Chapter 61 Obstructive Sleep Apnea
Clinical Features, Diagnosis, and Treatment*
Clinical Features
The clinical presentations of obstructive sleep apnea (OSA) are legion. Common clinical features can be divided into major and minor categories, as summarized in Box 61-1. Patients may present with daytime and/or nocturnal complaints, but commonly, spouses and living companions are the first to push for medical attention.
Excessive Daytime Sleepiness
Sleepiness is difficult to define and is a subjective feeling of impairment of concentration and increased craving for sleep. Excessive daytime sleepiness (EDS) is a nonphysiologic complaint that is not satisfied by a restorative sleep. Sleepiness is reported by 30% to 50% of the general population, so EDS alone is a poor predictor of OSA and requires a differential diagnosis to exclude many other medical conditions associated with this daytime symptom, such as depression, fibromyalgia, chronic insomnia, or hypothyroidism. Patients with OSA sometimes refer to EDS as abnormal daytime tiredness or lack of energy and convey the impression that they must make stringent efforts to remain alert and awake. It is believed that the loss of the restorative function of sleep due to repetitive arousals is the main mechanism to explain the presence of EDS in OSA. The Epworth Sleepiness Scale (Box 61-2) is the most popular tool for subjective evaluation (self-assessment) of EDS. A score above 10 of 24 is considered to be clinically relevant. The relationship between the Epworth score and the apnea-hypopnea index (AHI) (as a surrogate index of OSA severity) is relatively poor. In fact, 35% patients with severe OSA identified within sleep clinics do not complain of EDS. This discrepancy is partly due to underestimation or intentional underreporting the severity of sleepiness for personal gain, such as to avoid job loss. On the other hand, patients scoring high in the Epworth scale could have just mild to moderate OSA. EDS is the most disabling symptom among patients with OSA. One example is drowsiness while driving, which is associated with a three-fold increase in risk of accidents in patients with OSA. Poor school or job performance also is frequently reported.
Box 61-2 Epworth Sleepiness Scale
Patient Questionnaire
Sitting and reading __________
Sitting, inactive in a public place (e.g., a theater or a meeting) __________As a passenger in a car for an hour without a break __________
Lying down to rest in the afternoon when circumstances permit __________
Sitting and talking to someone __________
Sitting quietly after lunch without alcohol __________
In a car, while stopped for a few minutes in traffic __________
From Johns MW: A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale, Sleep 14:540–545, 1991.
Physical Examination 
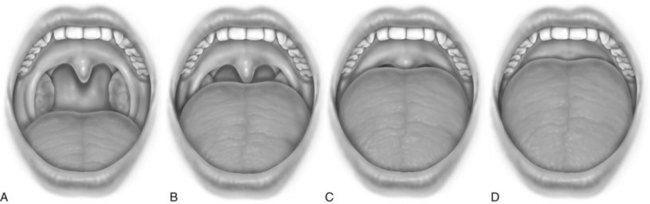
After a complete history has been obtained, a systematic examination should be performed. In nonobese patients (body mass index [BMI] less than 30 kg/m2), common physical findings can be observed in the craniofacial, nasal, pharyngeal, and dental areas (Box 61-3). Radiographic cephalometry is indicated if craniofacial abnormalities are suspected or if upper airway surgery is planned to treat the patient. The oropharynx must be examined, and the degree of oropharyngeal crowding can be scored using the Friedman Tongue Position, formerly called the modified Mallampati score. For this assessment, the patient is asked to open the mouth widely without protruding the tongue. The observer can assign scores as indicated in Figure 61-1. Higher scores suggest the presence of OSA.
Diagnosis
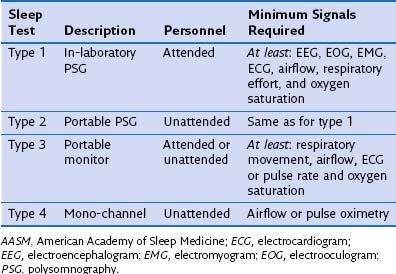
Once the diagnosis of OSA is suspected from the clinical or laboratory features, the presence and severity of the sleep-disordered breathing are confirmed and assessed by a sleep study. Patients should be referred for a sleep study as soon as possible if they have problems with work or driving because of daytime sleepiness. The American Academy of Sleep Medicine (AASM) classified four different levels of sleep testing, as summarized in Table 61-1). Inpatient sleep laboratory polysomnography (PSG) remains the “gold standard” modality for the diagnosis of OSA and the initiation of CPAP treatment. Nevertheless, the American Medicare Service recently has turned from full-night attended PSG to portable polygraphy to establish the need for CPAP as a reimbursable therapy for OSA.
