Obstructive Pulmonary Disease and Ventilatory Management
I Obstructive Pulmonary Diseases Are Characterized by Airflow Limitation
II Specific Diseases in This Category Include:
III In Obstructive Pulmonary Disease Airflow Limitation Results from:
C Ventilatory muscle dysfunction
D These are the major concerns affecting the approach and ease of providing ventilatory support to patients with obstructive pulmonary disease.
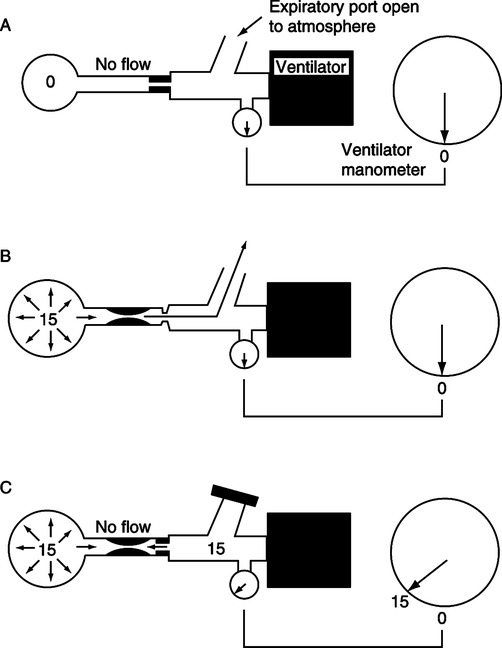
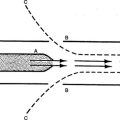
V Air Trapping (see Chapter 40) (Figure 21-1)
A The trapping of gas at end-exhalation in the lung parenchyma.
B This results in an increase in the functional residual capacity and an alteration in the end-expiratory position of the diaphragm.
C Air trapping results in the development of auto-positive end-expiratory pressure (PEEP).
D The level of auto-PEEP is determined by the tidal volume, respiratory system compliance, airway resistance, and expiratory time:
< ?xml:namespace prefix = "mml" />
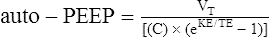 (1)
(1)E In obstructive lung disease auto-PEEP develops because of:
1. Increased compliance: All diseases except asthma
2. Increased airway resistance: All diseases but especially asthma
F With most obstructive diseases there is instability of the small airways. As a result these airways dilate during inspiration but narrow during expiration.
G This is referred to as dynamic airway obstruction.
H In most obstructive lung disease it is the combination of dynamic airway obstruction and increased respiratory system compliance that accounts for the development of auto-PEEP.
1. Auto-PEEP caused by this mechanism is usually not increased by the addition of applied PEEP until the applied PEEP level is ≥80% of the auto-PEEP level.
2. To trigger an assisted breath the patient must first decompress the auto-PEEP.
3. Appling PEEP in the presence of dynamic airway obstruction (see Figure 21-1) decreases the pressure threshold needed to trigger the ventilator.
I In asthma the limitation of airway flow is a result of increased fixed airway resistance caused by bronchospasm and edema.
1. In this setting auto-PEEP level is not beneficially affected by the application of PEEP.
2. For patients with asthma the application of PEEP usually does not offset the auto-PEEP as in dynamic airway obstruction. For those with asthma applied PEEP is normally additive to auto-PEEP.
J Beyond altered lung mechanics, minute ventilation has the greatest overall effect on the level of air trapping and auto-PEEP.
1. The greater the minute ventilation, the greater the auto-PEEP.
2. The lower the minute ventilation, the lower the auto-PEEP.
K Refer to Chapter 40 for a full discussion on identification of auto-PEEP level.
