyngeal size. The role of enlarged soft tissues has been demonstrated using MRI (Figure 60-5). In patients with OSA, pharyngeal lumen is smaller than normal and tissues are narrowed laterally, rather than in the anteroposterior dimension, especially at the retropalatal level, mainly owing to increased thickness of the muscular pharyngeal wall as well as an increase in tongue and total soft tissue volume. The interaction between the bony and soft tissue structures determines upper airway collapsibility. Nevertheless, increased airway length has been observed in subjects with OSA and has been shown to correlate with severity, especially in men. Usually, the longer the upper airway, the greater the risk of collapse.
Controversies and Pitfalls
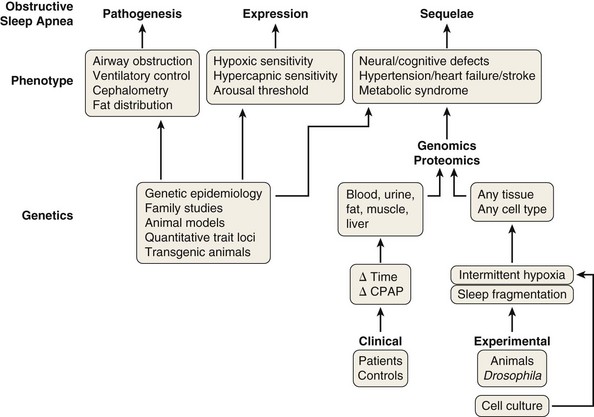
One of the most controversial issues in the epidemiologic approach to OSA is the difficulty of comparing the results of different studies, because they may use various methodologies and different definitions for the syndrome. Agreement is required on the definition of OSA and the type and applications of diagnostic devices, as well as a global consensus on the definition of hypopnea and what symptoms define OSA, not only in middle-aged men but also in women and elderly persons. More research should be dedicated to specific issues in these two populations: distinguishing between physiologic and pathologic situations in the number and type of respiratory events during sleep in the elderly and, in addition, the clinical presentation of OSA in women, which usually is different from the classical form. On the other hand, although general risk factors for OSA are fairly well known, an additional aspect merits further attention: the analysis of the genomic, proteomic, and metabolomic (metabolite-associated) aspects of the disease. Obstructive events during sleep result in changes in molecular processes that can be assessed, providing a potential molecular signature for the presence and consequences of OSA. Heterogeneity in biologic responses and their changes over time probably explain why some patients with OSA develop hypertension or experience more sleepiness than others, or why some affected persons suffer marked sleep fragmentation and others exhibit more intermittent hypoxia and its consequences. It is possible that developing and validating genetic and molecular signatures for OSA will provide not only diagnostic but also prognostic information, making the goal of personalized sleep medicine a reality in the near future (Figure 60-6).
Amardottir ES, Mackiewicz M, Gislason T, et al. Molecular signatures of obstructive sleep apnea in adults. A review and perspective. Sleep. 2009;32:447–470.
Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112.
Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–153.
Gaudette E, Kimoff RJ. Pathophysiology of OSA. Eur Respir Mon. 2010;50:31–50.
Larkin EK, Patel SR, Goodloe RJ, et al. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am J Respir Crit Care Med. 2010;182:947–953.
Launois SH, Pepin JL, Levy P. Sleep apnea in the elderly: a specific entity? Sleep Med Rev. 2007;11:87–97.
Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12:481–496.
Lindberg E. Epidemiology of OSA. Eur Respir Mon. 2010;50:51–68.
Patil SP, Schneider H, Schwartz AR, et al. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325–337.
Polotsky VY, O’Donnell CP. Genomics of sleep-disordered breathing. Proc Am Thorac Soc. 2007;4:121–126.
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143.