Polysomnography
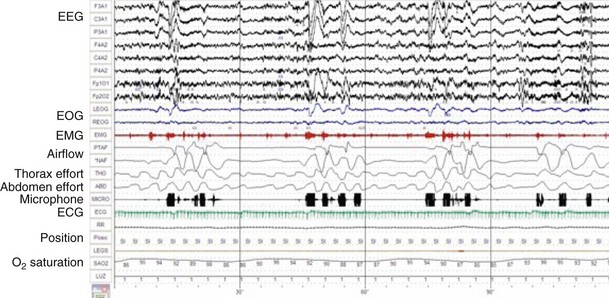
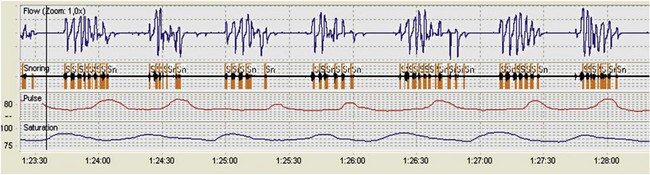
Polysomnography records neurophysiologic and cardiorespiratory signals, to assess sleep stage. Monitoring includes electroencephalography (EEG), electromyography (EMG) of the anterior tibialis, electrocardiography (ECG), oronasal airflow, snoring, thoracic, and abdominal movements, pulse oximetry, and body position (Figure 61-2). The PSG study must be performed in sleep laboratories by trained technicians and is therefore costly and time-consuming but provides the physician with information on both breathing- and non–breathing-related sleep disorders. PSG should be the preferred diagnostic sleep test for snorers with a lower likelihood of OSA, patients with EDS that could be caused by other non–breathing-related disorders during sleep (e.g., narcolepsy, restless legs syndrome), and patients with other medical comorbid conditions (e.g., neuromuscular, cardiac, and respiratory disorders). Apneas and hypopneas are identified, and the number of events per hour of sleep—the apnea-hypopnea index (AHI)—is determined. An AHI of less than 5 is normal. The severity of OSA is classified according to the AHI as mild (5 to 15 events/hour), moderate (15 to 30 events/hour), or severe (more than 30 events/hour). This disease severity classification is correlated with fatal and nonfatal cardiovascular outcomes.
Unattended Sleep Studies
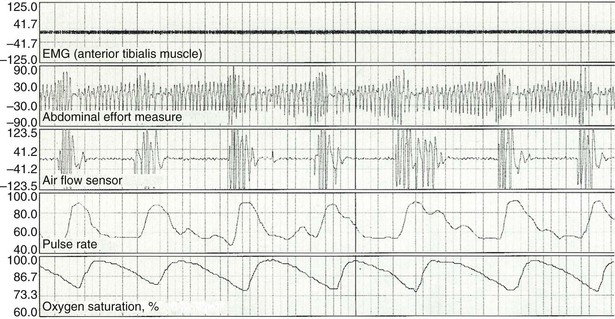
For hospitalized patients or for persons unable to attend a sleep laboratory, PSG can be performed as an unattended study (type 2 study of the AASM classification). Indications and technical requirements are the same as for type 1 (in-laboratory PSG). Type 3 patient monitors are increasingly used owing to the limited number and capacity of sleep laboratories, as well as the increasing number of persons suspected of having OSA. These devices do not monitor EEG and sleep stages but should record the minimum airflow, respiratory effort, and O2 saturation (Figure 61-3). They cannot evaluate sleep time or detect respiratory event-related arousals. As a result, they tend to underestimate the AHI. On the other hand, for most patients, home sleep studies offer greater convenience, and the study conditions allow close replication of the usual nighttime sleep pattern. The diagnosis of OSA is confirmed and severity determined using the same criteria as for PSG. Type 4 home monitors record continuous single or dual signals during sleep, including O2 saturation and/or airflow (Figure 61-4). Baseline values for SaO2, mean SaO2, nadir SaO2, and O2 desaturation index (number of episodes of desaturation greater than 3% as divided by recording time) can be collected very easily using home oximetry. Airflow recorders also assess the frequency of apneas and hypopneas, but differentiation between obstructive and central or mixed apneas cannot be achieved.
Diagnosis
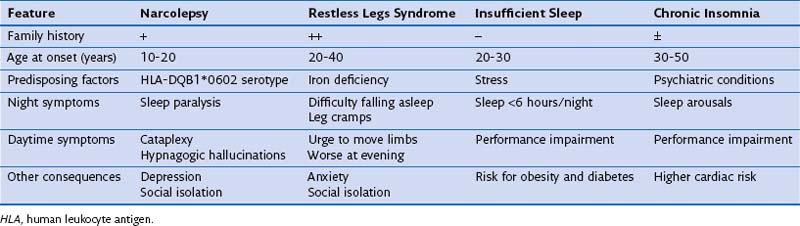
After snoring, EDS is the most common presenting complaint in persons with OSA. Bed partners should be asked about the usual pattern of the patient’s sleep, and the physician must consider other causes of EDS in the differential diagnosis (Table 61-2). Apart from OSA, sleep can be interrupted by many other medical conditions, all causing EDS. In particular, patients with asthma and COPD frequently wake up owing to increased nocturnal respiratory symptoms. Heart failure and gastroesophageal reflux must be ruled out as potential causes of poor sleep quality and EDS. As noted, a drug history to identify medications that can cause EDS (e.g., psychiatric drugs, antihistamines, beta blockers) also should be part of a complete evaluation of all patients reporting EDS.