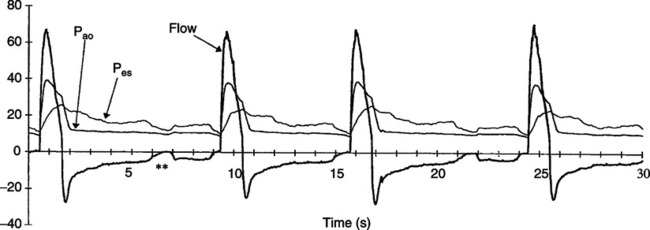
1. As noted in Figure 21-2 for spontaneously breathing patients triggering the ventilator, auto-PEEP is most commonly observed by a difference in the patients’ ventilatory rate and the ventilator response rate.
2. If the patient’s rate is higher than the ventilator response rate it is almost ensured that the cause is auto-PEEP.
3. To offset the effect of auto-PEEP on patient triggering (in dynamic airway obstruction) PEEP should be slowly applied in 1- to 2-cm H2O steps until every patient effort triggers the ventilator.
4. In some patients this may require applied PEEP as high as 15 cm H2O.
VI Increased Work of Breathing
A The biggest adverse impact of auto-PEEP for patients with obstructive lung disease is an increase in work of breathing.
B As discussed previously if a patient must decompress the auto-PEEP level to inspire, work of breathing increases. For example:
| 0.0 cm H2O | |
| 10.0 cm H2O | |
| 10.0 cm H2O | |
| 3.0 cm H2O | |
| 13.0 cm H2O |

C In patients with severe obstructive lung disease with air trapping and auto-PEEP, the diaphragm is flattened. This prevents its contraction from increasing the anterior-posterior diameter of the thorax and laterally expanding the lower rib cage. This frequently results in paradoxical breathing:
VII The Overall Indications for Ventilation in Patients With Chronic Pulmonary Disease Are:
VIII Noninvasive Positive Pressure Ventilation
For all patients with obstructive pulmonary disease requiring ventilatory support, except those with asthma, noninvasive positive pressure ventilation (NPPV) should be the first ventilatory option (see Chapter 43).
A The data clearly indicate that the use of NPPV in these patients results in:
1. A decreased need for intubation
2. Decreased length of mechanical ventilation
3. Decreased length of intensive care unit (ICU) stay
4. Decreased rate of nosocomial pneumonia and other infections
B NPPV should be applied with a ventilator that allows assessment of patient-ventilator synchrony; waveforms should be available (Box 21-1).
C Initially an oronasal mask should be used.
D Mode of ventilation should be pressure support or pressure assist/control (BIPAP).
E Peak airway pressure should not exceed 20 cm H2O to avoid gastric distention.
F PEEP should be set to minimize the effort to trigger inspiration as a result of auto-PEEP: approximately 3 to 10 cm H2O.
G Ventilatory pressures do not need to be elevated; for most patients a ventilating pressure (pressure support level) of 8 to 12 cm H2O is adequate to produce an appropriate Vt.
H Vt should only be approximately 4 to 8 ml/kg predicted body weight; excessive Vt increases dysynchrony.
I Respiratory rate should be up to the patient. Back-up rate should be set at approximately 8 to 10 breaths/min.
J Inspiratory time should be equal to the patient’s neuroinspiratory time, usually 0.6 to 1.0 second.
IX Invasive Ventilation of Obstructive Lung Disease (Box 21-2)
A Because of respiratory muscle dysfunction and increased work of breathing, assist/control and pressure support are the modes of choice.
B Volume or pressure targeting can be used, but pressure ventilation is preferred because it better ensures that gas delivery matches ventilatory demand.
C Vt is targeted or set at approximately 8 to 10 ml/kg provided plateau pressure is ≤25 cm H2O.
D If plateau pressure is >25 cm H2O, tidal volume should be approximately 6 to 8 ml/kg predicted body weight (PBW).
E PEEP is set to offset the effects of auto-PEEP and ensure all patient inspiratory efforts trigger an assisted breath, usually 5 to 10 cm H2O.