Treatment
General Measures
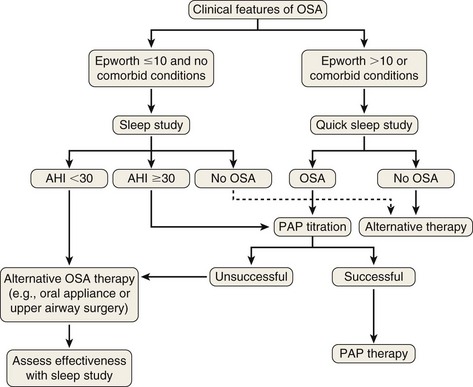
Treatment options should be offered in the context of the severity of the patient’s OSA, risk factors, associated comorbid conditions, and personal wishes. A flow diagram outlining a treatment algorithm is shown in Figure 61-5. All affected patients should avoid alcohol and sedatives, because these agents reduce upper airway muscle tone and increase severity of OSA.
Surgical Treatment
1. As a primary therapy for patients with mild OSA who have severe obstructive anatomy that is surgically correctible
2. As secondary treatment for OSA when CPAP or use of an oral appliance (OA) is poorly tolerated or contraindicated or provides inadequate correction or benefit
3. As an adjunctive therapy when obstructive anatomy or functional deficiency compromises other therapies, or to improve tolerance of other OSA treatment
Oral Appliances
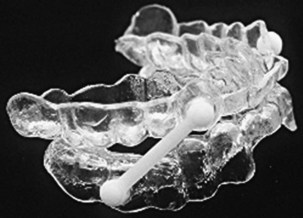
The aim with use of OAs is to increase upper airway patency and reduce its collapsibility by improving muscle tone. Most OA devices cover upper and lower teeth and hold the mandible in an advanced position with respect to the resting position. This positioning, in turn, pulls the tongue forward, making pharyngeal collapse with consequent obstruction much less likely to happen. An OA ideally should be custom-made for the patient after a dental consultation (Figure 61-6); when created using this approach, the device is much more likely to be beneficial.
Positive Airway Pressure
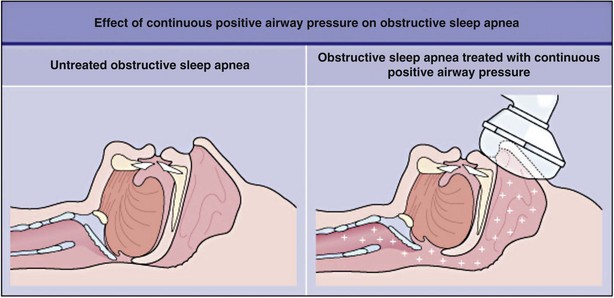
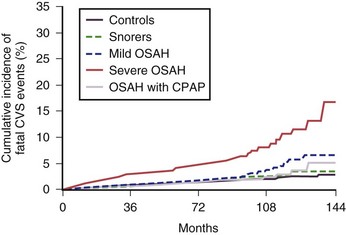
Positive airway pressure (PAP) currently is the management modality of choice for patients with OSA. PAP works by providing pneumatic splinting of the upper airway (Figure 61-7). In randomized trials, PAP delivered in continuous mode (i.e., CPAP) has been shown to improve quality of sleep, relieve daytime sleepiness, support maintenance and recovery of neurocognitive and driving abilities, decrease arterial hypertension, and help maximize quality of life in patients with OSA. It also reduces the frequency of cardiovascular events as long-term outcomes in prospective observational studies (Figure 61-8).
Controversies and Pitfalls
Centers for Medicare and Medicaid Services Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). Updated August 28, 2008 (online article) www.cms.gov accessed on March 28, 2012
Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academy Press, 2006.
Cross M, Vennelle M, Engleman HM, et al. Comparison of CPAP titration at home or the sleep laboratory in the sleep apnea hypopnea syndrome. Sleep. 2006;29:1451–1455.
Deegan PC, McNicholas WT. Predictive value of clinical features for the obstructive sleep apnea syndrome. Eur Respir J. 1996;9:117–124.
Douglas NJ. Home diagnosis of the obstructive sleep apnoea/hypopnoea syndrome. Sleep Med Rev. 2003;7:53–59.
Epstein LJ, Kristo D, Strollo P, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276.
Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea/hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053.
Pack AI, Platt AB, Pien GW. Does untreated obstructive sleep apnea lead to death? Sleep. 2008;31:1067–1068.
Sundaram S, Bridgman SA, Lim J, Lasserson TJ. Surgery for obstructive sleep apnea. Cochrane Database Syst Rev. 4, 2005. CD001004
Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041.