F Inspiratory time should be equal to the patient’s neuroinspiratory time. The more stressed the patient, the shorter the inspiratory time, usually 0.6 to 1.0 second.
G The inspiration-to-expiration (I:E) ratio is not set, but it should be as low as tolerated. The expiratory time should be lengthy to avoid air trapping, usually ≤1:3.
H The flow waveform in volume ventilation should be decelerating with a peak flow ≥60 liters per minute (LPM) to ensure that initial gas delivery will meet the patient’s inspiratory demand.
I FIO2 is set to ensure pao2 is between approximately 55 and 75 mm Hg. This usually requires an FIO2 ≤0.5.
J Respiratory rate is usually patient determined. However, many of these patients, after minor sedation, allow the ventilator to control rate. A back-up rate of 10 to 16 breaths/min generally maintains adequate ventilation.
K Adequate ventilation is a Paco2 equal to preventilation baseline, which may be 60 to 80 mm Hg. The key here is to maintain the pH at baseline values usually ≥7.30.
L Patients with bronchitis, emphysema, and cystic fibrosis are generally ventilated the same way as patients with chronic obstructive pulmonary disease (COPD).
X Ventilatory Management of Asthma (Box 21-3)
A All patients with severe asthma requiring ventilatory support develop air trapping and auto-PEEP.
B The air trapping occurs because of increased fixed airway resistance as a result of bronchospasm and edema. The accumulation of a large amount of secretions also causes a ball-valve obstruction, increasing air trapping.
C This high level of auto-PEEP coupled with an increased drive to breathe results in large intrathoracic pressure swings producing marked pulsus paradoxus.
D Mechanical ventilation is indicated when the asthmatic patient can no longer maintain adequate gas exchange.
1. This point is difficult to determine.
2. Because most patients are young, they can work to maintain a Paco2 ≤40 mm Hg until they are exhausted.
3. But once exhausted their Paco2 can increase rapidly.
4. As a result once Paco2 increases to >45 to 50 mm Hg and acidosis begins to develop, ventilatory support is indicated.
E Heliox: For many patients the administration of heliox (see Chapter 34) can markedly reduce the effort to breathe.
1. Unfortunately this does not occur for all patients.
2. At this time there is no method to determine who can be successfully treated with heliox and who cannot.
F NPPV has been used for some patients with severe asthma.
1. We have not found NPPV useful.
2. Patients alert and oriented and not sedated with severe asthma have a difficult time tolerating a tight-fitting mask.
G Two major concerns exist when initially ventilating a patient with severe asthma.
H Patients with asthma requiring intubation are markedly difficult to ventilate because of high airway resistance.
1. Large driving pressures are needed to move airflow past the obstruction.
2. Initially the only mode that can ventilate these patients is volume assist/control (VA/C).
3. In VA/C a high driving pressure can be achieved, but because of a low Vt, plateau pressures can still be maintained at <30 cm H2O.
4. Peak pressure may need to be 60 to 80 cm H2O to deliver a Vt of 4 ml/kg PBW ina1.5-second inspiratory time and still maintain a plateau pressure of <30 cm H2O.
5. Although a high peak pressure is not desirable, there is no evidence that it causes lung injury because it only represents the pressure driving gas to the obstruction, not the pressure affecting the peripheral lung.
6. Plateau pressure represents the peak alveolar distending pressure, which has been associated with marked alveolar damage.
7. Once reversal of some of the acute obstruction occurs, the mode can be charged to pressure assist/control (PA/C).
8. With PA/C peak pressure should be kept ≤30 cm H2O because with PA/C peak pressure is essentially equal to peak alveolar distending pressure.
9. The change from VA/C to PA/C should only occur when a peak pressure of ≤30 cm H2O in PA/C can deliver the desired tidal volume.
I Most patients with severe asthma require heavy sedation, and some patients also need paralysis to effectively provide mechanical ventilation.
1. During the initial phase of ventilation in those with severe asthma, spontaneously breathing patients fight the ventilator.
2. After initial stabilization, the least sedation that ensures adequate ventilation is indicated.
J As already stated Vt is set low, 4 to 8 ml/kg PBW.
K The actual Vt setting depends on the plateau pressure, which should be ≤30 cm H2O.
L Inspiratory time: For patients with severe asthma, time is needed to allow gas to enter the lung past obstructions. Inspiratory times ideally should be approximately 1 to 1.5 seconds. With shorter times effective ventilation may not be possible.
M Auto-PEEP is a major problem and more difficult to manage than in patients with COPD.
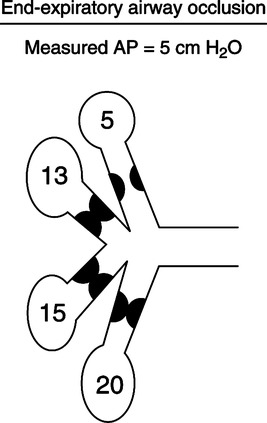
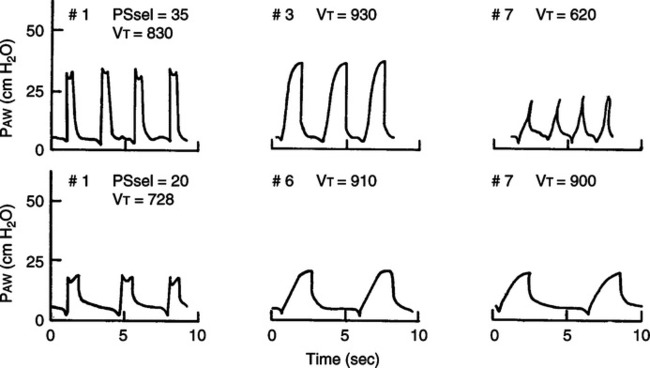
1. As illustrated in Figure 21-3 auto-PEEP cannot be effectively measured in those with severe asthma.
2. Obstruction is fixed and near complete in some airways; as a result at end exhalation some airways are completely obstructed, preventing communication of pressure with central airways.
3. As a result the auto-PEEP behind these obstructed areas is not included in the measurement of auto-PEEP.
4. This has been referred to as occult auto-PEEP.
5. When the measured auto-PEEP level is 8 cm H2O, the actual auto-PEEP level may be 15 cm H2O.
6. For patients with severe asthma it is better to monitor plateau pressure change as a reflection of changing auto-PEEP than auto-PEEP itself.
N Ideal respiratory rate for a given patient may vary widely in those with severe asthma.
1. It is difficult to identity a specific rate that results in the best ventilation and least auto-PEEP for all patients.
2. In some a low rate with a higher Vt results in the least auto-PEEP and best Pco2.
3. In others a higher rate with a smaller Vt results in the least auto-PEEP and best Pco2.
4. Ideal rates across patients can vary between approximately 8 and 20 breaths/min.
O Applied PEEP in most patients with severe asthma does increase the level of total PEEP (auto-PEEP + applied PEEP).
1. As a result, >5 cm H2O applied PEEP is not recommended.
2. If 5 cm H2O applied PEEP increases the plateau pressure it should be removed.
3. However, there are some asthmatic patients that do respond similarly to COPD patients. The application of applied PEEP decreases the measured auto-PEEP, but these are rare patients.
P Permissive hypercapnia: The first patients to experience permissive hypercapnia were asthmatic patients.
1. Because these are usually young otherwise healthy patients they frequently can tolerate a pH <7.20, sometimes a pH of 7.00.
2. The dilemma is which is more detrimental: the acidosis caused by the hypercapnia or the high plateau pressure needed to better ventilate.
3. If the patient’s hemodynamic status tolerates the acidosis, plateau pressure should not be increased to correct it.
Q FIO2 is adjusted to maintain the Pao2 >60 mm Hg.
R There are some data that indicate ventilating patients with heliox mixtures decreases plateau pressure and improves Paco2.
1. This is poorly defined in the literature, but we have observed improvement in select patients.
2. Heliox concentration should be at least 50% helium.
3. If the patient cannot tolerate ≤50% oxygen, heliox is not indicated.
4. Few ventilators function properly with heliox because of its density. Internal monitoring systems cannot accurately determine volume.
5. This prevents some ventilators from functioning properly.
6. If heliox is used, careful monitoring of plateau pressure is essential.
7. The readout of Vt will always be incorrect, underestimating the delivered Vt.
XI Use of New Modes of Ventilation (see Chapter 39)
A There are no data to indicate that the newer modes of ventilation improve outcome in patients with either COPD or asthma.
B Especially with asthma, patients are usually sedated to apnea, with VA/C and PA/C the modes of choice.
C For COPD patients assisted ventilation is common, but again none of the new modes has demonstrated any benefit.
D However, many COPD patients are ventilated in pressure support. Adjustment of rise time and expiratory cycling criteria is helpful to improve patient-ventilator synchrony.
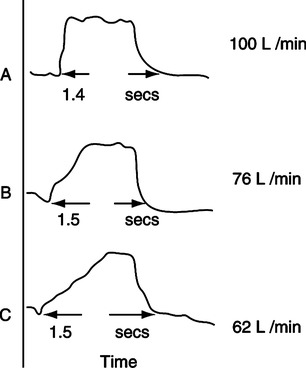
1. Rise time: Adjusts the slope of the pressure rise at the onset of pressure support (Figure 21-4).
2. The greater the patient’s demand, the higher (faster) should be the rise time setting.
3. Pressure should not exceed the set level at the onset of inspiration.
4. Pressure should not be concave at the onset of inspiration (Figure 21-5).
5. A more rapid rise time results in a greater initial flow rate.
1. This control varies the end-expiratory flow that terminates the pressure support breath.
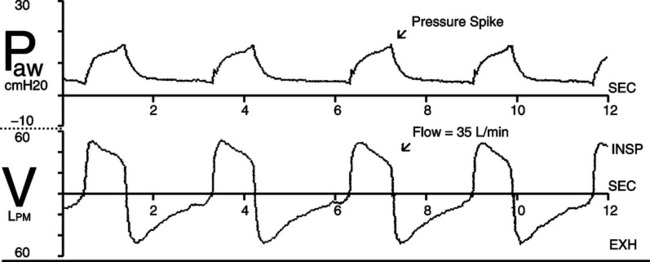
2. An increase in pressure above the set level (Figure 21-6) should not occur before exhalation in pressure support.
3. However, this problem is common in COPD patients if their end-expiratory flow rate is higher than the flow terminating the breath.
4. The expiratory cycling criteria should be increased (greater end-expiratory flow to cycle to exhalation) until no pressure spike is observed at end exhalation.
A COPD patients can be difficult to wean from ventilatory support.
1. With each exacerbation COPD patients’ baseline ventilatory function is more compromised.
2. The approach to weaning that has been shown to be most successful for COPD patients is a spontaneous breathing trial.
3. Most patients can successfully be evaluated by a T-piece trial.
4. However, those with high auto-PEEP levels may need spontaneous breathing evaluation while receiving 5 cm H2O continuous positive airway pressure.
5. Others with small endotracheal tubes or nasal intubation may need 5 cm H2O pressure support during evaluation of spontaneous breathing.
6. A small percentage of these patients will become ventilator dependent.
B Asthma patients do not well tolerate attempts at weaning.
1. Frequently when sedation is reversed the endotracheal tube itself can induce bronchospasm.
2. These patients once evaluated to be near baseline status can simply be extubated without a weaning trial.
3. As stated earlier patients are normally healthy and young, and once the severe asthma is reversed, they return to their baseline level.